Introduction
O-GlcNAcylation (O-GlcNAc) is the covalent
attachment of β-D-N-acetylglucosamine (GlcNAc) sugars to serine or
threonine residues of nuclear and cytoplasmic proteins. The
post-translational O-GlcNAc modification is reversible (1) and plays a critical role in regulating
a wide panel of cellular processes, such as apoptosis, cell stress
responses and signal transduction. O-GlcNAc transferase (OGT), an
enzyme that transfers GlcNAc from uridine diphosphate (UDP) to
serine/threonine residues of target proteins (2,3),
induced elevated expression of O-GlcNAc in tissues.
Since OGT and O-GlcNAcylation plays an important
role in normal biological process, aberrant regulation contributes
to the development of wide range of diseases, including cancer. OGT
and O-GlcNAcylation is elevated in breast cancer cell lines and
tissues, particularly in metastatic lymph nodes. Also it has been
demonstrated that O-GlcNAcylation could promote breast cancer
tumorigenesis and metastasis (4,5). In
colon and lung cancer, O-GlcNAcylation and OGT are also
upregulated, compared with that in the corresponding adjacent
tissues. Additionally, it demonstrated that O-GlcNAcylation
enhanced cell growth and invasion, and may play important roles in
lung and colon cancer formation and progression (6). In laryngeal cancer, OGT and
O-GlcNAcase (OGA) mRNA level was related to larger tumor size,
nodal metastases, higher grader and tumor size, their protein level
showed a trend of more advanced tumors to be more frequently OGT
and OGA positive, suggesting that O-GlcNAcylation may have an
effect on tumor aggressiveness (7).
Gastric cancer is the fourth most common cancer
worldwide and the second most frequent cause of cancer-related
death (8,9), with ~27% 5-year survival rate
(10). Although elevated OGT levels
were reported in various epithelial cancers, it remains unclear
whether OGT is upregulated and how OGT exerts its function in
gastric cancer. In the present study, we show that OGT mRNA levels
are upregulated in human gastric cancer compared with that in
adjacent tissues. Additionally, OGT function is analyzed. Silencing
OGT inhibits BCG-823 cell proliferation in vitro, and
reduces tumorigenicity in vivo. We demonstrated that OGT
silencing induces more cell apoptosis by increasing PUMA and
caspase-3 protein levels. Also, we screened the potential targets
of OGT using GBP array, our data suggest that silencing OGT
promotes apoptosis, which may also be through galectin and
hbgef.
Materials and methods
Sample
Human cancerous and adjacent normal parts of 7
gastric specimens were obtained from the First Hospital of China
Medical University as frozen tissues. All tissue specimens were
obtained with informed consent, and all investigations were
approved by the local Ethics Committee. Each sample was divided
into 2 parts, one for histopathological examination and the other
was stored at −80°C for protein extraction.
Cell culture
Gastric cancer cell line BCG-823 was maintained in
Dulbecco's modified Eagle's medium (DMEM; Invitrogen) supplemented
with 100 IU/ml penicillin and 100 µg/ml streptomycin, 2 mM
L-glutamine and 10% fetal bovine serum (FBS) in a 5% CO2
atmosphere at 37°C.
Knockdown (KD) of OGT by shRNA
shRNAs targeting OGT were synthesized and inserted
into pG-PU6/GFP/Neo vector by GenPharm Co. (Shanghai, China). A
scrambled sequence provided by GenPharm was used as a negative
control (shNC). The shRNA target sequence of OGT is GGATGCTTATATCA
ATTTAGG (4). The shRNA was
transfected into BGC-823 cells in 6-well culture plates using
Lipofectamine™ 2000 (Invitrogen). At 48 h after transfection, G418
was added and screened for 2 weeks. Then cells were lysed, qPCR and
western blotting were used to analyze the KD efficiency. The stable
cells were used for function analysis.
Reverse transcription (RT)-PCR and
quantitative real-time PCR (qRT-PCR)
Total RNA of tissues or cells was extracted using
the RNeasy Mini kit (Qiagen), according to the manufacturer's
protocol. For each RNA sample, 1 µg was reverse-transcribed
using a First Strand cDNA Synthesis kit (Invitrogen). Then, the
first-strand cDNA was used to amplify genes of interest with gene
specific primers. The number of PCR cycles was optimized for each
gene to ensure linear amplification. Gene-specific primers are as
follows: OGT forward, 5′-TTGCCTTCTGTGCATCCTCAT-3′ and OGT reverse,
5′-TATCCTACACGCAGCCGACC-3′; galectin-2 forward,
5′-TCTGTTCGGACAACTTCCTTCA-3′ and reverse,
5′-TTATTCTTTTAACTTGAAAGAGGA; HBEGF forward,
5′-CTCAGCCTTTTGCTTTGCTAAT-3′ and reverse,
5′-GGAACTCACTTTCCCTTGTGTC-3′; GAPDH forward,
5′-ATGGGGAAGGTGAAGGTCG-3′ and GAPDH reverse,
5′-GGGGTCATTGATGGCAACAATA-3′. RNA levels were normalized using
GAPDH.
The amplification program consisted of one cycle of
95°C for 10 min followed by 40 cycles of 95°C for 15 sec and 60°C
for 1 min. Relative quantity (RQ) of gene expression was normalized
to GAPDH and performed using the 2−ΔΔCt method.
Western blotting
Western blotting was performed as previously
described (11). Briefly, total
protein extracts of different cell lines were prepared using RIPA
buffer (Beyotime). Then, 20 µg of total proteins for each
sample was separated by SDS/PAGE (10% gels) and transferred to PVDF
membrane (Millipore). The membranes were blocked in 5% skimmed milk
in PBST for 2 h at room temperature. After blocking, the membrane
was probed with primary antibodies against the proteins of
interest. Finally, the proteins were further detected using the
horseradish peroxidase (HRP)-conjugated secondary antibody and
chemiluminescence HRP substrate kit (Millipore). The primary rabbit
anti-OGT and anti-O-GlcNAcylation were from Abcam. Caspase-3 and
PUMA were from Cell Signaling. Mouse anti-β-actin (clone 6G3) was
purchased from Tianjin Sungene.
Cell counting
Cells were treated with DMSO/OGT inhibitor
(Benzyl-2-acetamido-2-deoxy-α-D-galactopyranoside; Santa Cruz).
Cell number was measured with an automatic cell counter according
to the manufacturer's instructions. Briefly, cells were harvested
and suspended, and mixed with equal volume of 0.4% trypan blue.
Cell suspension (10 µl) was loaded onto TC20 system
(Bio-Rad) counting slides, and the number of viable cells was
quantified on a TC20 automated cell counter (Bio-Rad).
MTT assay
Cells (5×103) were seeded into a 96-well
culture plate and subsequently incubated with MTT reagent (0.5
mg/ml; Sigma) at 37°C for 2 h and MTT assay was then performed.
Xenograft
BALB/c nude mice (4 weeks old) were purchased from
the laboratory animal center of the Academy of Military Medical
Sciences. The maintenances and experimental animal procedures were
approved by the Animal Ethics Committee of China Medical
University. BALB/c nude mice (4 weeks old) were randomly assigned
to two groups (7 mice/group). BGC-823 cells (shOGT and shNC) were
trypsinized and resuspended at a final concentration of
1×107 cells/ml in phosphate-buffered saline (PBS). Then,
100 µl of cells were injected subcutaneously into the right
flank of the mice. Tumor growth was monitored twice every week and
recorded by measuring tumor length and width daily for 4 weeks, and
tumor volume was calculated using the formula: 1/2 (length ×
width2). After the experiments, mice were sacrificed and
tumors weighed.
Apoptosis analysis
Cell apoptosis was performed using the
allophycocyanin (APC)-Annexin V and 7-amino-actino-mycin D (7-AAD)
staining kit (Tianjin Sungene) following the manufacturer's
instructions. Briefly, stable cell line (shOGT and shNC) was plated
into a 6-well plate for 72 h with or without 5-fluorouracil (5-FU),
cells were then trypsinized and washed twice with ice-cold PBS and
then resuspended in binding buffer from the kit. APC-Annexin V and
7-AAD were added into the flow tube. Finally, flow cytometric
analysis was performed within 1 h using FACS Calibur (BD
Biosciences).
Glycan-binding protein (GBP) gene
microarray
Total RNA was extracted as described above. RNA
integrity was assessed by agarose gel electrophoresis and
spectrophotometric analysis. tcRNA was obtained by linear
amplification and labeled with Cy3/Cy5. Samples were hybridized to
the array. Data were analyzed by GenePix Pro 3.0. Differential
expression analysis was cut-off at fold-change of ±1.3. Heatmap was
produced using the R program.
Statistical analysis
Data are expressed as means ± SEM. Student's t-test
was used to evaluate the significance of differences between sample
means obtained from three independent experiments. Statistical
significance was defined as P<0.05.
Results
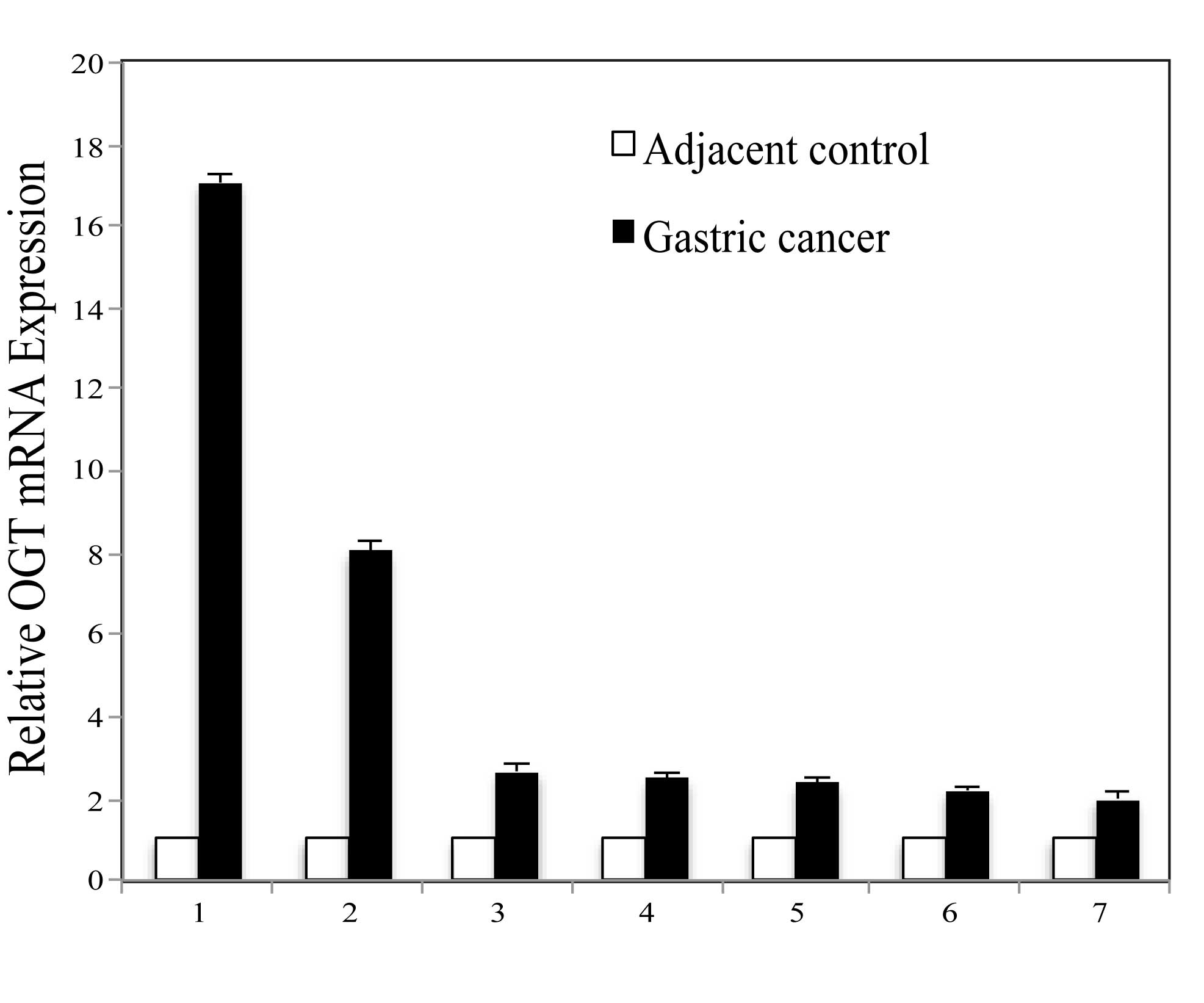
OGT is upregulated in gastric cancer
Highly expressed OGT in several types of cancer has
been reported, except in gastric cancer. In order to determine OGT
expression in gastric cancer, initial analysis was performed
comparing OGT expression levels between cancerous and paired
adjacent non-cancerous tissue mRNA derived from the same patient
with gastric cancer. Seven gastric cancerous and adjacent tissues
were used. Quantitative PCR results revealed differential
expression, although OGT mRNA was detectable in the normal tissues,
OGT is overexpressed in all cancerous tissues in comparison to the
normal groups (Fig. 1).
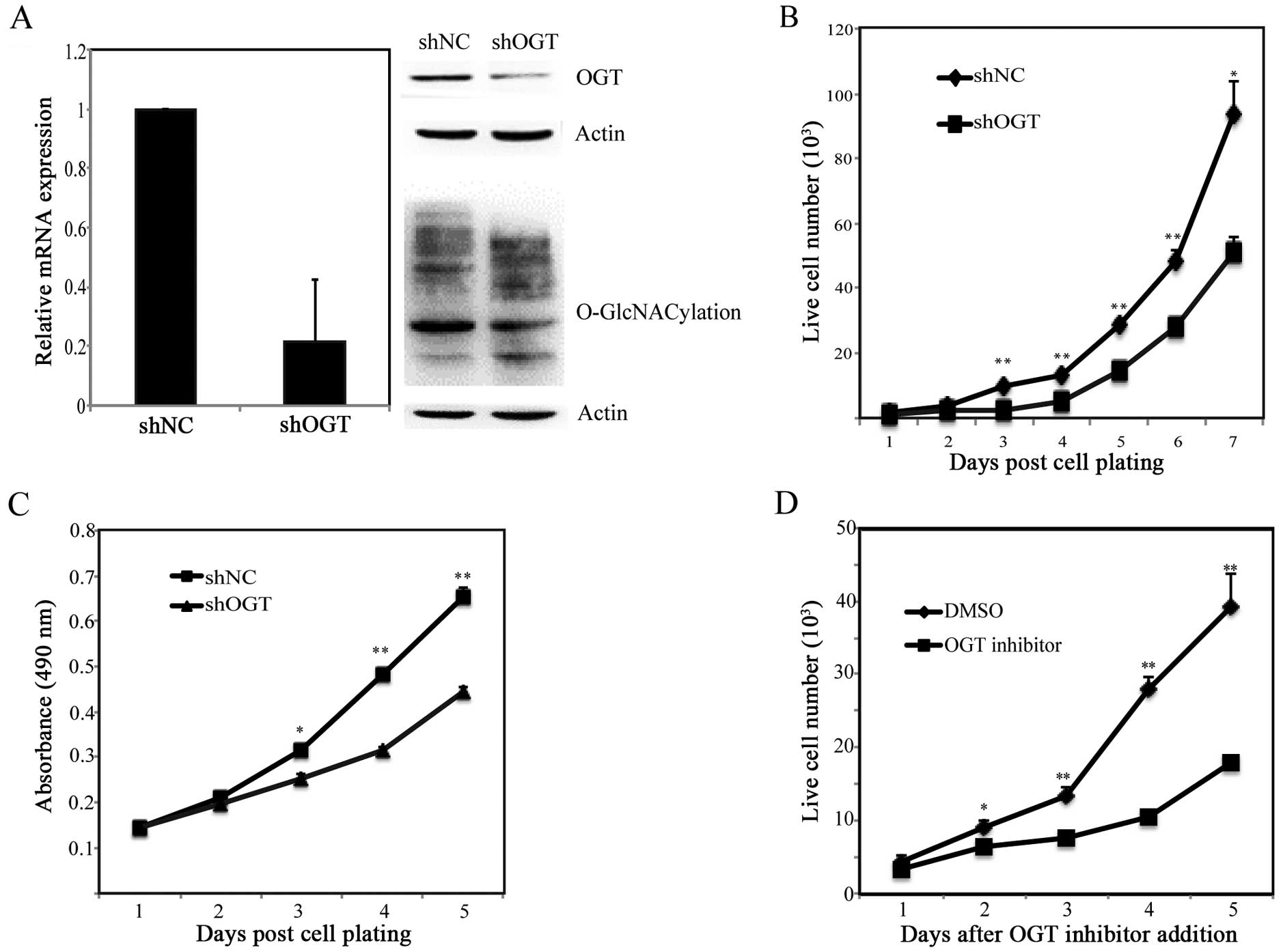
OGT KD in BGC-823 cells decreases cell
proliferation
To investigate the function of OGT in gastric
cancer, we used RNA interference strategy to knock down endogenous
OGT gene. Stable cells of control and OGT silencing group were
constructed with that in G418 selection. The efficiency of
inhibition of OGT was confirmed by qPCR and western blotting. OGT
mRNA and protein level were significantly reduced compared with
that in control cells (Fig. 2A). We
further analyzed whether the O-GlcNAcylation expression was changed
after OGT silencing. As expected, silencing OGT led to significant
reduction in global O-GlcNAcylation compared with control cells.
Therefore these stable cells were used for following functional
experiments. Proliferation of OGT KD cells was first assessed by
cell counting and MTT assay. Both results showed that OGT silencing
inhibited cell growth (Fig. 2B and
C). In addition, to confirm this result, inhibitor specifically
targeting OGT was used. Cell counting assay showed similar results
(Fig. 2D). Taken together, these
results indicated that OGT is required for gastric cancer cell
proliferation.
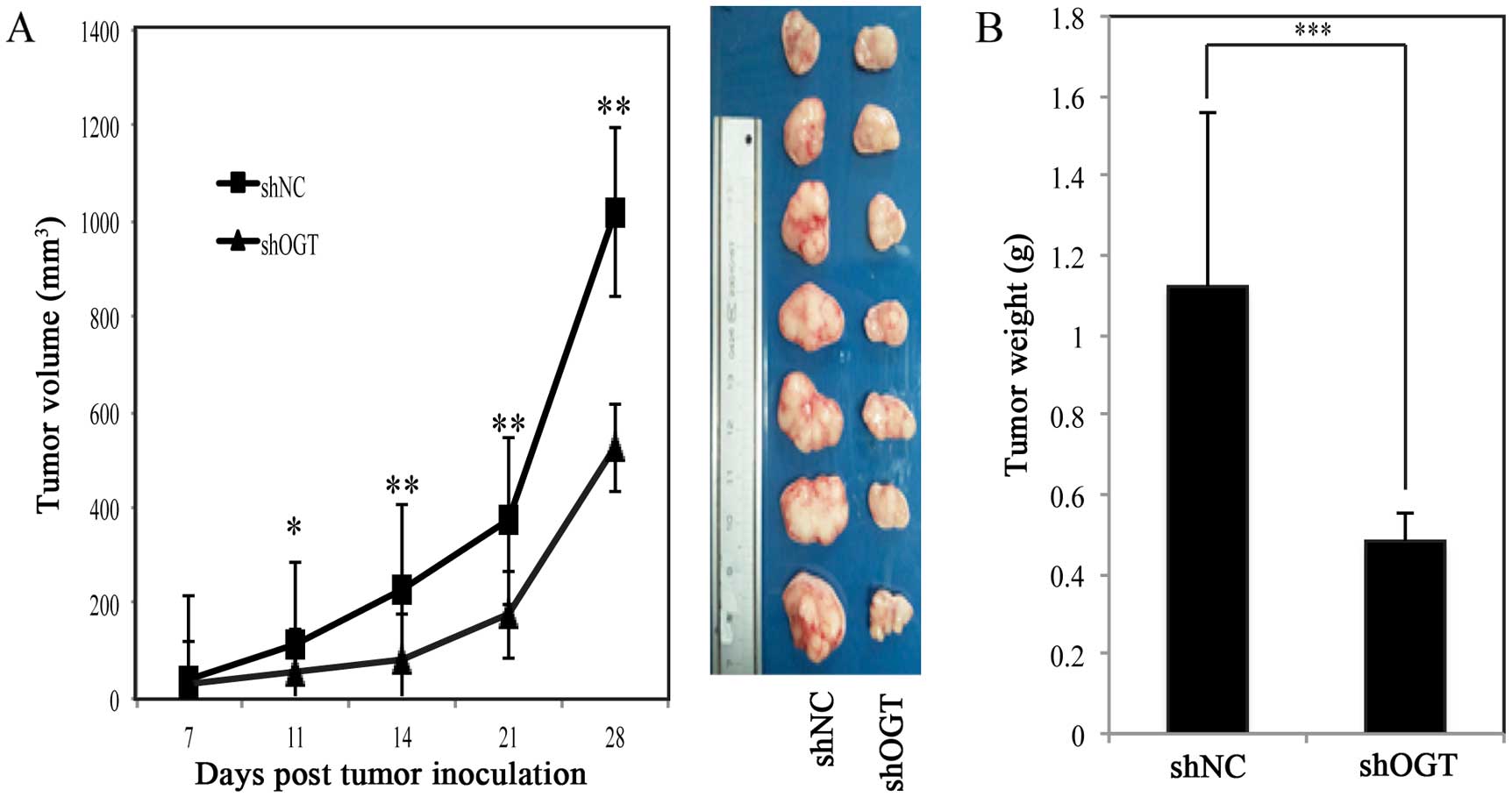
Silencing OGT inhibits tumor progression
in vivo
To examine the effects of OGT on tumorigenicity of
BGC-823 cells in vivo and explored the therapeutic potential
of OGT gene silencing in BGC-823, we compared the tumor growth in
immunocompromised nude mice after shNC/shOGT cell inoculation.
Tumor size was monitored twice weekly by cationic palpation. At the
end of the experiments, animals were sacrificed and the tumors
weighed. As shown in Fig. 3A,
silencing OGT could significantly suppress tumor growth. The tumors
harvested from the OGT KD group also weighed less (Fig. 3B), these suggesting that OGT could
be a therapeutic target in gastric cancer.
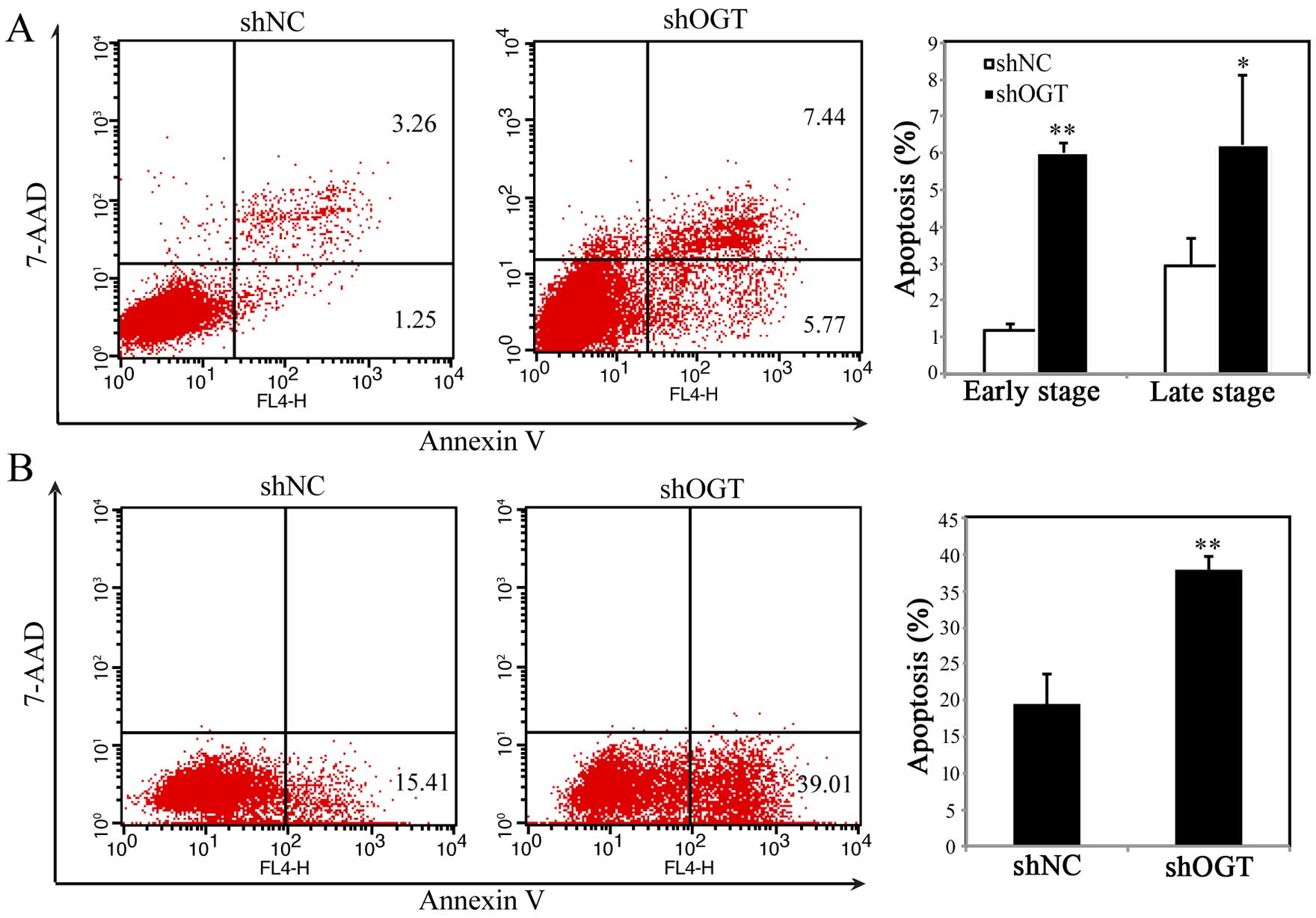
Suppression of OGT induces cell
apoptosis
To investigate whether the inhibition of
proliferation in OGT KD cells was due to cell apoptosis, we used
APC-Annexin V/7-AAD double staining kit followed by FACS analysis.
As shown in Fig. 4A, percentage of
APC-Annexin V+/7-AAD− (early apoptosis) and
APC-Annexin V+/7-AAD+ (late apoptosis) cells
markedly increased after OGT silencing, which indicated that OGT
has an anti-apoptosis role in gastric cancer cells. In addition,
OGT reversed anti-chemotherapeutic drug-mediated apoptosis. If
shOGT/shNC cells were treated with 5-FU, silencing OGT could
increase apoptosis, which suggested elevated level of OGT in
gastric cancer may be one of the drug-resistant mechanisms.
Silencing OGT induces cell apoptosis
through upregulation of PUMA and caspase-3
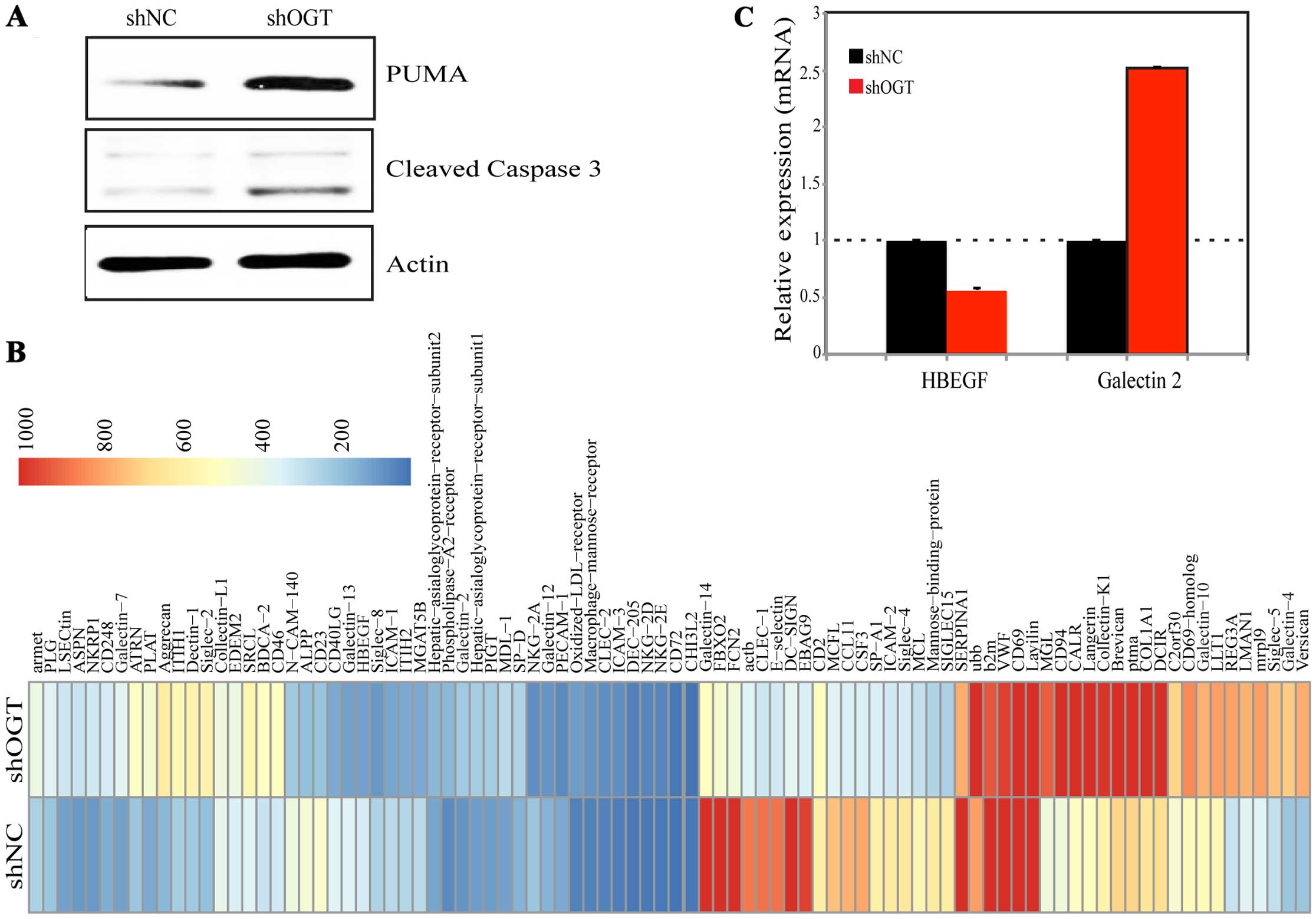
As silencing OGT promoted cell apoptosis, PUMA and
caspase-3 were detected by western blotting. Data showed that PUMA
and caspase-3 were significantly upregulated after OGT KD (Fig. 5A), which could be the reason of OGT
KD inducing apoptosis. In order to examine the downstream genes of
OGT, GBP gene microarray was performed. Ninety genes were assessed
in this array, they were well known as glycan-binding protein genes
based on the database of functional glycomics and uniprot. RNA from
shNC/shOGT was investigated. As shown in Fig. 5B, many apoptosis-related genes were
changed after OGT silencing. Of these, HBEGF were most
significantly reduced, whereas galectin 2 and
galectin 7 were increased after OGT suppression. qPCR was
used for validation, as shown in Fig.
5C. These genes have apoptotic-related function indicating that
OGT KD may target these proteins to modulate PUMA and
caspase-3.
Discussion
O-GlcNAc is the covalent addition of a GlcNAc moiety
to serine/threonine residues of cytosolic and nuclear proteins. OGT
could transfer GlcNAc form UDP-GlcNAc to substrate proteins,
whereas O-GlcNAcase (OGA) could remove GlcNAc. For O-GlcNAcylation
and OGT, the expression level has been examined in various types of
cancer, including breast (4,5,12),
prostate (13), lung, colorectal
(6), liver (14,15)
and non-solid cancers such as chronic lymphocytic leukemia
(15). However, the role of OGT in
gastric cancer was not reported.
The present study was designed to investigate the
expression and function of OGT in gastric cancer. We observed the
elevated expression of OGT at mRNA level in gastric cancerous
tissues compared with that in adjacent tissues. Silencing OGT
decreased cell proliferation both in vitro and in
vivo due to apoptosis induction. The reduction of OGT results
in pro-apoptosis effect in the presence or absence of 5-FU. In
addition, PUMA and caspase-3 were increased after OGT KD.
Furthermore, the GBP array results showed expression change of
various apoptosis-related genes after OGT KD, pointing to a tumor
genesis function of OGT in gastric cancer.
In order to determine the level of OGT in gastric
cancer, the mRNA level of 7 cases of gastric cancer and adjacent
non-cancer tissues was measured by qPCR. The results showed that
OGT is overexpressed in all cancer tissues when compare with that
in non-cancerous tissues. Due to the limit number of samples, the
protein level and whether OGT is associated with different stages
and patient survival are unknown, which need to be further
studied.
Several studies have shown that O-GlcNAcylation
plays a key role in cell growth, division and invasion. In ES
cells, OGT deletion is lethal (16), and OGT tissue-specific mutation
results in the loss of O-GlcNAcylation in specific tissues and
causes T-cell apoptosis (17).
Reduction of O-GlcNAcylation through RNA interference in breast
cancer cells leads to inhibition of tumor growth both in
vitro and in vivo. Reduced O-GlcNAcylation also
decreased lung and colon cancer invasion in a context-dependent
manner. Also, O-GlcNAcylation regulates cancer cell metabolism,
increasing glucose and glutamine uptake in cancer cells (18,19).
In order to investigate the function of OGT in
gastric cancer, the stable cell line expressing shRNA that targets
OGT was constructed. Cell counting and MTT assays showed OGT
suppression decreased cell proliferation. To confirm this result,
the inhibitor targeting OGT was used, similar results were shown.
Additionally, OGT KD cells showed less tumor growth in nude mice.
We demonstrated that OGT was necessary for the proliferation of
gastric cancer.
To explore the mechanism of silencing OGT in
inhibiting cancer cell proliferation, cell cycle was determined by
PI staining, but there was no change in these two cell lines (data
not shown). Apoptosis was then examined by Annexin V and 7-AAD
staining. Our results showed that OGT inhibition induced more cell
apoptosis, both early and late stage of apoptosis. Our data are
consistent with a previous study (17) showing that loss of OGT could cause T
cell apoptosis. Notably, OGT KD also increased the sensitivity of
5-FU on BCG-823 suggesting that cancer cell could use the
anti-apoptosis effect of OGT in drug-resistance.
Since OGT KD showed pro-apoptosis effect, we
examined apoptosis-related proteins by western blotting.
Importantly, the levels of PUMA and caspase-3 were significantly
upregulated in OGT KD cells, which are critical apoptosis proteins
(20). These results indicated that
the pro-apoptosis function of OGT KD may be through the
mitochondrial pathway, which was consistent with a recent study
(19). PUMA was regulated by p53,
and p53 was activated by survival signals (21). To determine which protein was
involved in OGT KD inducing apoptosis, we used a glycan-binding
protein gene array downstream of OGT. The results showed many genes
were altered after OGT silencing. Of these, HBEGF and
CCL11 are associated with survival or anti-apoptosis, which
were reduced (22). Whereas,
galectin 2, 4, 7 and 10 were increased, which have
pro-apoptosis effects (23–25). However, whether they are the direct
target of OGT or not needs to be further verified.
In summary, the present study has shed light on the
expression and function of OGT in gastric cancer. Our data showed
that OGT is overexpressed in gastric cancer tissues. Silencing OGT
inhibited cell growth in vitro and in vivo, due to
inducing cell apoptosis. Importantly, we showed that PUMA and
caspase-3 were upregulated after OGT silencing, which is the
mechanism of OGT suppression-induced apoptosis. In addition, we
found various candidate genes downstream of OGT. Further studies
need to be carried out, to investigate whether these genes were
associated with apoptosis in OGT KD cancer cells. Collectively our
results suggested that OGT is an oncogene in gastric cancer.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (no. 31300743), the Fund of the
Education Department of Liaoning province (no. L2012278) and
Science and Technology Plan Project of Liaoning Province (no.
2011404013-1, 2012225001, 2014225013).
References
|
1
|
Slawson C, Copeland RJ and Hart GW:
O-GlcNAc signaling: A metabolic link between diabetes and cancer?
Trends Biochem Sci. 35:547–555. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Haltiwanger RS, Holt GD and Hart GW:
Enzymatic addition of O-GlcNAc to nuclear and cytoplasmic proteins.
Identification of a uridine diphospho-N-acetylglucosamine:peptide
beta-N-acetylglucosaminyltransferase. J Biol Chem. 265:2563–2568.
1990.PubMed/NCBI
|
|
3
|
Dong DL and Hart GW: Purification and
characterization of an O-GlcNAc selective
N-acetyl-β-D-glucosaminidase from rat spleen cytosol. J Biol Chem.
269:19321–19330. 1994.PubMed/NCBI
|
|
4
|
Caldwell SA, Jackson SR, Shahriari KS,
Lynch TP, Sethi G, Walker S, Vosseller K and Reginato MJ: Nutrient
sensor O-GlcNAc transferase regulates breast cancer tumorigenesis
through targeting of the oncogenic transcription factor FoxM1.
Oncogene. 29:2831–2842. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gu Y, Mi W, Ge Y, Liu H, Fan Q, Han C,
Yang J, Han F, Lu X and Yu W: GlcNAcylation plays an essential role
in breast cancer metastasis. Cancer Res. 70:6344–6351. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mi W, Gu Y, Han C, Liu H, Fan Q, Zhang X,
Cong Q and Yu W: O-GlcNAcylation is a novel regulator of lung and
colon cancer malignancy. Biochim Biophys Acta. 1812:514–519. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Starska K, Forma E, Brzezińska-Błaszczyk
E, Lewy-Trenda I, Bryś M, Jóźwiak P and Krześlak A: Gene and
protein expression of O-GlcNAc-cycling enzymes in human laryngeal
cancer. Clin Exp Med. Oct 15–2014.PubMed/NCBI
|
|
8
|
Hartgrink HH, Jansen EP, van Grieken NC
and van de Velde CJ: Gastric cancer. Lancet. 374:477–490. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cunningham D, Jost LM, Purkalne G,
Oliveira J and Force EGT: ESMO Minimum Clinical Recommendations for
diagnosis, treatment and follow-up of gastric cancer. Ann Oncol.
16(Suppl 1): i22–i23. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Y, Wen T, Zhu M, Li L, Wei J, Wu X, Guo
M, Liu S, Zhao H, Xia S, et al: Glycoproteomic analysis of tissues
from patients with colon cancer using lectin microarrays and
nanoLC-MS/MS. Mol Biosyst. 9:1877–1887. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Krześlak A, Forma E, Bernaciak M,
Romanowicz H and Bryś M: Gene expression of O-GlcNAc cycling
enzymes in human breast cancers. Clin Exp Med. 12:61–65. 2012.
View Article : Google Scholar
|
|
13
|
Kamigaito T, Okaneya T, Kawakubo M,
Shimojo H, Nishizawa O and Nakayama J: Overexpression of O-GlcNAc
by prostate cancer cells is significantly associated with poor
prognosis of patients. Prostate Cancer Prostatic Dis. 17:18–22.
2014. View Article : Google Scholar
|
|
14
|
Zhu Q, Zhou L, Yang Z, Lai M, Xie H, Wu L,
Xing C, Zhang F and Zheng S: O-GlcNAcylation plays a role in tumor
recurrence of hepatocellular carcinoma following liver
transplantation. Med Oncol. 29:985–993. 2012. View Article : Google Scholar
|
|
15
|
Shi Y, Tomic J, Wen F, Shaha S, Bahlo A,
Harrison R, Dennis JW, Williams R, Gross BJ, Walker S, et al:
Aberrant O-GlcNAcylation characterizes chronic lymphocytic
leukemia. Leukemia. 24:1588–1598. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
O'Donnell N, Zachara NE, Hart GW and Marth
JD: Ogt-dependent X-chromosome-linked protein glycosylation is a
requisite modification in somatic cell function and embryo
viability. Mol Cell Biol. 24:1680–1690. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shafi R, Iyer SP, Ellies LG, O'Donnell N,
Marek KW, Chui D, Hart GW and Marth JD: The O-GlcNAc transferase
gene resides on the X chromosome and is essential for embryonic
stem cell viability and mouse ontogeny. Proc Natl Acad Sci USA.
97:5735–5739. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Marshall S: Role of insulin, adipocyte
hormones, and nutrient-sensing pathways in regulating fuel
metabolism and energy homeostasis: A nutritional perspective of
diabetes, obesity, and cancer. Sci STKE. 2006:re72006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ferrer CM, Lynch TP, Sodi VL, Falcone JN,
Schwab LP, Peacock DL, Vocadlo DJ, Seagroves TN and Reginato MJ:
O-GlcNAcylation regulates cancer metabolism and survival stress
signaling via regulation of the HIF-1 pathway. Mol Cell.
54:820–831. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ding WX, Ni HM, Chen X, Yu J, Zhang L and
Yin XM: A coordinated action of Bax, PUMA, and p53 promotes
MG132-induced mitochondria activation and apoptosis in colon cancer
cells. Mol Cancer Ther. 6:1062–1069. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nakano K and Vousden KH: PUMA, a novel
proapoptotic gene, is induced by p53. Mol Cell. 7:683–694. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yotsumoto F, Yagi H, Suzuki SO, Oki E,
Tsujioka H, Hachisuga T, Sonoda K, Kawarabayashi T, Mekada E and
Miyamoto S: Validation of HB-EGF and amphiregulin as targets for
human cancer therapy. Biochem Biophys Res Commun. 365:555–561.
2008. View Article : Google Scholar
|
|
23
|
Sturm A, Lensch M, André S, Kaltner H,
Wiedenmann B, Rosewicz S, Dignass AU and Gabius HJ: Human
galectin-2: Novel inducer of T cell apoptosis with distinct profile
of caspase activation. J Immunol. 173:3825–3837. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Paclik D, Danese S, Berndt U, Wiedenmann
B, Dignass A and Sturm A: Galectin-4 controls intestinal
inflammation by selective regulation of peripheral and mucosal T
cell apoptosis and cell cycle. PLoS One. 3:e26292008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kuwabara I, Kuwabara Y, Yang RY, Schuler
M, Green DR, Zuraw BL, Hsu DK and Liu FT: Galectin-7 (PIG1)
exhibits pro-apoptotic function through JNK activation and
mitochondrial cytochrome c release. J Biol Chem. 277:3487–3497.
2002. View Article : Google Scholar
|