Introduction
Primary central nervous system tumors account for
~2% of human malignancies (1). In
general, malignant glioma is the most common type of primary
intracranial tumor, and the incidence of malignant glioma is
increasing worldwide, primarily as a result of improvements in
diagnostic imaging (2). Despite the
comprehensive treatment regimen of surgery, radiotherapy and
chemotherapy, resistance to standard anti-proliferative treatment
with concomitant radiotherapy and chemotherapy is common and
manifests as an invasive cell population that leads to tumor
recurrence and death (3).
Therefore, there is an urgent need for the development of a novel
agent displaying glioma-specific toxicity.
Trichosanthin (TCS), a protein of ~27 kDa that is
extracted from the Chinese herb Trichosanthes kirilowii
Maxim, is a type I ribosome-inactivating protein (RIP) (4,5). It
has been used for centuries in China as an abortifacient during
early pregnancy (6). Many studies
have shown that TCS has enormous potential as a therapeutic drug
due to its suppression of the proliferation of various cancer cell
types. Research has demonstrated that TCS restricts human
choriocarcinoma cell proliferation by inducing reactive oxygen
species (ROS) production (7,8). In
addition, TCS suppresses the proliferation of HeLa cells by
blocking the PKC/MAPK signaling pathway (9) and induces the apoptosis of cervical
cancer cells by increasing the intracellular Ca2+
concentration (10) and by
regulating the expression of Smac (11). Moreover, previous studies have shown
that TCS suppresses the proliferation of breast cancer cells and
HepA-H cells by inducing cell cycle arrest and promoting apoptosis
(12–14). Furthermore, research suggests that
TCS induces the apoptosis of chronic myeloid leukemia cells via
endoplasmic reticulum stress, the mitochondrial-dependent apoptosis
pathway and the inhibition of PKC (15,16).
Studies have also revealed that TCS displays anti-HIV activity, as
TCS is cytotoxic to HIV-infected macrophages and lymphocytes and
decreases viral replication (17,18).
Recently, a new study showed that a peptide derived from TCS
suppresses the immune response by activating
CD8+CD28− regulatory T cells and serves as a
potential therapeutic agent for immunological diseases (19). TCS not only has inhibitory activity
against various tumor cells but also shows inhibitory activity
against several normal somatic cell types, including proximal
tubule epithelial cells, hepatocytes and antigen-specific T cells
(20–22). Previous studies have reported that
TCS can cause neurological reactions in HIV-infected patients and
that such toxicity may be due to the effect of TCS on HIV-infected
macrophages (23). However,
intravenous injections of TCS had no toxic effects on normal mouse
brain or pituitary cells (24). In
conclusion, since TCS displays anticancer activity in various
malignant tumors, we aimed to determine whether TCS exerts
antitumor effects on glioma cells. Here, we demonstrated the
anti-proliferative effects and antitumor mechanisms of action of
TCS on glioma cells. Our results suggest that TCS is a novel
chemotherapeutic agent that may target leucine-rich
repeat-containing G protein-coupled receptor 5 (LGR5) and the
Wnt/β-catenin pathway in human glioma cells.
Materials and methods
Materials
High glucose DMEM containing fetal bovine serum
(FBS), penicillin G and streptomycin was purchased from Gibco
(Carlsbad, CA, USA). The U87 and U251 human malignant glioma cell
lines were provided by the China Infrastructure of Cell Line
Resources, (Beijing, China). TCS was purchased from Shanghai
Jinshan Pharmaceutical (Shanghai, China). The primary antibodies
against LGR5, β-catenin, GSK-3β, c-myc and cyclin D1 used for
western blot analysis were provided by Santa Cruz Biotechnology
(Santa Cruz, CA, USA). The other reagents used in this study were
of analytical grade.
Cell culture
The U87 and U251 human malignant glioma cell lines
were cultured in high glucose DMEM containing 1% antibiotics and
10% FBS. The cells were cultured in an incubator at 37°C in 5%
CO2 and a humidified atmosphere.
Cell morphology
U87 and U251 cells were grown in culture flasks to
the logarithmic growth phase. Then, the cells were treated with TCS
(20 µM). Morphological changes in the cells were observed
using an inverted microscope, and the cells were photographed after
treatment for 24 h.
Cell viability assay
A Cell Counting Kit-8 (CCK-8) (Dojindo, Japan) assay
was utilized to evaluate cell viability. U87 and U251 cells were
resuspended in complete or serum-free medium at a density of
1×104 or 5×103 cells/well and were cultured
in 96-well plates. The cell samples were exposed to various
concentrations (2.5, 5, 10, 20, 40 or 80 µM) of TCS for 24,
48 or 72 h. After TCS treatment, the medium was removed from each
well, 10 µl of CCK-8 and 100 µl of serum-free medium
were added, and the cells were incubated for 1 h at 37°C. A
microplate reader was used to measure the absorbance of each well
at 450 nm.
Detection of apoptosis via flow
cytometry
The apoptosis of U87 and U251 cells was analyzed
using an Annexin V-FITC apoptosis detection kit (Sigma-Aldrich, St.
Louis, MO, USA). The cells were cultured with TCS (10, 20
µM) or vehicle (PBS) for 24 h, and then, the cells were
harvested at a density of 1×106 cells/ml. The cells were
washed twice with ice-cold PBS. Then, the cells (1×106)
were resuspended in 195 µl of binding buffer, and 5
µl of Annexin V-FITC and propidium iodide (PI) were added to
the cell suspension; subsequently, the cells were covered in
aluminum foil and incubated at room temperature for 20 min. After
this incubation, apoptosis was analyzed via flow cytometry.
DAPI staining
After U87 glioma cells were treated with TCS (10 or
20 µM) or PBS for 24 h, they were fixed in 4% formaldehyde
and washed three times with PBS. Then, the cells were stained with
DAPI staining solution (10 µg/ml) for 5 min and washed three
times with PBS. The morphologic changes in the nuclei were detected
using a fluorescence microscope.
Mitochondrial membrane potential
measurement based on
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-imidacarbocyanine iodide
(JC-1) staining
The decrease in mitochondrial membrane potential,
which is a hallmark of apoptosis, was detected using a JC-1
mitochondrial membrane potential assay kit (Cayman Chemical, Ann
Arbor, MI, USA). U87 glioma cells were treated with TCS (10 or 20
µM) or PBS for 24 h and then incubated in 1 ml of JC-1
working solution for 30 min at 37°C in an incubator. These cells
were washed twice with ice-cold PBS, and the fluorescence intensity
of the cells was then observed via fluorescence microscopy.
Decreased fluorescence of the cells was observed as the
mitochondrial membrane potential collapsed.
Terminal deoxynucleotidyl
transferase-mediated dUTP nick end-labeling (TUNEL) assay
DNA fragmentation was detected using a One-Step
TUNEL kit (Abnova Corporation, Taipei City, Taiwan) according to
the manufacturer's instructions. Briefly, after treatment with TCS
(10 or 20 µM) or PBS, U87 cells were fixed in 4%
paraformaldehyde for 30 min at room temperature. Then, the cells
were washed three times with PBS and permeabilized for 2 min on
ice. Next, the cells were resuspended in TUNEL working solution and
incubated in a humidified atmosphere shielded from light for 1 h at
37°C. Green fluorescence was detected in the FITC-labeled
TUNEL-positive cells under a fluorescence microscope.
Cell migration and invasion assays
Wound healing assays were used to assess the effects
of TCS on glioma cell migration. U87 cells were seeded in culture
plates and incubated until they reached confluency. Subsequently, a
scratch was made on the cell surface using a 10-µl pipette
tip. The cells were washed with PBS and treated with TCS (5
µM) or PBS for 24 h. The width of the wound was photographed
under a microscope at 0 and 24 h. In addition, the effect of TCS on
glioma cell invasion was determined using Matrigel-coated Transwell
assays. U87 cells were treated with DMEM containing TCS (5
µM) or PBS for 24 h. Then, the cells were trypsinized and
seeded into the upper chamber of a Transwell insert at a density of
1×105. Medium containing 20% FBS was added to the lower
chamber, and the cells were cultured for 24 h. The cells that
invaded into the outer surface of the Transwell insert were stained
with 0.5% crystal violet solution and counted using an inverted
microscope.
Western blot analysis
Cellular proteins were extracted from glioma cells
using lysis buffer [1 mM EDTA (pH 8.0), 50 mM Tris (pH 7.4), 150 mM
NaCl, 1% NP-40, 0.1% SDS, and 0.5% sodium deoxycholate] according
to the manufacturer's instructions for western blot analysis. A
bicinchoninic acid protein quantitation kit (BioVision, Palo Alto,
CA, USA) was used to measure the protein concentrations. The same
amounts of whole-cell protein extracts from each sample were
separated via SDS-PAGE and transferred to a PVDF membrane with a
pore size of 0.2 µm. Subsequently, the membrane was blocked
with 5% milk in TBS-T for 1 h at room temperature (RT), incubated
in a primary antibody overnight at 4°C, and then incubated in a
horseradish peroxidase-conjugated secondary antibody at room
temperature for 2 h. After washing three times with TBS, the
immunoreactive bands were detected using a gel imaging analysis
system after applying a chemiluminescence working solution to the
PVDF membrane according to the manufacturer's instructions.
Statistical analysis
SPSS version 19.0 was utilized for the statistical
analyses, and the data are presented as the mean ± standard
deviation (SD) of multiple independent experiments. Student's
t-test and ANOVA were used to evaluate the differences between the
means, and P<0.05 was considered to indicate a statistically
significant difference.
Results
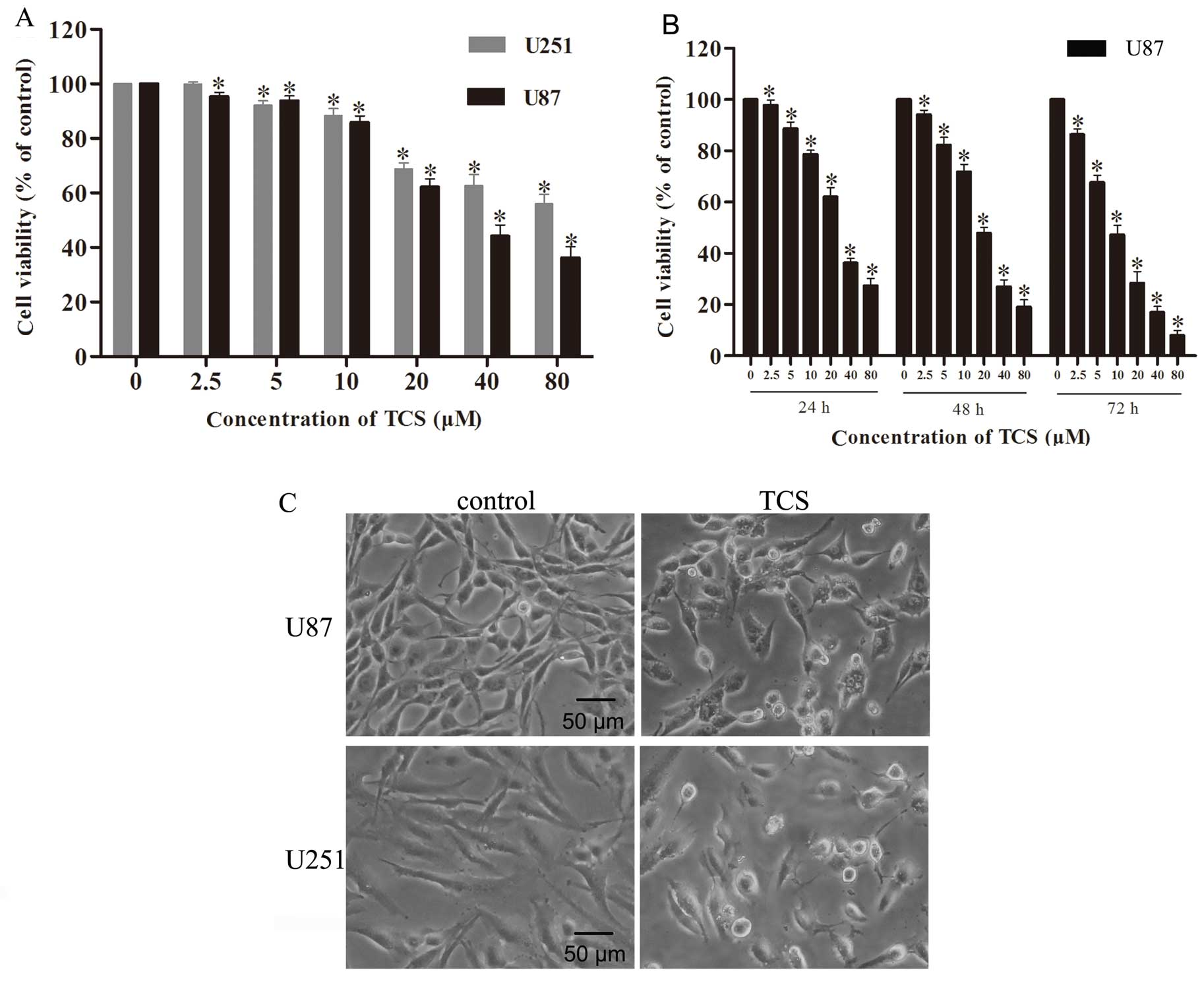
Cell viability assay
The CCK-8 assay was used to evaluate the impact of
TCS on glioma cell viability. U87 and U251 cells were treated for
24 h with TCS diluted to concentrations of 2.5, 5, 10, 20, 40 and
80 µM in complete medium; the assay results indicated that
relative to the control cells, U87 cells exhibited viabilities of
95.4, 93.8, 85.9, 62.2, 44.1 and 36.2%, respectively, and U251
cells exhibited viabilities of 99.9, 92.1, 88.3, 68.8, 62.7 and
56%, respectively (n=4; P<0.05) (Fig. 1A). In addition, the IC50
values for U87 and U251 were 40 and 51.6 µM, respectively.
We analyzed the change in cell viability of U87 cells after
treatment with TCS concentrations of 2.5, 5, 10, 20, 40 and 80
µM in serum-free medium; compared with the control cells
treated with PBS alone, U87 cells treated for 24 h exhibited
viabilities of 97.7, 88.7, 78.6, 62.1, 36.2 and 27.3%,
respectively, U87 cells treated for 48 h exhibited viabilities of
94.2, 82.3, 71.8, 47.8, 26.9 and 19.0%, respectively and U87 cells
treated for 72 h exhibited viabilities of 86.3, 67.7, 47.3, 28.4,
17.0 and 7.9%, respectively (n=4; P<0.05) (Fig. 1B). The IC50 values of U87
cells for 24, 48 and 72 h of TCS treatment were 30.2, 20.5 and 10.0
µM, respectively. The assay results indicated that in both
the presence and the absence of serum, TCS inhibited the viability
of glioma cells in a dose- and time-dependent manner.
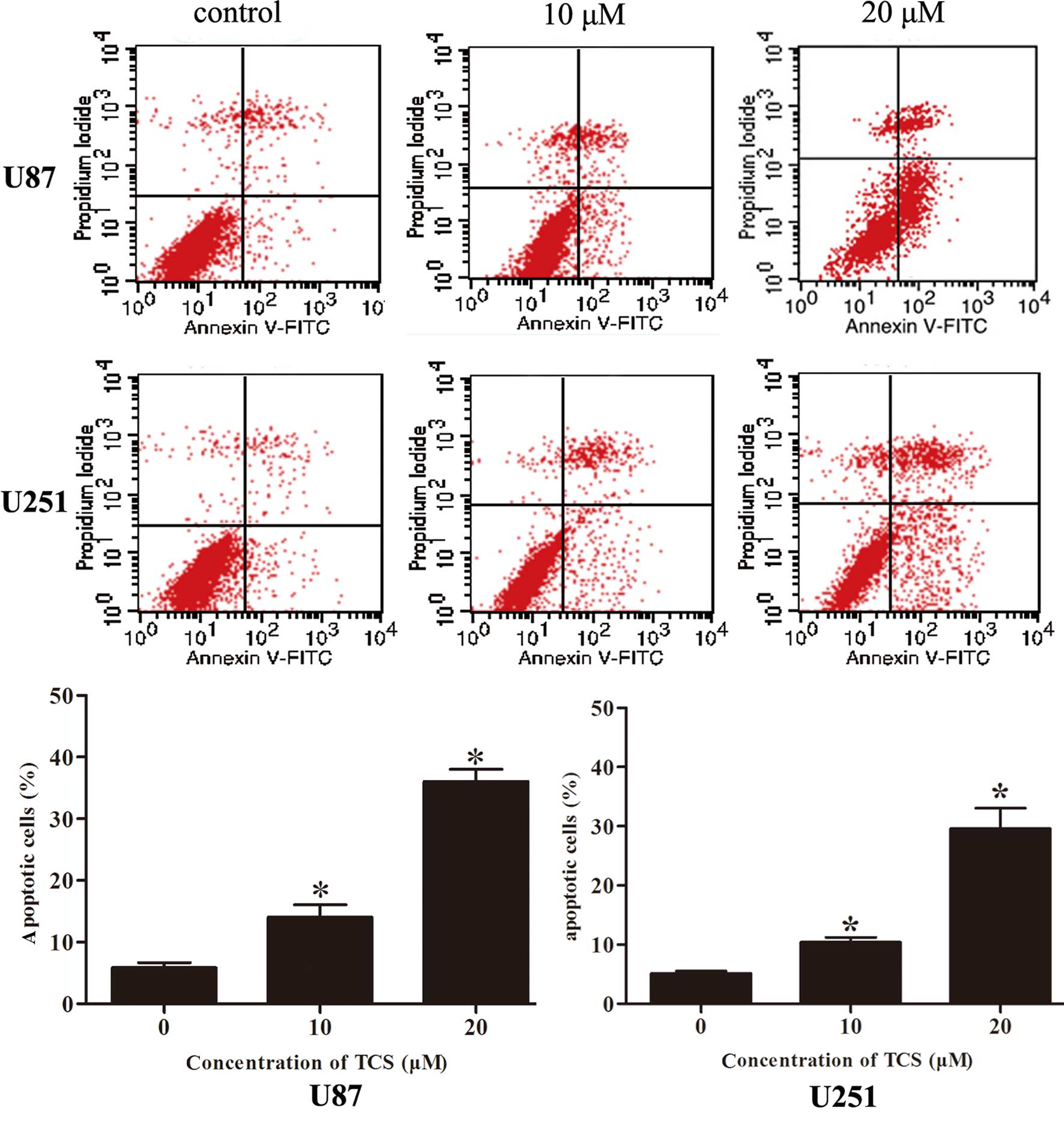
TCS induces the apoptosis of glioma
cells
To determine whether the induction of glioma cell
apoptosis is related to the inhibition of proliferation, the
apoptosis rate was analyzed via flow cytometry after labeling cells
with Annexin V-FITC/PI. As indicated in Fig. 2, the apoptotic percentages of the
U87 and U251 cells were 5.9 and 5.1%, respectively, in the
PBS-treated group; 14.1 and 10.4%, respectively, in the group
treated with 10 µM of TCS and 36.1 and 27.8%, respectively,
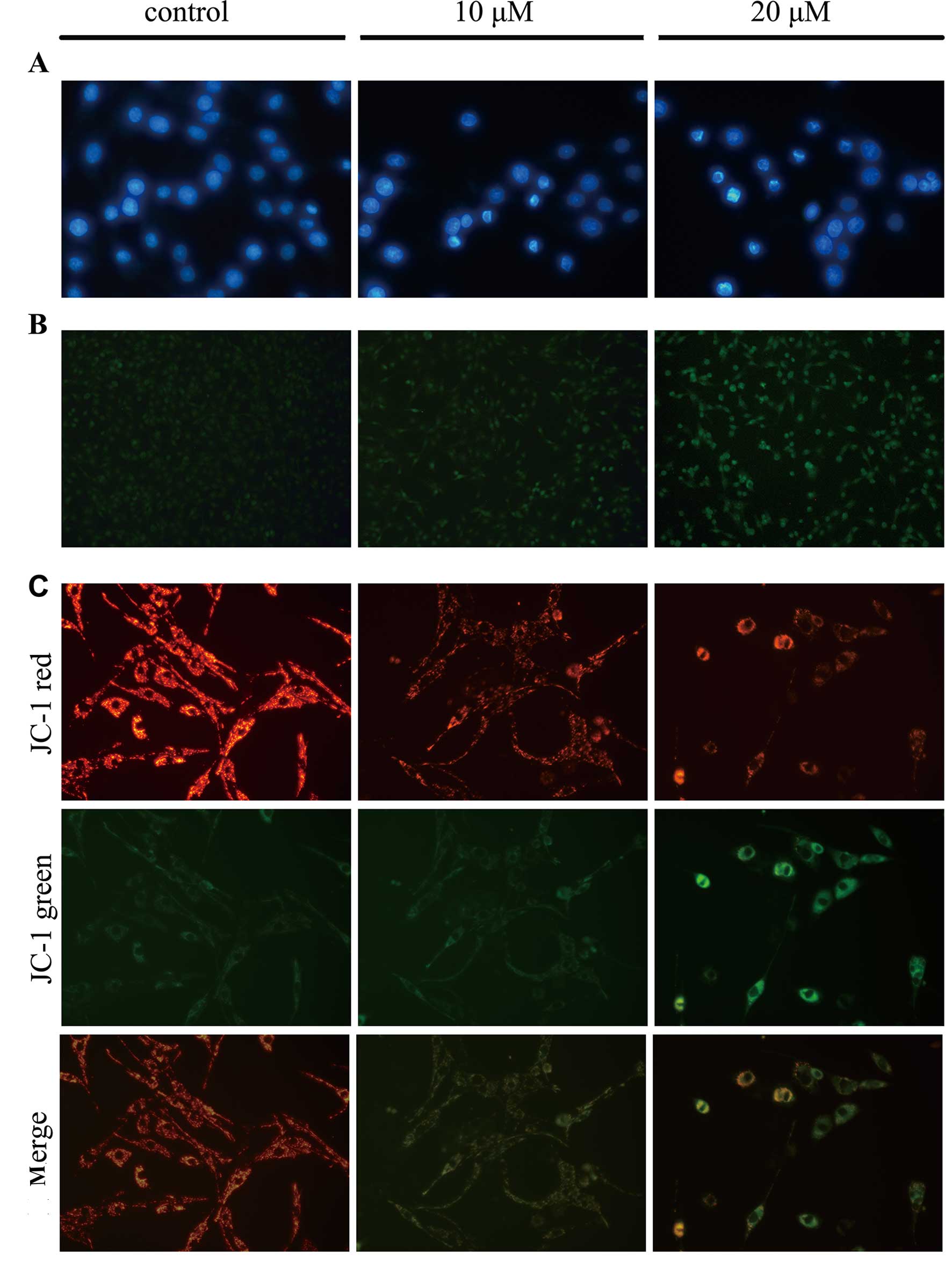
in the group treated with 20 µM of TCS. Moreover, DAPI
staining was used to observe morphological variations in the
nuclei, and the data showed that chromatin condensation and
fragmented nuclei which are typical characteristics of apoptosis,
were detected following DAPI staining when U87 cells were treated
with 10, 20 µM TCS for 24 h (Fig. 3A). Results also showed that the
number of TUNEL-positive U87 cells was significantly increased in
the TCS-treated group compared with the PBS treated group (Fig. 3B). Furthermore, JC-1 staining was
used to visualize mitochondrial depolarization in glioma cells
treated with TCS. The mitochondria of untreated JC-1-stained U87
cells emitted faint green fluorescence and strong orange–red
fluorescence, whereas the apoptotic cells emitted green
fluorescence. Compared with the control cells treated with PBS,
TCS-treated U87 cells exhibited significantly decreased
mitochondrial membrane potentials, as evidenced by these cells'
distinct green fluorescence (Fig.
3C).
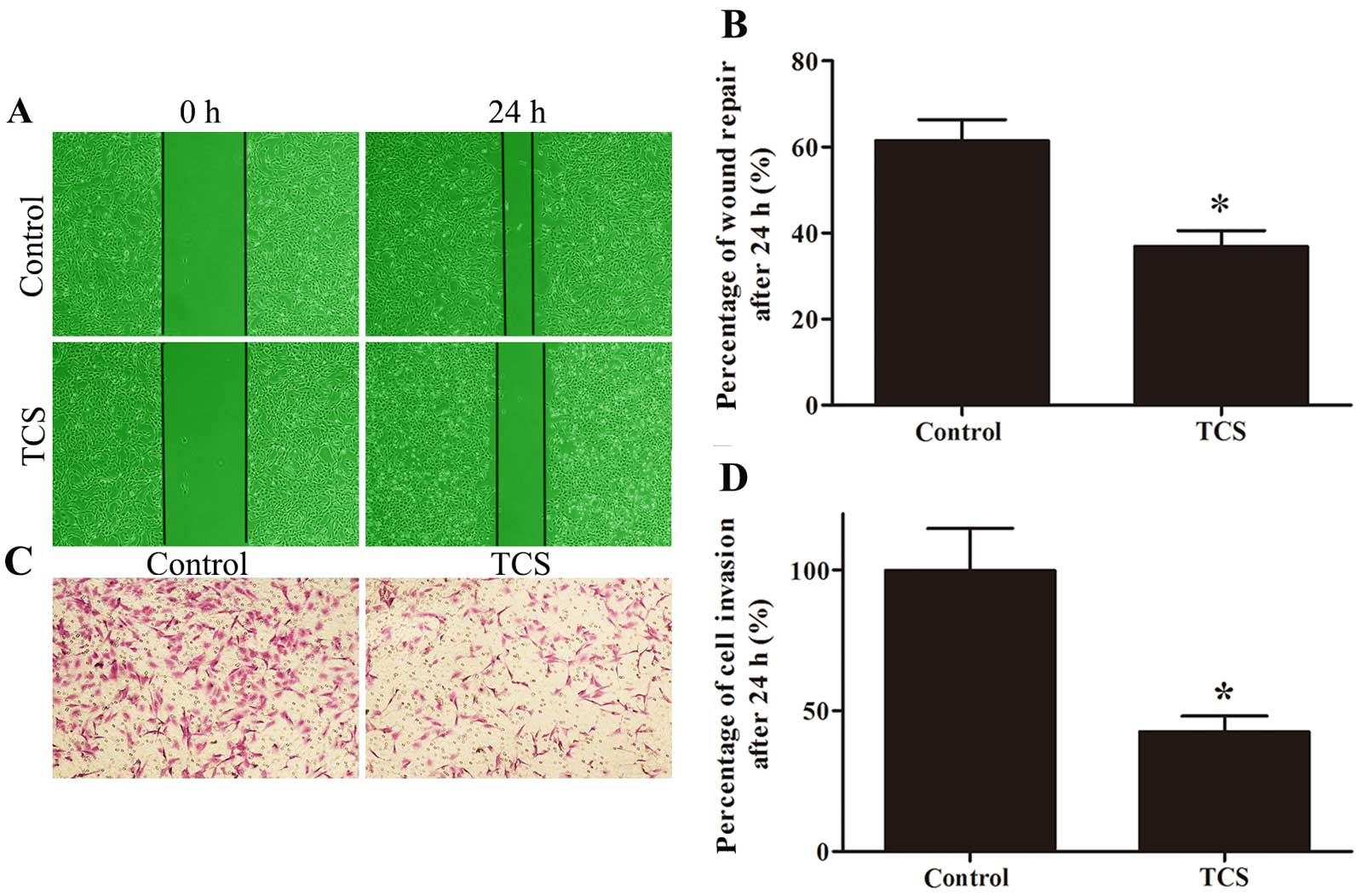
TCS inhibits glioma cell migration and
invasion
Wound healing and Matrigel-coated Transwell assays
were used to evaluate the effect of TCS on migration and invasion.
The percentage of wound repair decreased from 61.52 to 36.87% in
response to TCS, and the ratio of invading cells on the lower side
of the membrane was 42.57% after treatment with 5 µM TCS
compared with the control (set to 100%; Fig. 4).
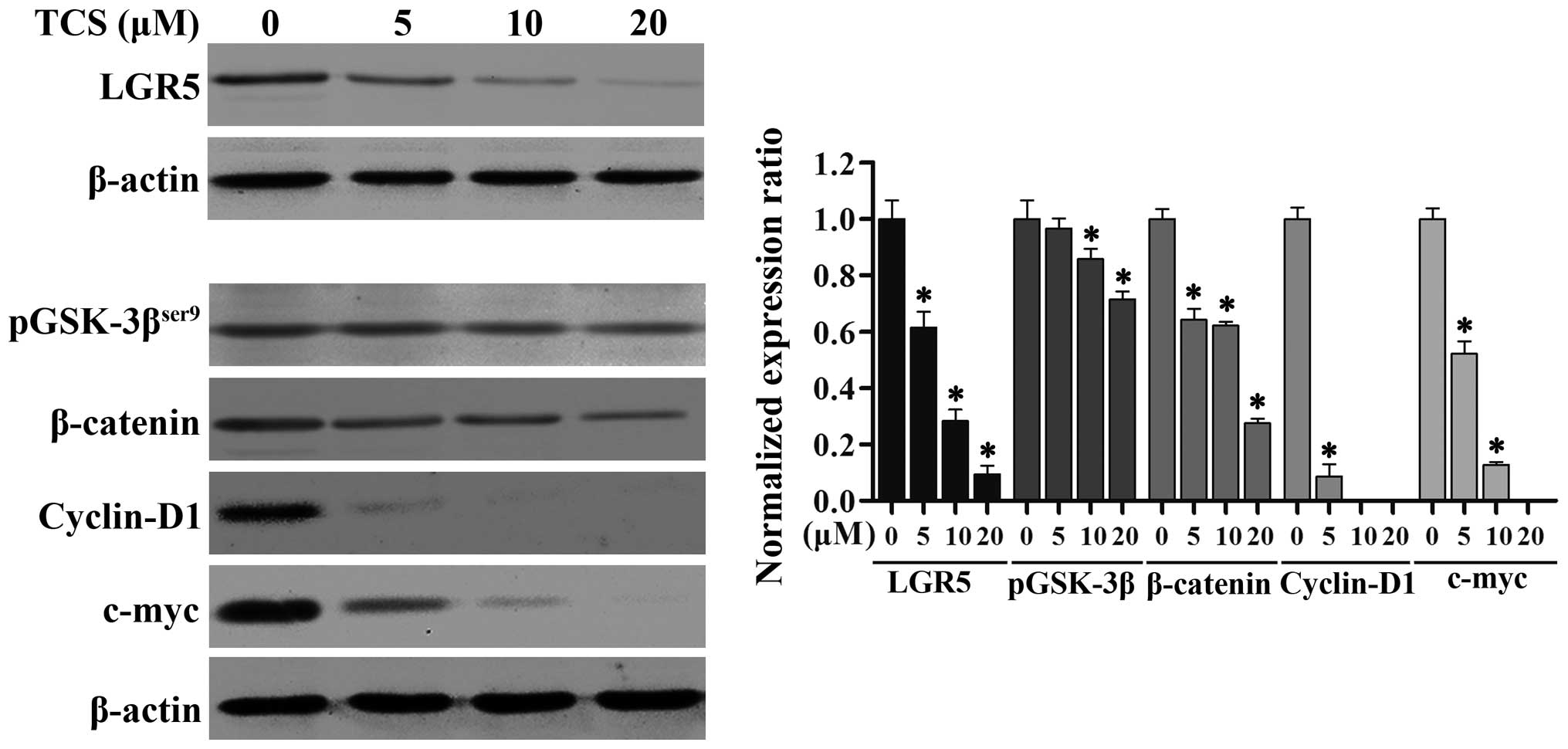
TCS inhibits the expression of LGR5 and
key proteins in the Wnt/β-catenin signaling pathway in glioma
cells
Studies have shown that LGR5 and the Wnt/β-catenin
signaling pathway play crucial roles in the proliferation,
differentiation and metastasis of cancers of varying origin
(25). To further explore the
suppressive mechanism of action of TCS in glioma cells, we examined
the expression levels of LGR5 by immunoblotting. As shown in
Fig. 5, as the TCS dose increased
(0–20 µM; 24 h), the levels of LGR5 protein expression in
the U87 cells markedly decreased. We also analyzed the protein
levels of phosphorylated glycogen synthase kinase-3β
(pGSK-3βser9), β-catenin, c-myc and cyclin D1, which are
key members of the Wnt/β-catenin signaling pathway, via western
blot analysis, and we found that increasing concentrations of TCS
(0–20 µM; 24 h) resulted in an evident downregulation of
these proteins (Fig. 5). Taken
together, these results demonstrated that TCS treatment reduces the
expression of LGR5 and key proteins in the Wnt/β-catenin signaling
pathway in a dose-dependent manner in glioma cells.
Discussion
Malignant gliomas account for the majority of
primary brain tumors (26). The
development of neuroimaging has allowed for the early diagnosis of
these gliomas, and standard treatment with surgery, chemotherapy
and radiation can be provided; however, patients with malignant
gliomas nonetheless have poor prognoses. According to research
data, the median survival duration for malignant glioma patients
after treatment is less than one year (27). Therefore, neurosurgical studies of
malignant gliomas have long focused on finding more effective
treatment approaches. As a newly discovered active constituent of
natural products, TCS has drawn a great deal of research attention
due to its antitumor effects. In the present study, we demonstrated
for the first time that TCS produces anti-glioma effects by
inhibiting cell viability, migration, and invasion and by inducing
apoptosis. Our results revealed that TCS significantly inhibited
the proliferation of the U87 and U251 cell lines, which contain
large numbers of glioma stem cells, in a dose- and time-dependent
manner. The IC50 values for U87 cells were lower than
the corresponding IC50 values for U251 cells, indicating
that U87 cells were more vulnerable than U251 cells to TCS.
Therefore, U87 cells were selected for additional
experimentation.
Prior research has demonstrated that inducing
apoptosis in tumor cells is an important mechanism of action for
many antitumor drugs. In our study, flow cytometry revealed that
apoptosis occurred in glioma cells after TCS treatment. Consistent
with this finding, different typical morphological characteristics
of apoptotic cells, including the condensation and clustering of
chromatin along the nuclear membrane, internucleosomal DNA
fragmentation and a decrease in mitochondrial membrane potential,
were detected in the TCS-treated U87 cells by DAPI staining, a
TUNEL assay, and JC-1 staining, respectively. These results suggest
that TCS inhibits the proliferation of malignant glioma cells by
inducing apoptosis.
However, the mechanism by which TCS induces
apoptosis in malignant glioma cells has not been well established.
LGR5, a member of the G protein-coupled receptor family (28), plays an important role in embryonic
development and is widely expressed in brain and spinal cord tissue
(29,30). Recent studies have revealed that the
overexpression of LGR5 promotes the survival and growth of
different types of tumors, including colorectal carcinomas, Ewing's
sarcomas and glioblastomas (31–33).
Furthermore, in our previous research, we found that LGR5
expression was closely associated with the pathologic grade of
gliomas; moreover, the knockdown of LGR5 was found to inhibit the
proliferation of U87 cells and suppresses the growth of xenografts
(34). In the present study, our
findings demonstrated that TCS treatment significantly reduce the
levels of LGR5, pGSK-3βSer9, β-catenin, c-myc and cyclin
D1 in the U87 cells. It has been well established that LGR5,
pGSK-3βSer9, β-catenin, c-myc and cyclin D1 are
important proteins of the Wnt/β-catenin pathway, which is
intimately involved in normal development and the regulation of
cancer stem cells (35). LGR5 binds
to Wnt, low-density lipoprotein receptor-related protein (LRP) and
frizzled (FZD) after interaction with the ligand R-spondin (RSPO);
the resulting complex promotes the phosphorylation of
GSK-3βSer9 and inhibits the phosphorylation of β-catenin
(36). Unphosphorylated β-catenin
translocates to the nucleus and interacts with transcription
factors, activating the expression of downstream genes such as
c-myc and cyclin D1 and ultimately promoting cell proliferation
(37). Research has reported that
in colorectal cancer cells, the depletion of LGR5 can decrease the
expression of c-myc and cyclin D1, which are downstream target
genes of the Wnt/β-catenin pathway, and induce apoptosis via an
intrinsic apoptosis pathway (38).
Therefore, we believe that suppression of LGR5 expression and of
the Wnt/β-catenin signaling pathway may be the mechanism by which
TCS induces apoptosis in U87 cells. However, understanding the
detailed mechanism will require further study.
Further studies of the efficacy of TCS in in
vivo models are needed to confirm our findings, although our
results have demonstrated that TCS can induce apoptosis and inhibit
the invasive/metastatic potential of glioma cells; thus, this study
has revealed a novel concept for the treatment of malignant
gliomas.
Acknowledgments
We thank Dr Weidong Yu and Mrs. Xin Yu for providing
technical assistance with western blotting, fluorescence microscopy
and FACS. We would also like to thank Dr Xiangjun He and Mrs. Mei
Li for providing laboratory equipment. We thank American Journal
Experts (AJE) for English language editing. This study was
supported by the Peking University People's Hospital Research and
Development Funds (no. RDB2011-14) and by the National Natural
Science Foundation of China (no. 81001009).
References
|
1
|
Jansen M, Yip S and Louis DN: Molecular
pathology in adult gliomas: Diagnostic, prognostic, and predictive
markers. Lancet Neurol. 9:717–726. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fortin Ensign SP, Mathews IT, Symons MH,
Berens ME and Tran NL: Implications of Rho GTPase signaling in
glioma cell invasion and tumor progression. Front Oncol. 3:2412013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maraganore JM, Joseph M and Bailey MC:
Purification and characterization of trichosanthin. Homology to the
ricin A chain and implications as to mechanism of abortifacient
activity. J Biol Chem. 262:11628–11633. 1987.PubMed/NCBI
|
|
5
|
Zhang XJ and Wang JH: Homology of
trichosanthin and ricin A chain. Nature. 321:477–478. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sha O, Niu J, Ng TB, Cho EY, Fu X and
Jiang W: Antitumor action of trichosanthin, a type 1
ribosome-inactivating protein, employed in traditional Chinese
medicine: A mini review. Cancer Chemother Pharmacol. 71:1387–1393.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang CY, Gong YX, Ma H, An CC and Chen
DY: Trichosanthin induced calcium-dependent generation of reactive
oxygen species in human choriocarcinoma cells. Analyst.
125:1539–1542. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang C, Gong Y, Ma H, An C, Chen D and
Chen ZL: Reactive oxygen species involved in trichosanthin-induced
apoptosis of human choriocarcinoma cells. Biochem J. 355:653–661.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang P, Chen LL, Yan H and Li JC:
Trichosanthin suppresses HeLa cell proliferation through inhibition
of the PKC/MAPK signaling pathway. Cell Biol Toxicol. 25:479–488.
2009. View Article : Google Scholar
|
|
10
|
Wang P, Xu S, Zhao K, Xiao B and Guo J:
Increase in cytosolic calcium maintains plasma membrane integrity
through the formation of microtubule ring structure in apoptotic
cervical cancer cells induced by trichosanthin. Cell Biol Int.
33:1149–1154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cui L, Song J, Wu L, Huang L, Wang Y,
Huang Y, Yu H, Huang Y, You CC and Ye J: Smac is another pathway in
the anti-tumour activity of trichosanthin and reverses
trichosanthin resistance in CaSki cervical cancer cells. Biomed
Pharmacother. 69:119–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dou CM and Li JC: Effect of extracts of
trichosanthes root tubers on HepA-H cells and HeLa cells. World J
Gastroenterol. 10:2091–2094. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fang EF, Zhang CZ, Zhang L, Wong JH, Chan
YS, Pan WL, Dan XL, Yin CM, Cho CH and Ng TB: Trichosanthin
inhibits breast cancer cell proliferation in both cell lines and
nude mice by promotion of apoptosis. PLoS One. 7:e415922012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zheng YT, Zhang WF, Ben KL and Wang JH: In
vitro immuno-toxicity and cytotoxicity of trichosanthin against
human normal immunocytes and leukemia-lymphoma cells.
Immunopharmacol Immunotoxicol. 17:69–79. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li J, Xia X, Ke Y, Nie H, Smith MA and Zhu
X: Trichosanthin induced apoptosis in HL-60 cells via mitochondrial
and endoplasmic reticulum stress signaling pathways. Biochim
Biophys Acta. 1770:1169–1180. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li J, Xia X, Nie H, Smith MA and Zhu X:
PKC inhibition is involved in trichosanthin-induced apoptosis in
human chronic myeloid leukemia cell line K562. Biochim Biophys
Acta. 1770:63–70. 2007. View Article : Google Scholar
|
|
17
|
Byers VS, Levin AS, Malvino A, Waites L,
Robins RA and Baldwin RW: A phase II study of effect of addition of
trichosanthin to zidovudine in patients with HIV disease and
failing antiretroviral agents. AIDS Res Hum Retroviruses.
10:413–420. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McGrath MS, Hwang KM, Caldwell SE, Gaston
I, Luk KC, Wu P, Ng VL, Crowe S, Daniels J and Marsh J: GLQ223: An
inhibitor of human immunodeficiency virus replication in acutely
and chronically infected cells of lymphocyte and mononuclear
phagocyte lineage. Proc Natl Acad Sci USA. 86:2844–2848. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang N, Li Z, Jiao Z, Gu P, Zhou Y, Lu L
and Chou KY: A Trichosanthin-derived peptide suppresses type 1
immune responses by TLR2-dependent activation of CD8(+)CD28(−)
Tregs. Clin Immunol. 153:277–287. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tang NL, Chan WL, Ke YO, Mak MK, Lai FM
and Tam SC: Acute renal failure and proximal tubule lesions after
trichosanthin injection in rats. Exp Mol Pathol. 64:78–89. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li F, Mei Y, Wang Y, Chen C, Tu J, Xiao B
and Xu L: Trichosanthin inhibits antigen-specific T cell expansion
through nitric oxide-mediated apoptosis pathway. Cell Immunol.
234:23–30. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ng TB, Liu WK, Tsao SW and Yeung HW:
Effect of trichosanthin and momorcharins on isolated rat
hepatocytes. J Ethnopharmacol. 3:81–87. 1994. View Article : Google Scholar
|
|
23
|
Garcia PA, Bredesen DE, Vinters HV,
Graefin von Einsiedel R, Williams RL, Kahn JO, Byers VS, Levin AS,
Waites LA and Messing RO: Neurological reactions in HIV-infected
patients treated with trichosanthin. Neuropathol Appl Neurobiol.
19:402–405. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ng TB, Kwong WH and Yeung HW: Intravenous
injections of the ribosome inactivating protein trichosanthin did
not affect methionine enkephalin and β-endorphin levels in the
mouse brain and pituitary. Biochem Mol Biol Int. 39:985–989.
1996.PubMed/NCBI
|
|
25
|
Rot S, Taubert H, Bache M, Greither T,
Würl P, Eckert AW, Schubert J, Vordermark D and Kappler M: A novel
splice variant of the stem cell marker LGR5/GPR49 is correlated
with the risk of tumor-related death in soft-tissue sarcoma
patients. BMC Cancer. 11:4292011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stewart LA: Chemotherapy in adult
high-grade glioma: A systematic review and meta-analysis of
individual patient data from 12 randomised trials. Lancet.
359:1011–1018. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bleehen NM and Stenning SP: The Medical
Research Council Brain Tumour Working Party: A Medical Research
Council trial of two radiotherapy doses in the treatment of grades
3 and 4 astrocytoma. Br J Cancer. 64:769–774. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hsu SY, Liang SG and Hsueh AJ:
Characterization of two LGR genes homologous to gonadotropin and
thyrotropin receptors with extracellular leucine-rich repeats and a
G protein-coupled, seven-transmembrane region. Mol Endocrinol.
12:1830–1845. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hsu SY, Kudo M, Chen T, Nakabayashi K,
Bhalla A, van der Spek PJ, van Duin M and Hsueh AJ: The three
subfamilies of leucine-rich repeat-containing G protein-coupled
receptors (LGR): Identification of LGR6 and LGR7 and the signaling
mechanism for LGR7. Mol Endocrinol. 14:1257–1271. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Barker N and Clevers H: Leucine-rich
repeat-containing G-protein-coupled receptors as markers of adult
stem cells. Gastroenterology. 138:1681–1696. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
McClanahan T, Koseoglu S, Smith K, Grein
J, Gustafson E, Black S, Kirschmeier P and Samatar AA:
Identification of overexpression of orphan G protein-coupled
receptor GPR49 in human colon and ovarian primary tumors. Cancer
Biol Ther. 5:419–426. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Scannell CA, Pedersen EA, Mosher JT, Krook
MA, Nicholls LA, Wilky BA, Loeb DM and Lawlor ER: LGR5 is expressed
by Ewing sarcoma and potentiates Wnt/beta-catenin signaling. Front
Oncol. 3:812013. View Article : Google Scholar
|
|
33
|
Nakata S, Campos B, Bageritz J, Bermejo
JL, Becker N, Engel F, Acker T, Momma S, Herold-Mende C, Lichter P,
et al: LGR5 is a marker of poor prognosis in glioblastoma and is
required for survival of brain cancer stem-like cells. Brain
Pathol. 23:60–72. 2013. View Article : Google Scholar
|
|
34
|
Wang D, Zhou J, Fan C, Jiao F, Liu B, Sun
P, Miao J and Zhang Q: Knockdown of LGR5 suppresses the
proliferation of glioma cells in vitro and in vivo. Oncol Rep.
31:41–49. 2014.
|
|
35
|
Wend P, Holland JD, Ziebold U and
Birchmeier W: Wnt signaling in stem and cancer stem cells. Semin
Cell Dev Biol. 21:855–863. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Glinka A, Dolde C, Kirsch N, Huang YL,
Kazanskaya O, Ingelfinger D, Boutros M, Cruciat CM and Niehrs C:
LGR4 and LGR5 are R-spondin receptors mediating Wnt/β-catenin and
Wnt/PCP signalling. EMBO Rep. 12:1055–1061. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Anna CH, Iida M, Sills RC and Devereux TR:
Expression of potential beta-catenin targets, cyclin D1, c-Jun,
c-Myc, E-cadherin, and EGFR in chemically induced hepatocellular
neoplasms from B6C3F1 mice. Toxicol Appl Pharmacol. 190:135–145.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hsu HC, Liu YS, Tseng KC, Tan BC, Chen SJ
and Chen HC: LGR5 regulates survival through mitochondria-mediated
apoptosis and by targeting the Wnt/β-catenin signaling pathway in
colorectal cancer cells. Cell Signal. 26:2333–2342. 2014.
View Article : Google Scholar : PubMed/NCBI
|