Introduction
Gliomas are one of the most lethal types of cancer
and are the most common types of brain tumor in the central nervous
system (1). Although various
therapeutic methods such as advanced surgical techniques, radiation
treatment and new combined chemotherapy are performed, survival is
low due to the extremely aggressive and invasive features of C6
glioma cells (2). Thus, the
regulation of invasion and migration represents an important
therapeutic target of cancer. Tumor cell invasion and migration is
a complex multi-step process and essentially requires degradation
of the extracellular matrix (ECM). The degradation enzymes of the
ECM include the matrix metalloproteinases (MMPs), a disintegrin and
metalloproteinase with thrombospondin motif proteases, and serine
proteases of the urokinase/plasmin-type (1). Among various degradation enzymes of
the ECM, MMPs are associated with tumor spreading and poor
prognosis (3).
MMPs have a signal peptide and a catalytic domain
that contains the highly conserved zinc binding site (4). MMPs, such as collagenases,
gelatinases, stromelysins and membrane-type matrix
metalloproteinases (MT-MMPs), can degrade most ECM types. They have
various roles in physiological and pathological processes, such as
tissue development, remodeling, and inflammation in cancer. The
overexpression of MMPs is correlated with metastasis and invasion
of malignant cancer cells (5,6). In
particular, MMP-9 and MMP-2 are the key enzymes involved in the
degradation of type IV collagen and the ECM (7), and are mainly associated with tumor
invasion, degradation of the blood-brain barrier,
neuro-degenerative processes, and angiogenesis in gliomas (8,9). MMP-9
and MMP-2 have structural and catalytic similarities. However,
MMP-9 can be increased by various agents, such as inflammatory
cytokines, growth factor, and phorbol myristate acetate (PMA),
whereas MMP-2 is continually expressed and is usually overexpressed
in malignant tumors. Particularly, PMA induces inflammation and
promotes tumor growth by increasing the invasion of various types
of cancer cells through MMP-9 activation (10).
Furthermore, PMA regulates MMP-9 expression by
regulating transcription factors including nuclear factor (NF)-κB
and activator protein-1 (AP-1) via mitogen-activated protein kinase
(MAPK) pathways (11,12). NF-κB and AP-1 are both important
transcription factors associated with the modulation of cell
migration and invasion (12). MAPK
is modulated by the focal adhesion kinase (FAK) pathway in various
types of cancers. FAK is a crucial target for the regulation of
tumor invasion and metastasis (13). It also modulates cell motility and
cell adhesion by transferring ECM signals from integrins to the
intracellular compartment (14).
Natural dietary phytochemicals are found in fruits,
spices, teas and vegetables. Among the various phytochemical
compounds, isothiocyanates (ITCs) are isolated from cruciferous
vegetables and are characterized as having a sulfur-containing
N=C=S functional group (15). ITCs
have shown biological and pharmacological activities in diseases,
including chronic-degenerative diseases, which include
cardiovascular diseases, neurodegeneration and diabetes (16,17).
Additionally, ITCs, including benzyl isothiocyanate (BITC),
phenethyl isothiocyanate (PEITC) and sulforaphane (SFN) have
preventative effects on various types of cancers (18). ITCs suppress myeloma, breast cancer,
and pancreatic cancer by inhibiting MMP-9 through NF-κB (19,20).
BITC was found to inhibit migration and invasion of human colon
cancer HT29 cells through the MAPK signaling pathway (21). PEITC inhibits the migration and
invasion of human gastric adenocarcinoma cells by suppressing the
NF-κB signaling pathways (22).
Moreover, SFN was found to sensitize TNFα-related
apoptosis-inducing ligand-mediated apoptosis by downregulating
extracellular signal-regulated kinase (ERK) in lung adenocarcinoma
A549 cells (23).
Therefore, we hypothesized that the anti-metastatic
activity of ITCs may function to modulate MMP-9 in C6 glioma cells.
In this study, the effects of BITC, PEITC and SFN on PMA-induced
MMP-9 expression were examined in C6 glioma cells. It was found
that ITCs suppressed PMA-induced MMP-9 expression by inhibiting
MMP-9 transcription levels by blocking FAK/JNK-mediated AP-1 and
NF-κB activation. Additionally, suppression of C6 glioma cell
invasion by ITCs was associated with inhibitory effects on MMP-9
expression.
Materials and methods
Cells and materials
The C6 rat glioma cell line was obtained from the
American Type Culture Collection (ATCC; USA). The culture medium
used in the experiments was Dulbecco's modified Eagle's medium
(DMEM; Thermo Scientific, Logan, UT, USA) containing 10% fetal
bovine serum (FBS) and 1% antibiotic-antimycotic. The cells were
incubated at 37°C in a humidified atmosphere of 5% Co2.
BITC, PEITC, SFN and N-methyl-β-phenethylamine (NMPEA) were
obtained from Sigma (St. Louis, MO, USA).
Cell viability assay
C6 cells were seeded in a 96-well plate and allowed
to attach for 24 h. Media were then discarded and replaced with 100
µl of new media containing various concentrations of ITCs
and cultured for 24 h.
3-[4,5-Dimethylthiazol2-yl]-2.5-diphenyltetrazolium bromide (MTT;
Roche Applied Science, Indianapolis, IN, USA) was added to each
well. The amount of formazan deposits was quantified according to
the supplier's protocol after 4 h of incubation with MTT reagent at
37°C in a 5% Co2 incubator. The half maximal inhibitory
concentrations (IC50) were determined as the
concentration of the test mixture that gave a 50% reduction in
absorbance compared to that of the control.
Gelatin zymography assay
Zymography was performed using the procedure
described by Lee et al with minor modification (24). C6 cells were seeded in 6-well
culture plates and incubated until they reached 80% confluency.
Fresh serum-free medium was then added with ITCs to each dish, and
further cultured for 24 h. Conditioned medium, so obtained, was
electrophoresed on polyacrylamide gels containing 0.1% (w/v)
gelatin. Gels were washed at room temperature for 30 min with 2.5%
Triton X-100 and then incubated at 37°C for 24 h in a buffer
containing 10 mM CaCl2, 0.01% NaN3 and 50 mM
Tris-HCl (pH 7.5). Gels were then stained with 0.2% Coomassie
Brilliant Blue (Bio-Rad, Hercules, CA, USA) and photographed on a
light box. Proteolysis was detected as a white zone in a dark blue
field.
Quantification of intracellular reactive
oxygen species (ROS)
The intracellular concentration of ROS in C6 glioma
cells was measured using an oxidation-sensitive fluorescent probe
dye, 2′,7′-dichlorodihydrofluorescein diacetate (DCF-DA; Invitrogen
Molecular Probes, Eugene, OR, USA). DCF-DA diffuses into cells,
where it is hydrolyzed by intracellular esterase to polar
2′,7′-dichlorodihydrofluorescein. This non-fluorescein analog gets
trapped inside the cells and is oxidized by intracellular oxidants
to a highly fluorescent 2′,7′-dichlorofluorescein level. C6 glioma
cells were treated with ITCs and PMA for 12 h, after which the
cells were incubated with 10 µM DCF-D at 37°C for 30 min.
The fluorescence of 2′,7′-dichlorofluores-cein was detected in
equivalent quantities of proteins using a multi-plate reader,
VICTOR3 (excitation, 530 nm, emission, 485 nm; Perkin-Elmer,
Waltham, MA, USA).
Western blot analysis
C6 cells were suspended in lysis buffer (50 mM Tris,
150 mM NaCl, 5 mM EDTA, 1 mM DTT, 1% Nonidet P-40, 100 µM
phenylmethylsulfonyl fluoride, 20 µM aprotinin and 20
µM leupeptin, adjusted to ph 8.0) at 4°C for 30 min,
followed by centrifugation at 12,000 rpm for 10 min. In addition,
to separate the proteins in cells buffer A [10 mM HEPES (pH 7.9),
1.5 mM MgCl2, 10 mM KCl, 0.5 mM DTT, 300 mM saccharose,
0.1% NP-10, 0.5 mM phenylmethylsulfonyl fluoride] was used. After
incubation for 5 min on ice, the samples were centrifuged at 1,000
rpm at 4°C for 1 min and the pellet was separated. A separate
pellet was dissolved in buffer B [20 mM HEPES (ph 7.9), 20%
glycerol, 100 mM KCl, 100 mM NaCl2, 0.2 mM EDTA, 0.5 mM
DTT, 0.5 mM phenylmethylsulfonyl fluoride]. After incubation for 15
min on ice, the samples were centrifuged at 1,000 rpm at 4°C for 5
min. Total protein concentration was determined using the Bradford
assay (Bio-Rad). Total protein (30 µg) was separated on 6 to
12% SDS-polyacrylamide gels and transferred to nitrocellulose
membranes (Schleicher & Schuell, Keene, NH, USA) using standard
SDS-polyacrylamide gel electrophoresis procedure. The membranes
were blocked in 5% skim milk in TBS-T for 1 h at room temperature.
The membranes were then incubated with the primary antibody
overnight at 4°C, washed three times with TBS-T, incubated with
goat anti-mouse IgG or goat anti-rabbit IgG secondary antibodies
for 1 h at room temperature and then washed with TBS-T three times.
Signals were detected using enhanced chemiluminescence (ECL;
Amersham Life Science Corporation, Arlington heights, IL, USA) film
and ChemiDOC XRS (Bio-Rad). Primary antibodies used in this study
were MMP-9 and MMP-2 purchased from Millipore (Billerica, MA, USA).
Phospho-ERK, phospho-JNK, phospho-p38, phospho-AKT, phospho-FAK,
NF-κB, c-Jun, and c-Fos were purchased from Santa Cruz
biotechnology, Inc. (Santa Cruz, CA, USA).
Reverse transcription-polymerase chain
reaction
Total RNA was extracted using TRIzol (Invitrogen,
Carlsbad, CA, USA), according to the manufacturer's instructions.
For RT-PCR, cDNA was synthesized from 1 µg of total RNA
using Bioneer AccuPower PCR PreMix kit (Daejeon, Korea) according
to the manufacturer's protocol. The cDNA was amplified by PCR with
the following primers: MMP-9, 5′-AAACCTCCAACCTCACGGAC-3′ (sense)
and 5′-GAAAGGCGTGTGCCAGTAGA-3′ (antisense); TIMP-1,
5′-CTGCAACTCGGACCTGGTTA-3′ (sense) and 5′-GTGCACAAATCTGGATTCCG-3′
(antisense); and β-actin, 5′-ATGTGGATAAAGCCGTCAGTGG-3′ (sense) and
5′-CTGGAGTGTCCATGGGACAG-3′ (antisense). PCR products were analyzed
by agarose gel electrophoresis and visualized by treatment with
ethidium bromide.
Transwell invasion assay
Matrigel-coated filter inserts (8-µm pore
size) that fit into 24-well migration chambers were obtained from
Becton-Dickinson (Franklin Lakes, NJ, USA). Cells were then plated
on the upper chamber. The lower chamber was filled with culture
media containing various drugs. Cells in the chamber were incubated
for 24 h at 37°C and cells that invaded the lower membrane surface
were fixed with methanol and stained with hematoxylin and eosin.
The cells that passed through the Matrigel and were located on the
underside of the filter were counted. Random fields were counted by
light microscopy (×400 magnification).
Wound-healing assay
This assay was performed using the procedure
described by Lin et al with minor modification (25). Cells were seeded in 6-well plates
and incubated until they reached 80% confluency. Monolayers were
scratched with a 200-µl pipette tip to create a wound, and
cells were then washed twice with serum-free culture media to
remove floating cells. Media were then replaced with fresh
serum-free media. Cells were subjected to the indicated treatment
for 24 h, and cells were photographed at 24 h.
Statistical analysis
All in vitro results are representative of at
least three independent experiments performed in triplicate. The
significance of differences between the experimental and control
values was analyzed by the Newman-Keuls test using SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA). P-values of <0.05 were
deemed to indicate a significant difference.
Results
ITCs inhibit PMA-induced MMP-9 expression
and activity
Before investigating the anticancer pharmacological
potential of ITCs on C6 glioma cells, the effect of ITCs on cell
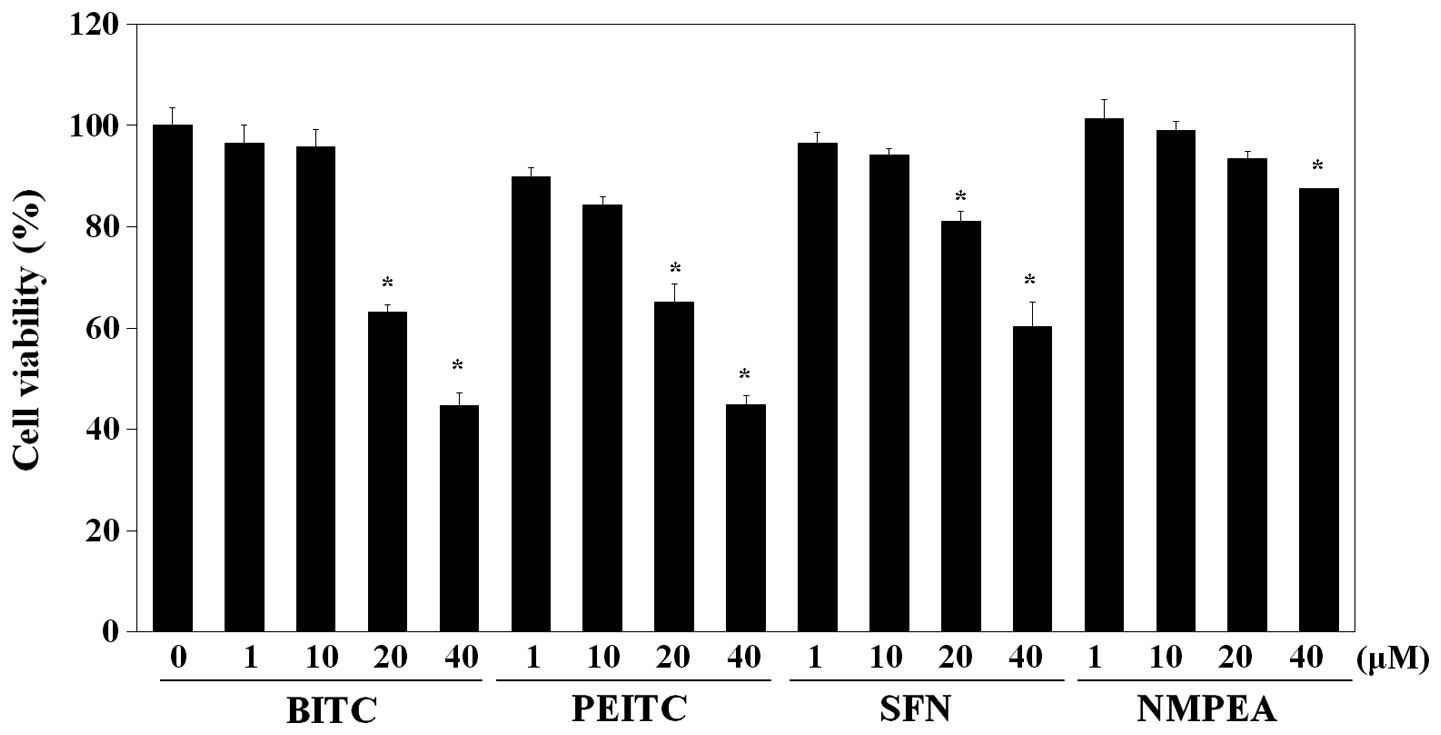
viability was first examined. As shown in Fig. 1, a cytotoxic effect on C6 glioma
cells was exhibited by BITC (IC50, 32.8 µM),
whereas PEITC (IC50, 34.3 µM) showed comparable
cytotoxic activity and SFN (IC50, 50.8 µM)
exhibited relatively less cytotoxicity. NMPEA, which is a
structural analog of PEITC without ITC functionality (26), was used as the negative control.
NMPEA did not significantly affect the cell viability below a
concentration of 20 µM. There was no obvious reduction in
the cell viability of the C6 glioma cells after treatment with all
ITCs drugs at doses <10 µM. Based on these results, a
concentration of 10 µM of the ITCs was used in the following
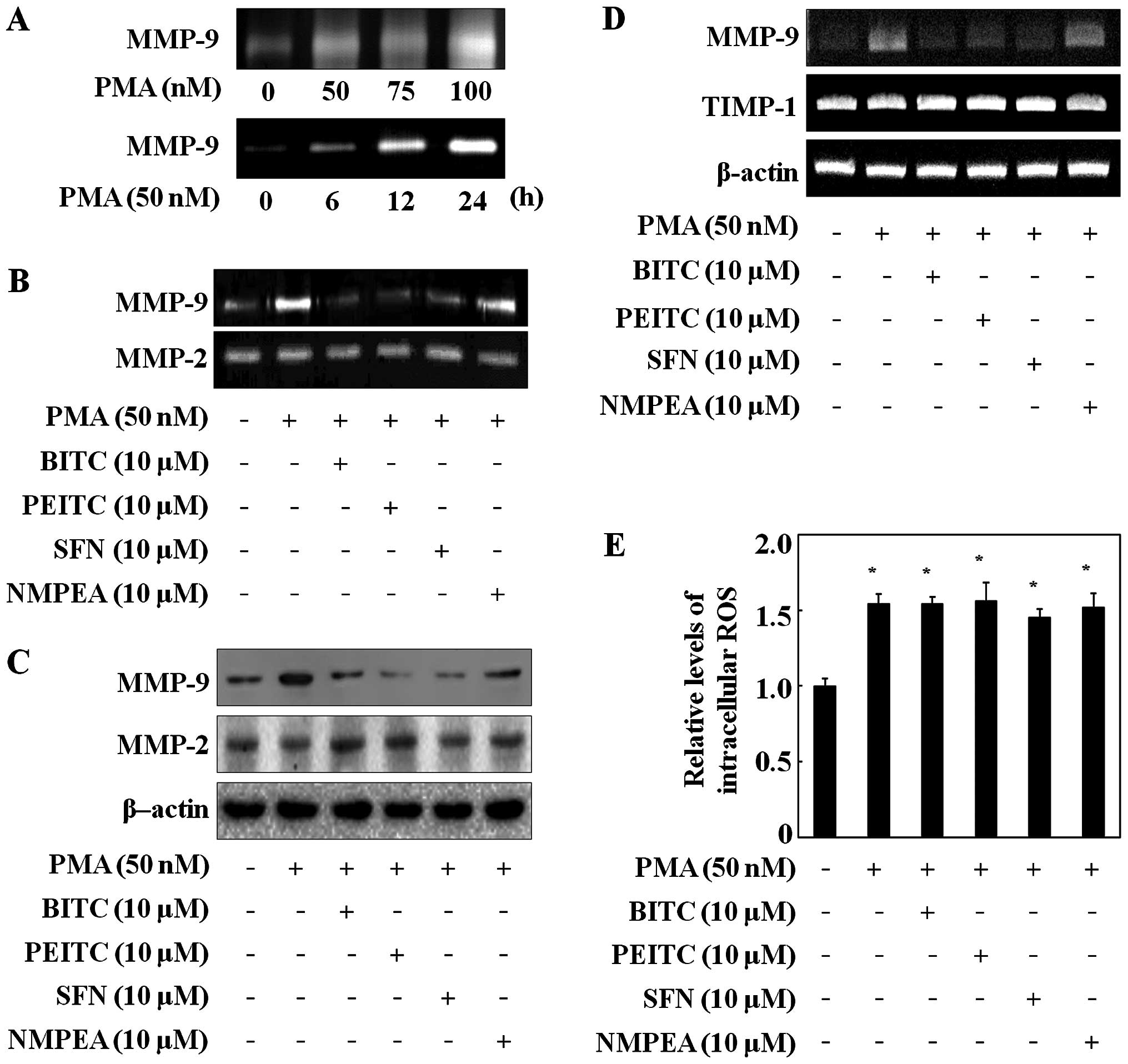
experiments. The MMP-9 secretion in the C6 glioma cells was induced
by PMA in a dose-dependent manner and a time-dependent manner
(Fig. 2A), whereas MMP-2 did not
change (data not shown). In the following experiments, the C6
glioma cells were treated with 50 nM PMA for 24 h. Then the
inhibitory effects of ITCs on MMP-9 and MMP-2 activity were
determined via gelatin zymography assay. ITCs at a concentration of
10 µM decreased the MMP-9 activity in the C6 glioma cells
but did not change MMP-2 activity (Fig.
2B).
To further confirm the influence of ITCs on MMP-9
expression, western blotting was performed. ITCs inhibited MMP-9
expression, whereas MMP-2 expression was not reduced in the C6
glioma cells (Fig. 2C). NMPEA had
no effect on both MMP-9 activity and expression (Fig. 2B and C). ITCs also reduced the
PMA-induced MMP-9 mRNA level in the C6 glioma cells, whereas NMPEA
had no affect (Fig. 2D). Moreover,
since the activity of MMP-9 is regulated by the endogenous tissue
inhibitor of metalloproteinase-1 (TIMP-1) (27), the expression level of TIMP-1 was
measured via RT-PCR. ITCs did not alter TIMP-1 mRNA expression.
These results suggest that the sulfur-containing functional group
of the ITCs directly inhibited the MMP-9 transcription level, as
NMPEA had no effect on PMA-induced C6 glioma cells. In addition,
since ROS generation is known to trigger PMA-mediated induction of
cell migration (28), the effects
of ITCs on intracellular ROS concentrations were measured. The C6
glioma cells exposed to PMA had increased ROS levels compared with
the untreated cells (Fig. 2E).
However, ITCs did not affect the PMA-induced ROS generation in the
C6 glioma cells, suggesting that the inhibitory effects of ITCs on
MMP-9 were not related to ROS generation.
ITCs reduce FAK-dependent JNK
phosphorylation in C6 glioma cells
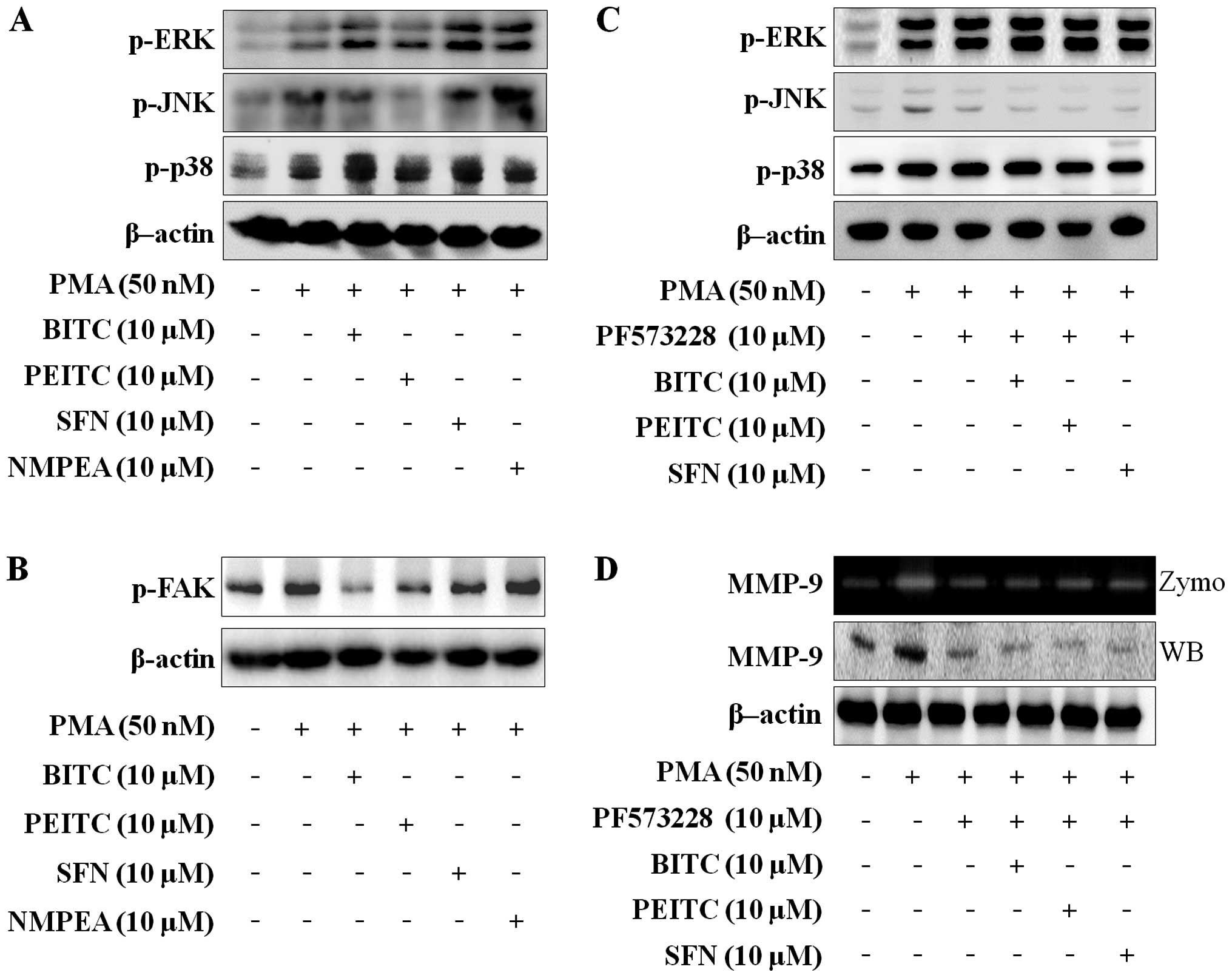
MAPK is one of the pathways involved in the
modulation of PMA-induced MMP-9 expression (29). To investigate the effects of ITCs on
the MAPK expression associated with migration and invasion in C6
glioma cells, western blotting was performed. As shown in Fig. 3A, the ITCs suppressed the
PMA-induced phosphorylation of JNK, but ERK and p38 were not
changed. As the FAK/JNK and FAK/ERK signaling pathways control MMP
secretion in carcinoma cells (30,31),
it was determined whether the ITCs suppress PMA-induced
phosphorylation of FAK in C6 glioma cells. ITCs with a
concentration of 10 µM inhibited the PMA-induced
phosphorylation of FAK (Fig. 3b),
and NMPEA had no effect. To confirm that FAK modulates the
PMA-induced MAPK pathways, C6 glioma cells were treated with 10
µM PF573228 (FAK inhibitor). The PMA-induced JNK
phosphorylation was decreased by PF573228. However, PMA-induced
phosphorylation of ERK and p38 were not changed (Fig. 3C). In addition, co-treatment with
ITCs and PF573228 only blocked PMA-induced JNK phosphorylation in
C6 glioma cells. We further analyzed the effect of PF573228 on
MMP-9 expression and activity in PMA-induced C6 glioma cells. The
MMP-9 expression and activity were blocked by PF573228 and
co-treatment with ITCs and PF573228 (Fig. 3D). We suggest that ITCs suppress
MMP-9 activity and expression via blocking FAK-dependent JNK
phosphorylation.
ITCs decrease nuclear translocation of
NF-κB and AP-1
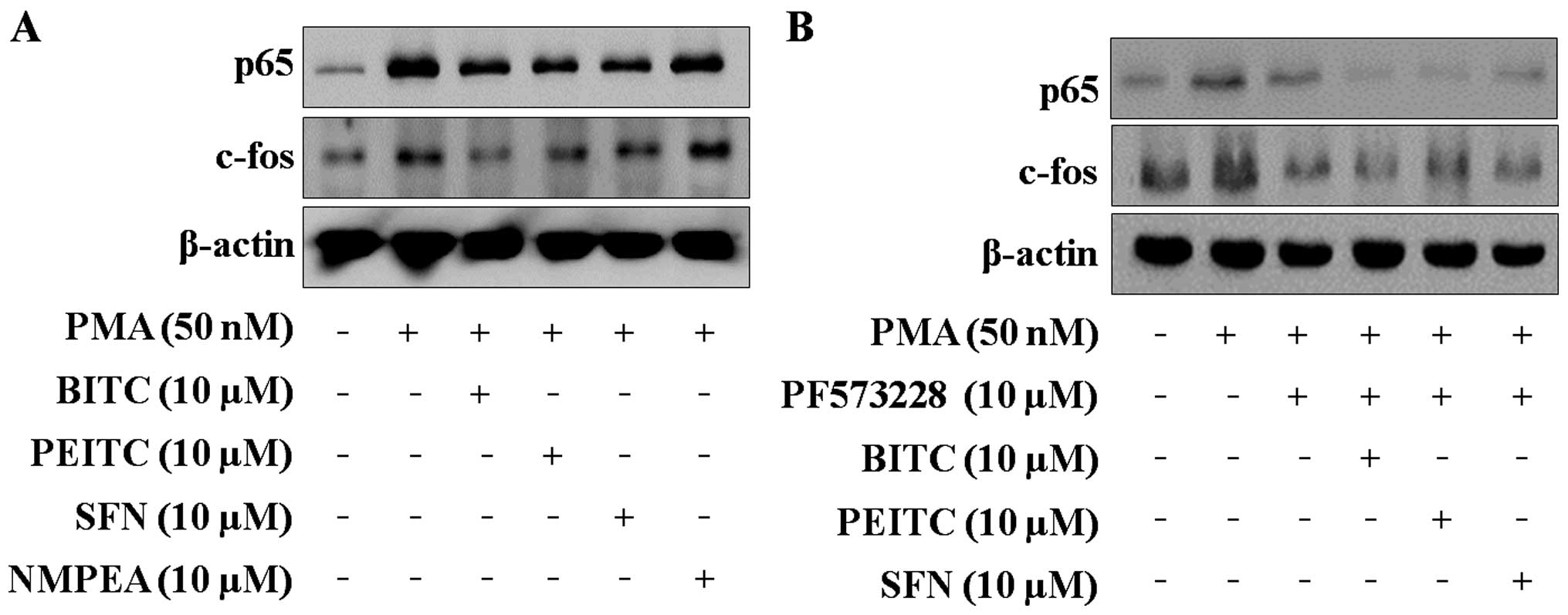
NF-κB and AP-1 are both important transcription
factors associated with the modulation of cell migration and
invasion (12). As shown in
Fig. 4A, PMA induced the nuclear
translocation of the NF-κB subunit p65 and the AP-1 subunit c-fos.
ITCs blocked the nuclear translocation of p65 and c-fos. It was
further confirmed that FAK regulates the nuclear translocation of
p65 and c-fos using PF573228. The nuclear translocation of p65 and
c-fos was blocked by PF573228, and co-treatment with ITCs and
PF573228 (Fig. 4B). These data
suggest that ITCs regulate the transcriptional activation of MMP-9
by reducing the PMA-induced nuclear translocation of the NF-κB
subunit p65 and the AP-1 subunit c-fos.
ITCs inhibit the migration and invasion
of C6 glioma cells
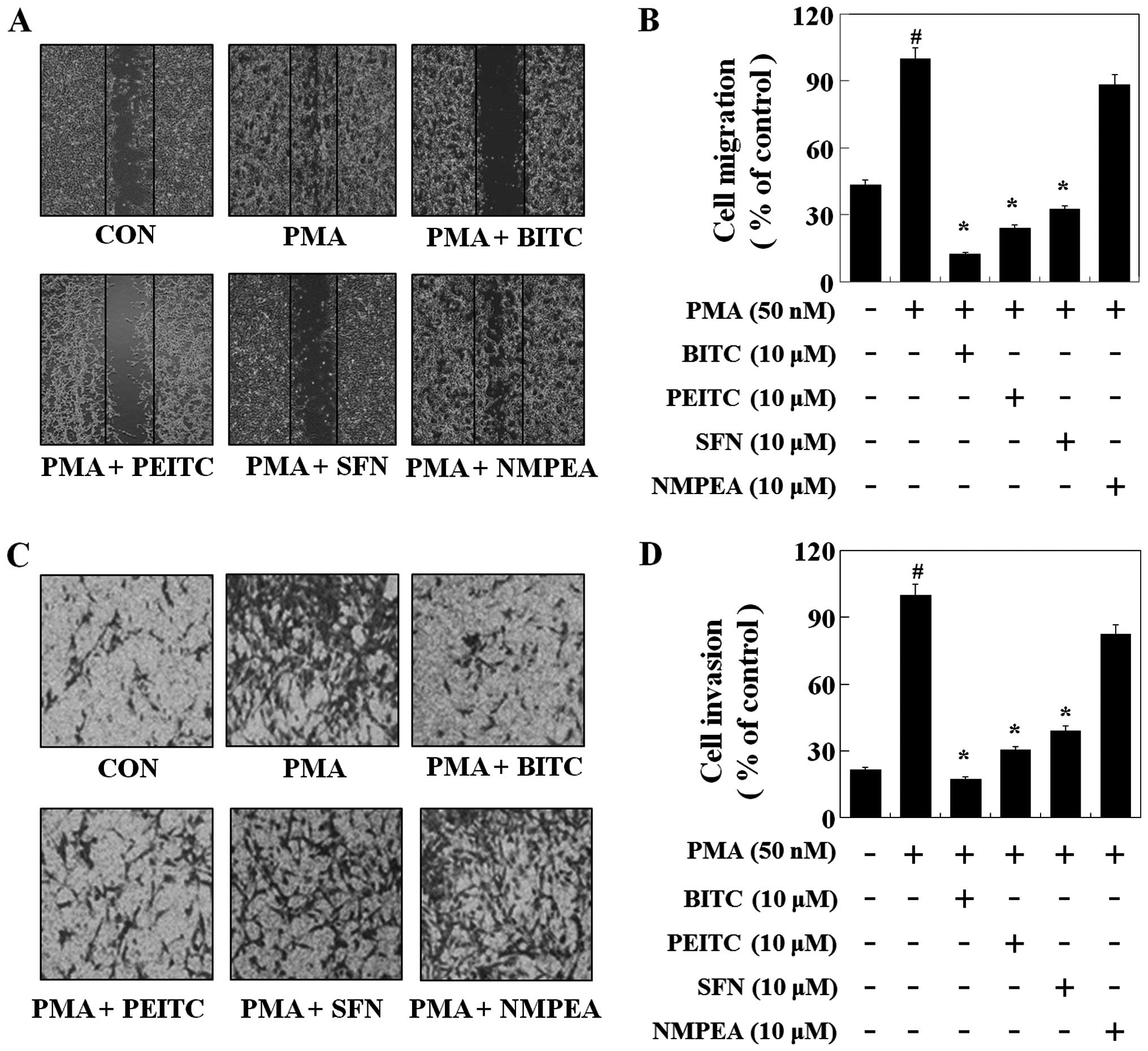
A wound-healing experiment was performed to evaluate
the effect of ITCs on C6 glioma cell migration. C6 glioma cells
were grown and then wounded by scraping. As illustrated in Fig. 5A and B, PMA induced the migration of
the C6 glioma cells. BITC, PEITC and SFN at the concentration of 10
µM decreased C6 glioma cell migration by 88, 76 and 68%
compared with PMA, respectively. To further determine the
inhibitory effect of ITCs on invasion, C6 glioma cells were treated
with 10 µM of ITCs, a Matrigel-based Transwell invasion
assay was performed. BITC, PEITC and SFN reduced the C6 glioma cell
invasion by 83, 70 and 61%, respectively (Fig. 5C and D). However, NMPEA did not
affect PMA-induced cell migration and invasion, suggesting that
ITCs suppressed the migration and invasion of C6 glioma cells by
inhibiting MMP-9 expression and the sulfur-containing functional
group plays an important role in the anti-metastatic effects of
ITCs.
Discussion
ITCs, including BITC, PEITC and SFN, are natural
phytochemicals. They have been reported to inhibit cancer
development, cardiovascular diseases, neurodegenerative diseases,
and other chronic-degenerative pathologies (16,17).
In detail, BITC was found to inhibit breast cancer stem cells
(32). PEITC suppressed
EGF-stimulated SAS human oral squamous carcinoma cell invasion by
targeting EGF receptor signaling (33). SFN was found to inhibit
MMP-9-activated human brain microvascular endothelial cell
migration and tubulogenesis (34).
In addition, ITCs were found to induce the growth inhibition and
apoptosis of human brain malignant glioma cells (35,36).
Furthermore, our study confirmed that ITCs inhibit the migration
and invasion of C6 glioma cells.
Gliomas are the most common brain tumors, which
originate in glial cells. They are difficult to cure since extreme
invasion recurs after surgical resection (1). Thus, the inhibitory effect on the
invasiveness of these cancer cells is an important therapeutic
target. Tumor metastasis is a multi-step process, that includes
changes in cell-ECM interaction, separation of intercellular
adhesion complexes, detachment of single cells from the solid tumor
mass, degradation of the ECM, and tumor cell migration into the
ECM. MMPs play a role in degrading all the components of the ECM
(37) and are reported to be major
proteinases involved in tumor growth, and associated with invasion
and migration (38). MMP-9 and
MMP-2 are involved in the invasion and migration of various types
of cancers, including gastric cancer (39), hepatocellular carcinoma (40), and glioblastoma multiform (41). In our study, ITCs reduced the
PMA-induced MMP-9 activity and expression but did not alter the
MMP-2 expression in PMA-induced C6 glioma cells. ITCs also
suppressed PMA-stimulated MMP-9 mRNA expression and did not affect
the TIMP-1 mRNA expression. These results suggest that ITCs
modulate PMA-induced MMP-9 activity by inhibiting MMP-9
transcription levels without altering TIMP-1. In addition, we found
that the ITCs did not affect PMA-induced ROS generation, which is a
potential inducer of cancer invasion and promotes apoptosis in
cancer cells (28). It has been
reported that PEITC induces ROS-mediated cancer cell death by
inhibiting oxidative phosphorylation (42). SFN also induced the growth
inhibition and apoptosis of neuroblastoma cells through an
ROS-dependent pathway (43). Based
on our results, however, non-cytotoxic concentrations of ITCs (10
µM) did not increase ROS generation. These results suggest
that the inhibitory effects of ITCs on PMA-induced MMP-9 were not
relevant to the apoptotic effects of ITCs.
The promoter of MMP-9 has cis-acting
regulatory elements for transcription factors that contain the
NF-κB site and the AP-1 site. The NF-κB transcription factor family
consists of five proteins: c-Rel, p105/p50 (NF-κB1), p100/52
(NF-κB2), p65 (RelA) and Relb. AP-1 is a transcriptional activator
composed of members of the Jun and Fos families (44). In this study, ITCs suppressed the
PMA-induced translocation of p65 and c-fos, suggesting that ITCs
decreased MMP-9 by inhibiting NF-κB and AP-1. Moreover, ITCs
suppressed PMA-induced C6 glioma cell migration and invasion. These
results showed that C6 glioma cell migration and invasion are
inhibited through MMP-9 suppression.
A previous study showed that the MAPK pathway
modulates MMP-9 expression by regulating transcription factors
(45). MAPK is composed of ERK,
JNK, and p38 and is found in various tumors, including the breast,
and may play a crucial role in tumor metastasis and progression
(46,47). In our study, ITCs suppressed the
PMA-induced phosphorylation of JNK in the C6 glioma cells. In
addition, FAK, a non-receptor kinase, was found to be overexpressed
in several tumors and regulates MMP-9 protein expression by
regulating the MAPK pathway (48).
It plays a major role in cell survival, proliferation, attachment,
migration and invasion (49,50).
ITCs decreased PMA-induced FAK phosphorylation in the C6 glioma
cells. The FAK inhibitor (PF573228) decreased the JNK
phosphory-lation and MMP-9 protein expression in the C6 glioma
cells. It also suppressed the transcription factors c-fos and p65.
These results suggest that ITCs suppress MMP-9 expression by
inhibiting the FAK/JNK pathway.
In conclusion, the present study showed that ITCs
have partial antitumor effects by inhibiting migration and invasion
through regulation of MMP-9 activation. We also found that the
sulfur-containing functional group is important to the
anti-metastatic effects of ITCs. Furthermore, it was demonstrated
that the inhibitory effects of ITCs on PMA-induced MMP-9 protein
expression are associated with the regulation of NF-κB and AP-1 by
suppressing the FAK/JNK signaling pathway. These results suggest
that ITCs are potential agents for the prevention of C6 glioma cell
migration and invasion.
Abbreviations:
|
ITCs
|
isothiocyanates
|
|
BITC
|
benzyl isothiocyanate
|
|
PEITC
|
phenethyl isothiocyanate
|
|
SFN
|
sulforaphane
|
|
ECM
|
extracellular matrix
|
|
MMPs
|
matrix metalloproteinases
|
|
PMA
|
phorbol myristate acetate
|
|
NF-κB
|
nuclear factor-κB
|
|
AP-1
|
activator protein-1
|
|
MAPK
|
mitogen-activated protein kinase
|
|
FAK
|
focal adhesion kinase
|
|
ERK
|
extracellular signal-regulated
kinase
|
Acknowledgments
The present study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Science, ICT and Future Planning
(no. 2014R1A2A1A11050776).
References
|
1
|
Mentlein R, Hattermann K and Held-Feindt
J: Lost in disruption: Role of proteases in glioma invasion and
progression. Biochim biophys Acta. 1825:178–185. 2012.PubMed/NCBI
|
|
2
|
Mangiola A, Anile C, Pompucci A, Capone G,
Rigante L and De Bonis P: Glioblastoma therapy: Going beyond
Hercules columns. Expert Rev Neurother. 10:507–514. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nakada M, Nakada S, Demuth T, Tran NL,
Hoelzinger DB and Berens ME: Molecular targets of glioma invasion.
Cell Mol Life Sci. 64:458–478. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Westermarck J and Kahari VM: Regulation of
matrix metalloproteinase expression in tumor invasion. FASEb J.
13:781–792. 1999.PubMed/NCBI
|
|
5
|
Kilian M, Gregor JI, Heukamp I, Hanel M,
Ahlgrimm M, Schimke I, Kristiansen G, Ommer A, Walz MK, Jacobi CA,
et al: Matrix metalloproteinase inhibitor RO 28-2653 decreases
liver metastasis by reduction of MMP-2 and MMP-9 concentration in
BOP-induced ductal pancreatic cancer in Syrian hamsters: Inhibition
of matrix metalloproteinases in pancreatic cancer. Prostaglandins
Leukot Essent Fatty Acids. 75:429–434. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Güllü IH, Kurdoğlu M and Akalin I: The
relation of gelatinase (MMP-2 and -9) expression with distant site
metastasis and tumour aggressiveness in colorectal cancer. Br J
Cancer. 82:2492000.PubMed/NCBI
|
|
7
|
Tsuchiya Y, Endo Y, Sato H, Okada Y, Mai
M, Sasaki T and Seiki M: Expression of type-IV collagenases in
human tumor cell lines that can form liver colonies in chick
embryos. Int J Cancer. 56:46–51. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rosenberg RN and Iannaccone ST: The
prevention of neurogenetic disease. Arch Neurol. 52:356–362. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bergers G and Coussens LM: Extrinsic
regulators of epithelial tumor progression: Metalloproteinases.
Curr Opin Genet Dev. 10:120–127. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ament SM, Gillissen F, Moser A, Maessen
JM, Dirksen CD, von Meyenfeldt MF and van der Weijden T:
Identification of promising strategies to sustain improvements in
hospital practice: A qualitative case study. BMC Health Serv Res.
14:6412014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hong S, Park KK, Magae J, Ando K, Lee TS,
Kwon TK, Kwak JY, Kim CH and Chang YC: Ascochlorin inhibits matrix
metallopro-teinase-9 expression by suppressing activator
protein-1-mediated gene expression through the ERK1/2 signaling
pathway: Inhibitory effects of ascochlorin on the invasion of renal
carcinoma cells. J Biol Chem. 280:25202–25209. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cho HJ, Kang JH, Kwak JY, Lee TS, Lee IS,
Park NG, Nakajima H, Magae J and Chang YC: Ascofuranone suppresses
PMA-mediated matrix metalloproteinase-9 gene activation through the
Ras/Raf/MEK/ERK- and Ap1-dependent mechanisms. Carcinogenesis.
28:1104–1110. 2007. View Article : Google Scholar
|
|
13
|
Son BH, Ahn SH, KO CD, Ka IW, Gong GY and
Kim JC: Significance of mismatch repair protein expression in the
chemotherapeutic response of sporadic invasive ductal carcinoma of
the breast. Breast J. 10:20–26. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao J, Zhang Y, Ithychanda SS, Tu Y, Chen
K, Qin J and WU C: Migfilin interacts with Src and contributes to
cell-matrix adhesion-mediated survival signaling. J Biol Chem.
284:34308–34320. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Melchini A and Traka MH: Biological
profile of erucin: A new promising anticancer agent from
cruciferous vegetables. Toxins (Basel). 2:593–612. 2010. View Article : Google Scholar
|
|
16
|
Cavell BE, Syed Alwi SS, Donlevy AM, Proud
CG and Packham G: Natural product-derived antitumor compound
phenethyl isothiocyanate inhibits mTORC1 activity via TSC2. J Nat
Prod. 75:1051–1057. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim MK and Park JH: Conference on
'Multidisciplinary approaches to nutritional problems'. Symposium
on 'Nutrition and health' Cruciferous vegetable intake and the risk
of human cancer: Epidemiological evidence. Proc Nutr Soc.
68:103–110. 2009. View Article : Google Scholar
|
|
18
|
Fimognari C, Turrini E, Ferruzzi L, Lenzi
M and Hrelia P: Natural isothiocyanates: Genotoxic potential versus
chemoprevention. Mutat Res. 750:107–131. 2012. View Article : Google Scholar
|
|
19
|
Brunelli D, Tavecchio M, Falcioni C,
Frapolli R, Erba E, Iori R, Rollin P, Barillari J, Manzotti C,
Morazzoni P, et al: The isothiocyanate produced from glucomoringin
inhibits NF-κB and reduces myeloma growth in nude mice in vivo.
Biochem Pharmacol. 79:1141–1148. 2010. View Article : Google Scholar
|
|
20
|
Kallifatidis G, Rausch V, Baumann B, Apel
A, Beckermann BM, Groth A, Mattern J, Li Z, Kolb A, Moldenhauer G,
et al: Sulforaphane targets pancreatic tumour-initiating cells by
NF-kappaB-induced antiapoptotic signalling. Gut. 58:949–963. 2009.
View Article : Google Scholar
|
|
21
|
Lai KC, Huang AC, Hsu SC, Kuo CL, Yang JS,
Wu SH and Chung JG: Benzyl isothiocyanate (BITC) inhibits migration
and invasion of human colon cancer HT29 cells by inhibiting matrix
metalloproteinase-2/-9 and urokinase plasminogen (uPA) through PKC
and MAPK signaling pathway. J Agric Food Chem. 58:2935–2942. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang MD, Lai KC, Lai TY, Hsu SC, Kuo CL,
Yu CS, Lin ML, Yang JS, Kuo HM, Wu SH, et al: Phenethyl
isothiocyanate inhibits migration and invasion of human gastric
cancer AGS cells through suppressing MAPK and NF-kappab signal
pathways. Anticancer Res. 30:2135–2143. 2010.PubMed/NCBI
|
|
23
|
Jin CY, Moon DO, Lee JD, Heo MS, Choi YH,
Lee CM, Park YM and Kim GY: Sulforaphane sensitizes tumor necrosis
factor-related apoptosis-inducing ligand-mediated apoptosis through
downregulation of ERK and Akt in lung adenocarcinoma A549 cells.
Carcinogenesis. 28:1058–1066. 2007. View Article : Google Scholar
|
|
24
|
Lee SR, Guo SZ, Scannevin RH, Magliaro BC,
Rhodes KJ, Wang X and Lo EH: Induction of matrix metalloproteinase,
cytokines and chemokines in rat cortical astrocytes exposed to
plasminogen activators. Neurosci Lett. 417:1–5. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin CW, Hou WC, Shen SC, Juan SH, Ko CH,
Wang LM and Chen YC: Quercetin inhibition of tumor invasion via
suppressing PKC delta/ERK/AP-1-dependent matrix metalloproteinase-9
activation in breast carcinoma cells. Carcinogenesis. 29:1807–1815.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mi L, Gan N and Chung FL: Isothiocyanates
inhibit proteasome activity and proliferation of multiple myeloma
cells. Carcinogenesis. 32:216–223. 2011. View Article : Google Scholar :
|
|
27
|
Hornebeck W, Lambert E, Petitfrère E and
Bernard P: Beneficial and detrimental influences of tissue
inhibitor of metallopro-teinase-1 (TIMP-1) in tumor progression.
Biochimie. 87:377–383. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu WS: The signaling mechanism of ROS in
tumor progression. Cancer Metastasis Rev. 25:695–705. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Woo CW, Lucarelli E and Thiele CJ: NGF
activation of TrkA decreases N-myc expression via MAPK path leading
to a decrease in neuroblastoma cell number. Oncogene. 23:1522–1530.
2004. View Article : Google Scholar
|
|
30
|
Xu HY, Qian AR, Shang P, Xu J, Kong LM,
Bian HJ and Chen ZN: siRNA targeted against HAb18G/CD147 inhibits
MMP-2 secretion, actin and FAK expression in hepatocellular
carcinoma cell line via ERK1/2 pathway. Cancer Lett. 247:336–344.
2007. View Article : Google Scholar
|
|
31
|
Jiménez E, Pérez de la Blanca E, Urso L,
González I, Salas J and Montiel M: Angiotensin II induces MMP 2
activity via FAK/JNK pathway in human endothelial cells. Biochem
Biophys Res Commun. 380:769–774. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kim SH, Sehrawat A and Singh SV: Dietary
chemopreventative benzyl isothiocyanate inhibits breast cancer stem
cells in vitro and in vivo. Cancer Prev Res (Phila). 6:782–790.
2013. View Article : Google Scholar
|
|
33
|
Chen HJ, Lin CM, Lee CY, Shih NC, Amagaya
S, Lin YC and Yang JS: Phenethyl isothiocyanate suppresses
EGF-stimulated SAS human oral squamous carcinoma cell invasion by
targeting EGF receptor signaling. Int J oncol. 43:629–637.
2013.PubMed/NCBI
|
|
34
|
Annabi B, Rojas-Sutterlin S, Laroche M,
Lachambre MP, Moumdjian R and Béliveau R: The diet-derived
sulforaphane inhibits matrix metalloproteinase-9-activated human
brain microvascular endothelial cell migration and tubulogenesis.
Mol Nutr Food Res. 52:692–700. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang TY, Chang WC, Wang MY, Yang YR and
Hsu YC: Effect of sulforaphane on growth inhibition in human brain
malignant glioma GBM 8401 cells by means of mitochondrial- and
MEK/ERK-mediated apoptosis pathway. Cell biochem Biophys.
63:247–259. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chou YC, Chang MY, Wang MJ, Harnod T, Hung
CH, Lee HT, Shen CC and Chung JG: PEITC induces apoptosis of human
brain glioblastoma GBM8401 cells through the extrinsic- and
intrinsic-signaling pathways. Neurochem Int. 81:32–40. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schnaper HW, Kopp JB, Poncelet AC, Hubchak
SC, Stetler-Stevenson WG, Klotman PE and Kleinman HK: Increased
expression of extracellular matrix proteins and decreased
expression of matrix proteases after serial passage of glomerular
mesangial cells. J Cell Sci. 109:2521–2528. 1996.PubMed/NCBI
|
|
38
|
Nagase H and Woessner JF Jr: Matrix
metalloproteinases. J Biol Chem. 274:21491–21494. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jin Y, Han HC and Lindsey ML: ACE
inhibitors to block MMP-9 activity: New functions for old
inhibitors. J Mol Cell Cardiol. 43:664–666. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen YJ, Wei YY, Chen HT, Fong YC, Hsu CJ,
Tsai CH, Hsu HC, Liu SH and Tang CH: Osteopontin increases
migration and MMP-9 up-regulation via alphavbeta3 integrin, FAK,
ERK, and NF-kappaB-dependent pathway in human chondrosarcoma cells.
J Cell Physiol. 221:98–108. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Velpula KK, Rehman AA, Chelluboina B,
Dasari VR, Gondi CS, Rao JS and Veeravalli KK: Glioma stem cell
invasion through regulation of the interconnected ERK, integrin α6
and N-cadherin signaling pathway. Cell Signal. 24:2076–2084. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Trachootham D, Zhou Y, Zhang H, Demizu Y,
Chen Z, Pelicano H, Chiao PJ, Achanta G, Arlinghaus RB, Liu J, et
al: Selective killing of oncogenically transformed cells through a
ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer
Cell. 10:241–252. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hsu YC, Chang SJ, Wang MY, Chen YL and
Huang TY: Growth inhibition and apoptosis of neuroblastoma cells
through ROS-independent MEK/ERK activation by sulforaphane. Cell
Biochem Biophys. 66:765–774. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Angel P and Karin M: The role of Jun, Fos
and the AP-1 complex in cell-proliferation and transformation.
Biochim Biophys Acta. 1072:129–157. 1991.PubMed/NCBI
|
|
45
|
Tseng HC, Lee IT, Lin CC, Chi PL, Cheng
SE, Shih RH, Hsiao LD and Yang CM: IL-1β promotes corneal
epithelial cell migration by increasing MMP-9 expression through
NF-κB- and AP-1-dependent pathways. PLoS one. 8:e579552013.
View Article : Google Scholar
|
|
46
|
Reddy KB, Nabha SM and Atanaskova N: Role
of MAP kinase in tumor progression and invasion. Cancer Metastasis
Rev. 22:395–403. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sivaraman VS, Wang H, Nuovo GJ and Malbon
CC: Hyper-expression of mitogen-activated protein kinase in human
breast cancer. J Clin Invest. 99:1478–1483. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen JS, Huang XH, Wang Q, Huang JQ, Zhang
LJ, Chen XL, Lei J and Cheng ZX: Sonic hedgehog signaling pathway
induces cell migration and invasion through focal adhesion
kinase/AKT signaling-mediated activation of matrix
metalloproteinase (MMP)-2 and MMP-9 in liver cancer.
Carcinogenesis. 34:10–19. 2013. View Article : Google Scholar
|
|
49
|
Siesser PM and Hanks SK: The signaling and
biological implications of FAK overexpression in cancer. Clin
Cancer Res. 12:3233–3237. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
McLean GW, Avizienyte E and Frame MC:
Focal adhesion kinase as a potential target in oncology. Expert
Opin Pharmacother. 4:227–234. 2003. View Article : Google Scholar : PubMed/NCBI
|