Introduction
Breast cancer is the most common malignant tumor
disease in women. Around 1.38 million newly diagnosed cancer occur
worldwide on an annual basis and among these, breast cancer
accounts for 23% of all cases (1).
The World Health Organization (WHO) estimates that about half of
breast cancer cases with 60% mortality are found in developing
countries. In China, the incidence and mortality of breast cancer
were increased from 169,452 and 44,908 in 2008 to 187,000 and
48,000 in 2012, respectively (GLOBOCAN 2012) (http://globocan.iarc.fr/). Currently, the early
detection of breast cancer relies on mammography and self breast
examination. Although several potential serum biomarkers of breast
cancer have been proposed, the clinical significance using
quantitative methods has barely been validated.
Nicotinamide phosphoribosyltransferase (NAMPT), also
known as visfatin or pre-B cell enhancing factor (PBEF), is an
insulin-mimetic adipocytokine highly expressed in and secreted by
visceral adipose tissue associated with obesity (2,3). The
overexpression of NAMPT has been found in the different types of
human malignant tumors, including colorectal, gastric, endometrial,
ovarian, breast, prostate and thyroid cancers, myeloma, melanoma,
astrocytomas/glioblastoma and other carcinomas (4–13).
Plasma NAMPT levels in patients with breast cancer are higher than
in healthy controls (14). Vascular
endothelial growth factor (VEGF), an angiogenic factor expressed in
the endothelial cells of blood vessels, plays a role in the process
of tumor angiogenesis. Many studies showed that circulating VEGF is
elevated in cancer patients. The high concentration of VEGF and its
soluble receptor in the serum of patients with breast cancer are
held responsible for the disease as they show positive correlations
with the clinical stages (15).
Elevated level of VEGF has been shown in early breast cancer
patients compared with healthy controls (16). Human epidermal growth factor
receptor-2 (HER2), an oncogene also known as Her-2/neu or
c-erbB-2, has been reported to have significantly high serum
levels in breast cancer patients compared with healthy controls and
in metastatic breast cancer patients compared with the
non-metastatic ones (17). Previous
report also showed that no correlation was found between
preoperative and perioperative serum VEGF and HER2 in patients with
early breast cancer (18). Thus, it
is speculated that NAMPT, VEGF and HER2 are three variables that
may collectively be useful as early diagnostic markers of breast
cancer. However, whether the absolute change of NAMPT is correlated
with VEGF and HER2 in benign and malignant breast tumors is
unknown. Furthermore, whether the circulating NAMPT/VEGF/HER2
triplets are associated with the clinicopathological
characteristics in patients with breast cancer is not yet
explored.
The present study was performed to examine the
expression of NAMPT, VEGF and HER2 in benign and malignant breast
tumors and to investigate whether the expression of NAMPT, VEGF and
HER2 is associated with the clinicopathological features of human
breast cancer. Moreover, we evaluated the serum levels of NAMPT,
VEGF and HER2 in breast cancer patients before and after tumor
removal. Finally, we analyzed the association of serum levels of
these biomarkers with their expression in the breast tissues of
cancer patients.
Materials and methods
Patients and tissue preparation
The study on human subjects was approved by the
Ethics Committee of Jinshan Hospital, Fudan University, Shanghai,
China. Samples from patients who were primarily diagnosed with
breast tumor at Jinshan Hospital from 2013 to 2014 were retrieved
for the present study. None of the patients had received
radiotherapy or chemotherapy before surgery. A total of 68
paraffin-embedded samples constituting 20 benign tumors and 48
malignant tumors were subjected to the histopathological
examination and immunohistochemistry. The adjacent normal tissues
were used as controls. The 10% formalin-fixed paraffin-embedded
breast tissue specimens were prepared. Four micrometer thick
sections of these specimens were stained by hematoxylin and eosin
(H&E) to confirm the histological characteristics. The
histological grades and clinical stages of tumor were classified by
experienced surgeons and pathologists based on the WHO
classification.
Immunohistochemical staining and
analysis
To evaluate the expression of NAMPT, VEGF and HER2
proteins in breast tumors, immunohistochemical staining was
performed as described previously (19). Briefly, after blocking, the sections
were incubated with a rabbit monoclonal anti-NAMPT, mouse
monoclonal anti-VEGF or anti-HER2 antibody (all 1:250 dilution;
Abcam, Cambridge, MA, USA) at 4°C overnight, followed by incubation
with biotinylated secondary antibody (1:150 dilution; Maixin Bio,
Fuzhou, China) at room temperature for 1 h. After washing, the
signal was detected using a DAB kit (diaminobenzidine; Maixin Bio).
Finally, the sections were counterstained with hematoxylin and
photographed under a light microscope (BX43; Olympus, Tokyo,
Japan).
Double blind scoring of NAMPT, VEGF and HER2
immunoreactive staining was performed independently by two
examiners who had no prior knowledge of patient's clinical status.
The sections were evaluated at original magnification, ×200. The
proportion of cells exhibiting protein expression was scored by the
extent of immunoreactive staining and was assigned to one of the
following categories as described previously (19). Briefly, the percentage of positive
cells was scored as follows: no reactivity as 0, ≤25% positive
cells as 1, 26–50% positive cells as 2, 51–75% positive cells as 3
and >75% positive cells as 4. The intensity of staining was
scored as follows: no staining as 0, weak staining as 1, moderate
staining as 2, and strong staining as 3. The final staining index
(SI) was developed based on the sum score of the positive staining
and intensity. The SI score was then clustered into four groups: 0,
≤2 sum points; 1, 3–4 sum points; 2, 5–6 sum points; 3, 7 sum
points. Finally, the cases were categorized based on the SI score
0–1 to be negative and 2–3 to be positive.
Blood sample collection
Blood samples were collected from 30 patients with a
malignant tumor on a day before operation (M-BO) and the 7th day
after operation (M-AO) as well as from 28 patients with benign
tumor. For comparison, 30 blood samples from age- and body mass
index (BMI)-matched healthy controls were obtained from the Health
Check-Up Center of Jinshan Hospital in 2014. The median age of
healthy controls and patients with malignant tumor was 50.00 and
52.00, respectively, and the median BMI of healthy controls and
patients with malignant tumor were 23.05 and 23.73, respectively.
All subjects (patients and healthy controls) who enrolled into the
study signed a consent form prior to the collection of blood
samples. Serum was prepared using a serum separator tube (SST)
which allowed samples to clot for 30 min before centrifugation for
15 min at 1,000 × g, aliquoted, and stored at −80°C until use. None
of the samples were previously thawed.
Enzyme-linked immunosorbent assay
Serum levels of NAMPT, VEGF and HER2 were determined
in patients and healthy individuals by enzyme-linked immunosorbent
assay (ELISA). NAMPT and HER2 kits were purchased from Wuhan
Xinqidi Biological Technology Co., Ltd. (Wuhan, Hubei, China),
whereas VEGF kit was purchased from R&D Systems (Human VEGF
Immunoassay, Quantikine® ELISA; R&D Systems, Inc.,
Minneapolis, MN, USA). Briefly, after adding 100 µl assay
diluent into each well, 100 µl standards and serum samples
were, respectively, added into the wells and incubated at room
temperature for 2 h. After washing away any unbound substances, 200
µl enzyme-linked polyclonal antibody specific to NAMPT,
VEGF, or HER2 was added into each well and incubated at room
temperature for 2 h. After washing 3 times, 200 µl substrate
solution was added into each well and incubated at room temperature
for 25 min. After adding 50 µl stop solution into each well,
the optical density of each well was determined within 30 min using
a microplate reader at 450 nm.
Statistical analysis
Statistical analyses were performed with SPSS
Statistics 21.0 software (SPSS, Chicago, IL, USA). Based on the SI
system, the categories on positivity and negativity were
classified. Statistical evaluation was performed using
χ2 test to analyze the association between the
expression of NAMPT/VEGF/HER2 and the clinicopathological
characteristics and to compare the positivity between benign and
malignant tumors. For comparison of each variable in breast
malignant tumors, a McNemar test was performed. For multiple group
comparison, an ANOVA was applied. Significant difference between 2
groups was analyzed by a Student's t-test. For testing correlation
between different serum markers, a linear regression was applied.
Data are presented as the mean ± standard deviation (SD) or
standard error of mean (SEM) as indicated. P<0.05 was considered
to indicate a statistically significant difference.
Results
NAMPT, VEGF and HER2 as breast tumor
progression markers
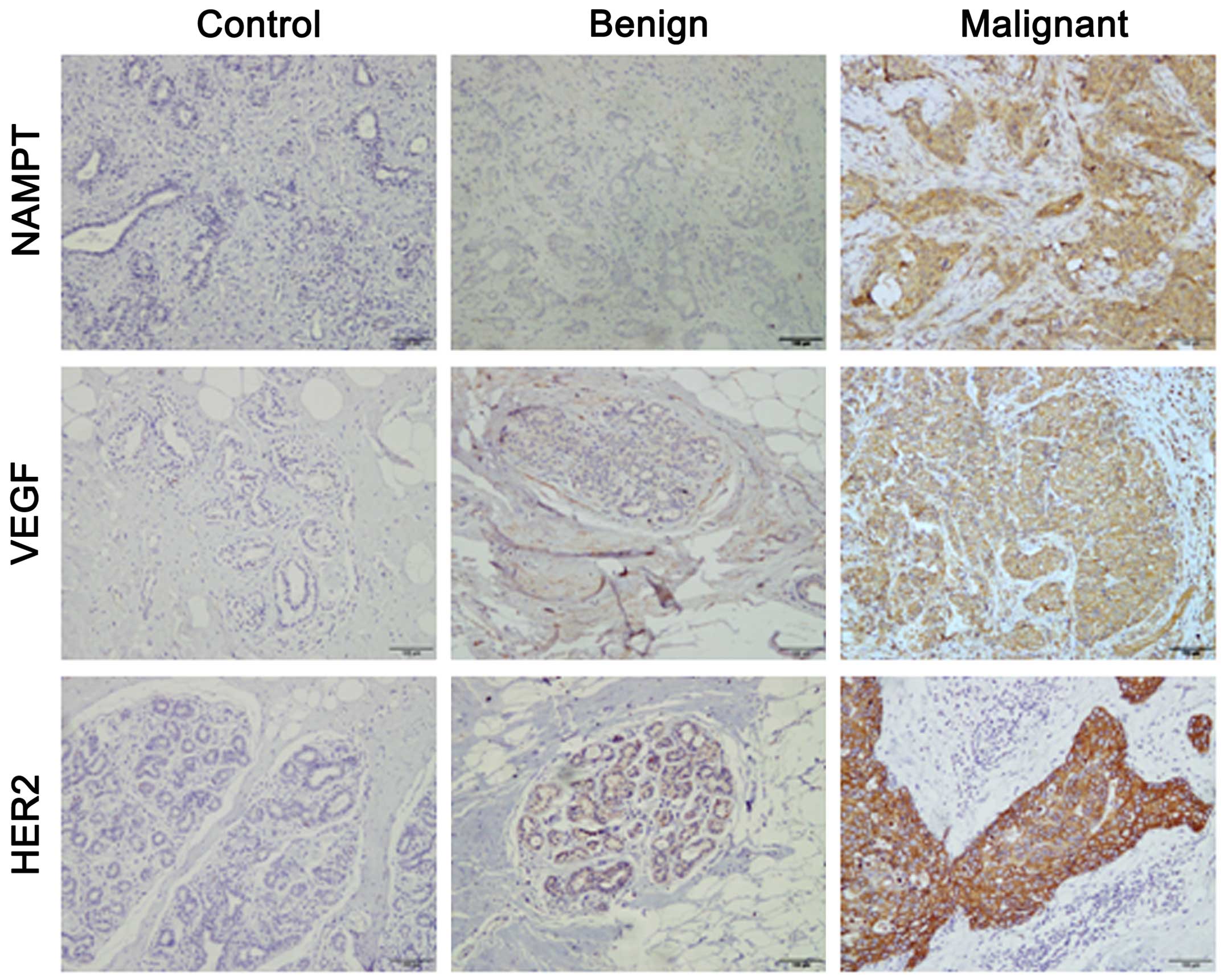
The expression of NAMPT, VEGF and HER2 in benign and
malignant breast tumors was detected by immunohistochemical
staining. The adjacent normal breast tissue was used as normal
control. We found that the expression of NAMPT, VEGF and HER2 was
undetectable or barely detectable in controls, whereas the aberrant
expression of NAMPT, VEGF and HER2 was noted in human benign and
malignant breast tumors (Fig. 1).
By comparison of all breast tissues, the highest degree of the
expression of NAMPT, VEGF and HER2 was observed in malignant
tumors. After the assessment of the SI score as indicated above, we
classified the expression level into positive and negative
categories. Compared with benign tumors, the positive rate of
NAMPT, VEGF and HER2 expression was significantly increased in
malignant tumors (all P<0.01) (Table
I). These data suggest that NAMPT, VEGF and HER2 may be the
indicators or progression markers of breast cancer development.
 | Table IExpression of NAMPT, VEGF and HER2 in
breast benign and malignant tumors. |
Table I
Expression of NAMPT, VEGF and HER2 in
breast benign and malignant tumors.
| Tumor | n | NAMPT
| VEGF
| HER2
|
|---|
| Positive n (%) | Negative n (%) | Positive n (%) | Negative n (%) | Positive n (%) | Negative n (%) |
|---|
| Benign | 20 | 2 (10.0) | 18 (90.0) | 3 (15.0) | 17 (85.0) | 5 (25.0) | 15 (75.0) |
| Malignant | 48 | 26 (54.2) | 22 (45.8) | 31 (64.6) | 17 (35.4) | 29 (60.4) | 19 (39.6) |
| P-value | | 0.001 | <0.001 | 0.008 |
Association of NAMPT, VEGF and HER2
expression with the clinicopathological characteristics of breast
cancer
To examine the association of the expression of
NAMPT, VEGF, and HER2 with the clinicopathological characteristics
of breast cancer, a Chi-square test was applied. All patient
information was gathered by reviewing medical charts and the
records of pathology. By comparison of 48 malignant tumors, we
found that the expression of NAMPT, VEGF and HER2 was not
significantly associated with age (≤50 vs. >50), tumor size (≤2
vs. >2 cm), lymph node metastasis (yes vs. no) and clinical
stage (0–II vs. III–IV) in patients with breast cancer (all
P>0.05) (Table II).
 | Table IIAssociation of the expression of
NAMPT, VEGF, and HER2 proteins with the clinicopathological
features of patients with breast cancer. |
Table II
Association of the expression of
NAMPT, VEGF, and HER2 proteins with the clinicopathological
features of patients with breast cancer.
| Clinicopathological
features | n | NAMPT
| VEGF
| HER2
|
|---|
| + | − | + | − | + | − |
|---|
| Age (years) at
diagnosis |
| ≤50 | 23 | 13 | 10 | 15 | 8 | 14 | 9 |
| >50 | 25 | 13 | 12 | 16 | 9 | 15 | 10 |
| P-value | | 0.753 | 0.930 | 0.951 |
| Tumor size |
| ≤2 cm | 19 | 10 | 9 | 11 | 8 | 12 | 7 |
| >2 cm | 29 | 16 | 13 | 20 | 9 | 17 | 12 |
| P-value | | 0.863 | 0.433 | 0.753 |
| LN metastasis |
| Yes | 17 | 11 | 6 | 14 | 3 | 8 | 9 |
| No | 31 | 15 | 16 | 17 | 14 | 21 | 10 |
| P-value | | 0.278 | 0.057 | 0.161 |
| Clinical stage |
| 0–II | 38 | 18 | 20 | 15 | 23 | 25 | 13 |
| III–IV | 10 | 8 | 2 | 2 | 8 | 4 | 6 |
| P-value | | 0.137a | 0.439a | 0.263a |
Correlation of the expression between
NAMPT, VEGF and HER2 in breast cancer
Next, we compared these triple variables with each
other to see whether the overexpression of NMAPT, VEGF and HER2 in
breast malignant tumor is correlated. Using a McNemar test, we
found that there was no significant difference among the
comparisons, such as NAMPT vs. VEGF, VEGF vs. HER2 and HER2 vs.
NAMPT (all P>0.05) (Table
III). These data in turn suggest that the expression of NAMPT,
VEGF and HER2 may be correlated. Further analysis showed that the
detection rate of NAMPT, VEGF and HER2 in combination was increased
in breast cancer patients. The detection rate of NAMPT, VEGF and
HER2 alone was 54.17, 64.58 and 60.42, respectively. The detection
rate of NMAPT plus VEGF, VEGF plus HER2, and HER2 plus NAMPT was
increased to 68.75, 87.50 and 81.25%, respectively. Finally, the
detection rate of collective triplets reached 89.58%.
 | Table IIICorrelation between NAMPT and VEGF
expression, Nampt and HER2 expression, and VEGF and HER2 expression
in breast malignant tumors. |
Table III
Correlation between NAMPT and VEGF
expression, Nampt and HER2 expression, and VEGF and HER2 expression
in breast malignant tumors.
| Positive (n) | Negative (n) | Total | P-value |
|---|
| VEGF
| | |
| NAMPT |
| Positive | 24 | 2 | 26 | |
| Negative | 7 | 15 | 22 | |
| Total | 31 | 17 | 48 | P=0.180 |
| HER2
| | |
| NAMPT |
| Positive | 16 | 10 | 26 | |
| Negative | 13 | 9 | 22 | |
| Total | 29 | 19 | 48 | P=0.678 |
| HER2
| | |
| VEGF |
| Positive | 18 | 13 | 31 | |
| Negative | 11 | 6 | 17 | |
| Total | 29 | 19 | 48 | P=0.839 |
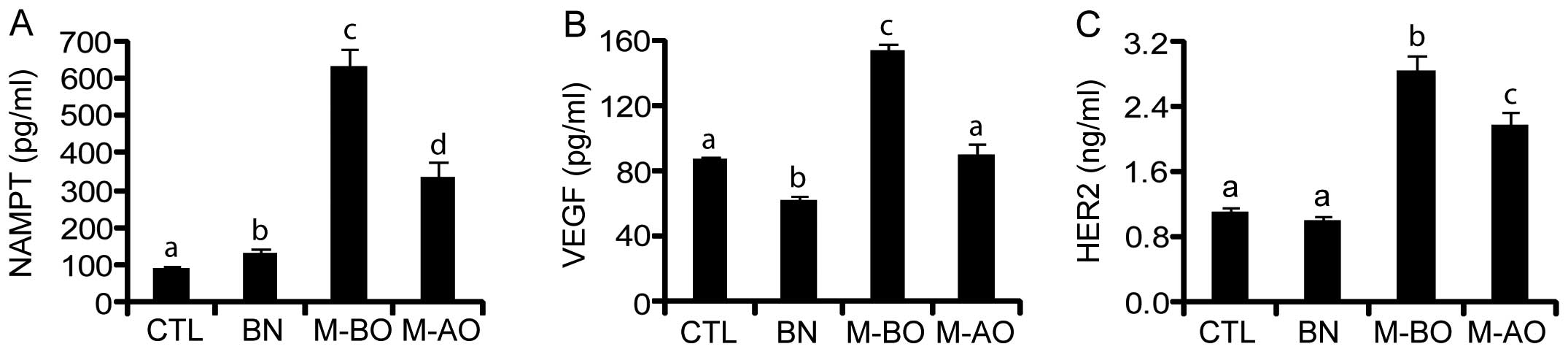
Evaluation of the serum concentrations of
NAMPT, VEGF and HER2 in healthy women and patients with breast
cancer
Since NAMPT, VEGF and HER2 are secretory proteins,
next we examined their serum levels using ELISA and compared the
difference of their concentrations in healthy controls and patients
with benign and malignant breast tumors before and after tumor
removal. Two factors, i.e., age and BMI, were considered when
comparisons were performed, but no significant difference was found
between healthy controls and breast cancer patients, indicating
that their age and BMI were matched between the two groups. The
basal levels of serum NAMPT, VEGF and HER2 in healthy controls
(CTL; n=30) were 94.90±4.24 pg/ml, 87.02±2.41 pg/ml and 1.12±0.04
ng/ml, respectively. The concentrations of serum NAMPT, VEGF and
HER2 in patients with benign tumor (BN; n=28) presented different
trends. In patients with a benign tumor, serum NAMPT was slightly
increased (P<0.05) (Fig. 2A),
whereas serum VEGF was slightly decreased (P<0.01) (Fig. 2B), compared with healthy controls.
However, no significant difference of serum HER2 was observed
between healthy controls and patients with a benign tumor
(P>0.05) (Fig. 2C). In patients
with a malignant tumor before operation (M-BO, n=30), the serum
levels of all three variables were significantly elevated (all
P<0.001) and there were 6.64-, 1.76-, and 2.52-fold increases of
the concentration of NAMPT, VEGF and HER2, respectively.
Interestingly after the operation (M-AO), the elevated serum NAMPT,
VEGF, and HER2 levels significantly declined 47, 41 and 23%,
respectively (all P<0.05), and that of VEGF almost returned to
the basal level of control (Fig.
2B).
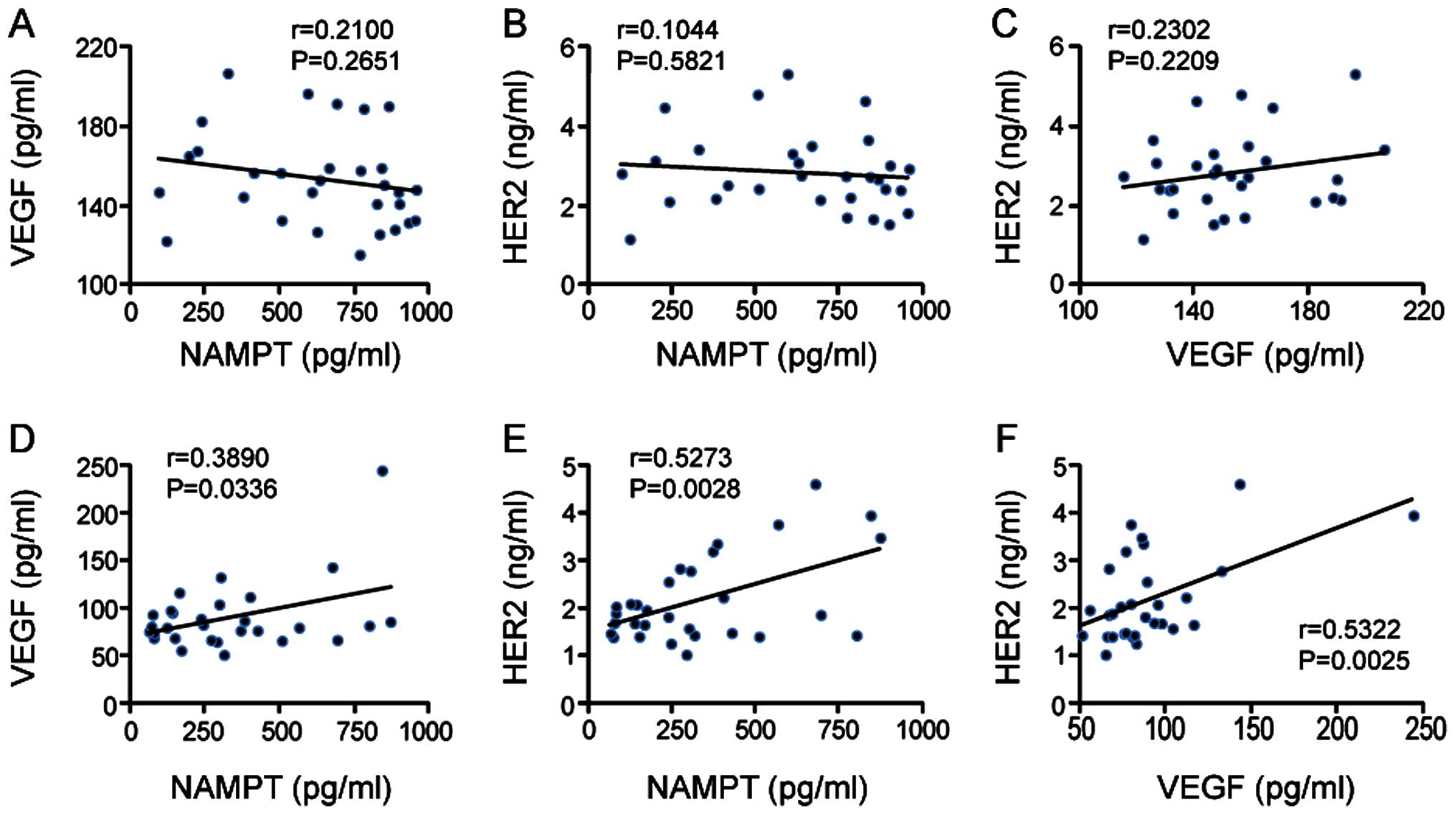
Correlation of serum levels of NAMPT,
VEGF and HER2 with each other in breast cancer patients
We further examined whether serum levels of NAMPT,
VEGF and HER2 are correlated with each other in patients with
malignant tumors. Before operation, no correlation was observed
between them (VEGF vs. NAMPT, NAMPT vs. HER2 and HER2 vs. VEGF; all
P>0.05) (Fig. 3A–C); however
after tumor removal, a significant correlation was found: VEGF vs.
NAMPT (P=0.0336) (Fig. 3D), NAMPT
vs. HER2 (P=0.0028) (Fig. 3E) and
HER2 vs. VEGF (P=0.0025) (Fig.
3F).
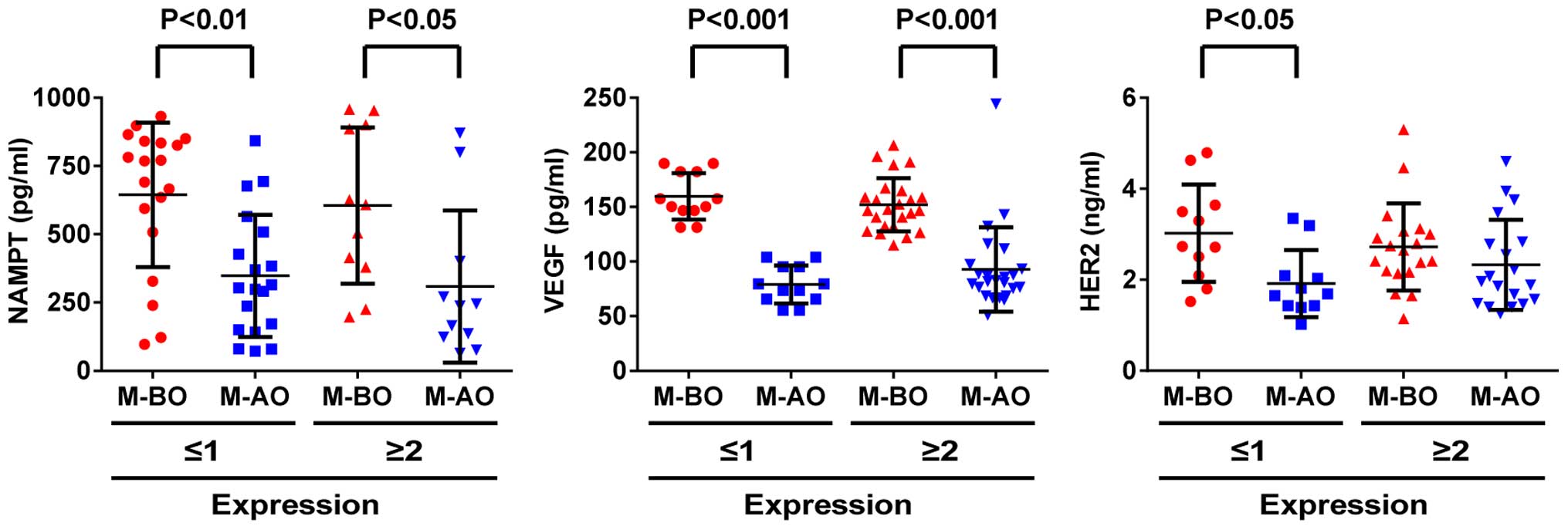
Association of serum concentration of
NAMPT, VEGF and HER2 with their tissue expression in patients with
a malignant tumor before and after operation
Since the presence and absence of a tumor seemed to
affect the serum levels of NAMPT, VEGF and HER2, next we examined
the association between serum levels and tissue expression of these
variables in patients with a malignant tumor before and after
operation. We found that the serum levels of NAMPT and VEGF were
higher in patients before operation than those after operation and
that serum levels were not associated with the positivity (SI score
≥2) and negativity (SI score ≤1) of their expression in the tumor
tissues (Fig. 4A and B). These data
suggest that serum variables are more sensitive than the tissue
counterparts. However, the change of the serum levels of HER2 was
different between negative and positive groups. In patients with
HER2-negative (SI score ≤1), the serum level of HER2 was
significantly decreased after tumor removal (M-AO vs. M-BO;
P<0.05), whereas in patients with HER2-positive (SI score ≥2),
no change of serum HER2 was observed after tumor removal
(P>0.05) (Fig. 4C).
Association of the concentrations of
serum NAMPT, VEGF and HER2 with the clinicopathological features of
breast cancer patients
Finally, the association of the serum levels of
NAMPT, VEGF and HER2 with the clinicopathological features of
breast cancer patients before and after tumor removal was examined.
The serum levels of NAMPT as well as VEGF were significantly
different in patients with breast cancer before and after operation
and were found to be higher in patients before surgery (P<0.05)
(Table IV), irrespective of the
clinicopathological features. The serum level of HER2 was also
higher in patients younger than 50, with tumor size ≤2 cm, without
lymph node metastasis in clinical stage of 0–II before operation
than those after operation (P<0.05). However in patients older
than 50, with tumor size >2 cm and lymph node metastasis in
clinical stage of III–IV, no significant difference of HER2 was
observed before and after operation (P>0.05). By comparing with
the clinicopathological features of patients, such as age (≤50 vs.
>50), tumor size (≤2 vs. >2 cm), lymph node metastasis (yes
vs. no), and clinical stage (0–II vs. III–IV), the serum levels of
NAMPT, VEGF and HER2 were similar (all P>0.05).
 | Table IVConcentration of serum NAMPT, VEGF
and HER2 associated with the clinicopathological features of breast
cancer patients. |
Table IV
Concentration of serum NAMPT, VEGF
and HER2 associated with the clinicopathological features of breast
cancer patients.
| Clinicopathological
features | Operation (n) | NAMPT (mean ± SD,
pg/ml)
| P-value | VEGF (mean ± SD,
pg/ml)
| P-value | HER2 (mean ± SD,
pg/ml)
| P-value |
|---|
| Before | After | Before | After | Before | After |
|---|
| Age (years) at
diagnosis |
| ≤50 | 14 | 564.32±276.52 | 277.79±216.65 | 0.008 | 161.95±22.06 | 104.42±44.00 | <0.001 | 3.13±1.08 | 2.28±0.88 | 0.011 |
| >50 | 16 | 687.95±255.68 | 382.34±258.41 | 0.004 | 146.08±23.46 | 77.56±20.92 | <0.001 | 2.57±0.86 | 2.08±0.96 | 0.128 |
| P-value | | 0.2167 | 0.2383 | | 0.0667 | 0.0513 | | 0.1329 | 0.5640 | |
| Tumor size |
| ≤2 cm | 14 | 645.86±230.78 | 401.48±276.80 | 0.005 | 153.60±24.31 | 87.06±46.60 | <0.001 | 2.85±0.85 | 2.08±1.00 | 0.013 |
| >2 cm | 16 | 616.59±304.40 | 274.11±196.00 | 0.004 | 153.38±24.20 | 92.75±24.02 | <0.001 | 2.81±1.13 | 2.27±0.85 | 0.106 |
| P-value | | 0.7673 | 0.1646 | | 0.9802 | 0.6853 | | 0.9218 | 0.5857 | |
| LN metastasis |
| No | 21 | 595.90±281.07 | 342.99±264.05 | 0.003 | 152.90±24.01 | 88.07±39.31 | <0.001 | 2.87±0.96 | 2.20±0.86 | 0.006 |
| Yes | 9 | 710.41±230.78 | 311.52±191.29 | 0.007 | 154.84±24.78 | 94.82±27.25 | <0.001 | 2.74±1.23 | 2.14±1.09 | 0.260 |
| P-value | | 0.2593 | 0.7179 | | 0.8456 | 0.5942 | | 0.7712 | 0.8916 | |
| Clinical stage |
| 0–II | 26 | 621.97±279.00 | 358.40±246.18 | 0.001 | 154.94±25.10 | 90.79±37.79 | <0.001 | 2.75±0.88 | 2.20±0.94 | 0.017 |
| III–IV | 4 | 684.07±207.59 | 172.01±138.85 | 0.018 | 144.04±10.06 | 85.57±21.08 | 0.003 | 3.34±1.63 | 2.03±0.80 | 0.146 |
| P-value | | 0.6199 | 0.0667 | 0.1514 | 0.6986 | | 0.5285 | 0.7189 | | |
Discussion
The present study demonstrated that NAMPT, VEGF and
HER2 were not only overexpressed in the tumor tissue, but also
increased in the circulation in patients with breast cancer. To our
knowledge this is the first report to propose a detection panel of
the NAMPT/VEGF/HER2 triplet for the diagnosis as well as prognosis
of human breast cancer.
It has been shown that the overexpression of NAMPT,
VEGF, or HER2 is found in several carcinomas, including breast
cancer (20–22). Previous studies showed that the
levels of plasma NAMPT were higher in Chinese patients with breast
cancer than in healthy controls (23), as well as that the levels of plasma
VEGF were higher in premenopausal patients with early breast cancer
than in normal premenopausal controls (16). It has also been reported that HER2
is a serum biomarker of breast cancer (24). However, these molecules are not
examined together in the same patient with breast tumor (either
benign or malignant). Importantly, the association of these
variables with the clinicopathological characteristics of breast
cancer has not been reported. In the present study we examined the
individual as well as cumulative expression of these variables in
the breast tissues from patients with tumors (benign or malignant)
and also determined their serum levels. Furthermore, we analyzed
the possible correlations between their expression in tissue and
serum concentration before and after the tumor removal by surgery.
To the best of our knowledge, current study is the first report to
evaluate them as a biomarker-triplet for breast cancer diagnosis
and also as an indicator of treatment effectiveness after tumor
removal.
Our immunohistochemistry analysis showed that the
expression of NAMPT, VEGF and HER2 was positive in most breast
cancer tissue while they were negative in adjacent normal tissue,
and their expression was higher in malignant tumors compared to the
benign ones. Further analysis showed that the rate of IHC
positivity for a single variable was ~60% in malignant tumor, which
was increased to ~79% for two variables and to 90% for all three
variables assessed together. These results imply a potential
clinical applicability in molecular pathology for these variables
to be used as a biomarker panel in the detection and prognosis of
breast cancer. The change of the expression level from negative in
healthy control to weak positive in benign tumor and to strong
positive in malignant tumor suggests that the NAMPT/VEGF/HER2
triplet presents the ability to be a progression biomarker for
tumorigenesis.
Compared with three variables in malignant tumors,
NAMPT was the most sensitive marker showing ~6.64-fold increase,
followed by HER2 (2.52-fold) and VEGF (1.76-fold). The most
important finding of the present study is that despite the
differences in concentrations, essentially all three variables
(NAMPT, VEGF and HER2) were found higher in the serum of breast
cancer patients, indicating that the elevated NAMPT/VEGF/HER2 can
be used as a diagnostic tool for human breast cancer. Furthermore,
the decrease in the serum level of this biomarker-triplet after
tumor removal suggests it to be a useful indicator of treatment
efficacy and prognosis. In terms of the association of HER2
expression, the serum level of HER2 detected by ELISA was
significantly decreased after tumor removal in patients
HER2-negative by ICH, but not in patients HER2-positive. These data
suggest that in HER2-positive patients, NAMPT and VEGF rather than
HER2 are most useful variables for monitoring the treatment
effectiveness after surgery.
Increasing evidence demonstrates that NAMPT is a
multifunctional enzyme which is important in metabolism and immune
response as well as in cancer. It can affect metastatic activities
and cell adhesive functions by regulating integrins in breast
cancer (25). Overexpression of
NAMPT is associated with aggressive pathological and molecular
features, such as estrogen receptor negativity and HER2-enriched
phenotypes (26), as well as
malignancy and poor prognosis (27,28).
VEGF, an angiogenic marker, is found to be overexpressed in primary
breast cancer (29) and plays a
role in breast cancer angiogenesis (30), whereas HER2 positivity and
negativity are related to the therapy of breast cancer (31,32).
Previous study also showed that the overexpression of HER2 was
significantly correlated to a higher expression of VEGF in breast
cancer (21), but the clinical
association of NAMPT/VEGF/HER2 with breast cancer has not been
previously reported. The present study provides evidence of the
relationship of these variables and their association with the
clinicopathological features.
In summary, the present study demonstrated that
NAMPT, VEGF and HER2 were overexpressed in breast tumors as well as
elevated in the serum of patients with breast cancer and their
circulating levels declined after tumor removal, suggesting a
clinical application of this triplet as a biomarker for breast
cancer diagnosis and as an indicator for treatment efficacy. The
combined measurement of the triplet may improve the sensitivity of
breast cancer diagnosis and potentially be used as a testing panel
for the detection of malignant tumors, the assessment of treatment
effectiveness, and the monitoring of the disease progression in
patients with breast cancer. Thus, we propose that the biomarker
triplet NAMPT/VEGF/HER2 can be used as a de novo detection
panel for the diagnosis and prognosis of human breast cancer.
Acknowledgments
The present study was supported by grants from
National Natural Science Foundation of China (81272880), the
Shanghai Committee of Science and Technology (124119b1300) and
Shanghai Municipal Health Bureau (2012–186) to G.X. and the
Committee of Science and Technology of Jinshan (2013-3-15) to Y.Z.
We thank Jimin Shi for pathological evaluation, and Jihong Zhang
and Xiaoqun Lv for technique assistance.
Abbreviations:
|
HER2
|
human epidermal growth factor
receptor-2
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
IHC
|
immunohistochemistry
|
|
M-AO
|
malignant tumor after operation
|
|
M-BO
|
malignant tumor before operation
|
|
NAMPT
|
nicotinamide
phosphoribosyltransferase
|
|
SD
|
standard deviation
|
|
SEM
|
standard error of mean
|
|
SI
|
staining index
|
|
VEGF
|
vascular endothelial growth factor
|
|
WHO
|
World Health Organization
|
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fukuhara A, Matsuda M, Nishizawa M, Segawa
K, Tanaka M, Kishimoto K, Matsuki Y, Murakami M, Ichisaka T,
Murakami H, et al: Visfatin: A protein secreted by visceral fat
that mimics the effects of insulin. Science. 307:426–430. 2005.
View Article : Google Scholar
|
|
3
|
Samal B, Sun Y, Stearns G, Xie C, Suggs S
and McNiece I: Cloning and characterization of the cDNA encoding a
novel human pre-B-cell colony-enhancing factor. Mol Cell Biol.
14:1431–1437. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bi TQ, Che XM, Liao XH, Zhang DJ, Long HL,
Li HJ and Zhao W: Overexpression of Nampt in gastric cancer and
chemopotentiating effects of the Nampt inhibitor FK866 in
combination with fluorouracil. Oncol Rep. 26:1251–1257.
2011.PubMed/NCBI
|
|
5
|
Wang B, Hasan MK, Alvarado E, Yuan H, Wu H
and Chen WY: NAMPT overexpression in prostate cancer and its
contribution to tumor cell survival and stress response. Oncogene.
30:907–921. 2011. View Article : Google Scholar
|
|
6
|
Hufton SE, Moerkerk PT, Brandwijk R, de
Bruïne AP, Arends JW and Hoogenboom HR: A profile of differentially
expressed genes in primary colorectal cancer using suppression
subtractive hybridization. FEBS Lett. 463:77–82. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Van Beijnum JR, Moerkerk PT, Gerbers AJ,
De Bruïne AP, Arends JW, Hoogenboom HR and Hufton SE: Target
validation for genomics using peptide-specific phage antibodies: A
study of five gene products overexpressed in colorectal cancer. Int
J Cancer. 101:118–127. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shackelford RE, Bui MM, Coppola D and
Hakam A: Over-expression of nicotinamide phosphoribosyltransferase
in ovarian cancers. Int J Clin Exp Pathol. 3:522–527.
2010.PubMed/NCBI
|
|
9
|
Folgueira MA, Carraro DM, Brentani H,
Patrão DF, Barbosa EM, Netto MM, Caldeira JR, Katayama ML, Soares
FA, Oliveira CT, et al: Gene expression profile associated with
response to doxorubicin-based therapy in breast cancer. Clin Cancer
Res. 11:7434–7443. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shackelford R, Hirsh S, Henry K,
Abdel-Mageed A, Kandil E and Coppola D: Nicotinamide
phosphoribosyltransferase and SIRT3 expression are increased in
well-differentiated thyroid carcinomas. Anticancer Res.
33:3047–3052. 2013.PubMed/NCBI
|
|
11
|
Tian W, Zhu Y, Wang Y, Teng F, Zhang H,
Liu G, Ma X, Sun D, Rohan T and Xue F: Visfatin, a potential
biomarker and prognostic factor for endometrial cancer. Gynecol
Oncol. 129:505–512. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maldi E, Travelli C, Caldarelli A,
Agazzone N, Cintura S, Galli U, Scatolini M, Ostano P, Miglino B,
Chiorino G, et al: Nicotinamide phosphoribosyltransferase (NAMPT)
is over-expressed in melanoma lesions. Pigment Cell Melanoma Res.
26:144–146. 2013. View Article : Google Scholar
|
|
13
|
Reddy PS, Umesh S, Thota B, Tandon A,
Pandey P, Hegde AS, Balasubramaniam A, Chandramouli BA, Santosh V,
Rao MR, et al: PBEF1/NAmPRTase/Visfatin: A potential malignant
astrocytoma/glioblastoma serum marker with prognostic value. Cancer
Biol Ther. 7:663–668. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dalamaga M, Archondakis S, Sotiropoulos G,
Karmaniolas K, Pelekanos N, Papadavid E and Lekka A: Could serum
visfatin be a potential biomarker for postmenopausal breast cancer?
Maturitas. 71:301–308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thielemann A, Baszczuk A, Kopczyński Z,
Kopczyński P and Grodecka-Gazdecka S: Clinical usefulness of
assessing VEGF and soluble receptors sVEGFR-1 and sVEGFR-2 in women
with breast cancer. Ann Agric Environ Med. 20:293–297.
2013.PubMed/NCBI
|
|
16
|
Byrne GJ, McDowell G, Agarawal R, Sinha G,
Kumar S and Bundred NJ: Serum vascular endothelial growth factor in
breast cancer. Anticancer Res. 27(5B): 3481–3487. 2007.PubMed/NCBI
|
|
17
|
Baskić D, Ristić P, Pavlović S and
Arsenijević N: Serum HER2 and CA 15-3 in breast cancer patients. J
BUON. 9:289–294. 2004.
|
|
18
|
Rocca A, Cancello G, Bagnardi V, Sandri
MT, Torrisi R, Zorzino L, Viale G, Pietri E, Veronesi P,
Dellapasqua S, et al: Perioperative serum VEGF and extracellular
domains of EGFR and HER2 in early breast cancer. Anticancer Res.
29:5111–5119. 2009.
|
|
19
|
Wang X, Gui L, Zhang Y, Zhang J, Shi J and
Xu G: Cystatin B is a progression marker of human epithelial
ovarian tumors mediated by the TGF-β signaling pathway. Int J
Oncol. 44:1099–1106. 2014.PubMed/NCBI
|
|
20
|
Dalamaga M, Karmaniolas K, Papadavid E,
Pelekanos N, Sotiropoulos G and Lekka A: Elevated serum
visfatin/nicotinamide phosphoribosyl-transferase levels are
associated with risk of postmenopausal breast cancer independently
from adiponectin, leptin, and anthropometric and metabolic
parameters. Menopause. 18:1198–1204. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Linderholm B, Andersson J, Lindh B,
Beckman L, Erlanson M, Edin K, Tavelin B, Grankvist K and
Henriksson R: Over-expression of c-erbB-2 is related to a higher
expression of vascular endothelial growth factor (VEGF) and
constitutes an independent prognostic factor in primary
node-positive breast cancer after adjuvant systemic treatment. Eur
J Cancer. 40:33–42. 2004. View Article : Google Scholar
|
|
22
|
Kirkpatrick K, Ogunkolade W, Elkak A,
Bustin S, Jenkins P, Ghilchik M and Mokbel K: The mRNA expression
of cyclo-oxygenase-2 (COX-2) and vascular endothelial growth factor
(VEGF) in human breast cancer. Curr Med Res Opin. 18:237–241. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li XY, Tang SH, Zhou XC, Ye YH, Xu XQ and
Li RZ: Preoperative serum visfatin levels and prognosis of breast
cancer among Chinese women. Peptides. 51:86–90. 2014. View Article : Google Scholar
|
|
24
|
Lam L, Czerniecki BJ, Fitzpatrick E, Xu S,
Schuchter L, Xu X and Zhang H: Interference-free HER2 ECD as a
serum biomarker in breast cancer. J Mol Biomark Diagn.
4:1512014.PubMed/NCBI
|
|
25
|
Santidrian AF, LeBoeuf SE, Wold ED,
Ritland M, Forsyth JS and Felding BH: Nicotinamide
phosphoribosyltransferase can affect metastatic activity and cell
adhesive functions by regulating integrins in breast cancer. DNA
Repair (Amst). 23:79–87. 2014. View Article : Google Scholar
|
|
26
|
Soncini D, Caffa I, Zoppoli G, Cea M,
Cagnetta A, Passalacqua M, Mastracci L, Boero S, Montecucco F,
Sociali G, et al: Nicotinamide phosphoribosyltransferase promotes
epithelial-to-mesenchymal transition as a soluble factor
independent of its enzymatic activity. J Biol Chem.
289:34189–34204. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lee YC, Yang YH, Su JH, Chang HL, Hou MF
and Yuan SS: High visfatin expression in breast cancer tissue is
associated with poor survival. Cancer Epidemiol Biomarkers Prev.
20:1892–1901. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dalamaga M: Nicotinamide
phosphoribosyl-transferase/visfatin: A missing link between
overweight/obesity and postmenopausal breast cancer? Potential
preventive and therapeutic perspectives and challenges. Med
Hypotheses. 79:617–621. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Iovino F, Ferraraccio F, Orditura M,
Antoniol G, Morgillo F, Cascone T, Diadema MR, Aurilio G,
Santabarbara G, Ruggiero R, et al: Serum vascular endothelial
growth factor (VEGF) levels correlate with tumor VEGF and p53
over-expression in endocrine positive primary breast cancer. Cancer
Invest. 26:250–255. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lissoni P, Fugamalli E, Malugani F,
Ardizzoia A, Secondino S, Tancini G and Gardani GS: Chemotherapy
and angiogenesis in advanced cancer: Vascular endothelial growth
factor (VEGF) decline as predictor of disease control during taxol
therapy in metastatic breast cancer. Int J Biol Markers.
15:308–311. 2000.
|
|
31
|
Ross JS and Fletcher JA: The HER-2/neu
oncogene in breast cancer: Prognostic factor, predictive factor,
and target for therapy. Stem Cells. 16:413–428. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Park IH, Lee KS, Kang HS, Kim SW, Lee S,
Jung SY, Kwon Y, Shin KH, Ko K, Nam BH, et al: A phase Ib study of
preoperative lapatinib, paclitaxel, and gemcitabine combination
therapy in women with HER2 positive early breast cancer. Invest New
Drugs. 30:1972–1977. 2012. View Article : Google Scholar
|