Introduction
Ovarian cancer is the fifth leading cause of cancer
death among women and is the primary cause of death from
gynecological malignancies (1). For
ovarian cancer in early phase, appropriate surgical staging and
adjuvant chemotherapy for selected cases will lead to survival
rates of 90–95% (2), while for
advanced phase, although most patients will achieve a complete
clinical response after maximal surgery together with chemotherapy
at first, nearly 50% of patients will eventually develop recurrent
disease (3) and the subsequent
acquired chemoresistance (4–6) cause
low cure rates and severely limit successful treatment (7). The difficulty in achieving an early
diagnosis and the aggressive nature of this type of cancer together
with the resistance limit the efficacy of surgical operation with
the 5-year survival rate approximately 45% (8). Therefore, finding of novel and
promising agents without resistance is urgent for the treatment of
ovarian cancer.
Sesquiterpene lactone compounds are known as
important botanical natural compounds have been widely used in
cancer clinical trials for breast, colorectal, kidney, prostate,
acute myeloid leukemia, acute lymphoblastic leukemia, non-small
lung cancer (9,10), gynecologic tumors (11) and pancreatic cancer (12). Isoalantolactone is a sesquiterpene
lactone isolated from the roots of Inula helenium L. that
possesses anti-inflammatory, anti-bacterial, anti-fungal,
anti-insecticidal activities with low toxicity (9,13,14).
Recent reports demonstrated that isoalantolactone inhibits growth
and induces apoptosis in pancreatic cancer cells in association
with increased generation of reactive oxygen species (12), and our high throughput screening
performance showed the anti-SKOV3 cell effect of
isoalantolactone. However, the anti-ovarian cancer potential of
isoalantolactone compounds and their mechanism of action have not
been fully elucidated.
Autophagy is a mode of cell death referred to as
type II programmed cell death compared with the apoptosis pathway
which is the type I programmed cell death (15), it is recognized as a cellular
process of lysosome-dependent cellular catabolic degradation when
cells are under various physiological and stress conditions, such
as hypoxia (16), nutritional
deprivation (17), radiation
(18,19), chemotherapeutic agents and viral
infection, and is characterized by the sequestration of bulk
cytoplasm and organelles in double-membrane autophagic vesicles and
has essential roles in survival, development and homeostasis.
Autophagy is also integral to human health and is involved in
physiology, development, lifespan and a wide range of diseases,
including cancer, neurodegeneration and microbial infection. Data
from several studies concerning the expression of PEA-15 in ovarian
cancer cells demonstrated that PEA-15 expression inhibited ovarian
cancer proliferation via induced autophagy but not via apoptosis
(20). PEA-15 is a 15 kDa small
non-catalytic protein that contains a death effector domain (DED)
with an irregularly structured C-terminal tail, regulating various
cellular processes, such as apoptosis, cell proliferation and
glucose metabolism (21).
Several natural compounds such as avicins, curcumin
and evodiamine (22) can induce
autophagic cell death. Recently, the induction of autophagic cell
death has been studied as a potential method for cancer therapy.
Therefore, we designed this study to investigate whether autophagy
is involved in the antitumor effects of isoalantolactone in human
ovarian cancer SKOV3 cells and to further elucidate
whether the antitumor activity of isoalantolactone is mediated by
the up-regulation of PEA-15, which induces autophagy via activation
of the ERK signaling pathway.
Materials and methods
Reagents
Isoalantolactone was purchased from Tauto Biotech
Company (Shanghai, China), purity >99% as determined by
analytical HPLC. Propidium iodide (PI), dimethylsulfoxide (DMSO),
acridine orange dye (AO), 3-meth-yladenine (3-MA), trypan blue dye,
cell culture media (McCoy's 5A medium, RPMI-1640 medium),
pentobarbital sodium, fetal bovine serum (FBS), penicillin and
streptomycin were purchased from Sigma-Aldrich (Beijing, China).
Cell-Light EdU imaging detection kit was purchased from RiboBio
(Guangzhou, China). On-TARGETplus SMARTpool siRNA for PEA-15 kit
was purchased from Dharmacon (Lafayette, CO, USA). Lipofectamine
2000 kit, TRIzol reagent and the Primescript™ reverse transcription
reagent kit were purchased from Invitrogen (Beijing, China). SYBR
Premix Ex Taq™ II was purchased from Takara (Dalian, China).
Polyclonal anti-human Beclin1, PEA-15, ERK, pERK, LC3 antibodies
were purchased from Cell Signaling Technology (Beverly, MA, USA).
Antibodies specific to β-actin, α-tubulin and horseradish
peroxidase-conjugated secondary antibodies were purchased from
Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cell culture
The human ovarian cancer cell line SKOV3
was purchased from the American Type Culture Collection (ATCC,
Manassas, VA, USA). The cells were maintained in McCoy's 5A medium
containing 10% FBS and 1% penicillin/streptomycin at 37°C in a
humidified atmosphere containing 5% CO2. Cell
detachments were achieved by rinsing with 0.05% trypsin/0.02% EDTA
solution. The cells were treated with different concentrations of
isoalantolactone dissolved in DMSO with a final concentration of 1%
for 24 h. DMSO-treated cells were used as a control.
Splenocytes isolation
Eight-week-old, C57/BL6 mice, weighing 20 g were
used. The mice were maintained in a specific pathogen-free grade
animal facility on a 12-h light/dark cycles at 22±2°C. The mouse
procedures were approved by the Experimental Animal Committee of
Liaoning Medical University. Mice were anesthetized using
pentobarbital sodium (65 mg/kg i.p.) and were perfused
transcardially with PBS. Following midline abdominal incision
spleen was removed and the freshly isolated splenocytes were
cultured in RPMI-1640 medium supplemented with 20% heat-inactivated
FBS and maintained at 37°C with 5% CO2 in a humidified
atmosphere.
Cell viability and proliferation
assay
For the in vitro cell viability experiments,
a trypan blue exclusion assay was performed using Vi-CELL series
cell viability analyzers (Beckman Coulter Inc., Brea, CA, USA). To
determine the inhibition effect of isoalantolactone on
SKOV3 cell growth, cells were incubated with 35 or 75
µM isoalantolactone for 24 h. Subsequently, the cells were
collected and washed with PBS and then incubated with 0.04% trypan
blue for 5 min at room temperature. Trypan blue only stains dead
cells. After washing, the cells were resuspended in PBS and the
percentage of living and dead cells were counted. The living cell
rate (%) = number of living cells/number of living cells + number
of dead cells) ×100.
To analyze cell proliferation for isoalantolactone
treatment, cells were treated as described above. Then cell
proliferation was examined by the 5-ethynyl-2-deoxyuridine (EdU)
incorporation assay using the Cell-Light EdU imaging detection kit
according to manufacturer's instructions.
Cell cycle analysis
SKOV3 cells were incubated with 35 and 75
µM isoalantolactone for 24 h. Then the cells were collected,
fixed, stained with PI staining solution (3.8 mM sodium citrate, 50
µg/ml PI in PBS) and 20 g/ml RNase A in the dark for 30 min.
Cell cycle distribution was assessed by flow cytometry (Beckman
Coulter, Epics XL). For the flow cytometric analysis, at least
10,000 cells were used for each sample. The data were analyzed
using Cell Quest software (Becton Dickinson, San Jose, CA, USA) to
measure the DNA content of cells in the G0/G1, S and G2/M
phases.
Acridine orange staining
Cells (2×106) were stained with AO
according to a published procedure (23). Briefly, after the cells were treated
with 35 and 75 µM isoalantolactone for 24 h, the cells were
incubated with 1 mg/ml AO for 20 min in the dark at 37°C.
Subsequently, the cells were washed twice with PBS. Images of the
cells were obtained by fluorescence microscopy.
Acidic vesicular organelle (AVO)
quantification and analysis
Cells were collected after treatment with 35 and 75
µM isoalantolactone in the presence or absence of the
autophagy inhibitor 3-methyladenine (3-MA 5 mM) for 24 h. Next, the
cells were incubated with 1 mg/ml AO for 20 min in the dark at
37°C. After washing, the cells were analyzed by flow cytometry and
CellQuest software. Red fluorescence emissions from 106
cells were analyzed, and quantified as percentage of cells
containing AVOs.
siRNA silencing of PEA-15
In all, 5×105 cells were seeded in 6-well
plates. After 24 h, the cells were transfected with siRNA using
Lipofectamine 2000. After 3 days, the transfected cells that were
treated with isoalantolactone or DSMO were collected for the
clonogenic survival assay and western blot analysis.
Immunoblot analysis
Cells were treated with isoalantolactone for 24 h,
washed twice with PBS, and lysed on ice with WIP cell lysis reagent
supplemented with 1% PMSF for 30 min. The insoluble protein lysate
was removed by centrifugation at 12,000 rpm for 15 min at 4°C. The
protein concentrations were determined using a NanoDrop 1000
spectrophotometer (Thermo Scientific, Waltham, MA, USA). Proteins
(20 µg) were electrophoresed using 10% SDS-PAGE gels and
transferred to a PVDF membrane. After blocking with 5% non-fat milk
and washing with a Tris-buffered saline/Tween solution (TBST), the
membranes were incubated overnight at 4°C with specific primary
antibodies and then with anti-rabbit IgG or anti-mouse IgG
secondary antibodies for 1 h at room temperature. Signals were
detected using the ECL plus chemiluminescence kit and X-ray film
(Millipore Billerica, MA, USA). All the bands obtained were
quantified by densitometry using ImageJ software.
Statistical analysis
The results are expressed as the means ± SEM.
Analyses were performed using GraphPad prism 5 software. One-way
analysis of variance (ANOVA) was used to analyze significant
differences between groups under different conditions. Student's
t-test was used to determine significance when only two groups were
compared and P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of isoalantolactone on the growth
inhibition on the SKOV3 cells and normal mouse
splenocytes
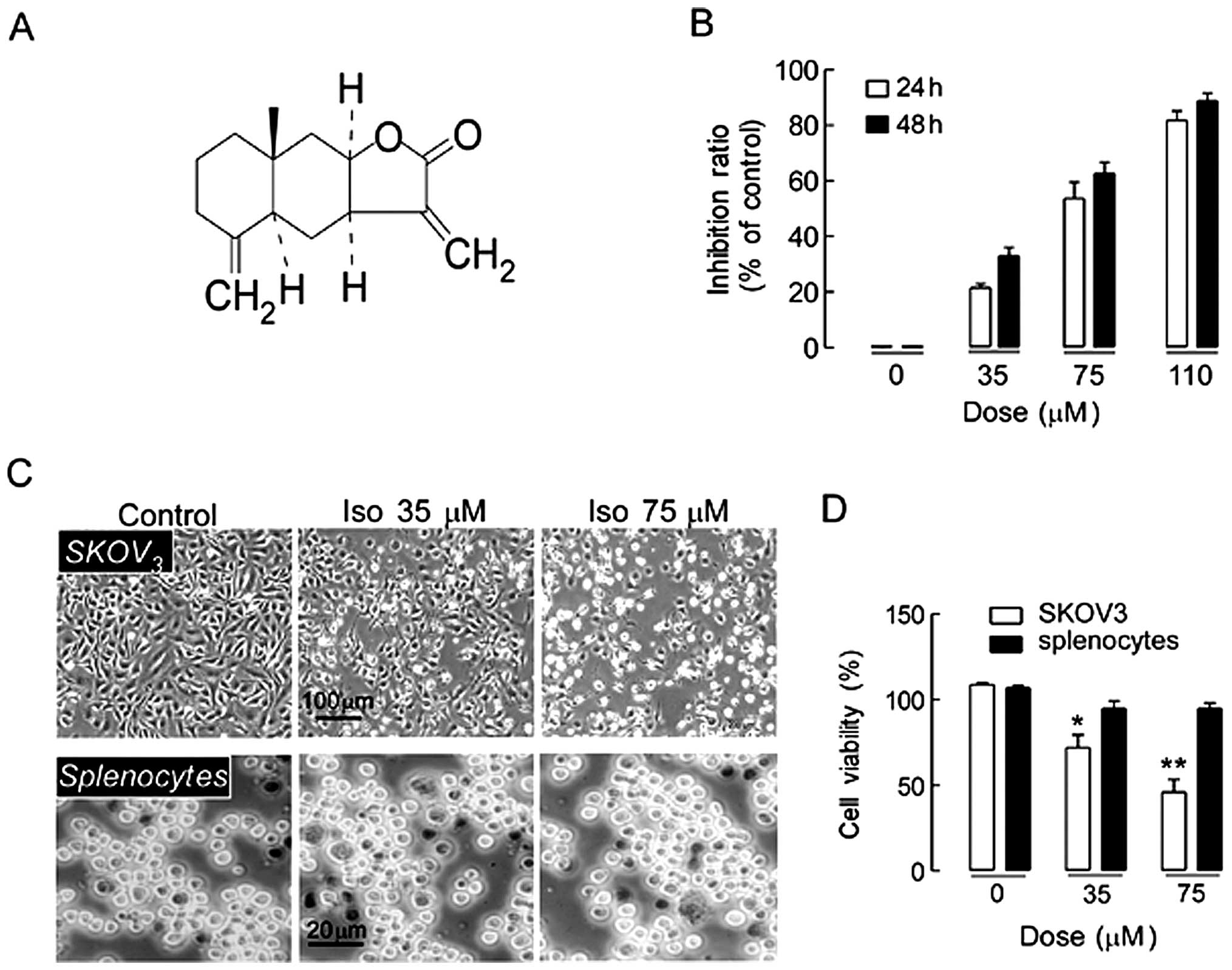
The chemical structure of isoalantolactone is shown
in Fig. 1A. SKOV3 cells
were treated with different doses of isoalantolactone (0, 35, 75,
or 110 µM) for 24 and 48 h. As shown in Fig. 1B, isoalantolactone significantly
inhibited the growth of SKOV3 cells in a dose- and
time-dependent manner. To further confirm the effect of
isoalantolactone on growth inhibition, cellular morphology changes
were observed using inverted phase contrast microscopy. The cells
became rounder and shrunken, and they floated in the culture medium
as the concentration of isoalantolactone increased (Fig. 1C), supporting the finding that
isoalantolactone inhibited SKOV3 cell growth in a
dose-dependent manner. The cytotoxic effect of isoalantolactone was
further evaluated by trypan blue staining on normal mouse
splenocytes. The data showed that fewer inhibitory effects were
observed in spleno-types treated with isoalantolactone at different
doses (Fig. 1C), suggesting that
SKOV3 cells are more sensitive than normal cells to
isoalantolactone. The effect of isoalantolactone at different
concentrations on the viability of SKOV3 cells was
assessed by live/death cells staining. As Fig. 1D shows, isoalantolactone inhibited
SKOV3 cells growth in a dose-dependent manner. The data
indicated that isoalantolactone was able to inhibit
SKOV3 cell growth selectively.
Effects of isoalantolactone on the cell
proliferation of SKOV3 cells
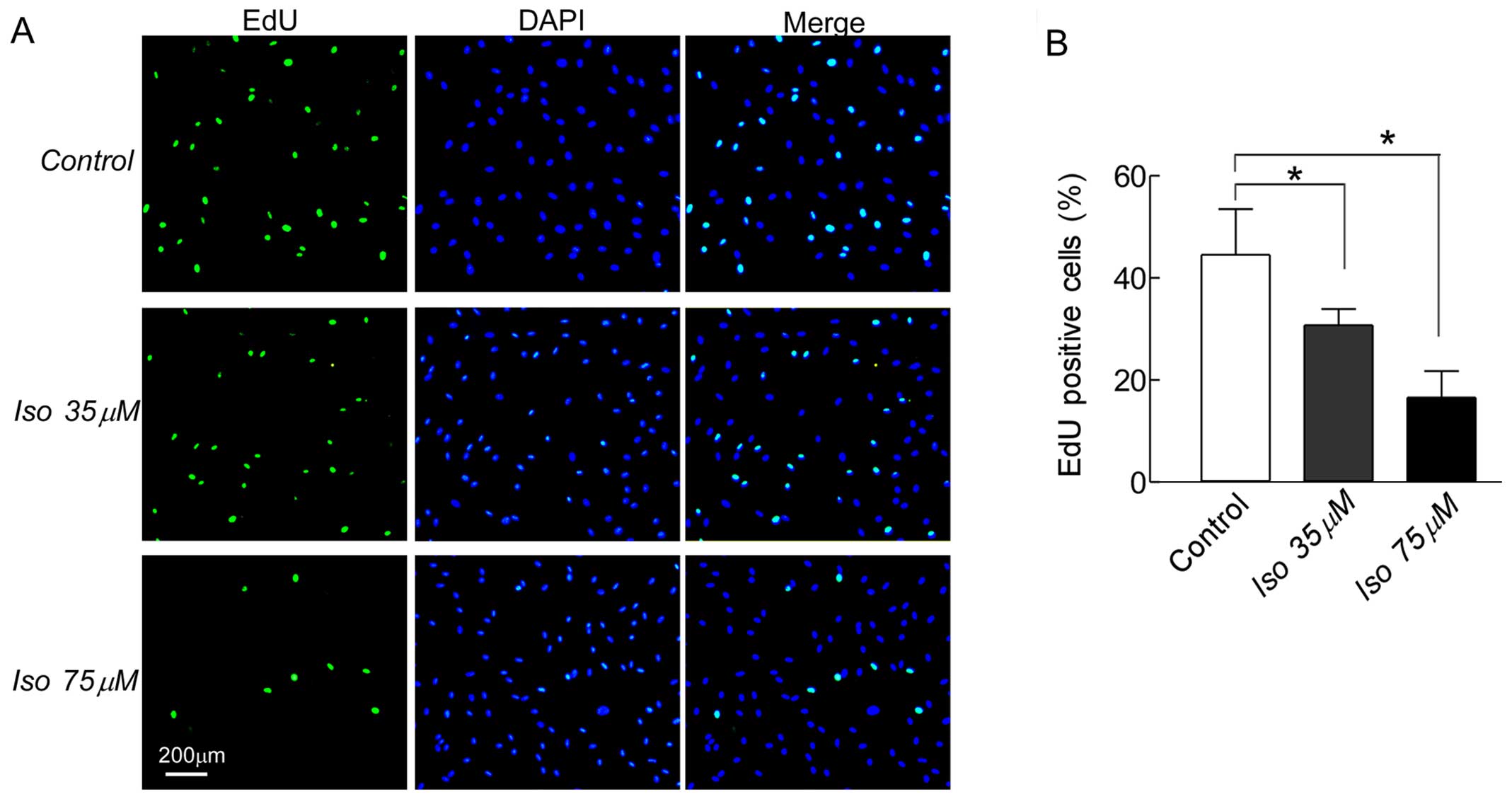
To further confirm that isoalantolactone inhibited
SKOV3 cell growth, we tested the cell proliferation
using the EdU incorporation assay. Fig.
2A shows the EdU-positive cells on SKOV3 cells
treated with 35 or 75 µM isoalantolactone for 24 h. Compared
to the control group (48.2%), the EdU-positive cells in the
isoalantolactone-treated groups at 35 (27.4%, P<0.05) or 75
µM (16.6%, P<0.05) were significantly reduced in a
dose-dependent manner (Fig. 2B).
Thus, isoalantolactone shows promise as an antitumor drug for human
ovarian cancer.
Effects of isoalantolactone on the cell
cycle and cell cycle-related proteins in SKOV3
cells
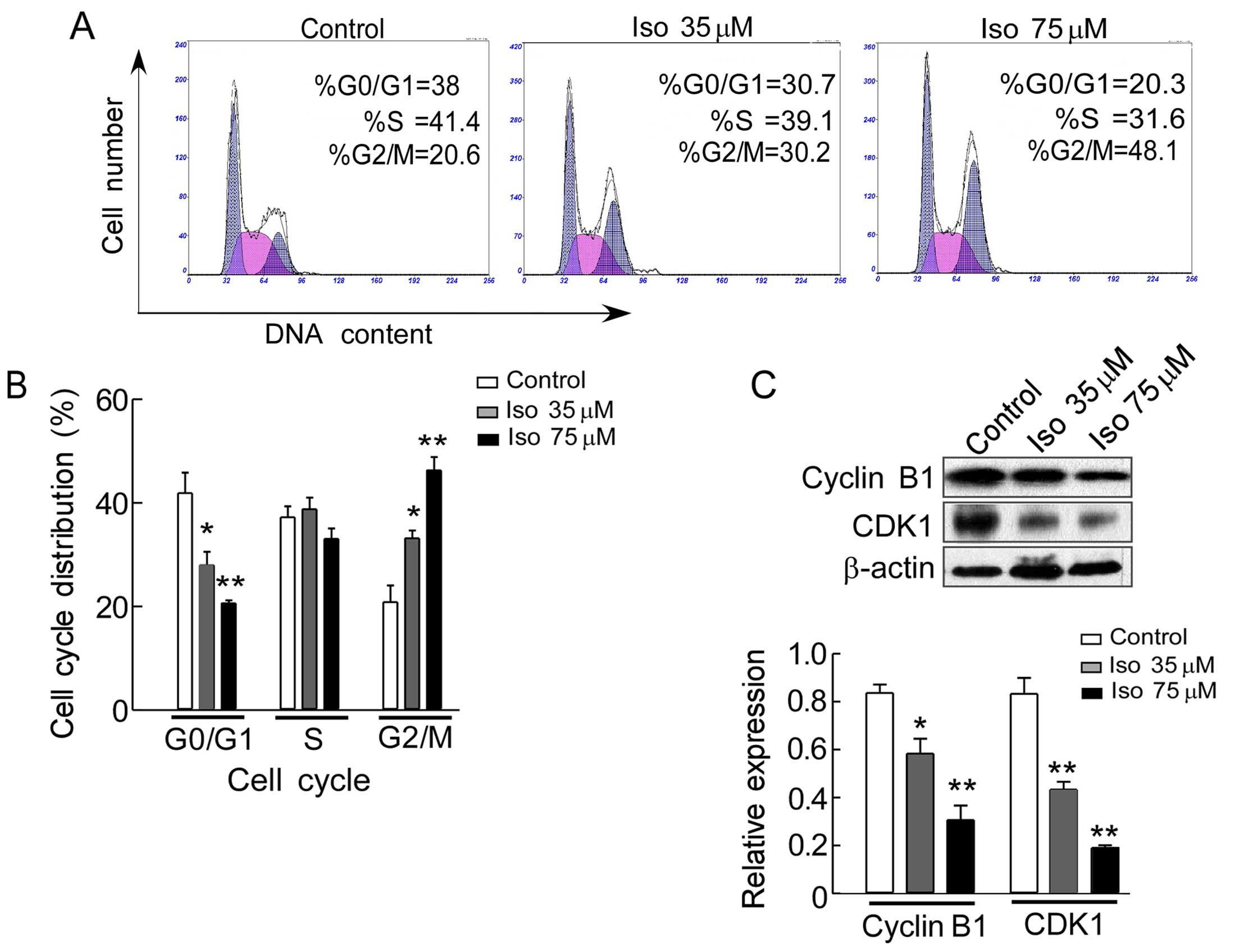
One of the major mechanisms underlying the
anti-proliferative effect of anticancer drugs is the prevention of
cell cycle progression. To explore the possible mechanism
underlying the inhibitory effect of isoalantolactone on the growth
of SKOV3 cells, cell cycle distribution was examined by
flow cytometry to analyze the DNA content of cell cycle phase. As
shown in Fig. 3A, isoalantolactone
induced a dose-dependent increase in the number of SKOV3
cells in G2/M phase, the percentage of accumulation of cells was
increased from 20.8% in the control group to 33.2% (P<0.05) and
46.3% (P<0.01) in the cells treated with 35 and 75 µM of
isoalantolactone, respectively for 24 h, leading to a decrease in
the proportion of cells in G0/G1 phase from 41.9% in the control
group to 27.9% (P<0.05) and 20.6% (P<0.01) in the cells with
the above-mentioned different concentration (Fig. 3B). We also investigated the
expression of cyclin B1 and CDK1 of cell cycle regulators by
western blot analysis. The results revealed that the expression of
cyclin B1 and CDK1 were gradually decreased with increasing
concentrations of isoalantolactone in a dose-dependent manner
(Fig. 3C), indicating that cell
cycle arrest at G2/M phase may be associated with the inhibition of
SKOV3 cell growth by isoalantolactone treatment.
Effects of isoalantolactone on AVO
formation in SKOV3 cells
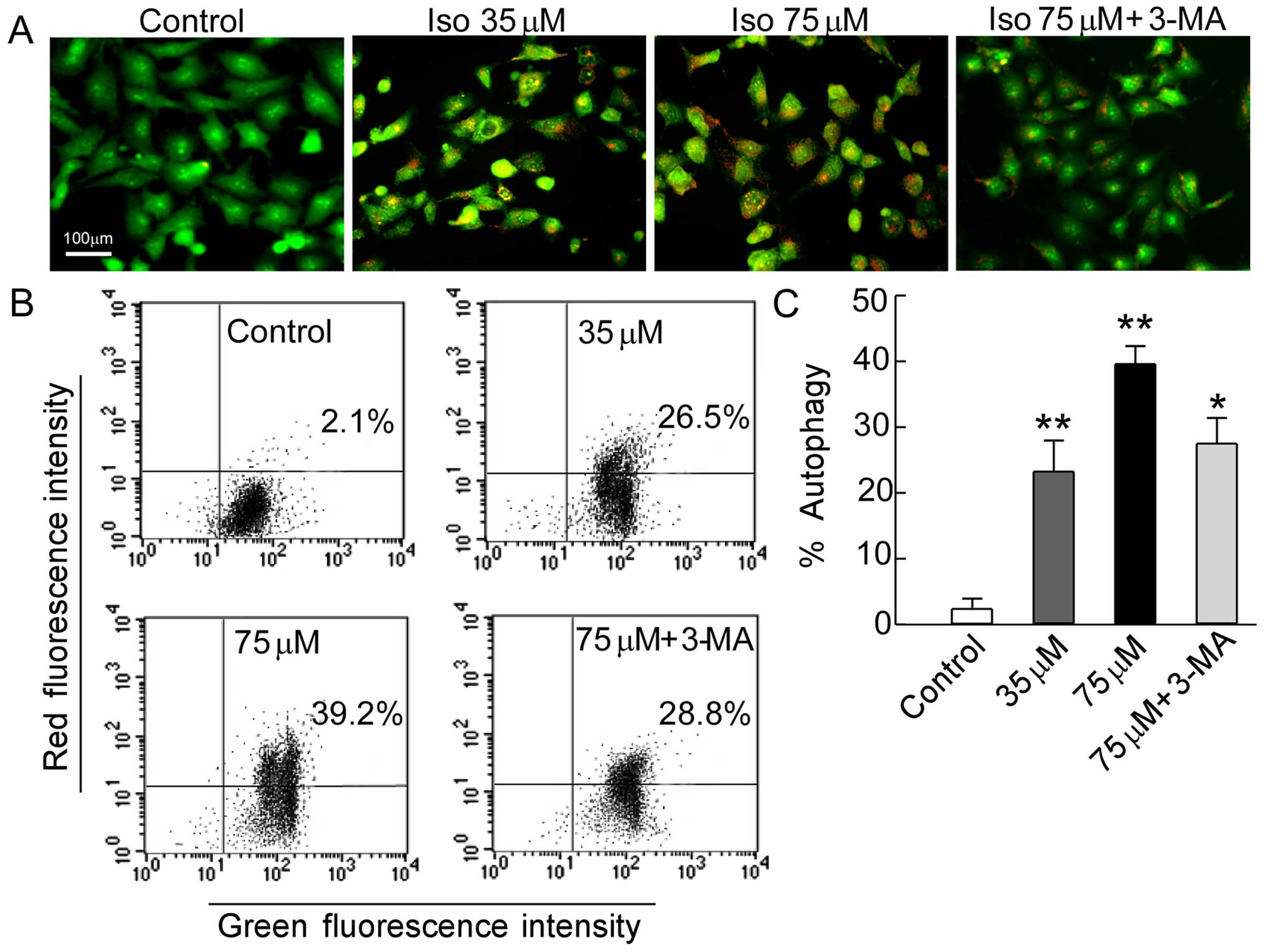
Autophagy is closely associated with tumors and
plays an important role in human tumor suppression. AVO formation
(autophagosomes and autolysosomes) is a characteristic feature of
autophagy (24). To investigate
whether autophagy was involved in the inhibitory effects of
isoalantolactone on SKOV3 cell growth, the accumulation
of AVOs was analyzed by AO staining and quantified by flow
cytometry (25). AO has a weak base
that freely passes across the plasma membrane in a neutral state
distinguished by green fluorescence. After entrance into acidic
compartments, AO changes into the protonated form which is
distinguished by bright red fluorescence while control cells
primarily showed green fluorescence. The intensity of the red
fluorescence was proportional to the degree of acidity. Thus, the
formation of AVOs could be quantified. As shown in Fig. 4A, after cell treatment with
isoalantolactone, the intensity of the bright red fluorescence was
markedly increased in a dose-dependent manner compared to the
control. We further quantified AVOs by flow cytometry. Fig. 4B illustrates significant formation
of AVOs in isoalantolactone-treated cells (26.5 or 39.2%,
P<0.01) compared to the control cells (2.1%). Accordingly, we
added the autophagy inhibitor 3-MA, which controls the autophagy
pathway at various points (26). We
found that isoalantolactone-induced AVO formation was suppressed
when the cells were treated in combination with the specific
autophagy inhibitor (28.8%, P<0.05) (Fig. 4C). These results show that
isoalantolactone-induced autophagy is involved in inducing death of
SKOV3 cells.
Effects of isoalantolactone on the
expression of autophagy proteins in SKOV3 cells
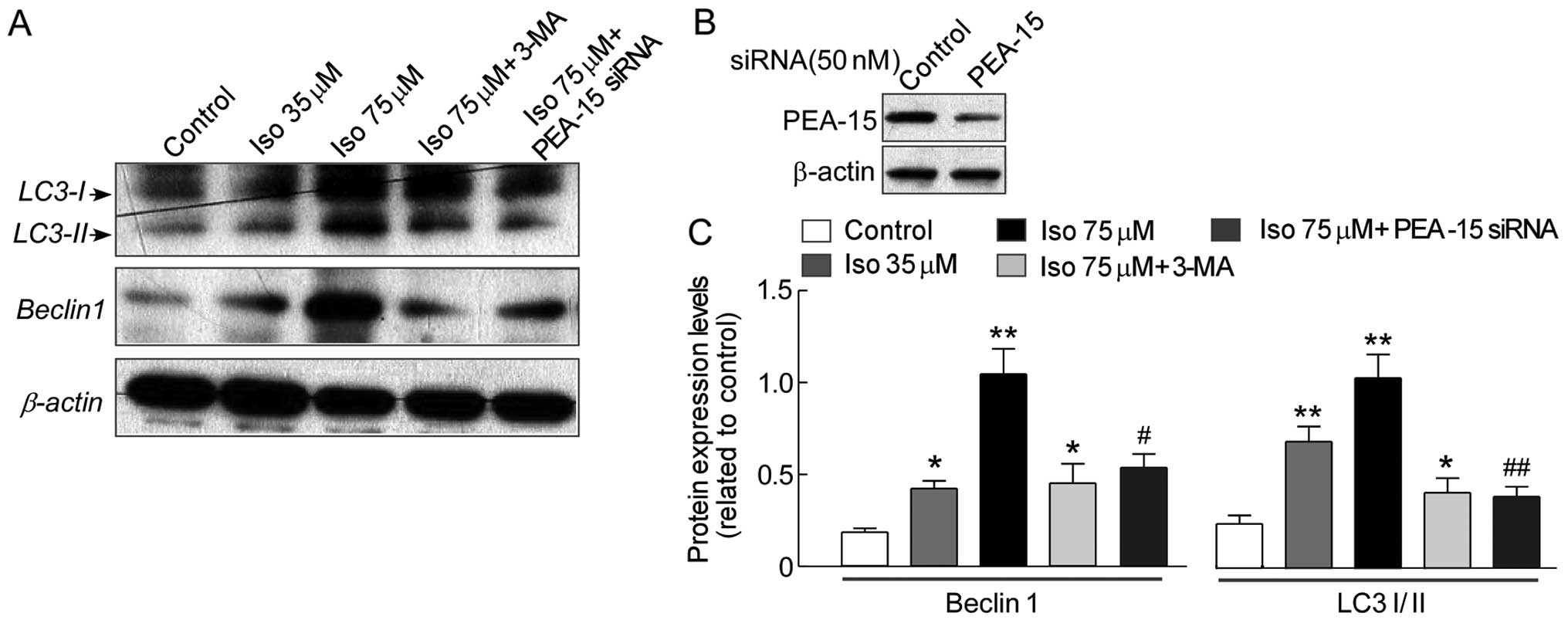
To further confirm involvement of autophagy in
isoalantolactone-induced SKOV3 cell death, Western blot
analysis was performed to evaluate the effects of isoalantolactone
on the autophagy protein expression of Beclin1, which was the
initiation factor of autophagosome formation, LC3-I and LC3-II
(autophagosome marker). As shown in Fig. 5, isoalantolactone activated Beclin1
expression and increased the ratio of LC3-I and LC3-II protein
expression in a dose-dependent manner, while markedly decreased in
cells pre-treated with the combination 3-MA and 75 µM
isoalantolactone compared to cells treated only with 75 µM
isoalantolactone, confirming that autophagy was involved in
isoalantolactone-induced cell death.
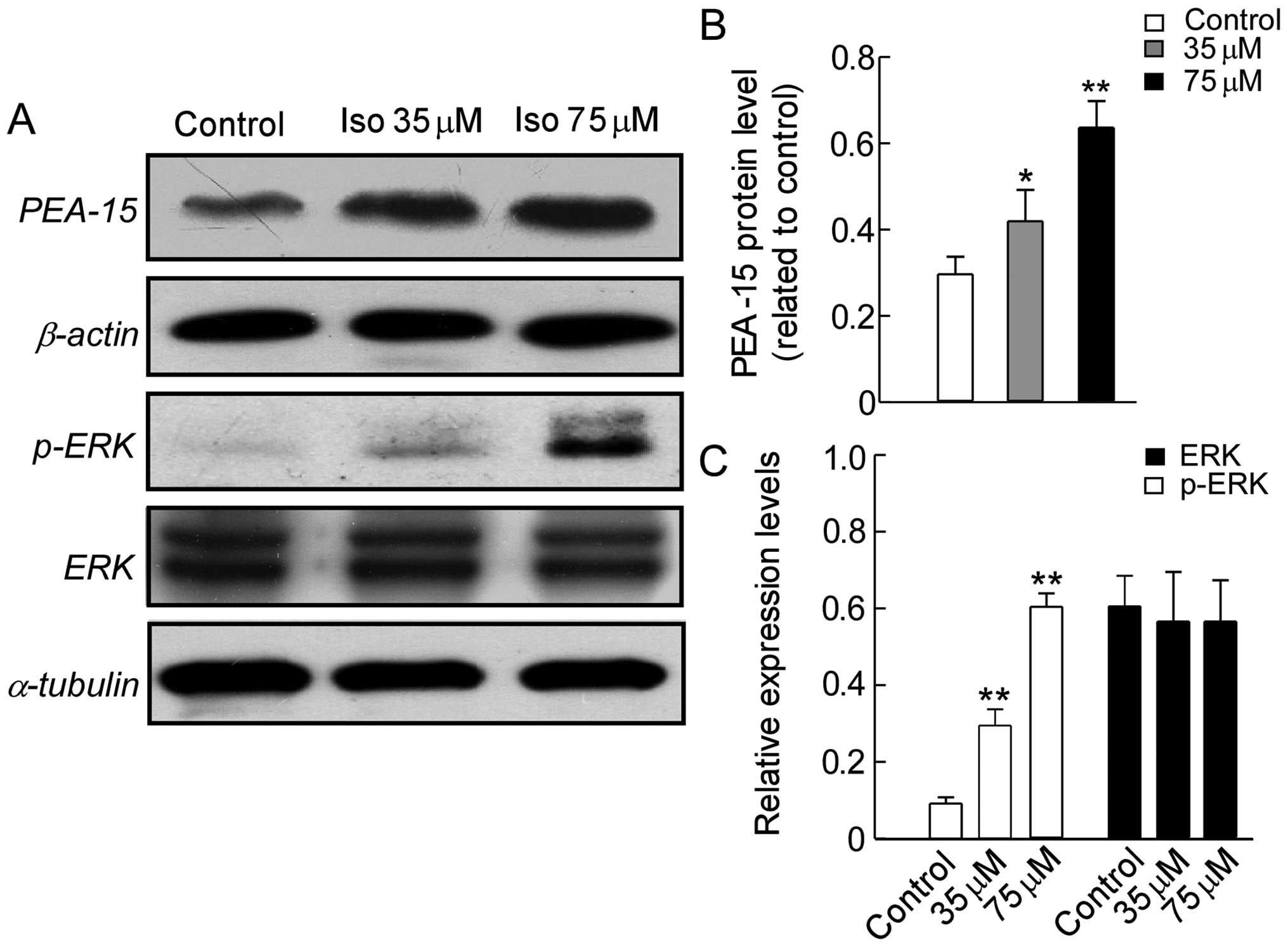
Effects of isoalantolactone on the
expression of autophagy regulators in SKOV3 cells
Autophagy is a complicated regulatory process, which
involves a great number of upstream regulating signaling pathways
(27,28). While ERK, the target of PEA-15, is
relatively new regulator studied of autophagy (20,29,30).
To elucidate the underlying mechanism of autophagy induced by
isoalantolactone in SKOV3 cells, we examined the
expression of PEA-15, ERK and pERK by western blot analysis. As
shown in Fig. 6A and B,
isoalantolactone greatly increased PEA-15 expression and activated
ERK phosphorylation in a dose-dependent manner compared to the
control cells, whereas no great change on ERK total protein level
was seen (Fig. 6C). These results
suggest that isoalantolactone-induced upregulation of PEA-15
expression and activation of ERK signaling pathway are associated
with autophagy in SKOV3 cells.
PEA-15 knockdown by siRNA depresses
autophagy of SKOV3 cells by isoalantolactone
induction
Based on the results described above, we further
confirm whether isoalantolactone induced autophagy on
SKOV3 cells through upregulation PEA-15 expression. The
PEA-15 knockdown was performed by siRNA. Indeed, PEA-15 knockdown
to ~50% of the original level significantly reduced AVO formation
of SKOV3 cells by isoalantolactone induction (Fig. 5B). Western blot analysis showed that
the expression of Beclin1, LC3-I and LC3-II protein levels on
SKOV3 cells were greatly decreased by PEA-15 knockdown
compared to cells treated with high-dose isoalantolactone
(P<0.05 or P<0.01) (Fig. 5C).
Thus, the PEA-15 levels correlated with isoalantolactone-induced
autophagy on SKOV3 cells.
Discussion
In the present study, high throughput screening of a
library of compounds derived from Chinese herbs was performed using
SKOV3 human ovarian cancer cells. Isoalantolactone, a
sesquiterpene lactone compound, was identified as a potent growth
inhibitor of SKOV3 cells during the screening process.
After the cells were treated with isoalantolactone for 24 h, the
IC50 value was 55 µM. Cell proliferation analysis
revealed that isoalantolactone inhibited SKOV3 cell
growth in a dose- and time-dependent manner, whereas, did not
display a significant toxic effect on normal mouse splenocytes
in vitro, which indicates the growth-inhibiting effect of
isoalantolactone was selective to tumor cells.
Cell cycle arrest, apoptosis and autophagy are main
causes of cell proliferation inhibition (30). Obstruction of cell cycle progression
in cancer cells is considered as one of the most effective
strategies for the control of tumor growth (31). We first investigated whether the
inhibitory effect of isoalantolactone on SKOV3 cell
growth was due to cell cycle arrest. Flow cytometry showed that
isoalantolactone blocked SKOV3 cells in the G2/M phase
of the cell cycle in a dose-dependent manner, suggesting that
retardation of cell cycle progression may be one of the mechanisms
underlying the antiproliferative effect of isoalantolactone. In
addition, we also analyzed cellular apoptosis by isoalantolactone
induction in SKOV3 cells. Flow cytometric analysis
showed that less apoptotic cells are observed by isoalantolactone
induction (data no shown). These data indicate that
isoalantolactone induced SKOV3 cell death through cell
cycle arrest in G2/M phase, but independently of the induction of
apoptosis.
Autophagy is a major pathway for bulk degradation of
proteins within the lysosome/vacuole compartments. This pathway
initiates the formation of the pre-autophagosome that enwraps part
of the cytoplasm to form a double membrane autophagosome, which is
then transported to the lysosome/vacuole for degradation (32,33).
The AVO formation is one of the characteristic features of cells
which pass through the process of autophagy after exposure to
different autophagy inducer agents (34). Beclin1 was part of the class III
PI3K complex that promotes autophagy, functions as a tumor
suppressor in mammalian cells and was essential for the
double-membrane autophagosome formation, which was required during
the initial steps of autophagy (35). The autophagic induction of Beclin1
facilitates the inhibition of tumorigenesis (28). The allelic loss of Beclin1 is
frequently seen in human breast, ovarian and prostate cancers
(36). LC3 (microtubule-associated
protein 1 light chain 3) was one of the most important mammalian
homologue of yeast Atg8 (37) which
was cleave by the protease ATG4 and then conjugated to the lipid
phosphatidyl ethanolamine via the activity of ATG7 and ATG3. While
the unprocessed form of LC3 (LC3I) was diffusely distributed
throughout the cytoplasm, the lipidated form of LC3 (LC3II)
specifically accumulates on nascent autophagosomes to promote
membrane fusion and thus represents a marker to monitor autophagy
(38).
Thus, we observed the effect of isoalantolactone
induction on the formations of AVOs in SKOV3 cells using
lysosomotropic agent AO staining and the expression of autophagy
related proteins using western blot analysis. Our results showed
accumulation of AVOs in the cytoplasm, and increased expression of
the Beclin1 protein as well as that ratio of the LC3-I, LC3-II
proteins occurred in a dose-dependent manner, whereas, respective,
the decrease was accompanied by treatment with an autophagy
inhibitor 3-MA following treatment with isoalantolactone,
demonstrating that autophagy was involved in
isoalantolactone-induced cell death in SKOV3 cells.
Accordingly, previous studies showed that ovarian
cancer cell proliferation was inhibited by the expression of
PEA-15, which blocks ERK-dependent proliferation by sequestering
ERK in the cytoplasm and preventing its entry into the nucleus
(39). Previous reports indicated
that the antitumor activity of PEA-15 in ovarian cancer cells was
due to induction of autophagy via activated pERK (20). Moreover, ERK was shown to
phosphorylate Gα-interacting protein to induce autophagy in human
colon cells, and the MAPK pathway is an important regulator of
autophagy (29,30). Thus further studies are needed to
investigate whether the anti-proliferative activity of
isoalantolactone occurs through upregulation of PEA-15 expression
to induce autophagy via activated ERK phosphorylation. Our results
showed that isoalantolactone-induced the upregulation of PEA-15
expression, the accumulation of AVOs in the cytoplasm, and the
increased expression of the Beclin1 protein as well as that ratio
of the LC3-I, LC3-II occurred in a dose-dependent manner, whereas
the respective decrease was accompanied by treatment with an
autophagy inhibitor or PEA-15 knockdown by siRNA. Taken together,
these results support the hypothesis that isoalantolactone
triggered autophagy and demonstrate that autophagy is involved in
the anti-tumor effects of isoalantolactone in SKOV3
cells.
In conclusion, our studies provide experimental
evidence that isoalantolactone significantly inhibits the
proliferation of SKOV3 cells and induces G2/M phase cell
cycle arrest. Furthermore, isoalantolactone-induced autophagy of
SKOV3 cells was through upregulation of the expression
of PEA-15 and activation of the phosphorylation of ERK. These
findings indicate that isoalantolactone is a potential antitumor
agent for treating ovarian cancer.
Acknowledgments
The present study was supported by the National
Natural Science Foundation of China (no. 31571184).
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bhoola S and Hoskins WJ: Diagnosis and
management of epithelial ovarian cancer. Obstet Gynecol.
107:1399–1410. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: The impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yagüe E, Arance A, Kubitza L, O'Hare M,
Jat P, Ogilvie CM, Hart IR, Higgins CF and Raguz S: Ability to
acquire drug resistance arises early during the tumorigenesis
process. Cancer Res. 67:1130–1137. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsuruo T, Naito M, Tomida A, Fujita N,
Mashima T, Sakamoto H and Haga N: Molecular targeting therapy of
cancer: Drug resistance, apoptosis and survival signal. Cancer Sci.
94:15–21. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Szakács G, Paterson JK, Ludwig JA,
Booth-Genthe C and Gottesman MM: Targeting multidrug resistance in
cancer. Nat Rev Drug Discov. 5:219–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ozols RF, Bookman MA, du Bois A, Pfisterer
J, Reuss A and Young RC: Intraperitoneal cisplatin therapy in
ovarian cancer: Comparison with standard intravenous carboplatin
and paclitaxel. Gynecol Oncol. 103:1–6. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ramirez I, Chon HS and Apte SM: The role
of surgery in the management of epithelial ovarian cancer. Cancer
Control. 18:22–30. 2011.PubMed/NCBI
|
|
9
|
Ghantous A, Gali-Muhtasib H, Vuorela H,
Saliba NA and Darwiche N: What made sesquiterpene lactones reach
cancer clinical trials? Drug Discov Today. 15:668–678. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang S, Won YK, Ong CN and Shen HM:
Anti-cancer potential of sesquiterpene lactones: Bioactivity and
molecular mechanisms. Curr Med Chem Anticancer Agents. 5:239–249.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Y, Ni ZY, Zhu MC, Dong M, Wang SM, Shi
QW, Zhang ML, Wang YF, Huo CH, Kiyota H, et al: Antitumour
activities of sesquiterpene lactones from Inula helenium and Inula
japonica. Z Naturforsch C. 67:375–380. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khan M, Ding C, Rasul A, Yi F, Li T, Gao
H, Gao R, Zhong L, Zhang K, Fang X, et al: Isoalantolactone induces
reactive oxygen species mediated apoptosis in pancreatic carcinoma
PANC-1 cells. Int J Biol Sci. 8:533–547. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pal HC, Sehar I, Bhushan S, Gupta BD and
Saxena AK: Activation of caspases and poly (ADP-ribose) polymerase
cleavage to induce apoptosis in leukemia HL-60 cells by Inula
racemosa. Toxicol In Vitro. 24:1599–1609. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Trendafilova A, Chanev C and Todorova M:
Ultrasound-assisted extraction of alantolactone and
isoalantolactone from Inula helenium roots. Pharmacogn Mag.
6:234–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Leist M and Jäättelä M: Four deaths and a
funeral: From caspases to alternative mechanisms. Nat Rev Mol Cell
Biol. 2:589–598. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Semenza GL: Mitochondrial autophagy: Life
and breath of the cell. Autophagy. 4:534–536. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shintani T and Klionsky DJ: Autophagy in
health and disease: A double-edged sword. Science. 306:990–995.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ito H, Daido S, Kanzawa T, Kondo S and
Kondo Y: Radiation-induced autophagy is associated with LC3 and its
inhibition sensitizes malignant glioma cells. Int J Oncol.
26:1401–1410. 2005.PubMed/NCBI
|
|
19
|
Paglin S, Hollister T, Delohery T, Hackett
N, McMahill M, Sphicas E, Domingo D and Yahalom J: A novel response
of cancer cells to radiation involves autophagy and formation of
acidic vesicles. Cancer Res. 61:439–444. 2001.PubMed/NCBI
|
|
20
|
Bartholomeusz C, Rosen D, Wei C, Kazansky
A, Yamasaki F, Takahashi T, Itamochi H, Kondo S, Liu J and Ueno NT:
PEA-15 induces autophagy in human ovarian cancer cells and is
associated with prolonged overall survival. Cancer Res.
68:9302–9310. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fiory F, Formisano P, Perruolo G and
Beguinot F: Frontiers: PED/PEA-15, a multifunctional protein
controlling cell survival and glucose metabolism. Am J Physiol
Endocrinol Metab. 297:E592–E601. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rasul A, Yu B, Zhong L, Khan M, Yang H and
Ma T: Cytotoxic effect of evodiamine in SGC-7901 human gastric
adenocarcinoma cells via simultaneous induction of apoptosis and
autophagy. Oncol Rep. 27:1481–1487. 2012.PubMed/NCBI
|
|
23
|
Kanzawa T, Kondo Y, Ito H, Kondo S and
Germano I: Induction of autophagic cell death in malignant glioma
cells by arsenic trioxide. Cancer Res. 63:2103–2108.
2003.PubMed/NCBI
|
|
24
|
Levine B and Yuan J: Autophagy in cell
death: An innocent convict? J Clin Invest. 115:2679–2688. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kanzawa T, Zhang L, Xiao L, Germano IM,
Kondo Y and Kondo S: Arsenic trioxide induces autophagic cell death
in malignant glioma cells by upregulation of mitochondrial cell
death protein BNIP3. Oncogene. 24:980–991. 2005. View Article : Google Scholar
|
|
26
|
Liang XH, Jackson S, Seaman M, Brown K,
Kempkes B, Hibshoosh H and Levine B: Induction of autophagy and
inhibition of tumorigenesis by Beclin 1. Nature. 402:672–676. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mathew R, Karantza-Wadsworth V and White
E: Role of autophagy in cancer. Nat Rev Cancer. 7:961–967. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen N and Karantza-Wadsworth V: Role and
regulation of autophagy in cancer. Biochim Biophys Acta.
1793:1516–1523. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Corcelle E, Nebout M, Bekri S, Gauthier N,
Hofman P, Poujeol P, Fénichel P and Mograbi B: Disruption of
autophagy at the maturation step by the carcinogen lindane is
associated with the sustained mitogen-activated protein
kinase/extracellular signal-regulated kinase activity. Cancer Res.
66:6861–6870. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ogier-Denis E, Pattingre S, El Benna J and
Codogno P: Erk1/2-dependent phosphorylation of Galpha-interacting
protein stimulates its GTPase accelerating activity and autophagy
in human colon cancer cells. J Biol Chem. 275:39090–39095. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Janssen A and Medema RH: Mitosis as an
anti-cancer target. Oncogene. 30:2799–2809. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mizushima N, Yamamoto A, Hatano M,
Kobayashi Y, Kabeya Y, Suzuki K, Tokuhisa T, Ohsumi Y and Yoshimori
T: Dissection of autophagosome formation using Apg5-deficient mouse
embryonic stem cells. J Cell Biol. 152:657–668. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Weidberg H, Shpilka T, Shvets E, Abada A,
Shimron F and Elazar Z: LC3 and GATE-16 N termini mediate membrane
fusion processes required for autophagosome biogenesis. Dev Cell.
20:444–454. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Daido S, Kanzawa T, Yamamoto A, Takeuchi
H, Kondo Y and Kondo S: Pivotal role of the cell death factor BNIP3
in ceramide-induced autophagic cell death in malignant glioma
cells. Cancer Res. 64:4286–4293. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cao Y and Klionsky DJ: Physiological
functions of Atg6/Beclin 1: A unique autophagy-related protein.
Cell Res. 17:839–849. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Aita VM, Liang XH, Murty VV, Pincus DL, Yu
W, Cayanis E, Kalachikov S, Gilliam TC and Levine B: Cloning and
genomic organization of beclin 1, a candidate tumor suppressor gene
on chromosome 17q21. Genomics. 59:59–65. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tanida I, Sou YS, Ezaki J,
Minematsu-Ikeguchi N, Ueno T and Kominami E:
HsAtg4B/HsApg4B/autophagin-1 cleaves the carboxyl termini of three
human Atg8 homologues and delipidates microtubule-associated
protein light chain 3- and GABAA receptor-associated
protein-phospholipid conjugates. J Biol Chem. 279:36268–36276.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kabeya Y, Mizushima N, Ueno T, Yamamoto A,
Kirisako T, Noda T, Kominami E, Ohsumi Y and Yoshimori T: LC3, a
mammalian homologue of yeast Apg8p, is localized in autophagosome
membranes after processing. EMBO J. 19:5720–5728. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Renault F, Formstecher E, Callebaut I,
Junier MP and Chneiweiss H: The multifunctional protein PEA-15 is
involved in the control of apoptosis and cell cycle in astrocytes.
Biochem Pharmacol. 66:1581–1588. 2003. View Article : Google Scholar : PubMed/NCBI
|