Introduction
Colorectal cancer (CRC) is one of the most common
malignant diseases, and the second leading cause of cancer-related
mortality in both developed and developing countries (1,2).
Metastasis contributes to the major cause of death for CRC and
10–25% of patients already present with liver metastasis at the
time of diagnosis (3,4). Cancer metastasis is a multifactor,
multistage and multistep biological process that includes cancer
cell migration, adhesion, invasion, growth, neovascularization,
specific organ homing and immune evasion (5). Although much progress has been
achieved, the detailed molecular mechanisms involved in primary
tumor cell invasion and metastasis still require further
investigation.
Nicotinic acetylcholine receptors (nAChRs) are a
family of integral membrane proteins responding to the binding of a
neurotransmitter acetylcholine (ACh) or tobacco extracts such as
nicotine. In humans, 16 different subunits of nicotinic
acetylcholine receptors (α1–7, α9–10, β1–4, δ, ε, γ) are expressed
on non-neuronal cells both within and outside the nervous system,
which consist of five subunits forming hetero- or homo-pentamers
that contain α (α1-α10), β (β1-β4), γ, δ or ε subunits (6). Genes encoding for individual nAChR
subunits are named CHRNA1, CHRNA2, CHRNA3, CHRNA4, CHRNA5, CHRNA6,
CHRNA7, CHRNA9 and CHRNA10 for the α subunits and CHRNB1, CHRNB2,
CHRNB3, and CHRNB4 for the β subunits. Both nAChR families are
expressed in cancer cells. Accumulated evidence indicates that
cancer might be triggered by altered signaling of nAChRs (7) and thus may play an important
regulatory role in cancer development and progression.
α7nAChR is a special subtype consisting of five
identical subunits which is expressed in many different
non-neuronal cells such as vascular and brain endothelial cells,
bronchial epithelial cells, keratinocytes, astrocytes,
synoviocytes, thymocytes, lymphocytes, bone marrow cells,
monocytes, macrophages, microglia and astrocytes (8–10) as
well as cancer cells including lung, pancreatic, gastric and colon
cancer (11–14). An unbalance of α7nAChR either at the
gene expression or protein expression level might be involved in
different diseases including Alzheimer and Parkinson disease
(15). Recent studies suggest that
nicotine and nicotinic derivatives could stimulate proliferation
and migration of colon cancer through α7nAChRs (14,16–18).
To date, it is not clear whether changes in the expression of
CHRNA7 affects colorectal cancer growth and metastasis. In this
study, we utilized LoVo human colorectal cancer cells to illustrate
the function of CHRNA7 in controlling tumor cell growth and
invasion. Based on the correlation of CHRNA7 expression with
colorectal cancer metastasis, overexpression of CHRNA7, which
resulted in abundant expression of α7nAChR, negatively controls
LoVo colorectal cancer cell invasion and metastasis via the
phosphatidylinositol 3-kinase/v-akt murine thymoma viral oncogene
homologue (PI3K/Akt) signaling pathway and thus has potential as a
therapeutic target for colorectal cancer metastasis. Thus, CHRNA7
may serve as either a diagnostic marker or a therapeutic target for
colorectal cancer metastasis.
Materials and methods
Materials
RPMI-1640 medium and fetal bovine serum (FBS) were
purchased from Gibco, (Gaithersburg, MD, USA) and Lipofectamine
2000 was from Invitrogen (Carlsbad, CA, USA). Plasmids for pLVX and
pLVX encoding the full length of acetylcholine receptor α7 gene
(pLVX-CHRNA7, NM_001190455) were constructed by GenScript
Corporation (Nanjing, China). The antibody against nicotinic
acetylcholine receptor α7 (anti-α7nAChR) was purchased from Abcam
(Oxford, UK) and antibodies against matrix metalloproteinase
(MMP)-1, -2, -3, -9 and -10 were obtained from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). Antibodies for
phospho-ERK1/2, phospho-Akt (Ser473), phospho-JNK, phospho-p38,
phospho-p65 (NF-κB), phospho-PI3K (p55/p85), ERK1/2, Akt, JNK, p38
and p65NF-κB were obtained from Cell Signaling Technology (Beverly,
MA, USA). The antibody for β-actin was purchased from Sigma-Aldrich
(St. Louis, MO, USA). PI3K inhibitor LY294002 was purchased from
Calbiochem (La Jolla, CA, USA). Goat anti-rabbit IgG antibody
conjugated to horseradish peroxidase (HRP) and goat anti-rat IgG
antibody conjugated to HRP were purchased from Santa Cruz
Biotechnology, Inc.
Cell culture and gene transfection
LoVo human colorectal adenocarcinoma cells were
obtained from the American Type Culture Collection (ATCC CCL-229™;
Manassas, VA, USA) and were grown in RPMI-1640 medium containing
10% heat-inactivated FBS, 2 mM glutamine, 50 U/ml penicillin and 50
µg/ml streptomycin and maintained at 37°C in a of 5%
CO2/95% air atmosphere. Cell viability was determined by
trypan blue exclusion assay. When growing at an exponential phase,
the cells were transfected with pLVX-CHRNA7 or pLVX-vector using
Lipofectamine 2000 according to the manufacturer's instructions.
After 48 h, the cells were harvested for subsequent
experiments.
Western blotting
Cells were lysed in 1X RIPA buffer containing 1 mM
phenylmethylsulfonyl fluoride (PMSF) and protease inhibitor
cocktail (all from Cell Signaling Technology, Danvers, MA, USA).
Protein concentration was determined by the Bradford protein assay.
Total proteins (40 µg) were separated by SDS-PAGE and
blotted onto PVDF membranes. After blocking with 5% non-fat milk,
the membranes were incubated overnight on ice with the primary
antibodies against α7nAChR (1:5,000), MMP-1, -2, -3, -9, -10,
phospho-ERK1/2, phospho-Akt, phospho-JNK, phospho-p38, phospho-p65
(NF-kB), phosph-PI3K (55/85), ERK1/2, Akt, JNK, p38, p65NF-κB and
β-actin (all dilutions 1:1,000). The next day, the membranes were
washed with TBST and incubated with HRP-conjugated anti-rabbit or
anti-rat secondary antibodies for 2 h at room temperature. After
the final washing, the membranes were incubated with ECL reagent
mixture and the signal was developed on a digital image system
(FluorChem E; Proteinsimple, Santa Clara, CA, USA).
Cell proliferation assay
Cells were seeded into 96-well plates at a density
of 2,000 cells per well, grown at 37°C overnight and then
transfected with pLVX-CHRNA7 or pLVX-vector. Cell growth was
analyzed using a WST-8 Cell Counting Kit-8 (CCK-8) (Dojindo
Laboratories, Kumamoto, Japan) at a frequency of every 24 h until 5
days after transfection. CCK-8 solution (10 µl) was added to
each well, and the cultures were incubated at 37°C for 1 h.
Absorbance at 450 nm was measured using an ultra microplate reader
(SpectraMax; Molecular Devices Corp., Sunnyvale, CA, USA).
Experiments were set in triplicates and repeated three times.
Transwell cell migration and invasion
assay
Transwell assays was used to estimate the effect of
CHRNA7 on migration and invasion of colon cancer cells. Cell
invasion and migration assays were carried out using modified
Boyden chambers consisting of Transwell (8-µm pore size;
Corning Costar Corp., Cambridge, MA, USA) membrane filter inserts
in 24-well tissue culture plates. For the invasion assay, 48 h
after transfection, the cells were trypsinized and resuspended in
serum-free RPMI-1640 medium at the density of 5×105
cells per ml. A total of 200 µl of cells were seeded into
each upper chamber of the Transwells, and 500 µl of medium
with 20% FBS was added in the lower chamber. The chambers were
incubated at 37°C in a humid atmosphere with 5% CO2.
After 48 h, the non-migrating cells on the upper surface of the
insert were removed with a cotton swab, and the cells that migrated
to the underside of the membrane were fixed with ice-cold methanol
and stained with crystal violet (0.1%). The cell surface of the
insert was then photographed under an inverted microscope (Olympus
CKX31; Olympus, Tokyo, Japan) for capturing images and quantified
by counting the number of cells in five fields at ×100
magnification per filter. For the invasion assay, an additional 50
µl of BD Matrigel (BD Biosciences, San Jose, CA, USA) was
added onto each upper chamber of the Transwells and the Transwells
were placed in a 37°C incubator for 6 h to solidify the Matrigel.
The tumor cell invasion capacity was then assessed in a way similar
to the migration assay.
Gelatin zymography
Gelatin zymography was used to determine the
activity of MMP-2 and MMP-9. Forty-eight hours after gene
transfection, cell culture supernatants of the LoVo cells were
collected and concentrated in Amicon Ulta-4 Centrifugal Filter
Devices (Millipore, Billerica, MA, USA). Equal amounts of total
protein were then mixed with SDS loading buffer and electrophoresed
on a 10% SDS polyacrylamide gel polymerized with 5 mg/ml gelatin.
After electrophoresis, the gels were re-natured by soaked for 30
min at room temperature in 2.5% Triton X-100. The gels were then
incubated in a developing buffer [50 mM Tris-HCl buffer (pH 7.4),
10 mM CaCl2] overnight at 37°C. The gels were stained
with 0.5% Coomassie Brilliant Blue R-250 and de-stained in washing
solution without dye. Gelatinolytic bands were observed as clear
zones against a blue background.
Statistical analysis
All assays were performed in triplicate, and
experiments were repeated at least three times. Data are presented
as mean ± SE. Statistical analysis was performed using the
Student's t-test to identify significant differences unless
otherwise indicated. P<0.05 was considered to indicate a
statistically significant difference. ImageJ software was used to
quantify the results of the western blotting. GraphPad Prism 5.0
software was used to create graphics.
Results
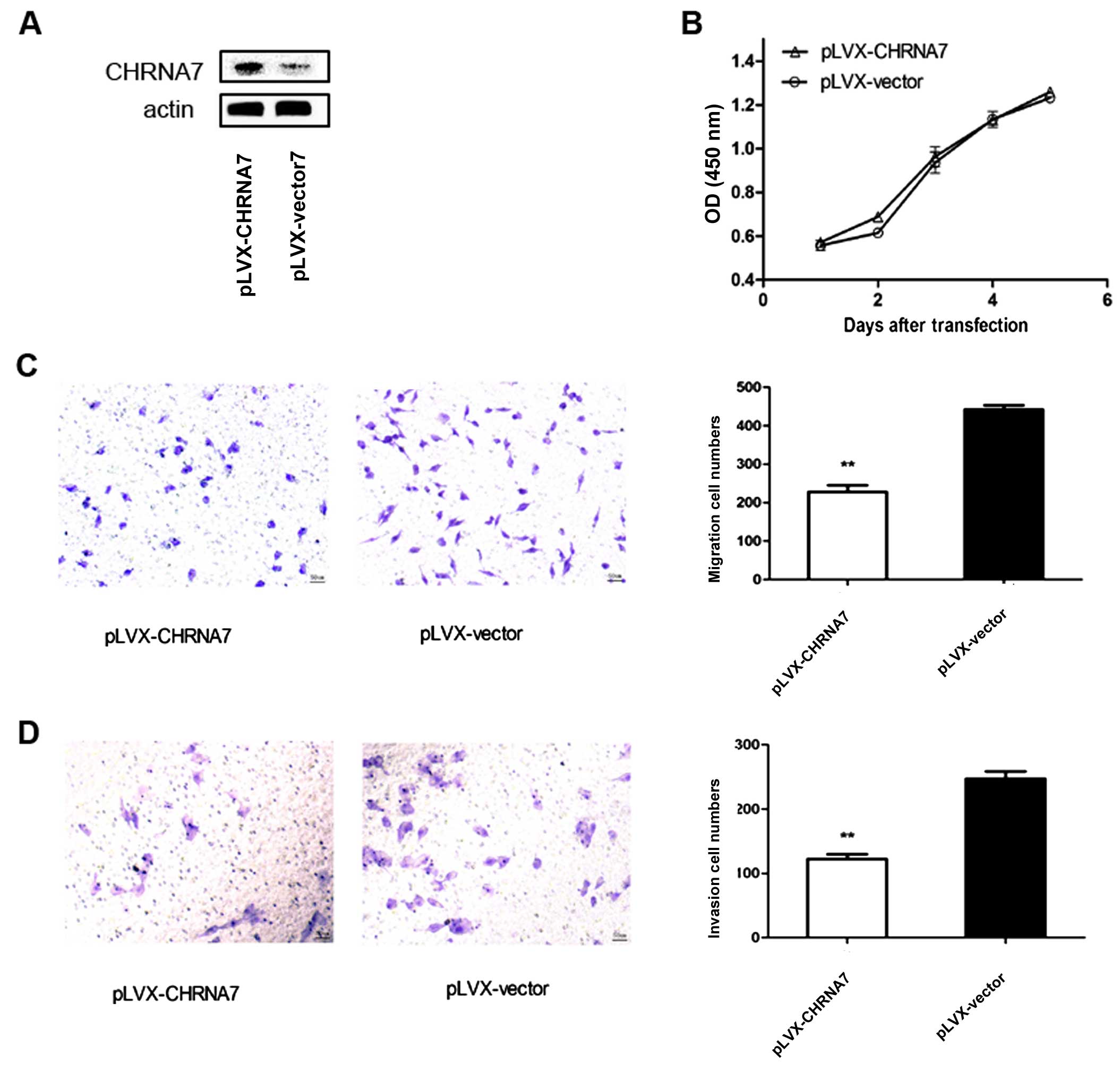
Overexpression of CHRNA7 does not affect
LoVo colorectal cancer cell proliferation
In order to evaluate the influence of CHRNA7, a
plasmid of pLVX-CHRNA7 was constructed and transfected into LoVo
cells. The transfection efficiency was ~80% as indicated by a
GFP-expression vector (data not shown). Western blotting revealed
the abundant expression of α7nAChR in the total cell lysate of the
LoVo cells 48 h after transfection (Fig. 1A).
Physiologically, we firstly measured the cell
proliferation of LoVo cells with or without CHRNA7 overexpression.
A CCK-8 cell proliferation assay was performed at different times
after LoVo cells were transfected with pLVX-CHRNA7 or pLVX-vector.
There was no difference between the two groups of cells from day 1
to 5 after transfection (Fig.
1B).
Overexpression of CHRNA7 inhibits LoVo
colorectal cancer cell migration and invasion
We then detected the influence of CHRNA7
overexpression on colon cancer cell metastasis. A Transwell assay
was conducted and BD Matrigel™ was used to imitate the
extracellular matrix. The migratory and invasive abilities were
evaluated based on the number of LoVo cells that passed through the
polycarbonate membrane of the Transwell invasion chamber. As shown
in Fig. 1C, the number of LoVo
cells that passed through the polycarbonate membrane in the
pLVX-CHRNA7 transfection group was significantly lower than that in
the pLVX-vector control group (P<0.01). The invasion assay
(Fig. 1D) showed similar results.
The number of LoVo cells in the pLVX-CHRNA7 transfection group was
also significantly lower than that in the pLVX-vector control group
(P<0.01).
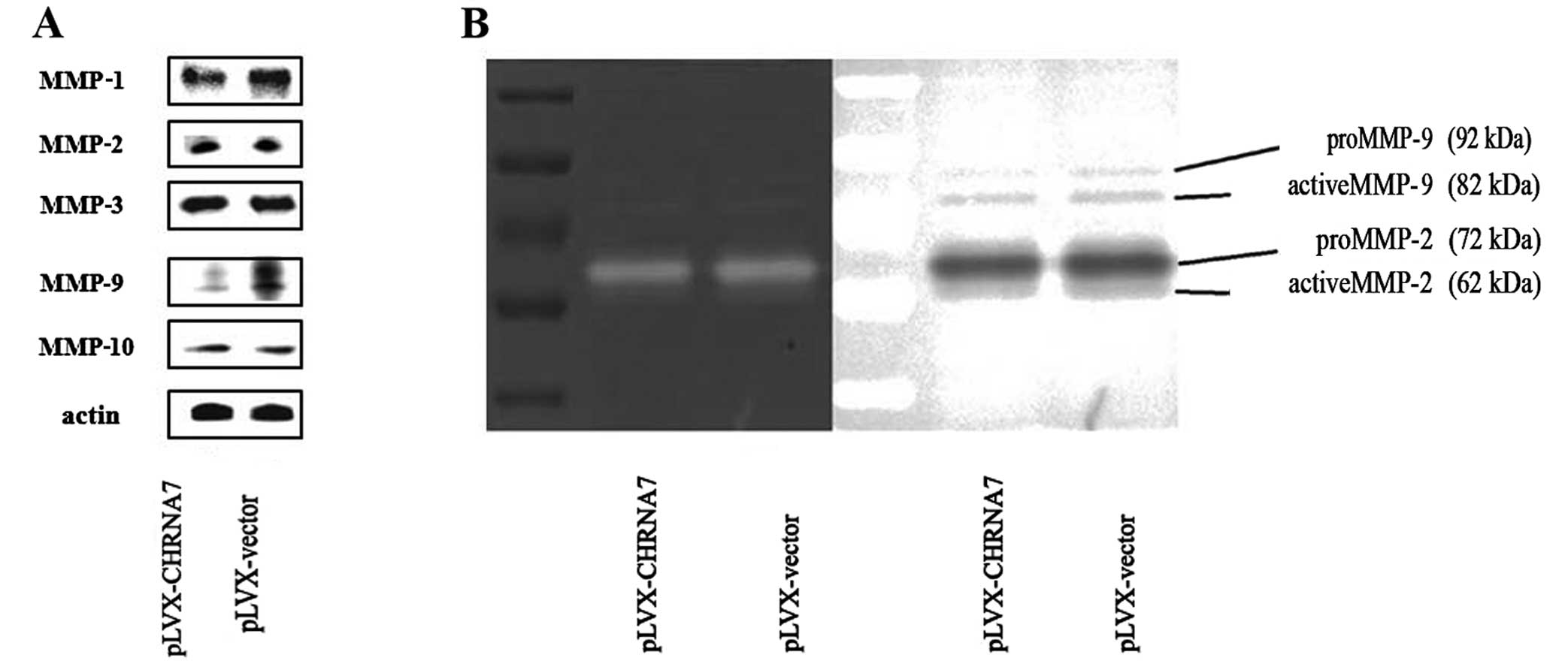
Overexpression of CHRNA7 inhibits MMP-1
and MMP-9 production and activity in LoVo colorectal cancer
cells
Next, we assessed whether the inhibition of
metastatic ability by CHRNA7/LoVo was associated with the
production of MMPs. Western blot analysis showed that levels of
MMP-1 and MMP-9 expression in the CHRNA7-transfected cells were
evidently less than these levels in the control vector-transfected
cells (Fig. 2A). There was no major
difference in MMP-2, -3 and -10 expression with or without CHRNA7
transfection. We also determined the enzymatic activities of the
MMPs by gelatin zymography using conditioned medium. Gelatin
zymography showed weaker lytic zones at the molecular masses
corresponding to MMP-9 in cells with elevated CHRNA7 expression and
had no effect on MMP-2 (Fig. 2B).
Consistently, the activity of MMP-9 was markedly decreased when
CHRNA7 was overexpressed. These data were consistent with the
results observed in the cell migration and invasion assays,
indicating that CHRNA7 inhibited the expression levels and
activities of MMP-1 and MMP-9, and thus suppressed the invasion of
colon cancer cells.
Overexpression of CHRNA7 activates
PI3K/AKT in LoVo colorectal cancer cells
We then investigated the activation of relevant
intracellular MAPK (JNK, p38 and ERK), NF-κB and PI3K/Akt signaling
pathways (19,20) in cells overexpressing CHRNA7.
Western blot assays were performed to examine the phosphorylation
of JNK, p38, ERK, NF-κB (p65), Akt and PI3K (p55/p85). As detailed
in Fig. 3, following comparison of
the CHRNA7-transfected cells and the control vector-trans-fected
cells, the CHRNA7-transfected cells exhibited higher
phosphorylation levels of Akt and PI3K p55. The phosphorylation of
ERK, JNK, P38 and NF-κB showed no difference between the
CHRNA7-overexpressing cells and the control cells.
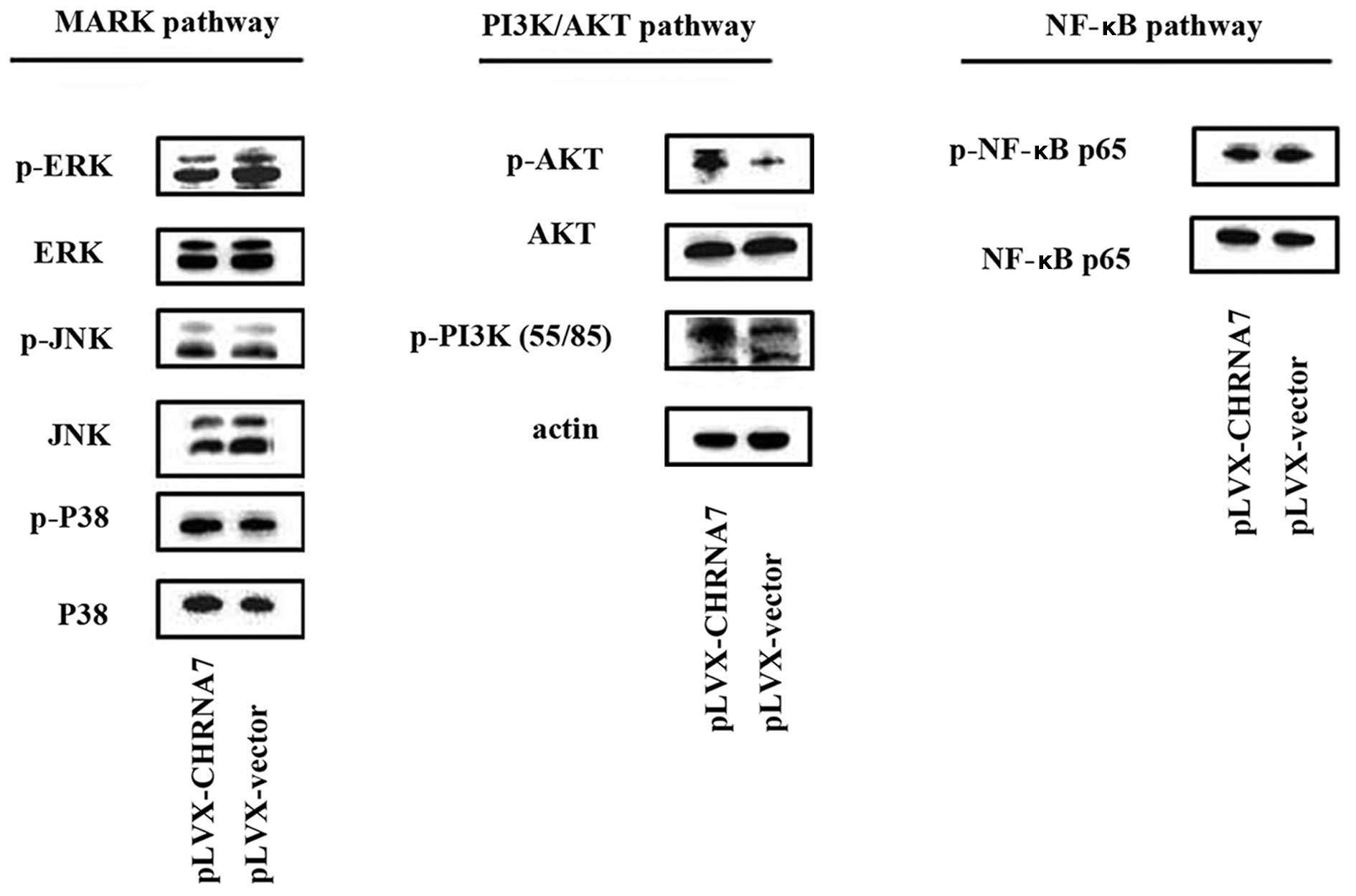 | Figure 3CHRNA7 activates the PI3K/AKT
signaling pathway in colon cancer LoVo cells. After transfection,
LoVo cells were harvested for protein extraction followed by
western blotting. Phosphorylation of key signaling molecules
representing the activation of relevant metastasis-related pathways
was assessed. Antibodies against p-JNK, JNK, p-p38, p-38, p-ERK,
ERK, p-NF-κBp65, NF-κBp65, p-AKT, AKT, p-PI3Kp85, p-PI3Kp55 and
β-actin were used. Results are representative of three independent
experiments. |
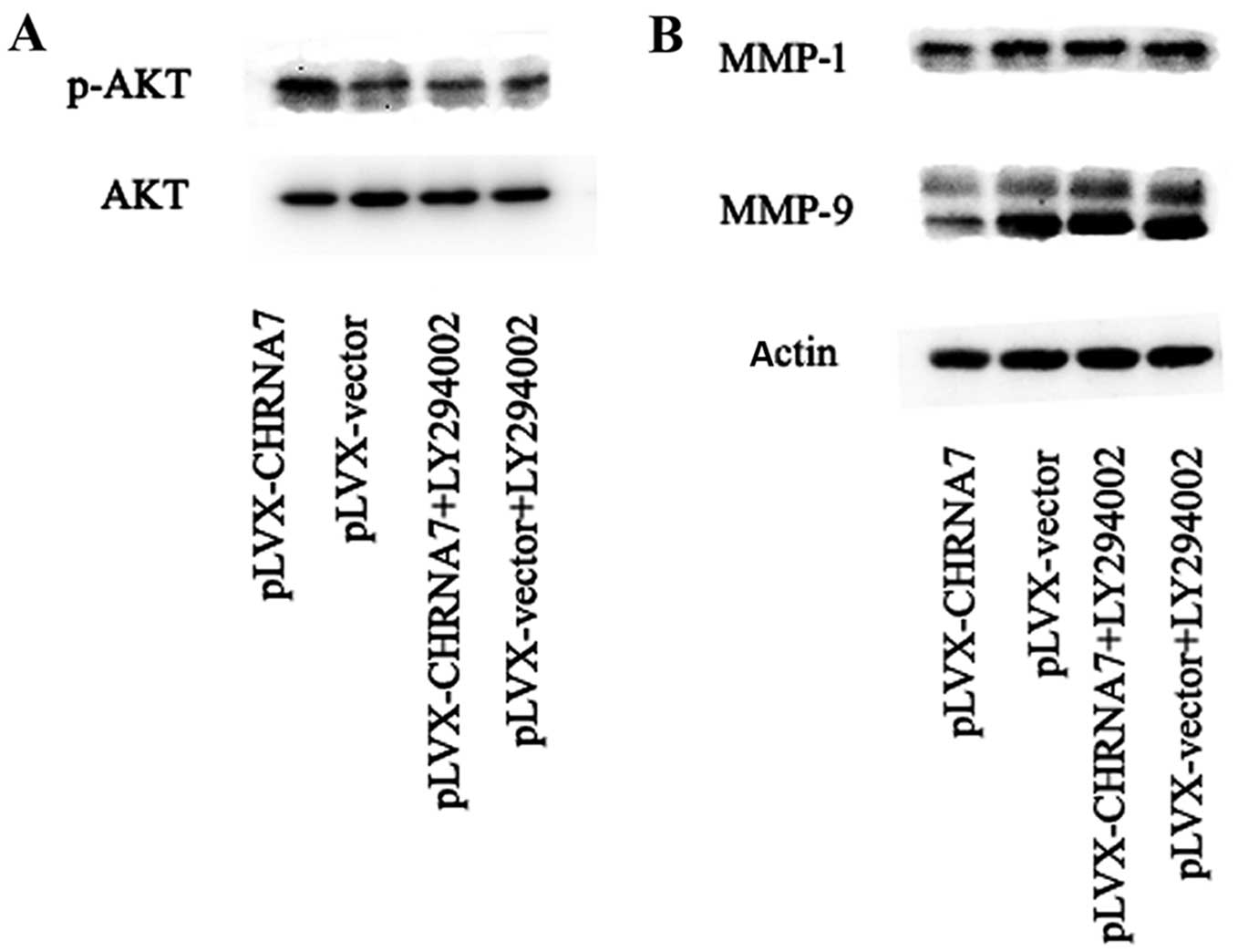
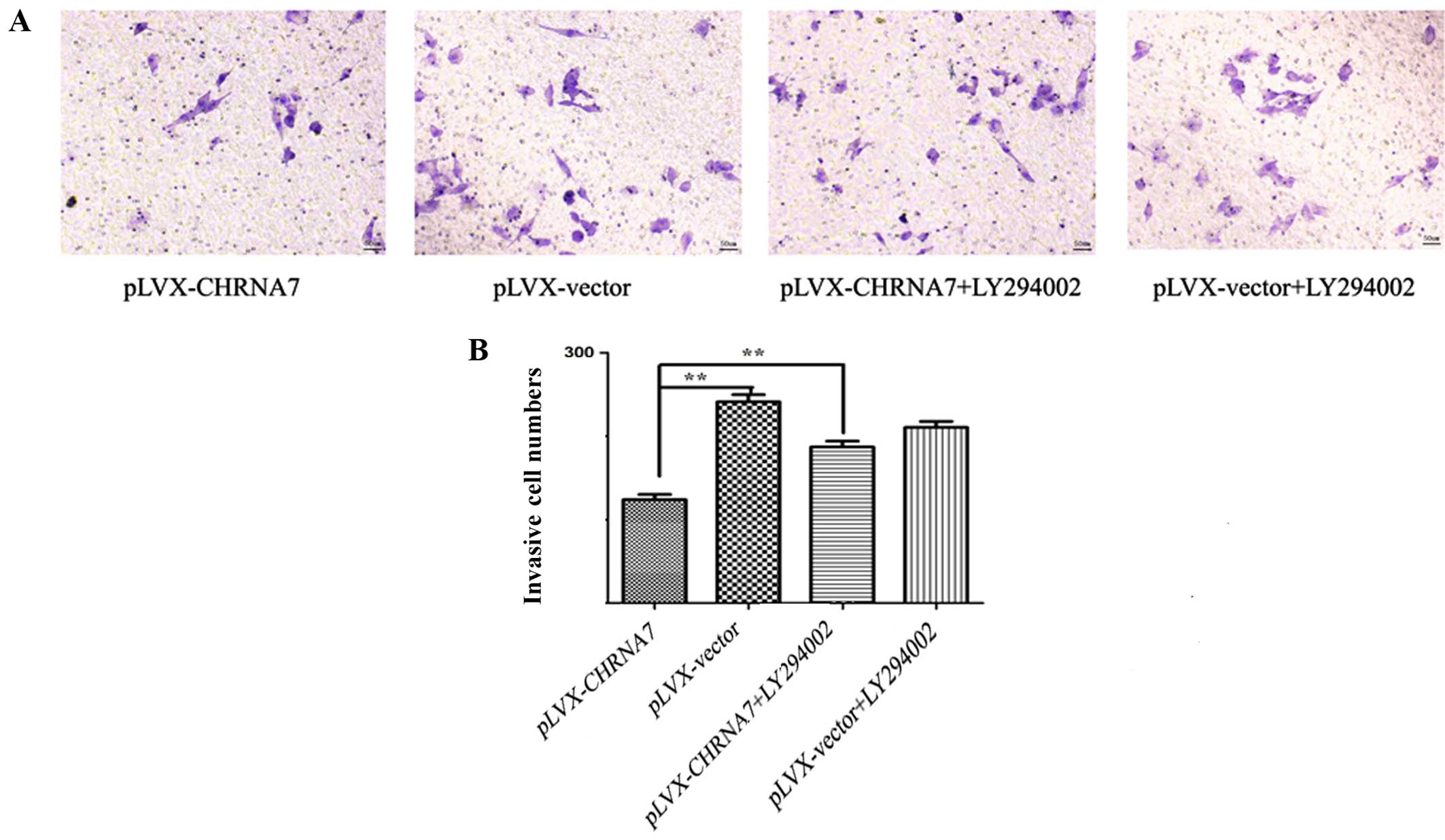
PI3K/AKT pathway inhibitor LY294002
abolishes the inhibitory effect of CHRNA7 on LoVo colorectal cancer
cell migration and invasion
To determine whether enhanced PI3K/AKT activation
may be involved in the inhibitory effect of CHRNA7 on the invasion
of LoVo cells through Matrigel, we included the PI3K/AKT pathway
inhibitor LY294002 into the experimental setting. Our results
indicated that LY294002 significantly suppressed the
phosphorylation of AKT in the CHRNA7-transfected cells (Fig. 4A), and reversed the inhibitory
effect of CHRNA7 on the expression of MMP-1 and MMP-9 (Fig. 4B). Furthermore, LY294002
significantly blocked the negative effects of CHRNA7 on cell
invasion of LoVo cells (Fig. 5).
Taken together, these results suggest that the CHRNA7 inhibition of
the invasion of colon cancer LoVo cells and MMP expression were
PI3K/AKT activation-dependent.
Discussion
The non-neuronal nAChRs have considerable
implications for a number of diseases such as cancers and
cardiovascular diseases. Thus, research has focused on the
activation of the non-neuronal nAChR signaling pathway induced by
tobacco use mimicked by nicotine binding (11,21).
The nAChRs, particularly α7nAChR, can mediate nicotine-dependent
upregulation of proliferative and survival genes that contribute to
the growth and progression of lung (22–25)
and colon cancer (14,16–18,
26). In this scenario, multiple
signaling pathways are related to nAChR coupled responses to
nicotine, i.e., protein kinase C (PKC) activation in breast cancer
cells (28), MAPK (ERK1/2) in
pancreatic carcinoma (28,29) and AKT/ERK pathways in human
malignant glioma cells (30). In
our previous studies, we revealed stimulation of nicotine
activities of MAPK/ERK, MAPK/p38 and PI3K(p55)/Akt signaling
cascades among which MAPK/p38 is responsible for the MMP-related
colon cell invasion and migration (unpublished data).
In addition to nicotine and other tobacco extracts,
there exists a broad range of stimuli and cell populations that may
be regulated by cholinergic receptors. Vukelic et al showed
that cholinergic neurotransmitters (ACh), similar to those released
through activation of a neural reflex, regulate responses to
products of the adaptive immune system, specifi-cally immune
complex (IC)-mediated activation of effector cells (31). In particular, in both lung cancer
and colon cancer, ACh acts as an autocrine growth factor for cancer
growth and development (32–34).
Since cancer cells both produce ACh and express nAChRs, these
receptors are believed to be involved in tumorigenesis, even
without stimulation with nicotine. In the present study, we
assessed the impact of endogenously expressed α7nAChR on colon
cancer cell growth and invasion in a 'non-smoking' environment. We
showed that overexpression of CHRNA7 did not affect LoVo colorectal
cancer cell proliferation but inhibited cell migration and
invasion. Reduced expression of MMP-1 and -9 and activation of the
PI3K/Akt signaling pathway were also noted.
PI3K/Akt is recognized as a key regulator for cell
biological behaviors including cell proliferation, angiogenesis,
migration and invasion. It is thus believed to be involved in tumor
occurrence, development (35) and
metastasis (36). Although
activation of PI3K/Akt is more likely associated with increased MMP
production and enhanced tumor cell invasion (37,38),
it is also true that activation of the PI3k/Akt pathway may be
associated with reduced MMP-2 and -9 production, as a result of
which the metastatic potency of colon carcinoma cells is decreased
(39).
It is believed that the overall cellular response to
stimulation with ACh is determined by the delicate balance between
multiple signals. A switch in the predominant nAChR subtype that is
expressed on the cell membrane occurs during malignant
transformation, which indicates that the effects of autocrine or
paracrine ACh on cancer cells might differ from the effects on
non-malignant cells, even if they are situated next to each other
in the same tissue (21,31). Moreover, the diversity of nAChRs
could be further increased by possible variants at the genomic or
mRNA level. These, together with the existence of polymorphisms in
the human neuronal nicotinic acetyl-choline receptor gene provide
another level of variability if they influence the expression or
amino acid sequence of the corresponding protein (40). In the present study, we evaluated
the consequence of overexpression of CHRNA7. Our results highlight
the need to dissect the physiological or pathophysiological roles
of CHRNA7 and/or α7nAChR in CRC patients with or without cigarette
smoking.
In summary, in the present study, we showed that
over-expression of α7nAChR in human colon cancer LoVo cells,
probably through the binding of cholinergic neurotransmitters i.e.,
ACh that are secreted by the cancer cells, activates the PI3K/AKT
pathway and inhibits colon cancer metastasis and invasion.
Therefore, our results indicate that CHRNA7 may serve as either a
diagnostic marker or a therapeutic target for colorectal cancer
metastasis. In addition, our results highlight the need for
investigating the physical and pathophysiological role of CHRNA7 or
α7nAChR in tumor factors other than cigarette smoking.
Acknowledgments
The present study was supported by grants from the
Key Project of the Natural Science Foundation of Zhejiang Province
(LZ6H160003 to W.-B. C.), the Qianjiang Talent Project of Zhejiang
(2013R10034 to J.Q.) and the National Natural Science Foundation of
China (30600596 to W.-B.C.).
Abbreviations:
|
CHRNA7
|
α7 neuronal nicotinic receptor
gene
|
|
α7nAChR
|
α7 nicotinic acetylcholine
receptor
|
|
CRC
|
colorectal cancer
|
|
MMP
|
metalloproteinase
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
Akt
|
v-akt murine thymoma viral oncogene
homologue
|
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang CC and Li J: An update on
chemotherapy of colorectal liver metastases. World J Gastroenterol.
18:25–33. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Meissner HI, Breen N, Klabunde CN and
Vernon SW: Patterns of colorectal cancer screening uptake among men
and women in the United States. Cancer Epidemiol Biomarkers Prev.
15:389–394. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mansouri D, McMillan DC, Crighton EM and
Horgan PG: Screening for colorectal cancer: What is the impact on
the determinants of outcome? Crit Rev Oncol Hematol. 85:342–349.
2013. View Article : Google Scholar
|
|
6
|
Millar NS and Harkness PC: Assembly and
trafficking of nicotinic acetylcholine receptors (Review). Mol
Membr Biol. 25:279–292. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schuller HM: Is cancer triggered by
altered signalling of nicotinic acetylcholine receptors? Nat Rev
Cancer. 9:195–205. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Papke RL, Bagdas D, Kulkarni AR, Gould T,
AlSharari SD, Thakur GA and Damaj MI: The analgesic-like properties
of the alpha7 nAChR silent agonist NS6740 is associated with
non-conducting conformations of the receptor. Neuropharmacology.
91:34–42. 2015. View Article : Google Scholar
|
|
9
|
Arias HR, Richards VE, Ng D, Ghafoori ME,
Le V and Mousa SA: Role of non-neuronal nicotinic acetylcholine
receptors in angiogenesis. Int J Biochem Cell Biol. 41:1441–1451.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Russo P and Taly A: α7-Nicotinic
acetylcholine receptors: An old actor for new different roles. Curr
Drug Targets. 13:574–578. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Egleton RD, Brown KC and Dasgupta P:
Nicotinic acetylcholine receptors in cancer: Multiple roles in
proliferation and inhibition of apoptosis. Trends Pharmacol Sci.
29:151–158. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Davis R, Rizwani W, Banerjee S, Kovacs M,
Haura E, Coppola D and Chellappan S: Nicotine promotes tumor growth
and metastasis in mouse models of lung cancer. PLoS One.
4:e75242009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shin VY, Jin HC, Ng EK, Yu J, Leung WK,
Cho CH and Sung JJ: Nicotine and
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone induce
cyclooxygenase-2 activity in human gastric cancer cells:
Involvement of nicotinic acetylcholine receptor (nAChR) and
beta-adrenergic receptor signaling pathways. Toxicol Appl
Pharmacol. 233:254–261. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wei P-L, Chang Y-J, Ho Y-S, Lee CH, Yang
YY, An J and Lin SY: Tobacco-specific carcinogen enhances colon
cancer cell migration through α7-nicotinic acetylcholine receptor.
Ann Surg. 49:978–985. 2009. View Article : Google Scholar
|
|
15
|
Di Paolo G and Kim T-W: Linking lipids to
Alzheimer's disease: Cholesterol and beyond. Nat Rev Neurosci.
12:284–296. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pettersson A, Nylund G, Khorram-Manesh A,
Nordgren S and Delbro DS: Nicotine induced modulation of SLURP-1
expression in human colon cancer cells. Auton Neurosci. 148:97–100.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wong HP, Yu L, Lam EK, Tai EK, Wu WK and
Cho CH: Nicotine promotes cell proliferation via alpha7-nicotinic
acetylcholine receptor and catecholamine-synthesizing
enzymes-mediated pathway in human colon adenocarcinoma HT-29 cells.
Toxicol Appl Pharmacol. 221:261–267. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cucina A, Dinicola S, Coluccia P, Proietti
S, D'Anselmi F, Pasqualato A and Bizzarri M: Nicotine stimulates
proliferation and inhibits apoptosis in colon cancer cell lines
through activation of survival pathways. J Surg Res. 178:233–241.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lemos C, Sack U, Schmid F, Juneja M and
Stein U: Anti-metastatic treatment in colorectal cancer: Targeting
signaling pathways. Curr Pharm Des. 19:841–863. 2013. View Article : Google Scholar
|
|
20
|
Chen J, Elfiky A, Han M, Chen C and Saif
MW: The role of Src in colon cancer and its therapeutic
implications. Clin Colorectal Cancer. 13:5–13. 2014. View Article : Google Scholar
|
|
21
|
Grando SA: Connections of nicotine to
cancer. Nat Rev Cancer. 14:419–429. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shi D, Guo W, Chen W, Fu L, Wang J, Tian
Y, Xiao X, Kang T, Huang W and Deng W: Nicotine promotes
proliferation of human nasopharyngeal carcinoma cells by regulating
α7AChR, ERK, HIF-1α and VEGF/PEDF signaling. PLoS One.
7:e438982012. View Article : Google Scholar
|
|
23
|
Dasgupta P, Rastogi S, Pillai S,
Ordonez-Ercan D, Morris M, Haura E and Chellappan S: Nicotine
induces cell proliferation by β-arrestin-mediated activation of Src
and Rb-Raf-1 pathways. J Clin Invest. 116:2208–2217. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dasgupta P, Rizwani W, Pillai S, Davis R,
Banerjee S, Hug K, Lloyd M, Coppola D, Haura E and Chellappan SP:
ARRB1- mediated regulation of E2F target genes in nicotine-induced
growth of lung tumors. J Natl Cancer Inst. 103:317–333. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin W, Hirata N, Sekino Y and Kanda Y:
Role of α7-nicotinic acetylcholine receptor in normal and cancer
stem cells. Curr Drug Targets. 13:656–665. 2012.PubMed/NCBI
|
|
26
|
Wei PL, Kuo LJ, Huang MT, Ting WC, Ho YS,
Wang W, An J and Chang YJ: Nicotine enhances colon cancer cell
migration by induction of fibronectin. Ann Surg Oncol.
18:1782–1790. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo J, Ibaragi S, Zhu T, Luo LY, Hu GF,
Huppi PS and Chen CY: Nicotine promotes mammary tumor migration via
a signaling cascade involving protein kinase C and CDC42. Cancer
Res. 68:8473–8481. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chipitsyna G, Gong Q, Anandanadesan R,
Alnajar A, Batra SK, Wittel UA, Cullen DM, Akhter MP, Denhardt DT,
Yeo CJ, et al: Induction of osteopontin expression by nicotine and
cigarette smoke in the pancreas and pancreatic ductal
adenocarcinoma cells. Int J Cancer. 125:276–285. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sullivan J, Blair L, Alnajar A, Aziz T, Ng
CY, Chipitsyna G, Gong Q, Witkiewicz A, Weber GF, Denhardt DT, et
al: Expression of a prometastatic splice variant of osteopontin,
OPNC, in human pancreatic ductal adenocarcinoma. Surgery.
146:232–240. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Khalil AA, Jameson MJ, Broaddus WC, Lin PS
and Chung TD: Nicotine enhances proliferation, migration, and
radioresistance of human malignant glioma cells through EGFR
activation. Brain Tumor Pathol. 30:73–83. 2013. View Article : Google Scholar
|
|
31
|
Vukelic M, Qing X, Redecha P, Koo G and
Salmon JE: Cholinergic receptors modulate immune complex-induced
inflammation in vitro and in vivo. J Immunol. 191:1800–1807. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Song P, Sekhon HS, Jia Y, Keller JA,
Blusztajn JK, Mark GP and Spindel ER: Acetylcholine is synthesized
by and acts as an autocrine growth factor for small cell lung
carcinoma. Cancer Res. 63:214–221. 2003.PubMed/NCBI
|
|
33
|
Song P, Sekhon HS, Lu A, Arredondo J,
Sauer D, Gravett C, Mark GP, Grando SA and Spindel ER: M3
muscarinic receptor antagonists inhibit small cell lung carcinoma
growth and mitogen-activated protein kinase phosphorylation induced
by acetylcholine secretion. Cancer Res. 67:3936–3944. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Song P, Sekhon HS, Fu XW, Maier M, Jia Y,
Duan J, Proskosil BJ, Gravett C, Lindstrom J, Mark GP, et al:
Activated cholinergic signaling provides a target in squamous cell
lung carcinoma. Cancer Res. 68:4693–4700. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Osaki M, Oshimura M and Ito H: PI3K-Akt
pathway: Its functions and alterations in human cancer. Apoptosis.
9:667–676. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sheng S, Qiao M and Pardee AB: Metastasis
and AKT activation. J Cell Physiol. 218:451–454. 2009. View Article : Google Scholar
|
|
37
|
Kim D, Kim S, Koh H, Yoon SO, Chung AS,
Cho KS and Chung J: Akt/PKB promotes cancer cell invasion via
increased motility and metalloproteinase production. FASEB J.
15:1953–1962. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang G, Wang F, Ding W, Wang J, Jing R, Li
H, Wang X, Wang Y, Ju S and Wang H: APRIL induces tumorigenesis and
metastasis of colorectal cancer cells via activation of the
PI3K/Akt pathway. PLoS One. 8:e552982013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li Q, Li M, Wang YL, Fauzee NJ, Yang Y,
Pan J, Yang L and Lazar A: RNA interference of PARG could inhibit
the metastatic potency of colon carcinoma cells via PI3-kinase/Akt
pathway. Cell Physiol Biochem. 29:361–372. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Weiland S, Bertrand D and Leonard S:
Neuronal nicotinic acetylcholine receptors: From the gene to the
disease. Behav Brain Res. 113:43–56. 2000. View Article : Google Scholar : PubMed/NCBI
|