Introduction
Currently, breast cancer is the most prevalent
oncological disease worldwide, with approximately 232,670 new cases
and 40,000 deaths among women living in the United States in 2012
(1). The most aggressive and
invasive tumor cells, including breast cancer cells, which often
reside in hypoxic environments, rely on extensive glycolysis to
meet their large demand for energy and biosynthetic precursors
(2,3). Once exposed to a hypoxic environment,
breast cancer cells subsequently exhibit enhanced angiogenesis and
tumor metastasis, which results in the poor survival rate of cancer
patients (4,5). Hence, investigation of hypoxia-induced
molecules will aid in the development of new tools for the
treatment of breast cancer.
MicroRNAs (miRNAs) are a type of endogenous and
small non-coding regulatory RNAs of 21–23 nucleotides, which mainly
recognize complementary sequences in the 3′-untranslated regions
(UTRs) of their target genes leading to mRNA degradation or
translation inhibition. Most animal miRNAs are evolutionarily
conserved and are often found in clusters (6,7).
Primary miRNAs with a stem-loop structure are transcribed by RNA
polymerases (RNA Pols) and processed in both the nucleus and
cytoplasm by Drosha as well as Dicer to generate mature miRNAs
(8). Existing studies have found
that miRNAs are involved in the regulation of multiple pathological
processes that contribute to tumorigenesis and metastasis, such as
tumor cell proliferation, differentiation, apoptosis, as well as
invasion (9). For example, a higher
level of miR-191 was reportedly found in several types of cancer,
including breast cancer, in which it was found to be hypoxia
inducible (10). In addition,
miRNA-10b (miR-10b) expression was increased in metastatic breast
cancer cells, along with positive regulation of cell migration and
invasion through the suppression of the homeobox D10
tumor-suppressor signaling pathway (11). In most solid cancers, miR-497 has
been found to be a tumor suppressor (12–14).
Such a function is exerted partly by anti-proliferative and
anti-growth potential (13–15). A previous study demonstrated that
miR-497 induced cell apoptosis by negatively regulating Bcl-2
protein expression at the post-transcriptional level in human
breast cancer (13). However, the
relationship between miR-497 and hypoxia remains unclear.
In the present study, we found that miR-497, as a
hypoxia responsive miRNA, was downregulated in human breast cancer
clinical samples and cell lines. Ectopic expression of miR-497
functioned as an angiogenesis suppressor both in vitro and
in nude mice by targeting VEGF and HIF-1α. Our results propose that
miR-497 may be a therapeutic target for breast cancer in the
development of angiogenesis inhibitors.
Materials and methods
Patients and tissue specimens
Forty-five human breast cancer clinical samples and
their corresponding normal breast tissues were obtained from breast
cancer patients who underwent surgery for breast cancer at The
Second Affiliated Hospital of Wenzhou Medical University between
October 2013 and October 2014. All cases were histologically
confirmed as invasive, ductal breast cancer by trained
pathologists. No patients received chemotherapy or radiotherapy
prior to surgery. All the specimens were obtained with informed
consent and approved by the Ethics Committee of Wenzhou Medical
University.
Cell culture
Human breast cancer cell lines T-74D, MCF-7,
MDA-MB-453, MDA-MB-468 and MDA-MB-435 were purchased from the
American Type Culture Collection (ATCC; Rockville, MD, USA).
Non-malignant breast epithelial cell line MCF-10A was preserved in
our laboratory. The cells were cultured in modified Eagle's medium
containing 10% fetal bovine serum (FBS), 0.5%
penicillin-streptomycin, and 1% glutamine at 37°C with 5%
CO2. HUVECs (obtained from ATCC) were cultured in
gelatin-coated plates with M199 medium containing 20% FBS,
endothelial cell growth supplement (50 μg/ml; Sigma) and
antibiotics, and incubated at 37°C in 5% CO2 in air. For
hypoxic stress, MCF-7 cells were placed in a hypoxia chamber
(Invivo200; Ruskin, UK) maintained at different pO2
conditions (ranging from 0.2 to 1% oxygen) and 5% CO2.
Cells in the logarithmic growth phase (~90% confluency) were
selected for the experiments.
RNA transfection
miR-497 mimics, inhibitors (anti-miR-497) and
AllStar negative control siRNA (NC) were synthesized by GenePharma
(Shanghai, China). The transfection was conducted using
Lipofectamine 2000 (Invitrogen, USA) according to the
manufacturer's instructions. Complete medium was changed 5 h after
transfection.
Preparation of tumor cell-conditioned
medium
After MCF-7 cells were transfected for 48 h, the
supernatant medium was replaced by serum-free medium and incubation
was carried out for another 24 h. The conditioned medium was
collected after centrifugation at 4°C and 2,000 rpm for 10 min and
was stored at −70°C for subsequent use. The cells were classified
as the negative control siRNA conditioned medium group (NC), the
miR-497 mimics conditioned medium group and the anti-miR-497
conditioned medium group, respectively.
Cell proliferation
HUVEC cells were seeded in each well of 96-well
culture plates (1,000 cells/well). After overnight incubation, the
medium was removed and replaced with fresh culture medium plus
equal amounts of the different conditioned medium. After 48 h of
incubation, the supernatant was discarded, and 10 μl CCK-8
solution (Dojin Laboratories, Kumamoto, Japan) was added. The cells
were then incubated at 37°C for 60 min, and the absorbance was
measured at 490 nm using a microplate spectrophotometer (Bio-Tek,
USA). This experiment was repeated twice.
Quantitative real-time PCR (RT-PCR)
Total RNA extraction was performed using TRIzol
reagent (Life Technologies, Carlsbad, CA, USA) according to the
manufacturer's instructions. Real-time qPCR was used to confirm the
expression levels of mRNAs. cDNA was produced according to the
protocol for PrimeScript™ RT reagent (Takara, Japan). The
expression levels of miRNAs were analyzed using TaqMan MicroRNA
Assay kits (Applied Biosystems, Foster City, CA, USA). Data
analysis was performed using the 2−ΔΔCt method. All
experiments were performed in triplicate.
Tube formation assay
For the capillary tube formation assay, Matrigel
(Becton Dickinson, USA) was dissolved at 4°C overnight, and each
well of prechilled 96-well plates was coated with 100 μl
Matrigel and incubated at 37°C for 45 min. HUVECs were transferred
to the 96-well plates with the different conditioned medium at the
density of 1×104/well for 12 h in a humidified 5%
CO2 atmosphere. Capillary-like structures of HUVECs were
photographed and the light micrograph images were stored in a
computer. Tubular structures were quantified by manual counting at
×100 magnification.
Western blot analysis
Cells were washed three times with ice-cold PBS and
lysed in NP-40 lysis buffer [20 mmol/l Tris-HCl (pH 7.4), 100
mmol/l NaCl, 1% NP-40, 0.5% sodium deoxycholate, 5 mmol/l
MgCl2, 0.1 mmol/l phenylmethylsulfonyl fluoride and 10
mg/ml of protease inhibitor mixture]. Protein was extracted using
Mammalian Protein Extraction reagent and its concentration was
determined by BCA assay (both from Pierce Inc., Rockford, IL, USA).
Total proteins (20–40 μg) from each sample were
electrophoresed on 8% SDS-PAGE gel, and transferred to a
nitrocellulose membrane. The membranes were blocked in 5% nonfat
milk and probed with the primary antibodies as indicated overnight
at 4°C, and then with the respective secondary antibodies. Band
signals were visualized using an enhanced chemiluminescence kit
(Pierce, Minneapolis, MN, USA). The same membrane was reprobed with
the anti-β-actin antibody, which was used as the internal
control.
In vivo tumorigenesis
In vivo experiments were conducted as
described previously (16).
Briefly, we used male athymic BALB/c nu/nu mice (4–6 weeks old)
that were maintained in standard mice plexiglass cages in a room
maintained at constant temperature and humidity under a 12 h light
and darkness cycle. A total of 1×107 logarithmically
growing cells from the different groups: a) MCF-7 cells; b) MCF-7
cells transfected with the negative control; c) MCF-7 cells
transfected with the miR-497 mimics were injected subcutaneously
into the mid-abdominal area, respectively. After 40 days of
observation, the mice were sacrificed and tumors were surgically
excised neatly and weighed. One part of the tissue was fixed in
formalin and another part was frozen in liquid nitrogen.
Immunohistochemical and microvessel
density (MVD) evaluation
Xenograft tumor samples were embedded in paraffin
and fixed with paraformaldehyde. After being washed in PBS, the
slides were blocked with protein block solution (DakoCytomation) to
block endogenous peroxidase activity. Such samples were then
incubated overnight with primary antibodies, involving
appropriately diluted CD34, VEGF and HIF-1α. Normal host serum was
used for negative controls, followed by staining with appropriate
HRP-conjugated secondary antibodies. The peroxidase was visualized
with 3-3′-diaminobenzidinetetrahydrochloride solution and then
counterstained with a weak hematoxylin solution stain. The stained
slides were dehydrated and visualized on an Olympus microscope
(Olympus, Japan). The stained sections were counted in the five
areas of highest vascular density at ×400 magnification, and the
MVD was expressed as the mean number of vessels in these areas.
Statistical analysis
Data are represented as mean ± SD for the absolute
values or percentage of the control. Statistical differences were
evaluated by one-way analysis of variance (ANOVA) followed by LSD
multiple comparison tests using SPSS software (version 17.0; SPSS,
Inc.). A value of P<0.05 was considered statistically
significant.
Results
Analysis of miR-497 in breast cancer cell
lines and clinical specimens
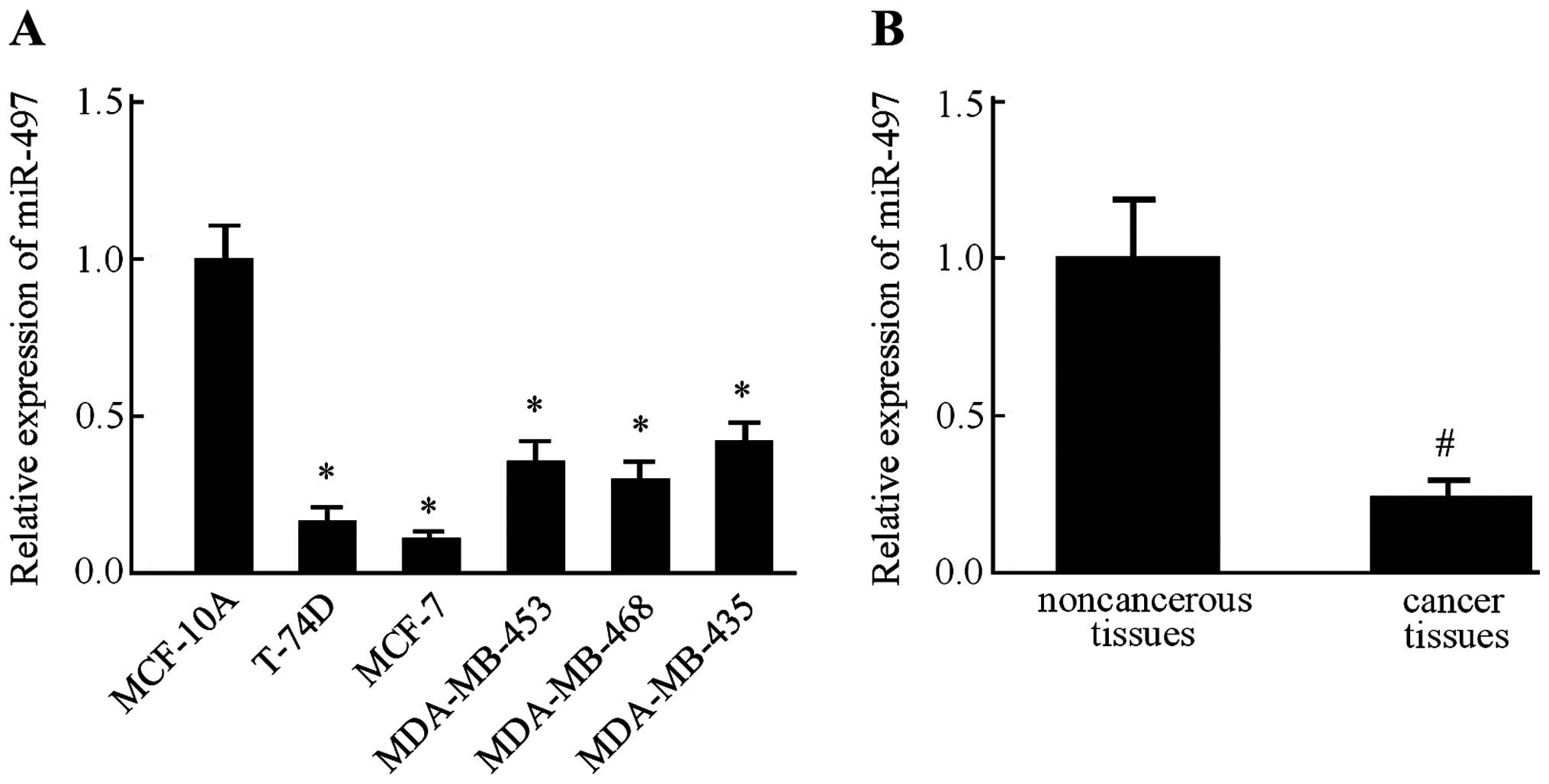
We examined the relative expression levels of
miR-497 in several breast cancer cell lines, namely T-74D, MCF-7,
MDA-MB-453, MDA-MB-468 and MDA-MB-435, along with MCF-10A, a
non-malignant breast epithelial cell line, by RT-PCR. In all five
breast cancer cell lines, the expression level of miR-497 was found
lower than that in the MCF-10A cells (Fig. 1A). Since expression of miR-497 in
the MCF-7 cells was much lower than the other four breast cancer
cell lines, this cell line was used in the subsequent experiments.
Next, we measured the miR-497 expression levels in 45 pairs of
ductal breast cancer tissues and the corresponding adjacent
non-cancerous tissues. Consistently, miR-497 was also downregulated
in the breast cancer tissues in comparison with the level in the
normal breast tissues (Fig. 1B).
These results indicated that miR-497 expression was significantly
suppressed in the breast cancer cell lines and tissue
specimens.
Hypoxia inhibits the expression of
miR-497 in MCF-7 cells
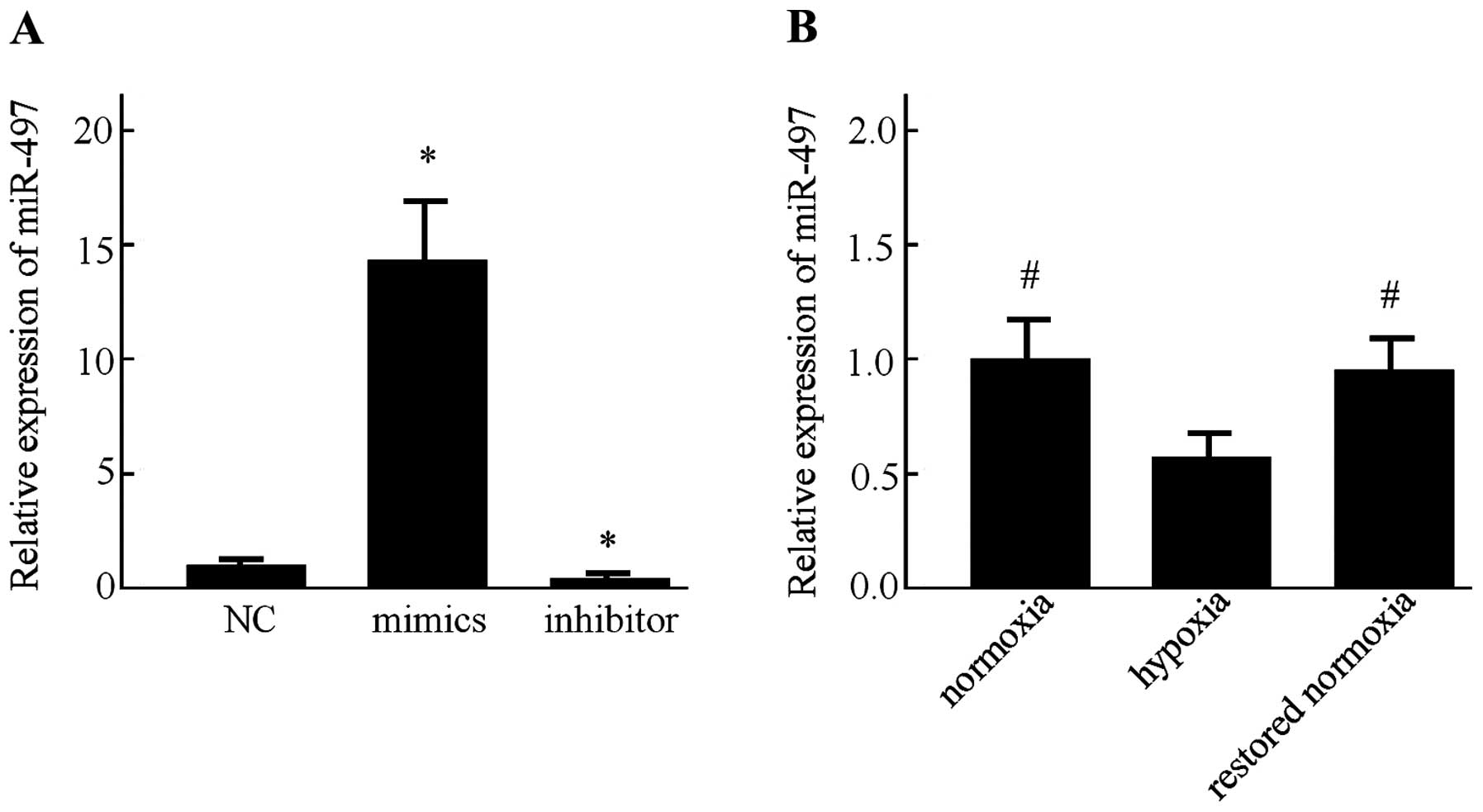
First, the miR-497 expression in the MCF-7 cells was
determined following transfection with miR-497 mimics, inhibitor or
the negative control for 48 h by RT-PCR. Transfection with the
miR-497 mimics resulted in a significant increase in its
expression. Moreover, the miR-497 level was markedly decreased
after the MCF-7 cells were transfected with the miR-497 inhibitor
(P<0.05) (Fig. 2A). Afterwards,
detection of miR-497 in the MCF-7 cells was performed by RT-PCR
under normoxic and hypoxic conditions. It was found that the
expression of miR-497 was significantly decreased under hypoxic
conditions. In addition, the expression of miR-497 was increased
after the MCF-7 cells were restored to a normoxic condition for 24
h (Fig. 2B).
Effects of conditioned medium on HUVEC
proliferation and tube formation
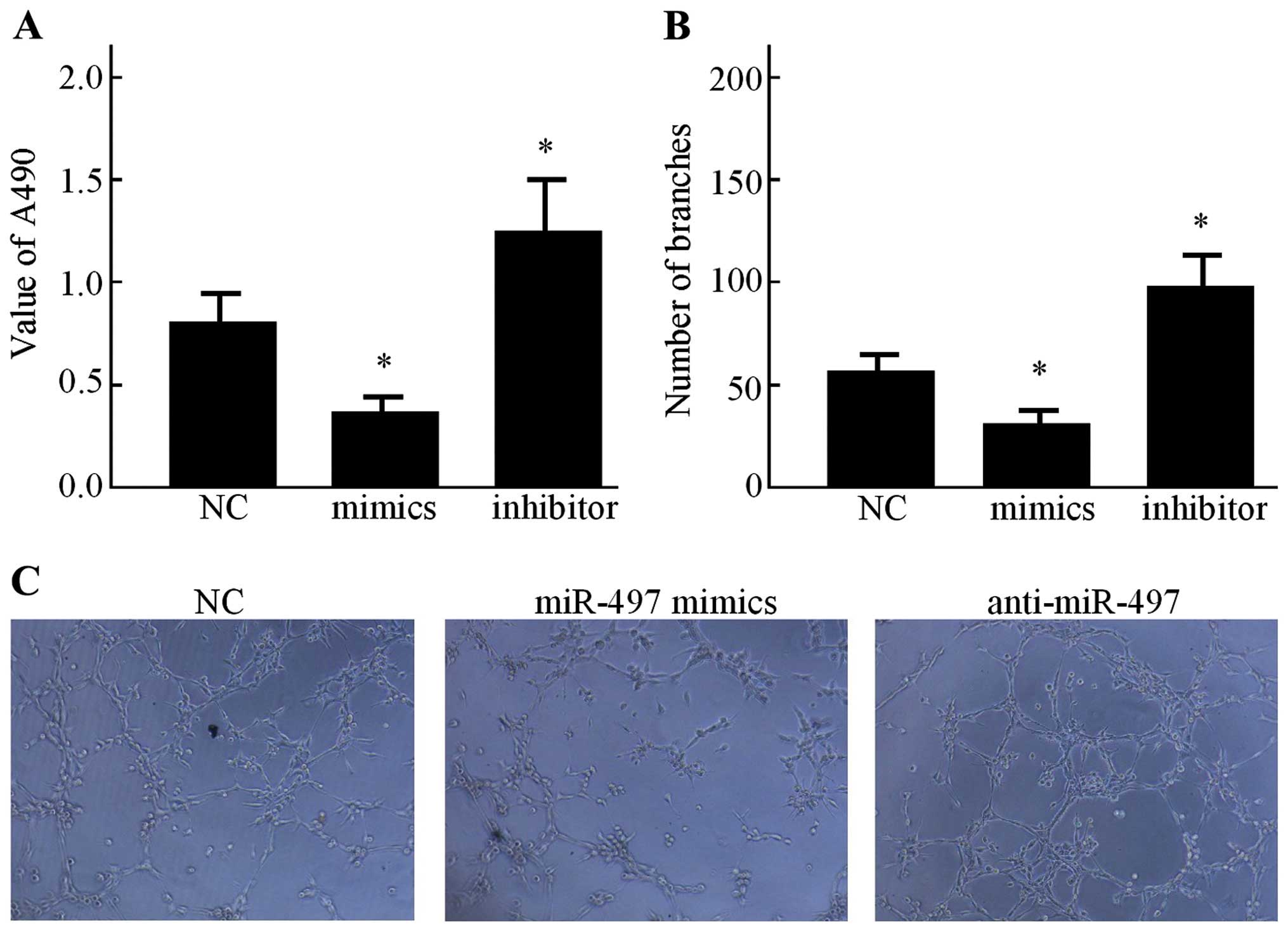
Secondly, the effects of conditioned medium on HUVEC
proliferation was evaluated. As shown in Fig. 3A, the CCK-8 assay indicated that
after being cultured in the conditioned medium from the miR-497
mimics group, the proliferation of HUVECs was relatively inhibited
compared with that of the NC group (P<0.05). However, in
comparison with the NC group, the miR-497 inhibitor increased the
growth rate of HUVECs (P<0.05). In order to explore the effect
of miR-497 on the capillary-like structure formation of HUVECs, an
in vitro capillary tube formation assay was performed using
the MCF-7 cell line. It was observed that the conditioned medium
from the miR-497 mimics group displayed strong ability to inhibit
the formation of capillary-like structures when compared to the NC
group (Fig. 3B), while the
anti-miR-497 conditioned medium resulted in significant promotion
of tubule formation of HUVECs on Matrigel (Fig. 3C).
VEGF is the direct target of miR-497 and
inhibits angiogenesis
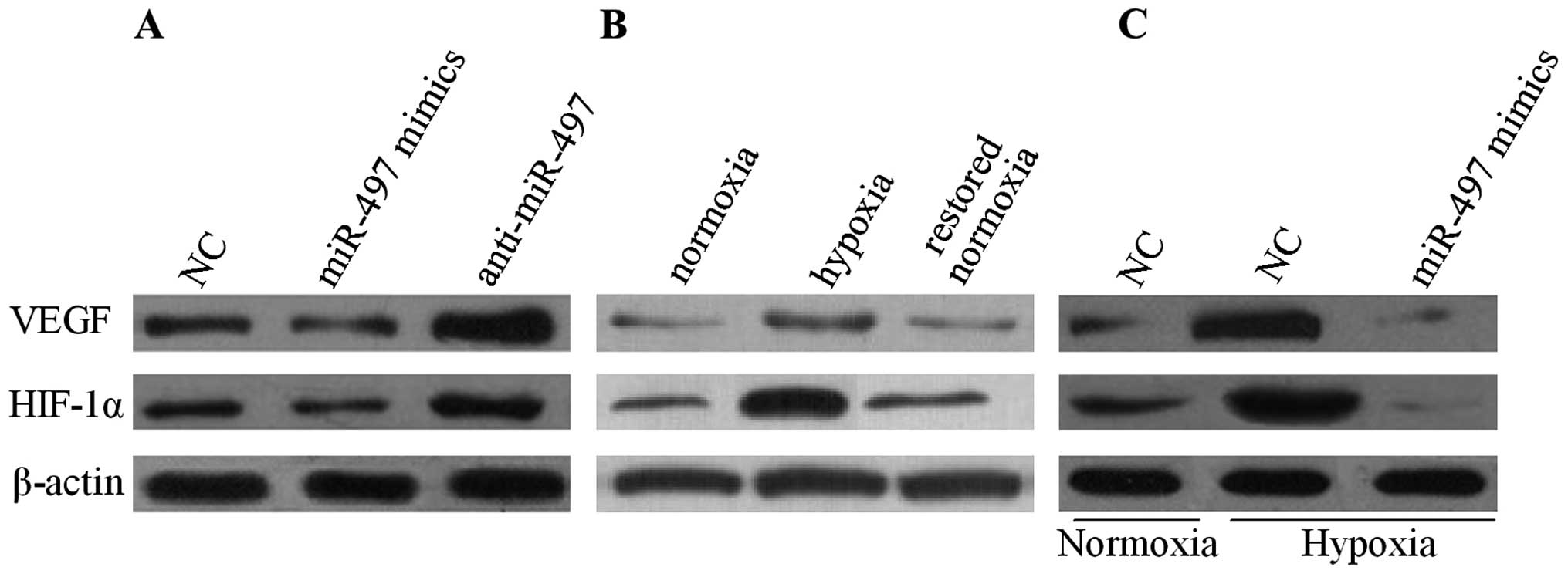
To unveil the underlying mechanism of the disrupted
angiogenesis mediated by miR-497, the expression of VEGF in MCF-7
cells was measured by western blot analysis. The result indicated
that the overexpression of miR-497 reduced the protein level of
VEGF while the suppression of miR-497 revealed an opposite result
under normoxic conditions (Fig.
4A). Next, the relationship between miR-497 and VEGF in
conditions of normoxia or hypoxia was elucidated. As shown in
Fig. 4B, in comparison with
normoxia, hypoxia upregulated the expression of VEGF in the MCF-7
cells. Moreover, the VEGF expression was significantly decreased
after the MCF-7 cells were restored to a normoxic condition. In
addition, the MCF-7 cells were transfected with miR-497 mimics or
anti-miR-497. The results revealed that VEGF secretion in the cell
culture medium was markedly decreased in the MCF-7 cells that were
transfected with the miR-497 mimics in a hypoxia condition
(Fig. 4C). To further investigate
the underlying mechanism, the expression of the transcription
factor HIF-1α, which is responsible for VEGF regulation, in MCF-7
cells was examined through western blot analysis. Fig. 4 shows that the expression of HIF-1α
was decreased in the MCF-7 cells after being transfected with the
miR-497 mimics, correlating with decreased VEGF production.
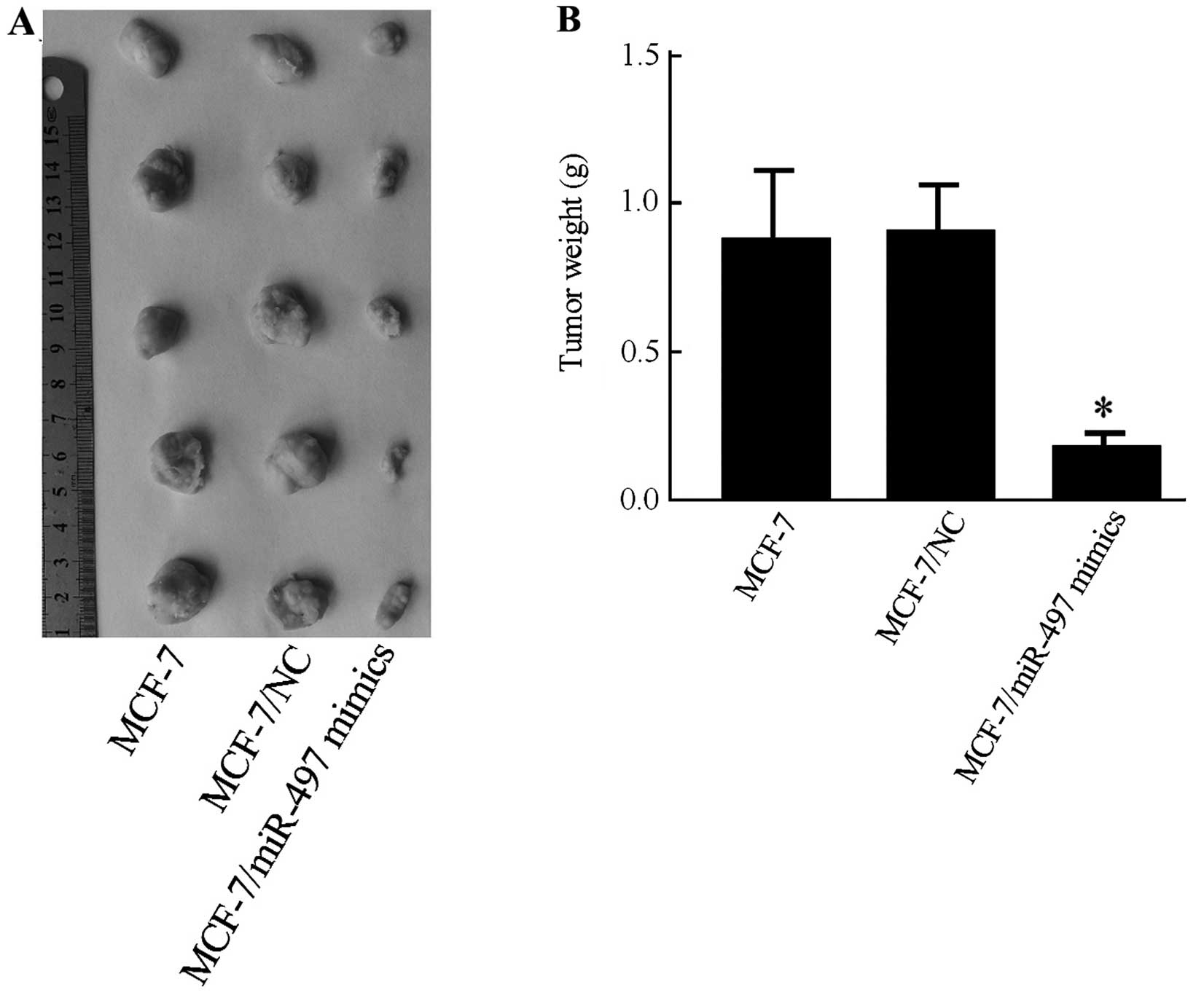
miR-497 inhibits tumor growth in nude
mice
To confirm the anti-tumorigenic effect of miR-497
in vivo, a xenograft model was performed to compare the
tumorigenesis of MCF-7 cells before and after miR-497 transfection.
Subcutaneous tumor nodes of different groups became palpable after
15 days of transplantation. The antitumor efficacy of miR-497 was
determined by considering mean tumor weight immediately following
euthanization. The final tumor weight showed a significant decrease
in the MCF-7/miR-497 mimics group compared with MCF-7 or with
MCF-7/NC groups (P<0.05) (Fig.
5A). The tumor weight in the MCF-7/NC group was not
significantly different than that in the MCF-7 group (P=0.723)
(Fig. 5B).
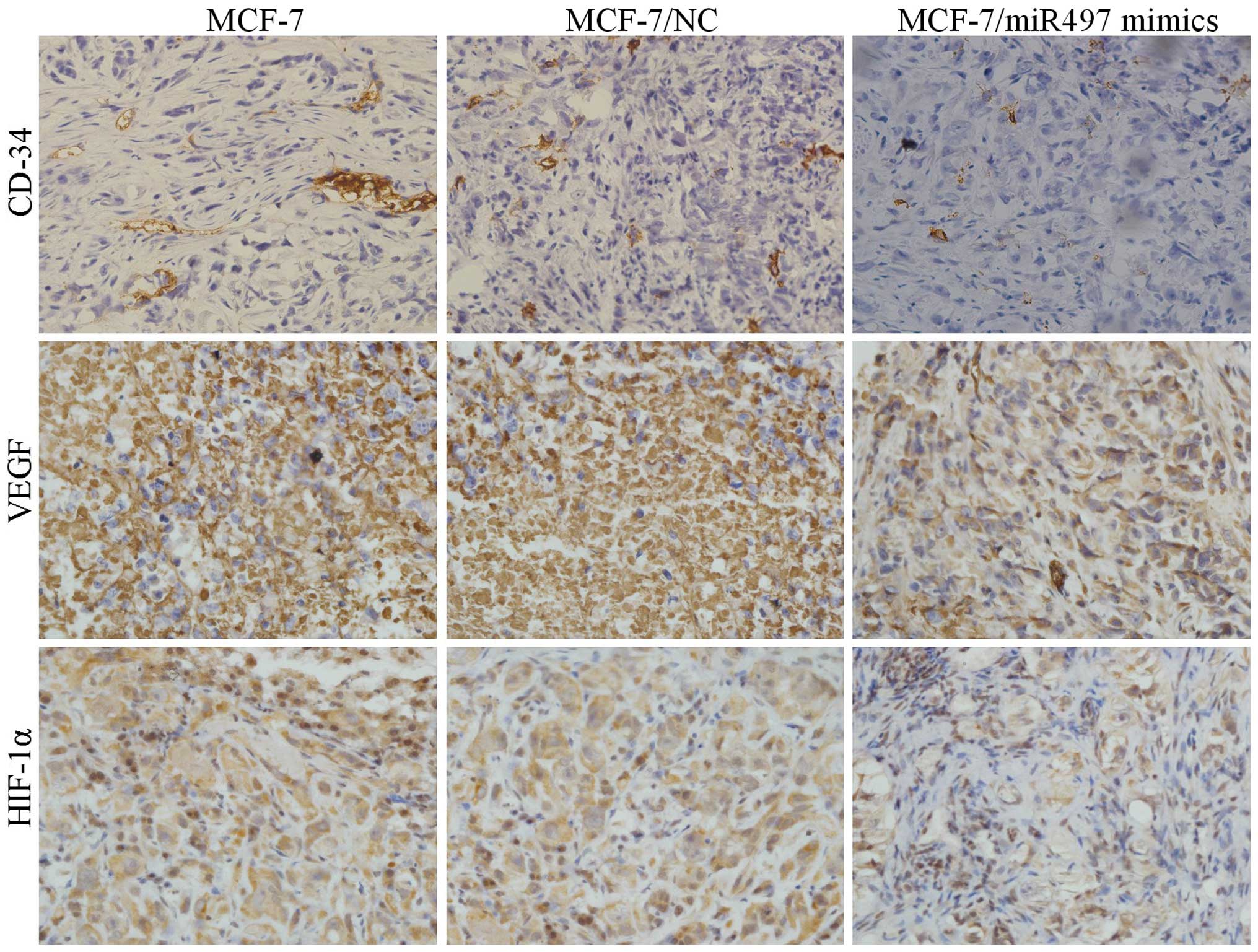
Tumor immunohistochemistry in vivo
As shown in Fig. 6,
MCF-7 cells transfected with miR-497 mimics resulted in a marked
reduction in vascularization microscopically compared with the
negative control. In addition, comparatively, the microvascular
density (MVD) was noticeably reduced in the MCF-7/miR-497 mimics
group. Subsequently, the expression of VEGF and HIF-1α in the tumor
tissues of all the three groups was examined by
immunohistochemistry. Expression levels of VEGF and HIF-1α were
significantly downregulated in the tumor tissues of the
MCF-7/miR-497 mimics group compared with these levels in the MCF-7
and MCF-7/NC groups, suggesting that miR-497 was directly involved
in the inhibition of angiogenesis as well as tumor growth in nude
mice.
Discussion
Solid tumors, such as breast carcinoma, are
characterized by their heterogeneous nature, metastatic potential,
regions of necrotic core and abnormal vasculature. Conventional
treatments include surgery, immunization therapy and chemotherapy.
Nevertheless, some patients who undergo chemotherapy experience
early recurrence or metastasis, leading to poor prognosis (4,5,17). The
identification of new therapeutic targets for breast cancer
treatment has become an intensive issue worldwide.
Over the past decades, research on the roles of
miRNAs in the development of malignant tumors have been a 'hot'
research topic. miRNAs are crucial players in many
pathophysiological processes owing to their promising potential of
being novel diagnostic and predictive markers for therapy.
Currently, our insight into their roles in the development of solid
tumors are rapidly accelerating (18). The majority of known and
characterized miRNAs may function as tumor suppressors or oncogenes
according to the cell type and tissue (19). Previous research demonstrated the
tumor-suppressive role of miR-497 in several cancer types,
including breast carcinoma (12–15).
In the present study, our RT-PCR results indicated that the
expression level of miR-497 in breast cancer tissues was markedly
lower than that in corresponding adjacent non-cancerous tissues,
which is consistent with a previous study (13). Moreover, our results showed that the
miR-497 expression was also generally suppressed in all five breast
cancer cell lines, in contrast to MCF-10A, which is a non-malignant
breast epithelial cell line. Thus, we hypothesized that miR-497 may
act as a valuable tumor suppressor in breast cancer and its
functional mechanism warrants further investigation to support its
significance. Accumulating evidence indicates that miR-497 induces
apoptosis as well as regulates cell growth and invasion in breast
cancer cells (13,20). Yet, the potential role of miR-497 in
angiogenesis remains unclear.
Recently, increasing evidence shows that
angiogenesis is essential for the growth of solid tumors and
research on tumor angiogenesis has become one of the most active
fields in tumor treatment (21).
Therefore, identifying anti-angiogenic targets is considered
effective in tumor treatment. A recent study demonstrated
angiogenesis-related markers in breast cancer (22). In the present study, we used the
breast cancer cell line MCF-7 transfected with the miR-497 mimics
to further investigate the effect of miR-497 on breast
cancer-associated angiogenesis. Since it is well recognized that
the process of tumor-derived angiogenesis is regulated by
pro-angiogenic and anti-angiogenic factors that are secreted by
tumor cells, we used the conditioned medium obtained from MCF-7
cells to eliminate the potential direct interaction between MCF-7
cells and HUVECs. Our results showed that the overexpression of
miR-497 inhibited the in vitro proliferation of HUVECs and
restrained angiogenesis in breast cancer cells by applying HUVECs.
Hypoxia is considered an important factor in promoting
angiogenesis, mainly by regulating pro-angiogenic and
anti-angiogenic factors, such as VEGF (23). A previous study suggested that
miR-210, as a hypoxia-inducible miRNA, augments the metastatic
potential of liver cancer cells (24). Therefore, to validate the hypothesis
that miR-497 can be induced by hypoxia, our results showed that the
expression of miR-497 was significantly decreased under hypoxia.
Additionally, as soon as the normoxic condition was restored, the
level of miR-497 was immediately increased. Thus, the data
suggested that hypoxia can reduce the expression of miR-497. To
date, this is the first study to illuminate the dynamic change of
miR-497 under normoxic and hypoxic conditions.
Furthermore, during the investigation of the
mechanism of miR-497 in breast cancer cell progression, it was
found that the overexpression of miR-497 reduced the protein level
of VEGF while suppression of miR-497 revealed an opposite result,
and the expression of VEGF in the cell culture medium decreased
markedly in the MCF-7 cells transfected with the miR-497 mimics,
even under a hypoxia condition. In addition, our in vivo
data disclosed that xenograft tumor tissues from the MCF-7/miR-497
mimics group displayed lower expression of CD34 and VEGF than the
MCF-7 and MCF-7/NC groups. Previous research has demonstrated that
the VEGF gene regulated by hypoxia is under the control of the
transcription factor HIF-1, which consists of the hypoxic response
factor HIF-1α and the constitutively expressed aryl hydrocarbon
receptor nuclear translocator HIF-1β/ARNT (25). Through the siRNA approach, the
present study demonstrated that the overexpression of miR-497
inhibited the expression of HIF-1α, corresponding to changes in the
expression of VEGF in vitro and in vivo. These
results indicated that miR-497 exerts an anti-angiogenic effect by
downregulating VEGF and HIF-1α in breast cancer cells. Such data
are consistent with those in the literature (13,25).
In conclusion, our present findings are consistent
with the hypothesis that miR-497 as a hypoxia-inducible miRNA,
suppresses the angiogenesis of breast cancer cells in vitro
as well as in vivo by inhibiting the expression of VEGF and
HIF-1α. These findings suggest that hypoxia-related markers in
breast cancer cells and the hypoxia/miR-497/HIF-1α pathway may
serve as a promising strategy for the treatment of breast
cancer.
Abbreviations:
|
miRNAs
|
microRNAs
|
|
VEGF
|
vascular endothelial growth factor
|
|
HIF-1α
|
hypoxia inducible factor-1α
|
|
HUVECs
|
human umbilical vein endothelial
cells
|
|
ATCC
|
American Type Culture Collection
|
|
NC
|
negative control
|
|
CCK-8
|
Cell Counting Kit-8
|
|
RT-PCR
|
real-time polymerase chain
reaction
|
|
PBS
|
phosphate-buffered saline
|
|
MVD
|
microvessel density
|
|
HRP
|
horseradish peroxidase
|
|
TUNEL
|
terminal deoxynucleotidyl
transferase-mediated dUTP nick-end labeling
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schulze A and Harris AL: How cancer
metabolism is tuned for proliferation and vulnerable to disruption.
Nature. 491:364–373. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parks SK, Chiche J and Pouysségur J:
Disrupting proton dynamics and energy metabolism for cancer
therapy. Nat Rev Cancer. 13:611–623. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zheng R, Yao Q, Xie G, Du S, Ren C, Wang Y
and Yuan Y: TAT-ODD-p53 enhances the radiosensitivity of hypoxic
breast cancer cells by inhibiting Parkin-mediated mitophagy.
Oncotarget. 6:17417–17429. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hamdan FH and Zihlif MA: Gene expression
alterations in chronic hypoxic MCF7 breast cancer cell line.
Genomics. 104:477–481. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Soares RJ, Cagnin S, Chemello F,
Silvestrin M, Musaro A, De Pitta C, Lanfranchi G and Sandri M:
Involvement of microRNAs in the regulation of muscle wasting during
catabolic conditions. J Biol Chem. 289:21909–21925. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Godnic I, Zorc M, Jevsinek Skok D, Calin
GA, Horvat S, Dovc P, Kovac M and Kunej T: Genome-wide and
species-wide in silico screening for intragenic MicroRNAs in human,
mouse and chicken. PLoS One. 8:e651652013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gromak N, Dienstbier M, Macias S, Plass M,
Eyras E, Cáceres JF and Proudfoot NJ: Drosha regulates gene
expression independently of RNA cleavage function. Cell Rep.
5:1499–1510. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhai H, Chen QJ, Chen BD, Yang YN, Ma YT,
Li XM, Liu F, Yu ZX, Xiang Y, Liao W, et al: Long noncoding RNA
MALAT1 as a putative biomarker of lymph node metastasis: A
meta-analysis. Int J Clin Exp Med. 8:7648–7654. 2015.PubMed/NCBI
|
|
10
|
Nagpal N, Ahmad HM, Chameettachal S,
Sundar D, Ghosh S and Kulshreshtha R: HIF-inducible miR-191
promotes migration in breast cancer through complex regulation of
TGFβ-signaling in hypoxic microenvironment. Sci Rep. 5:96502015.
View Article : Google Scholar
|
|
11
|
Chen W, Cai F, Zhang B, Barekati Z and
Zhong XY: The level of circulating miRNA-10b and miRNA-373 in
detecting lymph node metastasis of breast cancer: Potential
biomarkers. Tumour Biol. 34:455–462. 2013. View Article : Google Scholar
|
|
12
|
Du M, Shi D, Yuan L, Li P, Chu H, Qin C,
Yin C, Zhang Z and Wang M: Circulating miR-497 and miR-663b in
plasma are potential novel biomarkers for bladder cancer. Sci Rep.
5:104372015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wei C, Luo Q, Sun X, Li D, Song H, Li X,
Song J, Hua K and Fang L: microRNA-497 induces cell apoptosis by
negatively regulating Bcl-2 protein expression at the
posttranscriptional level in human breast cancer. Int J Clin Exp
Pathol. 8:7729–7739. 2015.PubMed/NCBI
|
|
14
|
Troppan K, Wenzl K, Pichler M, Pursche B,
Schwarzenbacher D, Feichtinger J, Thallinger GG, Beham-Schmid C,
Neumeister P and Deutsch A: miR-199a and miR-497 are associated
with better overall survival due to increased chemosensitivity in
diffuse large B-cell lymphoma patients. Int J Mol Sci.
16:18077–18095. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Han Z, Zhang Y, Yang Q, Liu B, Wu J, Zhang
Y, Yang C and Jiang Y: miR-497 and miR-34a retard lung cancer
growth by co-inhibiting cyclin E1 (CCNE1). Oncotarget.
6:13149–13163. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Peng L, Liu A, Shen Y, Xu HZ, Yang SZ,
Ying XZ, Liao W, Liu HX, Lin ZQ, Chen QY, et al: Antitumor and
anti-angiogenesis effects of thymoquinone on osteosarcoma through
the NF-κB pathway. Oncol Rep. 29:571–578. 2013.
|
|
17
|
Howell A, Anderson AS, Clarke RB, Duffy
SW, Evans DG, Garcia-Closas M, Gescher AJ, Key TJ, Saxton JM and
Harvie MN: Risk determination and prevention of breast cancer.
Breast Cancer Res. 16:4462014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rinck A, Preusse M, Laggerbauer B, Lickert
H, Engelhardt S and Theis FJ: The human transcriptome is enriched
for miRNA-binding sites located in cooperativity-permitting
distance. RNA Biol. 10:1125–1135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Raychaudhuri S: MicroRNAs overexpressed in
growth-restricted rat skeletal muscles regulate the glucose
transport in cell culture targeting central TGF-β factor SMAD4.
PLoS One. 7:e345962012. View Article : Google Scholar
|
|
20
|
Luo Q, Li X, Gao Y, Long Y, Chen L, Huang
Y and Fang L: MiRNA-497 regulates cell growth and invasion by
targeting cyclin E1 in breast cancer. Cancer Cell Int. 13:952013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu Y, Wondimu A, Yan S, Bobb D and
Ladisch S: Tumor gangliosides accelerate murine tumor angiogenesis.
Angiogenesis. 17:563–571. 2014. View Article : Google Scholar :
|
|
22
|
Retsky M, Bonadonna G, Demicheli R,
Folkman J, Hrushesky W and Valagussa P: Hypothesis: Induced
angiogenesis after surgery in premenopausal node-positive breast
cancer patients is a major underlying reason why adjuvant
chemotherapy works particularly well for those patients. Breast
Cancer Res. 6:R372–R374. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma J, Zhang L, Ru GQ, Zhao ZS and Xu WJ:
Upregulation of hypoxia inducible factor 1α mRNA is associated with
elevated vascular endothelial growth factor expression and
excessive angiogenesis and predicts a poor prognosis in gastric
carcinoma. World J Gastroenterol. 13:1680–1686. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dang K and Myers KA: The role of
hypoxia-induced miR-210 in cancer progression. Int J Mol Sci.
16:6353–6372. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Meng F, Dong B, Li H, Fan D and Ding J:
RNAi-mediated inhibition of Raf-1 leads to decreased angiogenesis
and tumor growth in gastric cancer. Cancer Biol Ther. 8:174–179.
2009. View Article : Google Scholar
|