Introduction
Cervical cancer is one of the most common female
cancers and is associated with a high mortality rate (1). In the last few years, there has been
great progress in the early diagnosis of cervical cancer along with
the improvement in biological detection technology. The universal
detection of cervical cancer and high-risk HPV for women of
childbearing age have greatly improved the rate of early diagnosis,
and the treatment efficiency of early stage cervical cancer is very
good. However, the treatment efficiency of advanced cervical cancer
is still very poor, and the 5-year survival rate is also very low.
Therefore, it is necessary to further explore the pathogenesis of
cervical cancer.
Cancer stem cells (CSCs) appear to be responsible
for tumor initiation, progression and resistance to conventional
treatment (2,3). Nanog is one of the core transcription
factors for maintaining the stemness of embryonic stem cells (ESCs)
(4). Abnormal expression of Nanog
is associated with several types of cancers, such as glioma
(5), breast cancer (6), embryonic carcinoma (7), and prostate cancer (8). Jeter et al (9) reported that cytoplasmic
Nanog+ stromal cells could promote cervical cancer
progression. Our previous study also indicated that a
TALEN-mediated Nanog gene knockout could attenuate the malignancy
of HeLa cells (10). These results
suggested that Nanog is a risk factor for cervical cancer. However,
whether Nanog is associated with cervical cancer cell
dedifferentiation and how cervical cancer cells acquire the ability
to invade surrounding tissues and metastasize are still
unclear.
In the present study, to elucidate the role of Nanog
in cervical cancer progression, we used mRNA synthesized in
vitro for the forced expression of Nanog. This synthetic mRNA
could bypass innate anti-viral responses induced in human cell
dedifferentiation and had kinetics substantially superior to
established viral protocols (11–13).
Furthermore, this method was non-mutagenic (2).
Materials and methods
Cell culture
HeLa cells (10)
were maintained in Dulbecco's modified Eagle's medium (DMEM)
containing 10% FBS.
Nanog mRNA synthesized in
vitro
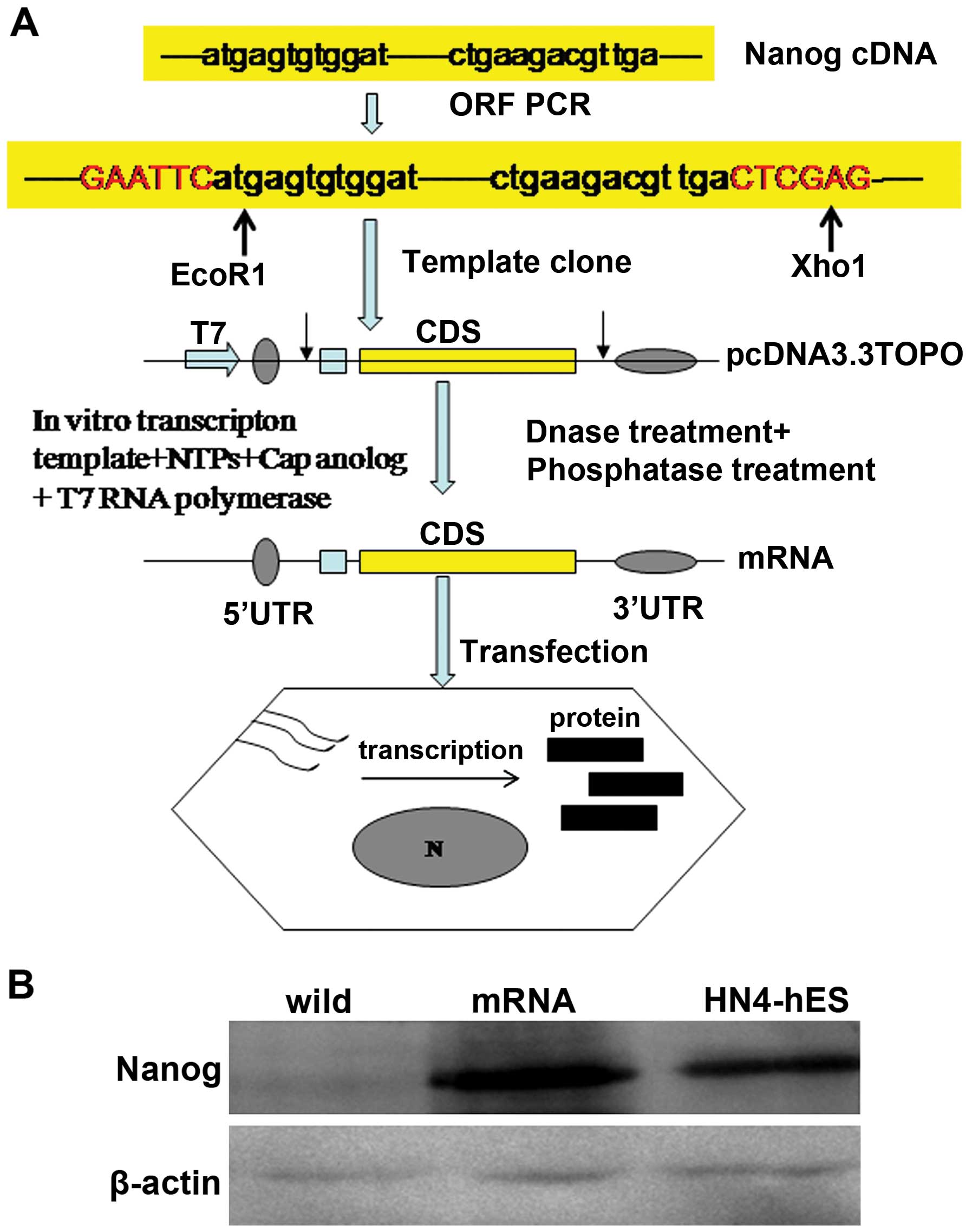
In vitro transcription template construction
and RNA synthesis is schematized in Fig. 1A according to introduction of Zangi
et al (13). The ORF of
Nanog was templated from the cDNA of HN4 human embryonic stem cells
(hESCs) (14). PCR reactions were
performed using PCR Master Mix (R300A; Takara) according to the
manufacturer's instructions. Splint-mediated ligations were carried
out using T4 DNA ligase (Oncogene). All intermediate PCR and
ligation products were purified using QIAquick spin columns
(Qiagen) before further processing. Template PCR amplifications
were sub-cloned using the pcDNA 3.3-TOPO TA cloning kit
(Invitrogen). Plasmid inserts were excised by restriction digestion
and recovered with Size Select gels (Invitrogen) and then used to
template tail PCRs.
RNA was synthesized with the MEgAscript T7 kit
(Ambion), using 1.6 µg of purified tail PCR product to
template each 40 µl reaction. Ribonucleoside blend, composed
of 3′-0-Me-m7G (5′) ppp (5′) G ARCA cap analog (New England
Biolabs), adenosine triphosphate and guanosine triphosphate (USB,
Cleveland, OH, USA), 5-methylcytidine triphosphate and
pseudouridine triphosphate (TriLink Biotechnologies, San Diego, CA,
USA), were used. Reactions were incubated for 5 h at 37°C and then
treated with antarctic phosphatase (New England Biolabs) for 2 h at
37°C to remove residual 5′-triphosphates. Treated RNA was purified
and quantitated using a Nanodrop (Thermo Scientific, Waltham, MA,
USA).
Nanog RNA transfection and stability
assay
RNA transfection was carried out using TransIT-mRNA
(Mirus Bio, Madison, WI, USA) cationic lipid delivery vehicles. For
TransIT mRNA transfections, ~1 µg mRNA was added to 100
µl Opti-MEM and mixed gently. BOOST reagent was added (1
µl/microgram of RNA) followed by TransIT-mRNA (1
µl/microgram of RNA), and the RNA-lipid complexes were added
to the culture media after a 2-min incubation at room temperature
(RT). Cells were cultured for an additional 36 to 48 h before
repeating the same mRNA transduction procedure. Three additional
mRNA transduction experiments were performed.
To determine the half-life of Nanog mRNA, total
protein of HeLa cells was extracted at different time-points (0, 6,
18, 30 and 42 h) after mRNA transfection. Nanog and β-actin gene
expression levels at each time-point were measured by western
blotting.
Tumor tissue species collection and
immunohistochemistry
Cervical cancer specimens (ten for high- and
low-grade group, respectively) were collected from patients who
underwent cervicectomy for cervical cancer between 2010 and 2014 at
Taihe Hospital, the Affiliated Hospital of Hubei University of
Medicine. The histological type of cervical cancer was determined
according to WHO classification criteria. Formalin-fixed,
paraffin-embedded sections of tumors and adjacent non-tumorous
tissues were used to detect the expression of Nanog. The present
study was approved by the Ethics Committee of Taihe Hospital.
Cisplatin and paclitaxel treatment and
cell viability assay
Cell viability was determined using an MTS kit
(Promega). Freshly disassociated cells were seeded in 96-well
plates (2×104/well; Corning), and the media were
replaced with HeLa cell medium containing 1 µg/ml cisplatin
or 20 ng/ml paclitaxel the next day. After treatment for 24 to 48
h, 20 µl MTS working solution was added to the medium and
incubated at RT for 1–4 h, and then the OD value at 490 nm was
recorded. Five replicate wells were used for each group.
Cell invasion ability detection
Transwell filters were used to analyze cell
migration in vitro (10).
The upper chamber of the polycarbonate membrane filter inserts
(8-µm; Corning, USA) were pre-coated with Matrigel, and then
a total of 5×104 cells was seeded into a 24-well plate
and cultured in 200 µl DMEM medium. The lower chamber was
filled with 500 µl of HeLa cell culture medium. After
incubation for 48 h, non-migrated cells in the upper chamber
surface were removed, and the migrated cells on the bottom side of
the membrane were fixed and then stained with 0.1% Crystal violet.
The stained membranes were observed, and the number of migrated
cells was counted.
Migration ability detection
The migration ability of HeLa cells was tested as
described in our previous study (10). A total of 5×105 cells was
seeded into 6-well plates, and a 10 µl pipette tip was used
to create a scratch on the cell monolayer the next day. Then, the
cells were cultured with DMEM and incubated at 37°C in 5%
CO2 atmosphere for 48 h. Pictures were taken at 0, 24
and 48 h. Distance from the scratch was measured from one side to
the other. Three replicate wells were used for each group.
Tumor sphere forming ability
detection
Cells were digested and resuspended in DMEM/F12
medium at a density of 1,000 cells/ml and seeded into 6-well plates
(2 ml/well). The serum-free culture medium consisted of DMEM/F12
(Sigma), 0.4% BSA, 2% B27 (Invitrogen), 20 ng/ml human recombinant
fibroblast growth factor 2 (FGF-2) and epidermal growth factor
(EGF) (both from Sigma). After 10–14 days of culture, sphere number
was measured. Each group was plated in 3 duplicate wells.
Colony-formation ability detection
The colony-formation assay was performed in 12-well
plates. Cells were seeded at 1,000 cells/well and cultured with
normal HeLa cell medium until colonies were large enough to be
visualized. Then, colonies were fixed and stained with 0.1% crystal
violet. Experiments were done in triplicate.
Subcutaneous xenograft
HeLa cells (1×106), transfected with or
without Nanog mRNA, were injected subcutaneously into nude mice
(n=3) as described in our previous study (10) and observed weekly until the mice
died.
Immunofluorescence
Cells were fixed with 4% paraformaldehyde followed
by permeabilization with 0.1% Triton X-100 and then blocked in 5%
BSA (both from Sigma). Afterwards, the cells were incubated with
primary Nanog (1:100) and CD133 (1:200) (both from Abcam)
antibodies overnight at 4°C and then with FITC-conjugated with rat
anti-mouse immunoglobulin (1:500) and Cy3-rat anti-mouse (1:500)
secondary antibody for 30 min at RT. Finally, the nuclei were
stained with 1 µg/ml Hoechst 33342 and visualized using a
Leica microscope.
Real-time (RT)-PCR and western blot
assay
Real-time (RT)-PCR, western blot assay and
statistical analysis were performed as described in our previous
study (10).
Results
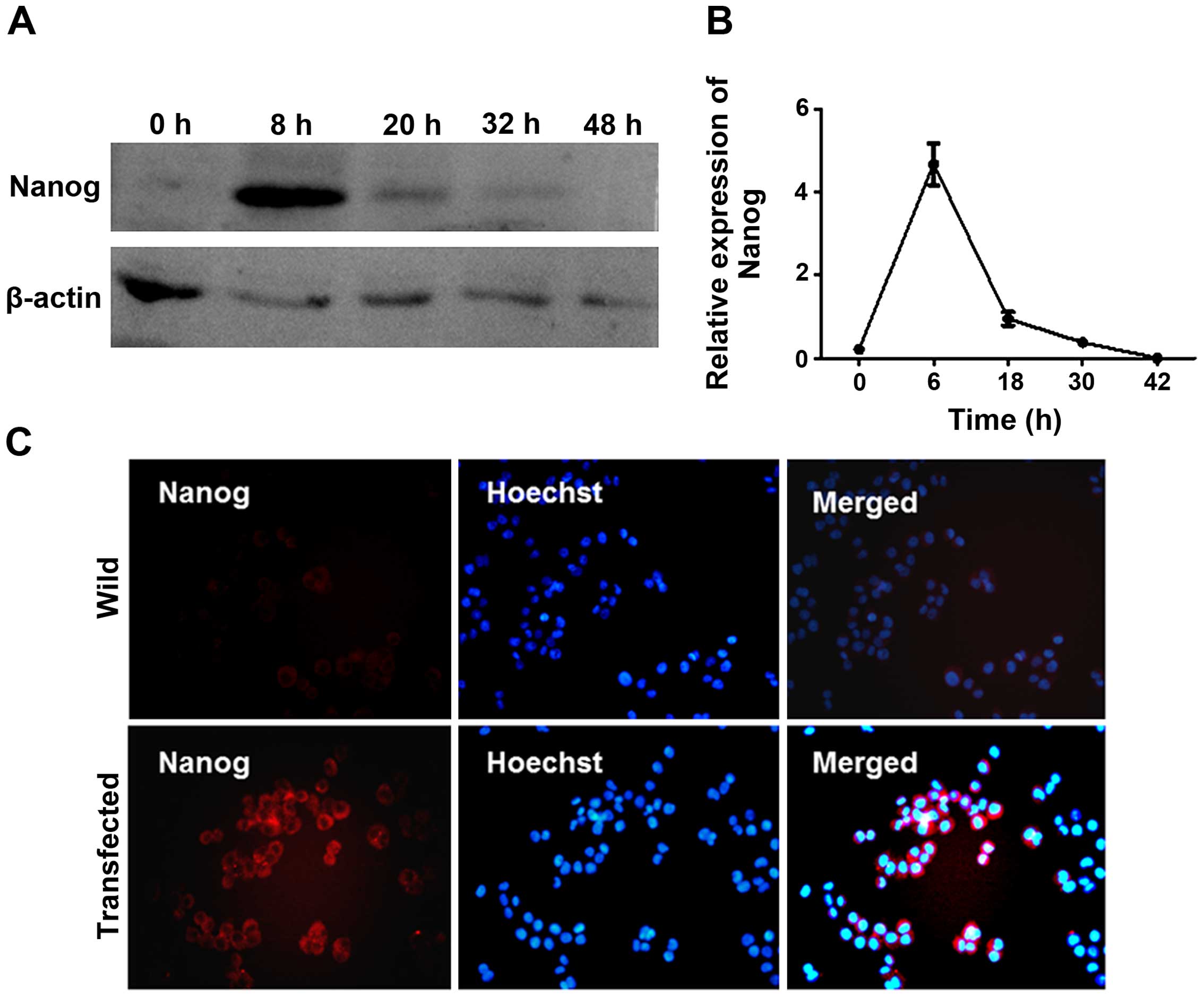
Nanog mRNA activated the expression of
endogenous Nanog
We transfected HeLa cells with Nanog mRNA,
synthesized in vitro, and detected the expression of
endogenous Nanog. First, we measured the protein level of Nanog in
HeLa cells at 6, 18, 30 and 42 h after transfection using western
blotting and immunofluorescence. Nanog protein expression was
evident 6 h after transfection and was maintained for up to 30 h.
Forty-two hours after transfection, Nanog levels returned to
baseline (Figs. 1B and 2). HeLa cells were treated with Nanog mRNA
4 times every other 32 h. Then the cells were cultured for an
additional 8–10 days, the protein level of Nanog was detected, and
it remained at a high level.
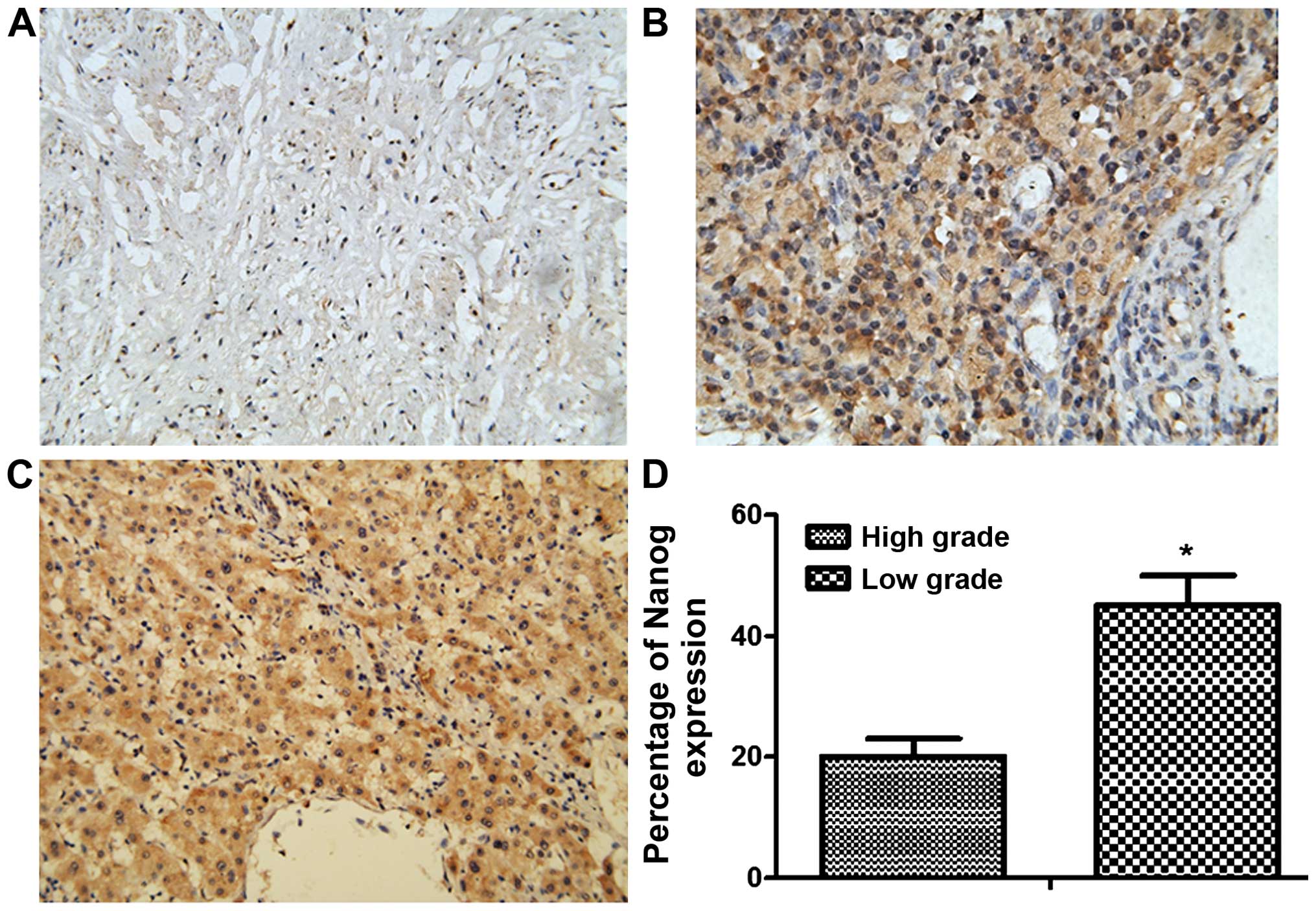
Nanog expression correlated with
malignancy and prognosis of cervical cancer patients
Nanog expression in cervical cancer specimens and
adjacent non-cancer tissues from 40 patients with cervical cancer
(20 each for low- and high-grade specimens) was detected by
immunohistochemistry. The expression of Nanog could hardly be
detected in non-cancer tissue specimens. On the contrary, Nanog
expression could be detected in ~20% of low-grade and 45% of
high-grade cervical cancer specimens. This indicated that the
expression of Nanog was positively correlated with clinical grading
and malignant degree of cervical cancer (Fig. 3).
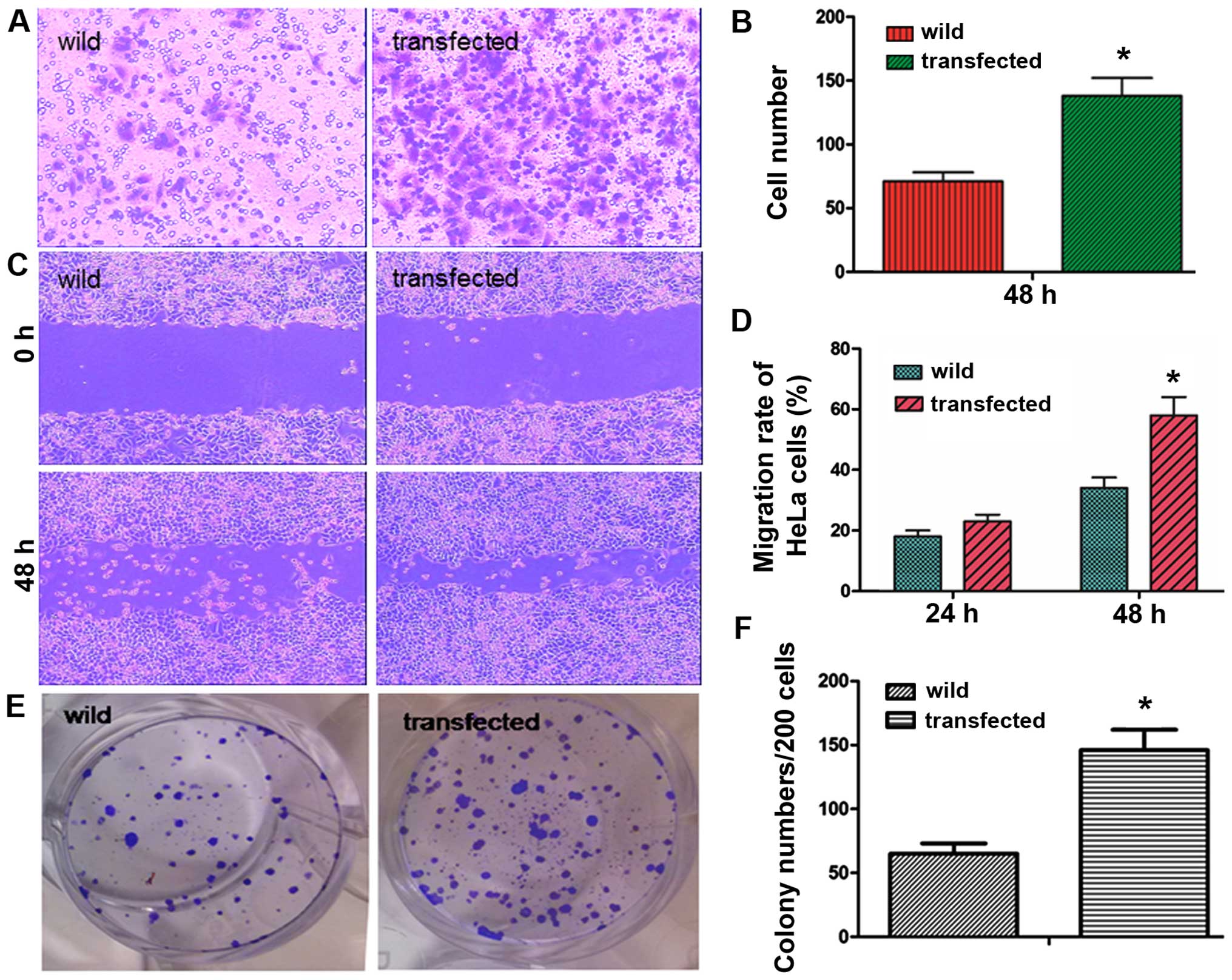
Nanog induces more aggressive biological
behavior
Transwell cell migration assays indicated that
forced expression of Nanog significantly increased the invasive
ability of HeLa cells. As shown in Fig.
3, after 48 h, the number of transfected cells that passed
through the Transwell was 138±16, but the number of passed cells in
the wild-type group was 62±7 (P<0.05, Fig. 4A and B), which was significantly
lower than in the transfected group.
Scratch assays indicated that Nanog overexpression
resulted in a significant increase in HeLa cell migration. The
migration rates of transfected cells after 24 and 48 h were
26.8±3.4 and 58.7±7.2%, respectively. However, the migration rates
in wild-type group were 18.9±2.3 and 33.2±4.1%, respectively
(P<0.05, Fig. 4C and D).
As shown in Fig. 4E and
F, clonogenicity of transfected HeLa cells was increased
according to the number of cell colonies, and the colony number of
transfected cells was 143±15, higher than the wild-type group,
which was 68±8 (P<0.05).
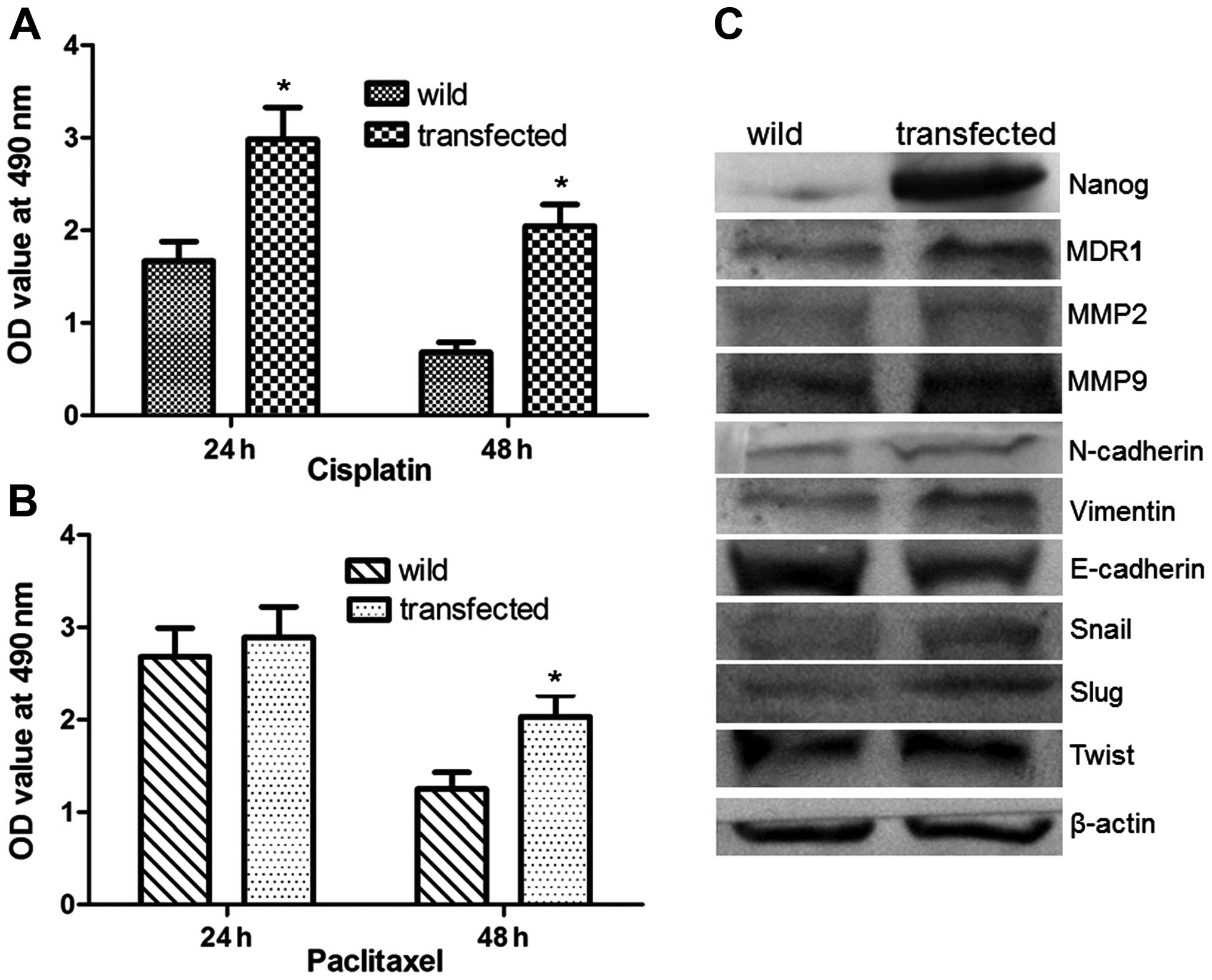
To evaluate the effect of Nanog on the sensitivity
of HeLa cells to chemotherapy, the wild and transfected cells were
exposed to cisplatin or paclitaxel. As shown in Fig. 5A and B, Nanog mRNA transfected HeLa
cells were less sensitive to cisplatin and paclitaxel than the
controls. These data indicated that the sensitivity of HeLa to
chemotherapy drugs was decreased by forced expression of Nanog.
To further evaluate the mechanism by which Nanog
affects the aggressive biological behavior and chemotherapy
sensitivity of HeLa cells, we investigated the expression of MDR1,
which is regarded as an important factor in drug resistance and
sensitivity of chemotherapy, MMP2, MMP9, and EMT associated
signals. As shown in Fig. 5C,
compared with the wild-type HeLa cells, the expression of MDR1,
MMP2, MMP9, N-cadherin, vimentin, Snail, Slug, and Twist were
significantly increased, but the expression of E-cadherin was
decreased in Nanog mRNA-transfected cells (P<0.05).
Nanog-induced dedifferentiation of
cervical cancer cells and its mechanism
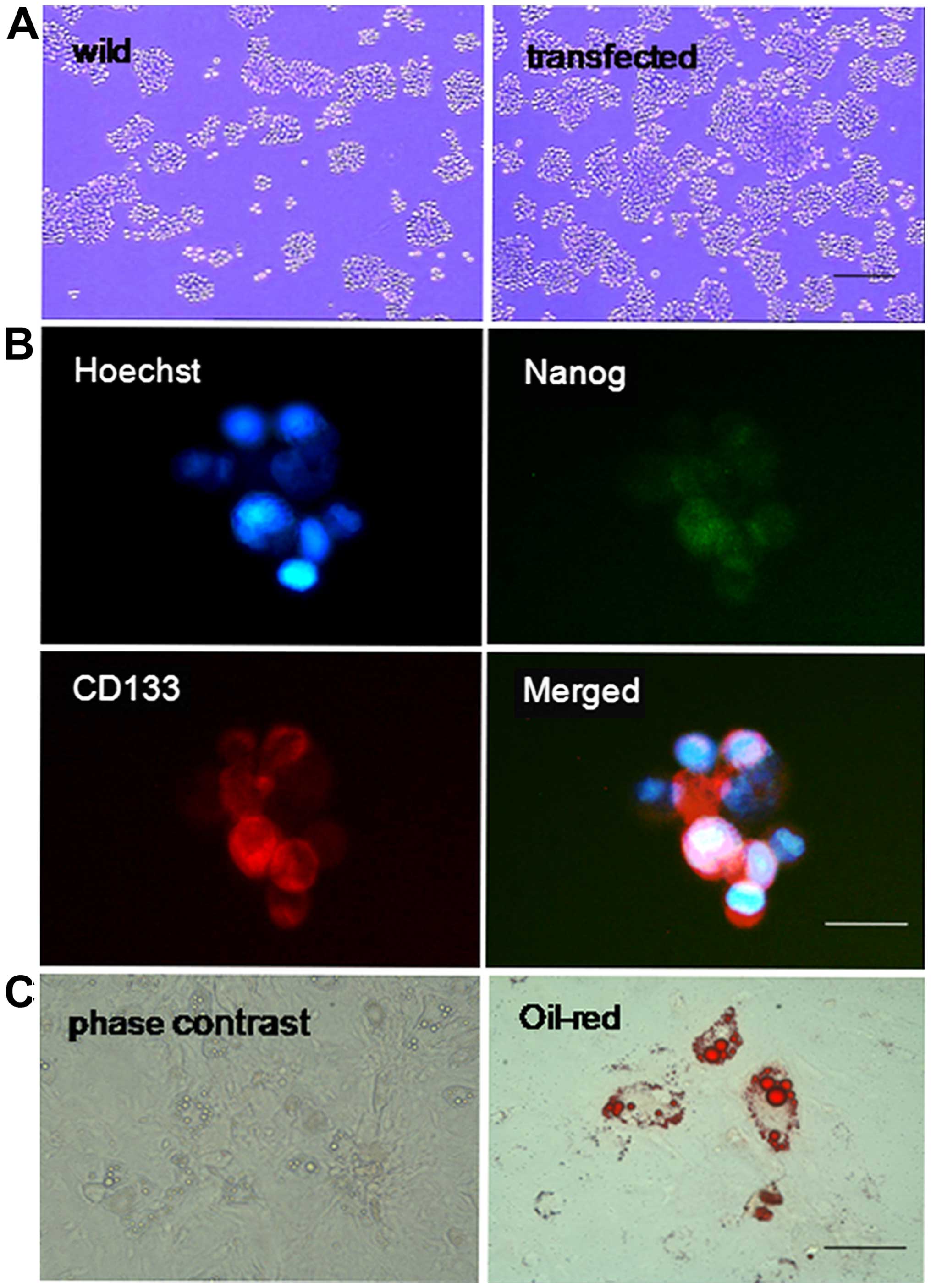
Cells transfected with and without Nanog mRNA were
cultured in tumor sphere-forming medium. After 14 days, the number
and size of the tumor spheres were analyzed. As shown in Fig. 6A, Nanog mRNA-transfected cells
formed significantly more tumor spheres than control cells.
Furthermore, the size of the tumor spheres was also significantly
larger in cells with forced Nanog expression.
Immunofluorescence was used to detect the expression
of Nanog and CD133 in sphere-forming cells, and it was found that
>90% of these cells expressed both markers (Fig. 6B). To further confirm their cancer
stemness, the tumor sphere HeLa cells were cultured in adipogenic
differentiation media for another 15 days and then stained with oil
red-O. As shown in Fig. 6C, the red
lipid droplets were observed in the differentiated cancer
cells.
RT-PCR detected the expression of other
transcription factors involved in somatic cell reprogramming. It
was found that the expression levels of endogenous Oct4, Sox2 and
FoxD3 were significantly increased, but Rex1, Klf4, Lin28 and c-Myc
were not increased (Fig. 7A and B).
Western blotting also detected increased expression of Oct4, Sox2
and CD133 in Nanog mRNA-transfected HeLa cells (Fig. 7C).
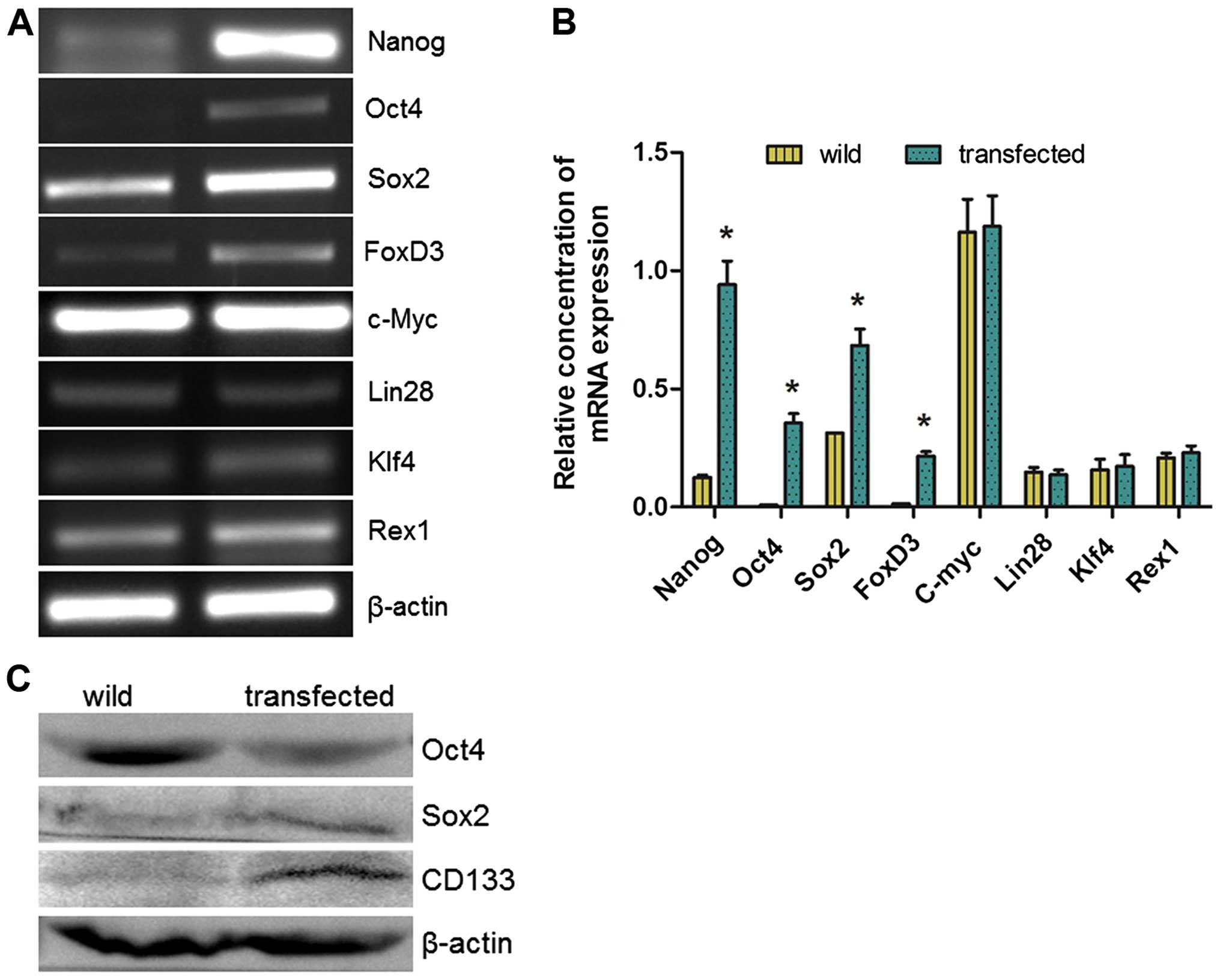 | Figure 7Mechanisms underlying Nanog-induced
differentiation. (A and B) Quantitative RT-PCR was performed for
Nanog, Oct4, Sox2, c-Myc, KLF4, Lin28, Rex1 and FoxD3 (n=3), and
showed significant increase of Nanog, Oct4, Sox2 and FoxD3
(*P<0.05), but not Rex1, c-Myc, Klf4 and Lin28. (C)
Western blot to detect the expression of Oct4, Sox2 and CD133 in
control and Nanog overexpressing HeLa cells. |
Increased tumorigenicity after
Nanog-induced dedifferentiation
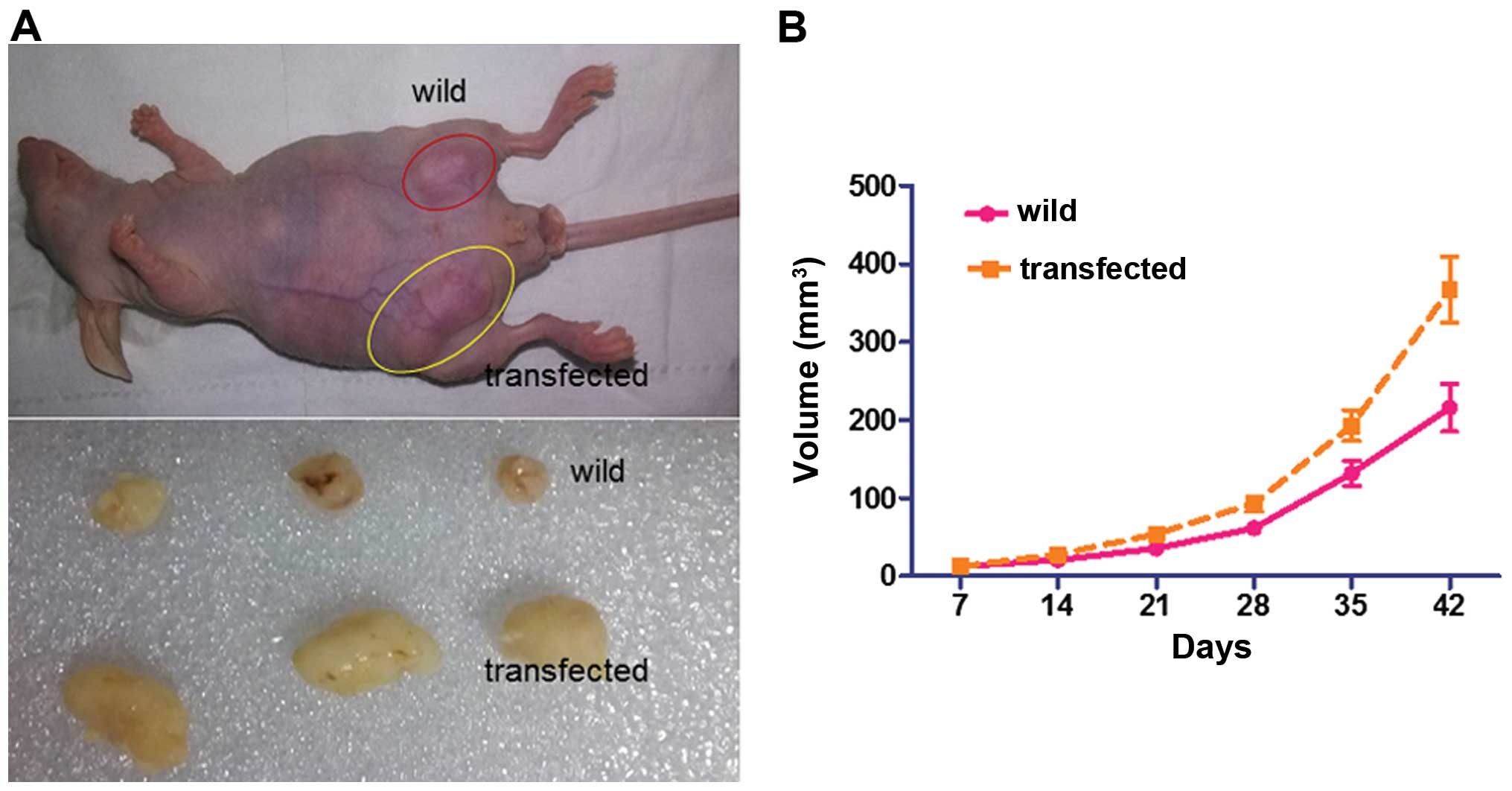
In order to verify the effect of Nanog-mediated
dedifferentiation in vivo, nude mice (n=3) were transplanted
with HeLa cells transfected with or without Nanog mRNA
(1×106) subcutaneously and were then monitored for tumor
growth. As shown in Fig. 8, the
tumors formed by Nanog mRNA-transfected cells were on average
larger than the control group (382±41 vs. 145±23 mm2,
P<0.05). These findings demonstrate that the transfected cells
have greater tumorigenicity than the wild HeLa cells.
Discussion
Malignancy is a complex process of multi-factors and
multi-stages. It involves widespread abnormal gene function.
Understanding the molecular mechanisms leading to invasiveness and
metastatic dissemination of carcinoma cells is important for the
development of new therapeutic strategies against cancer.
In the present study, mRNA synthesized in
vitro was transfected into HeLa cells to force expression of
Nanog, and it was found that both mRNA and protein levels of Nanog
were significantly increased in the Nanog mRNA-transfected HeLa
cells. Then, we examined the effect of forced Nanog expression on
the biological characteristics, such as colony formation capacity,
cell migration and invasive ability, of HeLa cells. It was shown
that the colony formation rate of HeLa cells with forced Nanog
expression was higher than the wild control. There were more
migrating and invasive transfected cells than in the wild controls.
These results indicated that the forced expression of Nanog could
increase the malignant behavior of HeLa cells.
CSCs play an important role in tumor progression
(3,15). To confirm whether the effect of
forced Nanog expression on HeLa cell malignant behavior is
associated with CSCs, we tested the tumor sphere forming ability of
HeLa-Nanog cells. It was shown that the Nanog mRNA alone was able
to increase the stemness (dedifferentiation) of cervical cancer
cells, and the expression of endogenous Oct4, Sox2, and FoxD3 was
significantly increased. This suggested that the pluripotency
factor circuitry in the HeLa cells is at least partially active,
allowing efficient dedifferentiation of HeLa cells by Nanog.
Studies have shown that the failure of chemotherapy
in many malignant tumors was partially associated with abnormal
expression of the MDR1 gene, which encodes the P-glycoprotein to
pump anticancer agents out of the cells (16,17).
In the present study, we examined the expression of MDR1 in HeLa
cells with or without Nanog mRNA transfection. We found that the
expression of MDR1 was increased along with the improved expression
of Nanog. This suggested that Nanog may be correlated with the
expression of the MDR1 gene and further altered the
chemosensitivity of human cervical cancer to cisplatin and
paclitaxel. Although the underlying mechanism of Nanog in
regulating MDR1 gene expression and chemoresistance remains
unclear, these results indicate that aberrant expression of Nanog
may be closely related to malignant characteristics, including
multidrug resistance of cervical cancer, and inhibition of Nanog
expression may be a new approach for sensitizing cervical cancer
cells to chemotherapeutic drugs to reverse MDR in cervical cancer
patients.
EMT plays a crucial role in promoting invasion and
metastasis during tumor progression and is a process which tumor
cells attenuate cell-cell adhesion to acquire a mesenchymal-like
phenotype and disseminate into neighboring or distant tissues
(18). Therefore, increased EMT
disrupts E-cadherin-mediated cell-cell adhesion and converts
epithetlial-like cells into mesenchymal-like cells during tumor
cell progression. In the present study, we found that the
overexpression of Nanog resulted in increased EMT, with elevated
N-cadherin and vimentin and decreased E-cadherin in HeLa cells. In
addition, the expression of Snail/Slug and Twist were also
increased in Nanog overexpressing HeLa cells. It has been reported
that Snail/Slug signaling regulates the EMT process (19). This evidence implies that Snail/Slug
and Twist participate in regulating the EMT process to influence
the metastasis of HeLa cells.
In conclusion, our present data suggested that the
Nanog mRNA synthesized in vitro could activate the
endogenous expression of Nanog and other transcription factors
associated with pluripotency, followed by induction of the
dedifferentiation of cervical cancer cells. This suggested that
Nanog may be a positive regulator of cervical cancer cell
dedifferentiation.
Acknowledgments
The present study was supported by the Hubei
University of Medicine Juvenile Scientific and Technological
Creativity team (2014 CXZ06), Educational Foundation of Hubei
Province (B2015484) and Major Science and Technology Projects of
infectious such as AIDS and viral hepatitis prevention and control,
China (2013ZX10001-004-002-005).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hu Y and Fu L: Targeting cancer stem
cells: A new therapy to cure cancer patients. Am J Cancer Res.
2:340–356. 2012.PubMed/NCBI
|
|
3
|
Gilbert CA and Ross AH: Cancer stem cells:
Cell culture, markers, and targets for new therapies. J Cell
Biochem. 108:1031–1038. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chambers I, Silva J, Colby D, Nichols J,
Nijmeijer B, Robertson M, Vrana J, Jones K, Grotewold L and Smith
A: Nanog safeguards pluripotency and mediates germline development.
Nature. 450:1230–1234. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zbinden M, Duquet A, Lorente-Trigos A,
Ngwabyt SN, Borges I and Ruiz i Altaba A: NANOG regulates glioma
stem cells and is essential in vivo acting in a cross-functional
network with GLI1 and p53. EMBO J. 29:2659–2674. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Freberg CT, Dahl JA, Timoskainen S and
Collas P: Epigenetic reprogramming of OCT4 and NANOG regulatory
regions by embryonal carcinoma cell extract. Mol Biol Cell.
18:1543–1553. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ben-Porath I, Thomson MW, Carey VJ, Ge R,
Bell GW, Regev A and Weinberg RA: An embryonic stem cell-like gene
expression signature in poorly differentiated aggressive human
tumors. Nat Genet. 40:499–507. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gu G, Yuan J, Wills M and Kasper S:
Prostate cancer cells with stem cell characteristics reconstitute
the original human tumor in vivo. Cancer Res. 67:4807–4815. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jeter CR, Liu B, Liu X, Chen X, Liu C,
Calhoun-Davis T, Repass J, Zaehres H, Shen JJ and Tang DG: NANOG
promotes cancer stem cell characteristics and prostate cancer
resistance to androgen deprivation. Oncogene. 30:3833–3845. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ding Y, Yu AQ, Li CL, Fang J, Zeng Y and
Li DS: TALEN-mediated Nanog disruption results in less
invasiveness, more chemosensitivity and reversal of EMT in Hela
cells. Oncotarget. 5:8393–8401. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Djurovic S, Iversen N, Jeansson S, Hoover
F and Christensen G: Comparison of nonviral transfection and
adeno-associated viral transduction on cardiomyocytes. Mol
Biotechnol. 28:21–32. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hoshijima M, Ikeda Y, Iwanaga Y,
Minamisawa S, Date MO, Gu Y, Iwatate M, Li M, Wang L, Wilson JM, et
al: Chronic suppression of heart-failure progression by a
pseudophosphorylated mutant of phospholamban via in vivo cardiac
rAAV gene delivery. Nat Med. 8:864–871. 2002.PubMed/NCBI
|
|
13
|
Zangi L, Lui KO, von Gise A, Ma Q, Ebina
W, Ptaszek LM, Später D, Xu H, Tabebordbar M, Gorbatov R, et al:
Modified mRNA directs the fate of heart progenitor cells and
induces vascular regeneration after myocardial infarction. Nat
Biotechnol. 31:898–907. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li B, Xu L, Lu WY, Xu W, Wang MH, Yang K,
Dong J, Ding XY and Huang YH: A whole-mechanical method to
establish human embryonic stem cell line HN4 from discarded
embryos. Cytotechnology. 62:509–518. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Landry JJ, Pyl PT, Rausch T, Zichner T,
Tekkedil MM, Stütz AM, Jauch A, Aiyar RS, Pau G, Delhomme N, et al:
The genomic and transcriptomic landscape of a HeLa cell line. G3
(Bethesda). 3:1213–1224. 2013. View Article : Google Scholar
|
|
16
|
Pérez-Tomás R: Multidrug resistance:
Retrospect and prospects in anti-cancer drug treatment. Curr Med
Chem. 13:1859–1876. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Goda K, Bacsó Z and Szabó G: Multidrug
resistance through the spectacle of P-glycoprotein. Curr Cancer
Drug Targets. 9:281–297. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Acloque H, Adams MS, Fishwick K,
Bronner-Fraser M and Nieto MA: Epithelial-mesenchymal transitions:
The importance of changing cell state in development and disease. J
Clin Invest. 119:1438–1449. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Casas E, Kim J, Bendesky A, Ohno-Machado
L, Wolfe CJ and Yang J: Snail2 is an essential mediator of
Twist1-induced epithelial mesenchymal transition and metastasis.
Cancer Res. 71:245–254. 2011. View Article : Google Scholar : PubMed/NCBI
|