Introduction
Acute lymphoblastic leukemia (ALL) is the most
common pediatric malignancy in childhood (0–14 years), constituting
slightly less than one-third of all childhood cancers diagnosed,
whereas it constitutes approximately 10% of the cancers diagnosed
among adolescents and young adults (15–19 years) (1).
Cytokines, which were originally identified as
products of immune cells, are small secreted proteins that have
specific effects on the interactions and communication between
cells. Cytokines are important mediators of immune responses that
are secreted in response to different infectious and noninfectious
stimuli. Different cytokines are able to stimulate or inhibit cell
growth, regulate cell differentiation, induce cell chemotaxis, and
modulate the expression of other cytokines. The balance among
different cytokines is very important for the maintenance of the
processes regulated by them, as they have been shown to participate
in both the initiation and progression of several pathological
processes, such as chronic inflammation and cancer (2).
Inflammation as a result of trauma,
ischemia-reperfusion injury, or chemically induced injury typically
occurs in the absence of microorganisms and has, therefore, been
termed 'sterile inflammation'. Sterile inflammation occurs in acute
conditions and is similar to microbial-induced inflammation in that
it is marked by the recruitment of neutrophils and macrophages and
the production of pro-inflammatory cytokines and chemokines, such
as tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1)
(3). The triggers of sterile
inflammation are still under investigation, and the pathways that
transduce sterile inflammatory signals have not been completely
clarified. Currently, most of the innate immune pathways that are
activated during infection have been implicated in sterile
inflammation, although distinct signaling pathways of sterile
inflammation exist (3,4). Whether immune pathology ensues after
acute sterile inflammation, depends on the balance between
pro-inflammatory and resolution pathways.
It is now evident that pattern recognition receptors
(PRRs) also recognize non-infectious material that can cause tissue
damage, as well as endogenous molecules that are released after
tissue injury or cell death. These endogenous molecules have been
termed damage-associated molecular patterns (DAMPs). DAMPs have
similar effects to pathogen-associated molecular patterns (PAMPs)
in terms of their ability to activate inflammatory pathways
(5).
In clinical practice, most patients with newly
diagnosed ALL present febrile episodes, which are usually assumed
to be associated with an infectious process. However, in 80% of
cases, no infection can be demonstrated (6,7). The
levels of circulating cytokines have been studied in patients with
ALL presenting fever and neutropenia in the presence of overt
infection (8,9); however, to date, no studies of
patients with ALL and fever without apparent infection have been
published. It is important to characterize the inflammatory profile
in patients with ALL presenting febrile episodes without clinically
apparent infection, as this may influence the medical management of
these patients. The aim of this study was to assess whether the
levels of inflammatory cytokines are increased in patients with ALL
without apparent infection.
Materials and methods
Study population
The study was conducted at the Hospital Infantil de
México Federico Gómez and at the Hospital Pediátrico Moctezuma in
Mexico City, and was approved by the ethics committees of both
institutions. Signed informed consent was obtained from all
parents, and assent was obtained from all children older than 9
years of age. The study included 99 children with ALL (newly
diagnosed) from the Department of Oncology of the two hospitals,
and 48 non-oncological patients (controls) who visited the
Department of Orthopedics and Ophthalmology of the Hospital
Infantil de México Federico Gómez for consultation and/or
preoperative analysis. We confirmed that patients with ALL included
in this study had no apparent clinical infection at diagnosis, the
point at which the blood sample used in this study was taken. We
conducted a detailed review of the clinical records to confirm that
the specimens of patients with ALL were negative for all
microbiological tests of blood, urine, and spinal fluid (LCR) by
culture and/or PCR. The exclusion criteria were: presence of any
evident infection at diagnosis, anti-inflammatory or antimicrobial
treatment, cancer-specific therapies, primary or secondary
immunodeficiencies, or recent blood-component transfusion.
Sample collection
Peripheral blood was collected in a Vacutainer tube
(BD Vacutainer®) without anticoagulant. After clotting,
the tubes were centrifuged at 1,000 x g for 10 min at 4°C. Serum
samples were stored at −80°C until analysis.
Determination of important cytokine and
chemokine levels in the serum
Cytokines and chemokines (IL-1β, TNF-α, IL-6, MCP-1,
IL-8, IL-10, IFN-γ, IL-12p70, IL-2, IL-4, IL-13 and IL-17) were
quantified in the sera of patients and controls using the multiplex
analyte profiling (xMAP) technology with the Millipore
HSCYTOMAG-60K kit (Luminex®, magnetic beads; Millipore,
Billerica, MA, USA), and were read on a MAGPIX apparatus
(Milliplex®; Millipore). All reagents were provided in
the kit and prepared according to the manufacturer's protocol. Each
sample was analyzed in duplicate, and the mean concentration of
each analyte was calculated using a parameter logistic fitted curve
generated from the standards.
TGF-β was determined in serum samples from 88
patients with ALL and 48 controls using an ELISA set (BD OptEIA™;
BD Biosciences) according to the manufacturer's protocol.
Statistical analyses
Values are plotted as the median with range.
Comparisons between groups were performed by comparing medians
using the Mann-Whitney U test. All statistical analyses were
performed using the SPSS program V22, and P<0.05 was considered
to indicate a statistically significant result.
Results
Clinical characteristics
The characteristics of the patients with ALL and the
non-oncological patients included in the study are described in
Table I. The ALL group included
both de novo precursor B-lineage and T-lineage leukemia
cases. Among the patients with ALL, 34% (34 patients) had fever at
admission, 50% of whom (17 out of 34 patients) had neutropenia. The
characteristics of the patients with ALL and fever and patients
with ALL without fever are described in Table II.
 | Table IDemographic characteristics of the
study population. |
Table I
Demographic characteristics of the
study population.
| ALL patients | Non-oncological
patients |
|---|
| No. of
subjects | 99 | 48 |
| Gender (F/M) | 40/59 | 23/25 |
| Age, mean (range)
in years | 7.5 (0.25–17) | 9.1 (5–17) |
| Diagnosis | ALL | Ophthalmologic,
orthopedic |
| Immunological
classification | | |
| Lineage B | 64 | |
| Lineage T | 14 | |
| Biphenotypic | 3 | |
| Not
classifiable | 18 | |
| With fever | 34 | |
 | Table IICharacteristics of the patients with
ALL with fever or without fever. |
Table II
Characteristics of the patients with
ALL with fever or without fever.
| Patients | Age (years) | Gender (F/M) | Leukocytosis
(103/µl) | Neutrophils
(total) |
|---|
| Fever (n=34) | 6.5±0.69 | (14/20) | 147.9±59.5 | 3,593±202.0 |
| No fever
(n=62) | 8.2±0.62 | (25/37) | 62.3±13.8 | 1,801±577.5 |
| P-value | | 0.085 | 0.076 | 0.289 |
After admission to the hospital, patients with ALL
with fever included in the study were treated with antimicrobial
and antipyretic drugs. No patient presented a positive microbial
culture during the following week and patients with ALL survived
for at least one week after admission.
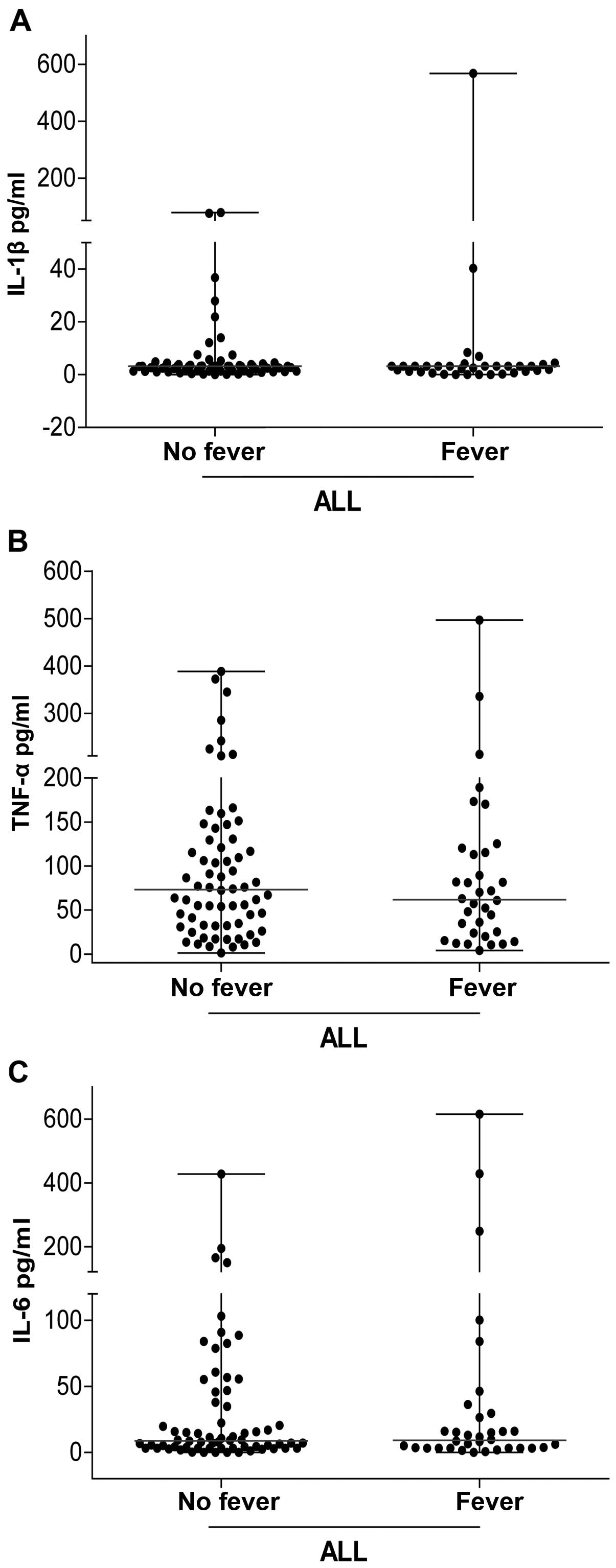
The concentration of circulating
pro-inflammatory cytokines associated with fever is similar between
patients with ALL and fever at diagnosis and those without
fever
We analyzed the levels of circulating
pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α). The
concentrations of IL-1β, IL-6, and TNF-α were not statistically
different between the two groups of patients (with and without
fever) (P>0.05).
We found no significant differences in the levels of
cytokines associated with fever (IL-1β, TNF-α, and IL-6) in the
sera of patients with ALL with and without fever at admission
(Fig. 1).
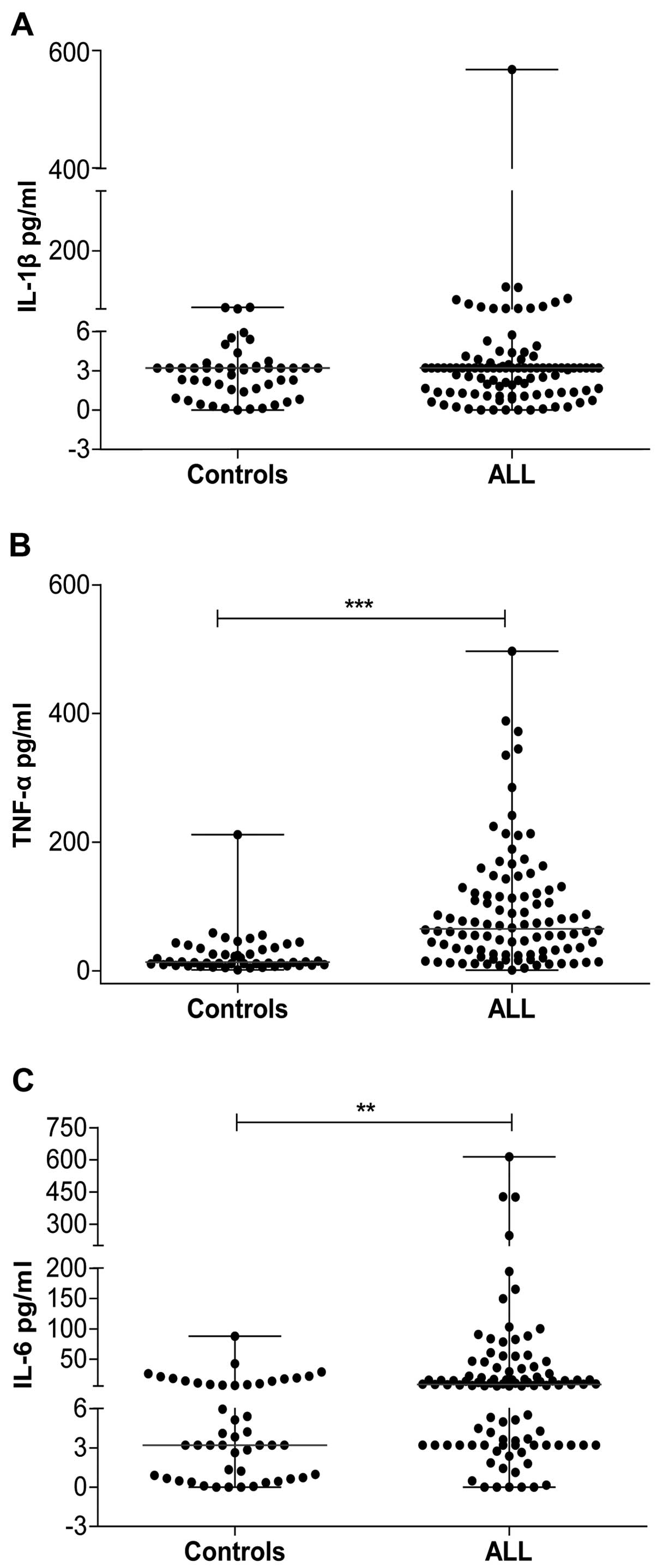
Patients with ALL show a pro-inflammatory
state at diagnosis
We analyzed the levels of circulating
pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) associated with
the production of fever; the concentrations of IL-6 and TNF-α were
higher in the group of patients with ALL than they were in the
control group (P<0.05). Although the concentration of IL-1β was
not statistically different between the two groups, it was observed
that the median concentration was higher in the ALL group than that
in the control group (3.2 vs. 3.2 pg/ml) (Fig. 2A). The median TNF-α concentration in
the control group was 13.60 pg/ml (range, 40–211.6 pg/ml), whereas
the median concentration in the ALL group was 65.40 pg/ml (range,
1.24–496 pg/ml) (Fig. 2B). The IL-6
concentration was 3.20 pg/ml (range, 0.00–87.85 pg/ml) in the
control group and 8.79 pg/ml (range, 0.00–615 pg/ml) in the ALL
group (Fig. 2C). These results
suggest the presence of an important pro-inflammatory profile in
patients with ALL at diagnosis in the absence of clinically
apparent infection.
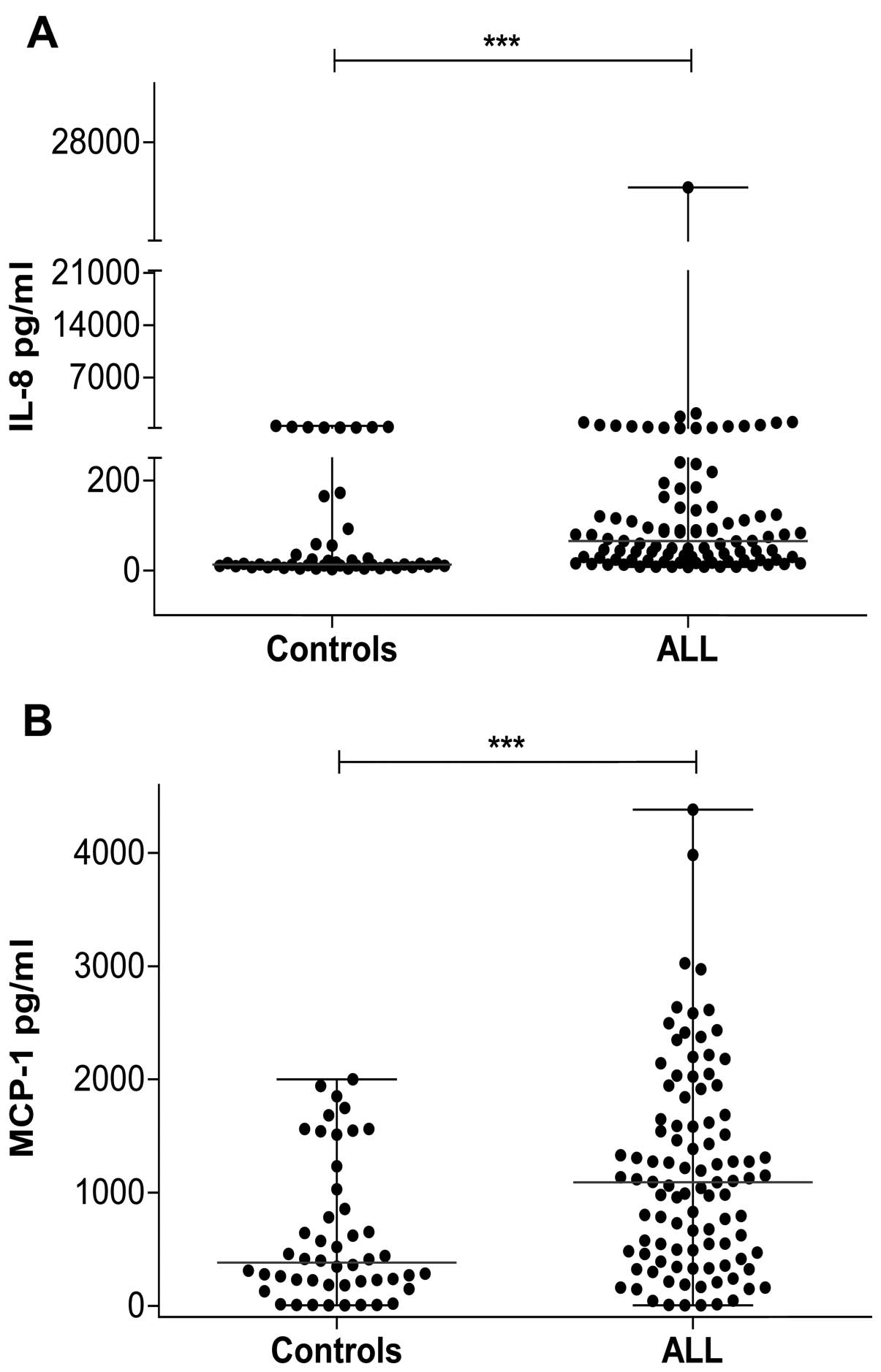
The concentration of circulating
pro-inflammatory chemokines is elevated in patients with ALL at
diagnosis
The pro-inflammatory cytokines IL-1β and TNF-α
induce the production of two important inflammatory chemokines that
participate in carcinogenesis, MCP-1 and IL-8. As we found elevated
levels of TNF-α in children with ALL, we were interested in
determining the levels of these chemokines in these patients. We
found that IL-8 levels were significantly higher in the ALL group
(median, 78.75 pg/ml; range, 4.80–24,937 pg/ml) than they were in
the control group (median, 13.03 pg/ml; range, 2.99–522 pg/ml)
(Fig. 3A). The concentration of
MCP-1 was also higher in the ALL group (median, 1,090 pg/ml; range,
4.28–4,382 pg/ml) compared with the control group (median, 381
pg/ml; range, 2.99–2,001 pg/ml) (Fig.
3B).
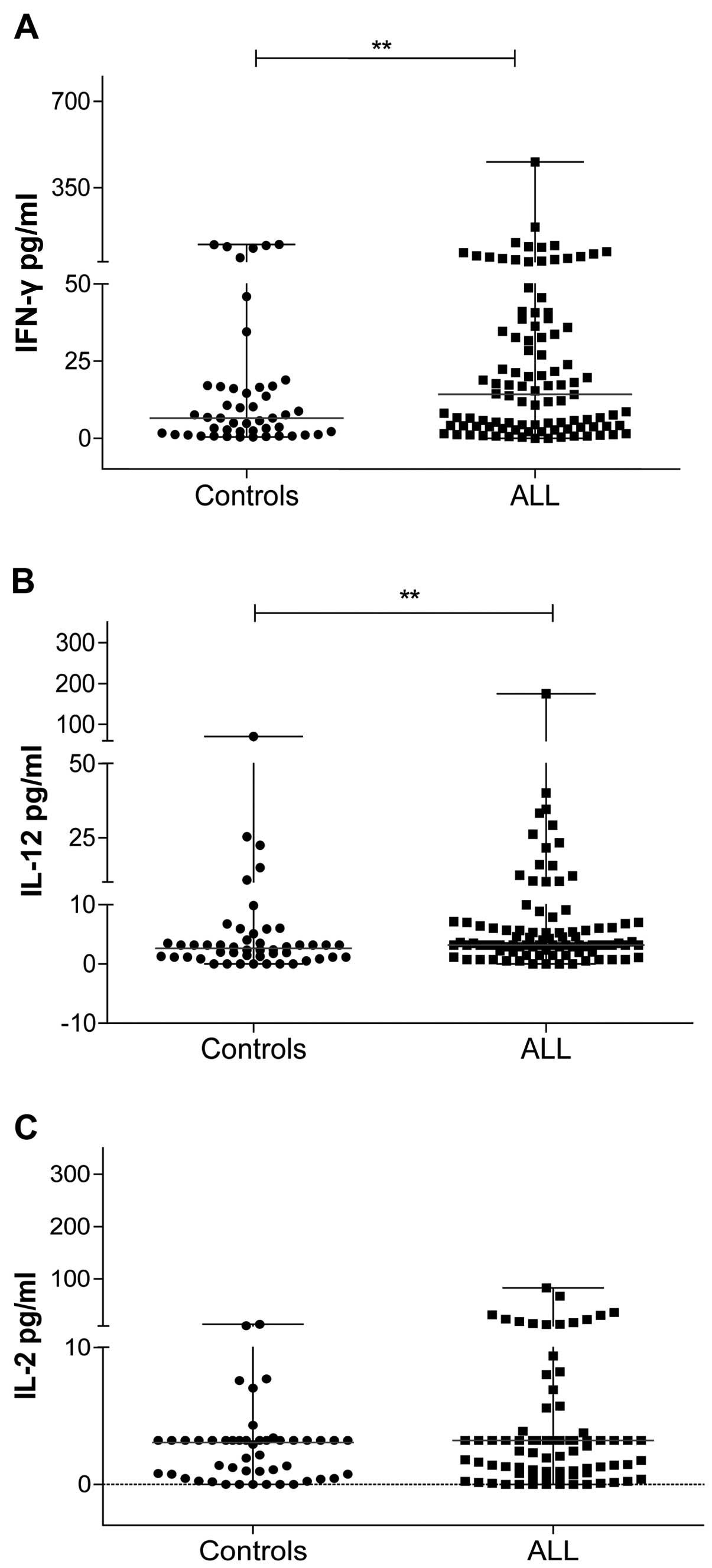
T-cell-polarizing cytokines in patients
with ALL at diagnosis
We also analyzed the levels of T-cell-polarizing
cytokines in patients with ALL without clinically apparent
infection. We analyzed Th1 cytokines such as IFN-γ, IL-12 and IL-2
(Fig. 4). The circulating levels of
IFN-γ and IL-12 were significantly higher in patients with ALL than
they were in the control group (IFN-γ in ALL: median, 6.50 pg/ml;
range, 0.0–456.60 pg/ml; IFN-γ in controls: median, 3.34 pg/ml;
range, 0.38–45.90 pg/ml; IL-12 in ALL: median, 3.20 pg/ml; range,
0.0–175.0 pg/ml; IL-12 in controls: median, 2.63 pg/ml; range,
0.0–70.56 pg/ml) (Fig. 4A and B).
The levels of IL-2 in patients with ALL (median, 3.20 pg/ml; range,
0.0–83.11 pg/ml) and in controls (median, 3.05 pg/ml; range,
0.0–13.61 pg/ml) were not significantly different (Fig. 4C).
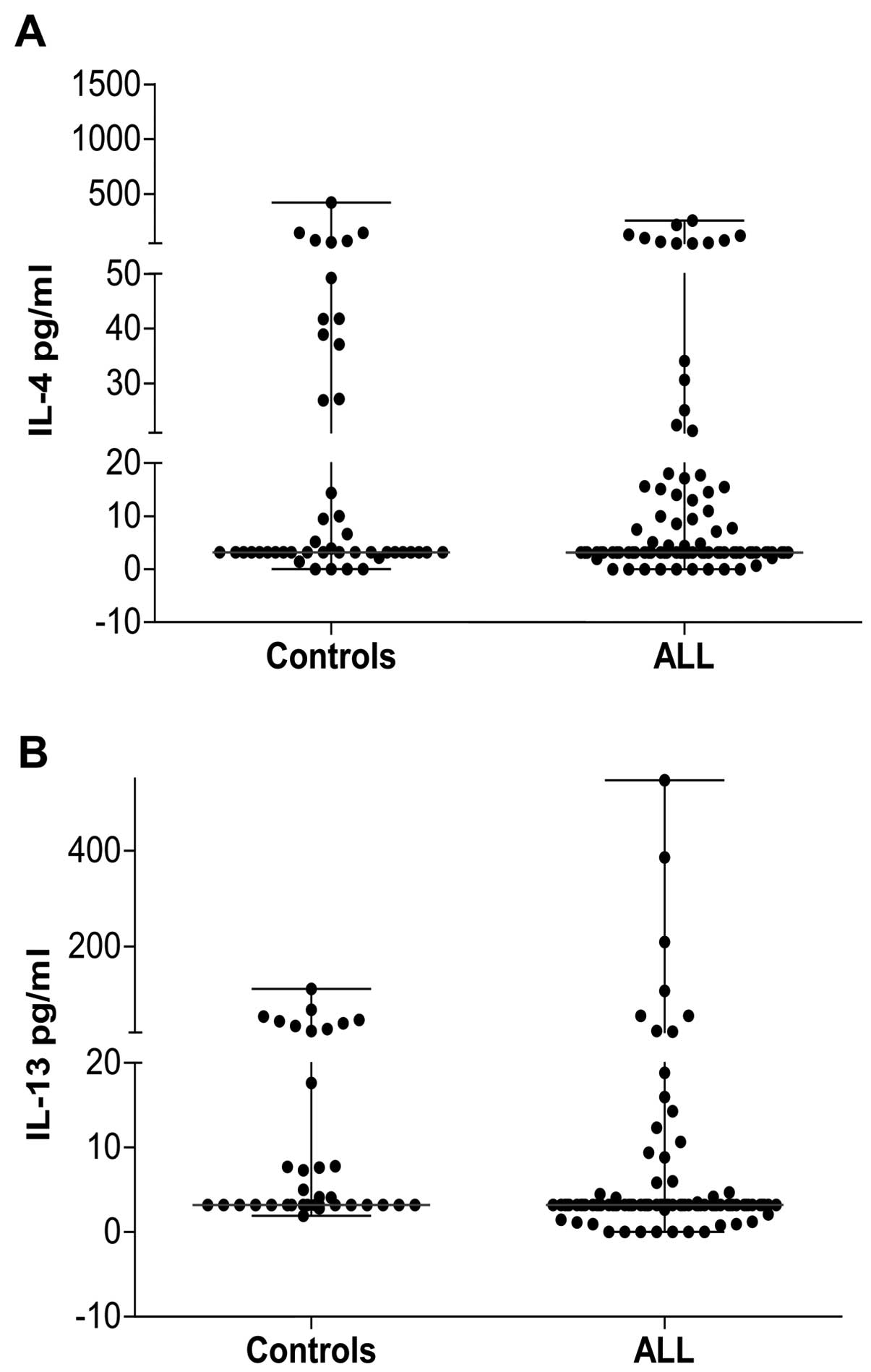
The levels of Th2 cytokines such as IL-4 and IL-13
were also measured to obtain an idea of the Th1/Th2 cytokine
balance in patients with leukemia. The level of IL-4 in patients
with ALL was not significantly different from that in the control
group. We found that the control and ALL groups had similar
circulating levels of this cytokine (Fig. 5A). Conversely, the level of IL-13
was lower in patients with ALL compared with the control group
(median, 3.20 vs. 3.20 pg/ml; range of IL-13 in patients with ALL,
0.0–547.10 pg/ml, but only three patient had a level >100 pg/ml;
range of IL-13 in the control group, 1.93–110.90 pg/ml) (Fig. 5B). The Th2 response in these
patients was decreased, thereby favoring the Th1 response.
Together, these results suggest an inclination toward a Th1 profile
in ALL, driven by increased Th1 and decreased Th2 cytokines in
patients with ALL.
Higher concentration of circulating
immunoregulatory cytokines in ALL at diagnosis
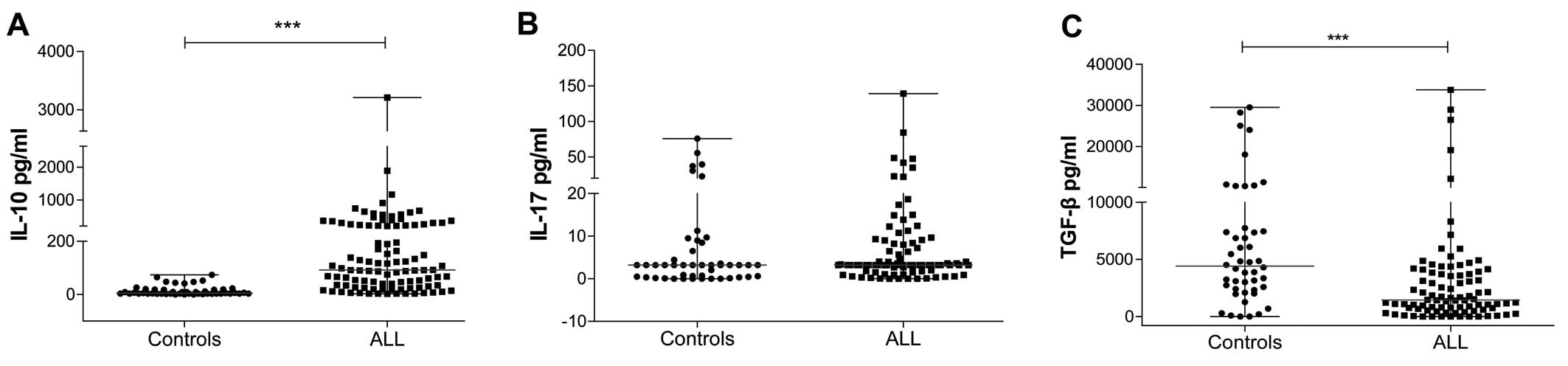
The circulating levels of IL-10 were higher in
patients with ALL than they were in the control group, with a
median of 91.81 vs. 5.15 pg/ml (range, 2.47–3,209 pg/ml for ALL vs.
0.0–73.63 pg/ml for the control group) (Fig. 6A). The IL-17 level was higher in ALL
than it was in the control group, with a median of 3.20 pg/ml
(range, 0.0–139.20 pg/ml) in ALL, while in the control group the
median was also 3.20 pg/ml but the range was 0.0–75.75 pg/ml
(Fig. 6B). However, TGF-β levels
were lower in patients with ALL than they were in the control group
(median, 1,438 vs. 4,407 pg/ml; range, 0.0–29,507 vs. 0.0–33,777
pg/ml) (Fig. 6C).
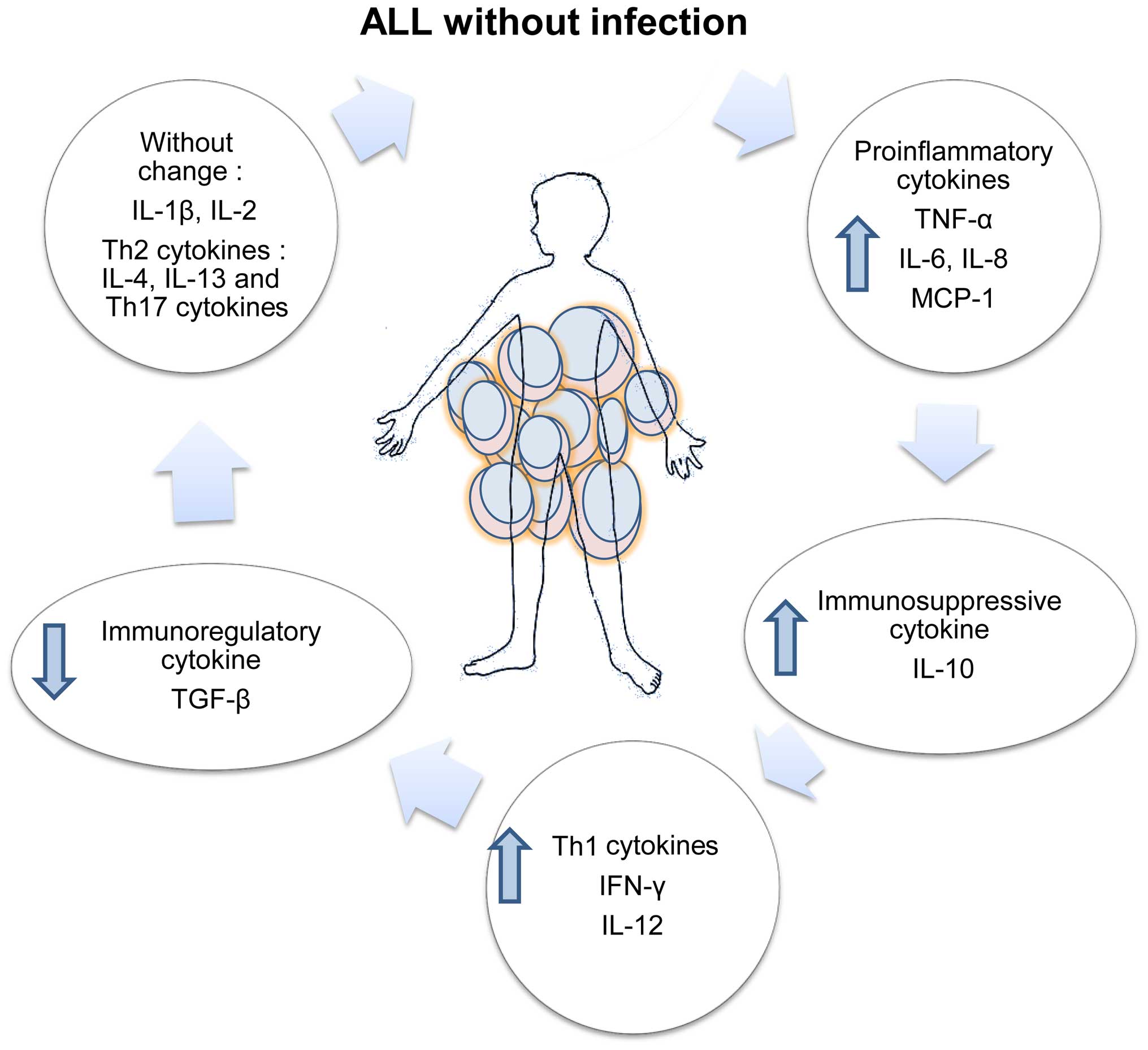
The balance of inflammatory, T cell-polarizing and
immunoregulatory cytokines produced in patients with ALL without
apparent infection was highly altered; a schematic representation
of this alteration is shown in Fig.
7.
Discussion
Cytokines are regulators of host responses to
infection, inflammation and trauma. Some cytokines promote
inflammation (pro-inflammatory cytokines), whereas others serve to
reduce inflammation and promote the healing of tissue
(anti-inflammatory cytokines). Thus, to maintain tissue homeostasis
and integrity, the levels of different cytokines are finely tuned
and controlled. The deregulation of cytokines plays an important
role in different pathologies, including cancer (10–12).
In this study, we showed that, at the systemic
level, there was an important pro-inflammatory condition at
diagnosis in children with ALL and no apparent infection, i.e.,
children for whom evidence of infection could not be obtained. We
report for the first time the higher concentrations of TNF-α and
IL-6 in patients with ALL with no infection, as elevated levels of
these cytokines have only been reported in the presence of
infectious processes (13,14), but not in sterile conditions. The
higher concentrations of TNF-α and IL-6 found in this research in
patients with ALL may be associated with the febrile state
presented by some of the children, possibly as a consequence of the
activation of the immune cells by DAMPs, which have the ability to
generate an immune response in the absence of infectious agents
(15). Different cellular signaling
pathways operate in response to varying levels of pro-inflammatory
cytokines, which can lead to genotoxic damage, cell apoptosis, or
cell growth. The increased concentration of pro-inflammatory
cytokines in the serum of patients with leukemia may favor a
neoplastic process. Moreover, IL-1β, TNF-α and IL-6 are
pro-inflammatory cytokines that can induce fever and can regulate
each other's levels, depending on their concentration (16).
Furthermore, we showed that this pro-inflammatory
state had characteristics of a Th1-polarized response. We found
that the concentrations of circulating TNF-α, IL-6, IL-8, MCP-1,
IL-12, IFN-γ and IL-10, were significantly increased in children
with ALL. In contrast, the concentrations of TGF-β were decreased
in ALL and those of IL-1β, IL-2, IL-4, IL-13 and IL-17 showed no
changes compared with the controls. This finding is significant
since it may reflect a sterile inflammation in ALL, as all children
included in this study did not show apparent infection at diagnosis
and had not received any antineoplastic treatment at the time of
collection of the blood samples. Recently, the levels of
circulating cytokines were studied in adult patients with ALL, and
IL-8 and MCP-1 were found to be elevated at diagnosis, thus
reflecting disease activity (17).
The results of our study suggest that the increases
in IL-6 and IL-8 in the absence of apparent infection are induced
by the activation of other molecules or mechanisms that are
independent of infectious microorganisms. IL-6 is important in the
maintenance of cancer stem cells in the neoplastic microenvironment
of leukemic cells (18) and has
been shown to stimulate the growth of AML (acute myeloid leukemia)
cells through signaling pathways (19).
Moreover, IL-6 has been reported as a sensitive
predictor of bacterial infection in neutropenic as well as
non-neutropenic febrile children with ALL (20); in addition, a study of the role of
IL-6 and IL-8 in a small group of patients with hematological
malignancies showed that they could be useful in excluding the
possibility of high-risk infection (21). IL-8 in the serum of patients with
ALL in association with Gram-negative infection was found to be
increased by 10-fold compared with the control group (22).
In the present study, we found that high levels of
the monocyte chemoattractant protein-1 (MCP-1/CCL2), which is a
member of the CC chemokine family, was significantly altered in
these patients and may contribute to a sterile inflammatory
environment. MCP-1 has previously been shown to play a major role
in the migration of monocytes toward human leukemic cells; however,
it was unable to increase the cytotoxic effects of monocytes on
human leukemic cells (23). A
higher level of MCP-1 in the cerebrospinal fluid of children with
ALL is associated with central nervous system involvement during
therapy (24).
There are reports of a high concentration of various
inflammatory cytokines in ALL associated with the presence of
infection or in response to chemotherapy (25–28).
However, there are no reports of an initial inflammatory process in
newly diagnosed patients with ALL without apparent infection.
The regulation of the immune response is important
in the process of carcinogenesis, as a deregulated pro-inflammatory
state may favor cancer, and inflammation also affects immune
surveillance. Cytokines such as IL-10, IL-17 and TGF-β have an
important function in immune regulation. The biological role of
IL-10 in cancer is quite complex; however, the presence of IL-10 in
advanced metastases and the positive correlation between serum
IL-10 levels and the progression of other diseases suggest a
critical role for IL-10 in the cancer micro-environment (29).
IL-17 is a cytokine that is produced by a subset of
T helper cells, called 'Th17 cells', which are generated in
response to signals from TGF-β, IL-6 and IL-23 (30,31).
IL-17 activates many of the same signaling events as do
inflammatory cytokines such as TNF-α and IL-1β, and is considered
to be an important bridging molecule between the innate and
adaptive immune systems (32). A
study of the role of this cytokine in leukemia showed that it is
increased in the blood and bone marrow in AML patients with poor
prognosis compared with healthy donors (33). However, in this study, we did not
find significant difference between patients with ALL and the
control group. The role of IL-12 in immunosurveillance has been
reported in cancer (34); in ALL,
the study of this cytokine has been focused on immunotherapy
(35,36). Our results indicate that IL-12 and
IFN-γ were elevated in patients with ALL compared with the control
group; thus, there was a Th1-biased immune response in patients
with this neoplasia.
Elevated systemic concentrations of these Th1
cytokines may promote an inflammatory microenvironment in the early
stages of neoplasia. Alterations in the Th1/Th2 ratio in cancer
patients is a common feature of a malignant process and may be the
result of a malfunction of Th1 cells, activation of Th2
lymphocytes, or both. We found no change in concentrations of IL-4
and IL-13 (Th2 cytokines) in patients with ALL vs. the controls,
which confirmed a predominantly Th1-type response in the
patients.
However, in patients with neutropenic fever, therapy
is and should remain restricted to antibiotics, even though it is
very likely that at least some of these patients do not have an
infectious process that causes fever, and that the
inflammatory-state-related cancer is the cause of the fever, which
opens the possibility of seeking a non-infectious marker that
induces cytokines associated with fever and with a strong
pro-inflammatory response in children with ALL. Further
investigation is needed to establish whether these alterations can
be used as a prognostic indicator in ALL.
The findings of this study have important
implications for understanding the inflammatory response in
patients with ALL and no clinically apparent infection, and the
bridge between the innate and adaptive immune responses through
T-cell polarization. Higher levels of cytokines may play a crucial
role in the pathogenesis of ALL and may be important therapeutic
targets and prognostic predictors in episodes of fever of unknown
origin and in strong inflammatory responses without apparent
infection.
Abbreviations:
|
ALL
|
acute lymphoblastic leukemia
|
|
TNF-α
|
tumor necrosis factor α
|
|
IFN-γ
|
interferon-γ
|
|
IL-1
|
interleukin-1
|
|
TGF-β
|
transforming growth factor β
|
|
MCP-1
|
monocyte chemoattractant protein-1
|
|
Th
|
T helper
|
Acknowledgments
This study was supported by Hospital Infantil de
México Federico Gómez, grant HIM/2011/22 and by Terry Fox
Fundation, grant HIM/2010/076. Erandi Pérez-Figueroa is a doctoral
student from Programa de Doctorado en Ciencias Biológicas,
Universidad Nacional Autónoma de México (UNAM) and received a
fellowship from CONACYT.
References
|
1
|
Gatta G, Botta L, Rossi S, Aareleid T,
Bielska-Lasota M, Clavel J, Dimitrova N, Jakab Z, Kaatsch P, Lacour
B, et al EUROCARE Working Group: Childhood cancer survival in
Europe 1999–2007: Results of EUROCARE-5 - a population-based study.
Lancet Oncol. 15:35–47. 2014. View Article : Google Scholar
|
|
2
|
Landskron G, De la Fuente M, Thuwajit P,
Thuwajit C and Hermoso MA: Chronic inflammation and cytokines in
the tumor microenvironment. J Immunol Res. 2014:1491852014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen GY and Nuñez G: Sterile inflammation:
Sensing and reacting to damage. Nat Rev Immunol. 10:826–837. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang Q, Shi Y, Yang Y, Lou G and Chen Z:
The sterile inflammation in the exacerbation of HBV-associated
liver injury. Mediators Inflamm. 2015:5086812015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Demaria S, Pikarsky E, Karin M, Coussens
LM, Chen YC, El-Omar EM, Trinchieri G, Dubinett SM, Mao JT, Szabo
E, et al: Cancer and inflammation: Promise for biologic therapy. J
Immunother. 33:335–351. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Agyeman P, Kontny U, Nadal D, Leibundgut
K, Niggli F, Simon A, Kronenberg A, Frei R, Escobar H, Kühne T, et
al: A prospective multicenter study of microbiologically defined
infections in pediatric cancer patients with fever and neutropenia:
Swiss Pediatric Oncology Group 2003 fever and neutropenia study.
Pediatr Infect Dis J. 33:e219–e225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khurana M, Lee B and Feusner JH: Fever at
diagnosis of pediatric acute lymphoblastic leukemia: Are
antibiotics really necessary? J Pediatr Hematol Oncol. 37:498–501.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Santolaya ME, Alvarez AM, Becker A, Cofré
J, Enríquez N, O'Ryan M, Payá E, Pilorget J, Salgado C, Tordecilla
J, et al: Prospective, multicenter evaluation of risk factors
associated with invasive bacterial infection in children with
cancer, neutropenia, and fever. J Clin Oncol. 19:3415–3421.
2001.PubMed/NCBI
|
|
9
|
Santolaya ME, Farfán MJ, De La Maza V,
Cociña M, Santelices F, Alvarez AM, Avilés CL, Becker A, O'Ryan M,
Román P, et al: Diagnosis of bacteremia in febrile neutropenic
episodes in children with cancer: Microbiologic and molecular
approach. Pediatr Infect Dis J. 30:957–961. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Barrera L, Montes-Servín E, Barrera A,
Ramírez-Tirado LA, Salinas-Parra F, Bañales-Méndez JL,
Sandoval-Ríos M and Arrieta Ó: Cytokine profile determined by
data-mining analysis set into clusters of non-small-cell lung
cancer patients according to prognosis. Ann Oncol. 26:428–435.
2015. View Article : Google Scholar
|
|
11
|
Mojtahedi Z, Khademi B, Erfani N, Taregh
Y, Rafati Z, Malekzadeh M and Ghaderi A: Serum levels of
interleukin-7 and interleukin-8 in head and neck squamous cell
carcinoma. Indian J Cancer. 51:227–230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sato T, Terai M, Tamura Y, Alexeev V,
Mastrangelo MJ and Selvan SR: Interleukin 10 in the tumor
microenvironment: A target for anticancer immunotherapy. Immunol
Res. 51:170–182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Buyukberber N, Buyukberber S, Sevinc A and
Camci C: Cytokine concentrations are not predictive of bacteremia
in febrile neutropenic patients. Med Oncol. 26:55–61. 2009.
View Article : Google Scholar
|
|
14
|
Fleischhack G, Cipic D, Juettner J, Hasan
C and Bode U: Procalcitonin-a sensitive inflammation marker of
febrile episodes in neutropenic children with cancer. Intensive
Care Med. 26(Suppl 2): S202–S211. 2000.
|
|
15
|
Castellheim A, Brekke OL, Espevik T,
Harboe M and Mollnes TE: Innate immune responses to danger signals
in systemic inflammatory response syndrome and sepsis. Scand J
Immunol. 69:479–491. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dinarello CA: Interleukin-1, interleukin-1
receptors and interleukin-1 receptor antagonist. Int Rev Immunol.
16:457–499. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Horacek JM, Kupsa T, Vasatova M, Jebavy L
and Zak P: Evaluation of serum levels of multiple cytokines and
adhesion molecules in patients with newly diagnosed acute
lymphoblastic leukemia using biochip array technology. Exp Oncol.
35:229–230. 2013.PubMed/NCBI
|
|
18
|
Zhao C, Chen A, Jamieson CH, Fereshteh M,
Abrahamsson A, Blum J, Kwon HY, Kim J, Chute JP, Rizzieri D, et al:
Hedgehog signalling is essential for maintenance of cancer stem
cells in myeloid leukaemia. Nature. 458:776–779. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Su YC, Li SC, Wu YC, Wang LM, Chao KS and
Liao HF: Resveratrol downregulates interleukin-6-stimulated sonic
hedgehog signaling in human acute myeloid leukemia. Evid Based
Complement Alternat Med. 2013:5474302013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Abrahamsson J, Påhlman M and Mellander L:
Interleukin 6, but not tumour necrosis factor-alpha, is a good
predictor of severe infection in febrile neutropenic and
non-neutropenic children with malignancy. Acta Paediatr.
86:1059–1064. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Diepold M, Noellke P, Duffner U, Kontny U
and Berner R: Performance of interleukin-6 and interleukin-8 serum
levels in pediatric oncology patients with neutropenia and fever
for the assessment of low-risk. BMC Infect Dis. 8:282008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu W, Jia Y, Du S, Tang H, Sun Y and Sun
L: Changes of sulfur dioxide, nuclear factor-κB, and interleukin-8
levels in pediatric acute lymphoblastic leukemia with bacterial
inflammation. Chin Med J (Engl). 127:4110–4113. 2014.
|
|
23
|
Civini S, Jin P, Ren J, Sabatino M,
Castiello L, Jin J, Wang H, Zhao Y, Marincola F and Stroncek D:
Leukemia cells induce changes in human bone marrow stromal cells. J
Transl Med. 11:2982013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eisenkraft A, Keidan I, Bielorai B, Keller
N, Toren A and Paret G: MCP-1 in the cerebrospinal fluid of
children with acute lymphoblastic leukemia. Leuk Res. 30:1259–1261.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bertolizio G, Stucchi R, Sahillioglu E,
Somaini M, Dander E, Biondi A, Jankovic M, D'Amico G and Ingelmo
PM: The effects of propofol and ketamine on the cytokine levels of
children with acute lymphoblastic leukemia. J Pediatr Hematol
Oncol. 35:e296–e300. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Du S, Jia Y, Tang H, Sun Y, Wu W, Sun L,
Du J, Geng B, Tang C and Jin H: Immune regulation of hydrogen
sulfide in children with acute lymphoblastic leukemia. Chin Med J
(Engl). 127:3695–3699. 2014.
|
|
27
|
Hatzistilianou M, Rekliti A, Athanassiadou
F and Catriu D: Procalcitonin as an early marker of bacterial
infection in neutropenic febrile children with acute lymphoblastic
leukemia. Inflamm Res. 59:339–347. 2010. View Article : Google Scholar
|
|
28
|
Matti BF, Saleem MA and Sabir SF:
Assessment of interleukin 1β serum level in different responder
groups and stages of chronic myeloid leukemia patients on imatinb
mesylate therapy. Indian J Hematol Blood Transfus. 30:247–252.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang S, Sun M, Gu C, Wang X, Chen D, Zhao
E, Jiao X and Zheng J: Expression of CD163, interleukin-10, and
interferon-gamma in oral squamous cell carcinoma: Mutual
relationships and prognostic implications. Eur J Oral Sci.
122:202–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Weaver CT, Hatton RD, Mangan PR and
Harrington LE: IL-17 family cytokines and the expanding diversity
of effector T cell lineages. Annu Rev Immunol. 25:821–852. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Weaver CT: Th17: The ascent of a new
effector T-cell subset. Preface Eur J Immunol. 39:634–636. 2009.
View Article : Google Scholar
|
|
32
|
Kolls JK and Lindén A: Interleukin-17
family members and inflammation. Immunity. 21:467–476. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Han Y, Ye A, Bi L, Wu J, Yu K and Zhang S:
Th17 cells and interleukin-17 increase with poor prognosis in
patients with acute myeloid leukemia. Cancer Sci. 105:933–942.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zitvogel L and Kroemer G:
CD103+ dendritic cells producing interleukin-12 in
anticancer immunosurveillance. Cancer Cell. 26:591–593. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lehmann D, Spanholtz J, Osl M, Tordoir M,
Lipnik K, Bilban M, Schlechta B, Dolstra H and Hofer E: Ex vivo
generated natural killer cells acquire typical natural killer
receptors and display a cytotoxic gene expression profile similar
to peripheral blood natural killer cells. Stem Cells Dev.
21:2926–2938. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pegram HJ, Purdon TJ, van Leeuwen DG,
Curran KJ, Giralt SA, Barker JN and Brentjens RJ: IL-12-secreting
CD19-targeted cord blood-derived T cells for the immunotherapy of
B-cell acute lymphoblastic leukemia. Leukemia. 29:415–422. 2015.
View Article : Google Scholar
|