Introduction
Multiple myeloma (MM) is a malignant disorder that
is characterized by the proliferation of a single clone of plasma
cells, which are derived from B cells in the bone marrow (1). The incidence of MM varies globally
from 1 per 100,000 people in China to approximately 4 per 100,000
people in most developed countries (2). Advances in therapies for MM, such as
high-dose therapy followed by autologous stem cell transplantation
(ASCT), have been shown to improve response rates, event-free
survival, and overall survival (3).
However, the molecular mechanisms by which the myeloma
microenvironment influences myeloma cell survival and its
responsiveness to therapy remain unclear. Previous investigations
have demonstrated alterations in the phosphatidylinositol 3-kinase
(PI3K)/Akt signaling cascades in MM cells and implicated the
pathway in clonal expansion (4,5).
Akt is a serine (Ser)/threonine (Thr) protein kinase
that resides within the cytosol in a catalytically inactive state
in quiescent or serum-starved cells (6). Activated Akt phosphorylates downstream
target molecules, including Bcl-2, caspase-9, and Bad, which
promote induction of its anti-apoptotic effects (5). The PI3K/Akt pathway is one of many
signaling pathways that play oncogenic roles in a wide spectrum of
human cancers (7). Several studies
have strongly suggested that Akt signaling mediates MM cell
resistance to conventional therapeutics, and biologically based
treatments targeting Akt may induce anti-MM activity in the bone
marrow microenvironment (8).
However, the specific role of the activation of Akt pathway in the
oncogenic processes involved in multiple myeloma is not completely
understood.
Hydrogen sulfide (H2S), a toxic gas that
smells like rotten eggs, forms with nitric oxide (NO) and carbon
monoxide (CO) a group of biologically active gases that are termed
gasotransmitters or gasomediators (9,10).
H2S is endogenously generated from L-cysteine by
pyridoxal-5′ phosphate-dependent enzymes, including cystathionine
β-synthase (CBS) and/or cystathionine γ-lyase (CSE), in mammalian
cells (11). Work over the last
decade has recognized the importance of endogenously produced
H2S in a variety of biological functions in the nervous,
cardiovascular, and immune systems (10,12).
There is currently no information available
regarding the effect of exogenous H2S on multiple
myeloma or its related mechanisms. The present study is aimed at
investigating whether H2S contributes to cancer progress
and at exploring whether its effects involve the amplification of
the Akt pathway in multiple myeloma cells.
Materials and methods
Patients
Twenty MM patients (11 males and 9 females) were
included in this study. Their median age was 57 years (range: 37–70
years). According to international staging system (ISS), 4 were
classified as stage I, 7 as stage II and 9 as stage III. Concerning
the types of monoclonal proteins present in the patients, 12 had
IgG, 5 had IgA and 3 had light chain disease. Fifteen age- and
gender-matched healthy subjects were the control group. This work
was performed in accordance with the guidelines of the Declaration
of Helsinki. This study was cleared by our Institutional Ethics
Review Board for human studies, and all subjects signed an informed
consent document. After informed consent was provided, peripheral
venous blood was collected in sterile tubes using EDTA as
anticoagulant and centrifuged at 1000 g for 10 min within 30 min of
collection. Plasma was extracted in the supernatant and stored at
−70°C for H2S determination.
Reagents
Sodium hydrosulfide (NaHS) and LY294002 were
obtained from Sigma-Aldrich (St. Louis, MO, USA). Freshly made NaHS
solution was used as the H2S donor. The Cell Counting
Kit 8 (CCK-8) was supplied by Dojindo Laboratories (Kumamoto,
Japan). Fetal bovine serum (FBS) and DMEM medium were obtained from
Gibco BRL (Grand Island, NY, USA). Anti- phosphorylated-Akt (p-Akt)
antibody, and Anti-total Akt (t-Akt) antibody, anti-Bcl-2 antibody
and anti-caspase-3 antibody were purchased from Cell Signaling
Technology (Danvers, MA, USA).
Cell culture
The human myeloma cell line NCI-H929 was supplied by
the Sun Yat-sen University Cancer Center (Guangzhou, Guangdong,
China) and maintained in DMEM medium supplemented with 10% FBS, 100
µg/ml streptomycin and 100 IU/ml penicillin at 37°C in a 5%
CO2 incubator. The NCI-H929 cells were treated with 500
µmol/l NaHS for 24 h or co-treated with 500 µmol/l
NaHS and 50 µmol/l LY294002 for 24 h.
Cell proliferation assay
NCI-H929 cells were cultured in 96-well tissue
culture plates (2×104 cells per well) in DMEM
supplemented with 10% FBS for 24 h. Then, the cells were exposed to
different concentrations of NaHS (500 and 1,000 µmol/l) for
24 h. Cell proliferation was measured by CCK-8 assay kit. Briefly,
10 µl CCK-8 solution was added to each well, and the plates
were incubated for an additional 2 h. Absorbance was measured using
a spectrometer at a wave length of 450 nm. The means of the optical
density (OD) of three wells in the indicated groups were used to
calculate the percentage of cells that were viable according to the
formula: cell viability (%) = (OD treatment group/OD control group)
×100%. The experiments were performed three times.
Cell cycle analysis by flow
cytometry
Cells were plated in 6-well plates at a density of
2×106 cells per well and grown in serum-free medium.
Then, the cells were exposed to different concentrations of NaHS or
co-treated with 500 µmol/l NaHS and 50 µmol/l
LY294002. After 24 h, the cell cycle analysis was performed. The
cells were harvested and fixed in 70% ethanol at 4°C overnight. The
fixed cells were washed twice with PBS, treated with RNase A (50
µg/ml) for 30 min at room temperature, and then stained with
propidium iodide. The stained cells were examined to analyze for
the cell cycle using a Beckman Coulter XL instrument (Beckman
Coulter, Brea, CA, USA).
Western blot analysis
As described above, the cells were harvested and
lysed using RIPA lysis buffer supplemented with protease
inhibitors. Total proteins were extracted and quantified using a
bicinchoninic acid (BCA) protein assay kit. Loading buffer was
added to the cytosolic extracts and the solution was then boiled
for 5 min. The same amount of supernatant was obtained from each
sample and fractionated using 10% sodium dodecyl
sulphate-polyacrylamide gel electrophoresis (SDS-PAGE), and the
total proteins were then transferred to polyvinylidene difluoride
(PVDF) membranes. The membranes were blocked in 5% fat-free milk in
fresh blocking buffer [0.1% Tween-20 in Tris-buffered saline
(TBS-T)] for 60 min at room temperature. They were then incubated
in either anti-t-Akt antibody (1:1,000), anti-p-Akt antibody
(1:1,000), anti-Bcl-2 antibody (1:1,000), or anti-caspase-3
antibody (1:1,000) in freshly prepared TBS-T containing 3% free-fat
milk overnight with gentle agitation at 4°C. The membranes were
washed three times for 5 min each with TBS-T and then incubated
with HRP-conjugated goat anti-rabbit secondary antibody (1:3,000)
in TBS-T containing 3% fat-free milk for 1.5 h at room temperature.
Then, the membranes were washed three times for 5 min each in
TBS-T. The immunoreactive proteins were visualized using ECL
reagent. To quantify protein expression levels, the X-ray film was
scanned and analyzed using ImageJ 1.47i software. The experiments
were performed 3 times.
ELISA assay
The concentrations of H2S in plasma
samples were determined using ELISA (Quantikine R&D System,
Minneapolis, MN, USA) according to the manufacturer's instructions.
Briefly, these assays involved the application of the quantitative
sandwich enzyme immunoassay technique. Monoclonal antibodies that
were specific for each assay were pre-coated onto microplates.
Standard controls and samples (100 µl of plasma) were
pipetted into the wells in duplicate. After H2S was
bound and the plates were washed, an enzyme-linked polyclonal
antibody that was specific for H2S was added to each
well. After the plates were thoroughly washed, a substrate solution
was added to the wells, and the color developed in proportion to
the amount of H2S that was bound during the first step.
The optical density of each well was determined using a microplate
reader at 450 nm. The value for the blank was subtracted from both
the standard controls and the samples. A standard curve was created
by plotting the logarithm of the mean absorbance of each standard
versus the logarithm of the known H2S concentration.
Concentrations are shown as picograms per milliliter. The
experiments were repeated 3 times.
Transwell migration assay
Human myeloma cells were harvested and washed twice
with PBS. After the cells were washed, 1×105 cells were
resuspended in 200 µl DMEM and added to the upper chamber of
the transwell membrane (Transwell Permeable Support with a
5.0-µm polycarbonate membrane, 6.5-mm insert, and 24-well
plate; Corning Costar, Tewksbury, MA, USA), and 600 µl of
10% FBS-DMEM was added to each bottom chamber. Four upper chamber
conditions were included in the assay: 1) control, 2) NaHS (500
µmol/l), 3) NaHS (500 µmol/l) + LY294002 (50
µmol/l), and 4) LY294002 (50 µmol/l). After 24 h of
incubation at 37°C, the cells that had migrated to the lower
chambers were counted. Triplicate experiments were performed for
each group, and the means and standard deviations were
calculated.
Statistical analysis
The results are expressed as mean ± SD. Differences
between groups were analyzed using one-way ANOvA followed by LSD
post-hoc comparison tests in SPSS 13.0 (SPSS, Chicago, IL, USA)
software. Significance was established at the P<0.05 level.
Results
Hydrogen sulfide concentration in the
serum of MM patients is significantly higher than in healthy
controls
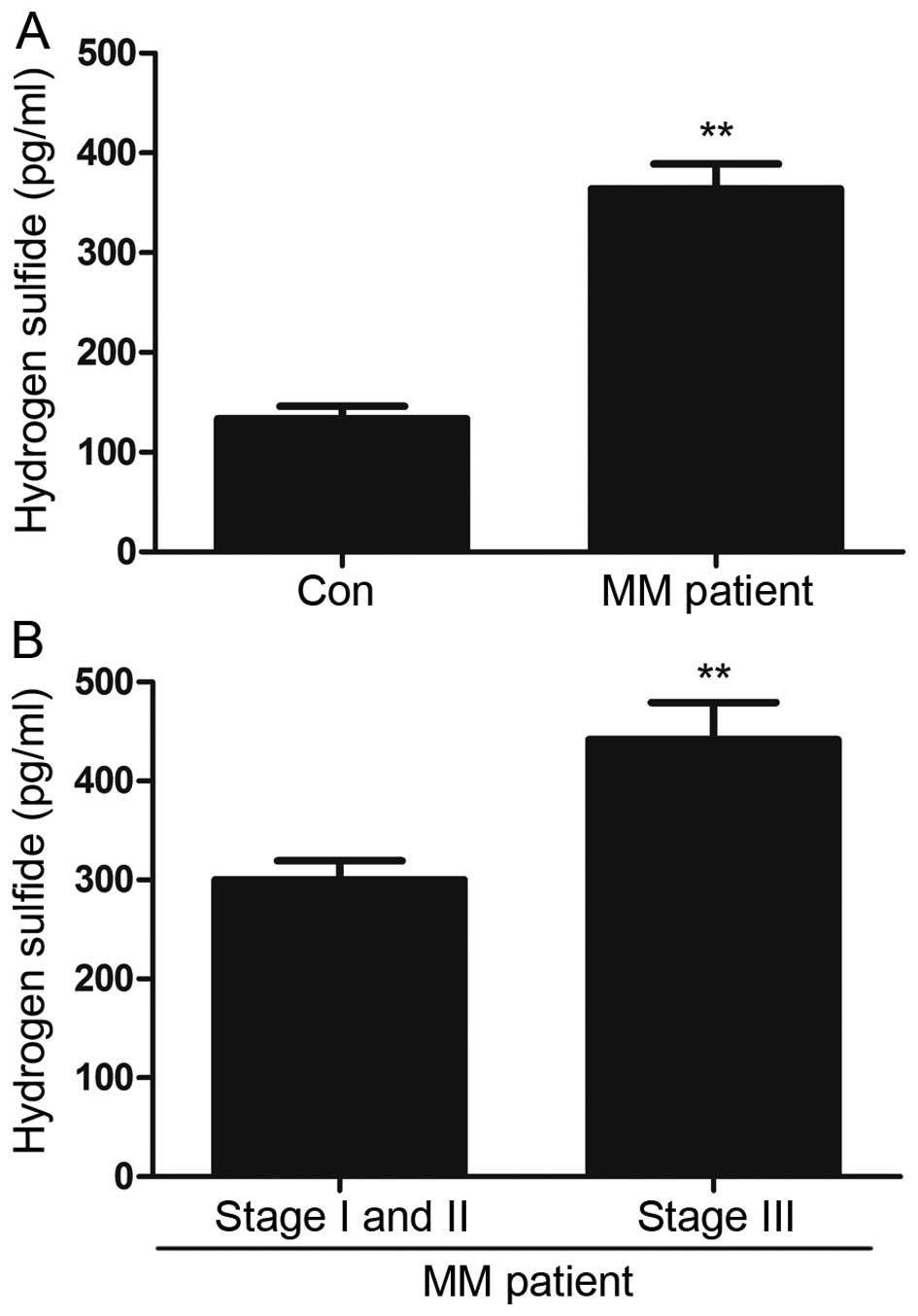
All measured parameters were significantly higher in
MM patients than in healthy controls. Furthermore, they increased
in parallel with disease progression, with higher values observed
in more advanced ISS stages. Higher serum levels of hydrogen
sulfide were observed in MM patients than in controls, and these
levels increased as the disease advanced. Serum H2S
concentrations in patients with MM were significantly elevated
compared to the controls (P<0.01) (Fig. 1A). Furthermore, statistically
significant differences were found in the levels of H2S
between the stage I-II and stage III MM patient groups (P<0.01)
(Fig. 1B).
NaHS promotes cell proliferation in
multiple myeloma cells
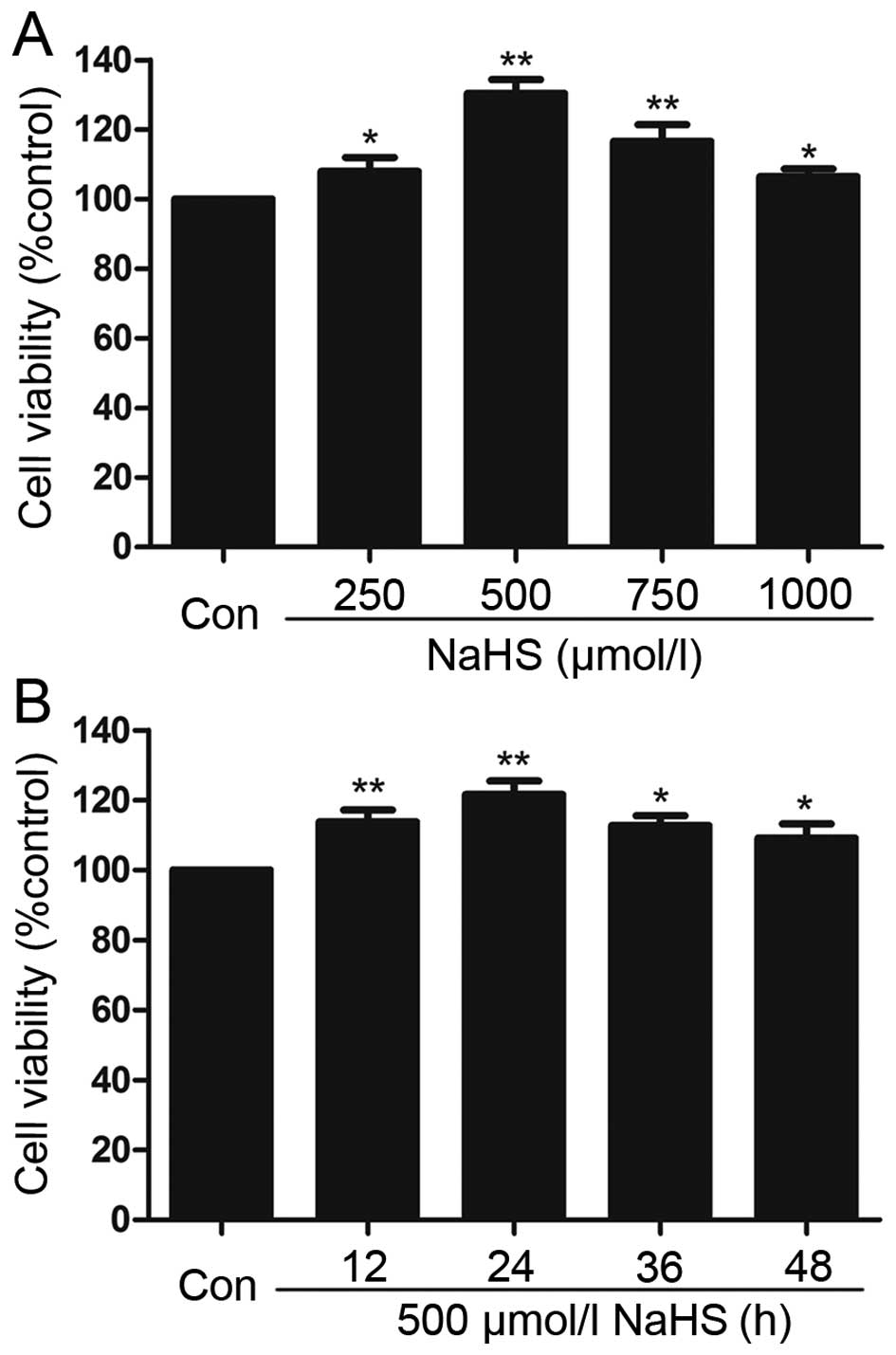
To test the effect of exogenous H2S on
human myeloma cell proliferation, a dose-response study was
performed using varying doses (250, 500, 750 and 1,000
µmol/l) of NaHS (a donor of H2S) for 24 h to
calculate the most effective dose of NaHS (Fig. 2A). The doses of NaHS ranging between
250 and 1,000 µmol/l markedly promoted cell proliferation,
leading to an increase in cell viability that reached a peak at 500
µmol/l. Therefore, 500 µmol/l NaHS was used in the
subsequent time-response study in which we analyzed the impact of
different treatment times (12, 24, 36 and 72 h). As shown in
Fig. 2B, treatment of myeloma cells
with 500 µmol/l NaHS for all of the indicated times markedly
promoted cell proliferation, which reached a maximal proliferative
effect at 24 h. Based on the above results, myeloma cells were
treated with 500 µmol/l NaHS for 24 h in all of the
following experiments.
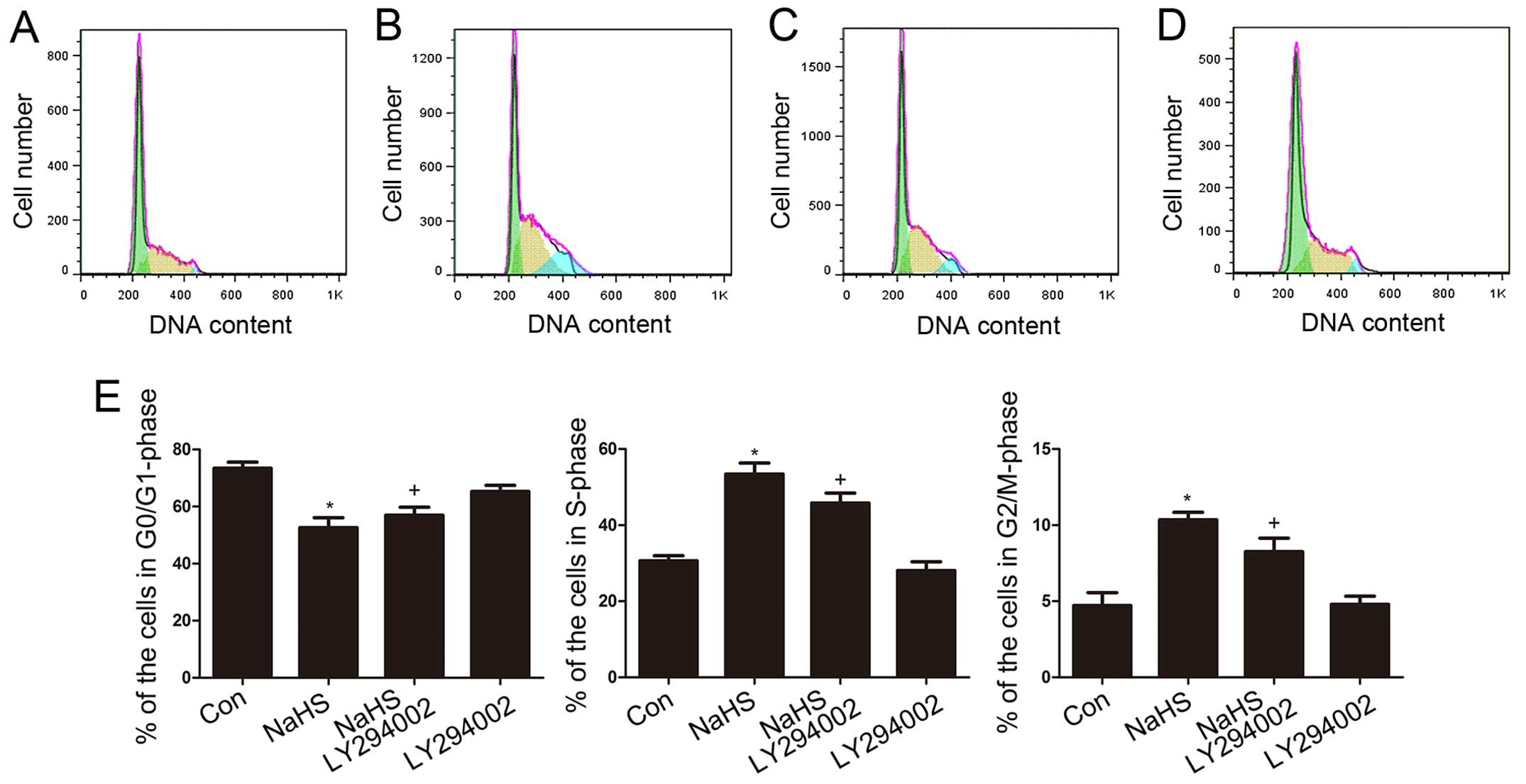
NaHS accelerates cell cycle progression
in multiple myeloma cells
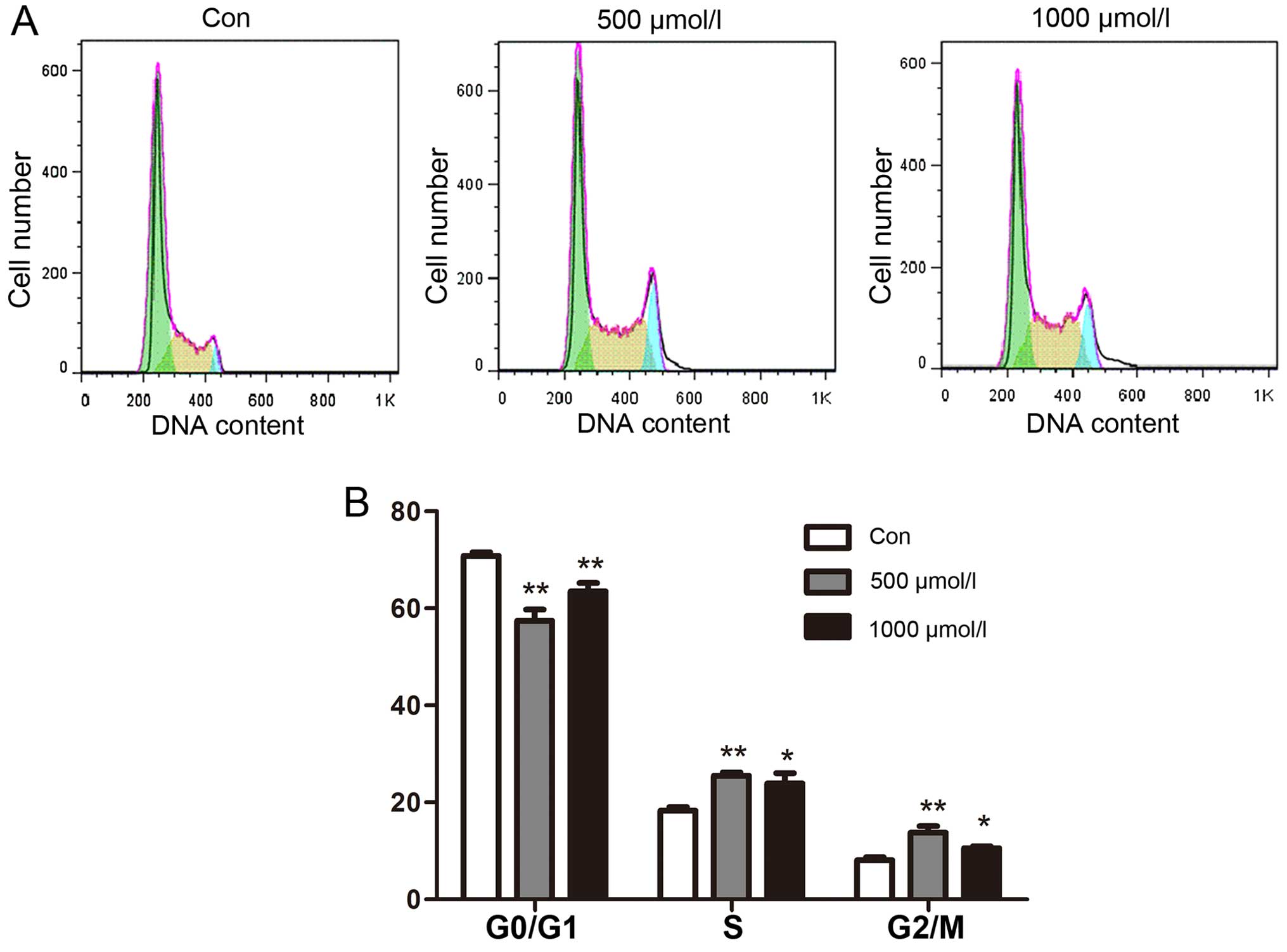
To investigate the effect of exogenous
H2S on cell cycle progression in human myeloma cells,
effects on the cell cycle were analyzed using flow cytometry. The
results showed that cell cycle progress was markedly altered when
the cells were treated with NaHS. The results indicated that in
myeloma cells, NaHS reduced cell cycle arrest in the G0/G1 phase
and increased the proportion of cells in the S and G2/M phases
(Fig. 3A).
To compare the experimental results more
intuitively, we drew histograms. The percentage of cells in the
different cell cycle phases after exposure to NaHS was compared to
the corresponding percentage in the untreated controls at the same
time point. The results revealed a dose-dependent trend in which
the proportion of cells in the G0/G1 phase decreased and the
proportion of cells in the S phase and G2/M phases increased as the
NaHS concentration increased. The most obvious change was observed
at the 24 h time point when cells were treated with 500
µmol/l NaHS (Fig. 3B).
NaHS amplifies the activation of Akt in
multiple myeloma cells
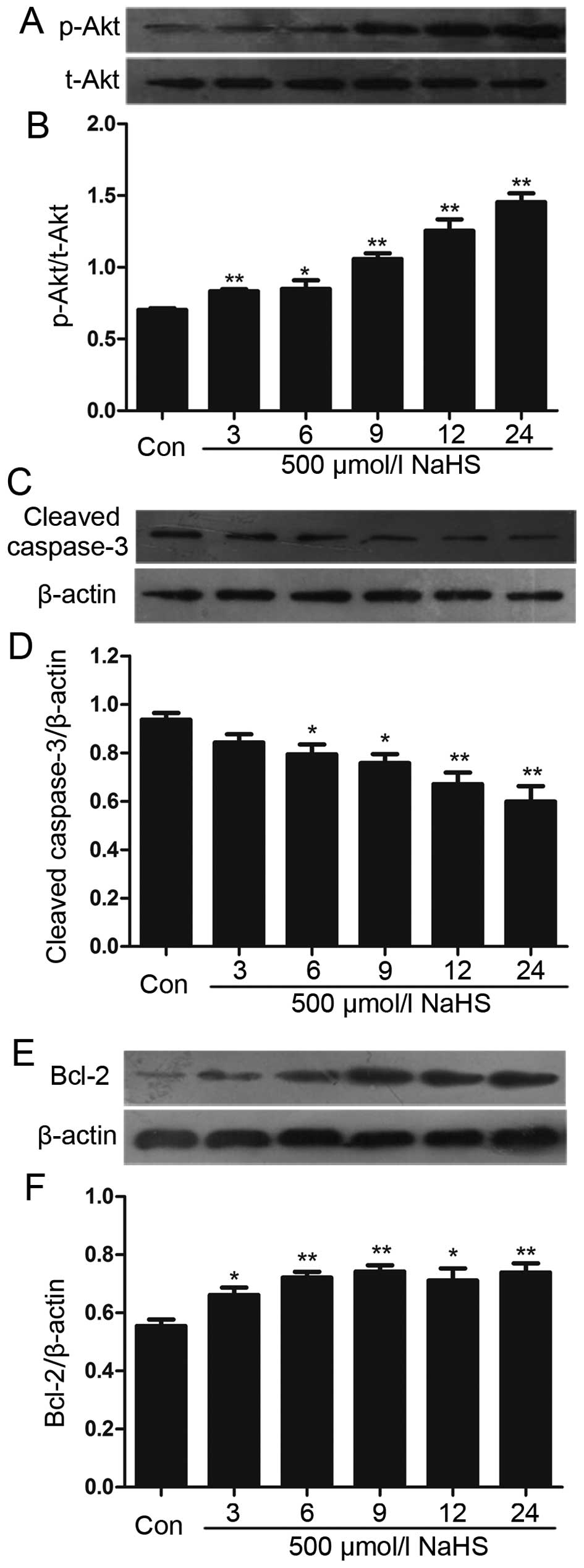
We analyzed the effects of NaHS on the level of Akt
phosphorylation. Multiple myeloma cells were exposed to 500
µmol/l NaHS for the indicated times (3, 6, 9, 12 and 24 h),
and the expression level of p-Akt was significantly upregulated,
reaching a peak at 24 h (Fig. 4A and
B).
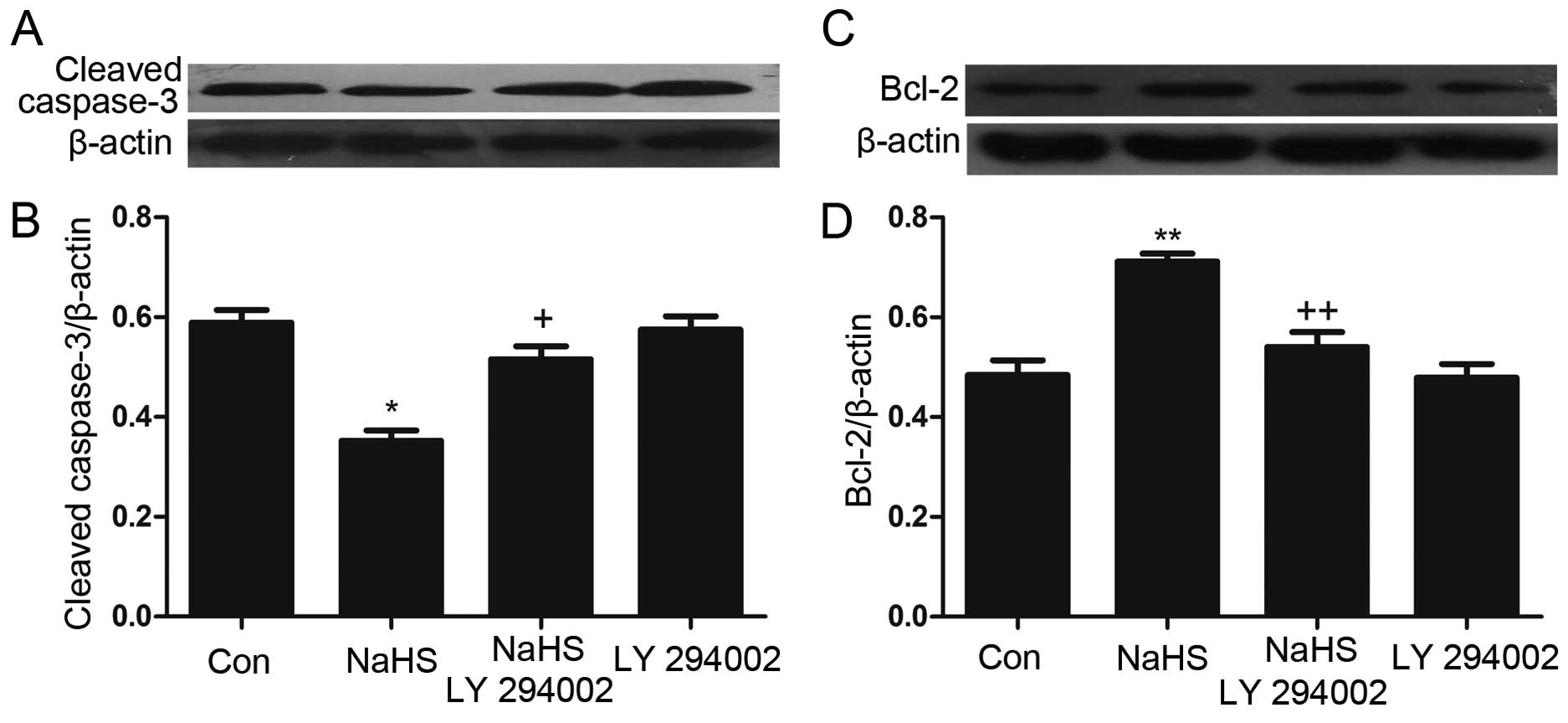
NaHS reduces the expression of caspase-3
and upregulates the level of Bcl-2 in multiple myeloma cells
To analyze the effect of NaHS on the expression of
caspase-3 and Bcl-2 in multiple myeloma cells, multiple myeloma
cells were exposed to 500 µmol/l NaHS for different period
of time (3, 6, 9, 12 and 24 h). As shown in Fig. 4, NaHS significantly enhanced the
expression of Bcl-2, which reached a peak at 9 h, and reduced the
expression of caspase-3.
LY294002 suppresses NaHS-induced
increased cell viability in multiple myeloma cells
Exposing multiple myeloma cells to 500 µmol/l
NaHS for 24 h significantly induced cell proliferation and
increased cell viability. However, the increased cell viability was
repressed by co-treatment with different doses of LY294002 (a
specific inhibitor of the Akt pathway) for 24 h. As shown in
Fig. 5, increasing the dose of
LY294002 from 1 to 10 µmol/l did not change cell viability.
However, increasing the dose of LY294002 from 50 to 200
µmol/l significantly suppressed cell proliferation, with the
decrease in cell viability reaching a minimum at 50 µmol/l.
In accordance with the above results, multiple myeloma cells were
co-treated with 500 µmol/l NaHS and 50 µmol/l
LY294002 for 24 h in all subsequent experiments.
LY294002 reduces the NaHS-induced
acceleration of cell cycle progression in multiple myeloma
cells
As shown in Fig.
6A–D, multiple myeloma cells were exposed to 500 µmol/l
NaHS for 24 h, the S-phase and G2/M-phase cells were significantly
increased. However, the G0/G1-phase population of cells was
markedly decreased. Co-treatment of multiple myeloma cells with 500
µmol/l NaHS and 50 µmol/l LY294002 for 24 h
substantially depressed the NaHS-induced increase in the
proportions of S-phase and G2/M-phase cells and significantly
decreased the number of G0/G1-phase cells. Treating the cells with
50 µmol/l LY294002 for 24 h did not alter cell cycle
progression.
LY294002 inhibits the NaHS-induced
increase in the expression of Bcl-2 and upregulates the
NaHS-induced decrease in caspase-3 expression in multiple myeloma
cells
As shown in Fig. 7,
multiple myeloma cells were exposed to 500 µmol/l NaHS for
24 h, and the expression of Bcl-2 was significantly increased.
However, the expression of caspase-3 was markedly decreased.
Notably, co-treatment of multiple myeloma cells with 500
µmol/l NaHS and 50 µmol/l LY294002 for 24 h
considerably depressed the NaHS-induced increase in the expression
of Bcl-2 while significantly upregulating caspase-3 expression.
Treating the cells with 50 µmol/l LY294002 for 24 h did not
alter the basal expression levels of Bcl-2 and caspase-3.
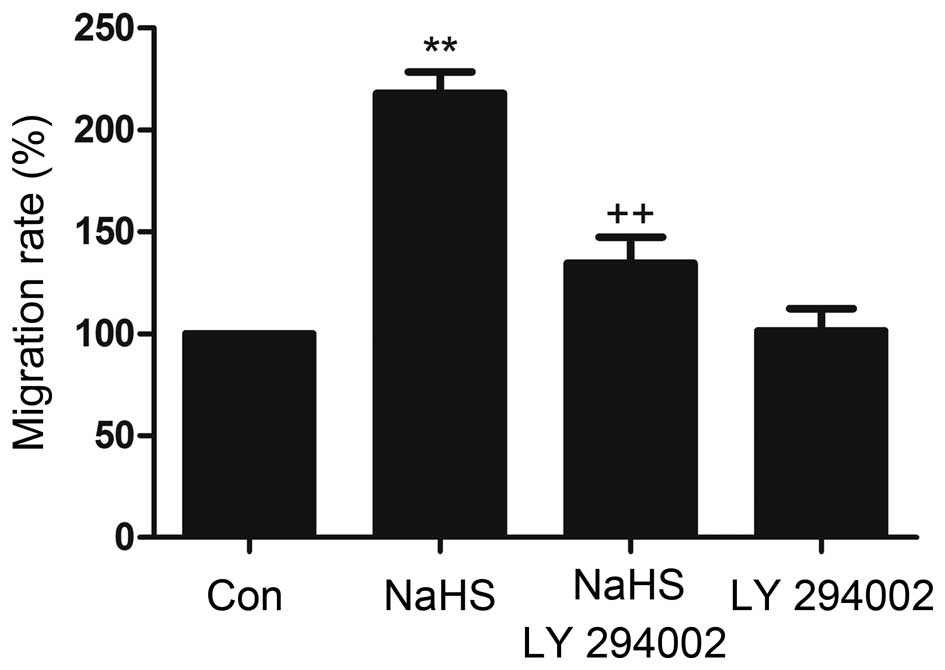
LY294002 inhibits NaHS-induced migration
in MM cells
In transwell migration assays, the migration rates
of MM cells (NCI-H929) toward conditioned medium that was collected
from NaHS was higher than the spontaneous migration rate,
indicating that NaHS induced migration in MM cells. This process
was inhibited by the Akt inhibitor.
LY294002 (Fig. 8).
These results show that LY294002 inhibits NaHS-induced migration in
MM cells and indicates that the Akt pathway may play an important
role in the process of NaHS-induced migration.
Discussion
H2S, the third gaseous transmitter
following NO and CO, modulates a range of cellular and molecular
mechanisms. Endogenous H2S in mammalian tissues is
mainly synthesized via the metabolism of L-cysteine by the
catalysis of two key enzymes, CBS and CSE (13,14).
Experiments have shown that plasma or blood H2S
concentrations range between 30 and 300 µM in humans,
depending on the method used for measurement and the age of the
donor (15). The role of
H2S, as a physiological molecule with pleiotropic
functions, is becoming increasingly apparent. Specifically,
H2S can elicit cardio-protective,
inflammation-preventing, anti-proliferative and anti-thrombotic
effects (16,17). There is an apparent paradox in the
effects of H2S on cancer. Many reports have shown that
inhibiting H2S biosynthesis exerts anticancer effects,
while other studies have shown that H2S donors of
various types exert anticancer actions both in vitro and
in vivo (18). In recent
years, an increasing number of studies have shown that hydrogen
sulfide (H2S) can mediate pathophysiological processes
during cancer cell growth, proliferation, migration, and invasion
(17,19,20).
Nevertheless, it was shown that H2S exerted potential
anticancer effects on gastric cancer cells (21), oral cancer cell lines (22), PLC/PRF/5 hepatoma cells (23), and colon cancer cells (24). Furthermore, an increasing amount of
evidence has shown that H2S is involved in
pathophysiological processes in tumors. The biological effects of
H2S that are relevant to cancer biology include the
regulation of vascular functions (vasorelaxation and the
stimulation of angiogenesis) (19)
and the regulation of intracellular signaling and cell death
(during which it acts as a direct and indirect antioxidant and
inhibits oxidative damage and cell death in response to diverse
stimuli) (25,26).
Based on the results of previous studies, we first
collected samples from patients to determine the levels of
H2S that are present in the serum. We found some
interesting results in this analysis. In our study, serum
H2S levels were significantly higher in the multiple
myeloma patient group than in the control group. Moreover, they
also increased in parallel with disease progression, with higher
levels of H2S observed in patients with advanced ISS
stages. We hypothesized that this gasotransmitter may be involved
in the progression of multiple myeloma. To verify this hypothesis,
we treated multiple myeloma cells with NaHS (a donor of
H2S that is actively being investigated because of the
above-described effects of H2S). Our findings
demonstrated that NaHS improved proliferation in multiple myeloma
cells when applied at concentrations ranging from 100 to 1,000
µmol/l. The optimal concentration of NaHS, at which it
induced its maximal effect on proliferation, was 500 µmol/l.
At this concentration, it led to increased cell viability,
indicating that H2S might contribute to multiple myeloma
growth. Then, the cells were co-treated with the indicated doses of
NaHS for 24 h to analyze its effects on the cell cycle. The results
showed that the proportion of G0/G1-phase cells was significant
decreased, while the proportions of S-phase cells and G2/M phase
cells were significantly increased. NaHS was therefore found to
accelerate cell cycle progression. Treatment of multiple myeloma
cells with 500 µmol/l NaHS for 24 h markedly diminished cell
apoptosis and decreased the expression of caspase-3, an apoptotic
factor. At the same time, the expression of Bcl-2, a protein that
protects against apoptosis, was increased. The Bcl-2 protein is a
component of a complex signaling system that controls apoptosis,
and its overexpression can prevent apoptosis in cells, potentially
leading to the continued division of mutated cells lines and
eventually to cancer. Additionally, the overexpression of Bcl-2 can
contribute to metastasis in certain cancers (27). These results demonstrate that the
Bcl-2 pathway is implicated in the NaHS-induced effects on MM cell
proliferation, apoptosis and migration. All of these data indicate
that H2S, when present at a relative high level, might
evoke proliferative and anti-apoptotic effects.
Many MM cell lines express high baseline levels of
p-Akt. Moreover, Akt is activated in the cells of MM patients
(4). The PI3K/Akt pathways plays a
crucial role during MM cell growth, survival and drug resistance
(28). We sought to explore the
mechanism underlying NaHS-induced pro-proliferative,
anti-apoptotic, angiogenic, cell cycle progression-accelerating,
and pro-migration effects in multiple myeloma cells. Here, we
studied the PI3/Akt pathway and found that NaHS markedly increased
the phosphorylation of Akt, which may have activated the Akt
pathway in multiple myeloma cells. Noteworthy, LY294002, an
inhibitor of PI3/Akt, blocked the NaHS-induced activation of
PI3/Akt and the NaHS-induced pro-proliferative, anti-apoptotic and
pro-migration effects in multiple myeloma cells by decreasing the
expression of Bcl-2, increasing the expression of caspase-3 and
reducing the NaHS-induced acceleration of cell cycle progression in
multiple myeloma cells. Our data show that NaHS markedly increased
cell migration in MM cells. However, the increase in the level of
migration was significantly suppressed by co-treating cells with LY
249002. These results suggest that the activation of Akt is
necessary for NaHS-induced cell progression in multiple myeloma and
the phosphorylation of Akt is required for NaHS-induced cell
proliferation. This result is consistent with the results of
previous reports that showed that NaHS promotes cancer cell
proliferation by up-regulating p-Akt (29).
In summary, H2S induced cell
proliferation, inhibited apoptosis, accelerated cell cycle
progression, and increased migration in multiple myeloma cells.
These effects might be mediated by the activation of the Akt
pathway, which leads to the overexpression of Bcl-2, the
down-regulation of caspase-3, an increase in cell viability and
migration rates, the acceleration of cell cycle progression and a
decrease in the number of apoptotic cells. In myeloma cells,
H2S is an endogenous tumor-promoting factor that plays a
deleterious role in multiple myeloma cell development. The
contribution of H2S to myeloma cell growth remains to be
further investigated. Further investigations into the biological
effects of H2S on multiple myeloma cells may advance our
knowledge of this novel gaseous transmitter and lead to a better
understanding of multiple myeloma development.
References
|
1
|
Kyle RA and Rajkumar SV: Criteria for
diagnosis, staging, risk stratification and response assessment of
multiple myeloma. Leukemia. 23:3–9. 2009. View Article : Google Scholar :
|
|
2
|
Raab MS, Podar K, Breitkreutz I,
Richardson PG and Anderson KC: Multiple myeloma. Lancet.
374:324–339. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hideshima T, Mitsiades C, Tonon G,
Richardson PG and Anderson KC: Understanding multiple myeloma
pathogenesis in the bone marrow to identify new therapeutic
targets. Nat Rev Cancer. 7:585–598. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hsu J, Shi Y, Krajewski S, Renner S,
Fisher M, Reed JC, Franke TF and Lichtenstein A: The AKT kinase is
activated in multiple myeloma tumor cells. Blood. 98:2853–2855.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hideshima T, Nakamura N, Chauhan D and
Anderson KC: Biologic sequelae of interleukin-6 induced PI3-K/Akt
signaling in multiple myeloma. Oncogene. 20:5991–6000. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang J, Li Y and Shen B: PI3-K/Akt
pathway contributes to IL-6-dependent growth of 7TD1 cells. Cancer
Cell Int. 3:1–4. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hsu JH, Shi Y, Hu L, Fisher M, Franke TF
and Lichtenstein A: Role of the AKT kinase in expansion of multiple
myeloma clones: Effects on cytokine-dependent proliferative and
survival responses. Oncogene. 21:1391–1400. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Whiteman M, Le Trionnaire S, Chopra M, Fox
B and Whatmore J: Emerging role of hydrogen sulfide in health and
disease: Critical appraisal of biomarkers and pharmacological
tools. Clin Sci (Lond). 121:459–488. 2011. View Article : Google Scholar
|
|
10
|
Kimura H: Hydrogen sulfide: Its
production, release and functions. Amino Acids. 41:113–121. 2011.
View Article : Google Scholar
|
|
11
|
Szabó C: Hydrogen sulphide and its
therapeutic potential. Nat Rev Drug Discov. 6:917–935. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vandiver M and Snyder SH: Hydrogen
sulfide: A gasotransmitter of clinical relevance. J Mol Med Berl.
90:255–263. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rose P, Moore PK, Ming SH, Nam OC,
Armstrong JS and Whiteman M: Hydrogen sulfide protects colon cancer
cells from chemopreventative agent beta-phenylethyl isothiocyanate
induced apoptosis. World J Gastroenterol. 11:3990–3997. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sen N, Paul BD, Gadalla MM, Mustafa AK,
Sen T, Xu R, Kim S and Snyder SH: Hydrogen sulfide-linked
sulfhydration of NF-κB mediates its antiapoptotic actions. Mol
Cell. 45:13–24. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Whiteman M and Moore PK: Hydrogen sulfide
and the vasculature: A novel vasculoprotective entity and regulator
of nitric oxide bioavailability? J Cell Mol Med. 13:488–507. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Papapetropoulos A, Pyriochou A, Altaany Z,
Yang G, Marazioti A, Zhou Z, Jeschke MG, Branski LK, Herndon DN,
Wang R, et al: Hydrogen sulfide is an endogenous stimulator of
angiogenesis. Proc Natl Acad Sci USA. 106:21972–21977. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Choi KS, Song H, Kim EH, Choi JH, Hong H,
Han YM and Hahm KB: Inhibition of hydrogen sulfide-induced
angiogenesis and inflammation in vascular endothelial cells:
Potential mechanisms of gastric cancer prevention by Korean red
ginseng. J Ginseng Res. 36:135–145. 2012. View Article : Google Scholar
|
|
18
|
Hellmich MR, Coletta C, Chao C and Szabo
C: The therapeutic potential of cystathionine β-synthetase/hydrogen
sulfide inhibition in cancer. Antioxid Redox Signal. 22:424–448.
2015. View Article : Google Scholar :
|
|
19
|
Szabó C and Papapetropoulos A: Hydrogen
sulphide and angiogenesis: Mechanisms and applications. Br J
Pharmacol. 164:853–865. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Attene-Ramos MS, Wagner ED, Plewa MJ and
Gaskins HR: Evidence that hydrogen sulfide is a genotoxic agent.
Mol Cancer Res. 4:9–14. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang L, Qi Q, Yang J, Sun D, Li C, Xue Y,
Jiang Q, Tian Y, Xu C and Wang R: An anticancer role of hydrogen
sulfide in human gastric cancer cells. Oxid Med Cell Longev.
2015:6364102015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Murata T, Sato T, Kamoda T, Moriyama H,
Kumazawa Y and Hanada N: Differential susceptibility to hydrogen
sulfide-induced apoptosis between PHLDA1-overexpressing oral cancer
cell lines and oral keratinocytes: Role of PHLDA1 as an apoptosis
suppressor. Exp Cell Res. 320:247–257. 2014. View Article : Google Scholar
|
|
23
|
Zhen Y, Pan W, Hu F, Wu H, Feng J, Zhang Y
and Chen J: Exogenous hydrogen sulfide exerts
proliferation/anti-apoptosis/angiogenesis/migration effects via
amplifying the activation of NF-κB pathway in PLC/PRF/5 hepatoma
cells. Int J Oncol. 46:2194–2204. 2015.PubMed/NCBI
|
|
24
|
Kodela R, Nath N, Chattopadhyay M, Nesbitt
DE, Velázquez-Martínez CA and Kashfi K: Hydrogen sulfide-releasing
naproxen suppresses colon cancer cell growth and inhibits NF-κB
signaling. Drug Des Devel Ther. 9:4873–4882. 2015.
|
|
25
|
Kolluru GK, Shen X, Bir SC and Kevil CG:
Hydrogen sulfide chemical biology: Pathophysiological roles and
detection. Nitric Oxide. 35:5–20. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang R: Physiological implications of
hydrogen sulfide: A whiff exploration that blossomed. Physiol Rev.
92:791–896. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fernández Y, Gu B, Martínez A, Torregrosa
A and Sierra A: Inhibition of apoptosis in human breast cancer
cells: Role in tumor progression to the metastatic state. Int J
Cancer. 101:317–326. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Younes H, Leleu X, Hatjiharissi E, Moreau
AS, Hideshima T, Richardson P, Anderson KC and Ghobrial IM:
Targeting the phosphatidylinositol 3-kinase pathway in multiple
myeloma. Clin Cancer Res. 13:3771–3775. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cai WJ, Wang MJ, Ju LH, Wang C and Zhu YC:
Hydrogen sulfide induces human colon cancer cell proliferation:
Role of Akt, ERK and p21. Cell Biol Int. 34:565–572. 2010.
View Article : Google Scholar : PubMed/NCBI
|