Introduction
Urothelial carcinoma of the bladder is an important
health issue worldwide. In the USA, bladder cancer is the fourth
most common malignancy in men and the eighth most common in women,
and it is estimated that 74,690 patients were diagnosed with
bladder cancer and 15,580 patients died from this malignancy in
2014 (1). Approximately 70% of
bladder cancers are diagnosed as non-muscle invasive bladder cancer
(NMIBC), including stages Ta and T1 (2,3). The
standard treatment for NMIBC is transurethral resection of bladder
tumor (TURBT) with or without adjuvant intravesical bacillus
Calmette-Guerin therapy, or chemotherapy with anthracyclines,
mitomycin, or gemcitabine (4).
Although Ta bladder cancer is associated with favorable
cancer-specific survival, T1 bladder cancer has a significant
potential to progress to muscle invasive bladder cancer (MIBC)
after initial treatment. One of the most significant issues is that
T1 (almost high-grade) bladder cancer can be a lethal disease with
varying degrees of aggressiveness and progression (5–8). T1
high-grade bladder cancer progresses to MIBC at a rate of 25–50%
(6,7,9). The
important goal of the clinical management for T1 high-grade bladder
cancer is to prevent progression of the cancer. Therefore, there is
an emerging need to discover novel biomarkers that can predict the
progression of the cancer accurately and become a clinically
available therapeutic target.
The α-Klotho (KLα) gene was identified as an
anti-aging gene in 1997 (10). The
authors reported that KLα-knockout mice developed a syndrome that
resembles ageing conditions, such as a short lifespan,
arteriosclerosis, and osteoporosis. Kurosu et al revealed
that KLα overexpression in mice extended their lifespan at a rate
of 20–30% (11). The KLα protein
exists in two forms: a membrane and a secreted form. Membrane KLα
functions as a co-receptor of fibroblast growth factor (FGF)23 to
regulate phosphate homeostasis (12,13).
Secreted KLα is a regulator of oxidative stress activity, multiple
growth factor receptors, and ion channels (14,15).
The β-Klotho (KLβ) gene was identified in 2000 (16), wherein the authors reported that the
amino acid sequence was 41.2% identical to that of KLα. Notably,
both KLα and KLβ lack glucosidase catalytic activity, and this
characteristic is the reason why both molecules are recognized as a
new and distinct protein family within the glycosidase family 1
superfamily. Whereas the role of KLβ is unclear, membrane KLβ is
specifically known to be a co-receptor of FGF19 and FGF21,
regulating the synthesis of bile acid and energy metabolism
(17–19). Recently, attention has been directed
toward the association between cancer and KLα/KLβ. In the case of
KLα, most of the studies have suggested it to be a tumor suppressor
(20–23). Doi et al reported that KLα
inhibited transforming growth factor-β1 signaling, which induced
epithelial-to-mesenchymal transition responses and suppressed
cancer metastasis in vivo (24). In contrast to KLα, the association
between cancers and KLβ expression has not yet been well
investigated. In the present study, we focused on the clinical
significance of KLα and KLβ, which could be regulators of the
cancer progression of urothelial carcinoma of the bladder.
Materials and methods
Human samples
We extracted tissue samples from 155 NMIBC and 6
MIBC patients who had undergone TURBT between April 2004 and March
2013. Patients who had undergone early cystectomy were excluded
from the study. The protocol for the research project was approved
by the Institutional Review Board for Clinical Studies (Medical
Ethics Committee ID: NMU-900), and informed consent was obtained
from all the patients.
Immunohistochemistry
To examine the expression levels of KLα and KLβ,
immunohistochemistry (IHC) was carried out. We used
paraffin-embedded tissues obtained from all 161 patients in the
study to examine the association between the KLα/KLβ expression
levels and clinicopathological variables. The paraffin blocks were
cut and placed on SuperFrost Plus Microscope Slides (Thermo Fisher
Scientific, Yokohama, Japan). The sections were deparaffinized and
antigen retrieval was carried out in citric acid buffer (pH 6.0) in
an autoclave. IHC staining was performed with the Histofine ABC kit
(Nichirei, Tokyo, Japan). Briefly, slides were treated with 1%
hydrogen peroxide in methanol to block endogenous peroxidase
activity. The slides were then incubated overnight at 4°C with
anti-KLα antibody (sc-22220, rabbit polyclonal, dilution 1/500) and
anti-KLβ antibody (sc-74343, rabbit polyclonal, dilution 1/200)
(both from Santa Cruz Biotechnology, Santa Cruz, CA, USA),
respectively. The slides were counterstained with hematoxylin,
dehydrated, and mounted on a cover slide. We evaluated each slide
using IHC scores (IHC score = intensity score + population score;
intensity: none, 0; low, 1; intermediate, 2; and high, 3;
population: none, 0; 0–25%, 1; 25–50%, 2; 50–75%, 3; and 75–100%,
4). The KLα/KLβ expression was categorized into low or high
according to the IHC score as follows: low, IHC score ≤4; high, IHC
score 5 or 6.
Cell lines
The human urothelial carcinoma cell lines MGH-U3,
J82, and UM-UC-3 were used in this study. MGH-U3 was a gift from Dr
H. LaRue (Laval University Cancer Research Centre, Quebec, Canada).
J82 and UM-UC-3 cells were purchased from the American Type Culture
Collection (ATCC; Manassas, VA, USA). The cell lines were
maintained in RPMI-1640 medium (Nacalai Tesque, Kyoto, japan),
supplemented with 10% fetal bovine serum (FBS; JRH, Tokyo, japan)
and 1% penicillin/streptomycin (Thermo Fisher Scientific) in a
standard humidified incubator at 37°C in an atmosphere of 5%
CO2.
Western blot analysis
Western blot analysis was performed using protein
extracted from the cultured cells. All proteins were extracted
using the RIPA lysis buffer (Sigma-Aldrich, St. Louis, MO, USA),
which contained 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1% NP-40, 1%
sodium deoxycholate, 2.5 mM sodium pyrophosphate, 1 mM
Na3VO4, 1 mM phenylmethylsulfonyl fluoride,
and 1 µg/ml leupeptin. Protein concentrations were
quantified using Protein Quantification kit-Wide Range (Dojindo,
Kumamoto, Japan). Total protein (20 µg) was diluted with
sodium dodecyl sulfate (SDS) loading buffer containing 2.5%
β-mercaptoethanol, boiled at 95°C for 5 min, and electrophoresed on
10% SDS-polyacrylamide gels using a Mini-PROTEAN Tetra
electrophoresis cell at 200 V for 35 min. The gels were then
transferred onto polyvinylidene difluoride membranes using a
semi-dry transfer apparatus (both from Bio-Rad, Hercules, CA, USA)
at 15 V for 45 min. After blocking overnight in Tris-buffered
saline (pH 7.6) containing 5% skim milk and 0.1% Tween-20, the
membranes were incubated for 1 h with the primary anti-KLα rabbit
polyclonal antibody (dilution 1/200), anti-KLβ rabbit antibody
(dilution 1/200), and anti-actin mouse monoclonal antibody
(dilution 1/10,000) as an internal loading control, followed by 1 h
with horseradish peroxidase-conjugated goat anti-rabbit IgG or
anti-mouse IgG antibody. The bound secondary antibody was detected
using a West Pico detection kit (Thermo Fisher Scientific). The
secondary antibodies (Santa Cruz Biotechnology) were used at
1:5,000, 1:5,000, and 1:10,000 dilutions, respectively.
Cell proliferation assays
Recombinant human KLβ was purchased from R&D
Systems (Minneapolis, MN, USA). The cell proliferation assay was
used to examine the effects of the exogenous KLβ. Each of the cell
lines was incubated with two different concentrations of KLβ (10 or
50 ng/ml) for 48 h in serum-free medium at 37°C in an atmosphere of
5% CO2.
Matrigel invasion assay and
transendothelial migration assay
We evaluated whether the cell migration ability
would increase with exogenous KLβ. At first, we performed the
Matrigel invasion assay using BioCoat Matrigel Invasion Chambers
(BD Biosciences, Piscataway, NJ, USA). Each cell line was incubated
at 37°C in an atmosphere of 5% CO2 with or without 50
ng/ml of KLβ. After 48 h of incubation, non-invading cells on the
upper chambers were removed and the invading cells in the lower
chambers were stained with calcein AM (PromoKine, Heidelberg,
Germany) and the cells were immediately examined under a
fluorescence microscope (Leica DMI 4000B).
We examined whether exogenous KLβ could increase the
ability of the cancer cells to invade the endothelial cell layer,
using human umbilical vascular endothelial cells (HUVECs; Lonza,
Tokyo, Japan). Using a FluoroBlok insert system, the insert
membrane chambers were coated with 30,000 HUVECs on fibronectin
(Wako, Osaka, Japan) for HUVEC adhesion and incubated at 37°C in an
atmosphere of 5% CO2. After 8 h of incubation,
low-concentrated colcemid (Nacalai Tesque) was added to the insert
membrane to inhibit the migration of endothelial cells. Then, each
cancer cell line was sprinkled onto the membrane. After a 24-h
incubation period, non-invading cells in the upper chambers were
removed and images of the invading cells on the membrane of the
lower chambers were captured and visualized under a fluorescence
microscope. The cells that attached to the bottom of the membrane
were stained and examined as aforementioned.
Soft agar colony formation assay
To examine for anchorage-independent growth, we
performed a soft agar colony formation assay. The base agar layer
was prepared using 1.2% agar solution (Difco, Franklin Lakes, NJ,
USA) mixed with an equal volume of 2X Dulbecco's modified Eagle's
medium (DMEM; Sigma-Aldrich)/20% FBS in a 24-well culture plate.
Cell suspensions (30,000 cells/ml) were prepared and mixed with
both the 1.2% agar solution and 2X DMEM/20% FBS in the same manner
as described above. Exogenous KLβ (50 ng/ml) or PBS as a control
was added into each well and incubation was carried out at 37°C in
an atmosphere of 5% CO2. A week after seeding, the
number of growing colonies was counted under a microscope.
Measurement of secreted KLβ by ELISA
Voided urine samples from 59 NMIBC patients and 10
MIBC patients were collected prior to surgery and stored at −80°C.
Urine was also obtained from four healthy volunteers as assay
controls. All 73 urine samples were thawed just before use and
analyzed for KLβ concentration with an enzyme-linked immunosorbent
assay (ELISA) kit (Cloud-Clone Corp., Houston, TX, USA), using a
Tecan microplate reader (Tecan Systems, Inc., San Jose, CA,
USA).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 5.0 (GraphPad Software, Inc., San Diego, CA, USA). The
figures were also constructed using GraphPad Prism 5.0. The
comparison between high and low KLβ expression was calculated using
the Mann-Whitney U test or the Student's t-test. The survival curve
was obtained using the Kaplan-Meier method and compared by the
log-rank test for each prognostic variable. A multivariate analysis
was performed using the Statistical Package for the Social
Sciences, version 19.0 (SPSS, Inc., Chicago, IL, USA). P<0.05
was considered statistically significant.
Results
The association of Klotho expression with
various clinicpathological variables
To investigate the association between KLα/KLβ and
various clinicopathological variables, we performed IHC analysis of
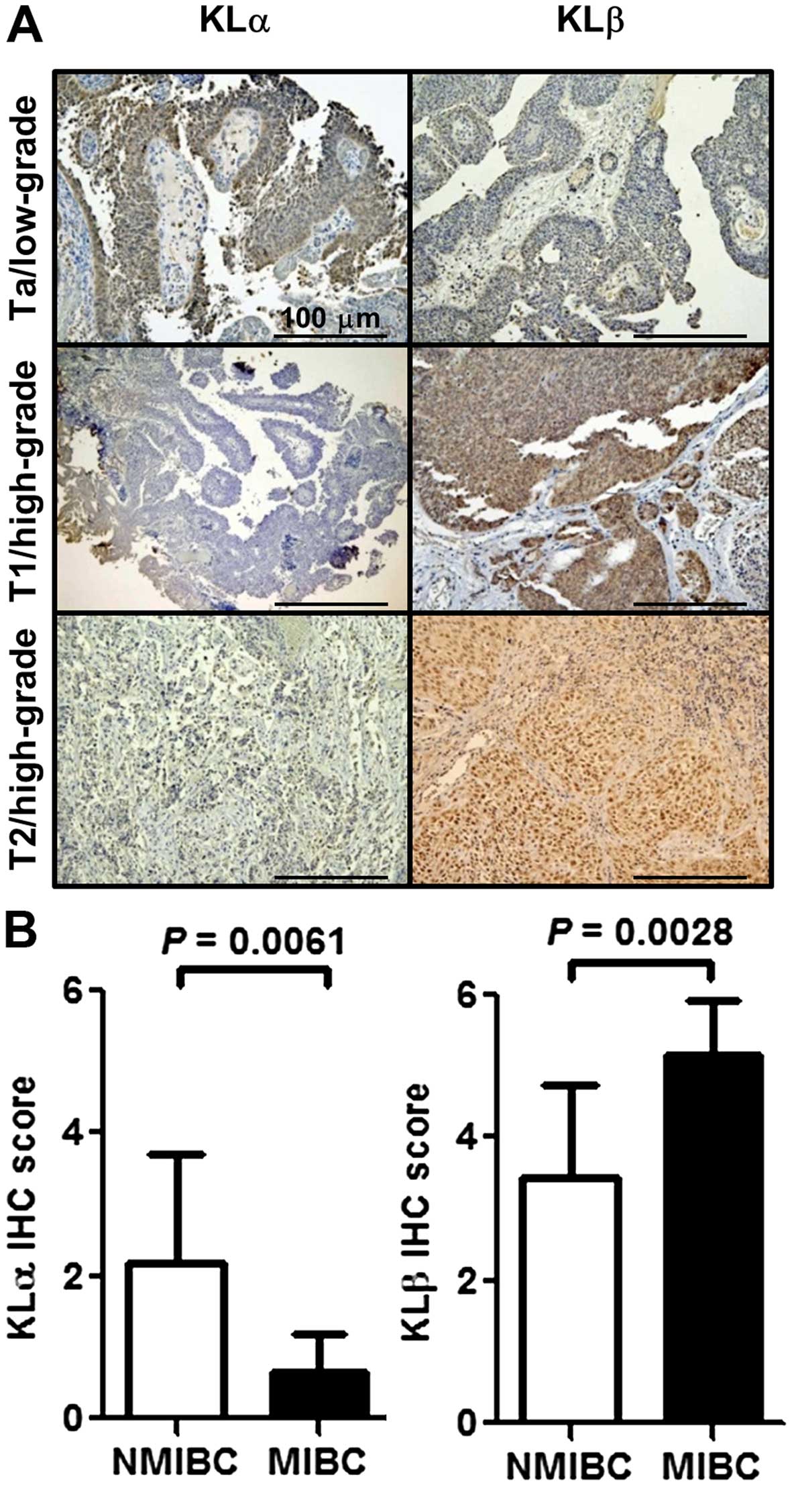
the KLα/KLβ expression levels. Fig.
1A shows representative images of weak, intermediate, and
strong expression of KLα (left panels) and KLβ (right panels). KLα
expression was higher in NMIBC than that in MIBC (P=0.0061;
Fig. 1B, left), whereas the
opposite was true for KLβ expression (P=0.0028; Fig. 1B, right). Table I lists the clinicopathological
background of the cohorts of 155 NMIBC patients and comparisons of
the variables with high and low KLα/KLβ expression. At initial
TURBT, the median age for this cohort was 71 years [interquartile
range (IQR) 34–94] and the median follow-up period was 53 months
(IQR 1–126). The pathological data, such as the tumor category,
tumor grade, and concomitant carcinoma in situ (CIS), were
significantly different between patients with low and high KLα
expression. In the case of KLβ, patients with high expression were
significantly of high stage and high grade, and had a high rate of
concomitant CIS and high presence of lymphovascular invasion (LVI)
compared with patients with low KLβ expression. KLα and KLβ showed
opposite trends for both invasiveness and tumor grade.
 | Table IPatient clinicopathological
background. |
Table I
Patient clinicopathological
background.
| Variables | No. of patients | KLα expression
| P-value | KLβ expression
| P-value |
|---|
| Low | High | Low | High |
|---|
| Total | 155 | 140 | 15 | | 117 | 38 | |
| Gender | | | | 0.67a | | |
1.0a |
| Male | 138 | 125 | 13 | | 105 | 33 | |
| Female | 17 | 15 | 2 | | 12 | 5 | |
| Age (at initial
TURBT) | | | | 0.73b | | |
0.19b |
| Median (IQR) in
years | 71 (34–94) | 71 (36–94) | 72 (34–85) | | 71 (34–94) | 72 (54–89) | |
| Tumor category | | | | 0.0006a | | | <0.01a |
| Ta | 68 | 56 | 12 | | 66 | 2 | |
| T1 | 73 | 73 | 0 | | 45 | 28 | |
| Tis | 14 | 11 | 3 | |
6 | 8 | |
| Tumor grade | | | | 0.0062a | | | <0.0001a |
| High | 84 | 81 | 3 | | 48 | 36 | |
| Low | 71 | 59 | 12 | | 69 | 2 | |
| Concomitant CIS
with Ta/1 (n=141) | | | | 0.0041a | | |
0.0002a |
| Yes | 51 | 51 | 0 | | 31 | 20 | |
| No | 90 | 78 | 12 | | 80 | 10 | |
| LVI with T1
(n=73) | | | | – | | |
0.0001a |
| Yes | 24 | 49 | 0 | |
7 | 17 | |
| No | 49 | 24 | 0 | | 38 | 11 | |
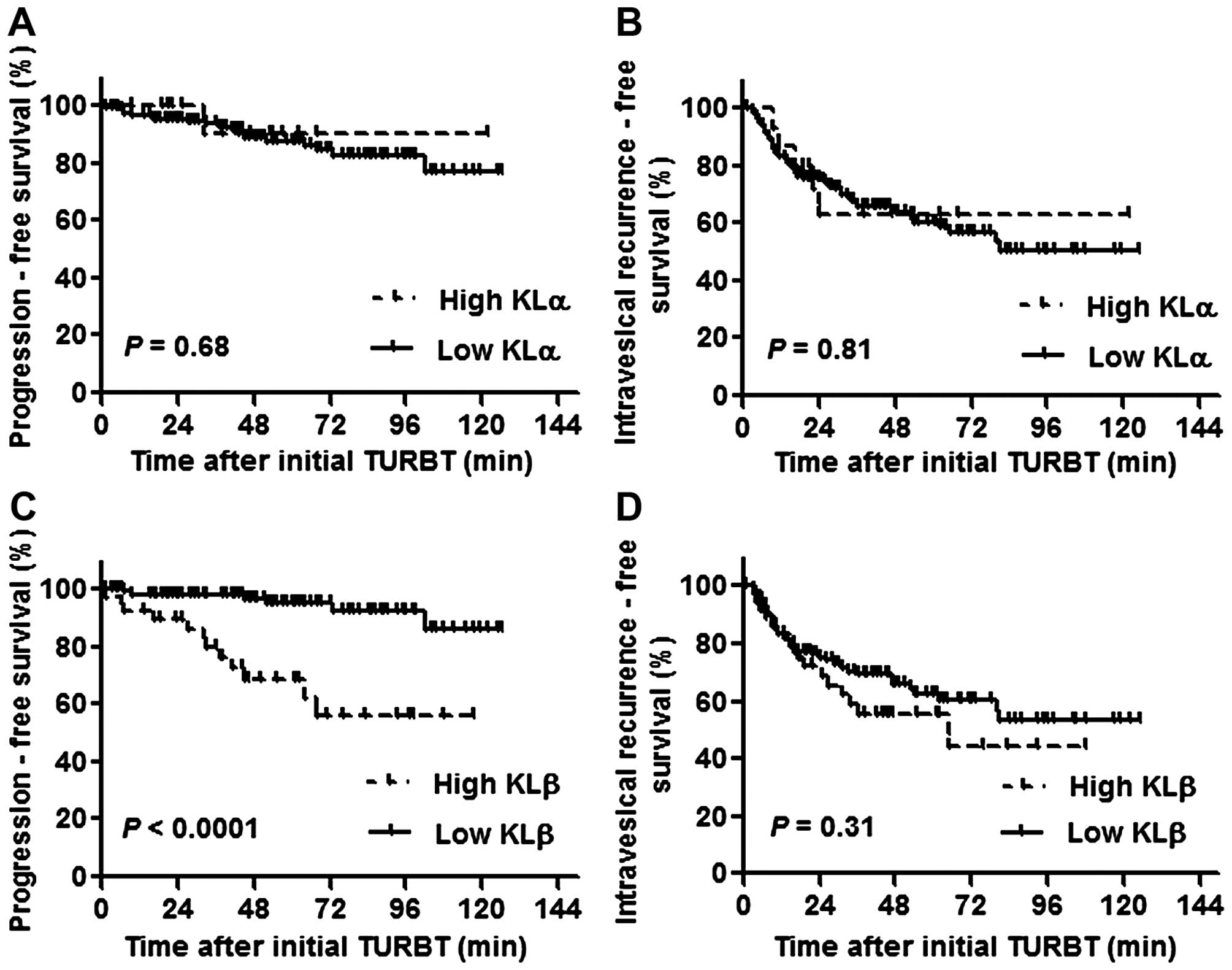
The expression level of KLβ, but not KLα,
is associated with disease progression to MIBC
Of the 155 NMIBC patients, 55 (35.5%) had an
intravesical recurrence at median 33.5 months after initial TURBT
and 18 patients (11.6%) progressed to muscle invasive tumors at
median 51 months after initial TURBT. With regard to the
intravesical progression- and recurrence-free survival in patients
with high or low KLα expression, there was no significant
difference between the two groups [P=0.68 (Fig. 2A) and P=0.81 (Fig. 2B), respectively]. In contrast, the
progression-free survival for the patients with high KLβ expression
was significantly shorter than that for the patients with low
expression (P<0.0001; Fig. 2C).
The intravesical recurrence-free survival was not significantly
different between the two groups (P=0.31; Fig. 2d).
Table II shows the
univariate analysis of prognostic factors for both progression- and
intravesical recurrence-free survival. The analysis revealed that
high KLβ expression was a poor prognostic factor for
progression-free survival [hazards ratio (HR) =13, 95% CI 4.2–38;
P<0.0001] but not for intravesical recurrence-free survival
(HR=1.4, 95% CI 0.73–2.6; P= 0.3). On the other hand, LVI was a
predictive factor for progression-free survival (HR=11, 3.1–37;
P=0.0002) but not for intravesical recurrence-free survival
(HR=1.8, 95% CI 0.82–3.7; P=0.14). The other factors such as T
category, tumor grade, and concomitant with CIS showed similar
results. Table III shows the
multivariate analysis of prognostic factors for progression-free
survival. The Cox proportional hazard model analysis identified
high KLβ expression (HR=6.9, 95% CI 2.6–18; P<0.0001) as an
independent prognostic factor of progression to muscle invasive
disease.
 | Table IIUnivariate analysis of prognostic
factors for progression- or intravesical recurrence-free
survival. |
Table II
Univariate analysis of prognostic
factors for progression- or intravesical recurrence-free
survival.
| Variables | Intravesical
recurrence-free survival
| Progression-free
survival
|
|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Gender |
| Male | 1 | | | 1 | | |
| Female | 0.99 | 0.39–2.5 | 0.29 | 3.4 | 0.60–19 | 0.17 |
| Tumor category |
| Ta | 1 | | | 1 | | |
| T1 | 0.82 | 0.48–1.4 | 0.5 | 3.8 | 1.1–14 | 0.042 |
| Tis | 0.47 | 0.14–1.6 | 0.22 | 7.1 | 1.6–32 | 0.011 |
| Tumor grade |
| Low | 1 | | | 1 | | |
| High | 0.9 | 0.52–1.6 | 0.73 | 3.5 | 1.4–8.8 | 0.0088 |
| CIS |
| No | 1 | | | 1 | | |
| Yes | 0.92 | 0.54–1.6 | 0.79 | 3.3 | 1.3–8.5 | 0.012 |
| LVI |
| No | 1 | | | 1 | | |
| Yes | 1.8 | 0.82–3.7 | 0.14 | 11 | 3.1–37 | 0.0002 |
| KLβ expression |
| Low | 1 | | | 1 | | |
| High | 1.4 | 0.73–2.6 | 0.3 | 13 | 4.2–38 | <0.0001 |
 | Table IIICox proportional models for
prognostic factors. |
Table III
Cox proportional models for
prognostic factors.
| Variables | Progression-free
survival
|
|---|
| HR | 95% CI | P-value |
|---|
| Gender |
| Male | 1 | | |
| Female | 2.2 | 0.60–7.7 |
0.24 |
| Tumor category |
| Ta | 1 | | |
| T1 | 0.87 | 0.14–5.4 |
0.89 |
| Tis | 3.3 | 0.58–19 |
0.18 |
| Tumor grade |
| Low | 1 | | |
| High | 0.83 | 0.07–9.7 |
0.88 |
| CIS |
| No | 1 | | |
| Yes | 1.2 | 0.32–4.2 |
0.81 |
| LVI |
| No | 1 | | |
| Yes | 2.3 | 0.78–6.5 |
0.13 |
| KLβ expression |
| Low | 1 | | |
| High | 6.9 | 2.6–18 | <0.0001 |
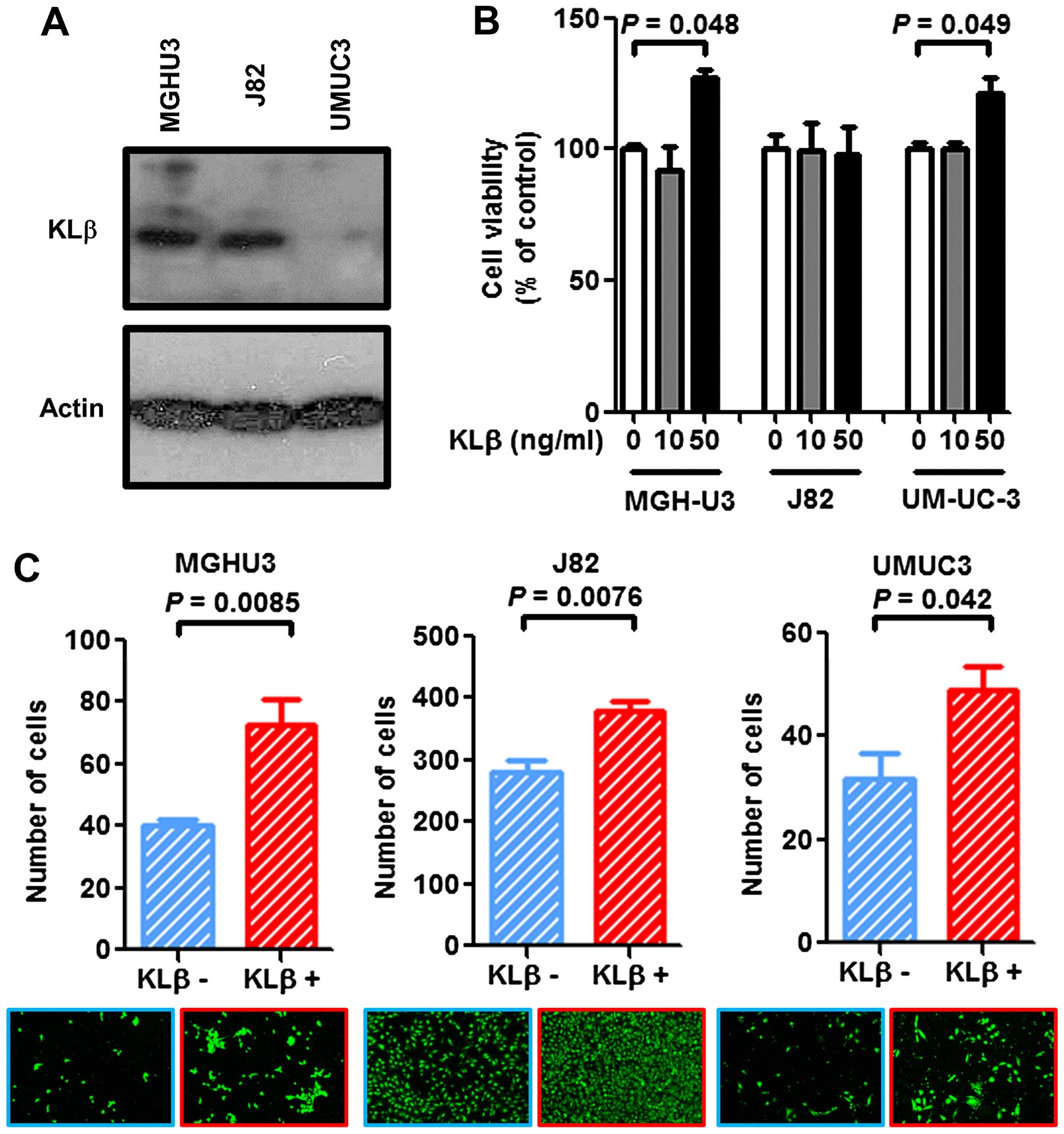
KLβ promotes the proliferation and tumor
invasiveness of urotherial cancer cells
To verify the expression level of KLβ in the three
urothelial cancer cell lines, western blot analysis was performed.
There were variations in the endogenous KLβ expression levels
(Fig. 3A), where UM-UC-3 expressed
the lowest level among the three cell lines. Exogenous KLβ
treatment at a concentration of 50 ng/ml promoted urothelial cancer
cell proliferation by 120–140% in the MGH-U3 and UM-UC-3 cells,
whereas no effect was observed in J82 cells (Fig. 3B). With regard to the Matrigel
invasion assay, KLβ treatment enhanced the invasiveness of all
three cell lines (Fig. 3C).
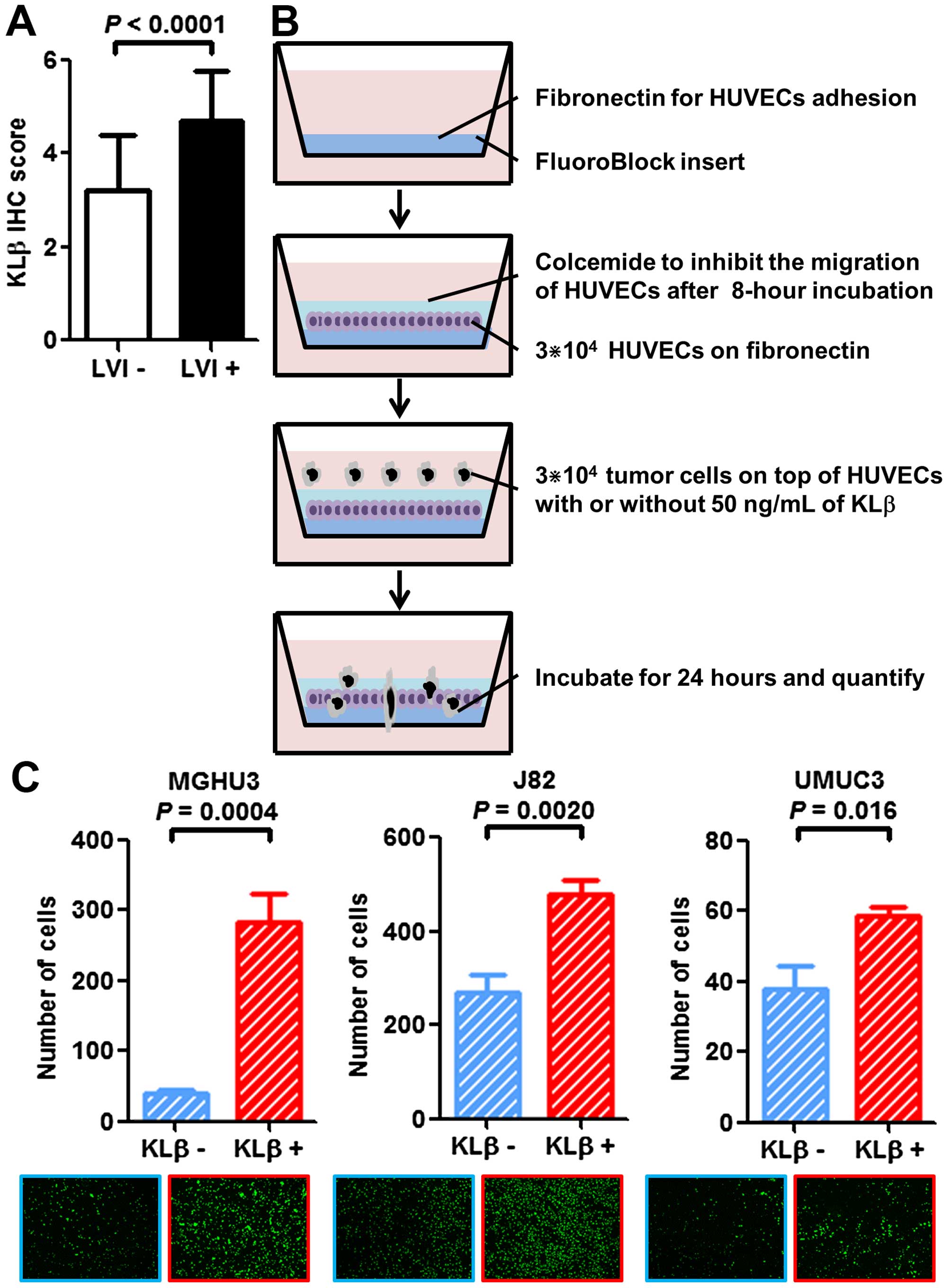
KLβ expression is associated with
LVI
We assessed the association between KLβ expression
and LVI status. Tumors with LVI had significantly higher KLβ
expression than LVI-negative tumors in the IHC analysis
(P<0.0001; Table I and Fig. 4A). To confirm this result with in
vitro experiments, we used the transendothelial migration assay
(Fig. 4B). Cells that broke through
the layer of the endothelial cells and migrated to the bottom of
the insert were quantified with a fluorescence-based
spectrophotometer and microscopy (Fig.
4C). Exogenous KLβ treatment enhanced the transendothelial
migration ability of all three cell lines. These findings suggest
that KLβ is highly related to disease progression via enhanced
tumor invasion through the vessel wall.
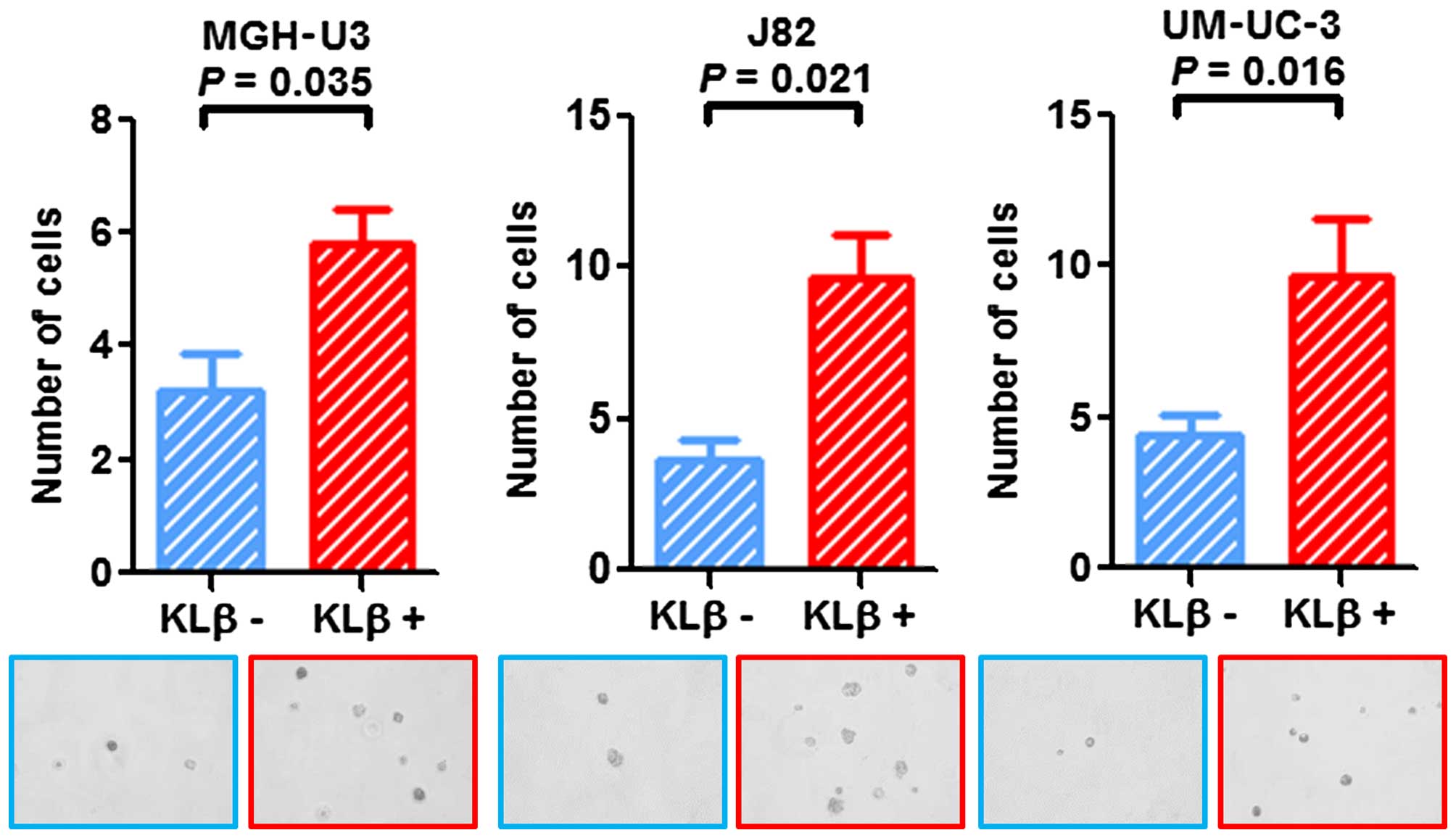
KLβ treatment enhances
anchorage-independent growth
Cells were suspended in soft agar and incubated with
or without KLβ. The number of colonies was counted 7 days after
seeding. Evaluation on day 7 showed a notable increase in the
colony formation ability of cells treated with KLβ in all three
cell lines (Fig. 5). The results
suggested that stimulation with KLβ enhanced the cell
anchorage-independent growth capability in vitro.
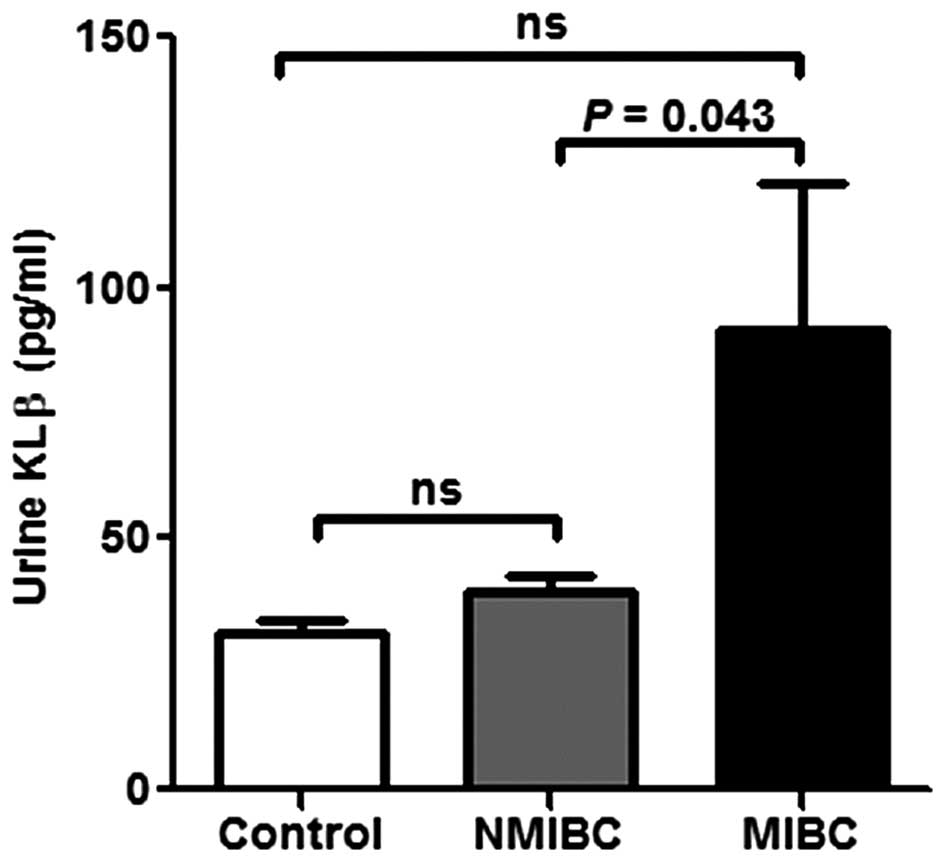
Urine KLβ concentration is increased in
MIBC patients
The preoperative voided urine KLβ concentration in
MIBC patients was significantly higher than that in NMIBC patients
(Fig. 6). However, there was no
significant difference between the healthy volunteers and the MIBC
patients and NMIBC patients, respectively (Fig. 6). To ascertain the association
between urine KLβ concentration and the result of urine cytology,
we categorized the studied patients into three groups: negative,
class I or II; suspicious, class III; and positive, class IV or V.
There were no significant differences in urine KLβ concentration
among the groups (data not shown).
Discussion
Our present results demonstrated the trend that KLα
is overexpressed in low-stage and low-grade tumors whereas KLβ is
overexpressed in high-stage and high-grade tumors of human bladder
cancer. The multivariate analysis revealed that a high KLβ
expression level is an independent prognostic factor for
progression to muscle invasive disease. However, KLα expression is
associated neither with intravesical recurrence- nor with
progression-free survival. Thus, we hypothesized that for
urothelial carcinoma of the bladder, KLα acts as a tumor suppressor
whereas KLβ acts as a tumor promotor.
In 2008, Wolf et al first reported that KLα
acted as a tumor suppressor in breast cancer (20). In their IHC study, the KLα
expression in normal breast tissue was higher than that in breast
cancer cells, and high KLα expression was associated with smaller
tumor size in breast cancer samples. In their in vitro
study, the cDNA or siRNA of KLα was transfected into the MCF-7 cell
line (which normally expresses KLα) and resulted in 60% reduction
or a 2.5-fold increase in cell growth, respectively. The authors
suggested the possibility of KLα having tumor-suppressing roles on
cell proliferation and survival, mediated by inhibition of the
IGF-1/insulin signaling pathway. Xie et al revealed that a
decreased level of KLα protein expression and an increased
methylation level of the KLα gene promoter region are poor
prognostic factors in hepatocellular carcinoma (HCC) (21). In the human lung cancer cell line
A549, KLα expression inhibited cancer proliferation and induced
apoptosis of the cells (22). In
the case of urogenital carcinoma, Zhu et al showed that the
survival rate for patients with high KLα expression was
significantly longer than that for patients with low expression,
and KLα suppressed the epithelial-to-mesenchymal transition and
migration and invasion of renal cell carcinoma cells (23). In the present study, although KLα
expression was significantly higher in NMIBC than in MIBC, KLα was
not a favorable prognostic factor of bladder cancer for
intravesical recurrence- and progression-free survival. Our IHC
study suggested that KLα expression possibly acts as a tumor
suppressor in bladder cancer. In order to verify this, we need to
increase the number of study cases or to examine a cohort of MIBC
patients.
Poh et al showed that elevated KLβ expression
contributed to HCC progression through the FGF4 signaling pathway
(25). On the contrary, Ye et
al reported the antiproliferative effect of KLβ by regulating
the Akt/GSK-3β/cyclin D1 signaling pathway in HCC (26). Therefore, the role of KLβ on
tumorigenesis and progression is still controversial. Feng et
al showed that FGF19 contributed to the promotion of prostate
cancer progression and KLβ possibly promoted the pathway
aforementioned (27). IHC analysis
in the present study showed that KLβ expression was associated with
tumor invasiveness and progression. However, we did not examine
other factors involved in KLβ, such as FGFs, so we were not able to
describe the possible mechanism for the tumor aggressiveness.
Similar to the situation in HCC (21), KLβ may regulate the phosphorylation
of ERK1/2, FRS2, and Akt, resulting in a progression-promoting cell
cycle or the inhibition of cancer cell apoptosis. Further
investigation is ongoing to elucidate the detailed mechanisms
associated with KLβ, using pathway assays related to FGFs.
KLβ treatment promoted cell proliferation, cell
invasiveness, and anchorage-independent growth in the human bladder
cancer cell lines. According to a meta-analysis by Kim et
al, the presence of LVI in TURBT specimens contributed to an
increase in the risk of pathological upstaging (28). Moreover, LVI was reported to be an
unfavorable prognostic factor for T1 bladder cancer (29). In our study, KLβ treatment increased
the invasion ability of all three human bladder cancer cell lines.
The IHC examination showed a significant relationship between KLβ
expression and LVI, indicating that KLβ stimulates LVI. Evaluation
of the anchorage-independent growth in a soft agar assay
demonstrated that exogenous KLβ treatment increased the colony
formation ability of all the human bladder cancer cell lines
studied. The cell cycle or apoptosis is possibly regulated by the
direct or indirect influence of KLβ.
The urine KLβ level measured by ELISA was
significantly higher in MIBC patients than in NMIBC patients in our
study. However, there was no significant difference in urine KLβ
levels between bladder cancer patients and healthy controls.
Although we expected urine KLβ to be a biomarker for discriminating
the malignant potential of bladder cancer, the differential results
in the present study did not reach statistical significance. There
was a trend of higher urine KLβ levels in patients with bladder
cancer than in the controls. However, we need to examine more cases
to make concrete conclusions. Notably, the urine KLβ level of the
MIBC patients was higher than that of the NMIBC patients.
Therefore, we may use pre-TURBT urine KLβ levels to clinically
distinguish MIBC from NMIBC, so as not to subject patients to
unnecessary examinations before TURBT. Moreover, we can select a
case with indication for adjuvant intravesical instillation therapy
after TURBT.
In conclusion, we postulate that KLβ acts as a tumor
promotor in human bladder cancer, and that the urine KLβ level is a
possible biomarker for distinguishing NMIBC from MIBC.
Acknowledgments
We would like to thank Dr Michihiro Toritsuka
(Department of Psychiatry, Nara Medical University, Nara, Japan)
for giving us substantial help with capturing the microscopic
images. The research study was supported by internal funding from
the Special Collaboration Studies grant, Nara Medical University,
and a grant in-part by the Ministry of Education, Culture, Sports,
Science and Technology (Japan).
Abbreviations:
|
KLα
|
α-Klotho
|
|
KLβ
|
β-Klotho
|
|
TURBT
|
transurethral resection of bladder
tumor
|
|
NMIBC
|
non-muscle invasive bladder cancer
|
|
MIBC
|
muscle invasive bladder cancer
|
|
FGF
|
fibroblast growth factor
|
|
IHC
|
immunohistochemistry
|
|
FBS
|
fetal bovine serum
|
|
CIS
|
carcinoma in situ
|
|
LVI
|
lymphovascular invasion
|
|
HCC
|
hepatocellular carcinoma
|
|
IQR
|
interquartile range
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
References
|
1
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miyake M, Fujimoto K, Anai S, Ohnishi S,
Nakai Y, Inoue T, Matsumura Y, Tomioka A, Ikeda T, Tanaka N, et al:
Clinical significance of heme oxygenase-1 expression in
non-muscle-invasive bladder cancer. Urol Int. 85:355–363. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nepple KG and O'Donnell MA: The optimal
management of T1 high-grade bladder cancer. Can Urol Assoc J.
3(Suppl 4): S188–S192. 2009.PubMed/NCBI
|
|
4
|
Hendricksen K and Witjes JA: Current
strategies for first and second line intravesical therapy for
nonmuscle invasive bladder cancer. Curr Opin Urol. 17:352–357.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kikuchi E, Fujimoto H, Mizutani Y, Okajima
E, Koga H, Hinotsu S, Shinohara N, Oya M and Miki T; Cancer
Registration Committee of the Japanese Urological Association:
Clinical outcome of tumor recurrence for Ta, T1 non-muscle invasive
bladder cancer from the data on registered bladder cancer patients
in japan: 1999–2001 report from the Japanese Urological
Association. Int J Urol. 16:279–286. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peyromaure M, Guerin F, Amsellem-Ouazana
D, Saighi D, Debre B and Zerbib M: Intravesical bacillus
Calmette-Guerin therapy for stage T1 grade 3 transitional cell
carcinoma of the bladder: Recurrence, progression and survival in a
study of 57 patients. J Urol. 169:2110–2112. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shahin O, Thalmann GN, Rentsch C,
Mazzucchelli L and Studer UE: A retrospective analysis of 153
patients treated with or without intravesical bacillus
Calmette-Guerin for primary stage T1 grade 3 bladder cancer:
Recurrence, progression and survival. J Urol. 169:96–100;
discussion 100. 2003. View Article : Google Scholar
|
|
8
|
Shelley MD, Jones G, Cleves A, Wilt TJ,
Mason MD and Kynaston HG: Intravesical gemcitabine therapy for
non-muscle invasive bladder cancer (NMIBC): A systematic review.
BJU Int. 109:496–505. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cambier S, Sylvester RJ, Collette L,
Gontero P, Brausi MA, van Andel G, Kirkels WJ, Silva FC,
Oosterlinck W, Prescott S, et al: EORTC nomograms and risk groups
for predicting recurrence, progression, and disease-specific and
overall survival in non-muscle-invasive stage Ta-T1 urothelial
bladder cancer patients treated with 1–3 years of maintenance
Bacillus Calmette-Guérin. Eur Urol. 69:60–69. 2016. View Article : Google Scholar
|
|
10
|
Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi
H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, et
al: Mutation of the mouse klotho gene leads to a syndrome
resembling ageing. Nature. 390:45–51. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kurosu H, Yamamoto M, Clark JD, Pastor JV,
Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M,
Kawaguchi H, et al: Suppression of aging in mice by the hormone
Klotho. Science. 309:1829–1833. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kuro-o M: Klotho. Pflugers Arch.
459:333–343. 2010. View Article : Google Scholar
|
|
13
|
Urakawa I, Yamazaki Y, Shimada T, Iijima
K, Hasegawa H, Okawa K, Fujita T, Fukumoto S and Yamashita T:
Klotho converts canonical FGF receptor into a specific receptor for
FGF23. Nature. 444:770–774. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kuro-o M: Klotho and aging. Biochim
Biophys Acta. 1790:1049–1058. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamamoto M, Clark JD, Pastor JV, Gurnani
P, Nandi A, Kurosu H, Miyoshi M, Ogawa Y, Castrillon DH, Rosenblatt
KP, et al: Regulation of oxidative stress by the anti-aging hormone
klotho. J Biol Chem. 280:38029–38034. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ito S, Kinoshita S, Shiraishi N, Nakagawa
S, Sekine S, Fujimori T and Nabeshima YI: Molecular cloning and
expression analyses of mouse betaklotho, which encodes a novel
Klotho family protein. Mech Dev. 98:115–119. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu MC and Moe OW: Klotho as a potential
biomarker and therapy for acute kidney injury. Nat Rev Nephrol.
8:423–429. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kurosu H, Choi M, Ogawa Y, Dickson AS,
Goetz R, Eliseenkova AV, Mohammadi M, Rosenblatt KP, Kliewer SA and
Kuro-o M: Tissue-specific expression of betaKlotho and fibroblast
growth factor (FGF) receptor isoforms determines metabolic activity
of FGF19 and FGF21. J Biol Chem. 282:26687–26695. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goetz R, Beenken A, Ibrahimi OA, Kalinina
J, Olsen SK, Eliseenkova AV, Xu C, Neubert TA, Zhang F, Linhardt
RJ, et al: Molecular insights into the klotho-dependent, endocrine
mode of action of fibroblast growth factor 19 subfamily members.
Mol Cell Biol. 27:3417–3428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wolf I, Levanon-Cohen S, Bose S, Ligumsky
H, Sredni B, Kanety H, Kuro-o M, Karlan B, Kaufman B, Koeffler HP,
et al: Klotho: A tumor suppressor and a modulator of the IGF-1 and
FGF pathways in human breast cancer. Oncogene. 27:7094–7105. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie B, Zhou J, Yuan L, Ren F, Liu DC, Li Q
and Shu G: Epigenetic silencing of Klotho expression correlates
with poor prognosis of human hepatocellular carcinoma. Hum Pathol.
44:795–801. 2013. View Article : Google Scholar
|
|
22
|
Chen B, Wang X, Zhao W and Wu J: Klotho
inhibits growth and promotes apoptosis in human lung cancer cell
line A549. J Exp Clin Cancer Res. 29:992010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu Y, Xu L, Zhang J, Xu W, Liu Y, Yin H,
Lv T, An H, Liu L, He H, et al: Klotho suppresses tumor progression
via inhibiting PI3K/Akt/GSK3β/Snail signaling in renal cell
carcinoma. Cancer Sci. 104:663–671. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Doi S, Zou Y, Togao O, Pastor JV, John GB,
Wang L, Shiizaki K, Gotschall R, Schiavi S, Yorioka N, et al:
Klotho inhibits transforming growth factor-beta1 (TGF-beta1)
signaling and suppresses renal fibrosis and cancer metastasis in
mice. J Biol Chem. 286:8655–8665. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Poh W, Wong W, Ong H, Aung MO, Lim SG,
Chua BT and Ho HK: Klotho-beta overexpression as a novel target for
suppressing proliferation and fibroblast growth factor receptor-4
signaling in hepatocellular carcinoma. Mol Cancer. 11:142012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ye X, Guo Y, Zhang Q, Chen W, Hua X, Liu
W, Yang Y and Chen G: βKlotho suppresses tumor growth in
hepatocellular carcinoma by regulating Akt/GSK-3β/cyclin D1
signaling pathway. PLoS One. 8:e556152013. View Article : Google Scholar
|
|
27
|
Feng S, Dakhova O, Creighton CJ and
Ittmann M: Endocrine fibroblast growth factor FGF19 promotes
prostate cancer progression. Cancer Res. 73:2551–2562. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim HS, Kim M, Jeong CW, Kwak C, Kim HH
and Ku JH: Presence of lymphovascular invasion in urothelial
bladder cancer specimens after transurethral resections correlates
with risk of upstaging and survival: A systematic review and
meta-analysis. Urol Oncol. 32:1191–1199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cho KS, Seo HK, Joung JY, Park WS, Ro JY,
Han KS, Chung J and Lee KH: Lymphovascular invasion in
transurethral resection specimens as predictor of progression and
metastasis in patients with newly diagnosed T1 bladder urothelial
cancer. J Urol. 182:2625–2630. 2009. View Article : Google Scholar : PubMed/NCBI
|