Introduction
Cancer is still a serious clinical issue with
significant social and economic impact on the human health care
system (1). Despite modern
advancements in diagnosis, prevention and therapy, cancer still
affects millions of patients worldwide, reduces their quality of
life and is one of the leading causes of mortality worldwide
(1,2). Natural products including plants,
microorganisms and marines provide rich resources for anticancer
drug discovery (3,4). Based on ancient and modern Chinese
herbal medicine books and Pharmacopoeia of China, there are many
natural anticancer plants or herbal formulations which provide a
guide, along with clinical evidence, for the identification of new
anticancer agents or a natural source of alternative cancer
therapy, and these have recently received increasing scientific
attention (5,6).
Corydalis yanhusuo, also named Rhizoma
Corydalis, is a well-known herbaceous plant commonly used in the
treatment of pain, injuries and coronary diseases in Traditional
Chinese Medicine (7,8). Alkaloids are important biological
active constituents of Corydalis yanhusuo (9). 13-Methyl-palmatrubine was isolated as
a natural protoberberine alkaloid from the methanol extract of the
tubes of Corydalis yanhusuo (10). The chemical structure of
13-methyl-palmatrubine is shown in Fig.
1A. One study found that 13-methyl-palmatrubine exhibited
antitumor activity in 3 types of human cancer cell lines (11). In our large scale screening for
suitable anticancer agents from herbal plants,
13-methyl-palmatrubine exhibited an antiproliferative effect on
various cell lines. Further experiments indicated that
13-methyl-palmatrubine induced apoptosis and arrested the cell
cycle in lung cancer A549 cells, suggesting that
13-methyl-palmatrubine may be an antitumor compound for lung cancer
treatment.
Materials and methods
Materials
RPMI-1640 medium, fetal bovine serum (FBS),
pancreatin, penicillin and streptomycin were obtained from Gibco
(Carlsbad, CA, USA). Terminal deoxynucleotidyl transferase
(TdT)-mediated dUTP nick end-labeling (TUNEL) Apo-Green Detection
kit was supplied by Roche (Roche, Basel, Switzerland). The Annexin
V and PI kit was purchased from Biotool (Selleck Chemicals,
Houston, TX, USA). Protein and RNA extraction kits, BCA protein
assay kit, propidium iodide (PI), caspase-3 and -9 activity kit,
dimethyl sulfoxide (DMSO), 4′,6-diamidino-2-phenylindole (DAPI) and
Hoechst 33243 were purchased from Beyotime Institute of
Biotechnology (Beyotime, Haimeng, China). 13-Methyl-palmatrubine
standard preparation (purity >98%) was purchased from the
National Institute for the Control of Pharmaceutical and Biological
Products (Beijing, China). The JC-10 Mitochondrion Membrane
Potential Assay Kit was obtained from AAT Bioquest (Sunnyvale, CA,
USA). ECL Advanced Detection kit was provided by Thermo Fisher
(Waltham, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-2H
tetrazolium bromide (MTT) and bovine serum albumin (BSA) was
purchased from Sigma-Aldrich (St. Louis, MO, USA). Primary
antibodies and HRP-labeled secondary anti-mouse/anti-rabbit
antibodies were provided by Cell Signaling Technology (CST;
Beverly, MA, USA). All other chemicals needed were of analytic
grade.
Cell lines and cell culture
The human cell lines used in the present study
included A549, a human lung cancer cell line; HCT116, a human colon
carcinoma cell line; MCF-7, a human breast cancer cell line;
MKN-45, a human cancer cell line; HepG2, a human hepatocellular
carcinoma cell line, L02, a human normal liver cell line; and
HEK293 a human kidney normal cell line obtained from the Cell Bank
of the Chinese Academy of Sciences (Shanghai, China). Cells were
grown in a humidified atmosphere of 5% CO2 at 37°C with
RPMI-1640 medium. Cells were supplemented with 10% fetal calf serum
containing antibiotics (100 IU/ml penicillin and 100 IU/ml
streptomycin).
Cytotoxicity assay in vitro
Cells were seeded and treated with
13-methyl-palmatrubine at increasing concentrations. The
13-methyl-palmatrubine standard was dissolved in DMSO at a
concentration of 300 µg/ml as the initial dose. Then, the
stock solution was maintained at −80°C. The solutions were diluted
to the desired concentrations when used. 13-Methyl-palmatrubine was
dissolved in DMSO with a final DMSO concentration <0.1%.
Cytotoxicity of the control and treated cells was measured using
the MTT assay. Cells (5×103) were cultured in 96-well
plates treated with the indicated concentratins for the indicated
times. The absorbance of the treated and control cells at 570 nm
was assessed using a microplate reader (PerkinElmer, Inc., Waltham,
MA, USA).
Cell cycle distribution assay by flow
cytometry
Cell cycle analysis was determined by PI staining.
Briefly, A549 cells were treated with 13-methyl-palmatrubine.
Treated and control cells were harvested, washed twice with
phosphate-buffered saline (PBS) and fixed with pre-cooled 70%
ethanol for 4 h at 4°C. Fixed cells were washed, pelleted,
re-suspended in 500 µl PBS containing 50 µg RNase A
at 37°C and then stained with 5 µg PI in the dark at room
temperature for 30 min. Finally, cell cycle distributions were
immediately assessed using a FACSCalibur cytometer (BD Biosciences,
San Jose, CA, USA).
Cell morphological assay
Hoechst 33342 staining
The non-small cell lung cancer (NSCLC) A549 cells
were cultured in 6-well plates and treated with increasing doses of
13-methyl-palmatrubine. The cells were fixed with 4%
paraformaldehyde and incubated with Hoechst 33342 (5 µg/ml)
for 10 min. After washing with cold PBS, the A549 cells after
treatment and the control cells were observed by inverted
fluorescence microscopy (D5100; Nikon, Tokyo, Japan).
TUNEL staining
The NCSLC A549 cells were cultured and treated on a
specific glass cover in 6-well plates and treated as mentioned
above. TUNEL kit was used to determine DNA fragmentation in
apoptotic cells according to the manufacturer's instructions. The
cells were stained by TUNEL and DAPI for image analysis. The
samples after treatment and the control cells were viewed by
Apo-Green fluorescence at 520 nm and blue DAPI at 460 nm using a
fluorescence microscope (D5100).
Apoptosis analysis with Annexin
V/PI
The A549 cells were cultured and treated as
mentioned above. A FITC-Annexin V/PI cell apoptosis assay was
conducted to evaluate the apoptosis ratio in the cells according to
the kit manufacturer's instructions. In brief, the untreated and
treated cells were washed and harvested in 100 µl 1X binding
buffer, and 5 µl Annexin V-FITC solution and 5 µl PI
staining solution were added to each 100 µl of cell
suspension. Then, the cells were incubated for 15 min. Binding
buffer (400 µl) was added to the cell suspensions before
determination by FACSCalibur flow cytometry (BD Biosciences).
Mitochondrial membrane potential (MMP)
assay
The AAT JC-10 assay kit was used to evaluate the
changes in MMP. The kit was used according to the manufacturer's
instructions. Briefly, A549 cells were seeded in a 96-well plate,
and 50 µl/well JC-10 solution was added into the cell
suspensions. Cell suspensions were incubate in a 37°C incubator
with 5% CO2 for 30 min. The cells were washed twice with
pre-cold PBS, re-suspended in assay buffer B and immediately
examined on a microplate reader under excitation filter of 490 nm
while emission filter of 525 and 590 nm, separately (PerkinElmer,
Inc.).
Animals
The present study was strictly conducted according
to the Declaration of Helsinki and the Guide for the Care and the
Use of Laboratory Animals as adopted and promulgated by the United
States National Institutes of Health. All animal experiments were
approved by the Institutional Animal Care and Use Committee of
FuDan University. Nude mice (6 weeks), half males and half females,
were provided by Shanghai SLAC Laboratory Animal Center. Healthy
A549 cells were harvested, washed 3 times, and suspended in cold
PBS. Each mouse was injected intraperitoneally with
1×106 A549 cells. The nude mice were then divided into
four groups: control, low dose, medium dose and high dose groups,
with 6 mice in each group. When the volume of the tumor reached 100
mm3, 13-methyl-palmatrubine was injected into the nude
mice, while saline was injected in the control group. All groups
were administered the injection every 3 days for a total of 7
treatments. The nude mice were sacrificed 24 h after the last
treatment, and the tumor xenografts were removed and measured.
Tumor sizes were measured every 3 days using micrometer calipers,
and tumor weights were calculated at the conclusion of the
experiments.
Immunohistochemistry
The paraffin-embedded implanted tumor samples were
stained using cleaved-caspase-3 and Ki67 antibodies separately for
immunohistochemistry according to the manufacture's instructions.
Images were captured by a fluorescence microscope (D5100).
Western blotting
A549 cells were planted and treated with the
designated concentrations of 13-methyl-palmatrubine for the desired
times. The cells were harvested, washed and lysed in RIPA lysis
buffer. Then, the lysate was centrifuged at 12,000 rpm for 10 min
at 4°C. The BCA kit was used to determine the protein level of the
supernatant. Proteins were separated with 8–15% SDS-PAGE,
transferred to polyvinylidene fluoride (PVDF) membranes, and
incubated with the respective primary antibody at 4°C overnight.
Then, the proteins were subsequently incubated with the secondary
antibody. The signals were determined by system (BD Biosciences).
The β-actin antibody was chosen as the control.
Analysis of data
Triplicate experiments were performed with
independent samples. The results are expressed as means ± standard
deviation (SD). The results were analyzed using ANOVA t-test to
assess statistical significance. A P-value at <0.05 was
considered to indicate a statistically significant result. All
statistical analyses were performed using commercially available
statistical software (SPSS 19.0; SPSS, Inc., Chicago, IL, USA).
Statistical differences were considered at the P<0.05, P<0.01
or P<0.001 level vs. the control group as indicated in the
figures and legends.
Results
13-Methyl-palmatrubine inhibits
proliferation and induces cell cycle arrest and apoptosis in the
A549 cells in vitro
To verify the effect of 13-methyl-palmatrubine on
cancer cell growth, cells were treated with various concentrations
of 13-methyl-palmatrubine for 48 h. MTT assay was used to assess
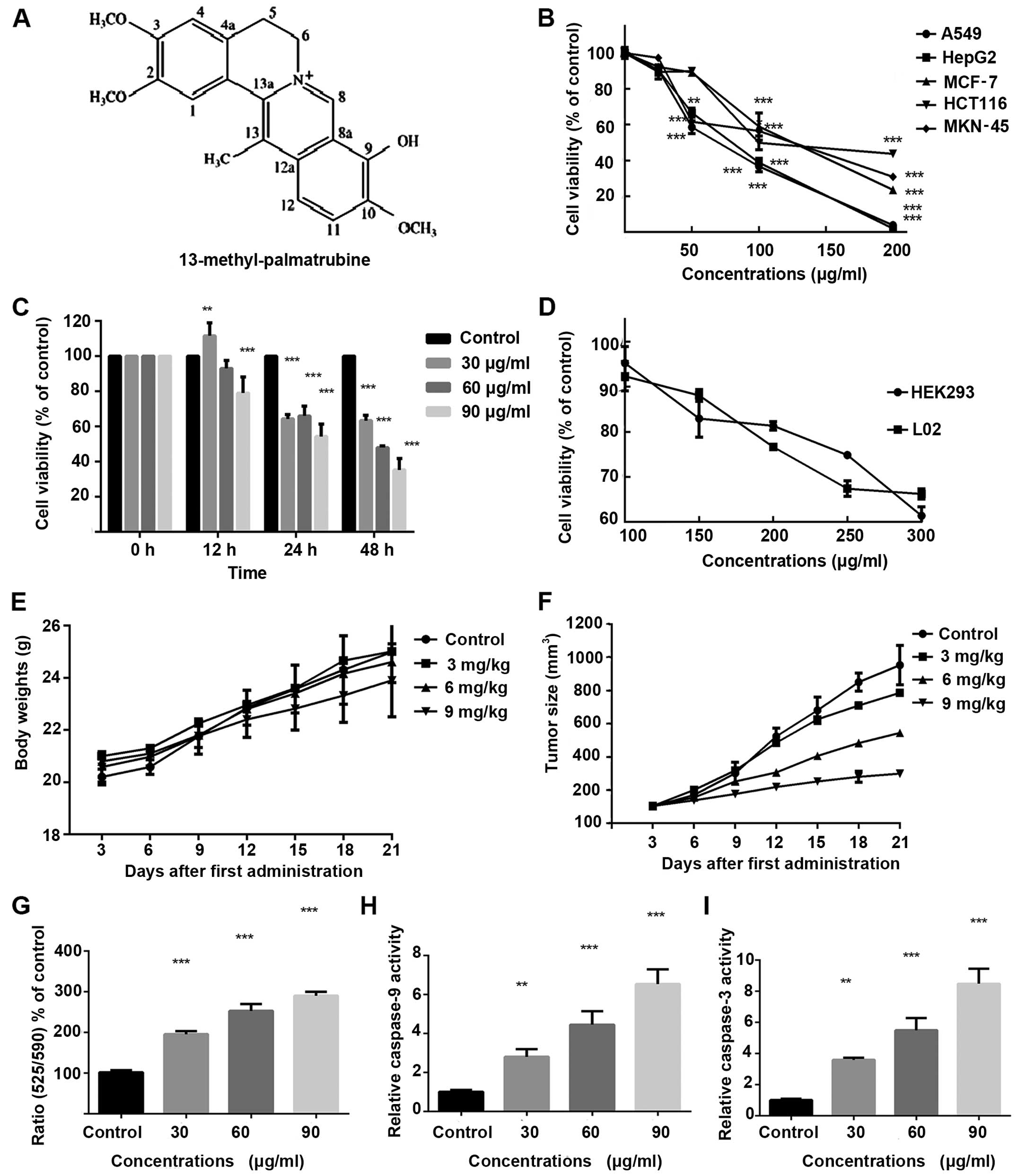
the cell viability after treatment. As shown in Fig. 1B, the viability of five cancer cell
lines was decreased in a concentration-dependent manner. Compared
with the A549 cells, the other human cancer cell lines demonstrated
more resistance to the 13-methyl-palmatrubine treatment, indicating
that A549 human lung cancer cells are more susceptible to
13-methyl-palmatrubine. Furthermore, cell-induced apoptosis by
13-methyl-palmatrubine in A549 cells was further confirmed in a
time- and dose-dependent manner, as shown in Fig. 1C. The most efficacious
13-methyl-palmatrubine treatment time was 48 h. The IC50
value of 13-methyl-palmatrubine in the A549 cells was 58.57±3.58
µg/ml at 48 h. Significantly higher IC50 values
for 13-methyl-palmatrubine in two normal human cell lines (L02 and
HEK293) are shown in Fig. 1D,
suggesting the relative safety of 13-methyl-palmatrubine.
In order to investigate the inhibitory effect on
proliferation induced by 13-methyl-palmatrubine treatment, multiple
assays were conducted to analyze whether 13-methyl-palmatrubine
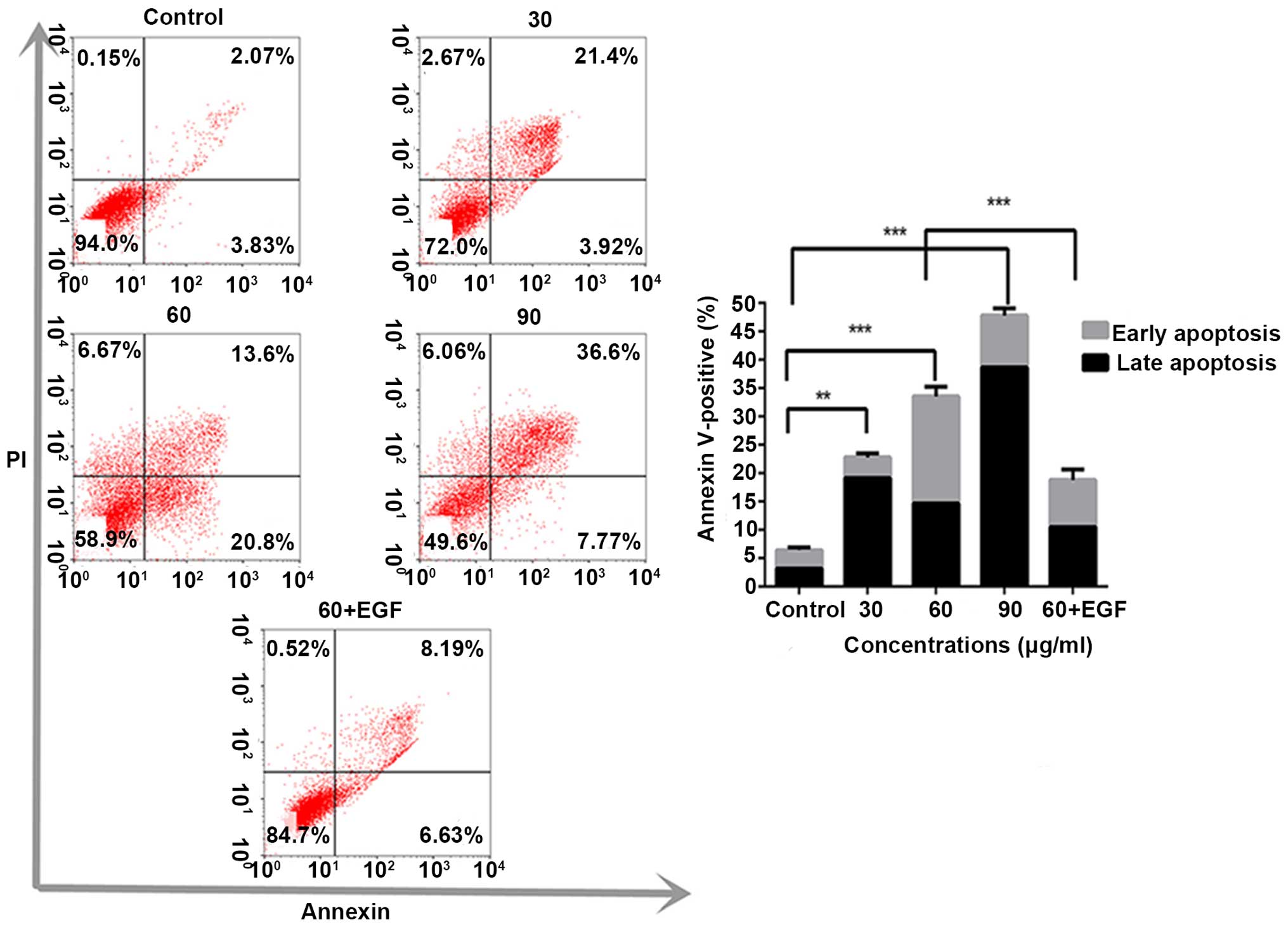
induces apoptosis in A549 cells. Based on the positive preliminary
MTT results, we conducted the Annexin V/PI assay to determine the
apoptotic population in the 13-methyl-palmatrubine-treated and
control cells. 13-Methyl-palmatrubine treatment significantly
increased the percentage of apoptotic cells when compared with the
control, as shown in Fig. 2.
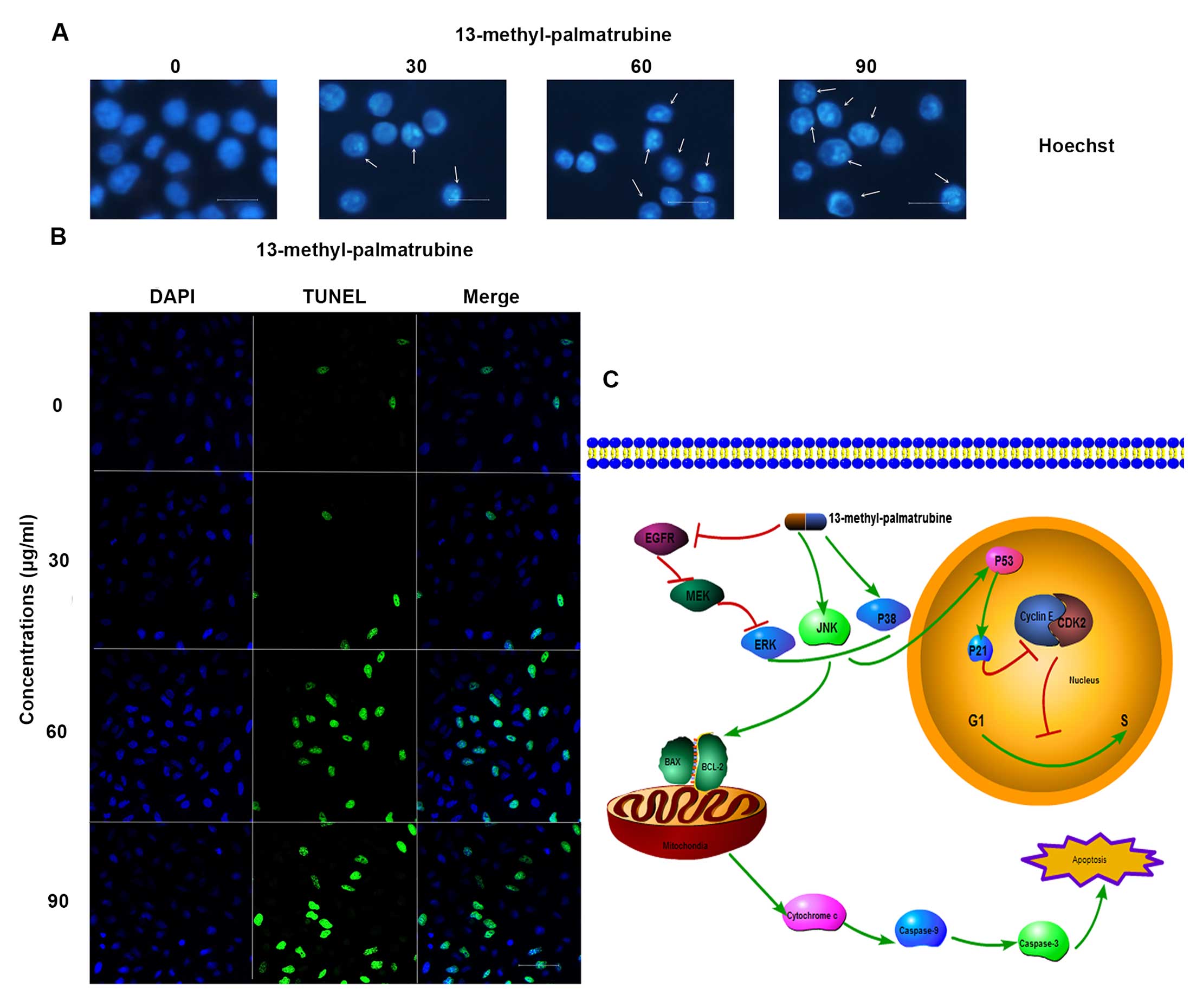
Hoechst staining exhibited differences in cell morphology displying
cell shrinkage, nuclear fragmentation and chromatin compaction
(Fig. 4A), which commonly
represents typical apoptosis in most apoptotic cases. Consistently,
TUNEL staining demonstrated an increased ratio of TUNEL (green) in
the 13-methyl-palmatrubine-treated cells (Fig. 4B).
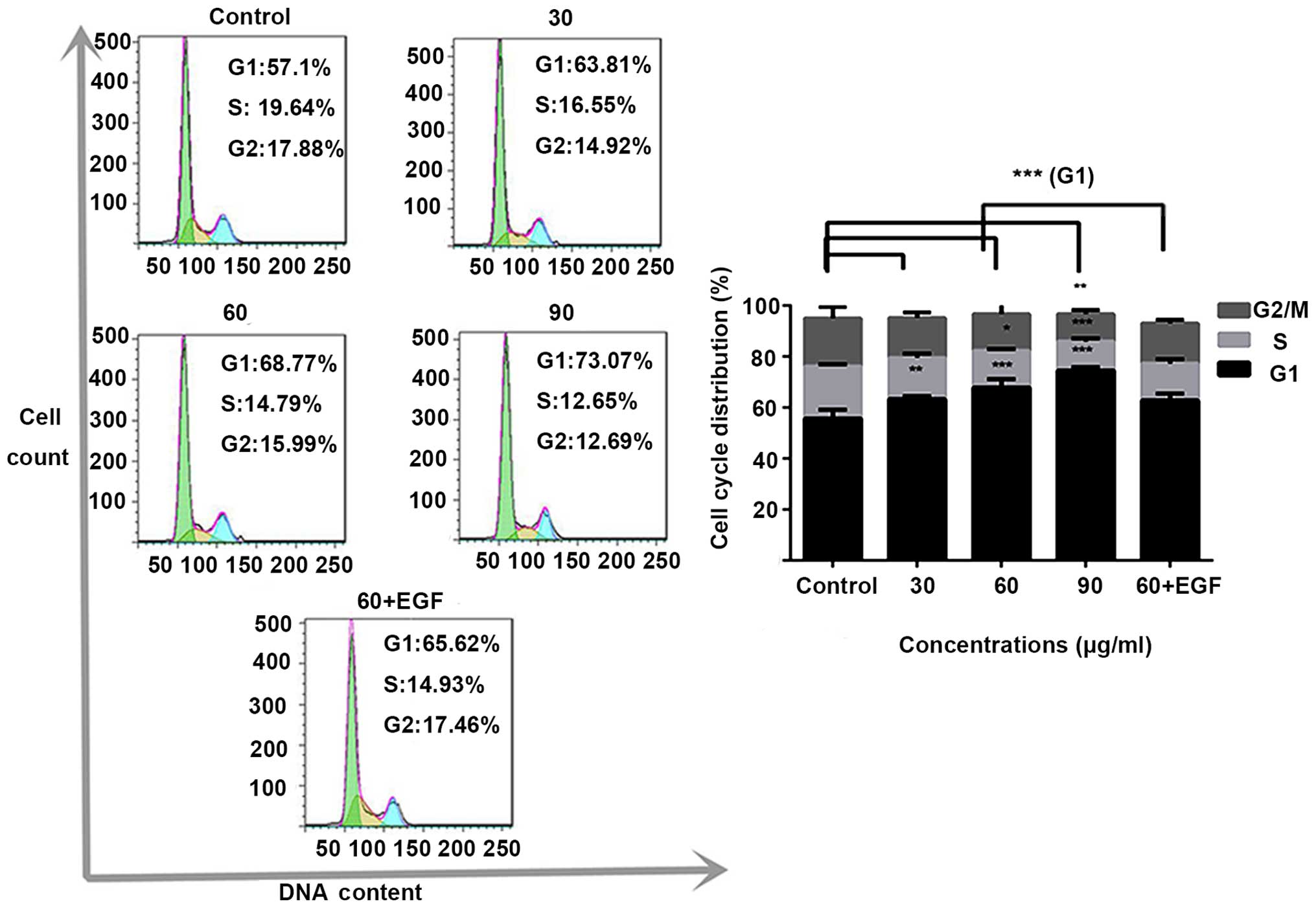
Subsequently, analysis of cell cycle distribution by
flow cytometry was performed. As shown in Fig. 3, 13-methyl-palmatrubine treatment at
increasing concentrations in the A549 cells led to a G1
phase accumulation of cells. These results showed that
13-methyl-palmatrubine treatment induced an accumulation of A549
cells in the G1/S phase in a dose-dependent manner.
Collectively, these results showed that 13-methyl-palmatrubine
inhibited A549 cell proliferation via G1/S cell cycle
phase arrest and apoptosis.
13-Methyl-palmatrubine inhibits
proliferation and induces cell cycle arrest and apoptosis in A549
cells in vivo
An in vivo study was conducted to evaluate
the antiproliferative effect of 13-methyl-palmatrubine. During the
study, no marked change in mouse body weight was noted (Table I and Fig. 1E). This implied that injection of
13-methyl-palmatrubine was not significantly toxic to the nude
mice. After treatment for 21 days, the tumors treated with
13-methyl-palmatrubine were smaller than that noted in the control
group (Table I and Fig. 1F). Therefore, we suggested that
13-methyl-palmatrubine may be a promising approach toward antitumor
treatment. The results were consistent with the in vitro
study.
 | Table IInhibitory effect of
13-methyl-palmatrubine on A549 implantation tumor growth in
BALB/c-nu mice. |
Table I
Inhibitory effect of
13-methyl-palmatrubine on A549 implantation tumor growth in
BALB/c-nu mice.
| Groups | Surviving animals
(n) | Body weight (g)
| Tumor weight
(g) | Inhibition rate
(%) |
|---|
| Start | End |
|---|
| Control | 6 |
20.2±0.30 | 24.98±1.17 | 1.40±0.18 | |
| Treatment | | | | | |
| 3 mg/kg | 6 | 20.88±0.36 | 24.58±1.22 |
0.91±0.23a | 34.59 |
| 6 mg/kg | 6 | 20.77±0.47 | 24.61±1.08 |
0.62±0.23c | 55.78 |
| 9 mg/kg | 6 | 20.58±0.58 | 23.90±1.40 |
0.38±0.16c | 73.18 |
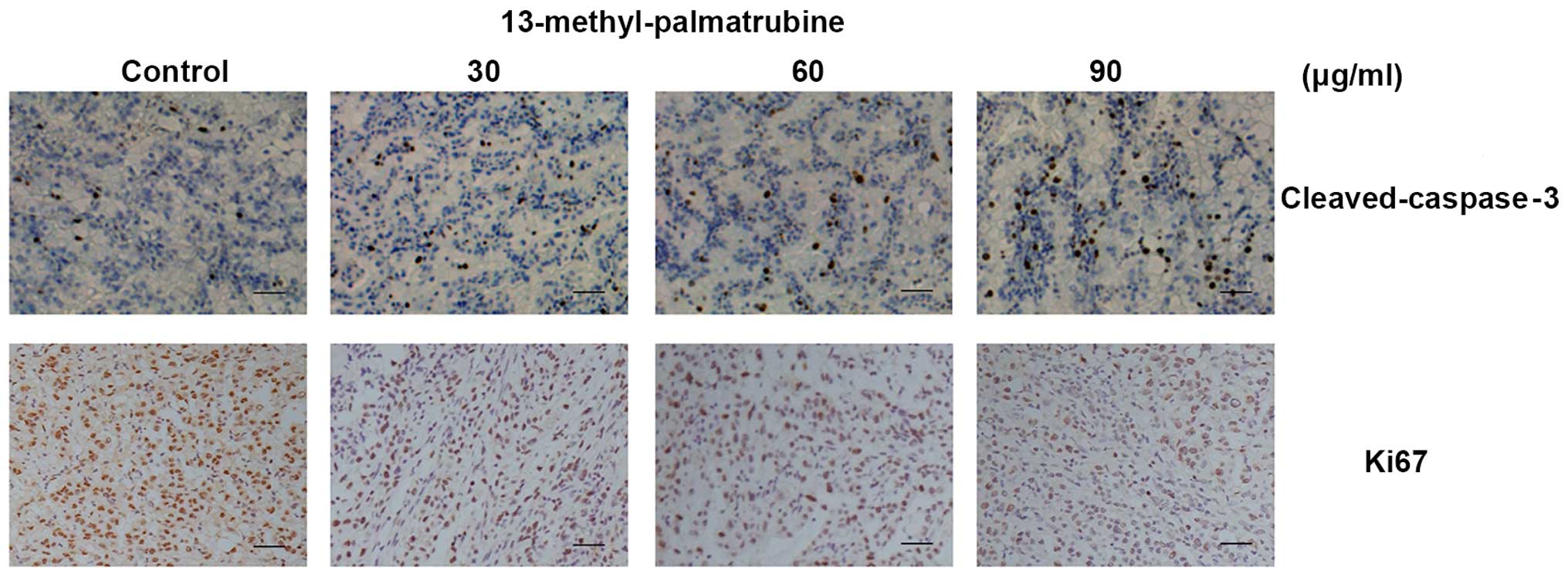
We found a significant decrease in the Ki67-positive
level in cells in the treatment group as compared with that noted
in the control group. Meanwhile, cleaved-caspase-3-positive cells
were increased in a concentration-dependent manner (Fig. 5), which indicated the apoptotic and
antiproliferative effects of 13-methyl-palmatrubine in
vivo.
The mechanism of 13-methyl-palmatrubine
in apoptosis
The JC-10 assay indicated the loss of ΔΨm after
13-methyl-palmatrubine treatment. Loss of ΔΨm is a typical event in
the early phase of the mitochondrial apoptotic pathway. Thus, we
choose 24 h as the experiment time in the JC-10 assay to
investigate the loss of MMP. As shown in Fig. 1G, our findings indicated that the
red fluorescence (normal cells) was decreased while the green
fluorescence (apoptotic cells) increased in the
13-methyl-palmatrubine treated cells. The decrease in the ratio of
red/green fluorescence indicated that 13-methyl-palmatrubine
induced the collapse of MMP (ΔΨm) in the A549 cells. Caspase-9 and
caspase-3 activity assay demonstrated activation of caspase-9 and
caspase-3. As shown in Fig. 1H and
I, 13-methyl-palmatrubine induced a significant activation of
caspase-3 and -9 in a dose-dependent manner, and there was a
significant difference between the control and
13-methyl-palmatrubine-treated cells.
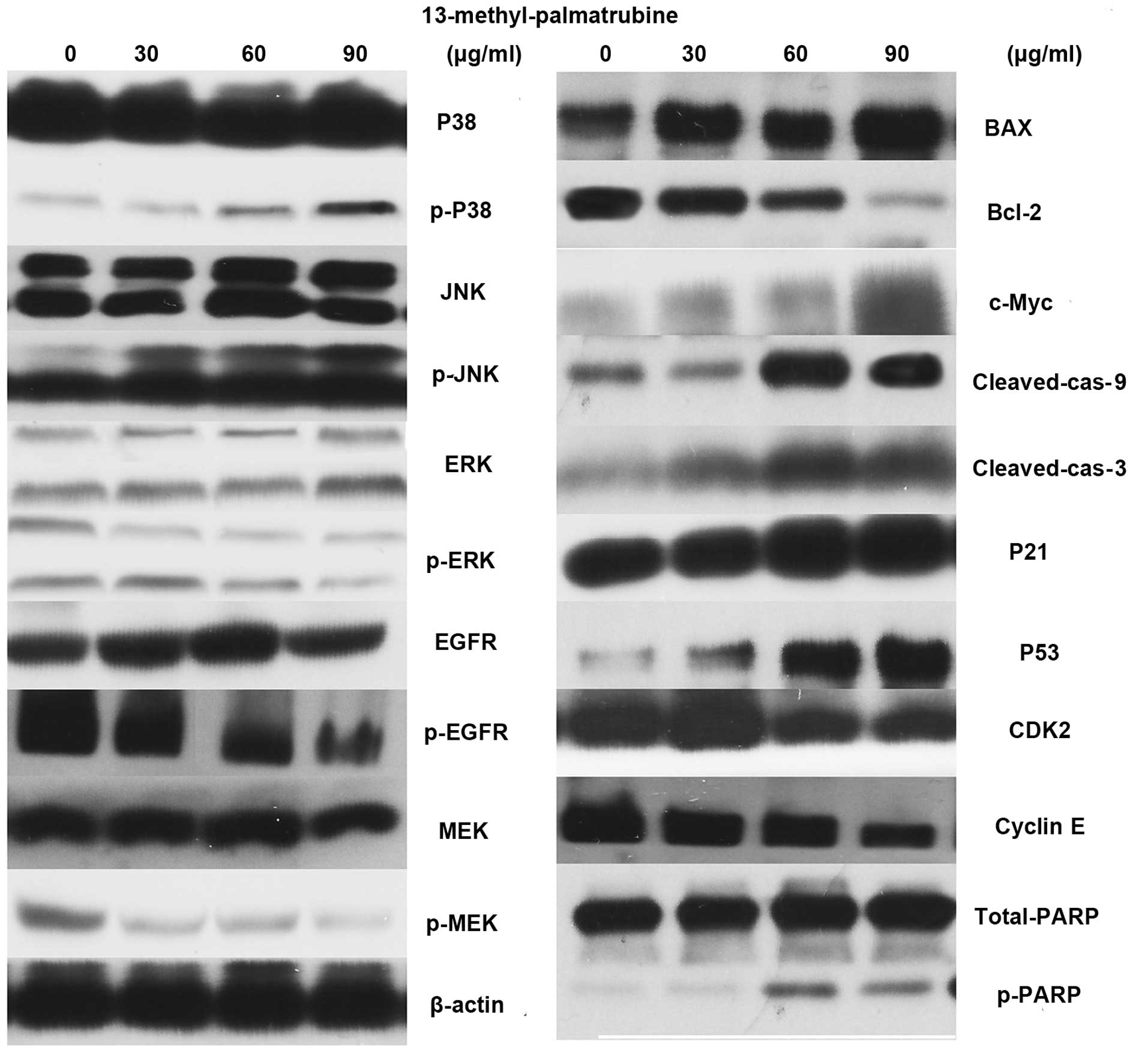
Western blotting of the
13-methyl-palmatrubine-treated A549 cells vs. the control cells
indicated the related apoptotic mechanism (Fig. 6). The epidermal growth factor
receptor (EGFR) pathway leads to numerous effects such as
anti-apoptosis, cell cycle arrest. MAPK is one of its multiple
downstream pathways. In the present study, 13-methyl-palmatrubine
treatment caused downregulation of EGFR, RAS and ERK. Notably,
there was marked upregulation in the MAPK expression level.
Phosphorylated (p)-P38 and p-JNK were increased in a
concentration-dependent manner, while there were no changes in the
total P38 and total JNK levels. The Bax/Bcl-2 ratio was increased.
The induction of apoptotic released c-Myc, and subsequent
activation of caspase-9 and -3 and cleaved PARP.
Mechanism of 13-methyl-palmatrubine in
cell cycle arrest
As above mentioned, there was an accumulation of
cells undergoing cell cycle progression in the G1/S
phase in a dose-dependent manner following 13-methyl-palmatrubine
treatment in the A549 cells. Then, we investigated whether
13-methyl-palmatrubine arrested the cell cycle through expression
of cyclin E and CDK2. Western blotting showed an appreciable
downregulation of cyclin E and upregulation of CDK2 with increasing
concentrations of 13-methyl-palmatrubine. Thus, we examined the
expression level of P53 and P21, which are two important proteins
that lie upstream of cyclin E/CDK2 and commonly result in cell
cycle arrest and apoptosis. Our results indicated that treatment
with 13-methyl-palmatrubine arrested the cell cycle in the
G1/S phase in a dose-dependent manner in the A549 cells
through upregulation of P53, P21 and inhibition of CDK2 and cyclin
E complex (Fig. 6).
Discussion
The antitumor effect of 13-methyl-palmatrubine was
reported 10 years ago. 13-Methyl-palmatrubine was found to exhibit
a moderate cytotoxic effect in several carcinoma cell lines
(11). The present study discovered
the antiproliferative effect of 13-methyl-palmatrubine in a panel
of cancer cell lines. Meanwhile, the IC50 value of
13-methyl-palmatrubine in normal cells was significantly higher
than this value noted in the cancer cell lines. These results
suggested that 13-methyl-palmatrubine may be a promising anticancer
agent. Thus, exploration of the mechanism of 13-methyl-palmatrubine
treatment was required.
The epidermal growth factor receptor (EFGR) protein
is a 170-kDa glycoprotein which consists of an extracellular
ligand-binding domain, a transmembrane domain containing a single
hydrophobic anchor sequence and an intracellular domain with
tyrosine. Phosphorylated EGFR initiates the activation of
downstream pathways, including Janus kinase (JAK) signal transducer
and activator of transcription (STAT), phosphatidylinositol
3-kinase (PI3K)/AKT and mitogen-activated protein kinase (MAPK)
cascades (12,13). Activation of the EGFR downstream
pathway leads to cell proliferation, migration, adhesion,
anti-apoptosis, angiogenesis and metastasis (13,14).
High expression of EGFR is closely associated with multiple
epithelial-derived tumors, for example, breast (15), colon (16), ovarian (17), gastric (18) and lung cancer (19).
At present, novel therapeutic approaches,
particularly antitumor agents, targeting the EGFR signaling pathway
family and their downstream pathways have been developed, such as
gefitinib (20) and afatinib
(21). In the present study, we
evaluated the impact of 13-methyl-palmatrubine treatment on the
EGFR pathway. 13-Methyl-palmatrubine at a moderate concentration
inhibited activation of the EGFR pathway with a downstream effect
on Ras/Raf/MEK/ERK. This effect was closely associated with cell
apoptosis and cell proliferation. The MAPK signaling pathway lies
downstream of EGFR and accepts the signal transmission of EGFR
(22). Numerous antitumor agents
focus on MAPK as a molecular target (23,24).
The ERK of MAPK is inhibited through mediation of the EGFR pathway
(17). Notably,
13-methyl-palmatrubine treatment induced phosphorylation of JNK and
P38 pathways which are other important signaling pathways in MAPK.
The combination effect of EGFR and MAPK led to a subsequent cascade
apoptotic reaction in 13-methyl-palmatrubine-induced cells.
MMPs play a crucial role in the apoptotic cascade
pathways (25). MMP collapse allows
release of cytochrome c from the space between the outer and
inner mitochondrial membranes into the cytosol, and therefore
subsequently triggers caspase activation and other apoptotic
processes (26,27). In the present study,
13-methyl-palmatrubine treatment elicited MMP collapse, and induced
the release of cytochrome c which is associated with the
activation of caspase-3 and -9, and cleavage of PARP. Thereby,
13-methyl-palmatrubine treatment triggers A549 cell death. The
present study suggested that 13-methyl-palmatrubine induced cells
to undergo apoptosis by initiating the intrinsic
mitochondrial-mediated pathway.
Serial study
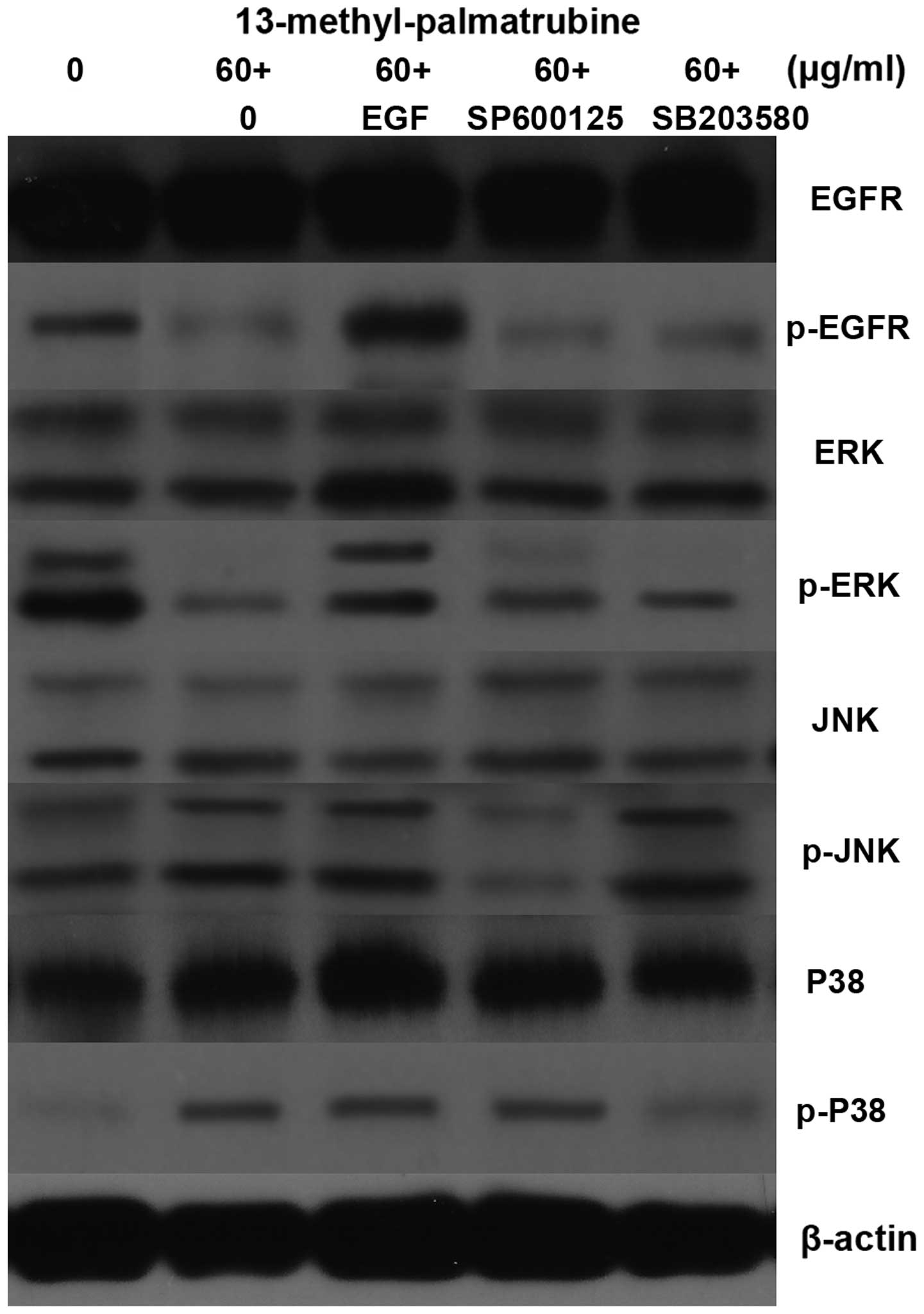
In addition, we conducted a serial study to confirm
the EGFR-MAPK signaling pathway activity in
13-methyl-palmatrubine-treated A549 cells. As known, EGF stimulates
activation of the EGFR signaling pathway (28). At first, the apoptosis and cell
cycle in the A549 cells treated with 13-methyl-palmatrubine at
medium concentrations followed by the addition of EGF to 100 ng/ml
were evaluated. The apoptosis in the 13-methyl-palmatrubine
combined with EGF group was decreased compared with the
13-methyl-palmatrubine only treated group, while the cell cycle was
also arrested (Figs. 2 and 3). The EGFR protein and downstream ERK
protein levels were upregulated in the combination group (Fig. 7). These results demonstrated that
the EGFR signaling pathway plays an important role in the activity
of 13-methyl-palmatrubine in the A549 cells.
Secondly, SP600125 and SB203580 are commonly used to
abolish JNK and P38 signaling pathway phosphorylation, separately.
Thus, they were employed to further investigation the role of the
MAPK signaling pathway in the 13-methyl-palmatrubine-treated A549
cells. As shown in Fig. 7, SP600125
suppressed JNK phosphorylation while it exerted no impact on other
signaling pathways. SB203580 inhibited P38 phosphorylation while it
elicited no impact on other signaling pathways. In conclusion, EGFR
inhibition, JNK activation and P38 activation may run separately
and contribute combination apoptotic effects.
P53 is a critical protein which causes a cellular
response to cell DNA damage in the apoptotic pathway (29). Meanwhile, P53 also plays a crucial
role in stimulating the transcription that arrests the cell cycle
(30). The regulation of the cell
cycle is also an important target of cancer therapy (31). Anticancer drugs usually arrest the
cell cycle at the G1/S or G2/M phase
(32,33). In the present study,
13-methyl-palmatrubine induced a significant increase in
G1/S arrest at increasing concentrations. P53 and its
downstream pathway genes, such as P21, are tightly linked to cell
proliferation, apoptosis and differentiation (34,35).
As mentioned above, western blot analysis demonstrated a
significant increase in P53 and P21 expression. The G1
phase to S phase cell progression is activated by phosphorylated Rb
which is affected by CDK2/cyclin E complexes. Our western blot
results showed that 13-methyl-palmatrubine inhibited the expression
of CDK2 and cyclin E.
In conclusion, in the present study,
13-methyl-palmatrubine was found to exert an antitumor effect via
induced apoptosis and cell cycle arrest. The EGFR signaling pathway
and downstream MAPK signaling pathway played important roles in the
13-methyl-palmatrubine-induced antitumor effect on the A549 cells
(Fig. 4C). In conclusion, the
results suggest that 13-methyl-palmatrubine may serve as a
potential therapeutic anticancer compound against human lung
tumors.
Acknowledgments
The State Administration of Traditional Chinese
Medicine 'Twelfth Five Year Plan' Key Specialty (Chinese Medicine
Geriatrics) and Shanghai 'XinLin New Star Plan' (ZY3-RCPY-2-2031)
provided financial support for the present study. The technological
support was provided by the Shanghai Key Laboratory of Clinical
Geriatric Medicine.
References
|
1
|
Saika K and Sobue T: Cancer statistics in
the world. Gan To Kagaku Ryoho. 40:2475–2480. 2013.In Japanese.
PubMed/NCBI
|
|
2
|
DeSantis C, Naishadham D and Jemal A:
Cancer statistics for African Americans, 2013. CA Cancer J Clin.
63:151–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Safarzadeh E, Sandoghchian Shotorbani S
and Baradaran B: Herbal medicine as inducers of apoptosis in cancer
treatment. Adv Pharm Bull. 4(Suppl 1): S421–S427. 2014.
|
|
4
|
Hiruma W, Suruga K, Kadokura K, Tomita T,
Sekino Y, Komatsu Y, Kimura M and Ono N: Antitumor effects of a
plant extract mixture. Yakugaku Zasshi. 133:487–491. 2013.In
Japanese. View Article : Google Scholar
|
|
5
|
Lu Y, Li CS and Dong Q: Chinese herb
related molecules of cancer-cell-apoptosis: A minireview of
progress between Kanglaite injection and related genes. J Exp Clin
Cancer Res. 27:312008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zheng S, Yang H, Zhang S, Wang X, Yu L, Lu
J and Li J: Initial study on naturally occurring products from
traditional Chinese herbs and vegetables for chemoprevention. J
Cell Biochem Suppl. 27:106–112. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Leung WC, Zheng H, Huen M, Law SL and Xue
H: Anxiolytic-like action of orally administered
dl-tetrahydropalmatine in elevated plus-maze. Prog
Neuropsychopharmacol Biol Psychiatry. 27:775–779. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sagare AP, Lee YL, Lin TC, Chen CC and
Tsay HS: Cytokinin-induced somatic embryogenesis and plant
regeneration in Corydalis yanhusuo (Fumariaceae) - a medicinal
plant. Plant Sci. 160:139–147. 2000. View Article : Google Scholar
|
|
9
|
Wu G, Qian Z, Guo J, Hu D, Bao J, Xie J,
Xu W, Lu J, Chen X and Wang Y: Ganoderma lucidum extract induces G1
cell cycle arrest, and apoptosis in human breast cancer cells. Am J
Chin Med. 40:631–642. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cheng XY, Shi Y, Zheng SL, Jin W and Sun
H: Two new protoberberine quaternary alkaloids from Corydalis
yanhusuo. J Asian Nat Prod Res. 10:1117–1121. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ikekawa T and Ikeda Y: Antitumor activity
of 13-methyl-berberrubine derivatives. J Pharmacobiodyn. 5:469–474.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lurje G and Lenz HJ: EGFR signaling and
drug discovery. Oncology. 77:400–410. 2009. View Article : Google Scholar
|
|
13
|
Uribe P and Gonzalez S: Epidermal growth
factor receptor (EGFR) and squamous cell carcinoma of the skin:
Molecular bases for EGFR-targeted therapy. Pathol Res Pract.
207:337–342. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hynes NE and Lane HA: ERBB receptors and
cancer: The complexity of targeted inhibitors. Nat Rev Cancer.
5:341–354. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Normanno N, Campiglio M, Maiello MR, De
Luca A, Mancino M, Gallo M, D'Alessio A and Menard S: Breast cancer
cells with acquired resistance to the EGFR tyrosine kinase
inhibitor gefitinib show persistent activation of MAPK signaling.
Breast Cancer Res Treat. 112:25–33. 2008. View Article : Google Scholar
|
|
16
|
LaBonte MJ, Wilson PM, Fazzone W, Russell
J, Louie SG, El-Khoueiry A, Lenz HJ and Ladner RD: The dual
EGFR/HER2 inhibitor lapatinib synergistically enhances the
antitumor activity of the histone deacetylase inhibitor
panobinostat in colorectal cancer models. Cancer Res. 71:3635–3648.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kandala PK, Wright SE and Srivastava SK:
Blocking epidermal growth factor receptor activation by
3,3′-diindolylmethane suppresses ovarian tumor growth in vitro and
in vivo. J Pharmacol Exp Ther. 341:24–32. 2012. View Article : Google Scholar :
|
|
18
|
Zhen Y, Guanghui L and Xiefu Z: Knockdown
of EGFR inhibits growth and invasion of gastric cancer cells.
Cancer Gene Ther. 21:491–497. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gadgeel SM, Ali S, Philip PA, Ahmed F,
Wozniak A and Sarkar FH: Response to dual blockade of epidermal
growth factor receptor (EGFR) and cycloxygenase-2 in nonsmall cell
lung cancer may be dependent on the EGFR mutational status of the
tumor. Cancer. 110:2775–2784. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kobayashi S, Boggon TJ, Dayaram T, Jänne
PA, Kocher O, Meyerson M, Johnson BE, Eck MJ, Tenen DG and Halmos
B: EGFR mutation and resistance of non-small-cell lung cancer to
gefitinib. N Engl J Med. 352:786–792. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huguet F, Fernet M, Giocanti N, Favaudon V
and Larsen AK: Afatinib, an irreversible EGFR family inhibitor,
shows activity toward pancreatic cancer cells, alone and in
combination with radiotherapy, independent of KRAS status. Target
Oncol. 11:371–381. 2016. View Article : Google Scholar
|
|
22
|
Dhillon AS, Hagan S, Rath O and Kolch W:
MAP kinase signalling pathways in cancer. Oncogene. 26:3279–3290.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang Y, Yan G, Song X, Wu K, Li Z, Yang C,
Deng T, Sun Y, Hu X, Yang C, et al: STIP overexpression confers
oncogenic potential to human non-small cell lung cancer cells by
regulating cell cycle and apoptosis. J Cell Mol Med. 19:2806–2817.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang G, Tang B, Tang K, Dong X, Deng J,
Liao L, Liao Z, Yang H and He S: Isoquercitrin inhibits the
progression of liver cancer in vivo and in vitro via the MAPK
signalling pathway. Oncol Rep. 31:2377–2384. 2014.PubMed/NCBI
|
|
25
|
Kasahara A and Scorrano L: Mitochondria:
From cell death executioners to regulators of cell differentiation.
Trends Cell Biol. 24:761–770. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ly JD, Grubb DR and Lawen A: The
mitochondrial membrane potential (deltapsi(m)) in apoptosis; An
update. Apoptosis. 8:115–128. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Estaquier J, Vallette F, Vayssiere JL and
Mignotte B: The mitochondrial pathways of apoptosis. Adv Exp Med
Biol. 942:157–183. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li L, Gao Y, Zhang L, Zeng J, He D and Sun
Y: Silibinin inhibits cell growth and induces apoptosis by caspase
activation, downregulating survivin and blocking EGFR-ERK
activation in renal cell carcinoma.
|
|
29
|
Danial NN and Korsmeyer SJ: Cell death:
Critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Seifrtová M, Cochlarová T, Havelek R and
Řezáčová M: Benfluron induces cell cycle arrest, apoptosis and
activation of p53 pathway in MOLT-4 leukemic cells. Folia Biol.
61:147–155. 2015.
|
|
31
|
Malumbres M: Cyclin-dependent kinases.
Genome Biol. 15:1222014. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhuo Z, Hu J, Yang X, Chen M, Lei X, Deng
L, Yao N, Peng Q, Chen Z, Ye W, et al: Ailanthone inhibits Huh7
cancer cell growth via cell cycle arrest and apoptosis in vitro and
in vivo. Sci Rep. 5:161852015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chien CC, Wu MS, Shen SC, Ko CH, Chen CH,
Yang LL and Chen YC: Activation of JNK contributes to
evodiamine-induced apoptosis and G2/M arrest in human
colorectal carcinoma cells: A structure-activity study of
evodiamine. PLoS One. 9:e997292014. View Article : Google Scholar
|
|
34
|
Ondrouskova E and Vojtesek B: Programmed
cell death in cancer cells. Klin Onkol. 27(Suppl 1): S7–S14.
2014.In Czech. View Article : Google Scholar
|
|
35
|
Lai CY, Tsai AC, Chen MC, Chang LH, Sun
HL, Chang YL, Chen CC, Teng CM and Pan SL: Aciculatin induces
p53-dependent apoptosis via MDM2 depletion in human cancer cells in
vitro and in vivo. PLoS One. 7:e421922012. View Article : Google Scholar : PubMed/NCBI
|