Periostin expression in cancer-associated fibroblasts of invasive ductal breast carcinoma
- Authors:
- Published online on: September 15, 2016 https://doi.org/10.3892/or.2016.5095
- Pages: 2745-2754
Abstract
Introduction
Breast cancer is the most prevalent malignant cancer in women in the world. It is highly heterogenic disease, both in terms of its molecular characteristics, as well as clinical course and prognosis (1). Despite of an improvement in early diagnosis and detection of early stages of breast cancer, the growing trend for morbidity and mortality due to this disease is still observed (1).
Periostin (POSTN) is multi-functional, homodimeric glycoprotein with molecular mass of about 93.3 kDa (2,3). POSTN, originally named as osteoblast-specific factor 2, (OSF-2) was first identified in 1993 as a putative cell adhesion protein for preosteoblasts in a mouse osteoblastic MC3T3-E1 cell line (2), and then classified as a new extracellular matrix (ECM) protein (4). The protein structure of POSTN is composed of an N-terminal secretory signal peptide followed by an EMI domain rich in cysteine, 4 internal repeated and conserved fascilin (FAS)-1 domains and a C-terminal variable hydrophilic domain (3,5,6). POSTN plays an important role in collagen fibrillogenesis (7), cell adhesion and wound healing process (8). It also takes part in ECM remodelling by interacting with other proteins, i.e. fibronectin, tenascin-C and type V collagen (4). It is believed that POSTN has also a key influence on the carcinogenesis process. POSTN interacts with multiple cell-surface receptors, most notably integrins (αvβ3, αvβ5, α6β4), and signals mainly via the PI3-K/Akt and other pathways to promote cancer cell survival, epithelial-mesenchymal transition (EMT), invasion, and metastasis (3,4,6,9–11).
There is also a number of studies indicating a significant role of tumour stroma in carcinoma progression (12). Tumour stroma, which is an essential part of the tumour, is formed by a stromal matrix in which cancer cells and the peritumoural stromal cells are embedded. One of the main cellular components of the tumour stroma, in addition to inflammatory cells and endothelial cells, are cancer-associated fibroblasts (CAFs), which may be an important source of POSTN. CAFs play several key functions, among others they promote tumour growth by secretion of growth factors (e.g. EGF, IGF-1, TGF-α). Some of the factors secreted by CAFs, i.e. hepatocyte growth factor (HGF/SF), significantly influence the migration of cancer cells (13). CAFs regulate the angiogenesis process and organise the stroma by producing ECM components (mainly collagen type I, III and V, fibronectin, laminin); simultaneously, they secrete metalloproteinases (MMPs), i.e. MMP-1 and MMP-3 are involved in proteolytic modifications and remodelling of ECM, which are crucial in the process of cell migration, angiogenesis and metastasis (12,14).
For several years studies have indicated increased expression of POSTN in various type of human cancer, among others in breast cancer (15–18), non-small cell lung carcinoma (NSCLC) (19–21), gastric cancer (22–25), colorectal cancer (26–28), ovary (29–31), prostate (32–34) and brain cancers (35–37). However, up till now, there are no data regarding expression of POSTN in stromal cells, the CAFs, either in pre-invasive (DCIS) or invasive (IDC) breast cancers. Taking into account the above-mentioned facts related to the potential role of POSTN in the promotion of cancer cell invasiveness and metastasis processes, it seems important to conduct studies to evaluate POSTN expression in CAFs in IDC and in DCIS and to correlate the results with clinicopathological parameters.
Materials and methods
Patients and clinical samples
The study was performed on archival material of 21 cases of FC (control group), 70 paraffin-embedded samples of IDC and 44 paraffin-embedded samples of DCIS diagnosed from 2000 to 2007. Paraffin blocks containing IDC were obtained from Lower Silesian Oncology Centre in Wroclaw, and samples of DCIS and FC were obtained from the Department of Tumour Pathology of the Maria Sklodowska-Curie Institute of Oncology in Krakow. The present study was approved by the Bioethics Commission at the Wroclaw Medical University. The patient clinicopathological data are listed in Table I. Histopathological evaluation of the hematoxylin and eosin (H&E) stained slides was used to determine the type and the malignancy grade of the tumours (G) according to WHO criteria (38). Most patients were treated by mastectomy or quadrantectomy, and a subsequent axillary lymph node resection. In 53 (75.7%) cases, adjuvant chemotherapy was applied. Only 1 (1.4%) patient received neoadjuvant chemotherapy prior to primary tumour resection. The mean patient age at diagnosis was 59±12.2 years. Molecular investigations were performed on frozen IDC fragments, sampled from 41 patients diagnosed from 2004 to 2006, including 11 cases that have been subjected to laser capture microdissection (LCM). All the samples were collected before treatment initiation.
Table IClinicopathological characteristics of the 70 invasive ductal breast carcinoma (IDC) patients. |
Immunohistochemistry
Immunohistochemical (IHC) reactions were performed using Dako Autostainer Link 48 (DakoCytomation, Glostrup, Denmark) equipment. Rabbit polyclonal antibody directed against Periostin (Novus Biologicals, Littleton, CO, USA) and murine monoclonal antibodies directed against D2-40 (ready-to-use, RTU, Dako), vimentin (Vim, RTU, Dako), α-smooth muscle actin (α-SMA, RTU, Dako), oestrogen receptor (ER) (clone 1D5; 1:100, Dako), progesterone receptor (PR) (clone 635; 1:100, Dako) were utilized.
The sections were first boiled in Target Retrieval Solution buffer using a Pre-Treatment link platform (in order to deparaffinise, rehydrate, and unmask the antigens) and subsequently cooled in a rinsing buffer (TBS/0.1% Tween). The activity of endogenous peroxidase was blocked by 5 min incubation with EnVision FLEX peroxidase-blocking reagent. EnVision FLEX System was used to visualize the antigens. After rinsing the slides in TBS/0.1% Tween buffer, the primary antibodies were applied for 20 min at room temperature. Next, the slides were incubated for 20 min with secondary antibodies, EnVision FLEX/HRP. To visualize the reaction, sections were incubated for 10 min with EnVision FLEX working solution, where 3,3′-diaminobenzidine (DAB) was used as a chromogen. All slides were counterstained with EnVision FLEX hematoxylin. Subsequently, the sections were dehydrated in alcohol and xylene, and then mounted in SUB-X mounting medium. HER-2 receptors were localised with the use of Hercept Test (Dako).
Evaluation of IHC reactions
All IHC sections were evaluated using a BX41 light microscope (Olympus, Tokyo, Japan) by two independent pathologists (P.D. and J.G.) who were blinded to the patient clinical data. POSTN expression in CAFs was assessed using the immunoreactive score (IRS) of Remmele and Stegner (39).
This scale evaluates the percentage of cells with positive reaction (A) and the intensity of the reaction (B). The final score represents the sum of the two values, ranging within the scope of 0–12 (AxB) (Table II). For the assessment of ER and PR expression a four grade scoring system based on tumour cell positivity in the whole tumour section was used: 0 (0% cells stained), 1 (1–10% cells stained), 2 (11–50% cells stained), 3 (51–100% cells stained). The evaluation of expression of HER2 receptors was performed with the use of a scale, in which both the intensity of colour of membrane reaction, as well as the percent of stained cancer cells are taken into account (40).
Laser capture microdissection (LCM)
Fresh frozen IDC specimens were obtained during resections in Lower Silesian Oncology Centre in Wroclaw from 11 patients. The obtained tissues were snap-frozen in liquid nitrogen and stored at −80°C until stromal and cancer cells LCM was performed. To this end, tissue sections (8 µm) were cut on a Leica CM1950 cryostat (Leica Microsytems, Wetzlar, Germany) and placed on a PET membrane slide (MMI, Glattbrugg, Switzerland). The slides were then fixed in 100% isopropyl alcohol and stained using the H&E staining kit Plus for LCM (MMI). LCM was performed using the MMI CellCut Plus system (MMI). Dissected samples were collected on the adhesive lids of 500 µl tubes (MMI).
Real-time PCR
Total RNA from 41 IDC cases was isolated with the use of RNeasy Mini kit (Qiagen, Hilden, Germany), according to manufacturer's instructions. Reverse transcription reactions were performed with the use of High-Capacity cDNA Reverse Transcription kits (Applied Biosystem, Foster City, CA, USA). In turn, total RNA from 11 microdissected stromal and cancer cells were isolated with the use of RNeasy Micro kit (Qiagen), according to manufacturer's instructions. QuantiTect Reverse Transcription kit (Qiagen) was used for cDNA synthesis. Expression of POSTN mRNA was determined by quantitative real-time PCR with using a 7500 Real-time PCR System and iTag Universal Probes Supermix (Bio-Rad, Hercules, CA, USA) according to the manufacturer's protocol. As reference gene β-actin (ACTB) was used. The primers and TaqMan probes used were: Hs00170815_m1 for POSTN, Hs99999903_m1 for β-actin (Applied Biosystem). All reactions were performed in triplicates under following conditions: initial denaturation at 94°C for 30 sec followed by 45 cycles of denaturation at 94°C for 15 sec, and annealing and elongation at 60°C for 60 sec. The relative mRNA expression levels of POSTN were calculated using the ΔΔCt method.
Statistical analysis
Prism 5.0 (Graphpad Software, La Jolla, CA, USA) statistical software was used to analyse the results. The non-parametric Mann-Whitney U test (for unpaired observations) and the Wilcoxon signed-rank test (for paired observations) were used to compare groups of data. The associations between clinical and pathological parameters and the expression of the studied IHC markers were analysed using χ2-test. Survival times were determined by the Kaplan-Meier method, and the significance of the differences was determined by a log-rank test. For each variable, the hazard ratio (HR) and the 95% confidence interval (95% CI) were estimated. In all the analyses, the results were considered statistically significant at p<0.05.
Results
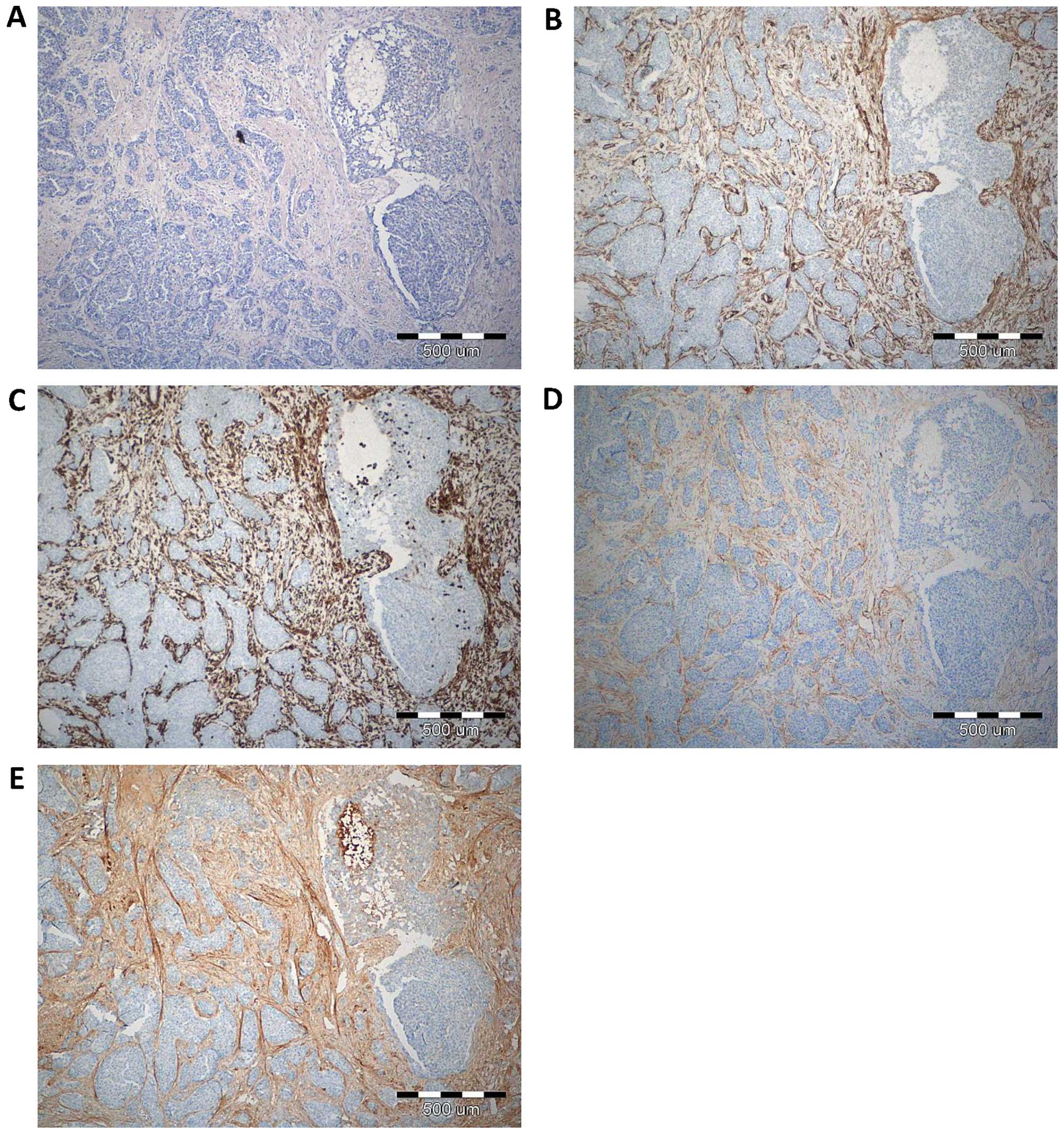
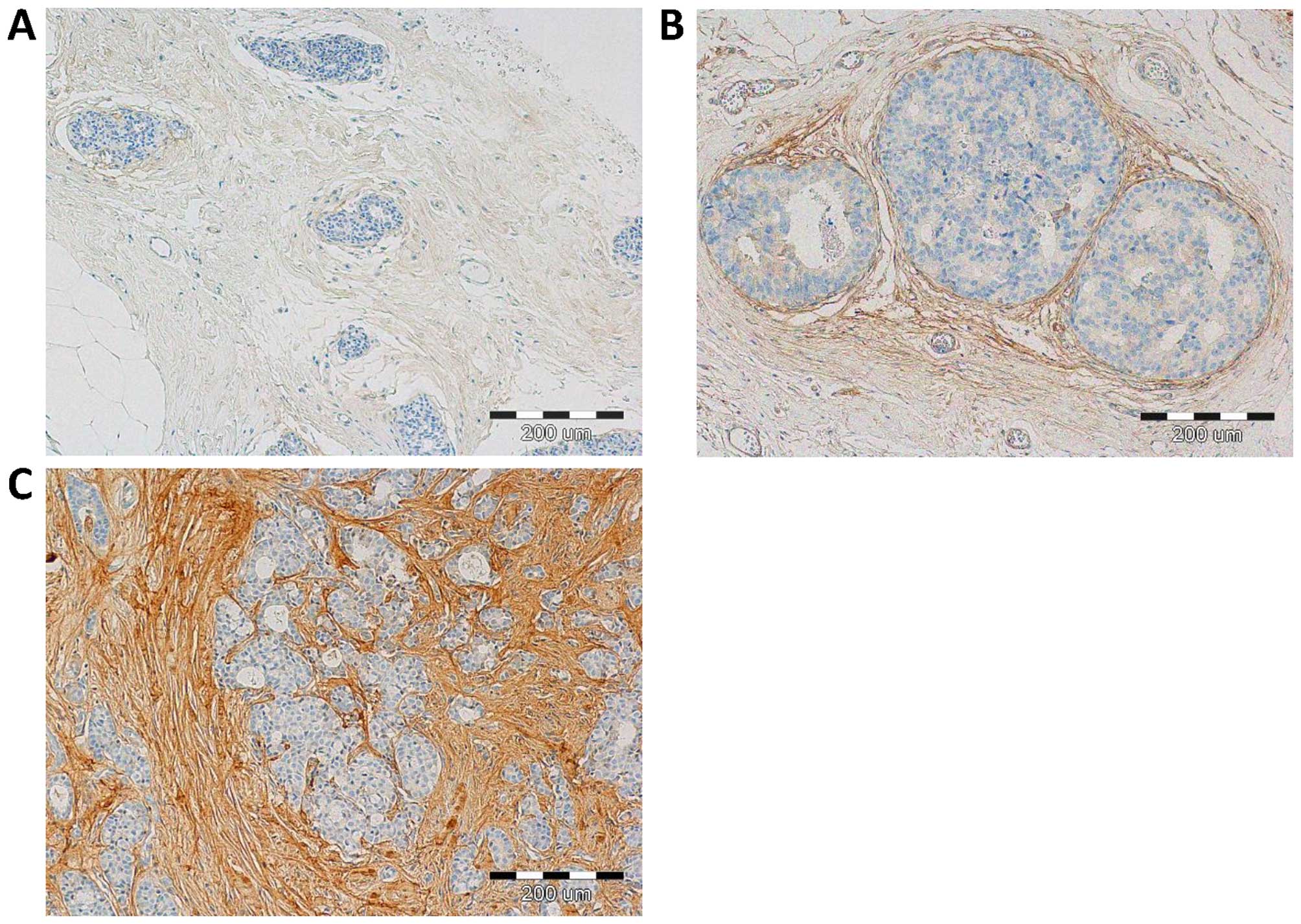
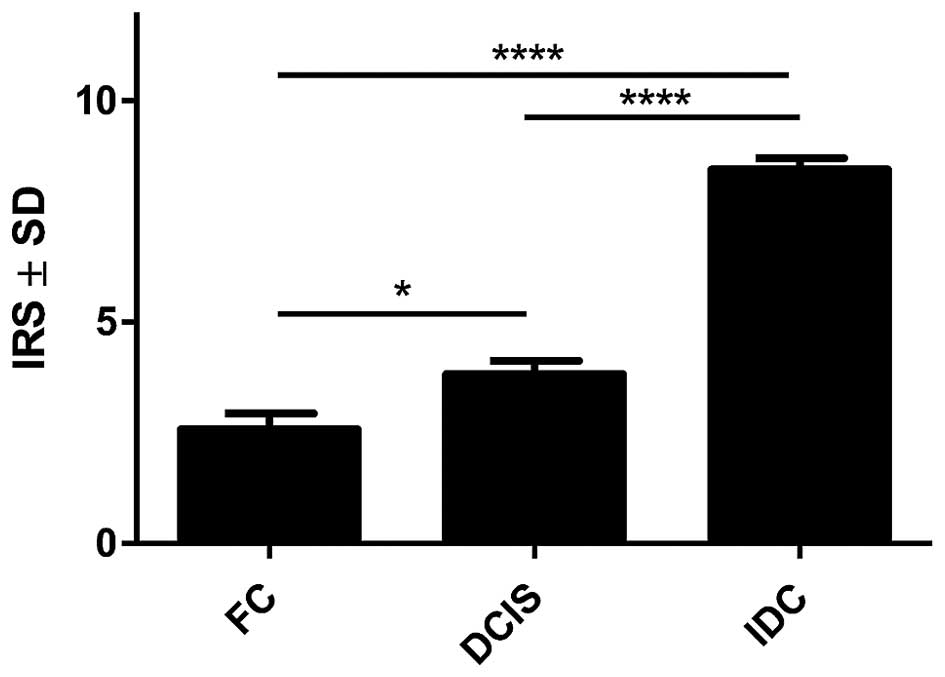
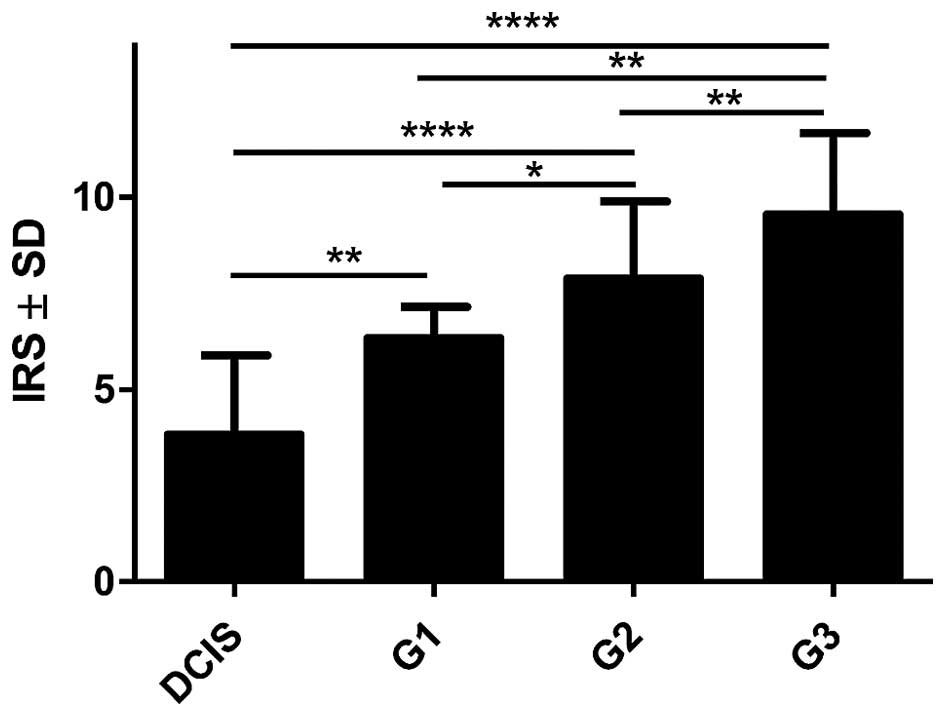
With the use of IHC, POSTN expression was found in 70 (100%) cases. Expression of POSTN was noted mainly in tumour stromal cells, i.e. CAFs, as evidenced by the positive IHC reaction for α-SMA, vimentin and podoplanin (D2-40), - a characteristic marker of CAFs, (Fig. 1). IHC reaction with POSTN expression in CAFs in IDC, DCIS and FC is shown in Fig. 2. Our results showed, that 70% of analysed tumour cases demonstrate high level of POSTN expression in CAFs, evaluated for 8–12 IRS points. The mean value of POSTN expression in CAFs was 8.41±2.21. Statistically significant higher expression level of POSTN in CAFs in IDC as compared to the cases of FC (p<0.0001 Mann-Whitney U test; Fig. 3) was observed. Additionally, statistically elevated POSTN expression in CAFs in IDC relative to the analysed cases of DCIS (p<0.0001) and significantly increased expression of POSTN in CAFs in DCIS in comparison to FC (p=0.0158, Mann-Whitney U test; Fig. 3) was also shown. Moreover, an increasing level of POSTN expression in CAFs with the growing malignancy grade of the tumours (G) was shown in the analysed group of IDC cases. Significant differences were noted using the Mann-Whitney U test between G1 tumours and those of G2 and G3 (p=0.0454 and p=0.0011, respectively). In addition, significant differences were observed between G2 and G3 tumours (p= 0.0036, Mann-Whitney U test, Fig. 4). Furthermore, significantly increased expression level of POSTN in CAFs in IDC in different grades of tumour malignancy (G) was found in comparison to the expression of POSTN in DCIS. Significant differences were noted between G1, G2 and G3 tumours relative to DCIS (p=0.0023, p<0.0001, p<0.0001, respectively, Mann-Whitney U test; Fig. 4).
The correlations between the presence of POSTN expressing CAFs in IDC and the clinicopathological parameters of the patients are summarized in Table III. With the χ2-test, significant correlations were found between high level of POSTN expression in CAFs in IDC (>8 IRS points) and tumour malignancy grade (G) (p=0.0070, Fisher's exact test). However, no significant correlation was found between POSTN expression in CAFs in IDC and the expression of ER, PR and HER2 receptor, the size of primary tumour (pT), metastasis to lymph nodes (pN), menopausal status or the age of patients.
Table IIICorrelations between periostin (POSTN) expression by CAFs and selected clinical pathological characteristics in 70 patients with invasive ductal breast carcinoma (IDC). |
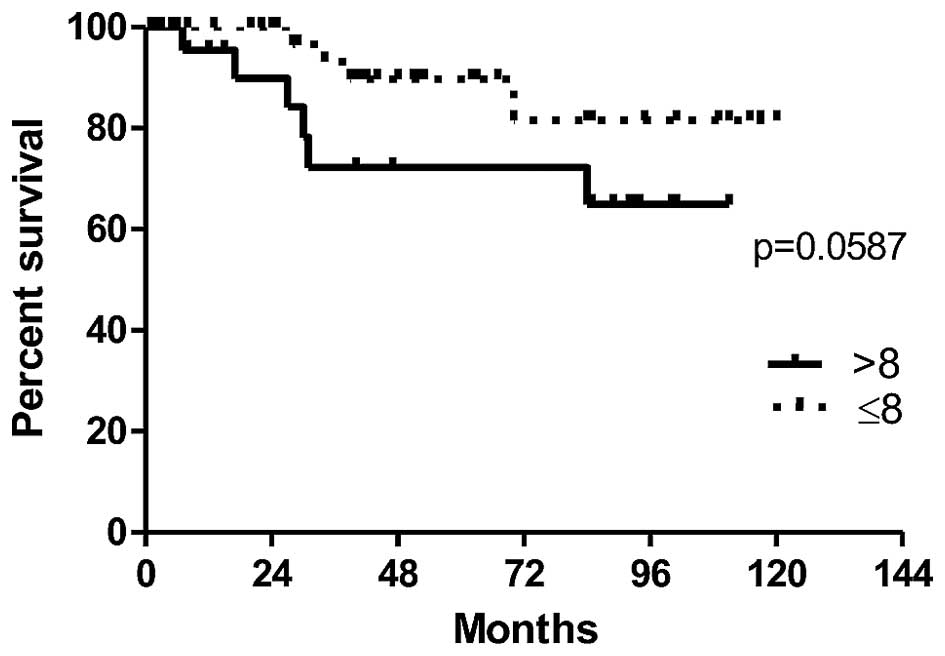
In order to evaluate the prognostic value of POSTN expression by CAFs in IDC cases, the survival of patients depending on the expression level of POSTN was analysed. The association between expression of POSTN in CAFs and overall patients survival time (OS) was found on the border of statistical significance (p=0.0587). The results demonstrate a clear tendency towards a shorter survival in a group of patients with high POSTN expression in CAFs in IDC (>8 IRS points), (Fig. 5). Moreover, univariate survival analysis in a studied group of patients showed that the presence of lymph node metastases (pN) (p=0.046) and the menopausal status (p=0.021) were associated with poor patient survival (Table IV).
Table IVUnivariate Cox proportional hazard analysis in 70 patients with invasive ductal breast carcinoma (IDC). |
Expression of POSTN in 41 cases was evaluated also at the mRNA level by using real-time PCR technique. Additionally, in the 11 of the above-mentioned IDC cases, POSTN mRNA expression was analysed in the microdissected stromal and cancer cells.
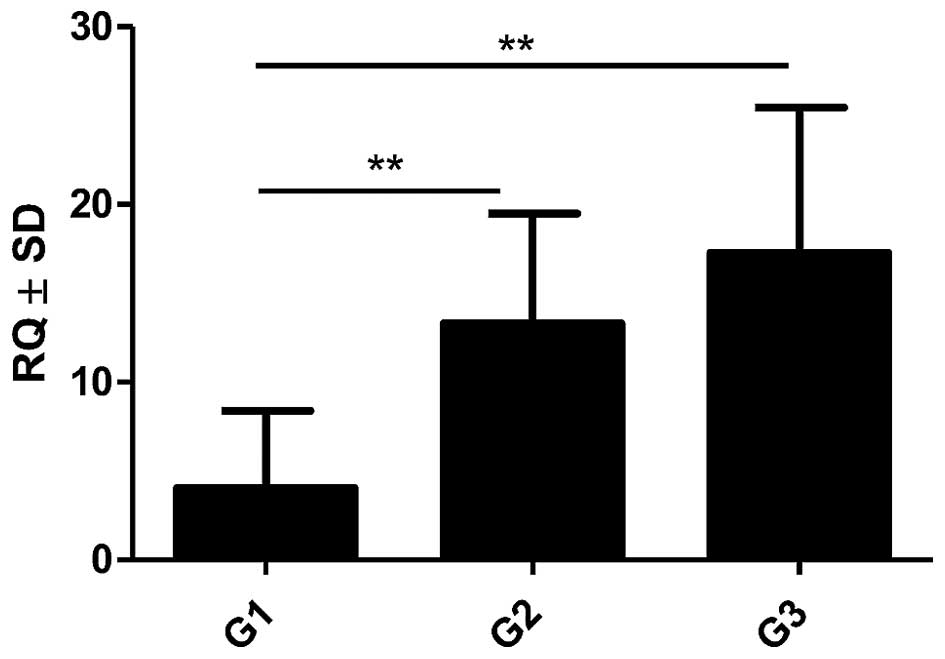
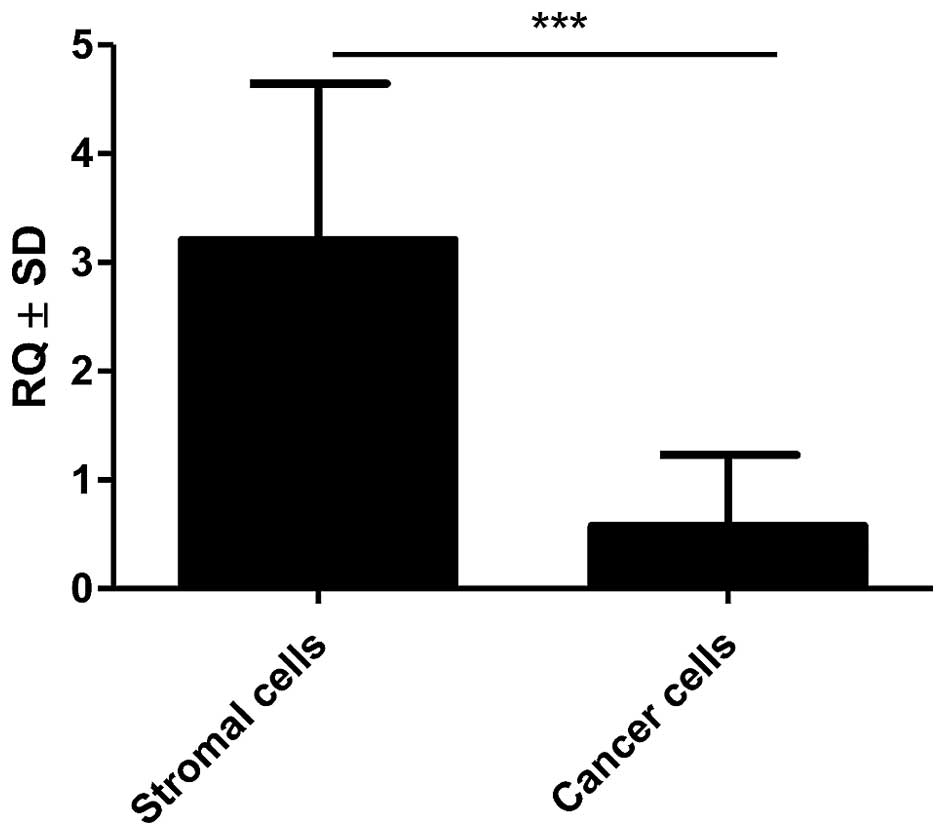
Expression of POSTN gene was noted in 100% (41) of analysed IDC cases. The obtained data showed increasing levels of POSTN mRNA expression which correlated to increased G of the tumours. A statistically higher mRNA expression of POSTN in G2 and G3 cases relative to G1 cases (p=0.0037 and p=0.0023, respectively, Mann-Whitney U test; Fig. 6) was found. Additionally, significantly higher expression of mRNA POSTN in stromal cells (CAFs) relative to cancer cells (p<0.001, Mann-Whitney U test; Fig. 7) was shown in the material from LCM.
Discussion
One of the main mechanisms responsible for cancer invasiveness and metastasis is the EMT process, characterised by the cells losing their epithelial phenotype (i.e. loss of intercellular adhesion, basal-apical polarity and depletion of E-cadherin expression) and acquiring mesenchymal features enabling their migration, invasion or metastasis, such as an increased expression of fibronectin, vimentin and N-cadherin (41,42). As shown in many studies, EMT may be initiated by signalling pathways activated by receptors with tyrosine or serine-threonine kinase activity (42). It is believed that POSTN is one of the factors influencing regulation of intracellular pathway related to 3 phosphatidylinositol kinase (PI3K) and serine-threonine AKT/PKB protein kinase (6,27,43).
POSTN is a glycoprotein that belongs to matricellular proteins (8). This protein plays an important role in numerous biological processes related to carcinogenesis, i.e. proliferation (26), migration (44), angiogenesis (45,46), invasion, and metastasis (45,47). It is believed that POSTN may be a marker for progression in various cancer types. However, the accurate mechanisms responsible for the influence of POSTN on cancer progression and metastasis still remain a subject of further studies.
So far, most research on the role of POSTN expression in breast cancer confirmed POSTN expression localized mainly in cancer cells and, in few cases, in tumour stroma (18). In our work, POSTN expression by stromal cells - CAFs, was shown for the first time, both in pre-invasive (DCIS) and invasive (IDC) tumours. The study confirming POSTN expression in CAFs is positive by IHC reaction performed on the serial sections for α-SMA, vimentin and D2-40, i.e. the CAFs, characteristic markers, was shown in our earlier studies (48).
In the present study, statistically increased expression of POSTN in CAFs in IDC in comparison to cases of FC was found with the use IHC method. It was also shown that the expression of POSTN was significantly higher in CAFs in IDC relative to cases of DCIS. POSTN expression by CAFs seems quite interesting taking into account that Lv et al (23) in their work on gastric cancer showed that the role of POSTN in tumorigenesis is strongly dependent on the type of cells it is derived from. Above-mentioned authors found that epithelial cell-derived POSTN can function as a tumour suppressor in gastric cancer through stabilizing p53 and E-cadherin proteins via the Rb/E2F1/p14ARF/Mdm2 signalling pathway (23).
On the other hand, Kikuchi et al (22) confirmed increased stromal POSTN expression with the increase of the stage of gastric cancer of both intestinal and diffuse type. Similarly to the results obtained by us it was noted that CAFs are the primary source of POSTN, which facilitates tumour cell invasion by inducing EMT and by establishing a neoplastic niche in gastric cancers (22). The research done by the same team in colorectal cancer with the use of IHC method and double immunofluorescent staining confirmed that POSTN is expressed by CAFs, which, is in line with our results (49). However, in contrast to the results of our study, these authors did not analyse the correlation between POSTN expression in CAFs and the tumour malignancy grade (G) (49). Similarly, underwood et al (50) showed a significant role of tumour stroma in oesophageal adenocarcinoma (EAC) progression. It was found that CAFs promote tumour cell invasion in vitro and growth in vivo by signalling to EAC cells via secretion of the ECM protein, POSTN (50).
Comparably to the results obtained by us, it was shown also in the studies conducted by Choi et al (31) that ovarian cancer CAFs are responsible for the deposition of POSTN in the stroma. It was also shown that POSTN expression in CAFs may have prognostic relevance in ovarian cancer (31). Similarly, Ryner et al (51) showed significant role of interactions between ovarian cancer and its microenvironment. The authors of the study emphasised that identification of reactive stromal components, including POSTN, may be helpful in the development of novel diagnostic and therapeutic strategies for overcoming chemoresistance in ovarian cancer (51). Li et al (52) also obtained comparable results in their studies and showed expression of POSTN mainly in the stroma of nasopharyngeal cancer. Additionally, with the use of LCM method and mass spectroscopy, the authors identified different protein expression profiles between the stroma of nasopharyngeal cancer and normal nasopharyngeal mucosa (52). Therefore, it is suggested that the stromal components, including POSTN, may play a crucial role in tumour metastasis and, potentially, they may become the target of pharmacotherapy (52).
The latest proteomic research by Reddy et al (53) also confirmed that the proteome of tumour-adjacent IDC stroma differs from that of tumour-distal stroma, which may have an important meaning in the understanding of the role of stromal cells, especially CAFs, in IDC progression process. It is believed that the studies on tumour stroma may set new directions for searching for target points for new therapies. In line with our results and with the use of IHC method, Zhang et al (16) also confirmed an increased expression of POSTN in breast cancer, which was observed mainly in the stromal compartment and, in few cases, in the cytoplasm of cancer cells. These authors showed also significantly increased expression of POSTN in breast cancers relative to corresponding normal tissues, which is consistent with the results obtained by us (16). Zhang et al (16) observed also positive association between expression of POSTN and the clinical stage of breast cancer, thus indicating an important role of POSTN in progression of this cancer.
Additionally, Puglisi et al (15) also described significantly increased expression of POSTN in breast cancer in comparison to the control group that included benign lesions of this organ. However, opposite to the results obtained by us, POSTN expression was localized mainly in the cytoplasm of cancer cells and, in 12% of cases, a nuclear reactivity was observed (15). Slightly different results of IHC were also obtained by Xu et al (17), where expression of POSTN was indicated mainly in the cytoplasm of breast cancer cells. Noteworthy are also studies by Ishiba et al (54). They showed significantly increased expression of POSTN in tumour phyllodes (mostly benign breast cancer), relative to fibroadenoma. POSTN expression was present mainly in tumour stroma, however, contrary to our results, no POSTN expression in CAFs was shown (54). Additionally, with the use of immunoprecipitation technique and mass spectrometry, the authors showed that in tumour phyllodes and in the in vitro studies POSTN forms a complex with decorin, small leucine-rich proteoglycan (54), which can delay tumour growth by blocking transforming growth factor β (TGF-β) (55) and by interaction with E-cadherin (55,56). It was also proved that knockdown of POSTN results in translocation of decorin from the cytoplasm to the extracellular space, leading to the inhibition of cancer cell migration and invasion. Therefore it is suggested that POSTN-decorin complex may become a potential target for anticancer therapy (54).
In the presented work, in order to confirm obtained results that determine the level of POSTN protein in IDC, the studies on the expression of POSTN gene (mRNA) with the use of quantitative method (real-time PCR) were also performed. In IDC, significant increase of expression of mRNA encoding for POSTN in parallel to growing malignancy grade (G) was found. High level of expression of mRNA POSTN reflected increased level of the protein in CAFs in IDC, as shown by us. Additionally, with the use of LCM method, we confirmed statistically significant higher expression of mRNA POSTN in stromal cells the CAFs in comparison to cancer cells. Significantly higher POSTN mRNA expression in breast cancer relative to normal breast tissue was also shown in the studies by Zhang et al (16) and Shao et al (46). The authors of these studies confirmed obtained results also with the use of IHC method and showed overexpression of POSTN in breast cancer in comparison to the corresponding control tissues, which is consequently in line with the results of our studies.
Moreover, prognostic impact of POSTN expression in stromal cells - CAFs was analysed regarding patient OS. The results showed the association of shorter overall survival time of patients with high expression of POSTN in CAFs, which was on the border of statistical significance. Therefore, it seems that high POSTN expression in CAFs may be associated with poorer prognosis, which was also confirmed by Nuzzo et al (18). Similarly, Hong et al (21) confirmed that in NSCLC, the 3-year overall survival rate for the patients with low levels of POSTN expression in tumour stroma was much higher than for those with high levels of POSTN expression. Such correlation was also observed by Ben et al (57) which showed that in pancreatic cancer, high expression of POSTN in tumour stroma and in cancer cells is associated with shorter patient survival time. The relationship between high POSTN expression in tumour stroma and shorter patient survival time was reported also in prostate cancer (32,33). Therefore it is suggested that in both breast cancer and in other types of cancers with epithelial origin, POSTN expression in tumour stroma, and particularly in CAFs, may be an unfavourable prognostic marker.
The results of our studies are the first that tackled the subject of POSTN expression in CAFs in IDC and DCIS. POSTN expression by CAFs was confirmed on protein and mRNA level with the use of LCM method. Additionally, we showed significantly increased expression of POSTN in CAFs IDC in comparison with FC cases. Significantly elevated expression of POSTN in CAFs in in IDC relative to DCIS was noted, which may indicate the role of POSTN expression by CAFs in the process of cancerous transformation. Moreover, POSTN expression correlated with increasing malignancy grade (G) of the analysed tumours, both on protein and on mRNA level.
In conclusion, POSTN may be a factor that plays an important role in the mechanism of cancer transformation and progression, which raises hope for the possible future usage of this glycoprotein as a target for therapy.
Acknowledgments
The study was subsidized from research funds from 2014 to 2016, as a research project no. Pbmn 141. This study was partially financially supported by the 'Wrovasc-Integrated Cardiovascular Centre' project, co-financed by the European Regional Development Fund, within the Innovative Economy Operational Program, 2007–2013.
References
|
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Takeshita S, Kikuno R, Tezuka K and Amann E: Osteoblast-specific factor 2: Cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem J. 294:271–278. 1993. View Article : Google Scholar : PubMed/NCBI | |
|
Nuzzo PV, Buzzatti G, Ricci F, Rubagotti A, Argellati F, Zinoli L and Boccardo F: Periostin: A novel prognostic and therapeutic target for genitourinary cancer? Clin Genitourin Cancer. 12:301–311. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Ruan K, Bao S and Ouyang G: The multifaceted role of periostin in tumorigenesis. Cell Mol Life Sci. 66:2219–2230. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF and Kudo A: Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res. 14:1239–1249. 1999. View Article : Google Scholar : PubMed/NCBI | |
|
Morra L and Moch H: Periostin expression and epithelial-mesenchymal transition in cancer: A review and an update. Virchows Arch. 459:465–475. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Norris RA, Damon B, Mironov V, Kasyanov V, Ramamurthi A, Moreno-Rodriguez R, Trusk T, Potts JD, Goodwin RL, Davis J, et al: Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J Cell Biochem. 101:695–711. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Hamilton DW: Functional role of periostin in development and wound repair: Implications for connective tissue disease. J Cell Commun Signal. 2:9–17. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Kudo Y, Siriwardena BS, Hatano H, Ogawa I and Takata T: Periostin: Novel diagnostic and therapeutic target for cancer. Histol Histopathol. 22:1167–1174. 2007.PubMed/NCBI | |
|
Michaylira CZ, Wong GS, Miller CG, Gutierrez CM, Nakagawa H, Hammond R, Klein-Szanto AJ, Lee JS, Kim SB, Herlyn M, et al: Periostin, a cell adhesion molecule, facilitates invasion in the tumor microenvironment and annotates a novel tumor-invasive signature in esophageal cancer. Cancer Res. 70:5281–5292. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Ratajczak-Wielgomas K and Dziegiel P: The role of periostin in neoplastic processes. Folia Histochem Cytobiol. 53:120–132. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Franco OE, Shaw AK, Strand DW and Hayward SW: Cancer associated fibroblasts in cancer pathogenesis. Semin Cell Dev Biol. 21:33–39. 2010. View Article : Google Scholar : | |
|
Pennacchietti S, Michieli P, Galluzzo M, Mazzone M, Giordano S and Comoglio PM: Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 3:347–361. 2003. View Article : Google Scholar : PubMed/NCBI | |
|
Kim HM, Jung WH and Koo JS: Expression of cancer-associated fibroblast related proteins in metastatic breast cancer: An immunohistochemical analysis. J Transl Med. 13:2222015. View Article : Google Scholar : PubMed/NCBI | |
|
Puglisi F, Puppin C, Pegolo E, Andreetta C, Pascoletti G, D'Aurizio F, Pandolfi M, Fasola G, Piga A, Damante G, et al: Expression of periostin in human breast cancer. J Clin Pathol. 61:494–498. 2008. View Article : Google Scholar | |
|
Zhang Y, Zhang G, Li J, Tao Q and Tang W: The expression analysis of periostin in human breast cancer. J Surg Res. 160:102–106. 2010. View Article : Google Scholar | |
|
Xu D, Xu H, Ren Y, Liu C, Wang X, Zhang H and Lu P: Cancer stem cell-related gene periostin: A novel prognostic marker for breast cancer. PLoS One. 7:e466702012. View Article : Google Scholar : PubMed/NCBI | |
|
Nuzzo PV, Rubagotti A, Zinoli L, Salvi S, Boccardo S and Boccardo F: The prognostic value of stromal and epithelial periostin expression in human breast cancer: Correlation with clinical pathological features and mortality outcome. BMC Cancer. 16:952016. View Article : Google Scholar : PubMed/NCBI | |
|
Sasaki H, Dai M, Auclair D, Fukai I, Kiriyama M, Yamakawa Y, Fujii Y and Chen LB: Serum level of the periostin, a homologue of an insect cell adhesion molecule, as a prognostic marker in nonsmall cell lung carcinomas. Cancer. 92:843–848. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Morra L, Rechsteiner M, Casagrande S, von Teichman A, Schraml P, Moch H and Soltermann A: Characterization of periostin isoform pattern in non-small cell lung cancer. Lung Cancer. 76:183–190. 2012. View Article : Google Scholar | |
|
Hong LZ, Wei XW, Chen JF and Shi Y: Overexpression of periostin predicts poor prognosis in non-small cell lung cancer. Oncol Lett. 6:1595–1603. 2013.PubMed/NCBI | |
|
Kikuchi Y, Kunita A, Iwata C, Komura D, Nishiyama T, Shimazu K, Takeshita K, Shibahara J, Kii I, Morishita Y, et al: The niche component periostin is produced by cancer-associated fibroblasts, supporting growth of gastric cancer through ERK activation. Am J Pathol. 184:859–870. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Lv H, Liu R, Fu J, Yang Q, Shi J, Chen P, Ji M, Shi B and Hou P: Epithelial cell-derived periostin functions as a tumor suppressor in gastric cancer through stabilizing p53 and E-cadherin proteins via the Rb/E2F1/p14ARF/Mdm2 signaling pathway. Cell Cycle. 13:2962–2974. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Li B, Wang L and Chi B: Upregulation of periostin prevents P53-mediated apoptosis in SGC-7901 gastric cancer cells. Mol Biol Rep. 40:1677–1683. 2013. View Article : Google Scholar | |
|
Liu Y and Liu BA: Enhanced proliferation, invasion, and epithelial-mesenchymal transition of nicotine-promoted gastric cancer by periostin. World J Gastroenterol. 17:2674–2680. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Tai IT, Dai M and Chen LB: Periostin induction in tumor cell line explants and inhibition of in vitro cell growth by anti-periostin antibodies. Carcinogenesis. 26:908–915. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Bao S, Ouyang G, Bai X, Huang Z, Ma C, Liu M, Shao R, Anderson RM, Rich JN and Wang XF: Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell. 5:329–339. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Xiao ZM, Wang XY and Wang AM: Periostin induces chemoresistance in colon cancer cells through activation of the PI3K/Akt/survivin pathway. Biotechnol Appl Biochem. 62:401–406. 2015. View Article : Google Scholar | |
|
Zhu M, Fejzo MS, Anderson L, Dering J, Ginther C, Ramos L, Gasson JC, Karlan BY and Slamon DJ: Periostin promotes ovarian cancer angiogenesis and metastasis. Gynecol Oncol. 119:337–344. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Zhu M, Saxton RE, Ramos L, Chang DD, Karlan BY, Gasson JC and Slamon DJ: Neutralizing monoclonal antibody to periostin inhibits ovarian tumor growth and metastasis. Mol Cancer Ther. 10:1500–1508. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Choi KU, Yun JS, Lee IH, Heo SC, Shin SH, Jeon ES, Choi YJ, Suh DS, Yoon MS and Kim JH: Lysophosphatidic acid-induced expression of periostin in stromal cells: Prognostic relevance of periostin expression in epithelial ovarian cancer. Int J Cancer. 128:332–342. 2011. View Article : Google Scholar | |
|
Tian Y, Choi CH, Li QK, Rahmatpanah FB, Chen X, Kim SR, Veltri R, Chia D, Zhang Z, Mercola D, et al: Correction: Overexpression of periostin in stroma positively associated with aggressive prostate cancer. PLoS One. 10:e01303332015. View Article : Google Scholar : PubMed/NCBI | |
|
Nuzzo PV, Rubagotti A, Zinoli L, Ricci F, Salvi S, Boccardo S and Boccardo F: Prognostic value of stromal and epithelial periostin expression in human prostate cancer: Correlation with clinical pathological features and the risk of biochemical relapse or death. BMC Cancer. 12:6252012. View Article : Google Scholar | |
|
Chen J, Xi J, Tian Y, Bova GS and Zhang H: Identification, prioritization, and evaluation of glycoproteins for aggressive prostate cancer using quantitative glycoproteomics and antibody-based assays on tissue specimens. Proteomics. 13:2268–2277. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou W, Ke SQ, Huang Z, Flavahan W, Fang X, Paul J, Wu L, Sloan AE, McLendon RE, Li X, et al: Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat Cell Biol. 17:170–182. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Mikheev AM, Mikheeva SA, Trister AD, Tokita MJ, Emerson SN, Parada CA, Born DE, Carnemolla B, Frankel S, Kim DH, et al: Periostin is a novel therapeutic target that predicts and regulates glioma malignancy. Neuro Oncol. 17:372–382. 2015. | |
|
Tian B, Zhang Y and Zhang J: Periostin is a new potential prognostic biomarker for glioma. Tumour Biol. 35:5877–5883. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Tavassoli FA, Ortiz-Hidalgo C, Baquera-Heredia J and Grassi P: Images in pathology: The hearts of a breast pathologist, a hematopathologist, and of a cytotechnologist. Int J Surg Pathol. 10:2952002. View Article : Google Scholar : PubMed/NCBI | |
|
Remmele W and Stegner HE: Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe. 8:138–140. 1987.In German. PubMed/NCBI | |
|
Mueller-Holzner E, Fink V, Frede T and Marth C: Immunohistochemical determination of HER2 expression in breast cancer from core biopsy specimens: A reliable predictor of HER2 status of the whole tumor. Breast Cancer Res Treat. 69:13–19. 2001. View Article : Google Scholar | |
|
Hugo H, Ackland ML, Blick T, Lawrence MG, Clements JA, Williams ED and Thompson EW: Epithelial-mesenchymal and mesenchymal-epithelial transitions in carcinoma progression. J Cell Physiol. 213:374–383. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Guarino M: Epithelial-mesenchymal transition and tumour invasion. Int J Biochem Cell Biol. 39:2153–2160. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Baril P, Gangeswaran R, Mahon PC, Caulee K, Kocher HM, Harada T, Zhu M, Kalthoff H, Crnogorac-Jurcevic T and Lemoine NR: Periostin promotes invasiveness and resistance of pancreatic cancer cells to hypoxia-induced cell death: Role of the beta4 integrin and the PI3k pathway. Oncogene. 26:2082–2094. 2007. View Article : Google Scholar | |
|
Gillan L, Matei D, Fishman DA, Gerbin CS, Karlan BY and Chang DD: Periostin secreted by epithelial ovarian carcinoma is a ligand for alpha(V)beta(3) and alpha(V)beta(5) integrins and promotes cell motility. Cancer Res. 62:5358–5364. 2002.PubMed/NCBI | |
|
Siriwardena BS, Kudo Y, Ogawa I, Kitagawa M, Kitajima S, Hatano H, Tilakaratne WM, Miyauchi M and Takata T: Periostin is frequently overexpressed and enhances invasion and angiogenesis in oral cancer. Br J Cancer. 95:1396–1403. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Shao R, Bao S, Bai X, Blanchette C, Anderson RM, Dang T, Gishizky ML, Marks JR and Wang XF: Acquired expression of periostin by human breast cancers promotes tumor angiogenesis through up-regulation of vascular endothelial growth factor receptor 2 expression. Mol Cell Biol. 24:3992–4003. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Kudo Y, Ogawa I, Kitajima S, Kitagawa M, Kawai H, Gaffney PM, Miyauchi M and Takata T: Periostin promotes invasion and anchorage-independent growth in the metastatic process of head and neck cancer. Cancer Res. 66:6928–6935. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Pula B, Jethon A, Piotrowska A, Gomulkiewicz A, Owczarek T, Calik J, Wojnar A, Witkiewicz W, Rys J, Ugorski M, et al: Podoplanin expression by cancer-associated fibroblasts predicts poor outcome in invasive ductal breast carcinoma. Histopathology. 59:1249–1260. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Kikuchi Y, Kashima TG, Nishiyama T, Shimazu K, Morishita Y, Shimazaki M, Kii I, Horie H, Nagai H, Kudo A, et al: Periostin is expressed in pericryptal fibroblasts and cancer-associated fibroblasts in the colon. J Histochem Cytochem. 56:753–764. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Underwood TJ, Hayden AL, Derouet M, Garcia E, Noble F, White MJ, Thirdborough S, Mead A, Clemons N, Mellone M, et al: Cancer-associated fibroblasts predict poor outcome and promote periostin-dependent invasion in oesophageal adenocarcinoma. J Pathol. 235:466–477. 2015. View Article : Google Scholar : | |
|
Ryner L, Guan Y, Firestein R, Xiao Y, Choi Y, Rabe C, Lu S, Fuentes E, Huw LY, Lackner MR, et al: Upregulation of periostin and reactive stroma is associated with primary chemoresistance and predicts clinical outcomes in epithelial ovarian cancer. Clin Cancer Res. 21:2941–2951. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Li M, Li C, Li D, Xie Y, Shi J, Li G, Guan Y, Li M, Zhang P, Peng F, et al: Periostin, a stroma-associated protein, correlates with tumor invasiveness and progression in nasopharyngeal carcinoma. Clin Exp Metastasis. 29:865–877. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Reddy LA, Mikesh L, Moskulak C, Harvey J, Sherman N, Zigrino P, Mauch C and Fox JW: Host response to human breast Invasive Ductal Carcinoma (IDC) as observed by changes in the stromal proteome. J Proteome Res. 13:4739–4751. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Ishiba T, Nagahara M, Nakagawa T, Sato T, Ishikawa T, Uetake H, Sugihara K, Miki Y and Nakanishi A: Periostin suppression induces decorin secretion leading to reduced breast cancer cell motility and invasion. Sci Rep. 4:70692014. View Article : Google Scholar : PubMed/NCBI | |
|
Yamaguchi Y, Mann DM and Ruoslahti E: Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 346:281–284. 1990. View Article : Google Scholar : PubMed/NCBI | |
|
Bi X, Pohl NM, Qian Z, Yang GR, Gou Y, Guzman G, Kajdacsy-Balla A, Iozzo RV and Yang W: Decorin-mediated inhibition of colorectal cancer growth and migration is associated with E-cadherin in vitro and in mice. Carcinogenesis. 33:326–330. 2012. View Article : Google Scholar : | |
|
Ben QW, Jin XL, Liu J, Cai X, Yuan F and Yuan YZ: Periostin, a matrix specific protein, is associated with proliferation and invasion of pancreatic cancer. Oncol Rep. 25:709–716. 2011.PubMed/NCBI |