Introduction
Gastroenteropancreatic neuroendocrine tumors
(GEP-NETs) originate from diffuse neuroendocrine cells that are
dispersed throughout the gastrointestinal tract and islets of
Langerhans in the pancreas. GEP-NETs are relatively rare,
accounting for ~0.5% of all human cancers (1). The estimated annual incidence is
~2–4.5 cases per 100,000 with an increasing trend over recent
decades, partly due to intensified awareness as well as the
application of advanced technology, such as new endoscopic and
imaging techniques (2–4).
These tumors, formerly named carcinoids, can involve
any part of the gastrointestinal tract and the endocrine pancreas,
and have the capability to synthesize and secrete neuropeptides and
hormones which play a key role in causing carcinoid syndrome. The
use of the term carcinoid has therefore been criticized due to its
emphasis on an implied benign behavior. However, it is now clear
that, despite a typically relatively indolent disease course, a
large percentage of these tumors have lethal malignant potential.
GEP-NETs include functional tumors, which secrete a variety of
peptide hormones, and non-functional tumors, which may be
asymptomatic and discovered by chance. Tumor extension,
histological differentiation, lymph node metastasis, lymphatic
invasion, and perineural invasion of the tumor are well known as
the accepted features for the pathological evaluation of GEP-NETs
as well as other malignant tumors of the gastrointestinal tract.
Recent epidemiological studies indicate that gastric and rectal
neuroendocrine neoplasms (NENs) are the most common forms of
GEP-NETs, while duodenal NETs are rare accounting for only 1–2% of
all GEP-NENs (1,5). Liver and lymphatic metastases are the
most common imaging findings at the time of the initial diagnosis
of gastrointestinal endocrine tumors, being detectable in up to 40%
of ileal and 80% of cecal lesions; furthermore, distant metastases
are present in up to 60–80% of pancreatic NETs (PNETs) at first
diagnosis (5).
At present, molecular markers as useful predictors
of malignant behavior have been investigated in more and more
studies. As neuroendocrine markers, synaptophysin (Syn) (a small
vesicle-associated marker) and chromogranin A (CgA) (a large
secretory granule-associated marker) are the mainstays, which may
be identified histologically in patients with GEP-NETs by
immunohistochemistry (IHC). In addition, the use of lymphatic
vessel density (LVD) and pro-lymphangiogenic mediators as
prognostic factors for tumor growth, differentiation and invasion
have been reported to have a significant impact on survival in
patients with GEP-NETs. In addition, the WHO proposed a grading
system for GEP-NETs in 2010, which is based on the Ki-67
proliferation index (G1, ≤2%; G2, 3–20%; G3, ≥20%) or mitotic
index. The Ki-67 proliferation index was found to be strongly
associated with tumor metastasis and prognosis in many studies
(6).
GEP-NETs consistently have a poor prognosis. For
example, 70–85% of non-functional PNETs present with unresectable
disease, often with liver metastases, and their 5-year survival
rate is 30–40%. With the lowest survival rate of the GEP-NETs,
PNETs show a median survival interval of only 24 months. Likewise,
75% of patients with small intestinal NETs either harbor liver
metastases at presentation, or will develop metastases during the
course of their disease. Due to the indolent disease course and
poor prognosis, accurate classification and prognostication are
critical for outlining the prognostic heterogeneity of this group
of tumors ensuring effective treatment. There still exists
controversy between pathologists and clinicians concerning the
nomenclature and classification of GEP-NETs. WHO updated the
classification of GEP-NETs in 2010 and all GEP-NETs were
categorized as malignant tumors. In contrast, according to the
International Classification of Diseases for Oncology, 3rd edition
(ICD-O-3), some GEP-NETs are categorized as benign or of uncertain
malignancy. Therefore, for a more accurate classification of
GEP-NETs, a greater understanding of their biological behavior is
required.
Timely therapeutic interventions for GEP-NET
patients are needed. Neuroendocrine tumors require not only
dedicated interventions to control their capacity to secrete
hormones, but also, antitumor growth strategies. Somatostatin
analog treatment remains a cornerstone of GEP-NET therapy (7). It has been found to be useful in
controlling clinical symptoms arising from hormone secretion and
slowing down disease progression. PNETs have been significantly
responsive to cytotoxic chemotherapy but current prospective data
are lacking (7). New treatment
options for GEP-NETs have become available, and highlight the
necessity of developing predictive biomarkers which will allow for
appropriate and individualized selection of therapy. Early
detection and surgical removal is currently the only reliable
curative treatment for GEP-NET patients, especially for patients
with poorly differentiated neuroendocrine carcinoma (8).
In the present research, we retrieved and analyzed
information concerning clinical characteristics and
metastasis-related risk factors, aiming to analyze the
clinicopathological characteristics of GEP-NETs and explore the
association between tumor metastasis and possible related risk
factors, which can benefit early diagnosis and treatment.
Materials and methods
Participants
This study was approved and monitored by the Ethics
Committee of the Second Xiangya Hospital of Central South
University, China. All participants provided written informed
consent. One hundred and forty-six GET-NET patients at the Second
Xiangya Hospital of Central South University, China, from January
2001 to January 2015, were enrolled in this retrospective study.
All of the data regarding these patients were recorded in a
structured manner that included patient demographic, clinical and
investigational parameters. The case notes of these patients were
reviewed, and information regarding their demography, disease
duration, clinical manifestations, radiological features,
endoscopic features, and treatment (medical, surgical, and
interventional therapy) was retrieved. According to the new WHO
classification and site-specific TMN staging (9–11),
patients in 0 and I stage were divided into the non-metastasis
group (n=96), while patients in II, III, IV stages were included in
the metastasis group (n=50).
We retrospected and summarized the clinical
features, tumor characteristics and IHC staining results of the
GEP-NETs, and collected 15 candidate risk factors for the GEP-NETs
(the 15 risk factors are listed in Table I for details). The risk factors
collected included patient general information, common clinical
manifestations, tumor characteristics and IHC staining results. We
evaluated which features act as metastasizing risk factors for
GEP-NETs below.
 | Table I.Screened risk factors for GEP-NET
metastasis. |
Table I.
Screened risk factors for GEP-NET
metastasis.
| Classification | Risk factors | Independent
variable |
|---|
| General
information | Gender | X1 |
|
| Age >40
years | X2 |
| Clinical
manifestation | Abdominal pain | X3 |
|
| Stool changes | X4 |
|
| Blood in stool | X5 |
| Common tumor | Tumor size | X6 |
|
characteristics | Tumor no. | X7 |
|
| Tumor site | X8 |
|
| Tumor type | X9 |
|
| Infiltration
depth | X10 |
| Pathological
IHC | CgA | X11 |
| parameters | Syn | X12 |
|
| Peritumoral
LVD | X13 |
|
| Intratumoral
LVD | X14 |
|
| Total LVD | X15 |
All procedures performed in studies involving human
participants were in accordance with the Ethical Standards of the
Institutional and/or National Research Committee and with the 1964
Helsinki Declaration and its later amendments or comparable ethical
standards.
Informed consent was obtained from all individual
participants included in the study. The patients volunteered to
participant in our study and they were informed in regards to the
goal and content of the study. The information of all patients was
kept confidential.
Tumor characteristics of the GEP-NETs. The
characteristics of the tumors, such as size, number, site, type,
tumor surface and infiltration depth, were assessed through the
combination of CT scans, MRI and endoscopic manifestation by two
experienced examiners. Tumor size, infiltration depth and presence
or absence of metastatic locoregional lymph nodes were best
assessed by endoscopic ultrasound (EUS). As for the patients who
underwent surgical resection, the characteristics of the tumors
were assessed directly through gross morphology by two
pathologists.
Immunohistochemistry
Sections for immunohistochemistry (IHC) were stained
using the avidin-biotin complex (ABC) method. Four-micrometer thick
sections were cut from the blocks, deparaffinized with xylene and
dehydrated through graded concentrations of alcohol. Endogenous
peroxidase activity was blocked with 3% hydrogen peroxide in
methanol for 10 min. After antigen retrieval, the sections were
then incubated with primary antibodies podoplanin (1:200 dilution,
sc-59347; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), CgA
(1:50, MAB-0202, Maxim) or Syn (1:50, RM-9111; Huaruikang Biotech
Science Co., Ltd, Wuhan, China) or Ki-67 (1:100, ab15580; Abcam,
Cambridge, UK) overnight at 4°C, with appropriate negative and
positive controls. Then the sections were incubated with the
secondary antibody (Universal HRP Multimer) for 8 min at 37°C.
Subsequently, the slides were treated with the DAB +
H2O2 substrate for 8 min followed by
counterstaining with hematoxylin and the bluing reagent at 37°C.
The reaction buffer (pH 7.6, Tris buffer) was used as a wash
solution.
Semi-quantitative methods were used to describe the
dyeing conditions of CgA or Syn positively stained tumor cells
according to the percentage and degree of staining of positive
cells. The extent of positive staining was semi-quantitatively
assessed as: 0, <5% staining; 1+, 5–25% staining; 2+, 26–50%
staining; 3+, 51–75% staining; and 4+, ≥75% staining. The Ki-67
proliferation index was determined by assessing the percentage of
positively staining tumor cell nuclei in 2,000 cells in areas with
the highest degree of nuclear labelling where possible. All slides
were evaluated the same day by two pathologists to minimize the
variability of the results.
LVD
LVD was assessed with digital image analysis,
applying podoplanin as a marker for lymphatic vessel endothelium
(12). Three most intensively
vascularized intratumoral fields were acquired. In each field,
vessels were marked manually and then counted automatically.
Eventually, LVD was calculated as the number of vessels in the most
vascularized field. A single immunoreactive endothelial cell or a
cluster of endothelial cells (brown in case of a lymphatic vessel)
separated from other vessels, was counted as a single vessel.
Vessels with and without lumen were counted.
Statistical analysis and the
establishment of the prediction equation
Clinical and pathological parameters including IHC
staining (measured semi-quantitatively) were analyzed using the
Chi-square test and Student's t-test. Logistic regression analysis
and Chi-square test were both used to conduct multivariate analyses
of metastasis-related risk factors of GEP-NETs. Statistical
significance was accepted at P<0.05. All analyses were performed
using SPSS version 17.0. Prediction equation: ln[P/(1-P)] = e +
β1X1 + β2X2 +
β3X3 + β4X4 +
β5X5 + β6X6 +
β7X7 + β8X8 +
β9X9 + β10X10 +
β11X11 + β12X12 +
β13X13 + β14X14 +
β15X15. We used a backward method to analyze
all 15 variables. The final equation was confirmed until no more
variables could be deleted from the current model. We also
estimated odds ratio for each variable and their 95% confidence
interval.
Results
Common characteristics of the
GEP-NETs
In our retrospective study, the total study
population included 96 males and 50 females with a mean age of
49.26±13.31 years. The composition of the cases enrolled in our
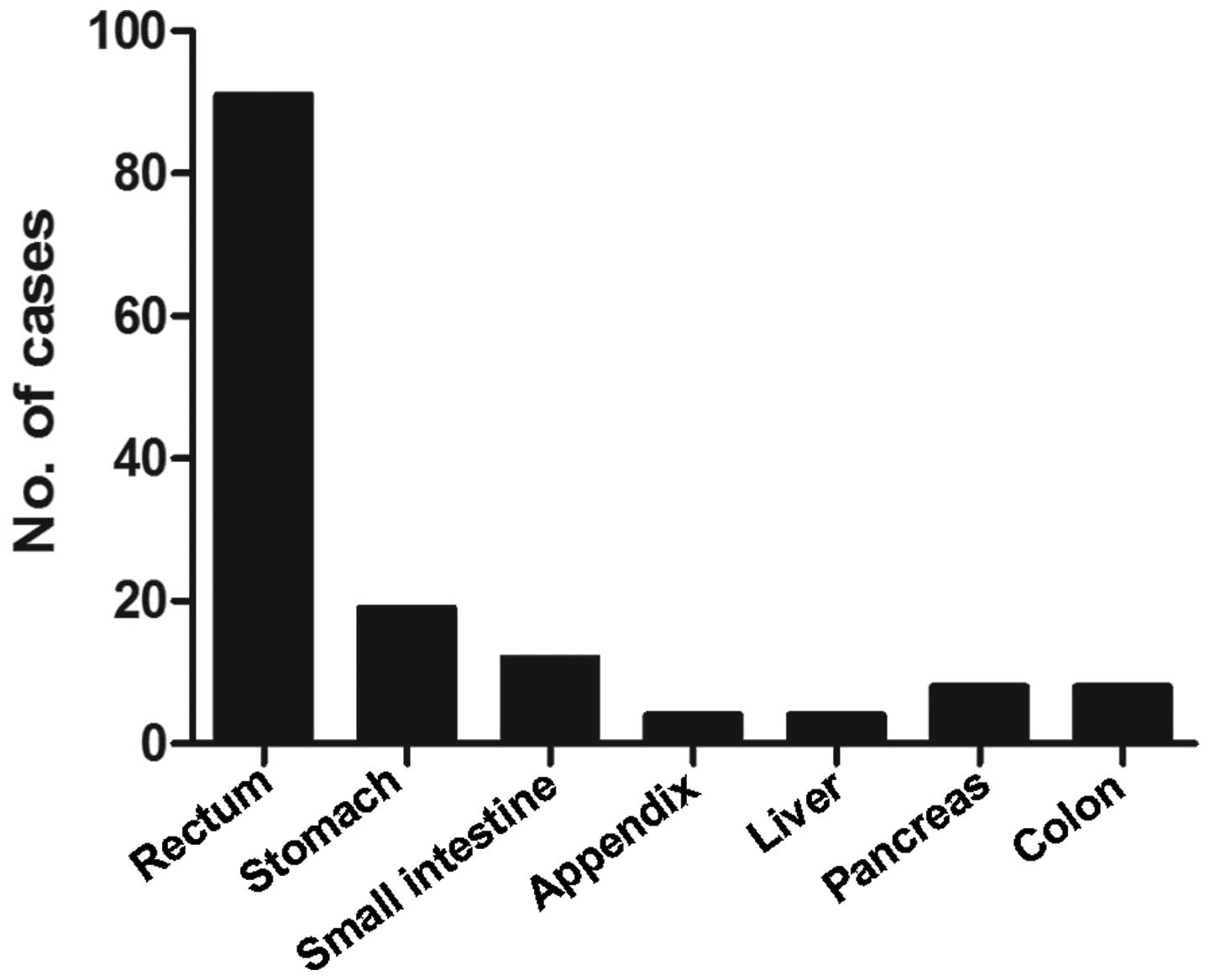
study is described in Fig. 1
according to tumor location. The most common underlying tumor sites
were the rectum (62.3%), stomach (13.0%), and small intestine
(8.2%). Abdominal pain (n=88), character change of stool (n=58) and
melaena (n=30) were the most common non-specific clinical
symptoms.
Clinical manifestation parameters
indicated for the metastasis of GEP-NETs
General information of the patients, such as age and
gender, and the three most common non-specific clinical symptoms
including abdominal pain, character change of stool and melaena,
were collected in our study. All the five candidate variables
evaluated in the retrospective cohort were analyzed by Chi-square
test (Table II). According to the
statistical results, tumor metastasis was more likely to occur in
the GEP-NET patients accompanied by change of stool and melaena.
Age, gender and abdominal pain were not determined to be metastasis
risk factors of GEP-NETs.
 | Table II.Univariate analysis of risk factors
for metastasis of GEP-NETs based on clinical manifestation
parameters. |
Table II.
Univariate analysis of risk factors
for metastasis of GEP-NETs based on clinical manifestation
parameters.
| Risk factors | No. | Non-metastasis
group | Metastasis
group | P-value |
|---|
| Gender |
|
|
| >0.05 |
|
Male | 96 | 59 | 37 |
|
|
Female | 50 | 37 | 13 |
|
| Age (years) |
|
|
| >0.05 |
| ≥40 | 112 | 66 | 46 |
|
| <40 | 34 | 30 | 4 |
|
| Abdominal pain |
|
|
| >0.05 |
|
Yes | 88 | 54 | 34 |
|
| No | 58 | 42 | 16 |
|
| Stool change |
|
|
| <0.05 |
|
Yes | 58 | 28 | 30 |
|
| No | 88 | 68 | 20 |
|
| Melaena |
|
|
| <0.05 |
|
Yes | 33 | 8 | 25 |
|
| No | 113 | 88 | 25 |
|
Ulcer formation and a larger size of
the lesion, not the infiltration depth of the tumor, participate in
higher metastasis risk of GEP-NETs
Tumor size, number, type, site and infiltration
depth were important parameters to evaluate the characteristics and
progression of tumors. Among the five candidate variables from the
common tumor characteristics evaluated in the retrospective cohort,
two were associated with GEP-NETs by Chi-square test and matching
Chi-square test (Table III): i)
tumor type: ulcerative type of neuroendocrine tumor metastasizes
more likely than the non-ulcer ones; and ⅱ) tumor size: the larger
the tumor, the higher the risk to transfer. The detailed
information such as tumor number, size, surface obtained by
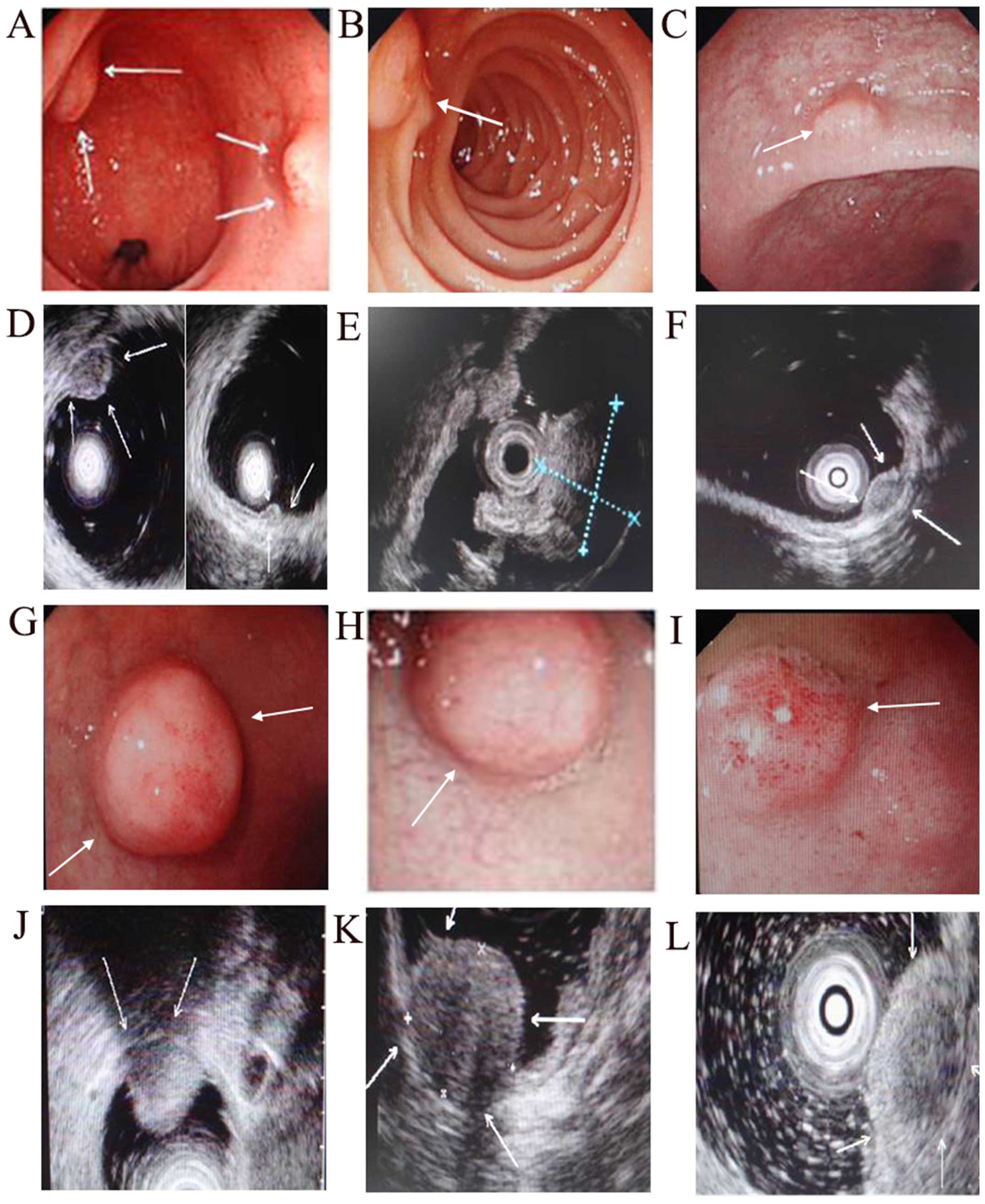
endoscopy is documented in Fig.
2.
 | Table III.Univariate analysis of risk factors
for metastasis of GEP-NETs based on common tumor
characteristics. |
Table III.
Univariate analysis of risk factors
for metastasis of GEP-NETs based on common tumor
characteristics.
| Risk factors | No. | Non-metastasis
group | Metastasis
group | P-value |
|---|
| Tumor size
(cm) |
|
|
| <0.05 |
|
<1 | 72 | 62 | 10 |
|
|
1–2 | 44 | 24 | 20 |
|
|
>2 | 30 | 8 | 22 |
|
| No. of tumors |
|
|
| >0.05 |
| 1 | 120 | 75 | 45 |
|
| ≥2 | 26 | 21 | 5 |
|
| Tumor type |
|
|
| <0.05 |
|
Ulcerative | 28 | 10 | 18 |
|
|
Non-ulcerative | 42 | 32 | 10 |
|
| Tumor site |
|
|
| >0.05 |
|
Stomach | 19 | 10 | 9 |
|
| Small
intestine | 12 | 6 | 6 |
|
|
Appendix | 4 | 3 | 1 |
|
|
Liver | 4 | 3 | 1 |
|
|
Pancreas | 8 | 1 | 7 |
|
|
Colon | 8 | 3 | 5 |
|
|
Rectum | 91 | 70 | 21 |
|
| Infiltration
depth |
|
|
| >0.05 |
|
Infiltration | 13 | 7 | 6 |
|
|
Non-infiltration | 31 | 20 | 11 |
|
When assessing the role of infiltration depth
achieved by endoscopic ultrasonography in tumor metastasis, there
was no statistical significance between the metastatic group and
the non-metastatic one. Performance under endoscopic
ultrasonography is shown in Fig. 3.
The pathological biopsy is the gold standard when it comes to
determining tumor metastases and endoscopy plays an important role
in tissue biopsy and endoscopic therapy.
CgA and Syn are not indicators for the
metastasis of GEP-NETs
CgA and Syn are considered as the most important
biomarkers for the pathological IHC index in diagnosing
neuroendocrine tumors. In the sections for IHC, CgA and Syn were
both sensitive and specific biomarkers for the presence of GEP-NETs
(Fig. 3), whereas they act more
like diagnostic biomarkers than risk factors for metastasis. The
study showed no statistically significant impact on metastasis for
CgA-positive (77/116) or Syn-positive (118/124) compared with
CgA-negative (39/116) or Syn-negative (6/124) samples by Chi-square
test (Table IV).
 | Table IV.Correlation between the expression of
CgA and Syn and GEP-NETs. |
Table IV.
Correlation between the expression of
CgA and Syn and GEP-NETs.
|
| N | n | χ2 | P-value |
|---|
| CgA |
|
| 1.635 | >0.05 |
| Positive | 77 | 30 |
|
|
| Negative | 39 | 8 |
|
|
| Syn |
|
| 0.001 | >0.05 |
| Positive | 118 | 41 |
|
|
| Negative | 6 | 2 |
|
|
Higher Ki-67 proliferation index
participates in higher risk of metastasis of GEP-NETs
The Ki-67 proliferation index is an important and
independent prognostic marker in GEP-NETs (Fig. 3). The prognosis of GEP-NETs is
closely related to the metastasis of the disease. The test
indicated that the Ki-67 proliferation index was significantly
correlated with tumor metastasis. Overall, 70 patients (66.67%) had
G1 tumors and G2 and G3 tumors were seen in 28 (26.67%) and 7
(6.67%) cases, respectively. When stratified according to grade,
metastasized tumors were noted in 14.29% (60/70) of the G1 cases,
85.71% (24/28) of the G2 cases, and 71.43% (5/7) of the G3 cases.
The metastasis of the disease was correlated with increased Ki-67
values (P<0.05) by Chi-square test (Table V).
 | Table V.Relationship between tumor grade and
metastasis of GEP-NETs. |
Table V.
Relationship between tumor grade and
metastasis of GEP-NETs.
| Grade | No. | Non-metastasis
group | Metastasis
group | P-value |
|---|
| G1 | 70 | 60 | 10 | <0.05 |
| G2 | 28 | 4 | 24 |
|
| G3 | 7 | 2 | 5 |
|
High tumor LVD is associated with a
higher risk of metastasis of GEP-NETs
LVD using podoplanin as a marker for lymphatic
vessel endothelium, which is often expressed at the leading
invasive edge of tumors, has been implicated in tumor progression.
Podoplanin immunonegativities and immunopositivities are presented
in Fig. 3. In our study, the
expression of podoplanin with brown staining was found in lymphatic
epithelial cells or surrounding tumor sites.
According to the test, there was statistical
significance between the lymph node metastasis group and without
lymph node metastasis group (P<0.05) (Table VI). However, when analyzing the
roles of peritumoral and intratumoral LVD in the tumor metastasis,
we found only peritumoral, not intratumoral LVD, to be
significantly associated with tumor metastasis (Fig. 4).
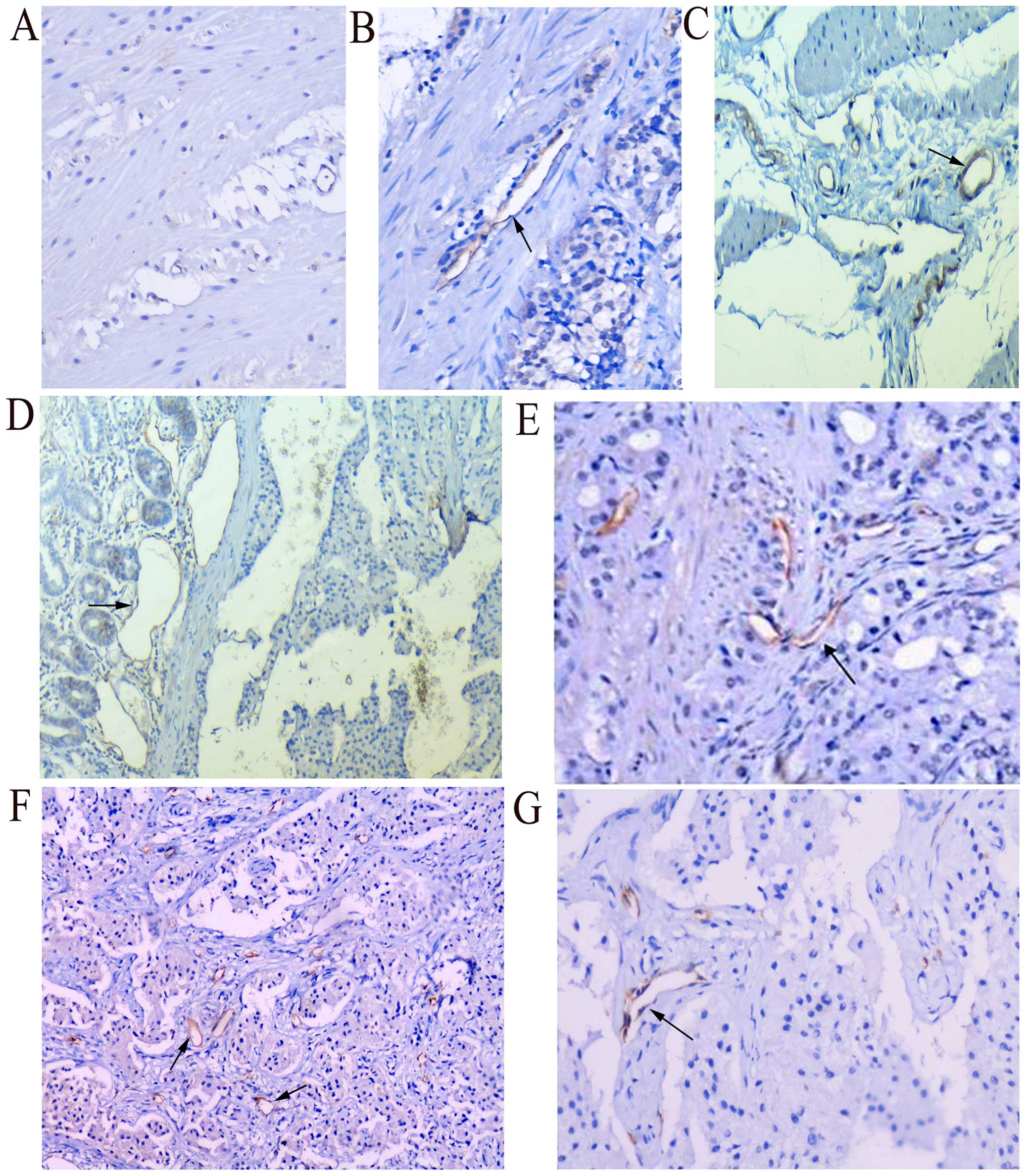 | Figure 4.Total LVD is a related risk factor
for metastasis. (A) No obvious positive expression of podoplanin
was noted in the normal rectal tissues (magnification, ×100). (B)
Moderate positive expression of podoplanin was noted in
non-metastatic rectal GEP-NETs, with endothelium of lymph vessels
staining brown (magnification, ×100). (C) Obvious positive
expression was noted in metastatic rectal GEP-NETs, with increased
endothelium of lymph vessels staining brown. LVD was greater than
that in the non-metastatic ones (magnification, ×100). (D and E)
Positive expression of podoplanin was noted in endothelium of
lymphatic vessels in non-metastatic duodenal neuroendocrine
carcinoma; (D) peritumoral lymphatic vessels appeared to be dilated
obviously (magnification, ×200); however (E) intratumoral lymphatic
vessels were flat and atretic (magnification, ×200). (F) More round
and dilated peritumoral lymphatic vessels were noted in duodenal
GEP-NETs with liver invasion (magnification, ×200), however (G)
intratumoral lymphatic vessels were flat and atretic; not
significant in number (magnification, ×200). LVD, lymphatic vessel
density; GEP-NETs, gastroenteropancreatic neuroendocrine
tumors. |
 | Table VI.Relationship between LVD and
metastasis of GEP-NETs. |
Table VI.
Relationship between LVD and
metastasis of GEP-NETs.
|
| No. | LVD | t | P-value |
|---|
| Lymph node
involvement |
|
| −2.373 | 0.021 |
|
Positive | 14 | 11.83±2.16 |
|
|
Negative | 47 | 10.65±1.31 |
|
| Peritumoral
LVD |
|
| −3.005 | 0.004 |
| Without
metastasis | 28 | 6.23±1.18 |
|
|
Metastasis | 33 | 7.33±1.51 |
|
| Intratumoral
LVD |
|
| 1.655 | 0.104 |
| Without
metastasis | 28 | 4.23±0.59 |
|
|
Metastasis | 33 | 4.00±0.45 |
|
| Total LVD |
|
| −1.949 | 0.056 |
| Without
metastasis | 28 | 10.46±1.21 |
|
|
Metastasis | 33 | 11.27±1.78 |
|
Metastasis-related variables
associated with GEP-NETs according to logistic regression
analysis
All the dependent variables in the table used
logistic regression to explore the association between tumor
metastasis and possible related risk factors. In our study,
logistic regression analysis using backward attribute selection
methods indicated that tumor size, tumor type, total LVD,
peritumoral LVD, melaena were significant and independent risk
factors (Table VII). Logistic
regression equation: ln[(P/(1-P)] = 7.283 + 2.277X6 -
3.092X9 + 4.129X13 - 3.061X15.
 | Table VII.Risk factors associated with GEP-NETs
according to logistic regression analysis. |
Table VII.
Risk factors associated with GEP-NETs
according to logistic regression analysis.
| Risk factors | B | P-value | Odds ratio |
|---|
| Tumor size | 2.277 | 0.037 | 9.752 |
| Total LVD | −3.061 | 0.036 | 0.047 |
| Peritumoral
LVD | 4.129 | 0.013 | 62.104 |
| Tumor type | −3.092 | 0.033 | 0.045 |
| Blood in stool | 3.333 | 0.089 | 28.020 |
| Constant | 7.283 | 0.275 | 1,455.043 |
Discussion
GEP-NETs are rare tumors, characterized by
heterogeneous biological behavior and clinical course. The
Surveillance, Epidemiology, and End Results (SEER) database
suggests that their prevalence has increased dramatically over the
last three decades, due to an increase in the actual number of
cases and/or more effective detection of this disease (13,14).
The prognosis of GEP-NETs mainly depends on whether the tumors
metastasize or not. Therefore, early detection and evaluation of
metastasis risk have been currently the most urgent task for
GEP-NET patients. To our best of our knowledge, among the 15
candidate variables in our retrospective cohort, four were
associated with GEP-NETs by Chi-square test and logistic
regression: i) tumor type, ⅱ) tumor size, ⅲ) peritumoral LVD, and
ⅳ) total LVD.
The clinical presentations of patients with GEP-NETs
depend on the hormonal activity of the tumors and on their location
and extent. Numerous tumors produce low levels of substances that
are clinically insignificant or secrete metabolically inactive or
inappropriately processed substances. Most GEP-NETs are
non-functional and present fairly late with mass effects, distant
metastasis, or both. Frequently, symptoms are vague and unspecific.
In our study, we found that the most common clinical manifestations
of non-functional GEP-NETs were abdominal pain (n=88), character
change of stool (n=58) and melaena (n=33), however, there was no
statistical significance between the symptoms above and metastasis.
Kuiper et al found that common symptoms of non-functional
neuroendocrine tumors were abdominal pain, weight loss, anorexia,
jaundice, nausea and vomiting and intra-abdominal haemorrhage
(15). None of the common symptoms
were reported to be specific to GEP-NETs. The classic syndromes
associated with functioning GEP-NETs include the carcinoid
syndrome, which is the result of the interaction of tumor factors
such as 5-hydroxytryptamine (5-HT) (serotonin), kinins, and
kallikrein entering the systemic circulation, leading to flush,
diarrhea, and other features of carcinoid syndrome. Occasionally,
carcinoid crisis, which is an overwhelming release of bioactive
amines, can develop in patients with foregut and midgut carcinoids,
and can present with hypotension (rarely hypertension),
arrhythmias, wheezing, and delirium. Recurrent hypoglycemia is a
typical symptom of insulinomas. These tumors manifest themselves in
adrenergic symptoms such as tachycardia, anxiety, sweating, and
palpitations, even loss of consciousness as a symptom. Recurrent
duodenal ulcer and gastroesophageal reflux are the primary symptoms
of duodenal or pancreatic gastrinoma.
Tumor characteristics, such as location, size, type,
number, infiltration depth, and the presence of metastases, were
assessed by CT scan, MRI and endoscopic procedures; the latter one
has been recommended for GI tract GEP-NENs, and plays a pivotal
role in the diagnostic work-up and the therapy of GEP-NENs. In our
study, tumor type and size were found to correlate significantly
with the metastasis of tumors. Ulcerative types are more likely to
transfer than the non-ulcerative ones. In addition, a size >2 cm
acts as a high risk factor which is consistent with what has been
reported in other research (16).
Schott et al reported that more than 80% of cases are <2
cm and are benign (17,18). All other tumors, such as
gastrinomas, glucagonomas, and VIPomas, and especially the numerous
non-functional NETs of the pancreas, are usually >2 cm and are
malignant. In a Japanese report, metastasis showed a significant
correlation with tumor size. Tumors <1 cm and confined to the
submucosa without vessel invasion did not show any metastasis.
According to the latest WHO classification and site-specific TMN
staging, the dimension recommended as a metastasis risk factor is
different owing to the different site. For the stomach, appendix,
colon and rectum tumors, lower size limits were defined based on
current information on the biology of tumors. The size limits
indicated for T1 (<1 cm) are those defined by the WHO for tumors
with ‘benign behavior’. Deeply invasive tumors are included over T2
(1–2 cm) according to site-specific clinicopathological
correlations (19,20). Unfortunately more and more cases of
small rectal carcinoids (even <5 mm) accompanied by multiple
liver metastasis have been reported in the literature (21,22).
As a rule, tumors confined to the pancreas, <2 cm in size, show
a benign behavior. However in the pancreas the size limit given for
T2 (2–4 cm) needs to be validated.
It is worth noting that location and infiltration
depth are considered to be high risk factors in other studies. The
relation between location, infiltration depth and metastasis
warrants more in-depth research. During the last decade, the
development of new and more sophisticated diagnostic and
therapeutic endoscopic instruments and tools have enriched the
armamentarium available to the endoscopist. GEP-NENs, however,
still represent a clinical challenge to the endoscopist because of
their small size, which may render their search very difficult. At
present, gastric, duodenal, and rectal NENs are diagnosed with
increased frequency due to the widespread use of diagnostic upper
and lower endoscopic examinations (23). Tumor size, depth of infiltration
within the GI wall, and presence or absence of metastatic
locoregional lymph nodes are important parameters, and can be
detected by endoscopy, especially EUS. EUS has a crucial role in
the search for GEP-NENs of the GI wall, since it provides
information on the size, depth of invasion and locoregional
metastasis. Also, EUS-guided fine-needle aspiration can also
provide a definite diagnosis and useful information for the correct
management of this type of lesion. For many years EUS has been
advocated as the best available technique for imaging the pancreas
and the extrahepatic biliary tree (10,24).
High resolution images of the main pancreatic duct and surrounding
parenchyma can be achieved and structures as small as 2–3 mm can be
distinguished thanks to the small distance between the transducer
and the gland. EUS can detect 45–60% of duodenal lesions and
90–100% of pancreatic lesions.
Pathological examination of biopsies or surgical
specimens reveals the verification of the neuroendocrine nature of
the tumor by IHC, for markers such as keratin, CgA, Syn,
neuron-specific enolase (NSE), grimelius, Ki67 and CD56, which
provides a promising new diagnostic method for NETs. Although
research into specific biomarkers to detect GEP-NETs is ongoing,
all the above-mentioned studies are non-conclusive, and further
research and validation studies are needed before these diagnostic
tools can be used in practice. In clinical study, CgA and Syn are
widely used in the diagnosis of neuroendocrine tumors. Although
non-conclusive, they have received the validation of many
researchers for their high sensitivity and specificity (25). We also speculate concerning the role
they play in tumor metastasis. In our study, we found that IHC of
biopsy specimens using a selected panel of markers, including CgA
and Syn could be used to help diagnose NETs with high sensitivity
and specificity. Our positivity rate of 66.4% for CgA in GEP-NETs
is similar to the results of a recent study (26). In contrast, the positivity rate of
Syn was 95.2% in our study, again similar to the results of
previous studies (27,28), confirming that Syn is superior to
CgA as an IHC marker. But both markers have no significant
relationship with the metastasis of NETs. We recommend CgA and Syn
as highly specific and sensitive neuroendocrine markers in the
diagnosis of NETs, but not markers for metastasis.
Ki-67 is an important marker of cell proliferation
which is active in the cell cycle phases G1, S and G2 and during
mitosis. A high Ki-67 proliferation index indicates abnormal
proliferation and the aggressiveness of a tumor. Many retrospective
studies have demonstrated that Ki-67 shows a good correlation with
tumor size, vessel and the behavior of neuroendocrine tumors.
Richards-Taylor et al provided a substantial body of
evidence related to the use of Ki-67 as a prognostic marker in
GEP-NETs (6). Özaslan et al
reported that the grade and stage of the disease increased in line
with a higher Ki-67 index (29). In
our study, the Ki-67 proliferation index (tumor grade) was
significantly correlated with the metastasis of GEP-NETs.
Lymph node metastasis is a common occurrence in
GEP-NETs. Malignant cells spread from their primary site to
regional lymph nodes via the lymphatics at an early stage in the
dissemination of the tumors (30).
Recent studies have indicated that tumor lymphangiogenesis, the
growth of new lymphatic vessels, is linked to the formation of
lymph node metastases when observing the LVD and the overexpression
of podoplanin (31–33). Our data used LVD as a marker of
lymphangiogenesis which was obtained or calculated from the
expression of PDPN. As we know, PDPN is a 38-kDa mucin-type
transmembrane glycoprotein with extensive O-glycosylation
and high sialic acid content, and has been implicated in tumor
progression. PDPN is expressed in lymphatic endothelial cells as
well as cancer cells. The mechanism underlying the impact of
lymphangiogenesis or LVD on tumor progression remains uncertain.
Previous studies have demonstrated a link between tumor-induced
lymphangiogenesis and enhanced tumor metastasis to sentinel lymph
nodes and remote metastasis based on the evidence that the
intratumoral vessels are newly proliferating and not trapped
pre-existing or hyperplastic lymph vessels. The main significance
of proliferating intratumoral lymph vessels is that they could
provide a possible route for the spread of tumors to local lymph
nodes. However, whether the effects of lymphangiogenesis on the
risk of metastasis are due to increased lymphatic permeability or
increased abundance of intratumoral and/or peritumoral lymphatics
remains controversial. Our study showed that total LVD was a
related risk factor for metastasis, and it was noted that
peritumoral, not intratumoral, LVD acted as a statistically
significant factor related to metastasis. We speculate that the
invasion of increased peritumoral LVD may play an important role in
tumor metastasis, similar to the results of Pastushenko et
al (34). Understanding the
mechanism of peritumoral lymphatic prolification and growth may
benefit the diagnosis and treatment of GEP-NETs.
In conclusion, the incidence of GEP-NET has shown a
marked increase during the last decade. Due to its indolent disease
courses and poor prognosis, early detection and timely assessment
of the risks of metastasis remain critical issues. We found that
tumor size, tumor type, Ki-67 proliferation index, peritumoral LVD
and total LVD may be metastasis-related risk factors for GEP-NET
patients. In addition, endoscope and neuroendocrine markers, such
as CgA and Syn, play important roles in identifying the
characteristics of tumors and aid in the diagnosis and treatment of
GEP-NETs.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 81172299) and the Research Program
from the Science and Technology Department of Hunan Province, China
(S2012F1023).
References
|
1
|
Meeker A and Heaphy C:
Gastroenteropancreatic endocrine tumors. Mol Cell Endocrinol.
386:101–120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pinchot SN, Holen K, Sippel RS and Chen H:
Carcinoid tumors. Oncologist. 13:1255–1269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Strosberg JR, Nasir A, Hodul P and Kvols
L: Biology and treatment of metastatic gastrointestinal
neuroendocrine tumors. Gastrointest Cancer Res. 2:113–125.
2008.PubMed/NCBI
|
|
4
|
Ramage JK, Ahmed A, Ardill J, Bax N, Breen
DJ, Caplin ME, Corrie P, Davar J, Davies AH, Lewington V, et al: UK
and Ireland Neuroendocrine Tumour Society: Guidelines for the
management of gastroenteropancreatic neuroendocrine (including
carcinoid) tumours (NETs). Gut. 61:6–32. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Klöppel G, Rindi G, Anlauf M, Perren A and
Komminoth P: Site-specific biology and pathology of
gastroenteropancreatic neuroendocrine tumors. Virchows Arch.
451:(Suppl 1). S9–S27. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Richards-Taylor S, Ewings SM, Jaynes E,
Tilley C, Ellis SG, Armstrong T, Pearce N and Cave J: The
assessment of Ki-67 as a prognostic marker in neuroendocrine
tumours: A systematic review and meta-analysis. J Clin Pathol.
69:612–618. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baudin E, Planchard D, Scoazec JY, Guigay
J, Dromain C, Hadoux J, Debaere T, Elias D and Ducreux M:
Intervention in gastro-enteropancreatic neuroendocrine tumours.
Best Pract Res Clin Gastroenterol. 26:855–865. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Knigge U and Hansen CP: Surgery for
GEP-NETs. Best Pract Res Clin Gastroenterol. 26:819–831. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li ZS and Li Q: The latest 2010 WHO
classification of tumors of digestive system. Zhonghua Bing Li Xue
Za Zhi. 40:351–354. 2011.(In Chinese). PubMed/NCBI
|
|
10
|
Yang Z, Tang LH and Klimstra DS:
Gastroenteropancreatic neuroendocrine neoplasms: Historical context
and current issues. Semin Diagn Pathol. 30:186–196. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Capelli P, Fassan M and Scarpa A:
Pathology - grading and staging of GEP-NETs. Best Pract Res Clin
Gastroenterol. 26:705–717. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Raica M, Cimpean AM and Ribatti D: The
role of podoplanin in tumor progression and metastasis. Anticancer
Res. 28:2997–3006. 2008.PubMed/NCBI
|
|
13
|
Gastrointestinal Pathology Study Group of
Korean Society of Pathologists, ; Cho MY, Kim JM, Sohn JH, Kim MJ,
Kim KM, Kim WH, Kim H, Kook MC, Park DY, Lee JH, et al: Current
trends of the incidence and pathological diagnosis of
gastroenteropancreatic neuroendocrine tumors (GEP-NETs) in Korea
2000–2009: Multicenter study. Cancer Res Treat. 44:157–165. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fraenkel M, Kim MK, Faggiano A and Valk
GD: Epidemiology of gastroenteropancreatic neuroendocrine tumours.
Best Pract Res Clin Gastroenterol. 26:691–703. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kuiper P, Verspaget HW, Overbeek LI,
Biemond I and Lamers CB: An overview of the current diagnosis and
recent developments in neuroendocrine tumours of the
gastroenteropancreatic tract: The diagnostic approach. Neth J Med.
69:14–20. 2011.PubMed/NCBI
|
|
16
|
Solcia E, Rindi G, Paolotti D, La Rosa S,
Capella C and Fiocca R: Clinicopathological profile as a basis for
classification of the endocrine tumours of the
gastroenteropancreatic tract. Ann Oncol. 10:(Suppl 2). S9–S15.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schott M, Klöppel G, Raffel A, Saleh A,
Knoefel WT and Scherbaum WA: Neuroendocrine neoplasms of the
gastrointestinal tract. Dtsch Arztebl Int. 108:305–312.
2011.PubMed/NCBI
|
|
18
|
Zhou X, Xie H, Xie L, Li J, Cao W and Fu
W: Endoscopic resection therapies for rectal neuroendocrine tumors:
A systematic review and meta-analysis. J Gastroenterol Hepatol.
29:259–268. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rindi G, Klöppel G, Alhman H, Caplin M,
Couvelard A, de Herder WW, Erikssson B, Falchetti A, Falconi M,
Komminoth P, et al: TNM staging of foregut (neuro)endocrine tumors:
A consensus proposal including a grading system. Virchows Arch.
449:395–401. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rindi G, Klöppel G, Couvelard A, Komminoth
P, Körner M, Lopes JM, McNicol A-M, Nilsson O, Perren A, Scarpa A,
et al: TNM staging of midgut and hindgut (neuro) endocrine tumors:
A consensus proposal including a grading system. Virchows Arch.
451:757–762. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsuboi K, Shimura T, Suzuki H, Mochiki E,
Haga N, Masuda N, Soda M, Yamamoto H, Asao T and Kuwano H: Liver
metastases of a minute rectal carcinoid less than 5mm in diameter:
A case report. Hepatogastroenterology. 51:1330–1332.
2004.PubMed/NCBI
|
|
22
|
Chun HJ, Jeen YT, Park SC, Keum B, Seo YS,
Um SH, Kim CD and Ryu HS: Multiple liver metastases from a rectal
carcinoid tumor. Gastrointest Endosc. 71:619–620. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Attili F, Capurso G, Vanella G, Fuccio L,
Delle Fave G, Costamagna G and Larghi A: Diagnostic and therapeutic
role of endoscopy in gastroenteropancreatic neuroendocrine
neoplasms. Dig Liver Dis. 46:9–17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
De Angelis C, Brizzi RF and Pellicano R:
Endoscopic ultrasonography for pancreatic cancer: Current and
future perspectives. J Gastrointest Oncol. 4:220–230.
2013.PubMed/NCBI
|
|
25
|
Chou WC, Hung YS, Hsu JT, Chen JS, Lu CH,
Hwang TL, Rau KM, Yeh KY, Chen TC and Sun CF: Chromogranin A is a
reliable biomarker for gastroenteropancreatic neuroendocrine tumors
in an Asian population of patients. Neuroendocrinology. 95:344–350.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Belli SH, Oneto A, Aranda C, O'Connor JM,
Domenichini E, Roca E, Méndez G, Bestani MC, Parma P, Giacomi N, et
al: Chromogranin A as a biochemical marker for the management of
neuroendocrine tumors: A multicenter study developed in Argentina.
Acta Gastroenterol Latinoam. 39:184–189. 2009.PubMed/NCBI
|
|
27
|
Gao W, Liu SM, Lu HZ, Liang J, Yuan YL and
Liu XY: Analysis of clinicopathological features of intestinal
neuroendocrine neoplasms. Zhonghua Zhong Liu Za Zhi. 34:450–456.
2012.(In Chinese). PubMed/NCBI
|
|
28
|
Li AF, Li AC, Hsu CY, Li WY, Hsu HS and
Chen JY: Small cell carcinomas in gastrointestinal tract:
Immunohistochemical and clinicopathological features. J Clin
Pathol. 63:620–625. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Özaslan E, Demir S, Karaca H and Güven K:
Evaluation of the concordance between the stage of the disease and
Ki-67 proliferation index in gastroenteropancreatic neuroendocrine
tumors. Eur J Gastroenterol Hepatol. 28:836–841. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang J, Guo Y, Wang B, Bi J, Li K, Liang
X, Chu H and Jiang H: Lymphatic microvessel density and vascular
endothelial growth factor-C and -D as prognostic factors in breast
cancer: A systematic review and meta-analysis of the literature.
Mol Biol Rep. 39:11153–11165. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Takahashi A, Ishii G, Kinoshita T, Yoshida
T, Umemura S, Hishida T, Yoh K, Niho S, Goto K, Ohmatsu H, et al:
Identification of prognostic immunophenotypic features in cancer
stromal cells of high-grade neuroendocrine carcinomas of the lung.
J Cancer Res Clin Oncol. 139:1869–1878. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Watanabe M, Tanaka H, Ohira M, Yoshii M,
Sakurai K, Toyokawa T, Kubo N, Yamamoto A, Muguruma K, Yamashita Y,
et al: Intranodal lymphangiogenesis precedes development of lymph
node metastasis and accelerates progression of gastric cancer. J
Gastrointest Surg. 18:481–490. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pasquali S, van der Ploeg AP, Mocellin S,
Stretch JR, Thompson JF and Scolyer RA: Lymphatic biomarkers in
primary melanomas as predictors of regional lymph node metastasis
and patient outcomes. Pigment Cell Melanoma Res. 26:326–337. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pastushenko I, Vermeulen PB, Carapeto FJ,
Van den Eynden G, Rutten A, Ara M, Dirix LY and Van Laere S: Blood
microvessel density, lymphatic microvessel density and lymphatic
invasion in predicting melanoma metastases: Systematic review and
meta-analysis. Br J Dermatol. 170:66–77. 2014. View Article : Google Scholar : PubMed/NCBI
|