Introduction
Tongue squamous cell carcinoma (TSCC) is the most
common type of oral cancer and is well-known for its high rate of
proliferation and nodal metastasis (1). Radiotherapy alone or in combination
with surgery, chemotherapy, or other targeted therapies, plays a
critical role in the treatment of patients with advanced stage TSCC
(2). However, TSCC is characterized
as moderately resistant to radiotherapy and the outcome of
radiotherapy is often not fully satisfactory due to radioresistance
(3). The mechanism of TSCC
radioresistance and the way to increase its radiosensitivity still
need to be explored.
There have been several studies on the application
of histone deacetylase (HDAC) inhibitors as anti-carcinogenic and
radiosensitizing agents (4–6). Trichostatin A (TSA), a pan-HDAC
inhibitor, has been shown to enhance the radiosensitivity of a
panel of human carcinoma cells (7–13).
However, its effect on TSCC cell radioresistance and the underlying
regulatory signal transduction pathways remain unclear.
Accumulating data have demonstrated that miRNAs,
such as miR-23a (14), miR-34b
(15), miR-153 (16), and miR-200c (17), play important roles in tumor
radiosensitivity by regulating DNA damage repair, cell-cycle
arrest, apoptosis, radiation-related signaling, and the tumor
microenvironment (18). Moreover,
it has been reported that epigenetic modulation such as histone
deacetylation contributes to the silencing of miR-375 (19), while TSA restores its expression
(20). Furthermore, our previous
study demonstrated that miR-375 has antitumor effects in TSCC
(21). This led us to speculate
that miR-375 is involved in the pharmacological regulation of
radioresistance by TSA.
Protein kinase B (AKT or PKB) is one of the major
intermediary molecules in the phosphatidylinositol-3-kinase
signaling pathway. It plays a crucial role in essential cellular
functions and contributes to tumorigenesis and tumor metastasis
(22). Previous studies have shown
the important role of AKT signaling pathway in the radioresistance
of cancer cells (3,23,24).
It has been demonstrated that miR-375 inhibits
3-phosphoinositide-dependent protein kinase 1 (PDK1) and
subsequently affects the AKT pathway (25–27).
Thus, we further hypothesized that TSA increases the
radiosensitivity of TSCC by miR-375 -mediated suppression of AKT
pathway.
In this study, we aimed to confirm the effects of
TSA on the radiosensitivity of TSCC cells, and to determine whether
the miR-375 mediates the pharmacological regulation of
radioresistance by TSA.
Materials and methods
Cell culture and treatments
The human tongue cancer cell lines SCC-15 and CAL27
were purchased from the American Type Culture Collection (ATCC,
Manassas, VA, USA) and cultured in Dulbecco's modified Eagle's
medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1%
antibiotics at 37°C in a humidified atmosphere of 5% CO2
and 95% air. Cells were irradiated using the HL-2000 HybriLinker
(UVP, Upland, CA, USA) at a wavelength of 254 nm. TSA was from
Sigma-Aldrich (St. Louis, MO, USA), dissolved in dimethyl sulfoxide
and stored at −80°C.
RNA oligoribonucleotides
The chemically-modified double-stranded miR-375
mimic and the corresponding miRNA mimic control; a
chemically-modified single-stranded miR-375 inhibitor and the
corresponding miRNA inhibitor control were from RiboBio Co.
(Guangzhou, China). The miR-375 mimic sequences were as follows:
sense, 5′-UUU GUU CGU UCG GCU CGC GUG A-3′, and antisense, 5′-AAA
CAA GCA AGC CGA GCG CAC U-3′; the miRNA mimic control sequences
were: sense, 5′-UUU GUA CUA CAC AAA AGU ACU G-3′, and antisense,
5′-AAA CAU GAU GUG UUU UCA UGA C-3′; the miR-375 inhibitor sequence
was: 5′-UCA CGC GAG CCG AAC GAA CAA A-3′; and the miRNA inhibitor
control sequence was: 5′-UCU ACU CUU UCU AGG AGG UUG UGA-3′.
Transient transfection
The cells were plated onto 6-well plates before
transfection. After reaching 80% confluence, the cells were
transfected with 30 nM miRNA mimic or 100 nM miRNA inhibitor using
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer's instructions.
RNA isolation and quantitative
reverse-transcription polymerase chain reaction (qRT-PCR)
Total RNA was extracted using the TRIzol reagent
(Invitrogen) then reverse-transcribed into complementary DNA using
a High Capacity cDNA Reverse Transcription kit (Applied Biosystems,
Foster City, CA, USA). Quantitative PCR was conducted at 95°C for
10 min followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min
using the ABI PRISM 7500 real-time PCR system (Applied Biosystems).
The primer used for miR-375 reverse transcription was as follows:
GTC GTA TCC AGT GCA GGG TCC GAG GTA TTC GCA CTG GAT ACG ACT CAC GC;
the primers for qRT-PCR of miR-375 were: sense, 5′-GTG CAG GGT CCG
AGG T-3′, and antisense, 5′-AGC CGT TTG TTC GTT CGG CT-3′; and the
primers for qRT-PCR of U6 (internal control for miRNA) were: sense,
5′-CTC GCT TCG GCA GCA CA-3′, and antisense, 5′-AAC GCT TCA CGA ATT
TGC GT-3′. The data were analyzed using the 2−∆∆Ct
relative expression quantity as described previously (28).
Western blot analysis
Western blot analysis was performed as described
previously (28). Briefly, cells
were harvested, washed with PBS, and lysed in RIPA buffer. Proteins
were separated by 12% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and transferred to nitrocellulose membranes.
Primary antibodies against phosphorylated AKT (p-AKT, Cell
Signaling Technology, Beverly, MA, USA), AKT (Cell Signaling
Technology), PDK1 (Cell Signaling Technology), and β-actin (Cell
Signaling Technology) were diluted at 1:1,000. The intensities of
the bands were quantified using ImageJ software (http://rsb.info.nih.gov/ij/). The background was
subtracted, and the signal of each target band was normalized to
that of the β-actin band.
Cell proliferation assays
Cell proliferation was measured using Cell Counting
Kit-8 (CCK-8, Dojindo, Kumamoto, Japan). Briefly, the cells were
plated onto 96-well plates (2×103 cells/well). After
treatment with TSA and/or exposure to a specific dose of radiation,
CCK-8 (10 µl) was added to each well at various time points and
incubated at 37°C for 3 h. The absorbance at 450 nm was measured
using a microplate spectrophotometer (Bio-Tek Instruments Inc.,
Winosski, VT, USA).
Colony formation
CAL27 cells (1×103 cells/well) seeded in
6-well plates were treated with TSA, and then exposed to the
indicated dose of radiation. The cells were cultured for 2–4 weeks,
and the colonies were detected by staining with crystal violet
(0.5% in 20% ethanol). Next, cells were harvested and measured by a
multifunctional micro-plate reader at 546 nm. The relative colony
number (relative survival cell number) = OD 546 administration
group/OD 546 control group, and the radiation survival curve was
drawn.
TUNEL staining
The cells were cultured on coverslips and treated
with TSA or transfected with miR-375 mimics. Next, the cells were
exposed to a specific dose of radiation. Then the cells were
subjected to TUNEL staining (one-step TUNEL Apoptosis Assay kit,
Beyotime, Jiangsu, China) according to the manufacturer's protocol.
Briefly, cells were fixed in 4% paraformaldehyde, treated with 0.1%
Triton X-100, and labeled with fluorescein-12-dUTP using terminal
deoxynucleotidyl transferase. Cell nuclei were stained with DAPI.
All fluorescent images were examined using a Leica DM3000
microscope (Leica, Solms, Germany).
Measurement of caspase-3/7
activity
The cellular enzymatic activity of caspase-3 and −7
was determined using a colorimetric assay (Caspase-Glo 3/7 Assay
Systems, Promega, Madison, MI, USA). Briefly, for each reaction,
cells were lysed and incubated with a luminogenic substrate
containing the DEVD sequence, which is cleaved by activated
caspase-3/7. After incubation at room temperature for one hour,
luminescence was quantified using a Centro XS3 LB 960 luminometer
(Berthold, Bad Wildbad, Germany).
Chromatin immunoprecipitation (ChIP)
assays
The acetylation of miR-375 promoter was assessed
using ChIP, which was performed using an EZ-Magna ChIP assay kit
(Merck Millipore, Darmstadt, Germany). Briefly, we sonicated the
crosslinked chromatin DNA to generate 200–1,000 bp DNA fragments.
DNA-protein complexes were immunoprecipitated with specific
antibodies against acetyl-histone H3 (ac-H3). Normal IgG was used
as the negative control. The protein/DNA complexes were then eluted
and reverse cross-linked. Spin columns were used to purify the DNA.
The precipitated DNA was quantified by qPCR. Relative enrichment
was calculated as the amount of amplified DNA normalized to the
input and relative to values obtained after normal IgG
immunoprecipitation. The primer specific for human GAPDH was used
as positive control. The sequences are as follows: sense, 5′-TAC
TAG CGG TTT TAC GGG CG-3′, and antisense, 5′-TCG AAC AGG AGG AGC
AGA GAG CGA-3′. The primer sequences for ChIP-1 are as follows:
sense, 5′-ACT ACA TCG CCT GGG TTT GA-3′, and antisense, 5′-TGC CAA
ATA TGC TGC TGG TT-3′; the ChIP-2 sequences are: sense, 5′-CAC CGC
CAG TAA AAG CAT CT-3′, and antisense, 5′-CCA TCC TTC CCT CTC AGA
GC-3′.
Statistical analysis
Statistical analyses were performed using SPSS 16.0
(SPSS, Chicago, IL, USA). The data are expressed as the mean ±
standard deviation (SD) from at least three independent
experiments. Differences between groups were analyzed using
Student's t-test. In cases of multiple-group testing, one-way
analysis of variance (ANOVA) was used. A two-tailed value of
P<0.05 was considered statistically significant.
Results
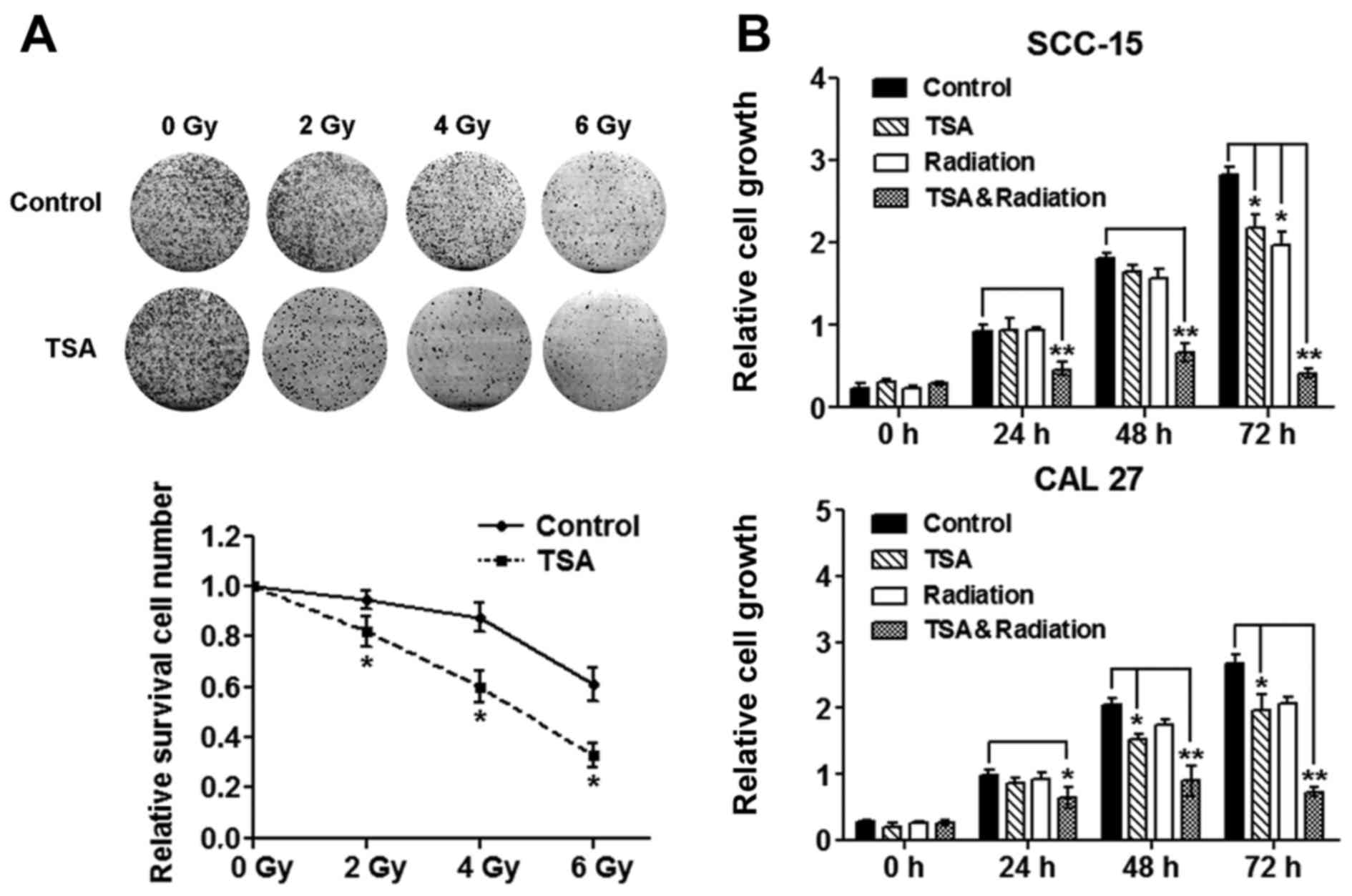
TSA inhibits TSCC cell proliferation
and sensitizes TSCC cells to radiation
To determine the dose of irradiation and TSA that
could be used without significantly affecting cell vitality, TSCC
cells were treated with different concentrations of TSA or
different dose of irradiation. Neither TSA (1 µg/ml) nor
irradiation (4 Gy) significantly affected the vitality of TSCC
cells (Fig. 1A), so we used these
non-cytotoxic doses in the subsequent experiments. We then used
CCK-8 assays to determine whether TSA can regulate radiosensitivity
in TSCC cells. Treatment with TSA or irradiation alone only
slightly reduced the cell proliferation until 72 h after treatment.
However, combination treatment with TSA and irradiation
significantly reduced the proliferation of SCC-15 and CAL27 cells
from 24 to 72 h after treatment (Fig.
1B). The colony formation ability of CAL27 cells was also
examined. The results showed that after a mild dose of irradiation
treatment (≤6 Gy), the colony formation of CAL27 cells was
gradually attenuated (Fig. 1A), and
pretreatment with TSA (1 µg/ml) further enhanced the activity of
irradiation (Fig. 1A).
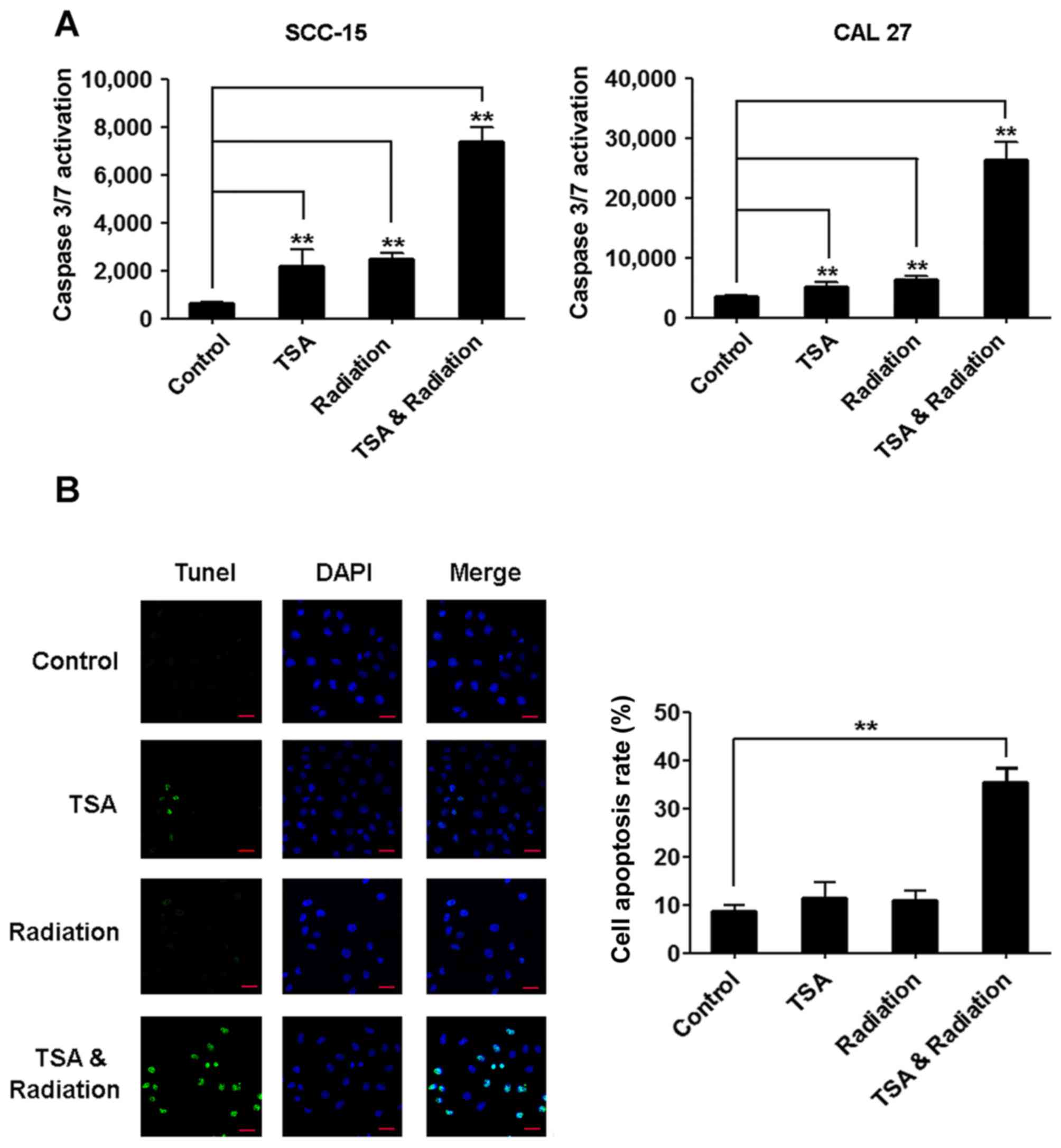
TSA enhances radiation-induced TSCC
cell apoptosis
To further confirm that TSA increases the
radiosensitivity of TSCC cells, we measured caspase-3/7 and
apoptosis after the treatments. The activity of caspase-3/7 was
upregulated after treatment with TSA or irradiation alone, and
further upregulated after the combinational treatment with TSA and
irradiation (Fig. 2A).
Consistently, the number of apoptotic cells revealed by TUNEL
staining was slightly increased by treatment with TSA or
irradiation alone, and was significantly increased by the
combinational treatment with TSA and irradiation (Fig. 2B).
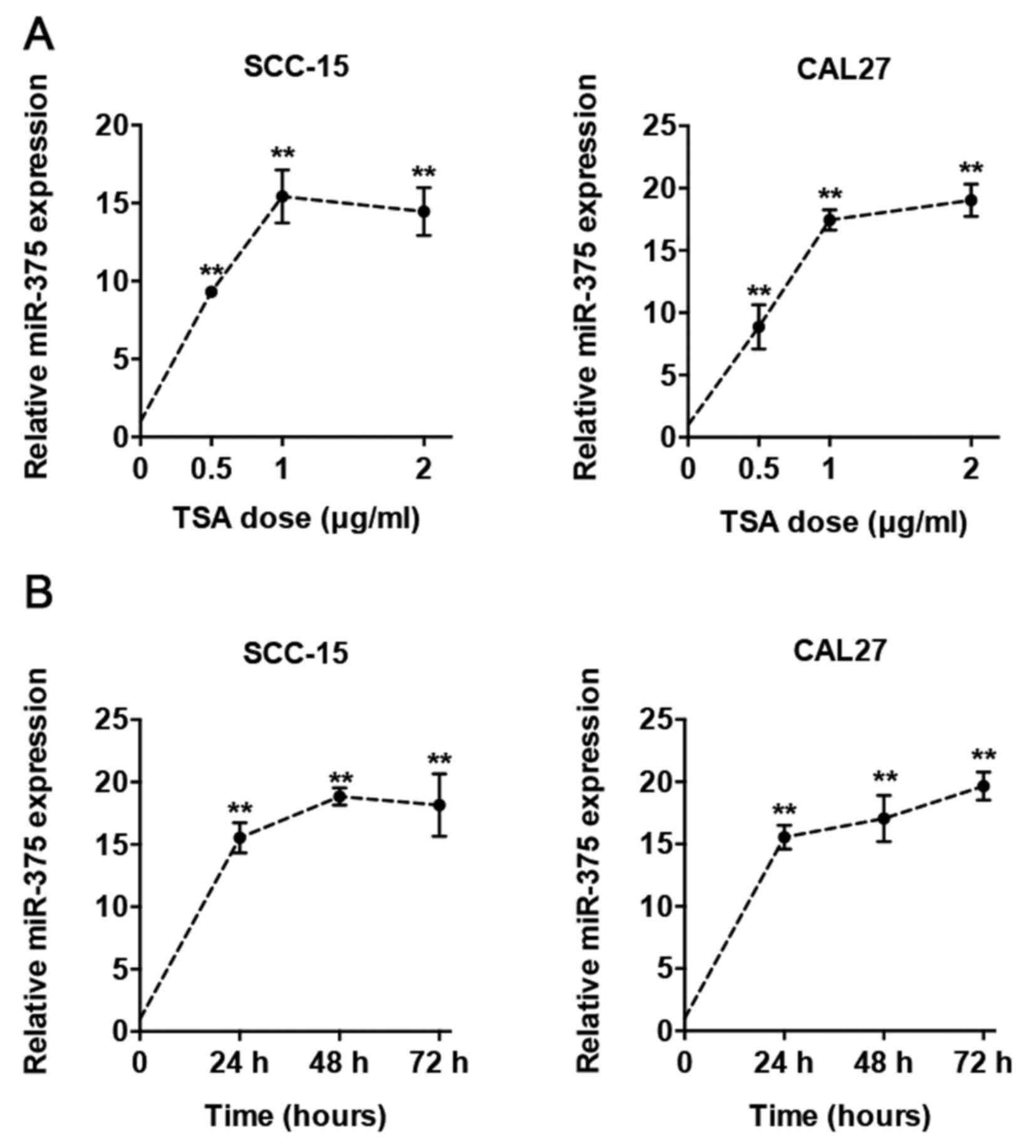
TSA induces miR-375 expression by
increasing histone acetylation of miR-375 promoter and subsequently
reduces AKT phosphorylation
To determine whether miR-375 is involved in the
regulation of radiosensitivity by TSA, we first treated the TSCC
cells with TSA in different concentrations (0.5–2 µg/ml) and time
(24–72 h), and found that TSA upregulated miR-375 expression
dose-dependently (Fig. 3A) and
time-dependently (Fig. 3B). Then,
we checked the histone acetylation in the miR-375 promoter region
by ChIP assays after TSA treatment. Two pairs of primers were
designed to analyze the status of ac-H3 in the promoter. We found
that TSA significantly increased the levels of ac-H3 in the
fragment containing ChIP-1 and ChIP-2 (Fig. 4A), suggesting that restoration of
histone acetylation in the promoter region induces miR-375
expression after TSA treatment. Further, we found that the level of
AKT phosphorylation was reduced after TSA treatment in TSCC cells,
and overexpression of miR-375 inhibited the expression of PDK1,
followed by the suppression of AKT phosphorylation without
significant change of total AKT levels (Fig. 4B).
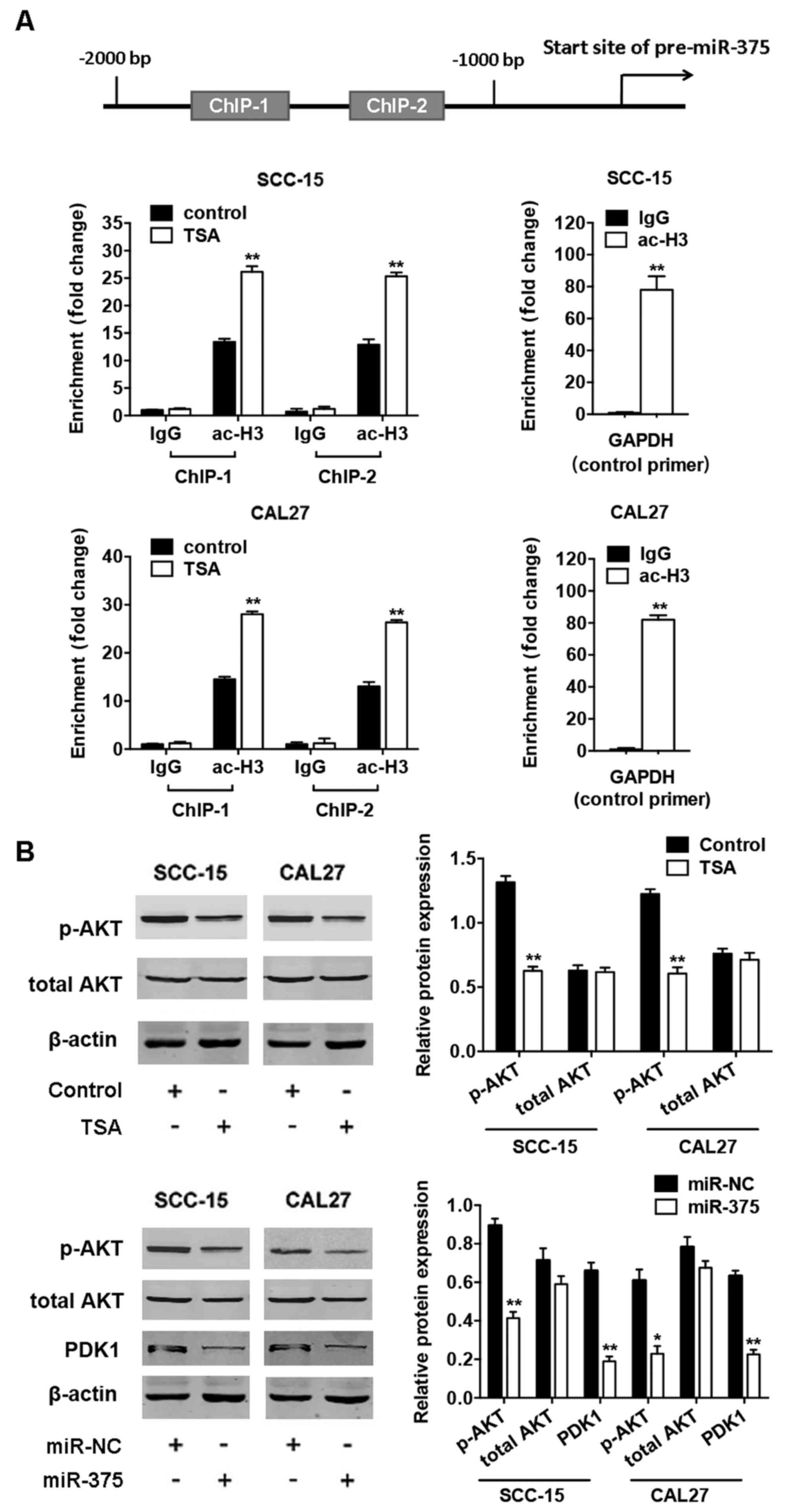 | Figure 4.TSA upregulates expression of miR-375
by histone acetylation of the miR-375 promoter and subsequently
downregulates phosphorylated AKT expression. (A) Upper, diagram of
the miR-375 promoter and location of the primers. Positions marked
are relative to the transcriptional start site. Soluble chromatin
from SCC-15 (middle) and CAL27 (lower) cells with or without TSA
was immunoprecipitated with anti-ac-H3 antibodies, and then
immunoprecipitated DNA was analyzed by quantitative real-time PCR.
Normal IgG served as negative control. The ac-H3 levels in the
promoter region of GAPDH served as positive control. (B) Upper,
western blot analysis of protein expression of p-AKT, total AKT,
and internal control β-actin in SCC-15 and CAL27 cells with TSA
treatment. Lower, western blot analysis of protein expression of
p-AKT, total AKT, PDK1, and β-actin in SCC-15 and CAL27 cells with
miR-375 overexpression. Histograms show the quantification of band
intensities. Results are presented as the mean ± SD (*P<0.05,
**P<0.01). |
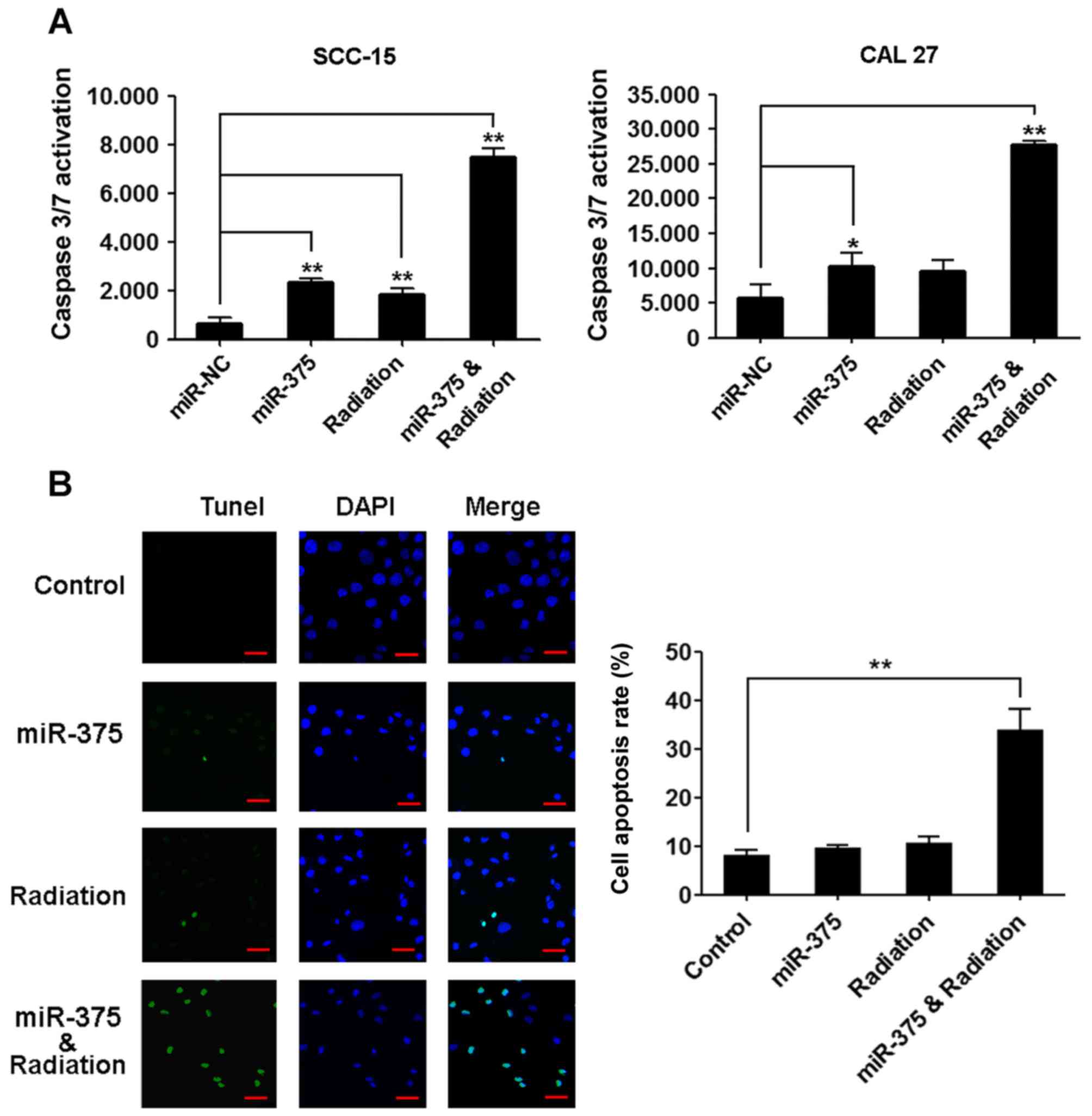
miR-375 increases TSCC cell
radiosensitivity
To determine whether miR-375 overexpression could
increase radiosensitivity of TSCC cells, SCC-15 and CAL27 cells
were transiently transfected with a control miRNA or miR-375 mimic,
and their radiosensitivity was determined. The TSCC cells treated
with irradiation or transfected with the miR-375 mimic did not
significantly change the percentage of apoptotic cells compared
with the control. However, combining overexpression of miR-375 with
irradiation induced significant TSCC cell apoptosis compared with
the control as revealed by the activity of caspase-3/7 (Fig. 5A) and TUNEL staining (Fig. 5B).
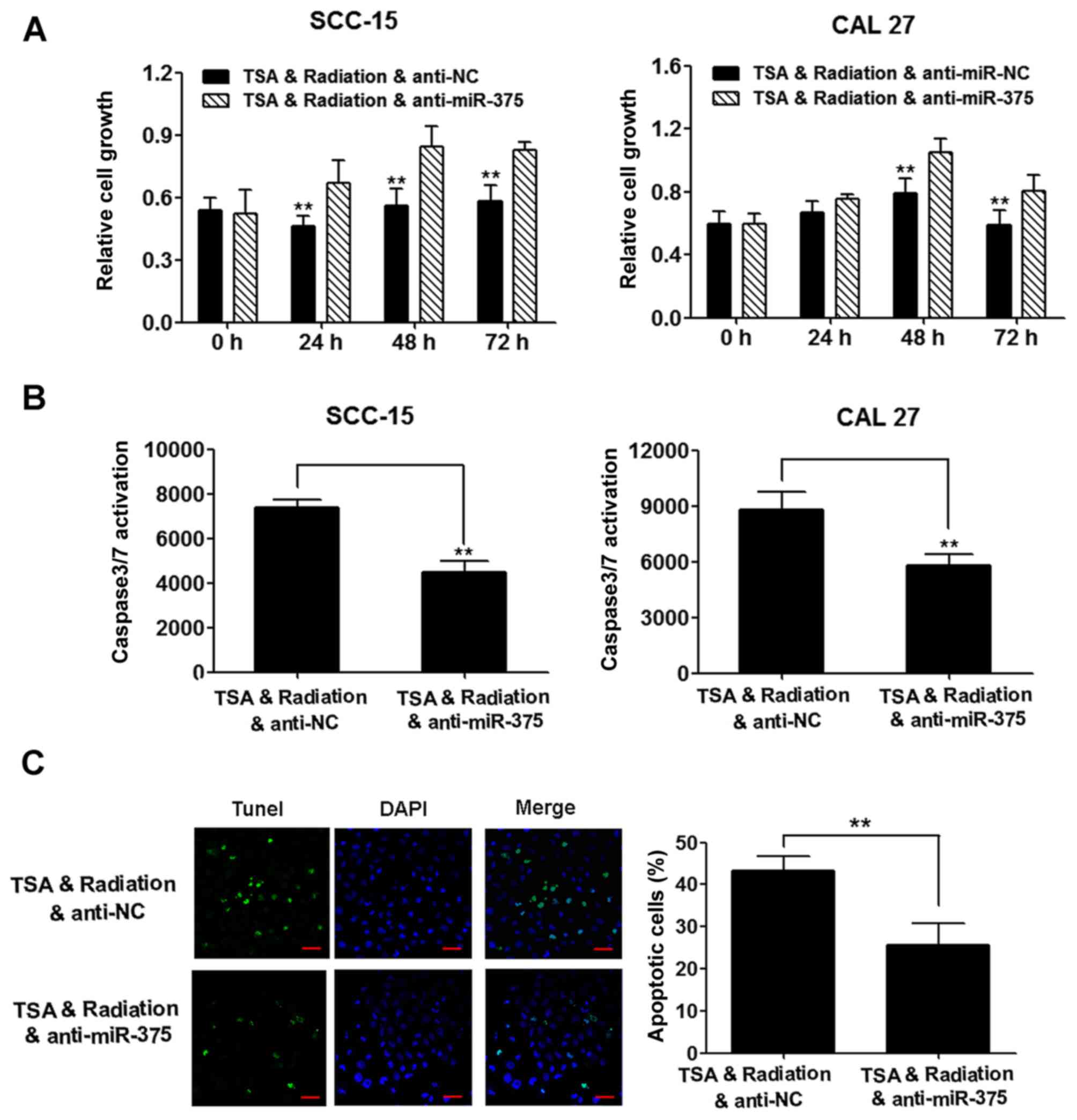
TSA-induced increase of
radiosensitivity is partially mediated by miR-375
To determine whether TSA-induced radiosensitivity is
mediated by miR-375, an inhibitor specific for miR-375 was used to
analyze the effects of miR-375 knockdown on the cellular
radioresponse. The inhibition of cell proliferation induced by
combining TSA with irradiation was relieved by miR-375 knockdown
(Fig. 6A), and the apoptosis
induced by the combined treatment was reduced by miR-375 knockdown
as revealed by the activity of caspase-3/7 (Fig. 6B) and TUNEL staining (Fig. 6C).
Discussion
In this study, we demonstrated that TSA sensitized
TSCC cells to radiation. TSCC is a common type of cancer from
squamous cell carcinomas of head and neck (HNSCCs) and is generally
deemed to have intermediate radiosensitivity (3). Our results demonstrated that TSA acted
as a powerful radiosensitizer in TSCC cells by inhibiting cell
growth and promoting apoptosis, consistent with previous studies in
non-small cell lung cancer (7),
cervical carcinoma (8), colon
cancer (9), and erythroleukemic
(10). The function of TSA
associated with the radiosensitivity of TSCC would advance the
development of drugs that increase the sensitivity of TSCC cells to
irradiation.
Moreover, we found that TSA increased the histone
acetylation of the miR-375 promoter and induced its expression in
TSCC cells. miR-375 overexpression increased TSCC cell
radiosensitivity, and the radiosensitivity increased by TSA was
reduced by knockdown of miR-375, indicating that TSA may at least
partially enhance TSCC cell radiosensitivity through miR-375.
Regulation of tumor radiosensitivity via miR-associated mechanisms
has attracted much attention. Several miRs (14–17)
are known to be involved in tumor radioresistance. Previous studies
have identified miR-375 as a tumor-suppressor gene in human HNSCC
(29–31) and it inhibits cell growth by
targeting Sp1 in TSCC (21).
However, the exact expression pattern and function of miR-375 in
radiosensitive and radioresistant TSCC remains to be explored. In
this study, we demonstrated that miR-375 enhanced the
radiation-induced TSCC cell apoptosis, which is consistent with a
recent study showing that miR-375 promotes the radiosensitivity of
cervical cancer cells and increases radiation-induced apoptosis
(32). Interestingly, a preclinical
study applied liposomal nanoparticles loaded with miRNA in
combination with radiotherapy in a lung cancer model and showed
that the miRNA delivery plus radiotherapy effectively inhibits
tumor growth (33). Thus, miRs-base
therapy may be well combined with irradiation to optimize treatment
plan in advance.
Furthermore, we found that miR-375 overexpression
downregulated PDK1 and phosphorylated AKT expression in TSCC cells,
consistent with previous studies demonstrating that miR-375
directly targets PDK1 and subsequently reduces the level of
phosphorylated AKT without significant change of total AKT level in
pancreatic cells (25,27) and gastric carcinoma (26). AKT pathway, which can be upregulated
by radiotherapy, is involved in the mechanisms of radiation
resistance (23,24). Especially in HNSCC, the AKT signal
transduction pathway is involved in the most important mechanisms
of radioresistance: intrinsic radioresistance, tumor-cell
proliferation, and hypoxia (3).
Several studies have showed that suppressing AKT activity
sensitizes cancer cells to irradiation (34–36).
Therefore, our study provided evidence that TSA induced apoptosis
in TSCC cells by upregulating miR-375 and subsequently suppressing
the AKT cascade. However, p53 (32), Sp1 (21), and Notch pathways (37) are also modulated by miR-375, and TSA
has also been shown to enhance cellular radiation sensitivity by
modulating BRCA1 (11), p53
(12), Bmi-1 (13), hypoxia-inducible factor 1-α, and
vascular endothelial growth factor expression (8), all of which are crucial for cell
vitality. From these results, we could not exclude the possibility
that other signaling pathways are also involved in the regulation
of TSCC cell radiosensitivity.
In conclusion, TSA and miR-375 increase TSCC
cellular radiosensitivity. TSA enhances radiation-induced TSCC cell
apoptosis at least partially by restoring the histone acetylation
of the miR-375 promoter and upregulating miR-375 expression,
leading to the suppression of PDK1-AKT pathway. Our results suggest
that TSA or miR-375, in combination with radiotherapy, may provide
a novel approach for the treatment of TSCC.
Acknowledgements
This study was supported by National Natural Science
Foundation of China (81472764 and 81402235), and Foundation of
Peking University School and Hospital of Stomatology
(PKUSS20140104).
References
|
1
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Calais G, Alfonsi M, Bardet E, Sire C,
Germain T, Bergerot P, Rhein B, Tortochaux J, Oudinot P and
Bertrand P: Randomized trial of radiation therapy versus
concomitant chemotherapy and radiation therapy for advanced-stage
oropharynx carcinoma. J Natl Cancer Inst. 91:2081–2086. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bussink J, van der Kogel AJ and Kaanders
JH: Activation of the PI3-K/AKT pathway and implications for
radioresistance mechanisms in head and neck cancer. Lancet Oncol.
9:288–296. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu L, Wu K, Ma S and Zhang S: HDAC
inhibitors: A new radiosensitizer for non-small-cell lung cancer.
Tumori. 101:257–262. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim IA, Kim IH, Kim HJ, Chie EK and Kim
JS: HDAC inhibitor-mediated radiosensitization in human carcinoma
cells: A general phenomenon? J Radiat Res (Tokyo). 51:257–263.
2010. View Article : Google Scholar
|
|
6
|
Zhang Y and Jung M, Dritschilo A and Jung
M: Enhancement of radiation sensitivity of human squamous carcinoma
cells by histone deacetylase inhibitors. Radiat Res. 161:667–674.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang F, Zhang T, Teng ZH, Zhang R, Wang
JB and Mei QB: Sensitization to gamma-irradiation-induced cell
cycle arrest and apoptosis by the histone deacetylase inhibitor
trichostatin A in non-small cell lung cancer (NSCLC) cells. Cancer
Biol Ther. 8:823–831. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yu J, Mi J, Wang Y, Wang A and Tian X:
Regulation of radiosensitivity by HDAC inhibitor trichostatin A in
the human cervical carcinoma cell line Hela. Eur J Gynaecol Oncol.
33:285–290. 2012.PubMed/NCBI
|
|
9
|
He G, Wang Y, Pang X and Zhang B:
Inhibition of autophagy induced by TSA sensitizes colon cancer cell
to radiation. Tumour Biol. 35:1003–1011. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Karagiannis TC, Smith AJ and El'Osta A:
Radio- and chemo-sensitization of human erythroleukemic K562 cells
by the histone deacetylase inhibitor Trichostatin A. Hell J Nucl
Med. 7:184–191. 2004.PubMed/NCBI
|
|
11
|
Zhang Y, Carr T, Dimtchev A, Zaer N,
Dritschilo A and Jung M: Attenuated DNA damage repair by
trichostatin A through BRCA1 suppression. Radiat Res. 168:115–124.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim IA, Shin JH, Kim IH, Kim JH, Kim JS,
Wu HG, Chie EK, Ha SW, Park CI and Kao GD: Histone deacetylase
inhibitor-mediated radiosensitization of human cancer cells: Class
differences and the potential influence of p53. Clin Cancer Res.
12:940–949. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dong Q, Sharma S, Liu H, Chen L, Gu B, Sun
X and Wang G: HDAC inhibitors reverse acquired radio resistance of
KYSE-150R esophageal carcinoma cells by modulating Bmi-1
expression. Toxicol Lett. 224:121–129. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qu JQ, Yi HM, Ye X, Li LN, Zhu JF, Xiao T,
Yuan L, Li JY, Wang YY, Feng J, et al: MiR-23a sensitizes
nasopharyngeal carcinoma to irradiation by targeting IL-8/Stat3
pathway. Oncotarget. 6:28341–28356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Balça-Silva J, Neves S Sousa, Gonçalves
AC, Abrantes AM, Casalta-Lopes J, Botelho MF, Sarmento-Ribeiro AB
and Silva HC: Effect of miR-34b overexpression on the
radiosensitivity of non-small cell lung cancer cell lines.
Anticancer Res. 32:1603–1609. 2012.PubMed/NCBI
|
|
16
|
Yang W, Shen Y, Wei J and Liu F:
MicroRNA-153/Nrf-2/GPx1 pathway regulates radiosensitivity and
stemness of glioma stem cells via reactive oxygen species.
Oncotarget. 6:22006–22027. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi L, Zhang S, Wu H, Zhang L, Dai X, Hu
J, Xue J, Liu T, Liang Y and Wu G: MiR-200c increases the
radiosensitivity of non-small-cell lung cancer cell line A549 by
targeting VEGF-VEGFR2 pathway. PLoS One. 8:e783442013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao L, Bode AM, Cao Y and Dong Z:
Regulatory mechanisms and clinical perspectives of miRNA in tumor
radiosensitivity. Carcinogenesis. 33:2220–2227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Isozaki Y, Hoshino I, Nohata N, Kinoshita
T, Akutsu Y, Hanari N, Mori M, Yoneyama Y, Akanuma N, Takeshita N,
et al: Identification of novel molecular targets regulated by tumor
suppressive miR-375 induced by histone acetylation in esophageal
squamous cell carcinoma. Int J Oncol. 41:985–994. 2012.PubMed/NCBI
|
|
20
|
Yin LH, Zheng XQ, Li HY, Bi LX, Shi YF, Ye
AF, Wu JB and Gao SM: Epigenetic deregulated miR-375 contributes to
the constitutive activation of JAK2/STAT signaling in
myeloproliferative neoplasm. Leuk Res. 39:471–478. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jia L, Huang Y, Zheng Y, Lyu M, Zhang C,
Meng Z, Gan Y and Yu G: miR-375 inhibits cell growth and correlates
with clinical outcomes in tongue squamous cell carcinoma. Oncol
Rep. 33:2061–2071. 2015.PubMed/NCBI
|
|
22
|
Manning BD and Cantley LC: AKT/PKB
signaling: Navigating downstream. Cell. 129:1261–1274. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dent P, Yacoub A, Contessa J, Caron R,
Amorino G, Valerie K, Hagan MP, Grant S and Schmidt-Ullrich R:
Stress and radiation-induced activation of multiple intracellular
signaling pathways. Radiat Res. 159:283–300. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Meng Z and Gan YH: Activating PTEN by
COX-2 inhibitors antagonizes radiation-induced AKT activation
contributing to radiosensitization. Biochem Biophys Res Commun.
460:198–204. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu S, Zhang M, Sun F, Ren L, He X, Hua J
and Peng S: miR-375 controls porcine pancreatic stem cell fate by
targeting 3-phosphoinositide-dependent protein kinase-1 (Pdk1).
Cell Prolif. 49:395–406. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsukamoto Y, Nakada C, Noguchi T, Tanigawa
M, Nguyen LT, Uchida T, Hijiya N, Matsuura K, Fujioka T, Seto M, et
al: MicroRNA-375 is downregulated in gastric carcinomas and
regulates cell survival by targeting PDK1 and 14-3-3zeta. Cancer
Res. 70:2339–2349. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou J, Song S, He S, Zhu X, Zhang Y, Yi
B, Zhang B, Qin G and Li D: MicroRNA-375 targets PDK1 in pancreatic
carcinoma and suppresses cell growth through the Akt signaling
pathway. Int J Mol Med. 33:950–956. 2014.PubMed/NCBI
|
|
28
|
Jia LF, Wei SB, Gong K, Gan YH and Yu GY:
Prognostic implications of micoRNA miR-195 expression in human
tongue squamous cell carcinoma. PLoS One. 8:e566342013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Harris T, Jimenez L, Kawachi N, Fan JB,
Chen J, Belbin T, Ramnauth A, Loudig O, Keller CE, Smith R, et al:
Low-level expression of miR-375 correlates with poor outcome and
metastasis while altering the invasive properties of head and neck
squamous cell carcinomas. Am J Pathol. 180:917–928. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nohata N, Hanazawa T, Kikkawa N, Mutallip
M, Sakurai D, Fujimura L, Kawakami K, Chiyomaru T, Yoshino H,
Enokida H, et al: Tumor suppressive microRNA-375 regulates oncogene
AEG-1/MTDH in head and neck squamous cell carcinoma (HNSCC). J Hum
Genet. 56:595–601. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jamali Z, Asl Aminabadi N, Attaran R,
Pournagiazar F, Oskouei S Ghertasi and Ahmadpour F: MicroRNAs as
prognostic molecular signatures in human head and neck squamous
cell carcinoma: A systematic review and meta-analysis. Oral Oncol.
51:321–331. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Song L, Liu S, Zeng S, Zhang L and Li X:
miR-375 modulates radiosensitivity of HR-HPV-positive cervical
cancer cells by targeting UBE3A through the p53 pathway. Med Sci
Monit. 21:2210–2217. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cortez MA, Valdecanas D, Niknam S, Peltier
HJ, Diao L, Giri U, Komaki R, Calin GA, Gomez DR, Chang JY, et al:
In vivo delivery of miR-34a sensitizes lung tumors to radiation
through RAD51 regulation. Mol Ther Nucleic Acids. 4:e2702015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kraus AC, Ferber I, Bachmann SO, Specht H,
Wimmel A, Gross MW, Schlegel J, Suske G and Schuermann M: In vitro
chemo- and radio-resistance in small cell lung cancer correlates
with cell adhesion and constitutive activation of AKT and MAP
kinase pathways. Oncogene. 21:8683–8695. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li X, Chen F, Zhu Q, Ding B, Zhong Q,
Huang K, Jiang X, Wang Z, Yin C, Zhu Y, et al: Gli-1/PI3K/AKT/NF-κB
pathway mediates resistance to radiation and is a target for
reversion of responses in refractory acute myeloid leukemia cells.
Oncotarget. 7:33004–33015. 2016.PubMed/NCBI
|
|
36
|
Luo XM, Xu B, Zhou ML, Bao YY, Zhou SH,
Fan J and Lu ZJ: Co-inhibition of GLUT-1 expression and the
PI3K/Akt signaling pathway to enhance the radiosensitivity of
laryngeal carcinoma xenografts in vivo. PLoS One. 10:e01433062015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang L, Song G, Liu M, Chen B, Chen Y,
Shen Y, Zhu J and Zhou X: MicroRNA-375 overexpression influences
P19 cell proliferation, apoptosis and differentiation through the
Notch signaling pathway. Int J Mol Med. 37:47–55. 2016.PubMed/NCBI
|