Introduction
Prostate cancer is the most frequently occurring
cancer and second leading cause of cancer-related deaths in men
(1). Although the five-year
relative survival rate has increased over the past 25 years,
prostate cancer is still the leading cause of cancer death in older
men (1). While delaying the
development of castrate resistant disease with the combination of
hormonal therapy and chemotherapy has been shown to significantly
improve overall survival (2), novel
and less toxic approaches for delaying the progression of prostate
cancer to castration independence would be of great benefit for
patients.
Atorvastatin and other statins inhibit
3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) reductase and are used
clinically as an effective treatment for the control of
hypercholesterolemia (3). Although
earlier epidemiological studies on statin use and overall prostate
cancer risk was not conclusive, large epidemiological studies
observed that statin use was associated with a reduced risk of
advanced prostate cancer (4–7). In
addition, clinical study found that statin use was associated with
a reduction in the risk of biochemical recurrence (8). Many studies indicate that COX-2 is
overexpressed in human prostate adenocarcinoma (9–12) with
consistently high levels observed in lymph node metastasis,
suggesting that in the prostate, COX-2 may act early in tumor
promotion and progression and potentially a target for drug therapy
in the management of the disease. Earlier studies from our
laboratory indicate that atorvastatin and celecoxib in combination
synergistically inhibited the growth and induced apoptosis in
cultured prostate cancer cells. This combination inhibited the
progression of androgen-dependent LNCaP tumors to androgen
independence and the growth of androgen-independent PC-3 prostate
tumors in SCID mice more effectively than either agent alone
(13,14). Clinically, however, cardiac toxicity
is a concern for celecoxib with long-term use increasing the risk
of cardiovascular events (15).
Therefore, investigation of other non-steroidal anti-inflammatory
drugs (NSAIDs) inhibiting COX-2 to use in combination with
atorvastatin to inhibit prostate cancer growth is appropriate.
Aspirin is one of the most commonly used NSAIDs and
has shown activity in a variety of cancers, including prostate
cancer (16–18). Although results from several
epidemiological studies for the association of aspirin use and
prostate cancer were controversial (19–21), a
recent meta-analysis of prospective and case-control cohort studies
including over 100,000 prostate cancer cases worldwide found that
aspirin was associated with a reduced risk of total prostate cancer
and prostate cancer-specific mortality (22). In addition, in men with
non-metastatic prostate cancer, post-diagnosis daily aspirin use
has been associated with lower prostate cancer-specific mortality
among men diagnosed with high-risk prostate cancer (≥T3 and/or
Gleason score ≥8) (23–25). Since aspirin has inhibitory effect
on COX-2 and anti-prostate cancer activities, we hypothesize that a
combination of aspirin with atorvastatin will strongly inhibit the
growth of prostate cancer.
In the present study, we evaluated the effects of
atorvastatin alone or in combination with aspirin on the growth and
apoptosis in LNCaP, VCaP and PC-3 cells. Additionally, we evaluated
the effect of these drugs alone or in combination on the growth of
prostate xenograft tumors in SCID mice. We found that the
combination of atorvastatin and aspirin strongly inhibited the
growth of PC-3 tumors and the combination strongly induced
apoptosis. We also found that the effects of the drug combination
on proliferation and apoptosis were associated with inhibition of
NF-κB and Stat3. These data provide a rationale for clinically
evaluating the combined use of atorvastatin and aspirin in the
treatment of prostate cancer.
Materials and methods
Cell culture and reagents
LNCaP, VCaP and PC-3 cells were obtained from the
American Type Culture Collection (ATCC, Rockville, MD, USA).
Aspirin, atorvastatin, propylene glycol, polysorbate 80, benzyl
alcohol, ethanol and DMSO were from Sigma (St. Louis, MO, USA).
Matrigel was obtained from BD Biosciences (Bedford, MA, USA).
RPMI-1640 tissue culture medium, penicillin-streptomycin,
L-glutamine and fetal bovine serum (FBS) were from Gibco (Grand
Island, NY, USA). The cells were maintained in RPMI-1640 culture
medium containing 10% FBS that was supplemented with penicillin
(100 U/ml)-streptomycin (100 µg/ml) and L-glutamine (300 µg/ml).
Cultured cells were grown at 37°C in a humidified atmosphere of 5%
CO2 and were passaged twice a week.
Determination of the number of viable
cells
The inhibitory effect of atorvastatin and aspirin
alone or in combination on human prostate cancer cells was
determined using the trypan blue exclusion assay. In dose-dependent
experiments, human prostate cancer LNCaP, VCaP (both are
androgen-dependent) and PC-3 (androgen-independent) cells were
treated with different concentrations of atorvastatin or aspirin
for 72 h. The number of viable cells after each treatment was
determined using a hemacytometer under a light microscope
(Optiphot-2, Nikon, Tokyo, Japan). Cell viability was determined by
the trypan blue exclusion assay, which was done by mixing 80 µl of
cell suspension and 20 µl of 0.4% trypan blue solution for 2 min.
Blue cells were counted as dead cells and the cells that did not
absorb dye were counted as live cells.
Assessment of apoptotic cells
Apoptosis was determined by morphological assessment
in cells stained with propidium iodide (PI) (26). Apoptotic cells were identified by
classical morphological features including nuclear condensation,
cell shrinkage, and formation of apoptotic bodies (26). Apoptosis was also determined by
caspase-3 immunostaining with an antibody that reacts with the
active form of caspase-3 (AF835, R&D Systems, Minneapolis, MN,
USA). In brief, cytospin slides were incubated with caspase-3
antibody for 30 min followed by incubation with a biotinylated
anti-rabbit secondary antibody for 30 min and incubation with
conjugated-avidin solution (ABC Elite® kit, Vector
Laboratories, Burlingame, CA, USA) for 30 min. Color development
was achieved by incubation with 0.02% 3,3′-diaminobenzidin
tetrahydrochloride containing 0.02% hydrogen peroxide for 10 min at
room temperature.
Western blotting
The levels of phospho-Erk1/2 and phospho-Stat3 were
determined by western blot analysis. PC-3 cells were seeded at a
density of 1×105 cells/ml of RPMI medium and incubated
for 24 h. The cells were then treated with atorvastatin (5 mM) and
aspirin (0.5 mM) alone or in combination for 24 h. After treatment,
the cell lysates were prepared as described earlier (27). Proteins were subjected to sodium
dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred to nitrocellulose membrane. After blocking nonspecific
binding sites with blocking buffer, the membrane was incubated
overnight at 4°C with primary antibodies (#9131 for phospho-Stat3
and #9101 for phospho-Erk1/2, both from Cell Signaling Co.,
Beverly, MA, USA). The β-actin detected by anti-β-actin antibody
(sc-47778, Santa Cruz Biotechnology Inc., Dallas, TX, USA) was used
as a loading control. Following removal of the primary antibody,
the membrane was washed three times with TBS (PBS containing 0.05%
Tween-20) buffer at room temperature and then incubated with
fluorochrome-conjugated secondary antibody (925–32211, LI-COR
Biotechnology, Lincoln, NE, USA). The membrane was then washed with
TBS three times. Final detection was done with an Odyssey infrared
imaging system (Li-Cor Biotechnology). The extent of protein
loading was determined by blotting for β-actin, and the levels of
phospho-Erk1/2 and phospho-Stat3 in the western blot was analyzed
by optical density measurement and normalized for actin.
NF-κB-dependent reporter gene
expression assay
NF-κB transcriptional activity was measured by the
NF-κB-luciferase reporter gene expression assay using the PC-3/N
cells (28). This cell line was
previously established in our laboratory by stable transfection of
PC-3 cells with an NF-κB luciferase construct (#CLS-013L;
SABiosciences, Valencia, CA, USA). A single stable clone, PC-3/N
was obtained and used in the present study. In brief, PC-3/N cells
were treated with aspirin or atorvastatin alone or in combination
for 24 h, and the NF-κB-luciferase activities were measured using
the luciferase assay kits from Promega (Madison, WI, USA). After
treatments the cells were washed with ice-cold phosphate
buffered-saline (PBS) and harvested in 1X reporter lysis buffer.
After centrifugation, 10 µl aliquots of the supernatants were
measured for luciferase activity by using a Luminometer from Turner
Designs Instrument (Sunnyvale, CA, USA). The luciferase activity
was normalized against known protein concentrations and expressed
as percent of luciferase activity in the control cells, which were
treated with DMSO solvent. The protein level was determined by
Bio-Rad protein assay kits (Bio-Rad, Hercules, CA, USA) according
to the manufacturer's instructions.
Formation and growth of PC-3 tumors in
immunodeficient mice
Male severe combined immunodeficient (SCID) mice
(6–7 weeks old) were obtained from Taco Farms Inc. (Germantown, NY,
USA). The animals were housed in sterile filter-capped
microisolator cages and provided with sterilized food and water.
Prostate cancer PC-3 cells (2×106 cells/0.1 ml/mouse)
suspended in 50% Matrigel (Collaborative Research, Bedford, MA,
USA) in RPMI-1640 medium were injected subcutaneously into the
right flank of the mice. After approximately 4 weeks, mice with
established xenograft tumors were injected with vehicle,
atorvastatin (5 mg/kg), aspirin (80 mg/kg), or atorvastatin (5
mg/kg) + aspirin (80 mg/kg) three times a week for 30 days. Each
group had 9 mice and all animals received the same amount of
vehicle (5 µl/g body weight) which consisted of propylene glycol,
polysorbate 80, benzyl alcohol, ethanol and water (40: 0.5: 1: 10:
48.5). Tumor size (length × width) and body weight were measured
three times a week. At the end of the study, mice were sacrificed,
tumors were excised, weighed and placed in phosphate-buffered
formalin at room temperature for 48 h and then placed in ethanol
for 48 h before preparing paraffin sections as previously described
(29). All animal experiments were
carried out under an Institutional Animal Care and Use Committee
(IACUC)-approved protocol (RU02-001).
Assay of tumor cell proliferation
Proliferation of the PC-3 xenograft tumors was
determined by measuring the expression of proliferating cell
nuclear antigen (PCNA) using immunohistochemical staining. In
brief, tumors were excised from each mouse and weighed at the end
of the experiment. Tumor tissues were fixed in buffered formalin
for 24 h and then with ethanol for 48 h. Paraffin blocks of tumor
tissues were prepared and paraffin sections of tumor tissues were
processed for immunohistochemical staining. The sections were
incubated with PCNA antibody (MAB424, Millipore Corp. Billerica,
MA, USA) for 1 h at room temperature. The sections were then
incubated with a biotinylated secondary antibody for 30 min
followed by incubation with horseradish peroxidase
conjugated-avidin solution for 30 min using the Elite ABC kit
(PK-6100, Vector Laboratories). PCNA staining in tumor cells (brown
color in nucleus) were examined under a microscope (Optiphot-2,
Nikon). At least 1000 cells were counted for each section.
Statistical analyses
Statistical analyses were done by using InStat
software (GraphPad Software, Inc., La Jolla, CA, USA). The analysis
of variance (ANOVA) was used for the comparison of growth
inhibition, apoptosis and NF-κB in the in vitro studies, and
for comparison of tumor size, body weight and PCNA positive cells
in the in vivo studies.
Results
Effects of atorvastatin and aspirin on
growth and apoptosis of human prostate cancer cells
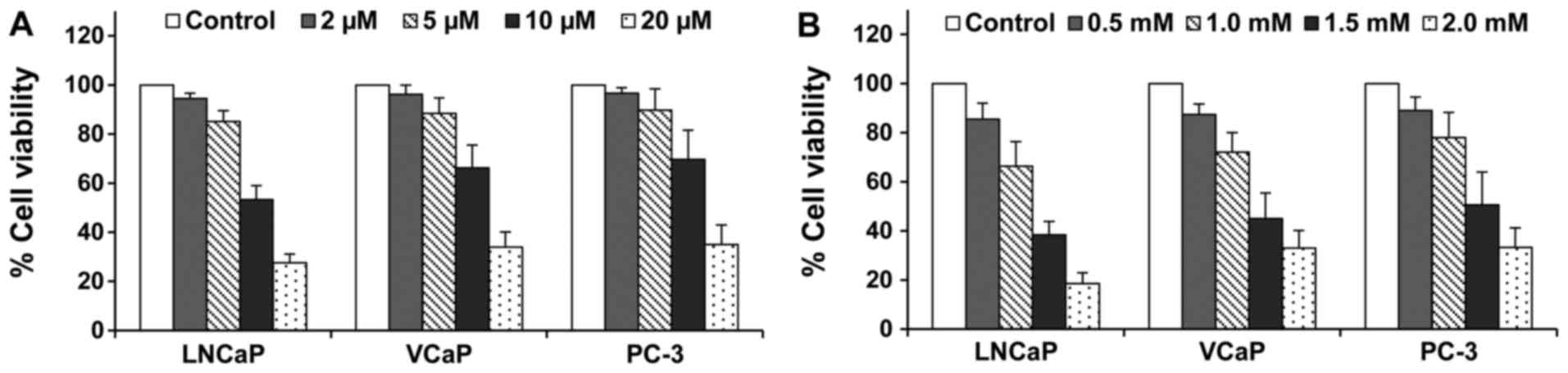
Treatment of different prostate cancer cells with
atorvastatin or aspirin inhibited the cell growth in a
concentration-dependent manner. The inhibitory effect of
atorvastatin or aspirin were similar among the three prostate
cancer cell lines tested. Treatment of the cells with atorvastatin
at 5 µM alone or aspirin at 0.5 mM alone resulted in 10–15%
decrease in the number of viable cells (Fig. 1). As shown in Table I, the combination of atorvastatin
and aspirin had a more potent effect for inhibiting the growth of
LNCaP, VCaP and PC-3 cells than either agent alone. Statistical
analysis using ANOVA with the Tukey-Kramer multiple comparison test
showed that the percentage of viable cells was significantly lower
in the atorvastatin and aspirin combination-treated group than in
the atorvastatin- or aspirin-treated groups.
 | Table I.Effects of atorvastatin and aspirin
alone or in combination on the growth and apoptosis in LNCaP, VCaP
and PC-3 cells. |
Table I.
Effects of atorvastatin and aspirin
alone or in combination on the growth and apoptosis in LNCaP, VCaP
and PC-3 cells.
|
|
| Apoptotic cells
% |
|---|
|
|
|
|
|---|
| Treatment | Viable cells (% of
control) | Morphological
assessment | Caspase-3
staining |
|---|
| LNCaP cells |
|
|
|
|
Control | 100 | 2.0±0.4 | 1.8±0.3 |
|
Atorvastatin (5 µM) | 86.7±4.4 | 9.3±1.1 | 10.0±0.9 |
| Aspirin
(0.5 mM) | 87.0±4.8 | 7.9±1.3 | 8.4±1.0 |
|
Atorvastatin (5 µM) + aspirin
(0.5 mM) |
60.1±10.7a |
22.3±5.5a |
25.1±5.4a |
| VCaP cells |
|
|
|
|
Control | 100 | 1.7±0.5 | 2.2±0.2 |
|
Atorvastatin (5 µM) | 90.3±2.7 | 7.8±2.2 | 8.8±1.0 |
| Aspirin
(0.5 mM) | 86.6±6.3 | 10.3±1.8 | 10.5±1.5 |
|
Atorvastatin (5 µM) + aspirin
(0.5 mM) |
63.4±8.2a |
24.3±7.1a |
26.0±6.4a |
| PC-3 cells |
|
|
|
|
Control | 100 | 1.1±0.5 | 1.6±0.3 |
|
Atorvastatin (5 µM) | 91.5±4.5 | 7.5±2.0 | 9.0±1.1 |
| Aspirin
(0.5 mM) | 87.6±7.0 | 8.2±2.0 | 9.5±2.1 |
|
Atorvastatin (5 µM) + aspirin
(0.5 mM) |
53.0±8.9b |
29.6±8.1a |
31.6±8.4a |
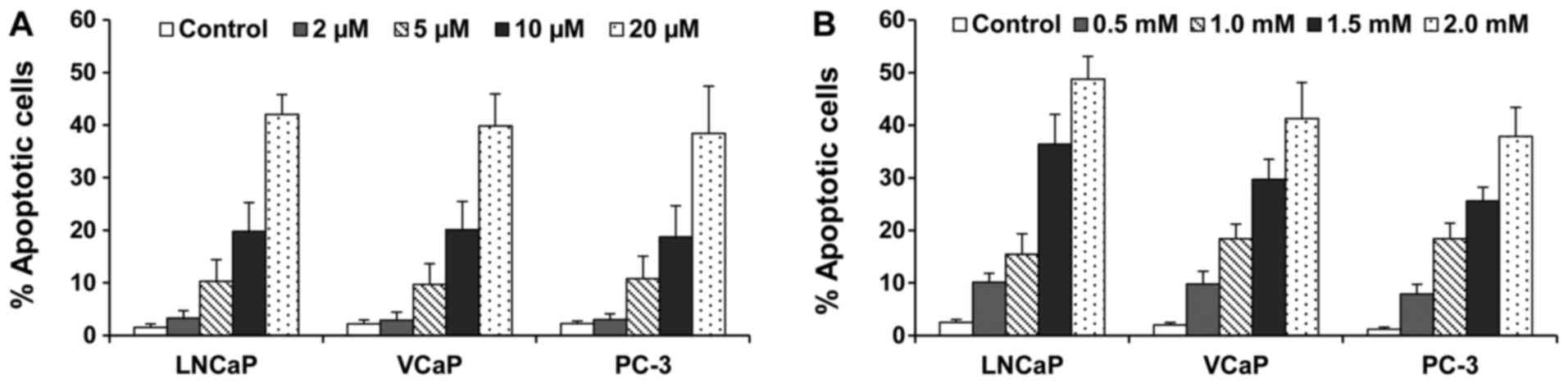
Treatment of different prostate cancer cells with
atorvastatin or aspirin resulted in a concentration-dependent
increase in the number of apoptotic cells (Fig. 2). As determined by morphological
assessment and caspase-3 staining, atorvastatin and aspirin in
combination had more potent stimulatory effect on apoptosis in
LNCaP, VCaP and PC-3 cells than with either agent alone.
Statistical analysis using ANOVA with the Tukey-Kramer multiple
comparison test showed that the percentage of apoptotic cells was
significantly higher in the atorvastatin + aspirin-treated group
than in the atorvastatin- or aspirin-treated groups (p<0.01).
Since the combination of atorvastatin and aspirin had stronger
effects on growth inhibition and apoptosis induction in the PC-3
cells, we chose the PC-3 line for further in vitro and in
vivo studies.
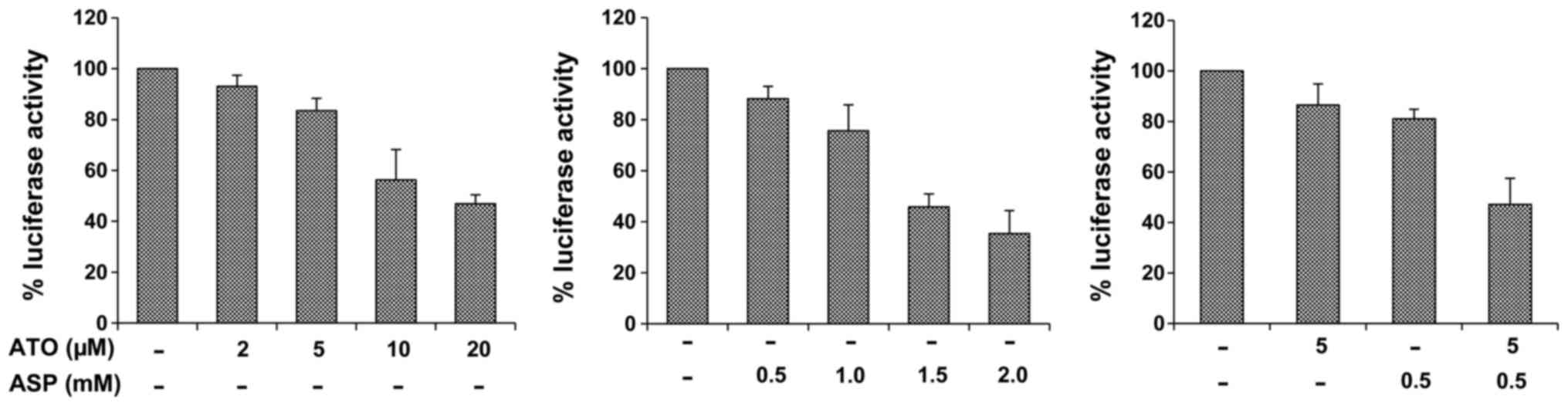
Inhibitory effect of atorvastatin and
aspirin on NF-κB transcriptional activity
Treatment of PC-3/N cells with atorvastatin or
aspirin dose-dependently decreased the luciferase activity in
PC-3/N cells indicating an inhibition of the NF-κB activation
(Fig. 3). The combination of
atorvastatin and aspirin had a much stronger effect than either
agent alone (Fig. 3). Statistical
analysis using ANOVA with the Tukey-Kramer multiple comparison test
showed significant differences for the luciferase activity between
the atorvastatin-treated group and the combination-treated group
(p<0.001), and between the aspirin-treated group and the
combination-treated group (p<0.01).
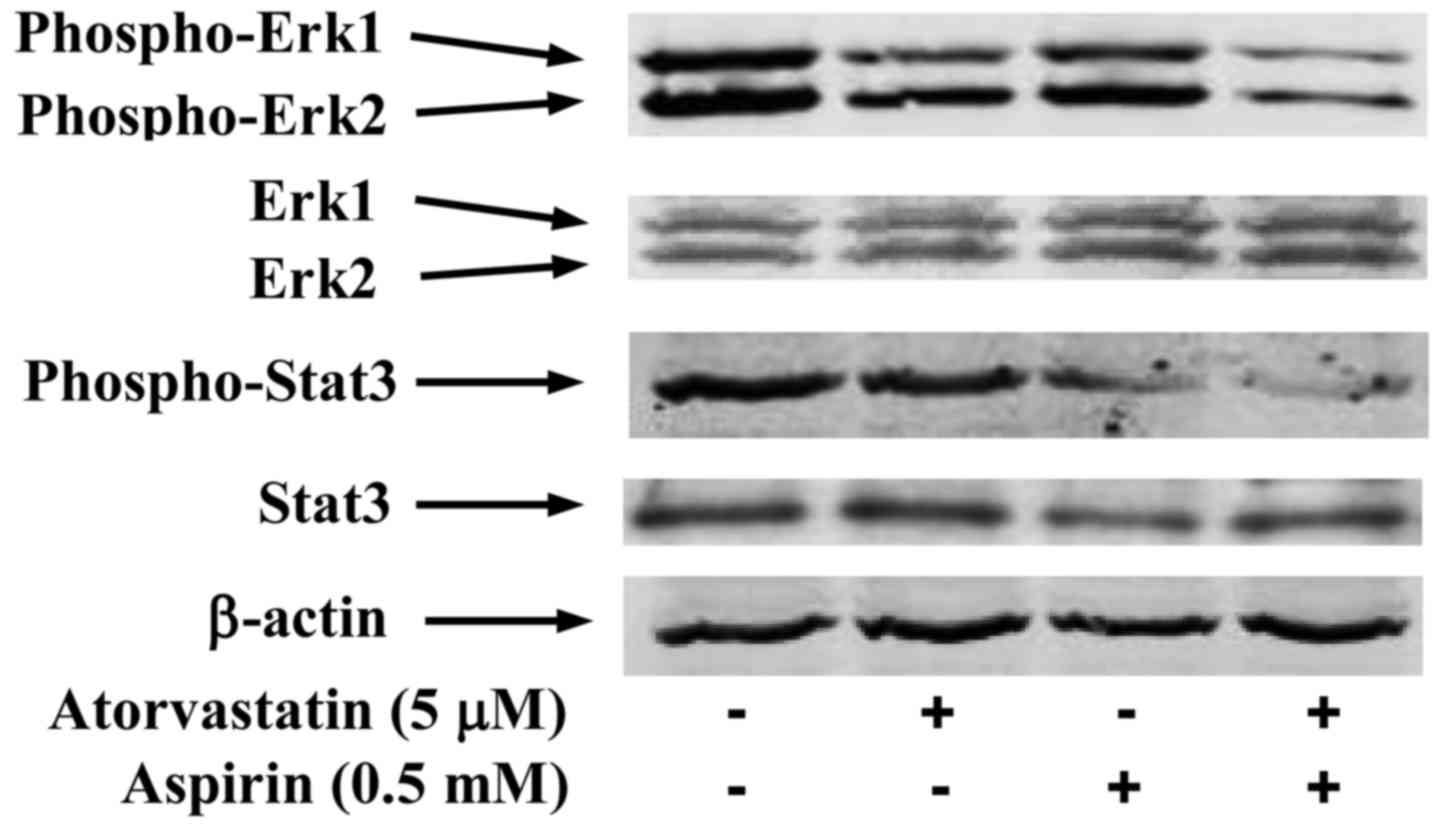
Effects of atorvastatin and aspirin on
the level of phospho-Stat3 and phospho-Erk1/2
Treatment of PC-3 cells with atorvastatin (5 µM)
resulted in a moderate decrease in the level of phospho-Erk1/2
while aspirin (0.5 mM) only had a small effect on decreasing the
level of this protein (Fig. 4). The
combination of atorvastatin (5 µM) and aspirin (0.5 mM) caused a
stronger decrease in the level of phospho-Erk1/2 than either agent
alone. The level of phospho-Erk1 relative to control (1.00) was
0.74 in cells treated with atorvastatin, 0.91 in cells treated with
aspirin and 0.22 in cells treated with the combination of
atorvastatin and aspirin. The level of Erk1 relative to control
(1.00) was 0.97 in cells treated with atorvastatin, 1.05 in cells
treated with aspirin and 1.06 in cells treated with the combination
of the two drugs. The relative level of phospho-Erk2 was 1.00 in
control, 0.87 in cells treated with atorvastatin, 0.93 in cells
treated with aspirin and 0.39 in cells treated with the combination
of atorvastatin and aspirin. The level of Erk2 relative to control
(1.00) was 1.02 in cells treated with atorvastatin, 1.05 in cells
treated with aspirin and 1.07 in cells treated with the combination
of the two drugs. As shown in Fig.
4, the drug combination also strongly decreased the level of
phospho-Stat3 in PC-3 cells. The level of phospho-Stat3 relative to
control (1.00) was 0.97 in cells treated with atorvastatin, 0.55 in
cells treated with aspirin and 0.12 in cells treated with the
combination of atorvastatin and aspirin. The relative level of
Stat3 was 1.00 in control, 1.04 in cells treated with atorvastatin,
0.97 in cells treated with aspirin and 1.02 in cells treated with
the combination of the two drugs.
In vivo effect of atorvastatin and aspirin on the
growth of PC-3 tumors. Treatment with atorvastatin alone (5 mg/kg)
resulted in a small inhibition on the growth of PC-3 tumors, while
aspirin alone (80 mg/kg) had a moderate inhibitory effect. The
combination of atorvastatin (5 mg/kg) and aspirin (80 mg/kg) had a
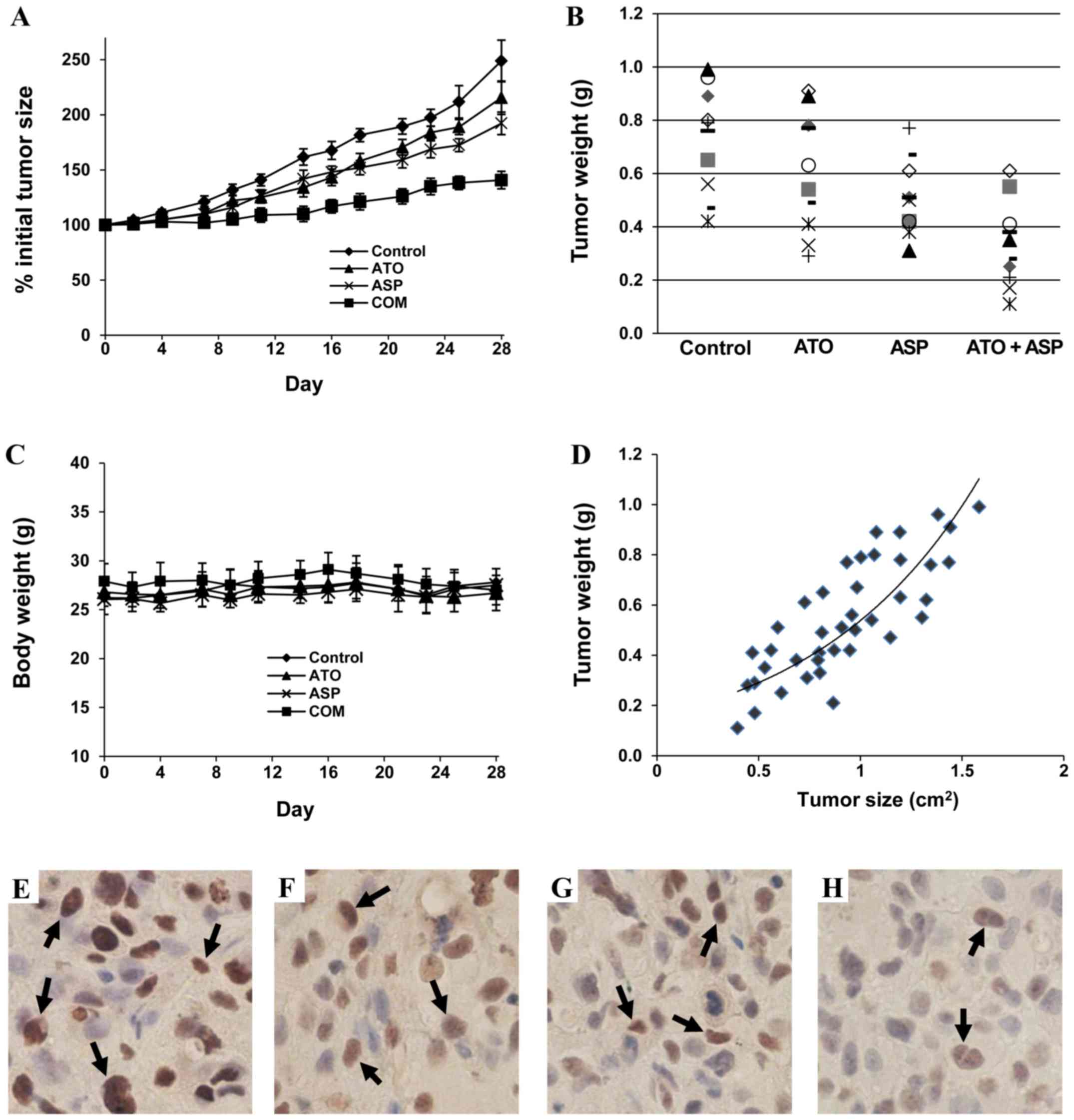
potent effect on inhibiting the growth of PC-3 tumors (Fig. 5A). The mean ± SE for percent initial
tumor size at the end of the experiment was 248.3±18.3 for the
control group, 215.4±14.0 for the atorvastatin-treated group,
192.3±9.9 for the aspirin-treated group, 140.8±8.1 for the animals
treated with combination of atorvastatin and aspirin. Statistical
analysis using ANOVA with the Tukey-Kramer multiple comparison test
showed statistically significant differences in the average tumor
size between the control group and the aspirin-treated group
(p<0.05), and between the control group and the
combination-treated group (p<0.001). The average tumor size in
the combination-treated group was significantly smaller than that
in the atorvastatin-treated group (p<0.01) and that in the
aspirin-treated group (p<0.05).
Tumor weights in each animal in the different
treatment groups at the end of the experiment is shown in Fig. 5B. The mean ± SE for tumor weight (g)
was 0.74±0.06 for the control group, 0.60±0.07 for the
atorvastatin-treated group, 0.53±0.04 for the animals treated with
aspirin, and 0.31±0.05 for the animals treated with the drug
combination. Statistical analysis using ANOVA with the Tukey-Kramer
multiple comparison test showed that the average tumor weight in
the combination group was significantly lower than that in the
atorvastatin-treated group (p<0.01) and that in aspirin-treated
group (p<0.05). We also determined the correlation between tumor
size and tumor weight, and found a good correlation (r=0.80)
between these two measurements (Fig.
5C). Treatment with atorvastatin and aspirin alone or in
combination did not affect the body weight of the mice (Fig. 5D). Statistical analysis using ANOVA
with the Tukey-Kramer multiple comparison test showed that the
differences in the percent of initial body weight between the
control group and any of the treatment group were not statistically
significant (p>0.05) (Fig.
5Z).
Effect of atorvastatin and aspirin on
the proliferation of PC-3 tumors
Immunohistochemical staining of PCNA in paraffin
sections of PC-3 tumors showed that treatment of SCID mice with
atorvastatin or aspirin alone resulted in a moderate decrease in
the number of PCNA positive cells (Fig.
5F and G). Treatment of the mice with the combination of
atorvastatin and aspirin resulted a potent decrease in the number
of PCNA positive cells (Fig. 5H).
The mean ± SD of percent PCNA positive cells was 70.3±8.5 in tumors
from the control group, 60.3±6.6 in tumors from the
atorvastatin-treated group, 54.9±7.5 in tumors from the
aspirin-treated group and 41.9±5.1 in tumors from the
combination-treated group. The differences in the number of PCNA
positive cells were statistically significant between the
combination-treated group and the atorvastatin-treated group
(p<0.001), and between the combination-treated group and the
aspirin-treated group (p<0.01).
Discussion
Recent large-scale epidemiological studies and a
meta-analysis have shown that aspirin use was associated with
reduced risk of overall and advanced prostate cancer risk (22,30).
In addition, post-diagnosis use of aspirin was associated with
lower prostate cancer specific mortality in patients with high-risk
disease (23–25). Use of the cholesterol lowering
statins has also been shown to have protective effects on prostate
cancer including decreased aggressiveness and significant decreases
in both all-cause and prostate cancer specific mortality (4–8). In
the present study, we demonstrated that atorvastatin in combination
with aspirin strongly inhibited the growth and induced apoptosis in
prostate cancer cells. Our study provided the first evidence that
the atorvastatin combined with aspirin had in vivo
anti-prostate cancer activity. The drug combination strongly
inhibited the growth of prostate xenograft tumors in SCID mice. Our
results, coupled with the epidemiologic evidence with each agent
alone, provide a strong rationale for clinically evaluating the
combination of atorvastatin and aspirin in prostate cancer
patients.
Multiple mechanisms for the growth inhibitory
effects of aspirin in prostate tumorigenesis have been suggested.
Aspirin is a noncompetitive inhibitor of the COX-enzymes 1 and 2
(16,31). COX-2 has an important role in tumor
angiogenesis and overexpression of this enzyme was found in
prostate cancer (9–12,32).
COX also produces ligands for peroxisome proliferator-activated
receptor gamma (PPAR-γ), a modulator of proliferation and apoptosis
in many cancer cell types (33). In
the present study, we found that aspirin inhibited activation of
NF-κB as determined by the luciferase reporter assay. Aspirin also
decreased the level of phospho-Stat3. Our results suggest that
aspirin inhibits multiple pathways in prostate cancer PC-3
cells.
The statin family of drugs inhibits
3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) reductase and is used
clinically as a safe and effective approach for the control of
hypercholesterolemia (34). Earlier
studies have indicated that in addition to the cholesterol-lowering
effect, statins have pleotropic activities that modulate other
biologic processes, such as cell proliferation and apoptosis by
inhibiting NF-κB and Erk1/2 (35).
HMG-CoA reductase produces farnesylpyrophosphate (FPP) and
geranylgeranylpyrophosphate (GGPP) (36). FPP and GGPP are involved in the
activation of Ras which is important for regulating cell growth and
apoptosis (37). Results of the
present study showed that atorvastatin decreased the level of Erk
which is a downstream target of the Ras pathway (37).
The mechanisms by which atorvastatin and aspirin in
combination strongly inhibited the growth and induced apoptosis in
prostate cancer cells are not clear. We found the combination of
atorvastatin and aspirin had more potent inhibitory effect on
activation of the transcription factor NF-κB than either drug used
alone. Strong inhibition of NF-κB activity by the drug combination
may lead to a strong down regulation of its anti-apoptotic genes
and contributed to the combined effect of atorvastatin and aspirin.
Atorvastatin alone had a moderate effect on inhibition of Erk1/2
and aspirin alone only caused small decrease in phosphor-Erk1/2
while the combination of these two drugs strongly decreased the
level of phosphor-Erk1/2. In addition, we found that the
combination more potently inhibited Stat3 than either drug alone.
Our unpublished preliminary study indicated that atorvastatin and
aspirin in combination inhibited androgen receptor (AR) activity as
measured by the luciferase reporter assay. However, the strong
combined effect of atorvastatin and aspirin on androgen-independent
PC-3 cells showed in our current study indicates that
AR-independent mechanisms were involved. The results of the
mechanistic studies described above indicate that the combination
of atorvastatin and aspirin target multiple signaling pathways
important in regulation of prostate cancer cell growth and
survival. Simultaneously inhibition of these important pathways may
lead to a strong inhibition in the growth and strong induction of
apoptosis in prostate cancer cells.
A recent retrospective case-control study in
Mediterranean men undergoing a prostate biopsy reported the use of
the combination of aspirin and statins resulted in significantly
less men diagnosed with prostate cancer compared to the untreated
group (p<0001) (38). The
investigators also evaluated the in vitro effects of
aspirin, simvastatin and the combination in LNCaP and PC3 cells.
The combination of aspirin and simvastatin significantly reduced
proliferation in both LNCaP and PC-3 cells compared to the
untreated control with no comparison to either drug alone (38). The in vivo effect of the drug
combination was not investigated in this study. In our present
study, we evaluated the effects of aspirin and atorvastatin alone
or in combination on growth inhibition and apoptosis in cultured
LNCaP, VCaP and PC-3 cells, and in PC-3 xenograft tumors in SCID
mice. In addition to the strong combined effect of aspirin and
atorvastatin on growth inhibition and apoptosis in cultured
prostate cancer cells, we showed that treatment of SCID mice with
atorvastatin and aspirin in combination more potently inhibited the
growth of PC-3 tumors than either drug along.
In the in vivo study, SCID mice bearing PC-3
xenograft tumors were treated with aspirin and atorvastatin alone
or in combination. The dose 5 mg/kg for atorvastatin and 80 mg/kg
for aspirin used in the present study was chosen based on the
effective doses of atorvastatin and aspirin in our previous studies
and other publications (13,14,39).
Atorvastatin or aspirin alone only had a moderate inhibitory effect
on the growth of PC-3 tumors. The combination of the two drugs more
potently inhibited the growth of PC-3 tumors than either drug used
alone. Treatment of SCID mice with i.p. injections of atorvastatin
(5 mg/kg body weight) and aspirin (80 mg/kg body weight) alone or
in combination did not cause body weight loss in the animals. In
addition, no abnormalities were found in the major organs at the
end of the experiment indicating that atorvastatin and aspirin at
the doses used in the present study were not toxic to the
animals.
In summary, we demonstrated in our current study
that the combination of atorvastatin with aspirin at therapeutic
concentrations potently inhibited the growth and stimulated
apoptosis in human prostate cancer cells. Strong effects of
atorvastatin and aspirin on growth inhibition and apoptosis
induction in prostate cancer cells were associated with inhibition
of NF-κB activation, decreased levels of phospho-Stat3 and
phospho-Erk1/2. Moreover, treatment of SCID mice with a combination
of atorvastatin and aspirin strongly inhibited the growth of
xenograft PC-3 tumors. The combination of atorvastatin and aspirin
may be an effective approach for delaying the progression of
prostate cancer and should be evaluated clinically.
Acknowledgements
The present study was supported by the Guangdong
Province Leadership Grant, China National Science Foundation Grants
(grant no. 81272452 and 21272043), and the Rutgers Cancer Institute
of New Jersey (CCSG P30-CA072720). The authors dedicate this study
to Dr Allan H. Conney, an outstanding and widely recognized cancer
researcher who passed away on September 10, 2013.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sweeney CJ, Chen YH, Carducci M, Liu G,
Jarrard DF, Eisenberger M, Wong YN, Hahn N, Kohli M, Cooney MM, et
al: Chemohormonal therapy in metastatic hormone-sensitive prostate
cancer. N Engl J Med. 373:737–746. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Malhotra HS and Goa KL: Atorvastatin: An
updated review of its pharmacological properties and use in
dyslipidaemia. Drugs. 61:1835–1881. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jacobs EJ, Rodriguez C, Bain EB, Wang Y,
Thun MJ and Calle EE: Cholesterol-lowering drugs and advanced
prostate cancer incidence in a large U.S. cohort. Cancer Epidemiol
Biomarkers Prev. 16:2213–2217. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Flick ED, Habel LA, Chan KA, Van Den Eeden
SK, Quinn VP, Haque R, Orav EJ, Seeger JD, Sadler MC, Quesenberry
CP Jr, et al: Statin use and risk of prostate cancer in the
California Men's Health Study cohort. Cancer Epidemiol Biomarkers
Prev. 16:2218–2225. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Murtola TJ, Tammela TL, Lahtela J and
Auvinen A: Cholesterol-lowering drugs and prostate cancer risk: A
population-based case-control study. Cancer Epidemiol Biomarkers
Prev. 16:2226–2232. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Platz EA, Leitzmann MF, Visvanathan K,
Rimm EB, Stampfer MJ, Willett WC and Giovannucci E: Statin drugs
and risk of advanced prostate cancer. J Natl Cancer Inst.
98:1819–1825. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cyrus-David MS, Weinberg A, Thompson T and
Kadmon D: The effect of statins on serum prostate specific antigen
levels in a cohort of airline pilots: A preliminary report. J Urol.
173:1923–1925. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Anai S, Tanaka M, Shiverick KT, Kim W,
Takada S, Boehlein S, Goodison S, Mizokami A and Rosser CJ:
Increased expression of cyclooxygenase-2 correlates with resistance
to radiation in human prostate adenocarcinoma cells. J Urol.
177:1913–1917. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Khor LY, Bae K, Pollack A, Hammond ME,
Grignon DJ, Venkatesan VM, Rosenthal SA, Ritter MA, Sandler HM,
Hanks GE, et al: COX-2 expression predicts prostate-cancer outcome:
Analysis of data from the RTOG 92-02 trial. Lancet Oncol.
8:912–920. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Richardsen E, Uglehus RD, Due J, Busch C
and Busund LT: COX-2 is overexpressed in primary prostate cancer
with metastatic potential and may predict survival. A comparison
study between COX-2, TGF-beta, IL-10 and Ki67. Cancer Epidemiol.
34:316–322. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ceylan Y, Lekili M, Muezzinoglu T, Nese N
and Isisag A: Predictive value of cyclooxygenase-2 over expression
for identifying prostate cancer from benign prostatic hyperplasia
in prostate biopsy specimens. Minerva Urol Nefrol. 68:255–262.
2016.PubMed/NCBI
|
|
13
|
Zheng X, Cui XX, Gao Z, Zhao Y, Lin Y,
Shih WJ, Huang MT, Liu Y, Rabson A, Reddy B, et al: Atorvastatin
and celecoxib in combination inhibits the progression of
androgen-dependent LNCaP xenograft prostate tumors to androgen
independence. Cancer Prev Res (Phila). 3:114–124. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zheng X, Cui XX, Avila GE, Huang MT, Liu
Y, Patel J, Kong AN, Paulino R, Shih WJ, Lin Y, et al: Atorvastatin
and celecoxib inhibit prostate PC-3 tumors in immunodeficient mice.
Clin Cancer Res. 13:5480–5487. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen J, Shen P, Zhang XC, Zhao MD, Zhang
XG and Yang L: Efficacy and safety profile of celecoxib for
treating advanced cancers: A meta-analysis of 11 randomized
clinical trials. Clin Ther. 36:1253–1263. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Patel MI, Subbaramaiah K, Du B, Chang M,
Yang P, Newman RA, Cordon-Cardo C, Thaler HT and Dannenberg AJ:
Celecoxib inhibits prostate cancer growth: Evidence of a
cyclooxygenase-2-independent mechanism. Clin Cancer Res.
11:1999–2007. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kashiwagi E, Shiota M, Yokomizo A,
Inokuchi J, Uchiumi T and Naito S: EP2 signaling mediates
suppressive effects of celecoxib on androgen receptor expression
and cell proliferation in prostate cancer. Prostate Cancer
Prostatic Dis. 17:10–17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pruthi RS, Derksen JE, Moore D, Carson CC,
Grigson G, Watkins C and Wallen E: Phase II trial of celecoxib in
prostate-specific antigen recurrent prostate cancer after
definitive radiation therapy or radical prostatectomy. Clin Cancer
Res. 12:2172–2177. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Murad AS, Down L, Smith G Davey, Donovan
JL, Lane J Athene, Hamdy FC, Neal DE and Martin RM: Associations of
aspirin, nonsteroidal anti-inflammatory drug and paracetamol use
with PSA-detected prostate cancer: Findings from a large,
population-based, case-control study (the ProtecT study). Int J
Cancer. 128:1442–1448. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu X, Plummer SJ, Nock NL, Casey G and
Witte JS: Nonsteroidal antiinflammatory drugs and decreased risk of
advanced prostate cancer: Modification by lymphotoxin alpha. Am J
Epidemiol. 164:984–989. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dasgupta K, Di Cesar D, Ghosn J, Rajan R,
Mahmud S and Rahme E: Association between nonsteroidal
anti-inflammatory drugs and prostate cancer occurrence. Cancer J.
12:130–135. 2006.PubMed/NCBI
|
|
22
|
Liu Y, Chen JQ, Xie L, Wang J, Li T, He Y,
Gao Y, Qin X and Li S: Effect of aspirin and other non-steroidal
anti-inflammatory drugs on prostate cancer incidence and mortality:
A systematic review and meta-analysis. BMC Med. 12:552014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Choe KS, Cowan JE, Chan JM, Carroll PR,
D'Amico AV and Liauw SL: Aspirin use and the risk of prostate
cancer mortality in men treated with prostatectomy or radiotherapy.
J Clin Oncol. 30:3540–3544. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Choe KS, Correa D, Jani AB and Liauw SL:
The use of anticoagulants improves biochemical control of localized
prostate cancer treated with radiotherapy. Cancer. 116:1820–1826.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jacobs EJ, Newton CC, Stevens VL, Campbell
PT, Freedland SJ and Gapstur SM: Daily aspirin use and prostate
cancer-specific mortality in a large cohort of men with
nonmetastatic prostate cancer. J Clin Oncol. 32:3716–3722. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang H, Cui XX, Chen S, Goodin S, Liu Y,
He Y, Li D, Wang H, Van Doren J, Dipaola RS, et al: Combination of
Lipitor and Celebrex inhibits prostate cancer VCaP cells in vitro
and in vivo. Anticancer Res. 34:3357–3363. 2014.PubMed/NCBI
|
|
27
|
Huang H, He Y, Cui XX, Goodin S, Wang H,
Du ZY, Li D, Zhang K, Kong AN Tony, DiPaola RS, et al: Potent
inhibitory effect of δ-tocopherol on prostate cancer cells cultured
in vitro and grown as xenograft tumors in vivo. J Agric Food Chem.
62:10752–10758. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wei X, Zhou D, Wang H, Ding N, Cui XX,
Wang H, Verano M, Zhang K, Conney AH, Zheng X, et al: Effects of
pyridine analogs of curcumin on growth, apoptosis and NF-κB
activity in prostate cancer PC-3 cells. Anticancer Res.
33:1343–1350. 2013.PubMed/NCBI
|
|
29
|
Ding N, Cui XX, Gao Z, Huang H, Wei X, Du
Z, Lin Y, Shih WJ, Rabson AB, Conney AH, et al: A triple
combination of atorvastatin, celecoxib and tipifarnib strongly
inhibits pancreatic cancer cells and xenograft pancreatic tumors.
Int J Oncol. 44:2139–2145. 2014.PubMed/NCBI
|
|
30
|
Huang TB, Yan Y, Guo ZF, Zhang XL, Liu H,
Geng J, Yao XD and Zheng JH: Aspirin use and the risk of prostate
cancer: A meta-analysis of 24 epidemiologic studies. Int Urol
Nephrol. 46:1715–1728. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vane JR and Botting RM: The mechanism of
action of aspirin. Thromb Res. 110:255–258. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yoshimura R, Matsuyama M, Kawahito Y,
Takemoto Y, Tsuchida K, Kuratsukuri K, Segawa Y, Shinnka T, Sano H
and Nakatani T: The effects of cyclooxygenase-2 inhibitors on
urological cancer cells. Int J Mol Med. 13:789–793. 2004.PubMed/NCBI
|
|
33
|
Michalik L, Desvergne B and Wahli W:
Peroxisome-proliferator-activated receptors and cancers: Complex
stories. Nat Rev Cancer. 4:61–70. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Farnier M and Davignon J: Current and
future treatment of hyperlipidemia: The role of statins. Am J
Cardiol. 82:3J–10J. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
McFarlane SI, Muniyappa R, Francisco R and
Sowers JR: Clinical review 145: Pleiotropic effects of statins:
lipid reduction and beyond. J Clin Endocrinol Metab. 87:1451–1458.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Goldstein JL and Brown MS: Regulation of
the mevalonate pathway. Nature. 343:425–430. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pruitt K and Der CJ: Ras and Rho
regulation of the cell cycle and oncogenesis. Cancer Lett.
171:1–10. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Olivan M, Rigau M, Colás E, Garcia M,
Montes M, Sequeiros T, Regis L, Celma A, Planas J, Placer J, et al:
Simultaneous treatment with statins and aspirin reduces the risk of
prostate cancer detection and tumorigenic properties in prostate
cancer cell lines. Biomed Res Int. 2015:7621782015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Stark LA, Reid K, Sansom OJ, Din FV,
Guichard S, Mayer I, Jodrell DI, Clarke AR and Dunlop MG: Aspirin
activates the NF-kappaB signalling pathway and induces apoptosis in
intestinal neoplasia in two in vivo models of human colorectal
cancer. Carcinogenesis. 28:968–976. 2007. View Article : Google Scholar : PubMed/NCBI
|