Introduction
Cervical cancer is a key factor associated with
morbidity and mortality in women worldwide (1). Infection with human papilloma viruses
(HPV) triggers carcinogenesis. Most of the precancerous lesions do
not progress to invasive carcinoma, suggesting that HPV is not the
only factor contributing to the development of cervical cancer
(2,3). However, persistent HPV infection
alters the pro-inflammatory cytokine profile, resulting in chronic
inflammation and recurrence of cervical cancer (4). Cancer stem cells (CSCs) play a vital
role in cancer initiation and metastasis (5). Metastasis results in treatment failure
and death (6).
Epithelial-mesenchymal transition (EMT) has been implicated as the
key factor in CSCs transformation (7,8). EMT
has been shown to induce reversion to a CSC-like phenotype, linking
CSCs and EMT (9,10).
NF-κB is a classic transcription factor activated by
inflammatory stimuli, such as LPS (11), TNF-α (12) and IL-10 (13). Activated NF-κB induces extensive
gene expression in immune response (TNF-α), angiogenesis (VEGF),
invasion (MMP-9) and EMT (Twist) (14–17).
Furthermore, NF-κB, a pleiotropic transcription factor, has been
implicated in EMT and metastasis (14–17).
In mammary epithelial cells, EMT is upregulated via overexpression
of NF-κBp65 (17).
The transcriptional factor TWIST mediates EMT and
cancer metastasis (18,19). In uterine cancers, Twist
overexpression promotes invasion and metastasis (20–23).
However, the role of NF-κB/Twist axis in cervical cancer has not
been investigated. In this study, we focused on the role of
NF-κB/Twist axis in vitro, by co-treatment of human cervical
cancer cell line HeLa with TNF-α and TGF-β.
Materials and methods
Reagents
DMEM was obtained from Gibco, FBS from PAA, trypsin
and penicillin-streptomycin from Invitrogen, and TGF-β and TNF-α
from Sino Biological (Beijing, China). Anti-E-cadherin and
anti-N-cadherin antibodies were supplied by Cell Signaling
Technology (Danvers, MA, USA). The following antibodies were
purchased from Abcam (Burlingame, CA, USA): anti-Bmi1, anti-Sox2,
anti-Oct4, anti-CD133, anti-CD44, anti-ALDH1, anti-NF-κBp65 and
anti-Twist1.
Cell culture and EMT morphology
HeLa cells were supplied by the Cell Bank of Chinese
Academy of Sciences (Shanghai, China), and cultured as described by
López et al (5). Cells were
incubated with TNF-α (10.0 ng/ml) for 24 h, TGF-β (5.0 ng/ml) for 6
days or TNF-α (10.0 ng/ml) for 24 h along with TGF-β (5.0 ng/ml)
for 6 days. EMT morphology were visualized under a light microscope
(Olympus, Japan). The regulation of gene expression was studied by
transducing cells with either NF-κBp65 shRNA and Twist1 shRNA or
NF-κBp65 and Twist1-carrying adenoviruses obtained
from Hanbio Biothechnology Co. Ltd. (1.0 ml, 1×1011
pfu/ml; Shanghai, China).
Wound healing assay
Wound healing was tested by loading cells
(5×105) in 6-well plates and grown until cells attained
90% confluence. A scratch was created using a 100-µl pipette tip
and rinsed with PBS. Photographs were obtained and analyzed at 24
h, and the migrating cell number was standardized with mock.
Sphere formation
Cells (1,000 cells/ml) were loaded on ultra-low
attachment 24-well culture plates (Corning, USA) in stem cell
conditional medium. Five days later, the number of spheroids in
each well was scored. The sphere formation rate was calculated as a
percentage of the total number of spheres among the viable
cells.
Western blot analysis
Whole cell lysates were prepared as previously
described (24). The primary
antibodies used for membrane incubation were as follows:
anti-E-cadherin, anti-N-cadherin, anti-Bmi1, anti-Sox2, anti-Oct4,
anti-CD133, anti-CD44, anti-ALDH1, anti-NF-κB and anti-Twist. The
membranes were further incubated with anti-mouse or anti-rabbit
secondary antibodies conjugated to horseradish peroxidase (HRP).
After incubation, the specific protein bands were visualized by
enhanced chemiluminescence, using β-actin as a loading control.
Statistical analysis
Experimental data were analyzed using SPSS 20.0 for
Windows (SPSS Inc, Chicago, IL, USA). Data representing mean ± SD
were subjected to one-way ANOVA. First, the homogeneity of variance
was determined. We used LSD to analyze pairwise comparisons among
the groups. In the event of incomplete variance, the control and
the experimental groups were analyzed with Tukey's test. A
probability of <0.05 suggested statistical significance.
Results
Treatment with TNF-α or TGF-β or both
induces EMT and CSCL properties in HeLa cells and increases NF-κB
and Twist levels
TNF-α induces proliferation of epithelial tumor
cells following exposure to TGF-β and EMT (17,19).
Morphological features ranging from cobblestone appearance to
spindle phenotypes were observed in HeLa cells after exposure to
pro-inflammatory cytokines TGF-β (5.0 ng/ml) or TNF-α (10.0 ng/ml)
or both (Fig. 1A). Western blot
analysis results were validated using antibodies targeting
EMT-related markers. As shown in Fig.
1B, the pro-inflammatory cytokines downregulated E-cadherin and
upregulated N-cadherin expression. Concurrently, we measured the
cell migration and self-renewal after treatment with the
inflammatory cytokines. The results showed that the combination of
pro-inflammatory cytokines enhanced migration (Fig. 1C) and self-renewal (Fig. 1D) of HeLa cells. The multipotent
stem cell factors Bmi1, Sox2 and Oct4 were overexpressed (Fig. 1E). Similarly, the stem cell markers
CD133, CD44 and ALDH1 were upregulated (Fig. 1F). NF-κB is a key regulator of
inflammation, and Twist plays an important role in EMT (17,25,26).
As illustrated in Fig. 1G, Western
blots revealed an overexpression of NF-κB and Twist1 in HeLa cells
after treatment with the different cytokines, either alone or in
combination.
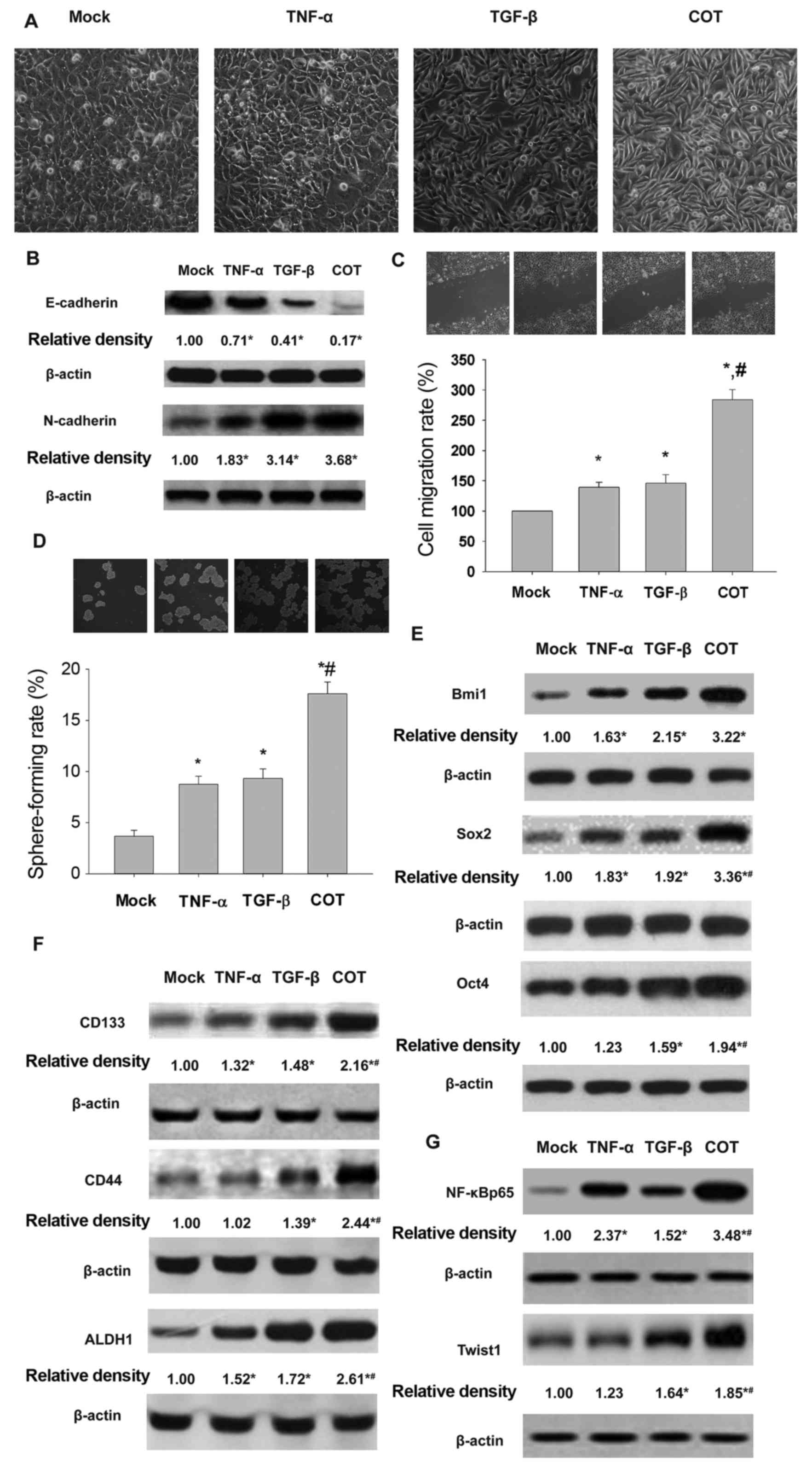 | Figure 1.Treatment with TGF-β and TNF-α alone
or in combination contributes to EMT and CSCL properties of HeLa
cells. HeLa cells exposed to TNF-α and TGF-β display mesenchymal
morphology (x20) (A), E-cadherin and N-cadherin expression (B),
cell migration (C), self-renewal (D), expression of Bmi1, Sox2 and
Oct4 (E), CD133, ALDH1 and CD44 (F) and NF-κB, and Twist1 proteins.
Mock, cells exposed to complete medium; TNF-α, cells exposed to
10.0 ng/ml of TNF-α for 24 h; TGF-β, cells were exposed to 5.0
ng/ml of TGF-β for 6 days; COT, cells were exposed to 10.0 ng/ml
TNF-α for 24 h and 5.0 ng/ml of TGF-β for 6 days. *P<0.05, vs
mock; #,*P<0.05, vs TNF-α or TGF-β. |
In this preliminary experiment, the characteristic
EMT phenotype was apparent in HeLa cells exposed to TNF-α alone for
24 h, and combined with TGF-β for 6 days (data not shown).
Silencing of NF-κBp65 downregulates
Twist and reverses EMT and CSCL features in HeLa cells exposed to
inflammatory cytokines
The expression of NF-κBp65 and Twist in
shNF-κBp65-expressing HeLa cells exposed to TGF-β and TNF-α was
significantly lower than in the control cells (Fig. 2A). EMT morphological changes and
relevant protein expression were detected. As shown in Fig. 2B and C, NF-κBp65 shRNA-expressing
HeLa cells exhibited cobble-stone-like morphology, while GFP
control cells displayed spindle shape. Furthermore, NF-κBp65
shRNA-expressing HeLa cells expressed higher levels of E-cadherin
in epithelial cells, and a lower level of N-cadherin. The wound
healing and sphere formation assays revealed that silencing of
NF-κBp65 expression in HeLa cells decreased migration and
self-renewal following co-treatment with TNF-α and TGF-β (Fig. 2D and E). Furthermore, compared with
control cells, knockdown of NF-κBp65 expression reduced the
expression of multi-functional proteins Bmi1, Sox2 and Oct4
(Fig. 2F), while CSC surface
markers CD133, CD44 and ALDH1 (Fig.
2G) were induced by co-treatment with TNF-α and chronic
exposure to TGF-β.
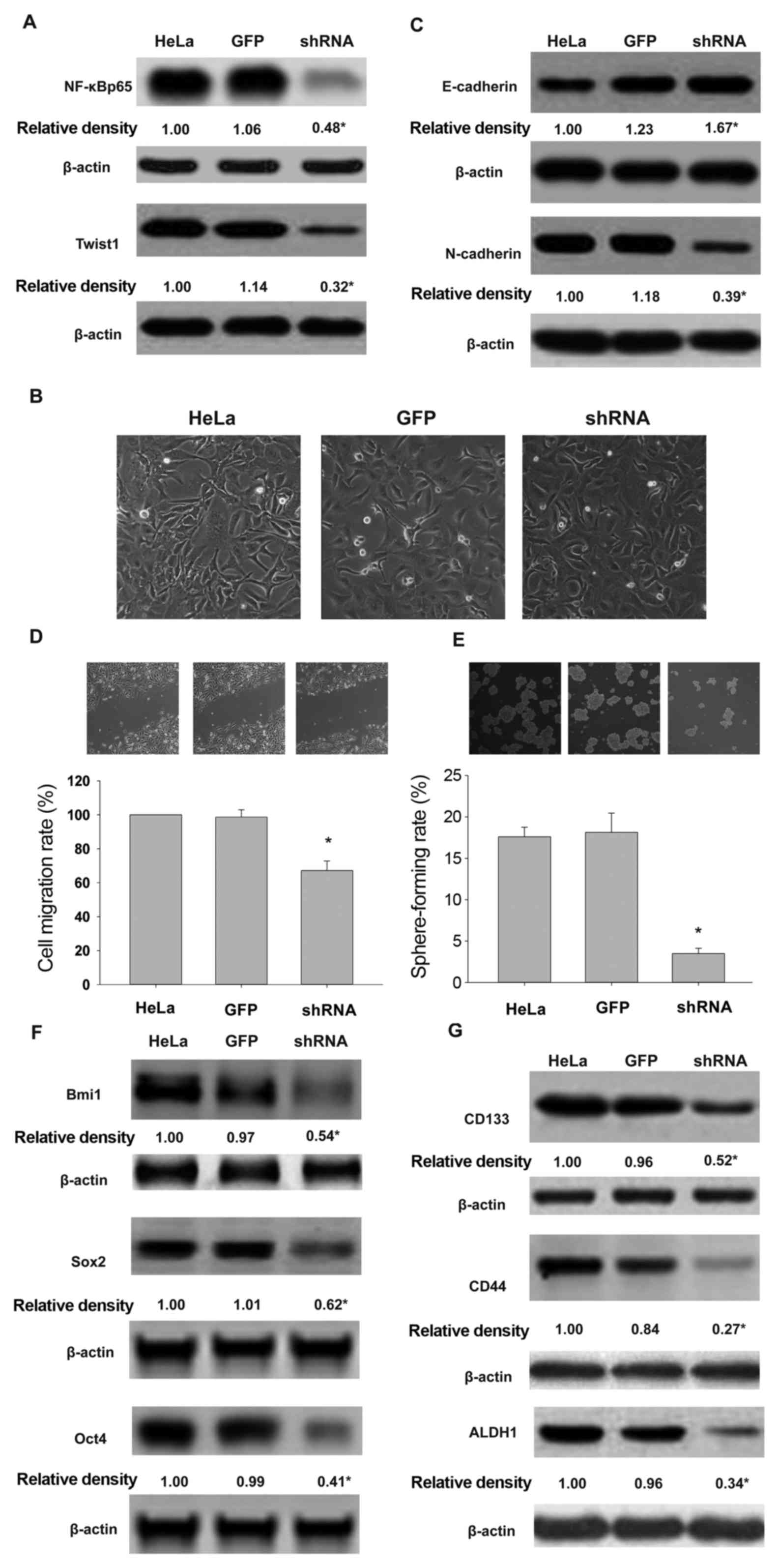 | Figure 2.Silencing of NF-κBp65 leads to Twist1
downregulation and attenuates the effect of TNF-α combined with
TGF-β on EMT and CSCL properties of HeLa cells. NF-κBp65 silencing
affects the expression of NF-κBp65 and Twist1 proteins (A), EMT
morphology (B), E-cadherin and N-cadherin profile (C), cell
migration (D), sphere formation (E), expression of Bmi1, Sox2 and
Oct4 (F), and CD133, CD44 and ALDH1 (G) in HeLa cells exposed to
inflammatory cytokines. HeLa, HeLa cells exposed to TNF-α and
TGF-β. GFP, cells transducted with adenovirus expressing GFP.
shRNA, cells transduced with adenovirus expressing shNF-κBp65.
*P<0.05, vs. HeLa or GFP. |
Overexpression of NF-κBp65 upregulates
Twist and promotes EMT and CSCL properties in HeLa cells exposed to
inflammatory cytokines
We evaluated the effect of overexpression of
NF-κBp65 on EMT and CSCL properties of HeLa cells exposed to TNF-α
and TGF-β. NF-κBp65-expressing adenovirus-infected HeLa cells
overexpressed NF-κBp65 and Twist1 (Fig.
3A). As illustrated in Fig. 3B and
C, increased expression of mesenchymal marker N-cadherin and
decreased expression of epithelial marker E-cadherin and spindle
shape were found in NF-κBp65-expressing HeLa cells. These findings
suggested that NF-κB mediated a significant switch from epithelial
to mesenchymal phenotypes in HeLa cells following exposure to TNF-α
and TGF-β. As shown in Fig. 3D and
E, NF-κBp65 overexpression results in increased cell migration
and self-renewal in HeLa cells. Furthermore, we found that NF-κBp65
expression modulated Bmi1, Sox2, Oct4 (Fig. 3F), and CD133, CD44 and ALDH1
(Fig. 3G) in HeLa cells exposed to
proinflammatory cytokines.
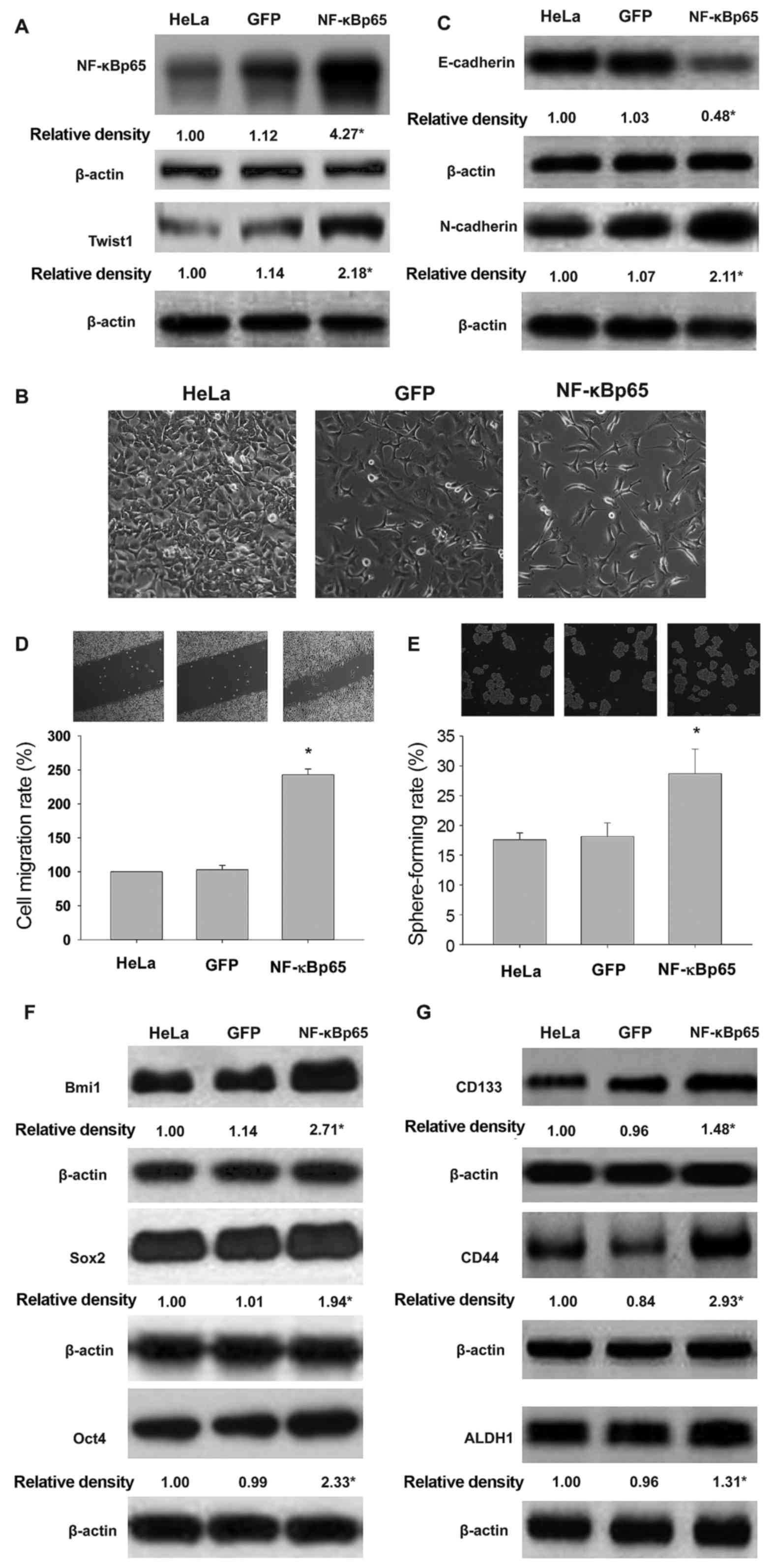 | Figure 3.Overexpression of NF-κBp65 increases
the effects of exposure to TNF-α and TGF-β on EMT and CSCL
properties in HeLa cells. Effects of NF-κBp65 gene
transduction on NF-κBp65 and Twist1 protein levels (A), EMT
morphology (B), E-cadherin and N-cadherin profile (C), cell
migration (D), sphere formation (E), Bmi1, Sox2 and Oct4 levels (F)
and the expression of CD133, CD44 and ALDH1 (G) in HeLa cells
exposed to TNF-α and TGF-β. HeLa, HeLa cells exposed to TNF-α and
TGF-β. GFP, cells transducted with GFP gene; NF-κBp65, cells
transduced with NF-κBp65 gene. *P<0.05, vs HeLa or
GFP. |
Knockdown of Twist1 has no effect on
NF-κB expression but reverses the EMT and CSCL properties in HeLa
cells
To elucidate the relationship between NF-κB and
Twist1 in HeLa cells, shTwist1-expressing adenovirus was used to
infect HeLa cells following exposure to TNF-α and TGF-β. Compared
with GFP-expressing HeLa cells, the knockdown of Twist1
downregulated the expression of Twist1 but not the expression of
NF-κBp65 (Fig. 4A). Twist1
knockdown resulted in cobble-stone-like cells (Fig. 4B). The expression of E-cadherin was
significantly upregulated while the mesenchymal marker N-cadherin
was downregulated (Fig. 4C).
Further, Twist1 silencing attenuated the migration and self-renewal
of HeLa cells exposed to TNF-α and TGF-β (Fig. 4D and E). Furthermore, Twist1
knockdown downregulated cancer stem-related multi-functional
proteins Bmi1, Sox2 and Oct4 (Fig.
4F) and surface markers CD133, CD44 and ALDH1 in HeLa cells
following co-treatment with TNF-α and TGF-β (Fig. 4G).
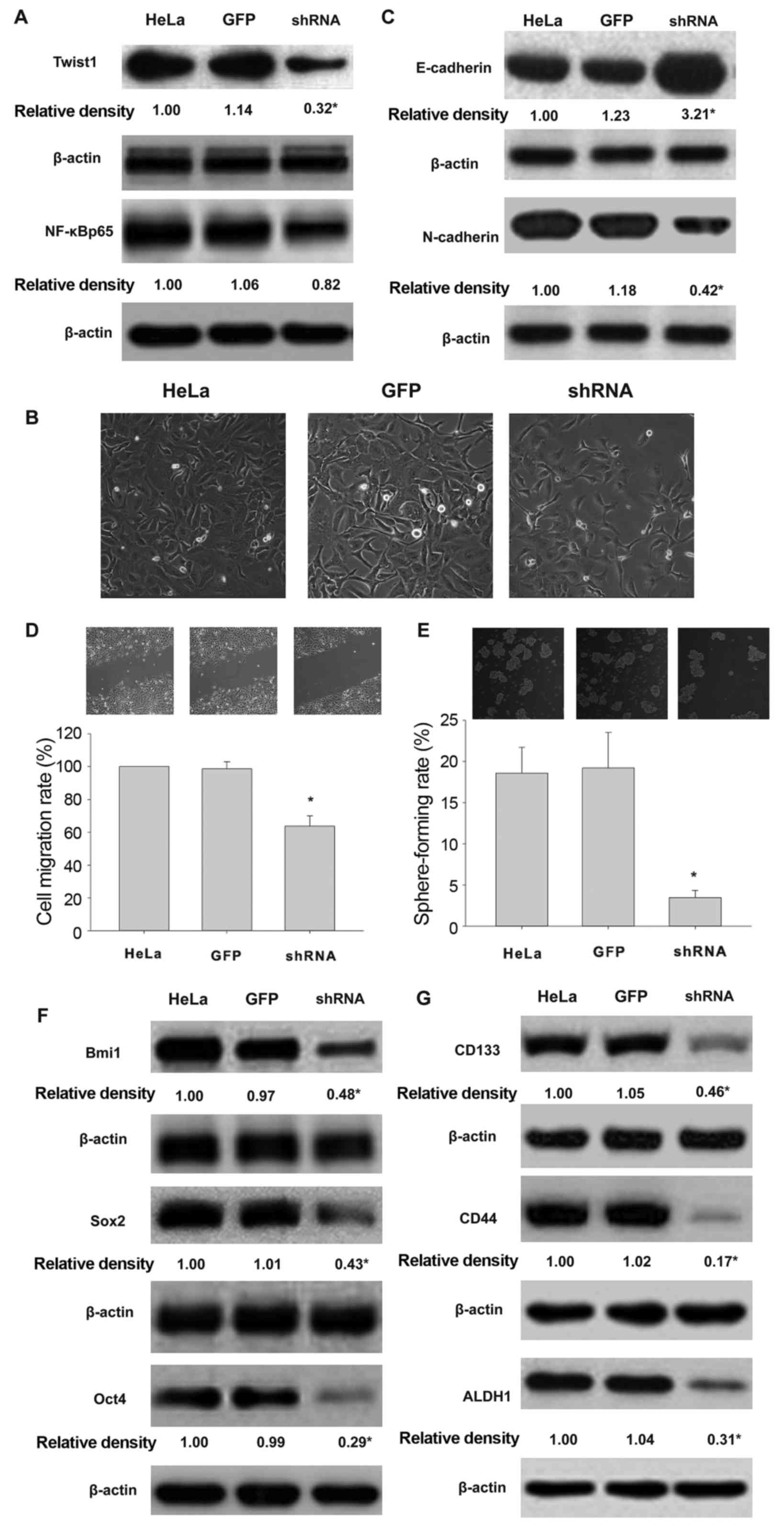 | Figure 4.Knockdown of Twist1 decreases the
impact of exposure to TGF-β and TNF-α on EMT and CSCL properties of
HeLa cells. Effects of Twist1 silencing on the expression of Twist1
and NF-κBp65 (A), EMT morphology (B), E-cadherin and N-cadherin
profile (C) cell migration (D), sphere formation (E), expression of
Bmi1, Sox2 and Oct4 (F) and CD133, CD44 and ALDH1 (G) in HeLa cells
exposed to TNF-α and TGF-β. HeLa, HeLa cells exposed to TNF-α and
TGF-β. GFP, cells transducted with GFP gene. shRNA, cells
transducted with sh Twist1 (*P<0.05). *P<0.05, vs HeLa or
GFP. |
Overexpression of Twist1 promoted EMT
and CSCL properties of HeLa cells exposed to TNF-α and TGF-β
Overexpression of Twist1 was not accompanied by
NF-κBp65 protein expression (Fig.
5A). As shown in Fig. 5B and C,
Twist1-expressing adenovirus infected HeLa cells overexpressing
mesenchymal marker N-cadherin and downregulated the epithelial
marker E-cadherin. Furthermore, overexpression of Twist1 increased
cell migration (Fig. 5D) and high
sphere formation rate (Fig. 5E).
Twist1 regulated the expression of Bmi1, Sox2, and Oct4 (Fig. 5F), and CD133, CD44 and ALDH1
(Fig. 5G) in HeLa cells following
co-treatment with TNF-α and TGF-β.
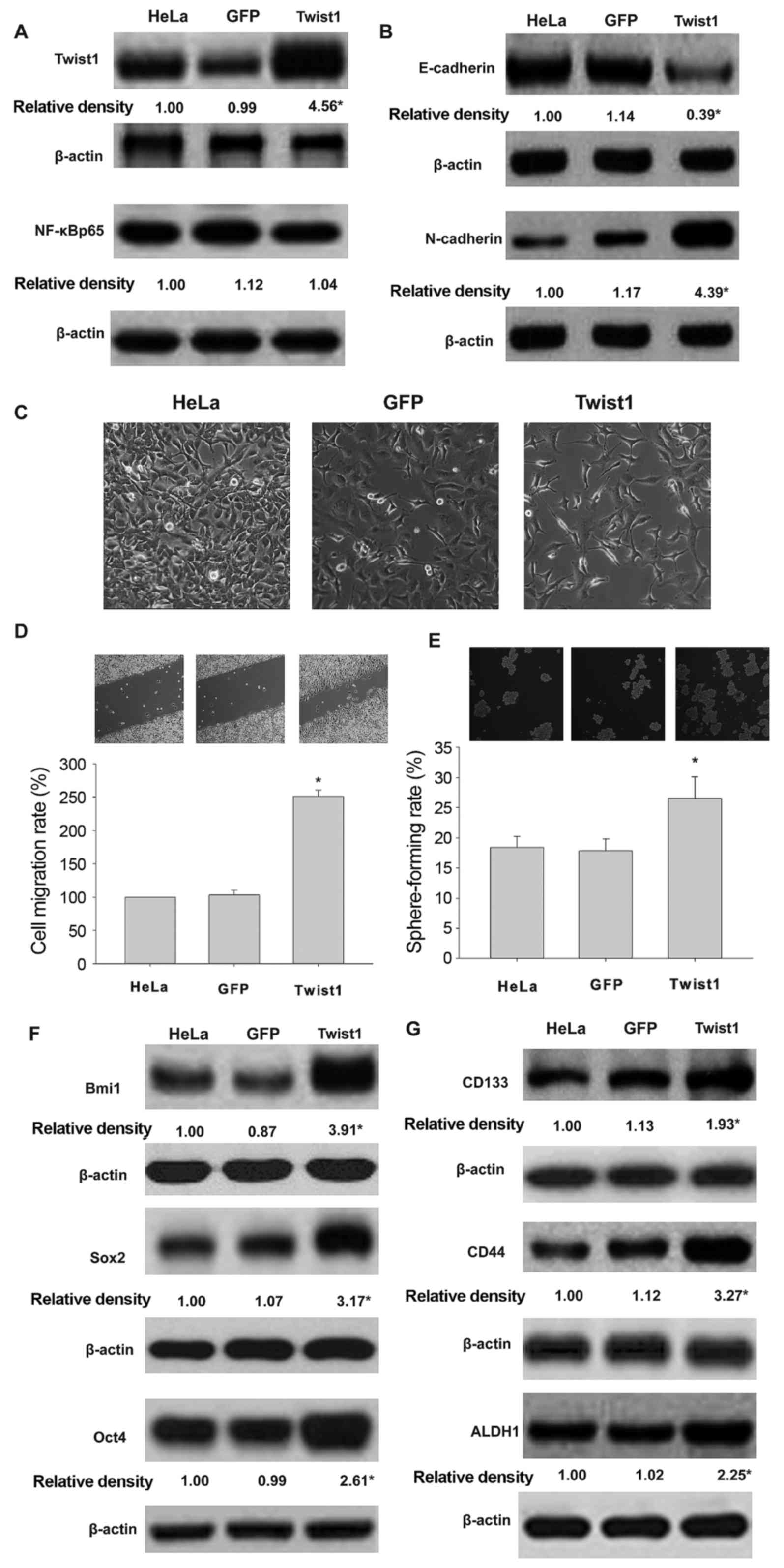 | Figure 5.Overexpression of Twist1
promotes EMT and CSCL properties in HeLa cells co-treated with
TGF-β and TNF-α. Effects of Twist1 gene transduction on the
expression of Twist1 and NF-κBp65 (A), EMT morphology (B),
E-cadherin and N-cadherin profile (C) cell migration (D), sphere
formation (E), expression of Bmi1, Sox2 and Oct4 (F) and CD133,
CD44 and ALDH1 (G) in HeLa cells exposed to TNF-α and TGF-β. HeLa,
HeLa cells exposed to TNF-α and TGF-β. GFP, the cells transducted
with GFP gene. Twist1, cells transducted with Twist1
gene (*P<0.05). *P<0.05, vs HeLa or GFP. |
Twist1 transduction rescues NF-κB
knockdown-related inhibition of Twist1 expression, and EMT and CSCL
properties in HeLa cells exposed to inflammatory cytokines
To elucidate the role of NF-κB/Twist axis in EMT and
CSCL properties of HeLa cells exposed to inflammatory cytokines, we
transducted Twist1 into NF-κBp65-silenced HeLa cells. As
shown in Fig. 6A, NF-κBp65 shRNA
downregulated NF-κBp65 and Twist protein expression. Transduction
of Twist1 upregulated the levels of Twist without affecting
the NF-κBp65 profile. Interestingly, Twist1 gene
transduction restored NF-κBp65 shRNA-downregulated Twist
expression. The shNF-κBp65-expressing HeLa cells overexpressed
epithelial markers E-cadherin and downregulated mesenchymal marker
N-cadherin. Under these conditions, Twist1 gene transduction
did not inhibit NF-κBp65, but rescued shNF-κBp65 downregulation of
Twist 1 expression (Fig. 6B and C).
Similarly, Twist1 gene transduction rescued the inhibitory
effects of shNF-κBp65 on migration (Fig. 6E) and self-renewal (Fig. 6F) and cancer stem cell-related
protein expression (Fig. 6G) in
HeLa cells following exposure to TNF-α and TGF-β.
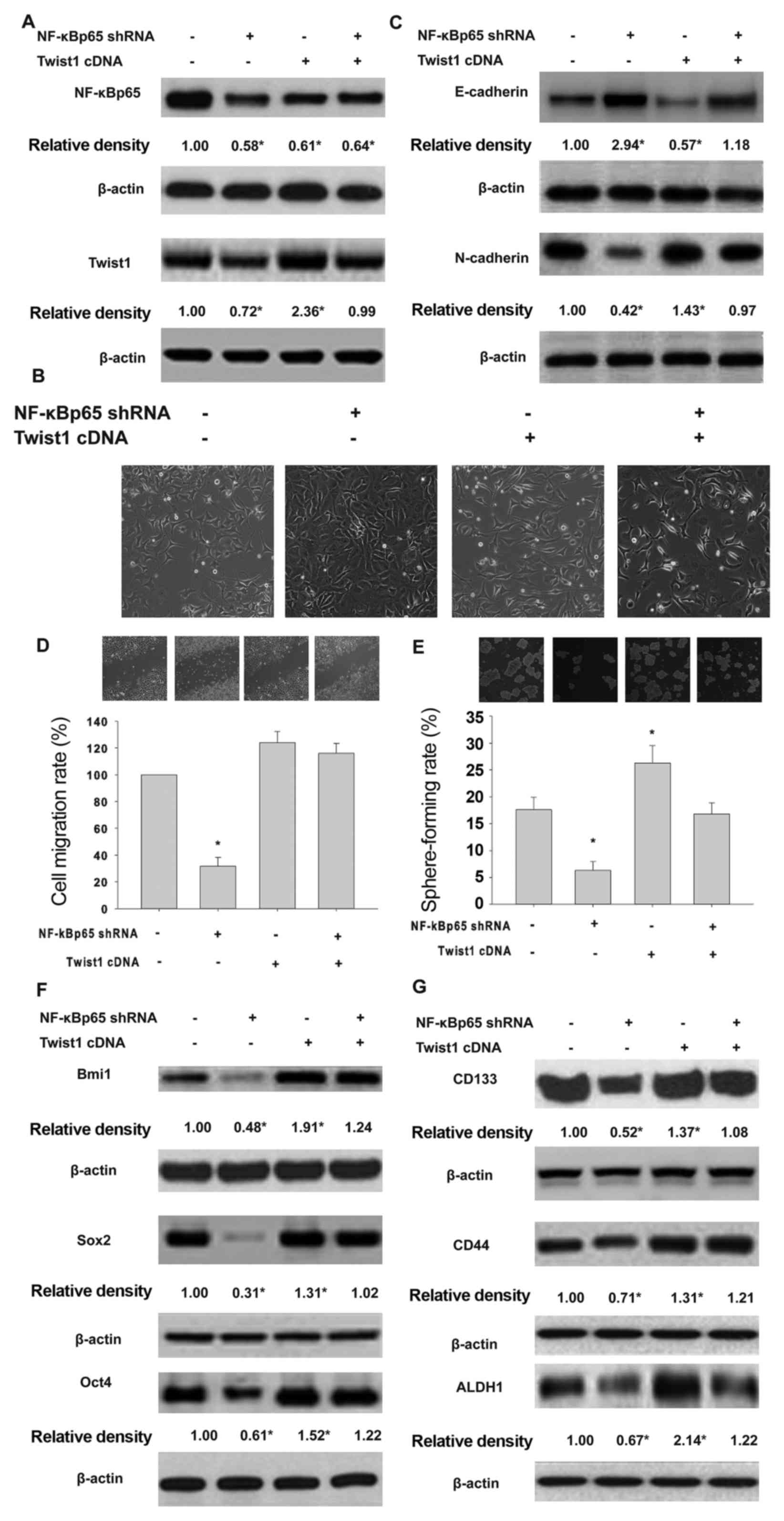 | Figure 6.Effects of Twist1 transduction
in HeLa cells expressing shNF-κBp65 on EMT and CSCL properties
following exposure to TNF-α and TGF-β. Effects of Twist1
gene transduction in HeLa cells expressing shNF-κBp65 on the
expression of Twist1 and NF-κBp65 proteins (A), EMT morphology (B),
E-cadherin and N-cadherin profile (C), cell migration (D), sphere
formation (E), expression of Bmi1, Sox2 and Oct4 (F), and the
expression of CD133, CD44 and ALDH1 (G) induced by exposure to
proinflammatory cytokines. HeLa, HeLa cells exposed to TNF-α and
TGF-β. NF-κBp65 shRNA, shNF-κBp65 transduction of HeLa cells
exposed to TNF-α and TGF-β. Twist1 cDNA, Twist1 gene
transduction of HeLa cells expressing NF-κBp65 shRNA induced by
TNF-α and TGF-β. *P<0.05 vs. HeLa or NF-κBp65 shRNA. |
Discussion
Chronic inflammation-induced carcinogenesis and
metastasis is a major challenge to cancer therapy, and is a key
factor contributing to mortality in many malignancies (11–17).
Understanding the mechanisms regulating the metastasis and
carcinogenesis induced by pro-inflammatory cytokines may lead to
novel therapeutic interventions (17). In this study, we demonstrated that
pro-inflammatory TNF-α and TGF-β synergistically induced EMT and
CSCL properties in HeLa cells via NF-κB/Twist axis. We
characterized the biological role of NF-κB and Twist1 in EMT and
cell migration, self-renewal and stem cell marker expression. We
demonstrated the role of NF-κB/Twist1 signal axis in HeLa cells
induced by exposure to TNF-α and TGF-β. Various studies suggest
that TNF-α induces a variety of epithelial cells and epithelial
tumor cell EMT morphology following chronic exposure to TGF-β
(17). In this study, we
constructed a chronic inflammation model, by co-treatment with
TNF-α and TGF-β to induce EMT phenotype in HeLa cells. Further, the
exposure to pro-inflammatory cytokines also leads to cell migration
and self-renewal, and CSC-related protein expression. NF-κBp65
knockdown or overexpression therefore, alters Twist1 protein
expression. However, Twist1 knockdown or overexpression has no
effects on NF-κBp65 expression. The results provide convinced
evidence supporting NF-κB location upstream of Twist1. Finally, we
demonstrated the role of NF-κB/Twist axis, using Twist and
shNF-κBp65 co-transduction rescue assay. The results show
that Twist1 overexpression almost reversed all the biological
effects of shNF-κBp65.
An increasing number of studies have shown that EMT
plays a decisive role in tumorigenesis, including local
infiltration and metastasis and spread through the circulatory
system (26). We monitored the cell
morphology and the expression of EMT-related proteins E-cadherin
and N-cadherin to determine the phenotype variation. E-cadherin
triggers epithelial intercellular adhesion. Cells devoid of
E-cadherin show increased N-cadherin expression (27). In this study, the role of
NF-κB/Twist signal axis in EMT phenotype acquisition by HeLa cells
was examined, and their overexpression promoted EMT.
Migration and CSL properties increase the risk of
malignant tumor metastasis (28–30).
Therefore, we investigated these phenomena along the NF-κB/Twist
axis, using scratch assay and sphere formation to detect migration
and self-renewal. NF-κB/Twist overexpression promotes HeLa cell
migration and self renewal. Similarly, NF-κB/Twist overexpression
upregulates the levels of CSC proteins Bmi1, Sox2 and Oct4 and CSC
surface proteins CD133, CD44, and ALDH1.
In conclusion, our results provide insight into the
mechanism of TNF-α-induced EMT and CSCL properties of HeLa cells
chronically exposed to TGF-β, and demonstrate that these effects
are mediated via NF-κB/Twist axis. Targeting NF-κB/Twist axis is a
potential treatment strategy to improve prognosis in patients with
cervical cancer.
Acknowledgements
This study was supported by the Projects of NSFC
(nos. 30760248, 81172375 and 31400311), the Project of Scientific
Research Fund of Hunan Provincial Education Department (no.
14C0707), the Project of Hunan Provincial Natural Science
Foundation (no. 13JJ3061) and the Scientific Research Fund of Hunan
Normal University (nos. 140668 and 140666).
Glossary
Abbreviations
Abbreviations:
|
CSCL
|
cancer stem cell-like
|
|
CSCs
|
cancer stem cells
|
|
EMT
|
epithelial-mesenchymal transition
|
|
HPV
|
persistent human papilloma virus
|
|
HRP
|
horseradish peroxidase
|
References
|
1
|
Diaz-Padilla I, Monk BJ, Mackay HJ and
Oaknin A: Treatment of metastatic cervical cancer: Future
directions involving targeted agents. Crit Rev Oncol Hematol.
85:303–314. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Erickson BK, Landers EE and Huh WK: Update
on vaccination clinical trials for HPV-related disease. Clin Ther.
36:8–16. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Murata T, Mizushima H, Chinen I, Moribe H,
Yagi S, Hoffman RM, Kimura T, Yoshino K, Ueda Y, Enomoto T, et al:
HB-EGF and PDGF mediate reciprocal interactions of carcinoma cells
with cancer-associated fibroblasts to support progression of
uterine cervical cancers. Cancer Res. 71:6633–6642. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Egawa N, Egawa K, Griffin H and Doorbar J:
Human papillomaviruses; epithelial tropisms, and the development of
neoplasia. Viruses. 7:3863–3890. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
López J, Poitevin A, Mendoza-Martínez V,
Pérez-Plasencia C and García-Carrancá A: Cancer-initiating cells
derived from established cervical cell lines exhibit stem-cell
markers and increased radioresistance. BMC Cancer. 12:482012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Iglesias M, Plowman GD and Woodworth CD:
Interleukin-6 and interleukin-6 soluble receptor regulate
proliferation of normal, human papillomavirus-immortalized, and
carcinoma-derived cervical cells in vitro. Am J Pathol.
146:944–952. 1995.PubMed/NCBI
|
|
7
|
Lin J, Liu X and Ding D: Evidence for
epithelial-mesenchymal transition in cancer stem-like cells derived
from carcinoma cell lines of the cervix uteri. Int J Clin Exp
Pathol. 8:847–855. 2015.PubMed/NCBI
|
|
8
|
Liu X, Wang D, Liu H, Feng Y, Zhu T, Zhang
L, Zhu B and Zhang Y: Knockdown of astrocyte elevated gene-1
(AEG-1) in cervical cancer cells decreases their invasiveness,
epithelial to mesenchymal transition, and chemoresistance. Cell
Cycle. 13:1702–1707. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Polyak K and Weinberg RA: Transitions
between epithelial and mesenchymal states: Acquisition of malignant
and stem cell traits. Nat Rev Cancer. 9:265–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang X, Li N, Shao H, Meng Y, Wang L, Wu
Q, Ioa Y, Li J, Bian J, Zhang Y, et al: Methane limit LPS-induced
NF-κB/MAPKs signal in macrophages and suppress immune response in
mice by enhancing PI3K/AKT/GSK-3β-mediated IL-10 expression. Sci
Rep. 6:293592016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen PJ, Wang YL, Kuo LM, Lin CF, Chen CY,
Tsai YF, Shen JJ and Hwang TL: Honokiol suppresses TNF-α-induced
neutrophil adhesion on cerebral endothelial cells by disrupting
polyubiquitination and degradation of IκBα. Sci Rep. 6:265542016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu X, Han C, Jin J, Qin K, Zhang H, Li T,
Li N and Cao X: Integrin CD11b attenuates colitis by strengthening
Src-Akt pathway to polarize anti-inflammatory IL-10 expression. Sci
Rep. 6:262522016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ramasamy S, Saez B, Mukhopadhyay S, Ding
D, Ahmed AM, Chen X, Pucci F, Yamin R, Wang J, Pittet MJ, et al:
Tle1 tumor suppressor negatively regulates inflammation in vivo and
modulates NF-κB inflammatory pathway. Proc Natl Acad Sci USA.
113:1871–1876. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mariani F, Sena P and Roncucci L:
Inflammatory pathways in the early steps of colorectal cancer
development. World J Gastroenterol. 20:9716–9731. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Korkaya H, Liu S and Wicha MS: Regulation
of cancer stem cells by cytokine networks: Attacking cancer's
inflammatory roots. Clin Cancer Res. 17:6125–6129. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li CW, Xia W, Huo L, Lim SO, Wu Y, Hsu JL,
Chao CH, Yamaguchi H, Yang NK, Ding Q, et al:
Epithelial-mesenchymal transition induced by TNF-α requires
NF-κB-mediated transcriptional upregulation of Twist1. Cancer Res.
72:1290–1300. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang J, Mani SA, Donaher JL, Ramaswamy S,
Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A and
Weinberg RA: Twist, a master regulator of morphogenesis, plays an
essential role in tumor metastasis. Cell. 117:927–939. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Liu J, Ying X, Lin PC and Zhou BP:
Twist-mediated epithelial-mesenchymal transition promotes breast
tumor cell invasion via inhibition of hippo pathway. Sci Rep.
6:246062016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fan Q, Qiu MT, Zhu Z, Zhou JH, Chen L,
Zhou Y, Gu W, Wang LH, Li ZN, Xu Y, et al: Twist induces
epithelial-mesenchymal transition in cervical carcinogenesis by
regulating the TGF-β/Smad3 signaling pathway. Oncol Rep.
34:1787–1794. 2015.PubMed/NCBI
|
|
21
|
Wushou A, Hou J, Zhao YJ and Shao ZM:
Twist-1 up-regulation in carcinoma correlates to poor survival. Int
J Mol Sci. 15:21621–21630. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang T, Li Y, Tuerhanjiang A, Wang W, Wu
Z, Yuan M and Wang S: Correlation of Twist upregulation and
senescence bypass during the progression and metastasis of cervical
cancer. Front Med. 8:106–112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu K, Chen L, Han X and Wang J and Wang
J: Short hairpin RNA targeting Twist1 suppresses cell proliferation
and improves chemosensitivity to cisplatin in HeLa human cervical
cancer cells. Oncol Rep. 27:1027–1034. 2012.PubMed/NCBI
|
|
24
|
Li J and Zhou BP: Activation of β-catenin
and Akt pathways by Twist are critical for the maintenance of EMT
associated cancer stem cell-like characters. BMC Cancer. 11:492011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li XW, Tuergan M and Abulizi G: Expression
of MAPK1 in cervical cancer and effect of MAPK1 gene silencing on
epithelial-mesenchymal transition, invasion and metastasis. Asian
Pac J Trop Med. 8:937–943. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lim W, Kim HE, Kim Y, Na R, Li X, Jeon S,
Choi H and Kim O: Association between cancer stem cell-like
properties and epithelial-to-mesenchymal transition in primary and
secondary cancer cells. Int J Oncol. 49:991–1000. 2016.PubMed/NCBI
|
|
27
|
Cao X, Ren K, Song Z, Li D, Quan M, Zheng
Y, Cao J, Zeng W and Zou H: 7-Difluoromethoxyl-5,4′-di-n-octyl
genistein inhibits the stem-like characteristics of gastric cancer
stem-like cells and reverses the phenotype of
epithelial-mesenchymal transition in gastric cancer cells. Oncol
Rep. 36:1157–1165. 2016.PubMed/NCBI
|
|
28
|
Choudhary KS, Rohatgi N, Halldorsson S,
Briem E, Gudjonsson T, Gudmundsson S and Rolfsson O: EGFR
signal-network reconstruction demonstrates metabolic crosstalk in
EMT. PLOS Comput Biol. 12:e10049242016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Moirangthem A, Bondhopadhyay B, Mukherjee
M, Bandyopadhyay A, Mukherjee N, Konar K, Bhattacharya S and Basu
A: Simultaneous knockdown of uPA and MMP9 can reduce breast cancer
progression by increasing cell-cell adhesion and modulating EMT
genes. Sci Rep. 6:219032016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kong L, Guo S, Liu C, Zhao Y, Feng C, Liu
Y, Wang T and Li C: Overexpression of SDF-1 activates the NF-κB
pathway to induce epithelial to mesenchymal transition and cancer
stem cell-like phenotypes of breast cancer cells. Int J Oncol.
48:1085–1094. 2016.PubMed/NCBI
|




















