Introduction
Prostate cancer is the most common cancer of the
male urogenital system and the second leading cause of
cancer-related mortality in the US. It remains the leading cause of
new cancer cases among men, accounting for ~26% of the new cases
diagnosed in 2015 (1,2). Asian countries have a substantially
lower incidence of prostate cancer, but a higher proportion of
advanced-stage or metastatic prostate cancer (3). For patients with early-stage prostate
cancer, androgen is the major regulator of cellular proliferation.
Nevertheless, 70–80% of androgen-independent prostate cancer
patients have no curative treatment options. Therefore, there is a
need to explore more effective anti-prostate cancer drugs and
therapeutic approaches.
The phosphoinositide-3 kinase/protein kinase B
(PI3K/Akt) cell proliferation and survival signaling pathway plays
a significant role in tumorigenesis in numerous types of cancer.
Dysregulation of the PI3K pathway commonly occurs in prostate
carcinogenesis (4). Fork head box O
(FOXO) transcription factor contains four members, FoxO1 (FKHR),
FoxO3a (FKHRL1), FoxO4 (AFX) and FoxO6, which function downstream
of the PI3K/Akt signaling pathway. FOXO proteins primarily function
as transcription factors in the nucleus by regulating the
expression of a large spectrum of tumor-suppressor genes.
Specifically, FoxO3a plays an important role in multiple cellular
processes including cell cycle arrest, cell death, DNA damage
repair, stress resistance and metabolism (5). Several anticancer drugs, including
imatinib, paclitaxel and doxorubicin, have been found to increase
FoxO3a by preventing oncogenic suppression of the protein functions
of FOXO (6). In a previous study,
during prostate cancer progression, increasing Akt activation led
to an increase in p-FoxO3a, and induced an increase in cytosolic
accumulation of FoxO3a, and binding with 14-3-3 (a chaperone
protein), which potentially affected transcriptional activity in an
age-dependent manner. Accumulated cytosolic FoxO3a is correlated to
Ser253 phosphorylation and accounts for FoxO3a nuclear exclusion;
these events lead to the regulation of FOXO-targeted genes, such as
pro- and anti-apoptotic proteins, and cell cycle regulatory
proteins (7,8).
Matrine, a major component extracted from a
traditional Chinese herb (Sophora flavescens), has a wide
range of clinical applications including cardiovascular protection,
anti-viral therapy for hepatitis and anti-inflammatory activity in
neuropathic pain (9,10). No apparent side-effects or toxicity
of matrine have been reported. Recently, the anticancer effect of
matrine has been explored, for instance, in gastric (11), breast (12) and cervical cancer (13). Its mechanisms against various types
of cancers include inducing cell cycle arrest, suppressing invasion
and metastasis, restraining angiogenesis, accelerating apoptosis,
inducing differentiation, reversing multi-drug resistance and
preventing or decreasing chemotherapy- or radiotherapy-induced
toxicity (14). However, a
systematic scientific evaluation of matrine and its anticancer
mechanisms in prostate cancer cell lines remains to be
performed.
Materials and methods
Cell lines, cell culture and
chemicals
Prostate epithelial cells RWPE1 and
androgen-independent prostatic carcinoma cells (PC-3) were used in
the present study. The PC-3 cell line was obtained from the Center
for Experimental Animals of Sun Yat-Sen University. The cells were
maintained in RPMI-1640 medium (Gibco, Grand Island, NY, USA) with
10% fetal bovine serum (FBS), supplemented with 1% penicillin and
streptomycin (Invitrogen, Carlsbad, CA, USA). The RWPE1 cell line
was maintained in complete keratinocyte serum-free medium,
supplemented with 50 mg/ml of bovine pituitary extract and 5 ng/ml
of epidermal growth factor (Gibco). Both cell lines were cultured
in a humidified incubator at 37̊C with an atmosphere of 5%
CO2. Matrine was purchased from Melonepharma (Dalian,
Liaoning, China). LY294002 was purchased from Cell Signaling
Technology (Carlsbad, CA, USA) and was dissolved in dimethyl
sulfoxide (DMSO) according to the manufacturer's instructions.
Antibodies against Akt, p-Akt, P27, CDK4 and Bim were purchased
from Cell Signaling Technology. Antibodies against CDK2, Bax, Bcl-2
and p-FoxO3a were purchased from Abcam (Cambridge, MA, USA).
Antibodies against FoxO3a were purchased from GeneTex (Irvine, CA,
USA).
Cell proliferation assay
The cell proliferation rate was determined using an
MTS assay (Promega Biosciences, LLC, San Luis Obispo, CA, USA)
according to the manufacturer's protocol. Briefly, 5×103
cells/well were seeded into 96-well plates (Corning, New York, NY,
USA) containing 100 µl of culture medium plus different
concentrations of matrine and were grown at 37̊C for 24, 48 and 72
h. Subsequently, the MTS reagent was added to each well and
incubated in the dark for 2 h and optical densities (ODs) at 490 nm
(OD490) were determined using a microplate reader (Multiskan MK3;
Thermo Scientific, Shanghai, China).
Cell apoptosis assay
The Annexin V-FITC/propidium iodide (PI) apoptosis
detection kit (eBioscience, Inc., San Diego, CA, USA) was used to
detect cell apoptosis according to the instructions of the
manufacturer. Cells were seeded into 6-well plates at
1.5×105 cells/well in a medium supplemented with 10% FBS
for 24 h, followed by the addition of matrine (1.5 g/l), LY294002
(10 µmol/l) or a combination of the two. After 48 h, treated cells
were collected and washed twice with chilled phosphate-buffered
saline (PBS). The cells were then resuspended in 400 µl of binding
buffer and divided into two tubes, adding 5 µl of Annexin V-FITC to
one tube according to the manufacturer's instructions. After
incubation for 15 min at room temperature in the dark, 10 µl of PI
was added. Finally, the stained cells were examined by BD
FACSCalibur flow cytometer equipped with CellQuest software (both
from BD Biosciences, Franklin Lakes, NJ, USA).
Cell cycle arrest assay
Following treatment with matrine or LY294002 under
the same conditions as the apoptosis assay, the cells were
resuspended at a concentration of 1.5×105 cells/ml.
Subsequently, the cells were fixed in 70% ethanol and stored
overnight at 4̊C. The fixed cells were suspended in PI containing
RNase A, and then incubated at 37̊C for 1 h. DNA content was
analyzed using a fluorescence-activated cell sorter and CellQuest
software.
Western blot analysis
Following treatment with matrine (1.5 or 2.5 g/l)
and/or LY294002, cells were washed twice with ice-cold PBS, lysed
with RIPA lysis buffer and complete protease inhibitor for 30 min
on ice, and then cleared by centrifugation at 12,000 rpm at 4̊C for
another 30 min. The total protein concentration in the extracts was
assessed utilizing a BCA protein assay kit (Beyotime Biotechnology,
Shanghai, China). Equal amounts of protein were separated by
SDS-PAGE and transferred to polyvinylidene fluoride (PVDF)
membranes (Millipore, Billerica, MA, USA). The membranes were
blocked with 5% BSA or non-fat dry milk in Tris-buffered saline and
Tween-20 (TBST), for 1 h and then probed with antibodies against
Akt (9272), p-Akt (2965), P27 (2552), CDK4 (12790), Bim (2933)
(1:1,000; Cell Signaling Technology); CDK2 (ab32147), Bax
(ab32503), Bcl-2 (ab59348) and p-FoxO3a (ab154786) (1:1,000; Abcam)
and FoxO3a (GTX79072) (1:500; GeneTex) at 4̊C with gentle shaking
overnight. The membranes were then incubated with HRP-conjugated
anti-rabbit or anti-mouse secondary antibodies (1:20,000; Cell
Signaling Technology, Beverly, MA, USA) for 1 h at room temperature
followed by detection using a chemiluminescence ECL kit
(Millipore).
RNA isolation and semi-quantitative
RT-PCR
According to the manufacturer's instructions, total
RNA was extracted from treated cell samples using TRIzol reagent,
before being reverse-transcribed into cDNA using PrimeScript RT
Master Mix (both from Takara, Dalian, China). GAPDH was used as an
internal control. RT-PCR and data collection were performed on the
CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA).
The results were calculated using the 2−ΔΔCt method. All
results are expressed as the mean ± SEM of three independent
experiments. Primers were synthesized by Shenggong Biological
Engineering Co., Ltd. (Shanghai, China). The results were
normalized with the value detected for GAPDH. Real-time PCR primers
were as follows: FoxO3a forward (5′-GCACCTCATCCTTCTTTCAA-3′) and
reverse (5′-CATGCGTCACCATCTCTTTT-3′); PI3K forward
(5′-AAAGGCGGCTTGAAAGGT-3′) and reverse
(5′-GACGATCTCCAATTCCCAAA-3′); and GAPDH forward
(5′-TGGTCGTATTGGGCGCCTGGT-3′) and reverse
(5′-TCGCTCCTGGAAGATGGTGA-3′).
Statistical analysis
Statistical analyses were performed using the SPSS
software package (version 19.0) and GraphPad Prism Software. Data
are expressed as the mean ± standard deviation (SD) and analyzed
using one-way ANOVA. All of the p-values were two-sided and
p<0.05 was considered to indicate a statistically significant
result.
Results
Matrine effectively inhibits the
proliferation of prostate cancer cells in a concentration- and
time-dependent manner
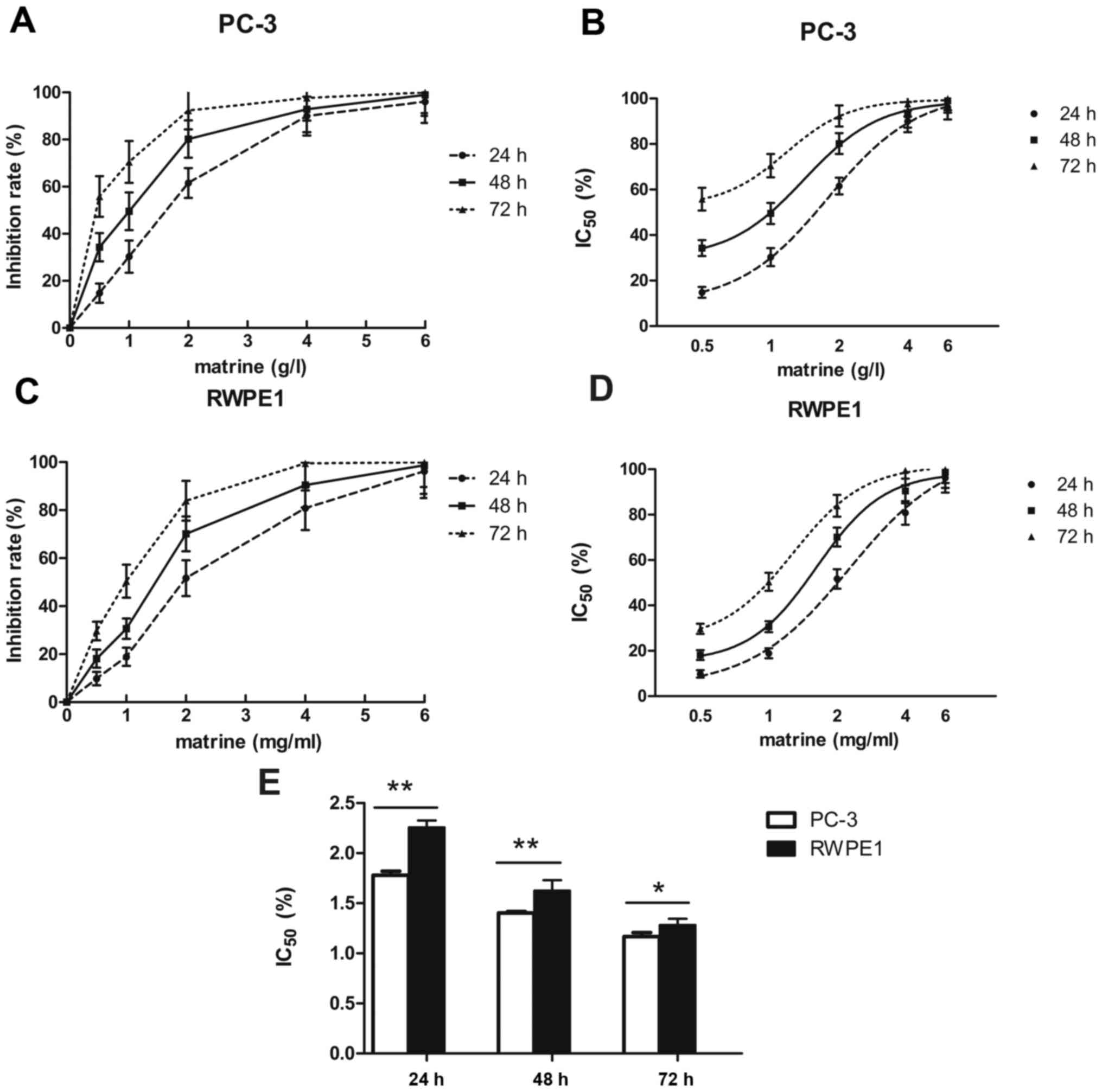
The proliferation of prostate cancer cells was
assessed using the MTS assay. Matrine inhibited the proliferation
of PC-3 cells, with an IC50 value of 1.78±0.023 g/l at
24 h, 1.40±0.008 g/l at 48 h and 1.17±0.023 g/l at 72 h (Fig. 1A and B). Therefore, the
concentration of 1.5 g/l was appropriate for the subsequent
experiments. To determine whether matrine affects the proliferation
of RWPE1, an MTS assay was performed following matrine treatment at
various concentrations. As shown in Fig. 1C and D, similar proliferation
inhibition was observed in the RWPE1 cell line. Matrine inhibited
the proliferation of RWPE1 cells, with an IC50 value of
2.26±0.039 g/l at 24 h, 1.63±0.061 g/l at 48 h and of 1.28±0.053
g/l at 72 h. The sensitivity of matrine in the PC-3 cells was
higher than that in the RWPE1 cells (Fig. 1E).
Matrine triggers prostate cancer cell
cycle arrest at the G0/G1 phase by
upregulating P27 and downregulating CDK4 and CDK2
To further determine whether the antiproliferation
effect of matrine on prostate cancer cells was due to cell cycle
arrest, cultured prostate cancer cells were treated with matrine,
LY294002 or a combination of the two for flow cytometric analysis.
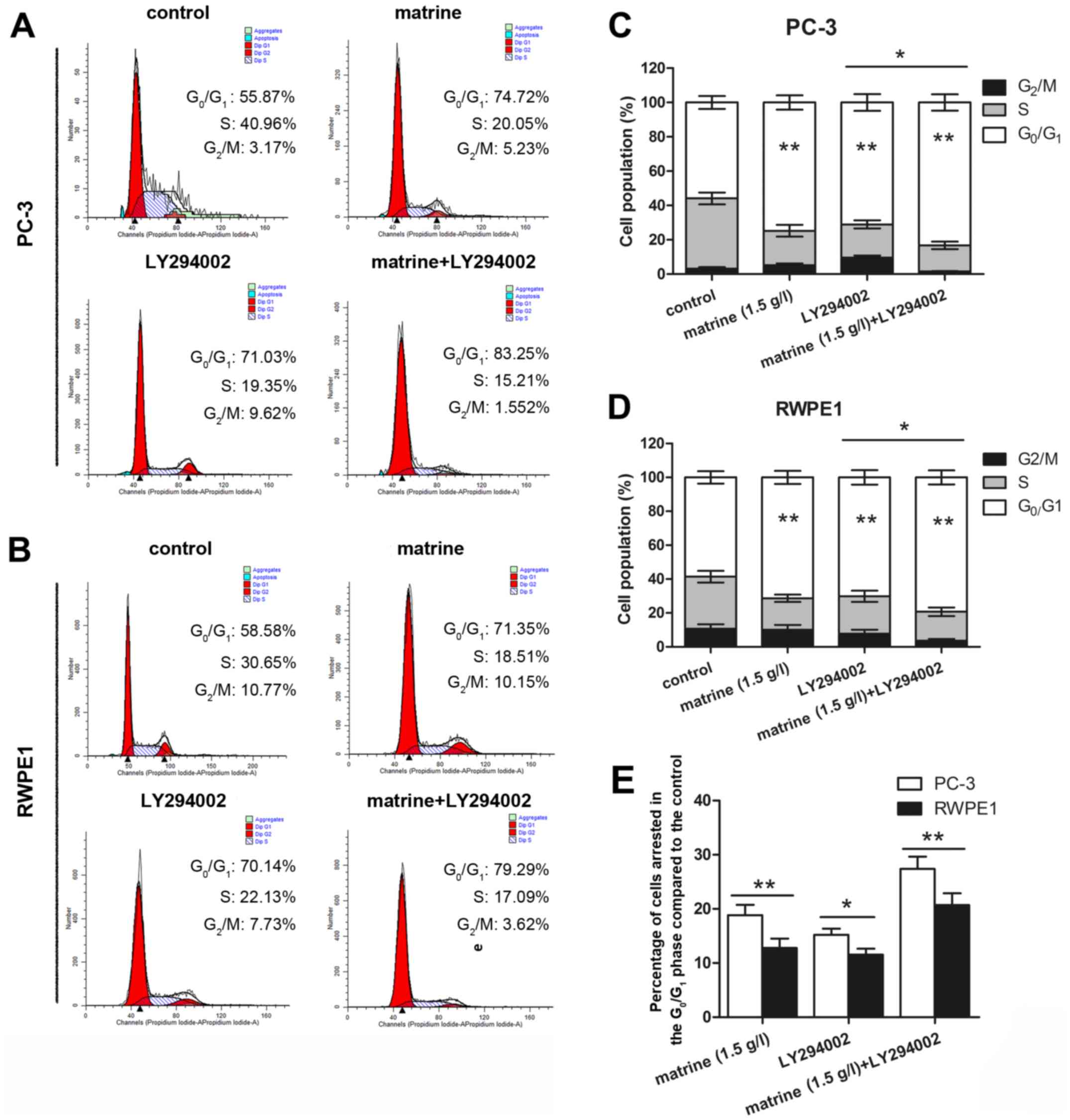
As shown in Fig. 2A-D, matrine
treatment resulted in an appreciable arrest of PC-3 (Fig. 2A and C) and RWPE1 cells (Fig. 2B and D) in the
G0/G1 phase of the cell cycle at 74.72 and
71.35%, respectively, after 48 h of treatment compared to the
untreated controls (55.87 and 58.58%, respectively). The decrease
in the percentage of cells in the S and G2/M phases of
the cell cycle was accompanied by a concomitant increase in the
G0/G1 cell population. Matrine combined with
LY294002 treatment of PC-3 (Fig. 2A and
C) and RWPE1 cells (Fig. 2B and
D) induced 83.25 and 79.29% arrest in the
G0/G1 phase of the cell cycle, respectively,
compared to the LY294002 control (71.03 and 70.14%, respectively).
Compared to the control, the increasing percentage of cell arrest
in the G0/G1 phase in the PC-3 cells was
higher than that in the RWPE1 cells (Fig. 2E).
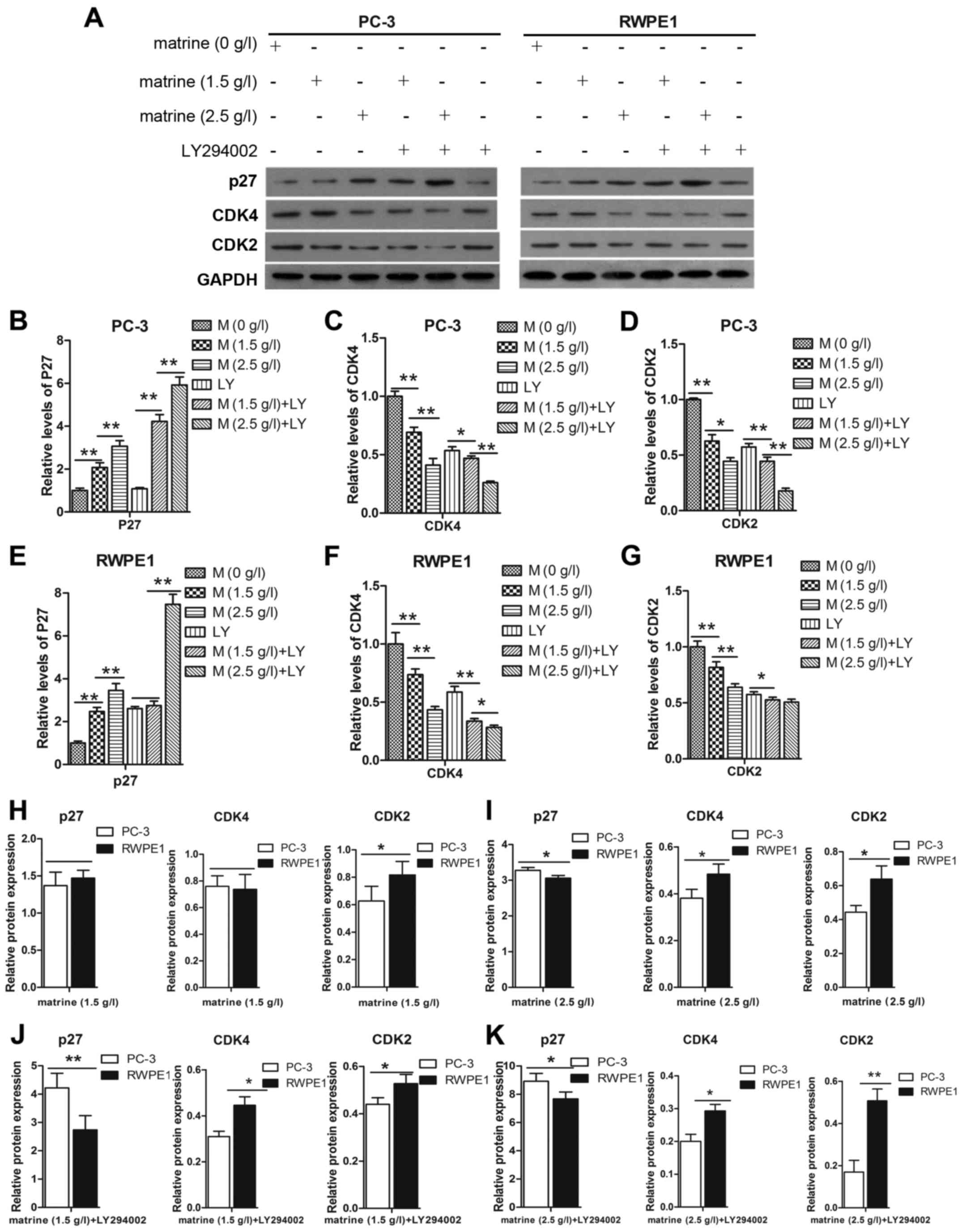
We assessed the effect of matrine on the expression
of P27, CDK2 and CDK4, to determine whether matrine-induced cell
cycle arrest of prostate cancer cells was dependent on the
regulation of P27, CDK2 and CDK4. The expression levels of CDK2 and
CDK4 were decreased in the PC-3 and RWPE1 cell lines after
treatment with matrine (Fig. 3A-G).
Notably, activation of P27 in cells was accompanied by a parallel
decrease in the expression of CDK2 and CDK4. This was particularly
evident when compared to cells treated with LY294002, in which
matrine triggered prostate cancer cell cycle arrest at the
G0/G1 phase by upregulating P27 and
downregulating CDK4 and CDK2 relative to the protein expression of
the PC-3 cells compared to the RWPE1 cells (Fig. 3H-K). The increased expression level
of P27 and decreased expression levels of CDK4 and CDK2 in the PC-3
cells were higher than those in the RWPE1 cells.
Matrine induces cell apoptosis by
increasing Bim and Bax and decreasing Bcl-2 protein levels in
prostate cancer cell lines
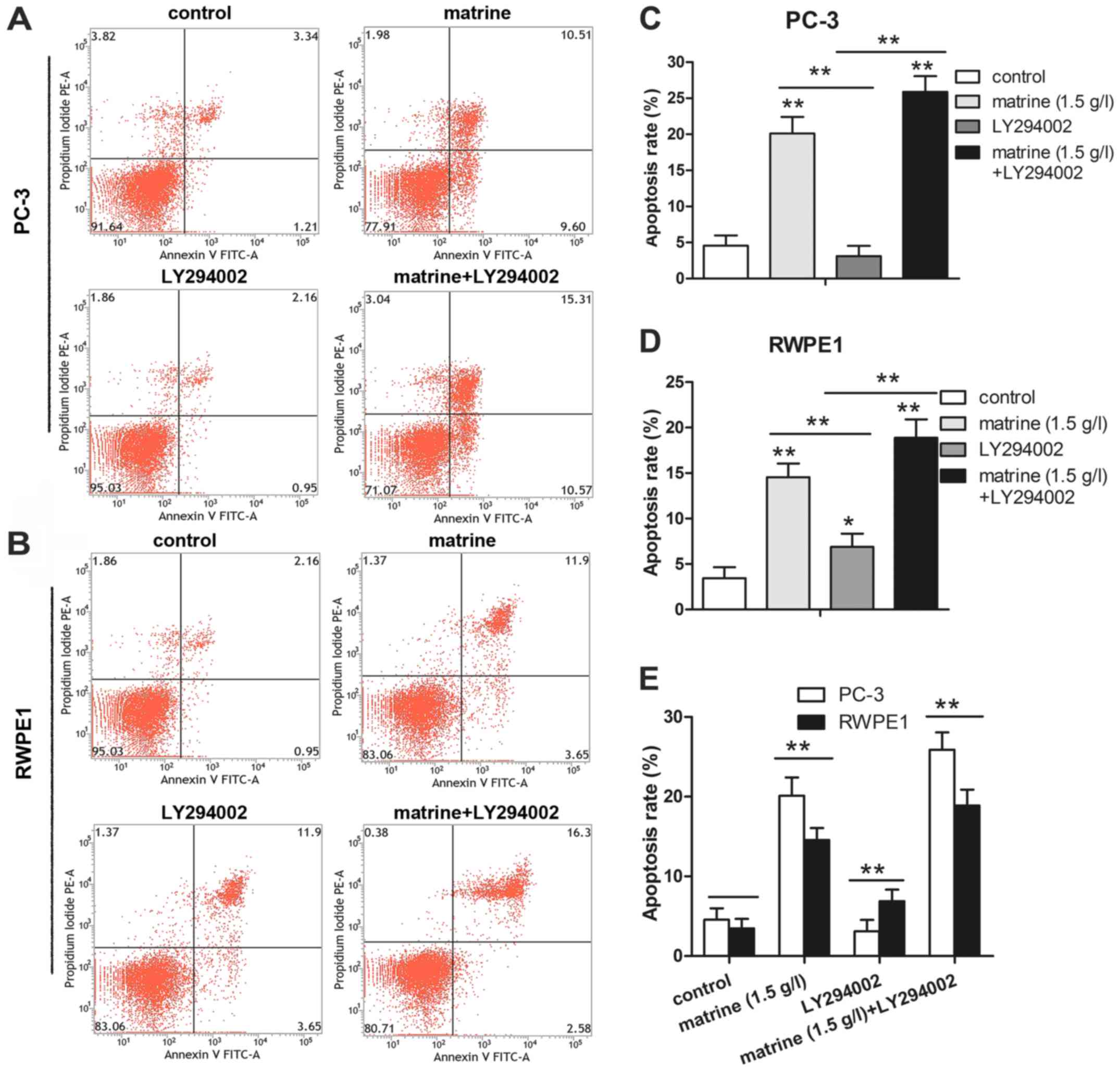
To gain further insight into the dynamic progression
from apoptosis to eventual cell death induced by matrine, Annexin
V-FITC/PI double staining was used to assess the cell population
undergoing apoptosis after treatment with matrine, LY294002 or a
combination of the two for 48 h. The percentage of apoptotic cells,
including early (Annexin V+/PI−) and late
(Annexin V+/PI+) apoptotic cells, was 20.11%
in the PC-3 cells and 15.55% in the RWPE1 cells after treatment
with matrine (Fig. 4A-D). However,
<5% of the untreated cells underwent apoptosis under the same
conditions (Fig. 4A-D). PC-3 and
RWPE1 cells treated with matrine combined with LY294002 resulted in
25.88% cell apoptosis in the PC-3 cells and 18.88% in the RWPE1
cells, compared to 3.11 and 6.89%, respectively, with LY294002
treatment only (Fig. 4A-D).
Compared to the RWPE1 cells, the total apoptosis in the PC-3 cells
was higher (Fig. 4E).
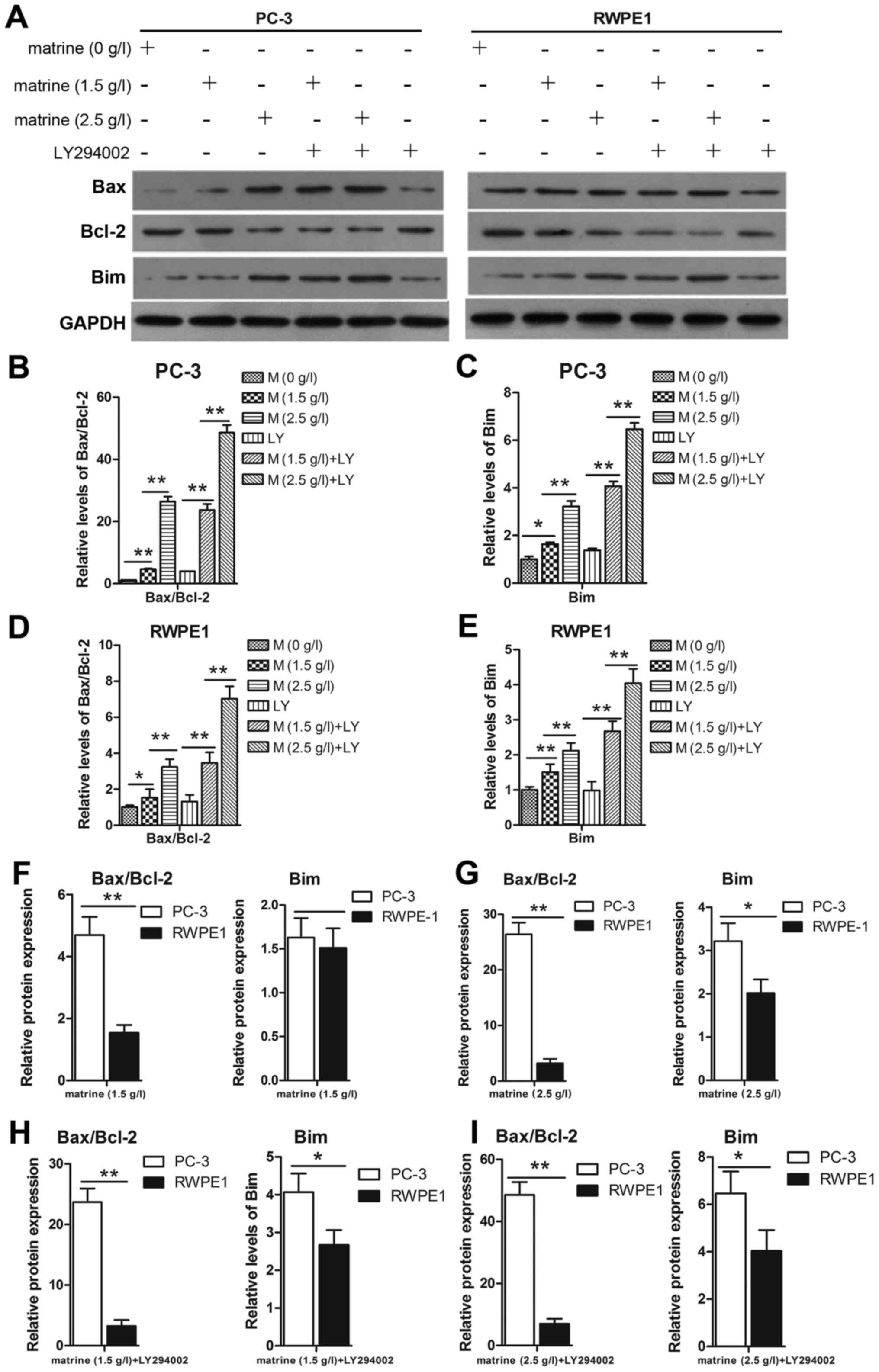
To further demonstrate that matrine induced cell
apoptosis in prostate cancer cells, the proteins Bim, Bax and Bcl-2
were studied. Bim can neutralize pro-survival members such as Bcl-2
and activate Bax, which can finally induce apoptosis. The prostate
cancer cell line PC-3 expressed a high Bcl-2 and low Bax level, but
the level of Bcl-2 decreased while the level of Bax and Bim
increased under the effect of matrine (Fig. 5A). Thus the ratio of the Bcl-2/Bax
(Fig. 5B and D) protein was
markedly downregulated, which corresponded to the upregulation of
Bim (Fig. 5A, C and E). For further
verification, cells were treated with matrine combined with
LY294002 to confirm that Bim may contribute, at least in part, to
the induction of prostate cancer cell apoptosis by regulating the
Bax and Bcl-2 relative protein expression in PC-3 cells compared to
RWPE1 cells (Fig. 5F-I). The
increased expression of Bim and the ratio of Bax/Bcl-2 in the PC-3
cells were higher than those in the RWPE1 cells.
Matrine suppresses the activity of the
PI3K/Akt signaling pathway in prostate cancer cells
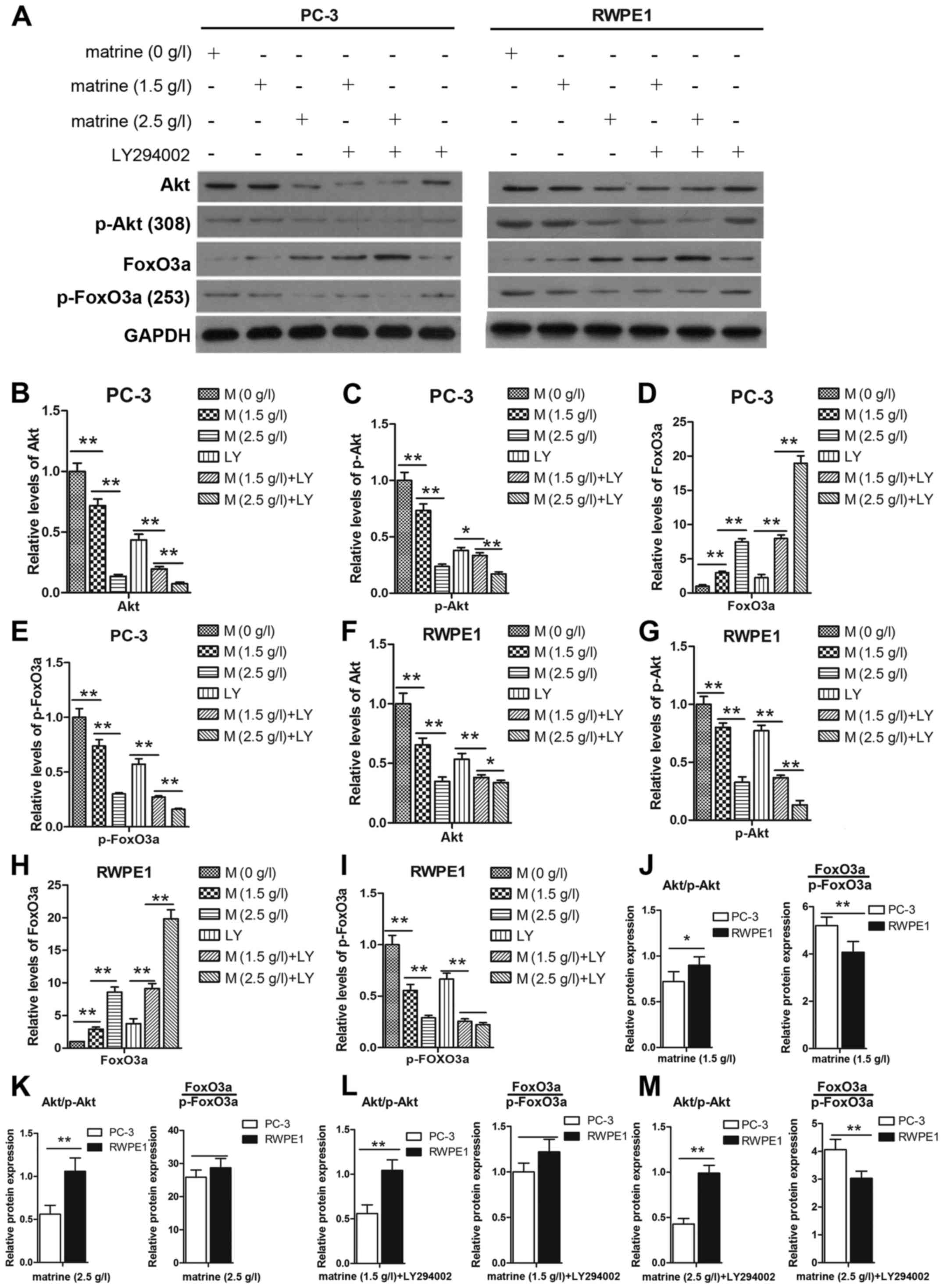
Activation of the Akt signaling pathway plays a
critical role through which cancer cells promote cell survival. We
were therefore interested in determining whether matrine treatment
has an effect on the Akt pathway. The expression levels of p-Akt
and Akt were decreased after treatment with matrine for 48 h
(Fig. 6A). To further confirm this
finding, LY294002 was used to treat the cells for 1 h prior to
matrine treatment. As shown in Fig. 6B
and C, and F and G the expression levels of p-Akt and Akt were
decreased after treatment with matrine and LY294002 compared to the
matrine- or the LY294002-only group. A previous study demonstrated
that an increase in p-Akt induced increased p-FoxO3a and its
dysregulation (15). To determine
whether matrine targets the expression of FoxO3a and its
phosphorylation in prostate cancer cells, we determined the
expression of FoxO3a and p-FoxO3a at the transcriptional (Fig. 7B and D) and translational levels
(Fig. 6D and E, and 6H and I). The
decreased expression levels of Akt/p-Akt and increased levels of
FoxO3a/p-FoxO3a in the PC-3 cells were more obvious than in the
RWPE1 cells (Fig. 6J-M).
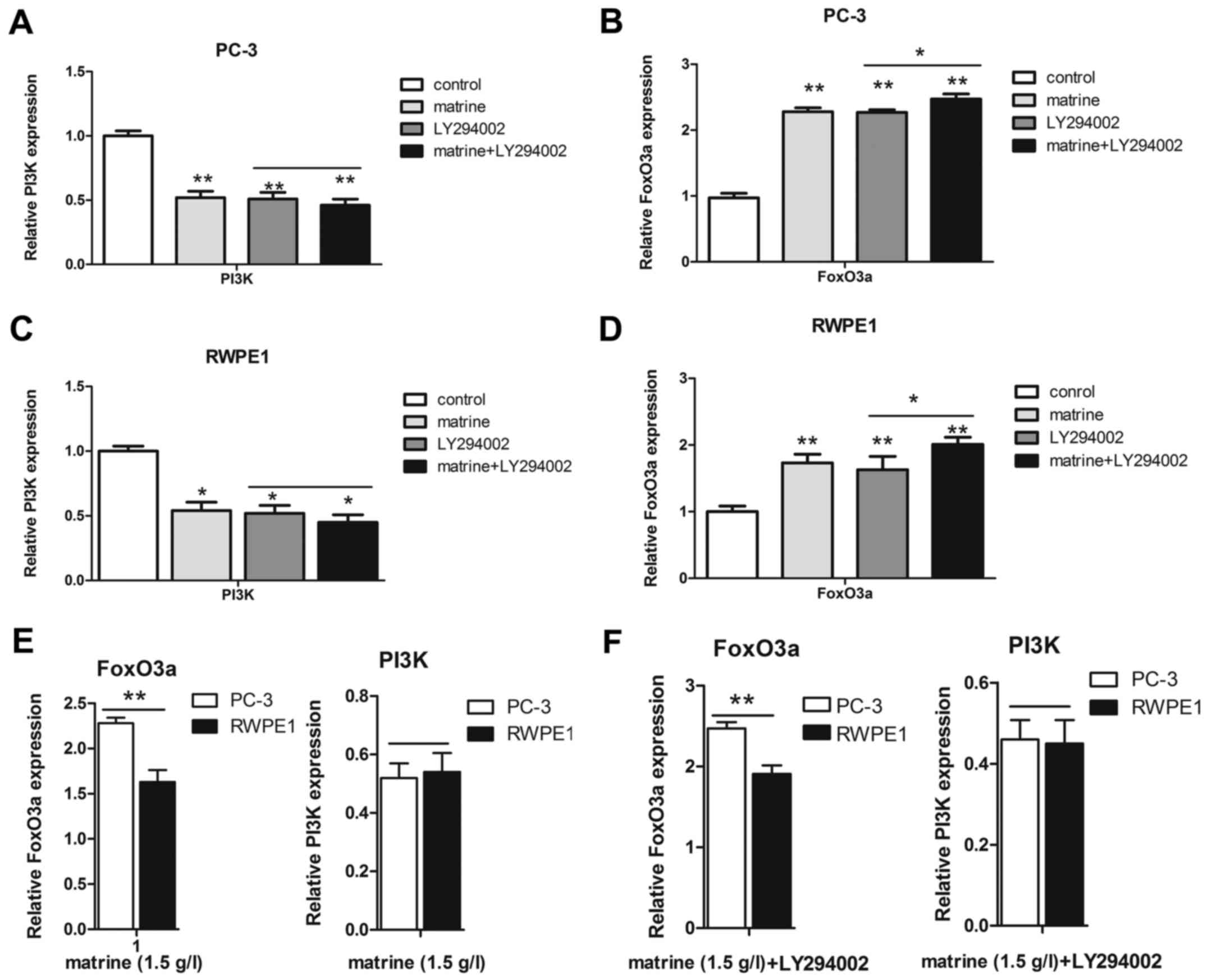
Furthermore, we also detected the expression of PI3K at the
transcriptional level in the two prostate cell lines (Fig. 7A and C). The increase in the mRNA
levels of FoxO3a in the PC-3 cells was higher than that in the
RWPE1 cells, but PI3K was not (Fig. 7E
and F). Unfortunately, the exact mechanism causing the
different transcriptional and translational levels remains unclear.
However, matrine treatment resulted in an increase in FoxO3a and a
decrease in p-FoxO3a and PI3K. Furthermore, LY294002 enhanced the
effect of matrine, increasing FoxO3a with a concomitant decrease in
p-FoxO3a via the PI3K/Akt signaling pathway.
Discussion
Our previous studies showed that matrine could be
considered as a potential candidate for the treatment of prostate
cancer (16). However, the exact
underlying mechanisms of the effect of matrine on the inhibition of
cancer cell growth are not fully understood. The results of the
present study demonstrated that matrine effectively inhibited the
proliferation of PC-3 and RWPE1 cells, which was associated with
cell cycle arrest and apoptosis. Matrine decreased cell cycle
progression by promoting a G0/G1 phase block,
the effects of which were accompanied by the upregulation of the
level of P27 and the downregulation of the levels of CDK4 and CDK2
proteins. Concomitantly, matrine induced cell apoptosis as
evidenced by the Bim, Bax and Bcl-2 protein level analysis. Our
data also suggested that the proliferation of tumor cell
suppression by matrine was linked to the inhibition of PI3K/Akt and
FoxO3a activation. In the present study, LY294002 was used as a
positive control since it has demonstrated antiproliferation
properties in a variety of cell types and significantly
downregulates FoxO3a phosphorylation (17). To the best of our knowledge, this is
the first study demonstrating the involvement of the Akt and FoxO3a
signaling pathways in matrine-mediated prostate cancer
suppression.
The PI3K/Akt signaling pathway is activated in
various human cancers, including prostate cancer (18,19).
Decreased PTEN expression and loss of heterozygosity have been
observed to cause hyperactive Akt in human prostate cancer
(20). Several mechanisms involved
in the overall responses of matrine in the inhibition of growth and
induction of apoptosis in cancer or normal cells have been reported
(12,21). Consistent with this, our results
demonstrated that activation of FoxO3a was also implicated in the
effect of matrine on the regulation of the PI3K/Akt signaling
pathway. FoxO3a plays an important role in multiple cellular
processes involving cell cycle arrest, cell death, DNA damage
repair, stress resistance and metabolism, and is a vital regulation
point of the downstream PI3K/Akt signaling pathway (5). Persistently activated Akt-mediated
phosphorylation of FoxO3a is known to be associated with 14-3-3
protein, thereby leading to the transport of FoxO3a out of the
nucleus and its retention in the cytoplasm, thus, preventing the
transcriptional activity of FoxO3a (20). We detected the expression of FoxO3a
at the translational level to verify whether the inhibition of
p-Akt by matrine led to the retention of FoxO3a and sequentially
increased the transcription of the downstream target genes of
FoxO3a such as Bim and P27. Our results indicated that the levels
of p-FoxO3a were decreased after matrine intake, resulting in
increased levels of Bim and P27.
To date, there have been two major pro-apoptosis
pathways: the mitochondrial (intrinsic pathway) and the death
receptor pathway (extrinsic pathway) (22,23).
In the intrinsic pathway, apoptosis is controlled by a balance
between the pro-apoptotic (Bax, Bak, Bim and Bad) and
anti-apoptotic (Bcl-2 and Bcl-xL) members of the Bcl-2 family
(24). Bim induces mitochondrial
outer membrane permeability to release cytochrome c during
apoptosis, neutralizes pro-survival members such as Bcl-2 and
activates Bax (25). Consistent
with this, our data revealed that Bim was upregulated after
matrine-mediated decreased phosphorylation of p-Akt (Thr308) and
p-FoxO3a (Ser253), thereby inducing increased levels of Bax and
decreased levels of Bcl-2. Previous studies demonstrated that the
loss of mitochondrial membrane potential cannot only lead to the
release of cytochrome c, but also subsequent activation of
caspases (26). Unfortunately, the
present study did not clarify our understanding of caspases,
however, our findings revealed that Bim may contribute, at least in
part, to the induction of prostate cancer cell apoptosis by
matrine. P27 from the Cip/Kip family is a well-defined substrate
for the ubiquitin ligase activity of SKP2/CUL1/F-box (SCF) complex
(27), and plays a central role in
restraining the G1 phase initiation and G1/S
transition (28). The upregulation
of P27, as shown in the present study at the protein level in the
matrine intake group, is consistent with the low levels of P27 in
the control group. A similar result was obtained with LY294002
treatment alone and in combination with matrine. In the past,
activation of the PI3K/Akt pathway was implicated in the regulation
of P27 expression in diverse cell types (29). Collectively, these results suggest
that a G0/G1 phase arrest of the cell cycle
following inhibition of p-Akt by matrine can be attributed to a
significant increase in P27 protein expression in prostate
cancer.
In conclusion, the data presented here demonstrated
that matrine treatment in prostate cancer can activate FoxO3a and
that its accumulation can induce the expression of downstream
target proteins Bim and P27, resulting in cell cycle arrest at the
G0/G1 phase and triggering apoptosis in
prostate cancer. We also demonstrated a synergistic effect of
matrine and LY294002 on prostate tumors. In light of the different
effects of matrine on PC-3 and RWPE1 cells, a lack of in-depth
research remains. However, these findings suggest that matrine
could be used as a potential preventive agent in the management of
castration-resistant prostate cancer in humans.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (nos. 81472382 and 81672550),
the National Natural Science Foundation of China for Young
Scientists Grant (no. 81101947), the Guangdong Province Natural
Science Foundation (no. 2014A030313079), the Fundamental Research
Funds for the Central Universities (no. 14ykpy19), the Guangdong
Province Science and Technology for Social Development Project
(nos. 2013B021800107 and 2013B021800095), the 2015 Guangzhou City
Scientific Research Projects (no. 201510010298), and the
International Science and Technology Cooperation Project of
Guangdong Province Science and Technology Plan (no
2016A050502020).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fowke JH, McLerran DF, Gupta PC, He J, Shu
XO, Ramadas K, Tsugane S, Inoue M, Tamakoshi A, Koh WP, et al:
Associations of body mass index, smoking, and alcohol consumption
with prostate cancer mortality in the Asia Cohort Consortium. Am J
Epidemiol. 182:381–389. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sarker D, Reid AHM, Yap TA and de Bono JS:
Targeting the PI3K/AKT pathway for the treatment of prostate
cancer. Clin Cancer Res. 15:4799–4805. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shukla S: FOXO3a: A potential target in
prostate cancer. Austin J Urol. 12014.
|
|
6
|
Daitoku H, Sakamaki J and Fukamizu A:
Regulation of FoxO transcription factors by acetylation and
protein-protein interactions. Biochim Biophys Acta. 1813:1954–1960.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arrendale A, Kim K, Choi JY, Li W, Geahlen
RL and Borch RF: Synthesis of a phosphoserine mimetic prodrug with
potent 14-3-3 protein inhibitory activity. Chem Biol. 19:764–771.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rinner O, Mueller LN, Hubálek M, Müller M,
Gstaiger M and Aebersold R: An integrated mass spectrometric and
computational framework for the analysis of protein interaction
networks. Nat Biotechnol. 25:345–352. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang W, You RL, Qin WJ, Hai LN, Fang MJ,
Huang GH, Kang RX, Li MH, Qiao YF, Li JW, et al: Anti-tumor
activities of active ingredients in Compound Kushen Injection. Acta
Pharmacol Sin. 36:676–679. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou Y, Wu Y, Deng L, Chen L, Zhao D, Lv
L, Chen X, Man J, Wang Y, Shan H, et al: The alkaloid matrine of
the root of Sophora flavescens prevents arrhythmogenic effect of
ouabain. Phytomedicine. 21:931–935. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guo B, Zhang T, Su J, Wang K and Li X:
Oxymatrine targets EGFRp-Tyr845 and inhibits
EGFR-related signaling pathways to suppress the proliferation and
invasion of gastric cancer cells. Cancer Chemother Pharmacol.
75:353–363. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xie M, He G, Wang R, Shi S, Chen J, Ye Y,
Xie L, Yi X and Tang A: Matrine-induced apoptosis of human
nasopharyngeal carcinoma cells via in vitro vascular endothelial
growth factor-A/extracellular signal-regulated kinase1/2 pathway
inactivation. Horm Metab Res. 46:556–560. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang L, Wang T, Wen X, Wei Y, Peng X, Li
H and Wei L: Effect of matrine on HeLa cell adhesion and migration.
Eur J Pharmacol. 563:69–76. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Y, Xu Y, Ji W, Li X, Sun B, Gao Q and
Su C: Anti-tumor activities of matrine and oxymatrine: Literature
review. Tumour Biol. 35:5111–5119. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shukla S, Bhaskaran N, Babcook MA, Fu P,
Maclennan GT and Gupta S: Apigenin inhibits prostate cancer
progression in TRAMP mice via targeting PI3K/Akt/FoxO pathway.
Carcinogenesis. 35:452–460. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Q, Lai Y, Wang C, Xu G, He Z, Shang X,
Sun Y, Zhang F, Liu L and Huang H: Matrine inhibits the
proliferation, invasion and migration of castration-resistant
prostate cancer cells through regulation of the NF-κB signaling
pathway. Oncol Rep. 35:375–381. 2016.PubMed/NCBI
|
|
17
|
Wang D, Yang Y, Huang R, Zhang Z and Lin
X: Myostatin activates the ubiquitin-proteasome and
autophagy-lysosome systems contributing to muscle wasting in
chronic kidney disease. Oxid Med Cell Longev. 2015:6849652015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Majumder PK and Sellers WR: Akt-regulated
pathways in prostate cancer. Oncogene. 24:7465–7474. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carnero A: The PKB/AKT pathway in cancer.
Curr Pharm Des. 16:34–44. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Trotman LC, Alimonti A, Scaglioni PP,
Koutcher JA, Cordon-Cardo C and Pandolfi PP: Identification of a
tumour suppressor network opposing nuclear Akt function. Nature.
441:523–527. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang P, Wang Z, Chong T and Ji Z: Matrine
inhibits proliferation and induces apoptosis of the
androgen-independent prostate cancer cell line PC-3. Mol Med Rep.
5:783–787. 2012.PubMed/NCBI
|
|
22
|
Yaoxian W, Hui Y, Yunyan Z, Yanqin L, Xin
G and Xiaoke W: Emodin induces apoptosis of human cervical cancer
hela cells via intrinsic mitochondrial and extrinsic death receptor
pathway. Cancer Cell Int. 13:712013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nam YJ, Mani K, Ashton AW, Peng CF,
Krishnamurthy B, Hayakawa Y, Lee P, Korsmeyer SJ and Kitsis RN:
Inhibition of both the extrinsic and intrinsic death pathways
through nonhomotypic death-fold interactions. Mol Cell. 15:901–912.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Heath-Engel HM and Shore GC: Regulated
targeting of Bax and Bak to intracellular membranes during
apoptosis. Cell Death Differ. 13:1277–1280. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Desagher S and Martinou JC: Mitochondria
as the central control point of apoptosis. Trends Cell Biol.
10:369–377. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Takuma K, Baba A and Matsuda T: Astrocyte
apoptosis: Implications for neuroprotection. Prog Neurobiol.
72:111–127. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Morimoto M, Nishida T, Honda R and Yasuda
H: Modification of cullin-1 by ubiquitin-like protein Nedd8
enhances the activity of SCFskp2 toward
p27kip1. Biochem Biophys Res Commun. 270:1093–1096.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ray A, James MK, Larochelle S, Fisher RP
and Blain SW: p27Kip1 inhibits cyclin D-cyclin-dependent
kinase 4 by two independent modes. Mol Cell Biol. 29:986–999. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stahl M, Dijkers PF, Kops GJ, Lens SM,
Coffer PJ, Burgering BM and Medema RH: The forkhead transcription
factor FoxO regulates transcription of p27Kip1
and Bim in response to IL-2. J Immunol. 168:5024–5031. 2002.
View Article : Google Scholar : PubMed/NCBI
|