Introduction
Chemotherapy plays a significant role in the
treatment of liver cancer since the majority of patients diagnosed
with liver cancer are terminal, and the tumors cannot be completely
removed. At this stage, liver cancer has low susceptibility to
chemotherapeutic drugs, an issue that may relate to multidrug
resistance (MDR) genes. The commonly used clinical chemotherapeutic
drugs for liver cancer therapy include cisplatin (PDD), doxorubicin
(DOX), mitomycin (MCC), 5-fluorouracil (5-FU) and bleomycin (BLM).
However, application of these drugs is limited to some extent due
to their serious side effects. DOX is the most widely used drug in
the treatment of liver cancer, but its cardiac toxicity is also the
most severe among the chemotherapeutic drugs. Therefore, searching
for new targets and screening for novel anti-liver cancer drugs is
crucial.
Hydrogen sulfide (H2S) is named as the
third gasotransmitter signaling molecule alongside nitric oxide
(NO) and carbon monoxide (CO), and plays important roles in many
physiological processes (1–6). Recently studies on H2S have
revealed its roles in cancer therapy and have shown that
H2S exhibits both pro-apoptotic and anti-apoptotic
effects in cancer cells (7–9). Moreover, tumor-derived endogenous
H2S contributes to angiogenesis and cytoprotection
(10,11).
Endogenous H2S is mainly generated by
pyridoxal-5-phosphate (PLP)-dependent enzymes,
cystathionine-β-synthase (CBS), cystathionine γ-lyase (CSE), and
pyridoxal 5-phosphate (PLP)-independent enzyme, 3-mercaptopyruvate
sulfurtransferase (MST) (12).
Recent studies have demonstrated that endogenous H2S
produced by CBS promotes the proliferation of human ovarian and
colon cancer cells (10,11). However, CBS primarily localizes in
liver and brain tissues in humans and it is unclear whether it
contributes to liver cancer progression. In the present study, we
investigated the role of CBS in human liver cancer cells and the
inhibitory effects of a novel inhibitor on CBS in these cells.
Materials and methods
Cell lines
Human liver cancer cell lines SMMC7721, HepG2,
BEL-7404 and human normal liver cells HL7702 and QSG7701 were
procured from the American Type Culture Collection (ATCC; Manassas,
VA, USA), cultivated in Roswell Park Memorial Institute (RPMI)-1640
or Dulbecco's modified Eagle's medium (DMEM) (HyClone, Logan, UT,
USA) supplemented with 10% fetal bovine serum (FBS) and 1%
penicillin/streptomycin in a 37̊C incubator with 5%
CO2.
siRNA transfection
CBS-specific siRNA (sense,
5′-CCAAGUGUGAGUUCUUCAAdTdT-3′ and antisense,
5′-UUGAAGAACUCACACUUGGdTdT-3′) was designed to target the open
reading frame (ORF) region of the CBS mRNA. The cells were seeded
to reach 30–40% confluency at the point of transfection. Forward
transfection was carried out using Lipofectamine 2000 (Invitrogen,
Carlsbad, CA, USA) according to the manufacturer's protocol.
Western blot analysis
Cells were harvested and lysed using RIPA buffer (50
mM Tris-HCl, pH 8.0; 150 mM sodium chloride; 1.0% NP-40; 0.5%
sodium deoxycholate; and 0.1% SDS) with 10 µg/ml protease inhibitor
phenylmethanesulfonyl fluoride (PMSF; Sigma-Aldrich, St. Louis, MO,
USA). Cell lysates were clarified by centrifugation at 12,000 × g
for 10 min and protein concentration in the supernatant was
determined. Total cellular proteins (40 µg) were separated using
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and transferred to polyvinylidene difluoride membranes
(Millipore Corporation, Bedford, MA, USA). After blocking with 5%
milk or 5% bovine serum albumin (BSA) for 2 h at room temperature,
the membranes were incubated overnight at 4̊C with the primary
antibodies. After incubation with the secondary antibody, the
proteins were detected using AlphaImager chemiluminescence system
(ProteinSimple, San Jose, CA, USA). The primary antibodies used
included CBS rabbit polyclonal (1:1,000; Abcam, Cambridge, MA,
USA), Bax rabbit monoclonal (1:1,000), Bcl-2 rabbit monoclonal
(1:1,000), caspase-3 rabbit monoclonal (1:1,000), cleaved caspase-3
rabbit monoclonal (1:1,000), PARP rabbit monoclonal (1:1,000),
cleaved PARP rabbit monoclonal antibodies (1:1,000) (all from Cell
Signaling Technology, Danvers, MA, USA), HMOX1 (HO-1) rabbit
polyclonal (1:600) and β-actin rabbit polyclonal antibodies
(1:2,000) (both from Proteintech, Chicago, IL, USA). Secondary
antibody was peroxidase-conjugated AffiniPure goat
anti-rabbit/mouse (1:5,000; Proteintech).
H2S detection
Production of H2S from cancer cells was
spectrophotometrically measured. Briefly, SMMC7721 cells were
transfected with CBS siRNA or exposed to aminooxyacetate (AOAA;
Sigma-Aldrich) or QICs (QIC0, QIC1, QIC2 and QIC3,
quinolone-indolone conjugates (13), synthesized chemically by Professor
Quoqiang Hu, College of Pharmacy, Henan University) and treated
with 2 mM L-cysteine and 0.5 mM pyridoxal phosphate. Meanwhile 1%
(w/v) zinc acetate (500 µl) was added to the filter papers adhered
to the tissue culture plate cover to absorb H2S. After
48 h, the filter papers were placed in tubes containing 0.2% (w/v)
N,N-dimethyl-p-phenylenediamine
dihydrochloride dye (500 µl), 10% (w/v) ammonium ferric sulfate (50
µl) and 3 ml H2O, and incubated for 20 min at room
temperature. Absorbance at 670 nm was subsequently monitored.
Production of H2S was determined using a standard curve
for NaHS (0–1 mM; R2=0.9995) and presented as
nmol/min/1×106 cells.
MTS and EdU assays
3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS) and 5-ethynyl-2-deoxyuridine (EdU) assays were used to assess
cell viability and cell proliferation. SMMC7721 cells were plated
into 96-well plates, and treated with CBS siRNA, CBS inhibitor
aminooxyacetic acid (AOAA), CBS-overexpression plasmid and QICs
(QIC0, QIC1, QIC2 and QIC3) for 48 h, respectively. Cell viability
was evaluated by determining the number of cells with MTS
(Sigma-Aldrich). The assay was carried out in triplicate for three
independent experiments. DOX and sunitinib served as positive
controls. The effects of CBS knockdown, CBS overexpression and QIC2
on cell proliferation were tested by the EdU assay kit (Ruibobio,
Guangzhou, China). Briefly, cells were cultured in 96-well plates
and transfected with CBS siRNA or the CBS-overexpression plasmid
and exposed to AOAA or QICs for 48 h. Then, the cells were
incubated with 50 µm of EdU for 2 h at 37̊C. Cells were fixed with
4% formaldehyde for 30 min, incubated with glycine (2 mg/ml) for 5
min and treated with 0.5% Triton X-100 for 10 min to permeabilize
the cells. After being washed with phosphate-buffered saline (PBS)
for 5 min, cells were incubated with Apollo for 30 min and treated
twice with 0.5% Triton X-100. DNA was stained with Hoechst 33342
stain for 30 min and visualized with fluorescence microscopy. Five
groups of cells in the images were randomly selected.
Flow cytometric assay
CBS siRNA- or scramble siRNA (Sc siRNA)-transfected
cells, empty vector- or CBS-overexpressing plasmid-transfected
cells and QIC2-treated or untreated cells (3×105
cells/well) were harvested at 48 h post-transfection or
post-treatment. Apoptosis was determined via dual staining with
Annexin V-FITC/PI (Biyuntian, Shanghai, China). Briefly, the cells
were harvested and washed with PBS twice, and resuspended in 195 µl
binding buffer. Annexin V-FITC (5 µl) was firstly added and gently
mixed. Then, 10 µl PI was added, mixed gently and incubated for
10–20 min in the dark and analyzed using the FACSCalibur system (BD
Biosciences, Franklin Lakes, NJ, USA). The assays were carried out
in triplicate.
Measurements of intracellular reactive
oxygen species (ROS) and reduced glutathione (GSH)
Production of intracellular ROS was quantified using
the DCFH-DA assay according to the instructions included in the
Reactive Oxygen Species Assay kit (Biyuntian). For the CBS siRNA-
or Sc RNA-transfected cells, empty vector- or CBS-overexpression
plasmid-transfected cells and QIC2-treated or untreated cells in a
96-well plate, old media were aspirated out and incubated in new
RPMI-1640 medium containing 10 µM dichlorofluorescein diacetate
(DCFH-DA) at 37̊C in a 5% CO2 atmosphere for 20 min.
Then, the medium was removed gently and replaced with 200 µl of 1X
PBS followed by a wash with 1X PBS. The fluorescence released was
read using a microplate spectrofluorometer with excitation/emission
wavelengths set at 488/525 nm. Reduced GSH was determined using a
GSH and GSSG assay kit (Biyuntian). The cells pretreated with QIC2
(0, 0.25, 0.5 and 1 µM) were collected and washed with 1X PBS. The
pellet was immediately resuspended with three times volume of
protein removal reagent M. Then, the sample was rapidly frozen and
thawed twice using liquid nitrogen and 37̊C water bath. The
suspension of cells was transferred into a microfuge tube and
centrifuged at 10,000 g/min for 10 min at 4̊C. The clarified
supernatant was collected for the assay according to the protocol.
The assays were carried out in triplicate.
Statistical analysis
Statistical analyses were performed with the SPSS
17.0 software (SPSS, Inc., Chicago, IL, USA). The results are
expressed as the mean ± SD. Differences between two groups were
analyzed using the Student's t-test. p<0.05 was considered to
indicate a statistically significant difference.
Results
Endogenous CBS/H2S pathway
modulates the proliferation of human hepatoma cells
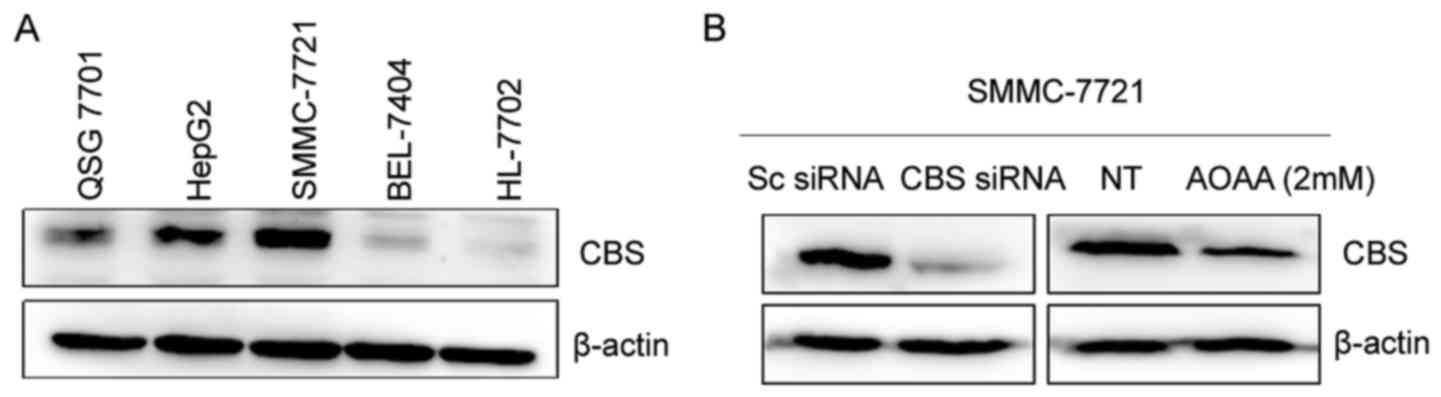
To explore the relationship between CBS expression
and the proliferation of hepatoma cells, we firstly checked the
intrinsic levels of CBS protein in hepatoma cell lines including
SMMC-7721, HepG2 and BEL-7404 as well as the normal liver cell
lines HL-7702 and QSG-7701. As shown in Fig. 1A, CBS protein levels in the human
hepatocellular carcinoma SMMC-7721 and HepG2 cells were higher than
levels in the human normal liver HL-7702 and QSG-7701 cells.
However, BEL-7404 cells had a low CBS protein level. CBS levels
were reported to be coordinately regulated with proliferation in
human cells and may represent a novel marker for both
differentiation and proliferation in certain cell types (14). Previous research has also shown that
the rise in H2S released from hepatoma HepG2 cells is
correlated with an increase in cystathionine β-synthase (CBS)
(15). The high CBS expression in
SMMC-7721 and HepG2 cells suggests that CBS is mainly responsible
for the production of endogenous H2S in the cells and is
closely related to cell proliferation. The low CBS level in
BEL-7404 cells may imply that other enzymes are mainly responsible
for the catalysis of the generation of endogenous H2S in
these cells or that endogenous H2S is not relevant to
the survival and growth of BEL-7404 cells since previous studies
have shown that CSE is also present in liver cancer cells (6). To explore the biological significance
of high CBS expression in SMMC-7721 and HepG2 cells, we knocked
down CBS using siRNA transfection or inhibited the activity of CBS
protein by AOAA in SMMC-7721 cells and analyzed the changes in the
endogenous H2S level and cell proliferation ability.
Surprisingly, we found that inhibition of endogenous
CBS/H2S significantly reduced the cell viability and
inhibited the growth of SMMC-7721 cells (Fig. 1B-F). Moreover, EdU assay showed that
CBS siRNA transfection also distinctly inhibited the proliferation
of SMMC-7721 cells (Fig. 1G). These
results indicate that the CBS/H2S system is closely
related to the cell survival and proliferation of human liver
cancer cells and CBS downregulation is conducive to the treatment
of liver cancer.
CBS knockdown induces cell apoptosis
and increases oxidative stress in hepatoma cells
Apoptosis is one of the major
mechanisms of inhibiting cell proliferation or inducing cell
death
To explore how the CBS/H2S pathway
regulates the proliferation of hepatoma cells, we investigated the
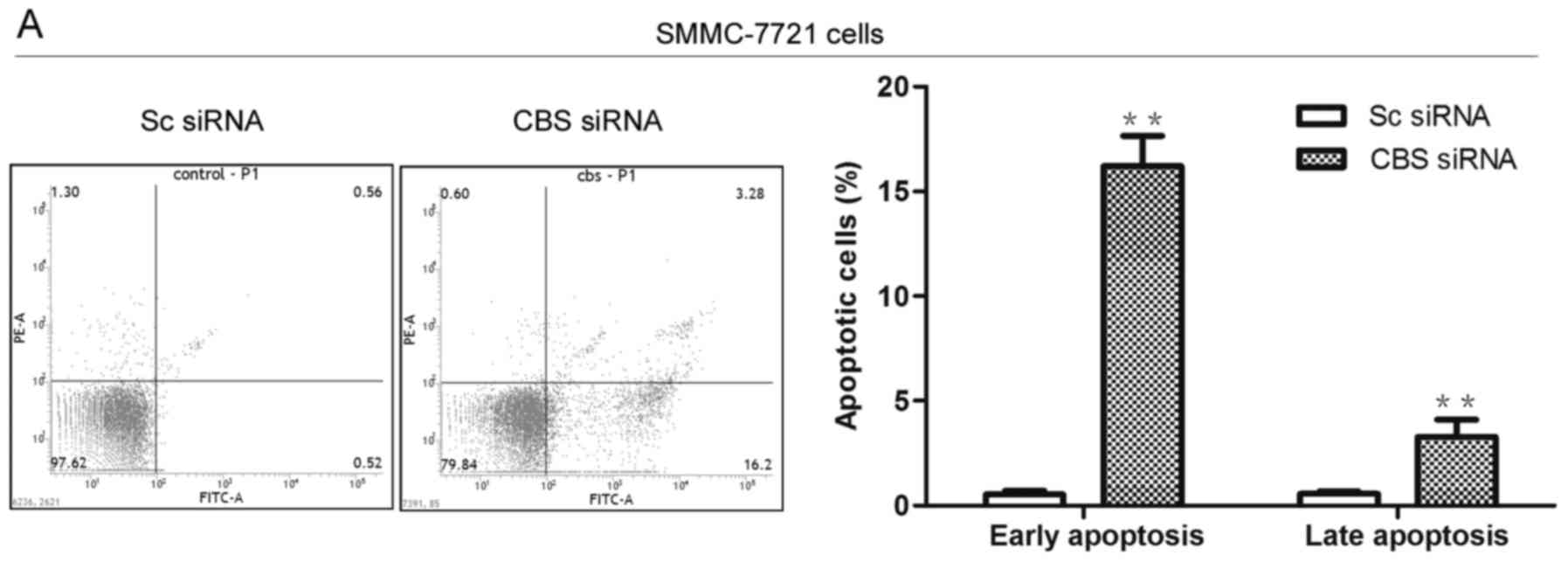
effect of CBS knockdown on cell apoptosis. As shown in the flow
cytometric results (Fig. 2A), CBS
siRNA transfection caused an increased apoptosis rate in the
SMMC-7721 cells. We also examined changes in apoptosis-related
proteins and found that CBS knockdown caused not only a distinct
increase in the Bax/Bcl-2 ratio, but also activation of caspase-3
and PARP (Fig. 2B). Cancer cell
survival is known to be related with the ability of cancer cells to
counteract oxidative stress, which may be associated with Heme
oxygenase-1 (HO-1), a microsomal enzyme that plays the role of an
antioxidant to resist oxidative stress. HO-1 is overexpressed in
hepatoma cells. It is also known that H2S regulates
resistance to oxidative stress in many organs and systems and plays
an important regulatory role in the expression of HO-1 (16). However, whether or not the
CBS/H2S system maintains hepatoma survival by modulating
HO-1 expression and oxidative stress remains unclear. To address
this issue, we investigated the correlation between the
CBS/H2S system and HO-1 expression in hepatoma cells.
Our data showed that HO-1 protein expression was significantly
decreased in the CBS siRNA-transfected SMMC-7721 cells compared
with the Sc siRNA-transfected group (Fig. 2C). Meanwhile, increased ROS level
was also observed in the CBS siRNA-transfected SMMC-7721 cells
(Fig. 2D). These results indicate
that the CBS/H2S system regulates cell apoptosis and is
closely related to the oxidative stress in hepatoma cells.
QIC2 inhibits cell proliferation and
promotes cell apoptosis by downregulating CBS expression in human
hepatoma cells
AOAA, an inhibitor of CBS, is unable to be developed
into a powerful anticancer drug, due to the cytotoxicity of AOAA in
human normal liver HL-7702 cells and the high dose of AOAA needed
in liver cancer cells (Fig. 3A).
Thus, to screen more effective and less harmful CBS inhibitors for
liver cancer therapy, we investigated the inhibitory effect of
quinolone-indolone conjugates (QIC0, QIC1, QIC2 and QIC3) on the
expression of CBS protein and cell viability in SMMC-7721 cells. As
shown in Fig. 3B, the results
clearly showed that a significant reduction in the CBS protein
level was achieved under QIC2 treatment. However, treatments with
QIC0, QIC1 and QIC3 did not exhibit obvious effects on CBS protein
expression. In terms of cell growth, QIC2 yielded the strongest
inhibitory effect on the SMMC-7721 cells compared with QIC0
(p=0.018), QIC1 (p=0.008) and QIC3 (p=0.0009) (Fig. 3C). QIC2 was thus used for further
evaluation of the anticancer activity and relevant molecular
mechanisms. Following treatment of QIC2, SMMC-7721 cell viability
was significantly reduced in a dose-dependent manner. The
inhibitory effect was stronger than that of the positive control
sunitinib but slightly weaker than the positive control DOX
(Fig. 3D). However, QIC2
demonstrated the lowest toxicity to human normal liver HL-7702
cells compared with both controls sunitinib and DOX (Fig. 3E). Meanwhile, we also observed the
inhibition of cell proliferation (Fig.
3F and G) and induction of cell apoptosis (Fig. 3H and I). To demonstrate whether QIC2
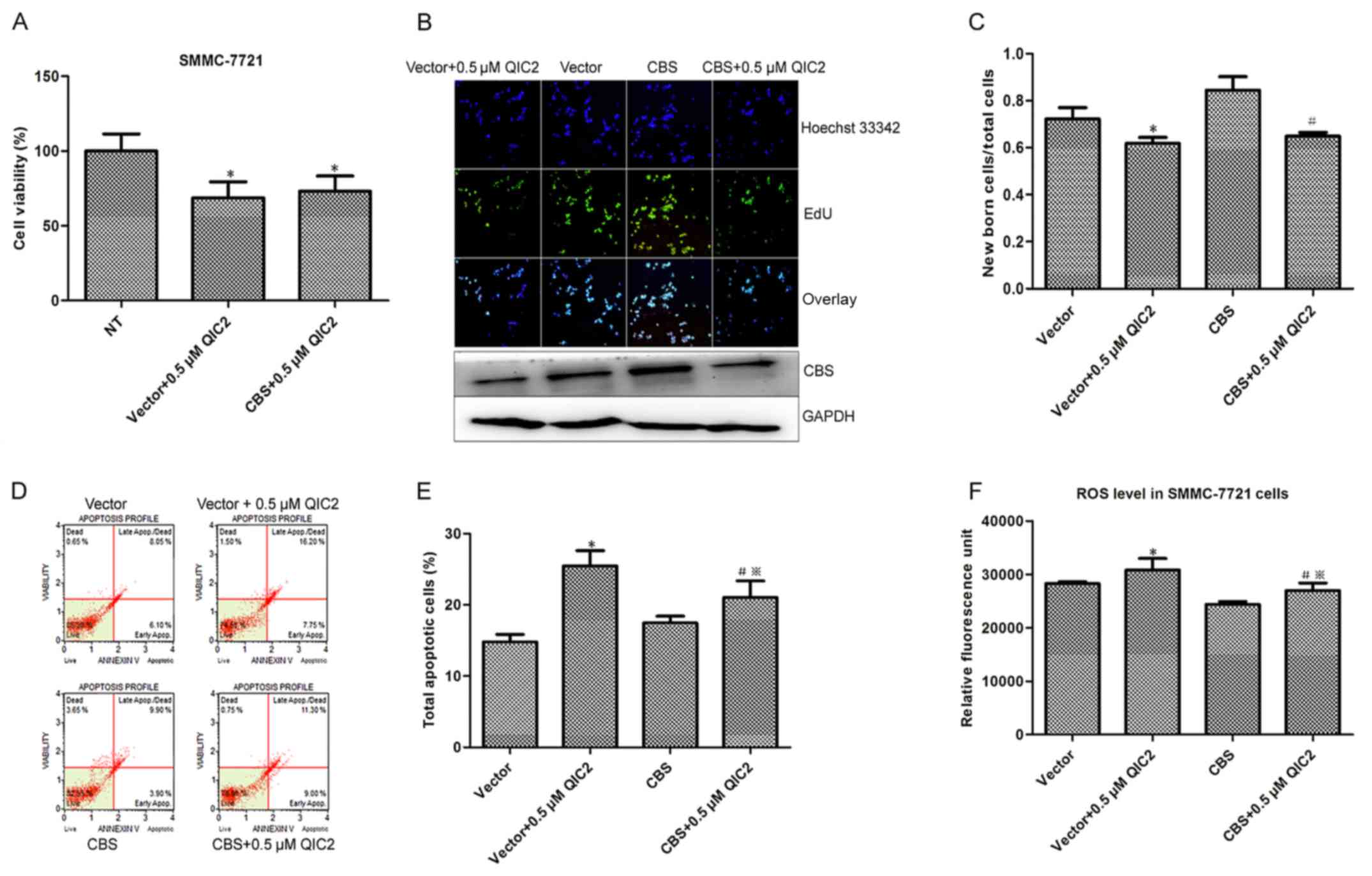
acts through CBS, we transfected the CBS-overexpressing plasmid
into SMMC-7721 cells and determined the activities and roles of
QIC2 in the cells. Increased cell proliferation and decreased ROS
level were observed in the cells transfected with the CBS plasmid
compared with the cells transfected with the empty vector (Fig. 4), which further manifests the roles
of CBS in hepatoma cells. Meanwhile, the decreased cell viability
and newborn cells caused by QIC2 were not distinct between the CBS
plasmid- and empty vector-transfected groups (Fig. 4A-C), suggesting that CBS
overexpression did not abrogate the inhibitory activity of QIC2 on
cell growth and proliferation. However, the increase in the
percentage of apoptotic cells and elevated ROS levels induced by
QIC2 were significantly reduced in the CBS-transfected group when
compared with the vector-transfected group (Fig. 4D-F). This infers that CBS
overexpression rescues the effects of QIC2 on cell apoptosis and
QIC2 acts by targeting CBS.
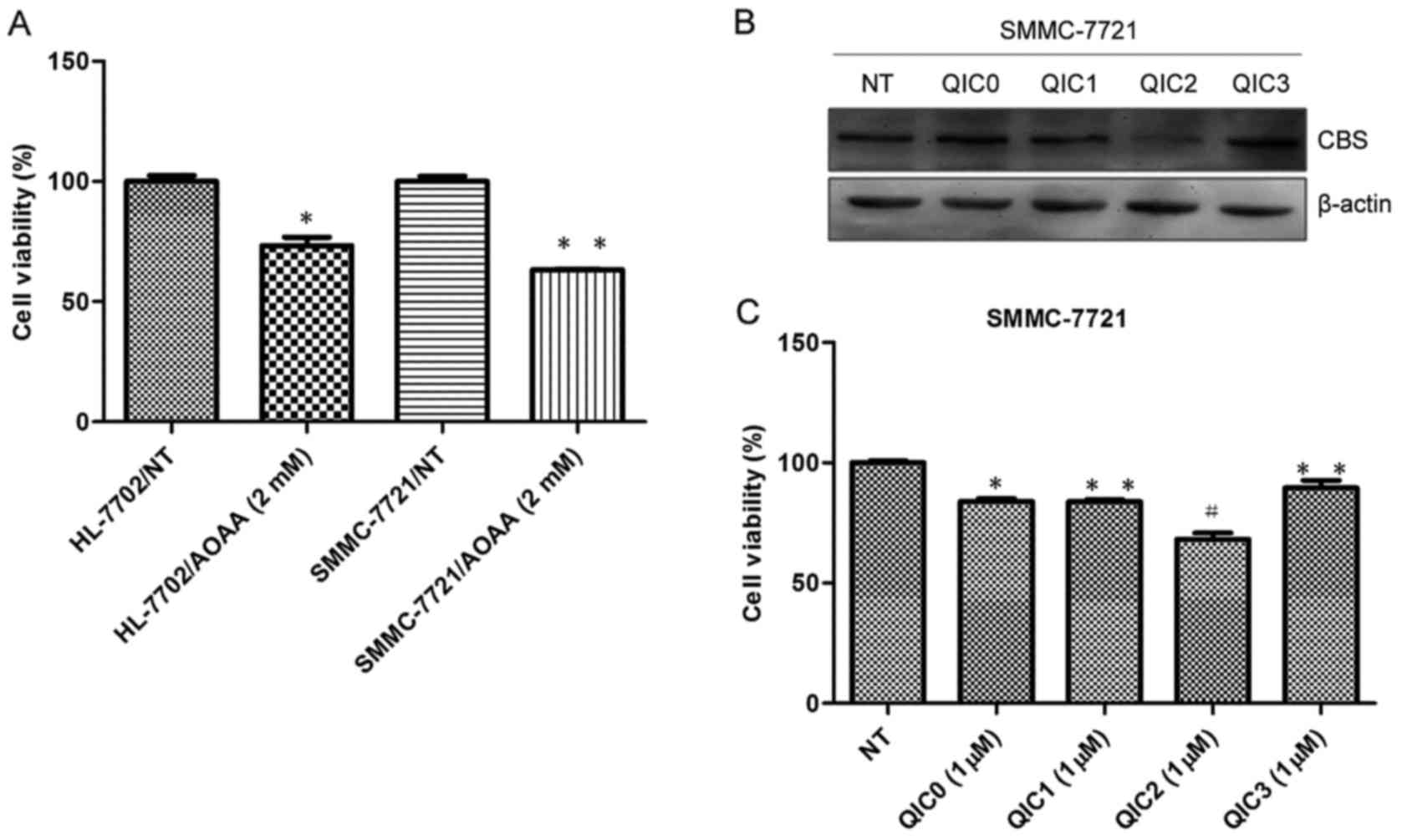 | Figure 3.The effect of QIC2 with CBS inhibitory
activity on cell proliferation and apoptosis in SMMC-7721 cells.
(A) AOAA caused growth inhibition in human normal liver HL-7702
cells and hepatoma SMMC-7721 cells. Cell viability was evaluated in
triplicate by a microplate spectrofluorometer. Error bars indicate
SD (n=3); *p<0.05 vs. the HL-7702/NT (no treatment) groups,
**p<0.01 vs. the SMMC-7721/NT (no treatment) groups. (B and C)
Screening of effective and safe CBS inhibitors from
quinolone-indolone conjugates (QICs). Error bars indicate SD (n=3);
#p<0.01 vs. the NT (no treatment) groups, *p<0.05
vs. the QIC2 groups, **p<0.01 vs. the QIC2 groups. (D and E)
Effects of QIC2 on cell growth in human liver cancer SMMC-7721 and
normal liver HL-7702 cells. (F and G) Analyses of cell
proliferation. EdU assays were used to test the effect of QIC2 on
proliferation in SMMC-7721 cells (magnification, ×20). EdU,
5-ethynyl-2′-deoxyuridine. Error bars indicate SD (n=3); *p<0.05
vs. the 0 µM QIC2 groups, **p<0.01 vs. the 0 µM QIC2 groups. (H
and I) Analyses of cell apoptosis. Flow cytometric assay with
Annexin V/7-amino-actinomycin D double staining. Error bars
indicate SD (n=3); *p<0.05 vs. the 0 µM QIC2 groups, **p<0.01
vs. the 0 µM QIC2 groups. AOAA, aminooxyacetate; DOX, doxorubicin;
QIC2, quinolone-indolone conjugate 2. |
QIC2 inhibits the CBS/H2S
system, causes oxidative stress and activates the caspase-3 cascade
in human hepatoma cells
To further investigate the anticancer mechanism of
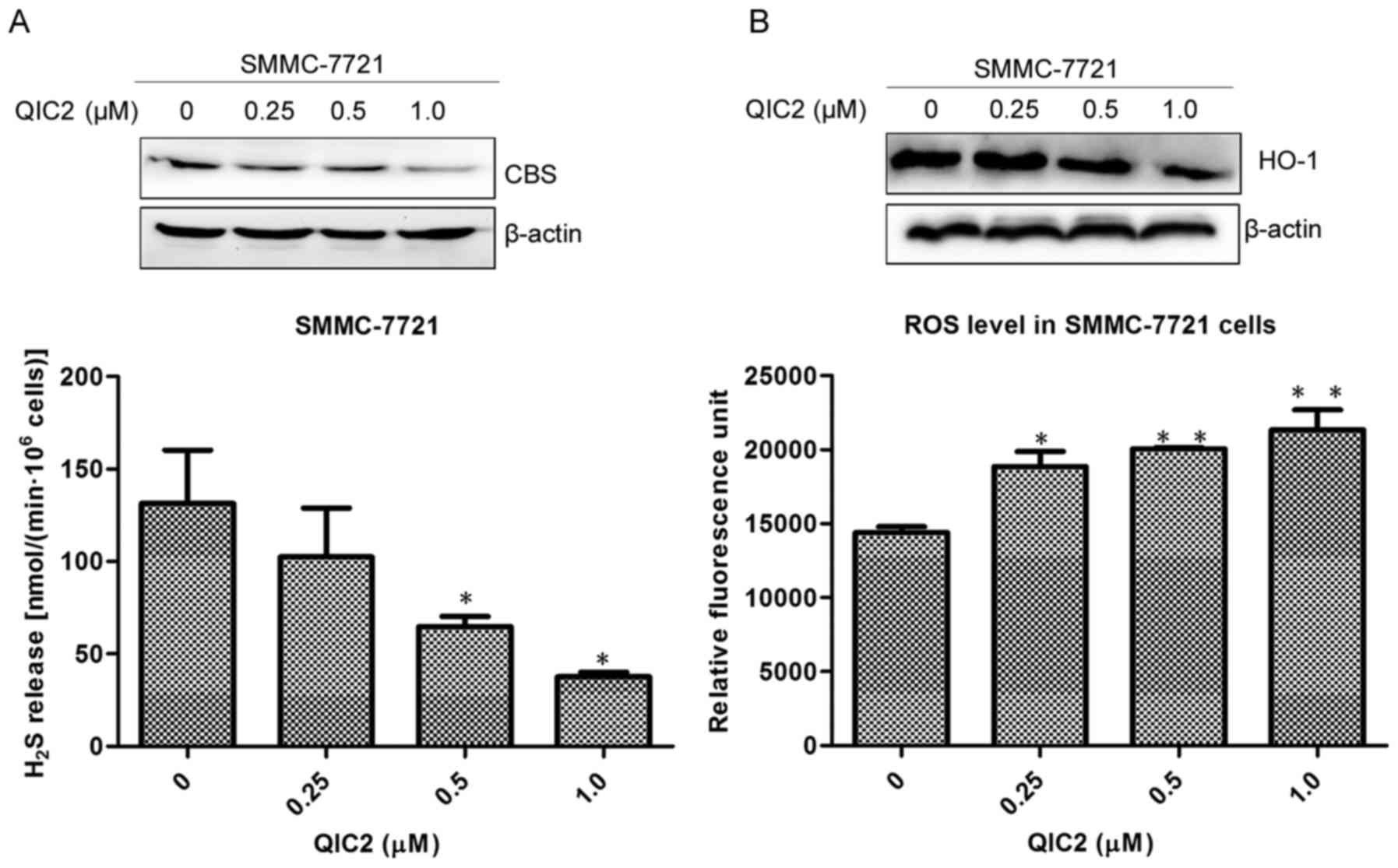
QIC2, we evaluated changes in the CBS/H2S system, HO-1
protein expression, ROS and GSH levels in the SMMC-7721 cells.
Downregulated CBS/H2S system, decreased HO-1 protein
level while increased ROS level were found in the SMMC-7721 cells
treated with QIC2 (Fig. 5A and B).
This was accompanied by a decreased GSH level (Fig. 5C), suggesting that QIC2 causes
oxidative stress in SMMC-7721 cells. Additionally, QIC2 was found
to activate caspase-3 and PARP in the SMMC-7721 cells (Fig. 5D). These results indicate that QIC2
induces human hepatoma cell apoptosis via increasing oxidative
stress and activating the caspase-3 cascade due to inhibition of
the CBS/H2S system.
Discussion
The CBS/H2S system is strongly expressed
in various ovarian and colon cancer cell lines and contributes to
cancer progression. In the present study, we demonstrated that CBS
protein was also highly expressed in human hepatoma cell lines
HepG2 and SMMC-7721. This phenomenon led us to believe that
CBS/H2S is involved in the proliferation of HCC cells.
Indeed, inhibition of the CBS/H2S pathway by AOAA or CBS
siRNA strongly suppressed the proliferation of the HepG2 and
SMMC-7721 cells. This additionally suggests that the
CBS/H2S pathway contributes to the excessive growth of
hepatoma cells with high intrinsic CBS expression levels.
Apoptosis is one of the major mechanisms involved in
the inhibition of cell proliferation or induction of cell death
(17–21), and involves proteomic variations,
including the Bcl-2 family, Bax and the caspase-3 cascade. In our
experiments, inhibition of the CBS/H2S system caused by
CBS siRNA induced the apoptosis of SMMC-7721 cells, pointing to the
involvement of the CBS/H2S pathway in modulating
hepatoma cell proliferation. In addition, we found that CBS
knockdown caused a distinct increase in the Bax/Bcl-2 ratio. While
in CBS-knockdown cells, caspase-3 and PARP activities were
significantly upregulated. All these observations were consistent
with the molecular indices of cell apoptosis. Therefore, we
conclude that inhibition of the CBS/H2S pathway causes
apoptosis via increasing the ratio of Bax/Bcl-2 and activating the
caspase-3 cascade.
Linking the CBS/H2S system with HO-1
expression and oxidative stress in cancer cells has not been
illustrated previously. Cancer cell apoptosis involves oxidative
stress while HO-1 plays vital antioxidant and anti-apoptosis roles.
Therefore, we assume that the CBS/H2S system is involved
in HO-1 expression and oxidative stress in hepatoma cells. Indeed,
we found that the downregulation of the CBS/H2S system
inhibited the expression of HO-1 and increased the oxidative stress
level in SMMC-7721 cells. Previous studies have shown that ROS
display a ‘two-faced’ character in cancer cells (22–24).
On the one hand, a basal level of ROS induces and sustains the
oncogenic phenotype of cancer cells. On the other hand, an
increased ROS level inhibits cancer progression. Oxidative stress
induced by increased ROS is a proverbial inducer of apoptosis in
human cancer cell lines (25–28).
In human hepatoma cells, we observed that inhibition of
CBS/H2S increased ROS production, a result similar to
the inhibition of CSE/H2S (29). These findings suggest that the
CBS/H2S system regulates oxidative stress and
consequently induces the apoptosis of hepatoma cells.
Thus, we demonstrated that the CBS/H2S
system with high intrinsic CBS expression levels is vital in
maintaining the proliferation of hepatoma cells. It is expected
that inhibition of the system restrains the excessive growth of
hepatoma cells and induces the process of mitochondrial apoptosis.
Based on CBS inhibitor screening assay, we identified a
quinolone-indolone conjugate compound QIC2 with a strong
suppressive effect on both CBS protein expression and hepatoma cell
proliferation. In terms of the mechanism involved in the modulation
of proliferation, QIC2 was found to induce cell apoptosis by
increasing oxidative stress and activating the caspase-3 cascade
via inhibition of the CBS/H2S system.
In conclusion, the CBS/H2S pathway is
vital for maintaining the proliferation of hepatoma cells. The
novel compound QIC2 can potently suppress the CBS/H2S
system to inhibit the excessive growth and proliferation of the
cells. Further investigation of the roles of CBS in liver tumors
and its relationship with pathological features of liver cancer may
advance the development of CBS and its inhibitors into effective
therapeutic approaches against liver cancer.
Acknowledgements
The authors would like to acknowledge the financial
assistance provided by a grant from the Key Science and Technology
Fund of Henan Province (nos. 142300410128 and 14A310025) in
China.
References
|
1
|
Coletta C, Papapetropoulos A, Erdelyi K,
Olah G, Módis K, Panopoulos P, Asimakopoulou A, Gerö D, Sharina I,
Martin E, et al: Hydrogen sulfide and nitric oxide are mutually
dependent in the regulation of angiogenesis and
endothelium-dependent vasorelaxation. Proc Natl Acad Sci USA.
109:9161–9166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Szabo C, Coletta C, Chao C, Módis K,
Szczesny B, Papapetropoulos A and Hellmich MR: Tumor-derived
hydrogen sulfide, produced by cystathionine-β-synthase, stimulates
bioenergetics, cell proliferation, and angiogenesis in colon
cancer. Proc Natl Acad Sci USA. 110:12474–12479. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kimura Y, Goto Y and Kimura H: Hydrogen
sulfide increases glutathione production and suppresses oxidative
stress in mitochondria. Antioxid Redox Signal. 12:1–13. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sheng J, Shim W, Wei H, Lim SY, Liew R,
Lim TS, Ong BH, Chua YL and Wong P: Hydrogen sulphide suppresses
human atrial fibroblast proliferation and transformation to
myofibroblasts. J Cell Mol Med. 17:1345–1354. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Popov D: An outlook on vascular hydrogen
sulphide effects, signalling, and therapeutic potential. Arch
Physiol Biochem. 119:189–194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yin P, Zhao C, Li Z, Mei C, Yao W, Liu Y,
Li N, Qi J, Wang L, Shi Y, et al: Sp1 is involved in regulation of
cystathionine γ-lyase gene expression and biological function by
PI3K/Akt pathway in human hepatocellular carcinoma cell lines. Cell
Signal. 24:1229–1240. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baskar R, Li L and Moore PK: Hydrogen
sulfide-induces DNA damage and changes in apoptotic gene expression
in human lung fibroblast cells. FASEB J. 21:247–255. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sivarajah A, Collino M, Yasin M, Benetti
E, Gallicchio M, Mazzon E, Cuzzocrea S, Fantozzi R and Thiemermann
C: Anti-apoptotic and anti-inflammatory effects of hydrogen sulfide
in a rat model of regional myocardial I/R. Shock. 31:267–274. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cao Y, Adhikari S, Ang AD, Moore PK and
Bhatia M: Mechanism of induction of pancreatic acinar cell
apoptosis by hydrogen sulfide. Am J Physiol Cell Physiol.
291:C503–C510. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bhattacharyya S, Saha S, Giri K, Lanza IR,
Nair KS, Jennings NB, Rodriguez-Aguayo C, Lopez-Berestein G, Basal
E, Weaver AL, et al: Cystathionine beta-synthase (CBS) contributes
to advanced ovarian cancer progression and drug resistance. PLoS
One. 8:e791672013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Szabo C, Coletta C, Chao C, Módis K,
Szczesny B, Papapetropoulos A and Hellmich MR: Tumor-derived
hydrogen sulfide, produced by cystathionine-β-synthase, stimulates
bioenergetics, cell proliferation, and angiogenesis in colon
cancer. Proc Natl Acad Sci USA. 110:12474–12479. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang R: Hydrogen sulfide: The third
gasotransmitter in biology and medicine. Antioxid Redox Signal.
12:1061–1064. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu YH, Wei XL, Hu GQ and Wang TX:
Quinolone-indolone conjugate induces apoptosis by inhibiting the
EGFR-STAT3-HK2 pathway in human cancer cells. Mol Med Rep.
12:2749–2756. 2015.PubMed/NCBI
|
|
14
|
Maclean KN, Janosík M, Kraus E, Kozich V,
Allen RH, Raab BK and Kraus JP: Cystathionine beta-synthase is
coordinately regulated with proliferation through a redox-sensitive
mechanism in cultured human cells and Saccharomyces
cerevisiae. J Cell Physiol. 192:81–92. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sanokawa-Akakura R, Ostrakhovitch EA,
Akakura S, Goodwin S and Tabibzadeh S: A H2S-Nampt
dependent energetic circuit is critical to survival and
cytoprotection from damage in cancer cells. PLoS One.
9:e1085372014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hua W, Chen Q, Gong F, Xie C, Zhou S and
Gao L: Cardioprotection of H2S by downregulating iNOS
and upregulating HO-1 expression in mice with CVB3-induced
myocarditis. Life Sci. 93:949–954. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Danial NN and Korsmeyer SJ: Cell death:
Critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fulda S and Debatin KM: Sensitization for
anticancer drug-induced apoptosis by the chemopreventive agent
resveratrol. Oncogene. 23:6702–6711. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu Z, Li D, Zheng X, Wang E and Wang J:
Selective induction of apoptosis: Promising therapy in pancreatic
cancer. Curr Pharm Des. 19:2259–2268. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kaufmann SH and Earnshaw WC: Induction of
apoptosis by cancer chemotherapy. Exp Cell Res. 256:42–49. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shan T, Ma Q, Zhang D, Guo K, Liu H, Wang
F and Wu E: β2-adrenoceptor blocker synergizes with gemcitabine to
inhibit the proliferation of pancreatic cancer cells via apoptosis
induction. Eur J Pharmacol. 665:1–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Circu ML and Aw TY: Reactive oxygen
species, cellular redox systems, and apoptosis. Free Radic Biol
Med. 48:749–762. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kuranaga Y, Yamada N, Kashiwaya M,
Nakamura M, Cui L, Kumazaki M, Shinohara H, Sugito N, Taniguchi K,
Ito Y, et al: Anti-oncogenic gem-dihydroperoxides induce
apoptosis in cancer cells by trapping reactive oxygen species. Int
J Mol Sci. 17:E712016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
He G, He G, Zhou R, Pi Z, Zhu T, Jiang L
and Xie Y: Enhancement of cisplatin-induced colon cancer cells
apoptosis by shikonin, a natural inducer of ROS in vitro and in
vivo. Biochem Biophys Res Commun. 469:1075–1082. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Manna A, Saha P, Sarkar A, Mukhopadhyay D,
Bauri AK, Kumar D, Das P, Chattopadhyay S and Chatterjee M:
Malabaricone-A induces a redox imbalance that mediates apoptosis in
U937 cell line. PLoS One. 7:e369382012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ahamed M, Akhtar MJ, Raja M, Ahmad I,
Siddiqui MK, AlSalhi MS and Alrokayan SA: ZnO nanorod-induced
apoptosis in human alveolar adenocarcinoma cells via p53, survivin
and bax/bcl-2 pathways: Role of oxidative stress. Nanomedicine.
7:904–913. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mileo AM and Miccadei S: Polyphenols as
modulator of oxidative stress in cancer disease: New therapeutic
strategies. Oxid Med Cell Longev. 2016:64756242016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee G, Oh TI, Um KB, Yoon H, Son J, Kim
BM, Kim HI, Kim H, Kim YJ, Lee CS, et al: Small-molecule inhibitors
of USP7 induce apoptosis through oxidative and endoplasmic
reticulum stress in cancer cells. Biochem Biophys Res Commun.
470:181–186. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pan Y, Ye S, Yuan D, Zhang J, Bai Y and
Shao C: Hydrogen sulfide (H2S)/cystathionine γ-lyase
(CSE) pathway contributes to the proliferation of hepatoma cells.
Mutat Res. 763–764:10–18. 2014. View Article : Google Scholar
|