Introduction
Angiogenesis, the growth of new capillary blood
vessels, is a normal and vital process in growth, development and
wound healing. However, it is also a fundamental step in the growth
of tumours. Nutrients and oxygen, supplied by the blood vessels
into the tumours, are essential for the growth and progression of
malignant tumours beyond the size of 1–2 mm3 (1). Newly formed blood vessels can
facilitate cells escaping through leakage from primary tumour sites
to metastasize, the major cause of mortality for cancer patients.
Anti-angiogenesis has been recognized as valuable therapy in
treatment of various metastatic cancers since the theory was first
proposed by Folkman (2,3). Tumour angiogenesis is believed to be
regulated by the interactions between pro-angiogenic and
anti-angiogenic factors in the tumour microenvironment (4). Angiogenic response is a dynamic
process requiring a series of fine-tuned angiogenic signalling and
molecular events. Hypoxia is the common inducer of angiogenesis in
the core of large tumours stimulating the release of pro-angiogenic
factors to promote endothelial cell proliferation, migration,
differentiation and self-assembly into vascular-like structures.
Subsequently, perivascular cells are recruited to form mature and
stable vessels (5).
The interaction between endothelial cells (ECs) and
pericytes (PVCs) has gained increasing attention as a central
process in the regulation of blood microvascular structures as well
as in their stabilization and maturation. Aberrant interplays
between the two cell types have been observed in a multitude of
human pathological conditions, including cancer angiogenesis
(6). Targeting one cell type,
either ECs or PVCs may produce limited effects. The most effective
therapies will probably involve targeting multiple mediators, and
will require improving the efficiency of drug delivery to the
tumour microenvironment (7,8). Therefore, improving the therapeutic
response will require consideration of various signalling pathways
and cell types involved in the vascular component of cancer.
Hypoxia-inducible factor-1 (HIF-1α) dependent
hypoxia-induced response is tightly controlled by HIF-prolyl
hydroxylase domain (PHD) which targets HIF-1α for degradation.
Oxygen-dependent PHDs negatively regulate HIFs and, crucially,
confer its oxygen sensitivity. In the presence of oxygen, PHD2
hydroxylates HIF-1α on two specific proline residues, which results
in its destruction. In hypoxia, PHD2 is missing its co-substrate
(oxygen), rendering it inactive. HIF-1α then becomes stabilized,
and results in the upregulation of angiogenic factors such as
vascular endothelial growth factor (VEGF), fibroblast growth factor
(FGF)-2 and angiopoietin-2 thereby promoting neovascularization.
Hypoxia also inactivates PHDs, causing accumulation of HIF-1α which
in turn further transactivates PHDs. This feedback loop ensures the
homeostasis of HIF-1α activity caused by hypoxia (9,10).
After VEGF is released from tumour cells, it binds
to two cognate VEGF receptors, VEGF receptor 1 (VEGFR1) and VEGF
receptor 2 (VEGFR2/KDR/flk-1), which are expressed on local
vascular ECs (11). Signalling
through VEGFR1/2 drives the process of angiogenesis, which involves
dissolution of the vascular basement membrane, endothelial cell
proliferation and formation of new blood vessels (12). The binding to VEGF receptor is a
crucial step in initiation of EC proliferation, migration and
differentiation during angiogenesis (13,14).
The VEGF signalling system has been suggested as a highly
‘druggable’ target and potent inhibitors of the VEGF signalling
pathway have been used clinically including bevacizumab, sunitinib
and sorafeib (15–17). To date, targeting the HIF/VEGF-VEGFR
axis has been a promising strategy for cancer therapy (18). Anti-VEGF-VEGFR therapies may also
have immunological effects (19).
Since HIF signalling contributes to the acquisition of resistance
against anti-VEGF therapy, the combined blockade of VEGF and HIF-1α
is being explored as a cancer treatment strategy (5). Despite the indisputable success of
anti-angiogenic drugs in the clinical treatment for some advanced
solid cancers, there are difficulties with regard to the control of
the activities of these drugs and of the identification of patients
who are sensitive to them (20).
Epidemiological studies have demonstrated that
cruciferous vegetables can reduce the risk of various types of
cancers in humans (21,22). Sulforaphane (SFN) is one of the most
extensively studied isothiocyanates (ITCs) from broccoli and
cauliflower. SFN has been found to suppress tumour cell growth
through multiple molecular mechanisms including induction of cell
cycle arrest and apoptosis in many types of tumour cells (23–26).
SFN has been shown to inhibit ECs proliferation via apoptosis and
autophagy (27–29) and suppress VEGF and MMP-2 expression
(30,31), the latter being associated with the
inhibition of FOXO1/AKT pathways (32). Moreover, SFN is a known inducer of
both thioredoxin reductase (TrxR1) and thioredoxin (26,33),
which are involved in inflammation, apoptosis, angiogenesis,
embryogenesis and cardiovascular disease involved in angiogenesis
(34). The molecular mechanisms of
SFN suppression of angiogenesis, in particular the effects on
signalling pathways between ECs and pericytes in response to SFN
treatment, are not fully understood. In the present study a
coculture of primary human umbilical vein endothelial cells
(HUVECs) and pericytes in a 3D collagen gel model were used to
dissect the mechanism by which SFN interacts with crosstalk between
these two key cell types in angiogenesis. Understanding the
interactions between HUVECs and pericytes under SFN treatment may
contribute to the development of novel agents in anti-angiogenetic
therapy.
Materials and methods
Reagents
SFN (4-methylsulfinylbutyl isothiocyanate) was
purchased from Toronto Research Chemicals, Inc. (North York, ON,
Canada). High concentration rat tail type-I collagen solution and
purified mouse anti-human CD31/PECAM (555444) antibody were both
purchased from BD Biosciences (Oxford, UK). Polyclonal donkey
anti-mouse Cy3 was purchased from Abcam (Cambridge, UK). Growth
factors PDGF-BB and bFGF were obtained from Gibco/Life Technologies
(Paisley, UK). The primary antibodies against HIF-1α (ab2185), VEGF
(sc7269), Flk1 (sc6251), TrxR1 (sc20147 were purchased from Santa
Cruz Biotechnology (Heidelberg, Germany). The primary antibodies
against PHD1 (ab80361) and PHD2 (ab83560) were purchased from
Abcam. Secondary antibodies were from Santa Cruz Biotechnology.
siRNA for TrxR1 and AllStars (AS) negative control were all
purchased from Qiagen (West Sussex, UK). Electrophoresis and
western blotting supplies were obtained from Bio-Rad Laboratories
(Hemel Hempstead, UK) and the chemiluminescence kit was from GE
Healthcare (Little Chalfont, UK).
Cell culture
HUVECs were obtained from TCS Cellworks and used
between passages 2 and 8 for all experiments. The cells were grown
in plastic flasks pre-treated with 10 µg/ml type-I collagen (BD
Biosciences) in phosphate-buffered saline (PBS) for 30 min in 37°C
incubator. Endothelial growth medium-EGM2 (C22011; PromoCell,
Birmingham, UK) with supplements was used as culture medium for
HUVECs according to the manufacturers protocols. Murine
perivascular cells (PVC) were isolated as previously described and
used between passages 32 and 38 (35). Pericytes were routinely incubated in
Dulbeccos modified Eagles medium (DMEM) containing 10% fetal bovine
serum (FBS). All cells were incubated at 37°C in 95% humidified air
containing 5% CO2.
Cell viability assay
The MTT assay was used to examine the toxicity of
SFN in HUVECs and pericytes. Cells were seeded in 96-well plates
and cultured in an incubator at 37°C to ~70–80% confluence.
Cultured cells were treated with concentrations (1.25–160 µM) of
SFN or DMSO (0.1% as control) for 24 h with four replicate wells
per treatment. After all treatments, the medium was removed and
fresh medium (100 µl) was added together with 10 µl MTT solution (5
mg/ml), then incubated at 37°C for 1 h to allow the MTT to be
metabolized. The formazan produced was re-suspended in 100 µl of
dimethyl sulfoxide (DMSO)/well. The final absorbance in the wells
was quantified using a microplate reader (BMG Labtech Ltd.,
Aylesbury, UK) at a test wavelength of 570 nm and a reference
wavelength of 670 nm. Viability of treated cells was expressed as a
percentage of control as follows: (A570 nm-A670 nm) sample/(A570
nm-A670 nm) control×100. The IC50 was determined using
CalcuSyn software version 2.0 (Biosoft, Cambridge, UK).
3D co-culture in collagen gel
Capillary-like tube formation in 3D collagen
matrices of co-culture with HUVEC and pericytes was used to test
the angiogenic effects of SFN. Collagen type I gels (2 mg/ml) were
prepared in 1X DMEM medium from concentrated rat tail type I
collagen solution (>8 mg/ml in 0.02 M acetic acid; BD
Biosciences) at 4°C, supplemented with final concentrations of 2%
FBS, 22.5 mM NaHCO3, 1 mM sodium pyruvate and
neutralized with 0.1N NaOH according to the suppliers instructions.
Cells were trypsinised, washed with PBS, counted and desired cell
numbers were collected by centrifugation. Cells were suspended in
the collagen I gel solution at 4°C and 400 µl suspension added per
well into 24-well plates. After an initial incubation at 37°C for
20 min, 400 µl EGM2 culture medium containing SFN (0.6–20 µM) or
0.1% DMSO (control) was added to solidified collagen I gels with
supplements to achieve final concentrations of 10 ng/ml for VEGF
and PDGF, respectively, and 250 µg/ml ascorbic acid phosphate.
Typical experiments contained 2.5×105 HUVEC and/or
0.5×105 PVC/well, unless otherwise stated. Medium was
changed every 24 h and cultures were maintained for up to 5
days.
Immunostaining of HUVEC-pericyte
coculture model
Immunohistochemical analyses of cell cultures were
performed as described by Brachvogel et al (36) and Zhou et al (37). Whole mount immunohistochemistry of
3D collagen cultures was performed as described by Bader et
al (38). Briefly, gels were
washed in PBS, fixed with 80% methanol/20% DMSO for 30 min at 20°C
(or 16 h at 4°C), then rehydrated in 50% methanol/PBS, 20%
methanol/PBS and PBS-T (PBS, 0.1% Tween-20) for 1 h each and then
incubated with blocking buffer (10% FBS, 5% BSA in PBS) for 2–4 h
at room temperature or 16 h at 4°C. Gels were incubated with
primary antibodies in blocking buffer for 16 h at 4°C and then
washed 7 times for 1 h each in TBS-T (TBS, 01% Tween-20) followed
by incubation with fluorescently labelled secondary antibodies in
blocking buffer for 2–16 h and then washed again as described
above. After nuclear staining, samples were mounted in Gelvatol and
examined by fluorescence microscopy (SteREO LumarV12 and Axioplan2;
Carl Zeiss, Oberkochen, Germany). Pictures of fluorescent signals
were captured by a black-white camera and colour-coded by
AxioVision software (version 4.5).
Western blot analysis of protein
expression
HUVECs and PVC cells were treated with SFN (1–20 µM)
or DMSO (0.1% as control) at 70–80% confluence. Total protein was
extracted by washing cells twice with ice-cold PBS and harvested by
scraping in 20 mM Tris-HCl (pH 8.0), 150 mM NaCl, 2 mM EDTA, 10%
glycerol, 1% Nonidet P-40 (NP-40) containing mini-complete
proteinase inhibitor. The cell suspension/lysate was placed in an
ice bath for 20 min and then centrifuged at 12,000 × g for 15 min
at 4°C. Protein concentrations were determined using the Brilliant
Blue G dye-binding assay of Bradford using BSA as a standard.
Equivalent aliquots of protein were mixed with 4X SDS-PAGE sample
buffer and DTT reducing agent (to 50 nmol/l) and were heated to
95°C for 5 min. Equal amounts of samples were loaded onto SDS-PAGE
gel and subsequently transferred to PVDF (polyvinylidene
difluoride) membranes (Bio-Rad Laboratories). The membrane was
washed three times for 45 min with PBST and then incubated with the
secondary antibody diluted with 5% milk in PBST for 1 h. After
further washing the membrane three times for 45 min with PBST,
antibody binding was determined by a chemiluminescence detection
kit and densitometry was measured by Fluor ChemImager (Alpha
Innotech, San Leandro, CA, USA).
Statistical analysis
All experiments were independently repeated at least
three times. Data are means ± SD. Students t-test was applied for
differences between groups using SPSS software. Significant
differences among groups were calculated and P<0.05 was viewed
as statistically significant.
Results
Effect of SFN on cell proliferation
and viability in HUVECs and PVC
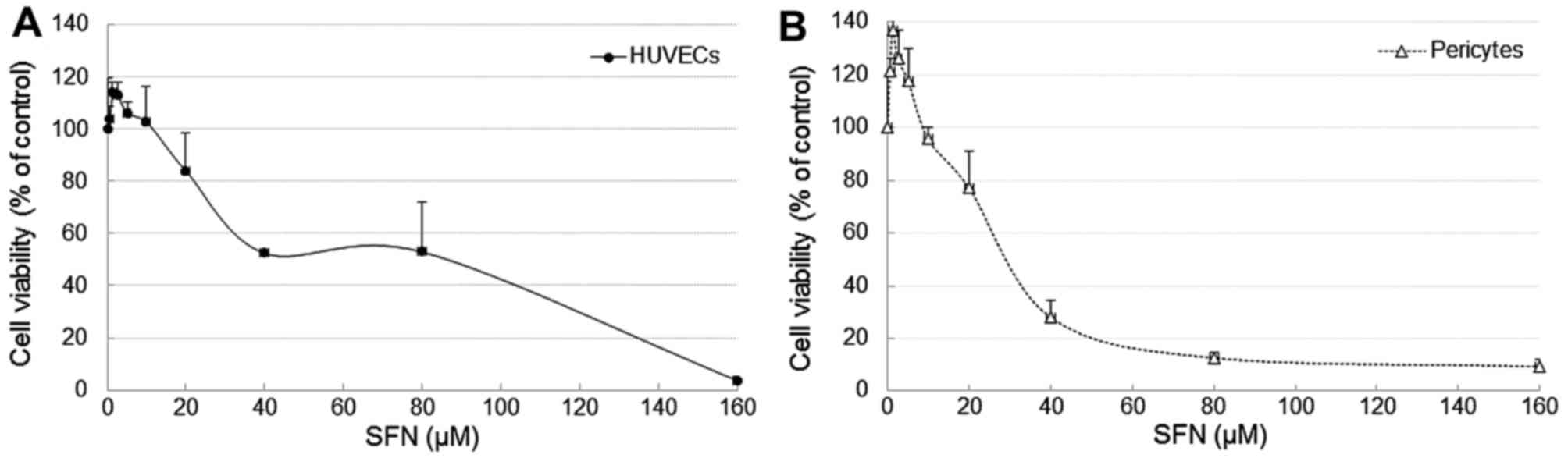
As a viable and adequate population of HUVECs and
PVC is essential for angiogenesis, the effect of SFN on cell
proliferation and viability of both cell types were measured by
treating the cells with SFN over a range of concentrations
(0.625–160 µM) for 24 and 48 h followed by an MTT assay. At low
doses, from 0.625 to 5 µM, SFN has no toxic effect on cell
viability. SFN at 2.5 µM (24 h) promoted cell proliferation to 116
and 136% in HUVECs and PVC, respectively (Fig. 1). However, a dose-dependent effect
on cell viability was observed following treatments with SFN
between 20 and 160 µM, i.e. cell viability decreased to 83.8% and
<10% respectively, IC50, 46.7 µM. In parallel, the
influence of SFN on PVC was also determined, and a similar
dose-dependent effect on cell viability was observed. In both cell
types, there was no significant difference in cell viability after
exposure to 10 µM SFN at 24 and 48 h although the PVCs are slightly
more sensitive to SFN with IC50, 32.4 µM. Based on these
results, 10 µM SFN was chosen as an optimum dosage for the tube
formation experiments and 1–20 µM were used for mechanistic
studies.
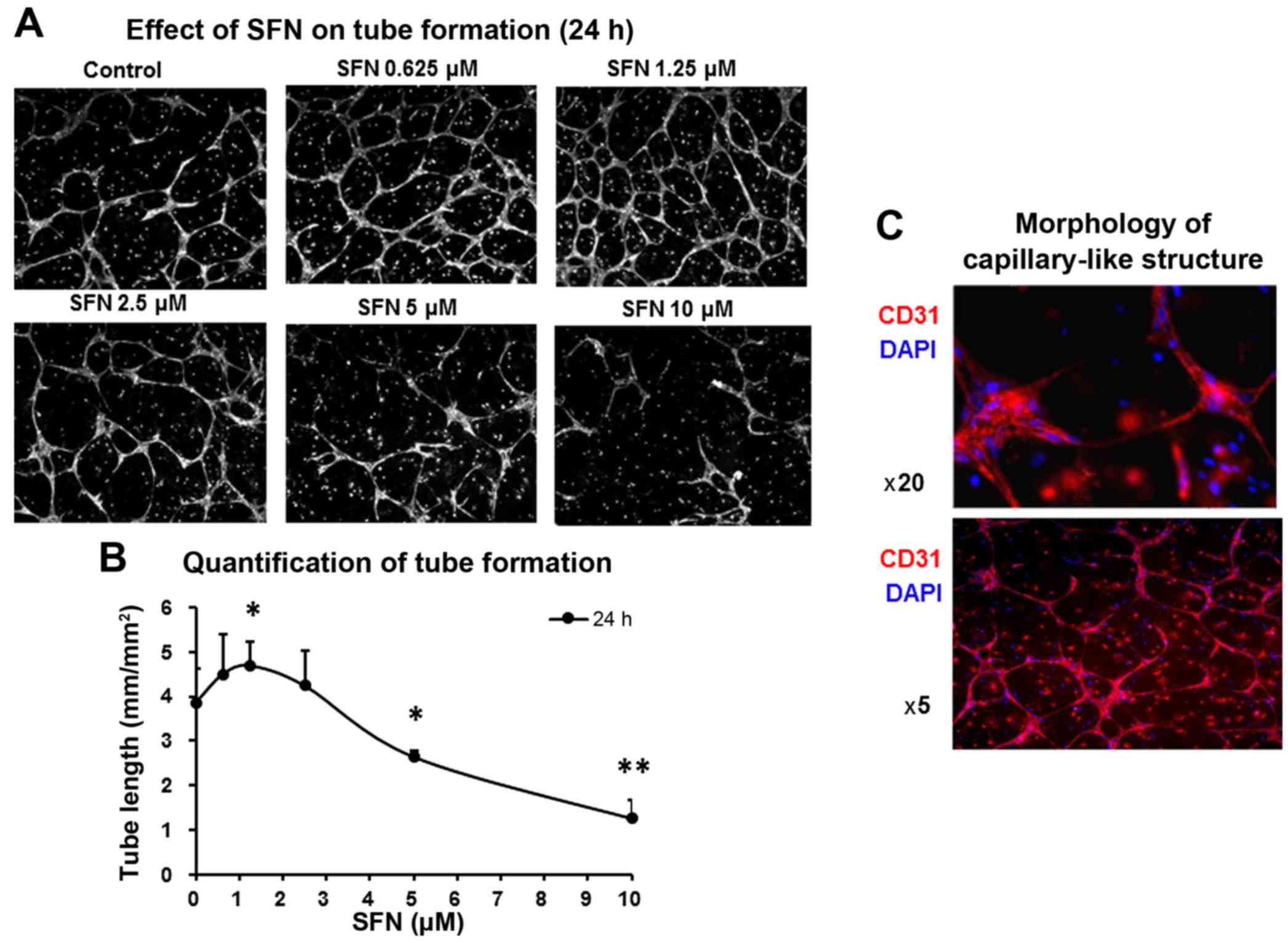
SFN suppresses capillary formation in
3D collagen model of HUVECs and PVC co-culture
The tube formation assay in 3D collagen gel is a
well-established procedure for the evaluation of angiogenic
capacities. To detect the angiogenic effect of SFN, a co-culture
model consisting of HUVECs and pericytes was used to mimic more
realistically the in vivo angiogenic process. Angiogenic
growth factors were present in the collagen matrix in order to more
closely represent a tumour microenvironment to promote
capillary-like tube formation through ECs alignment with supporting
pericytes. In the vehicle control (0.1% DMSO), HUVECs and pericytes
formed robust capillary structures. The addition of SFN to the
growth medium of the co-culture collagen gel, led to a
concentration-dependent disruption of the tube structure. The
formation of tube structures were only partially inhibited at
moderate SFN concentrations (2.5–10 µM) whilst higher dosages (20
µM) completely inhibited the formation of tube structures (Fig. 2A). Notably, SFN at low
concentrations from 0.625 to 1.25 µM promoted the formation of
tubes by 115–120% of the control, i.e. total tube length was 3.86
mm/mm2 in control and 4.69 mm/mm2 in SFN
(1.25 µM) treated cells (Fig. 2B).
Immunostaining of control cells with anti-CD31 and DAPI showed
capillary-like morphology in the untreated HUVECs and PVCs
(Fig. 2C).
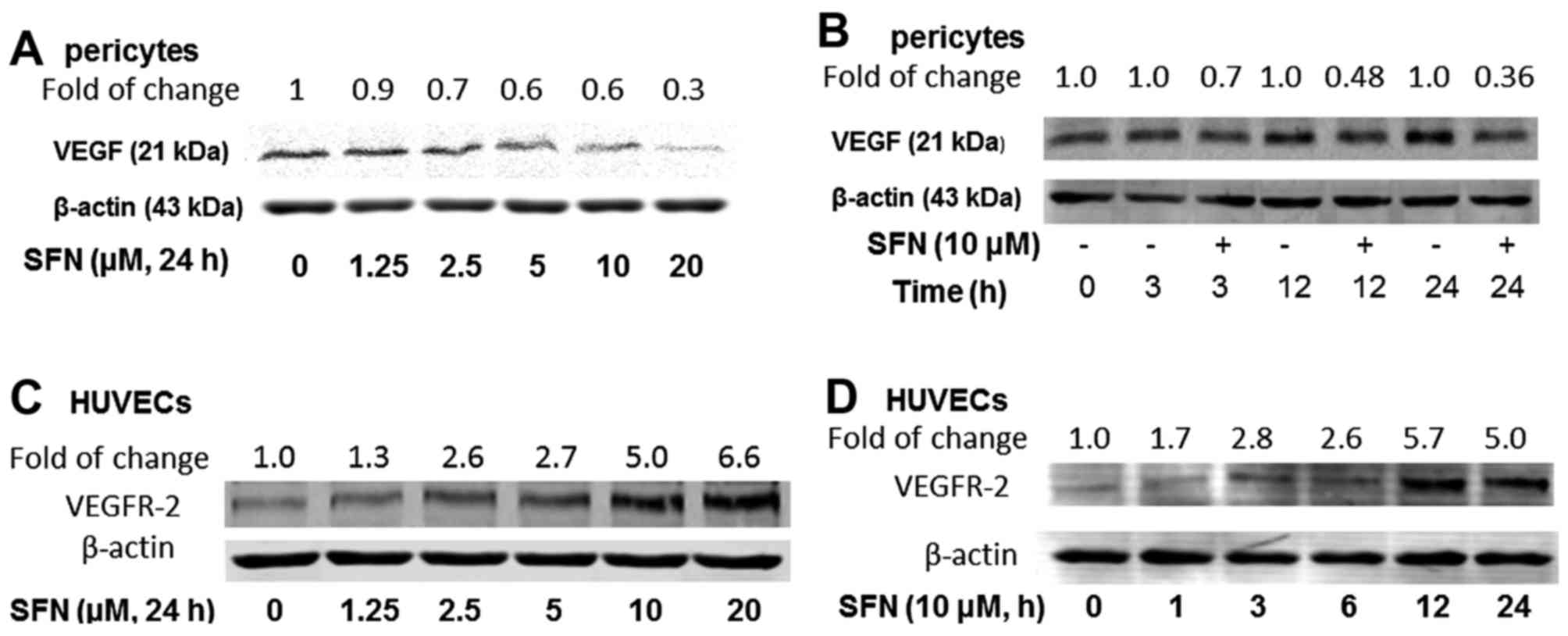
Effect of SFN on VEGF and VEGFR2
expression
To evaluate whether SFN inhibits VEGF expression,
PVC were cultured in EGM2 medium for 24 h followed by a
dose-dependent (1.25–20 µM), or a time course (0–24 h) of treatment
with SFN (10 µM). Western blot analysis for VEGF protein expression
demonstrated that SFN inhibits VEGF in PVC in a dose- and
time-dependent manner (Fig. 3A and
B). SFN inhibited VEGF expression to 60 and 30% of the control
after the treatments with 5 and 20 µM SFN (24 h), respectively. An
inhibitory effect of 10 µM SFN was also observed after 3 h (70% of
control), 12 h (48% of control) and 24 h (36% of control) treatment
(Fig. 2B). However, VEGF was not at
detectable level in similarly treated HUVECs (data not shown). To
further investigate VEGF and its receptor signalling pathway, the
effect of SFN on VEGFR2 protein expression was determined. Not
surprisingly, PVCs do not express detectable levels of VEGFR2 (data
not shown), whilst HUVECs showed a significant upregulation of
VEGF-R2 level after SFN treatment (Fig.
3C and D).
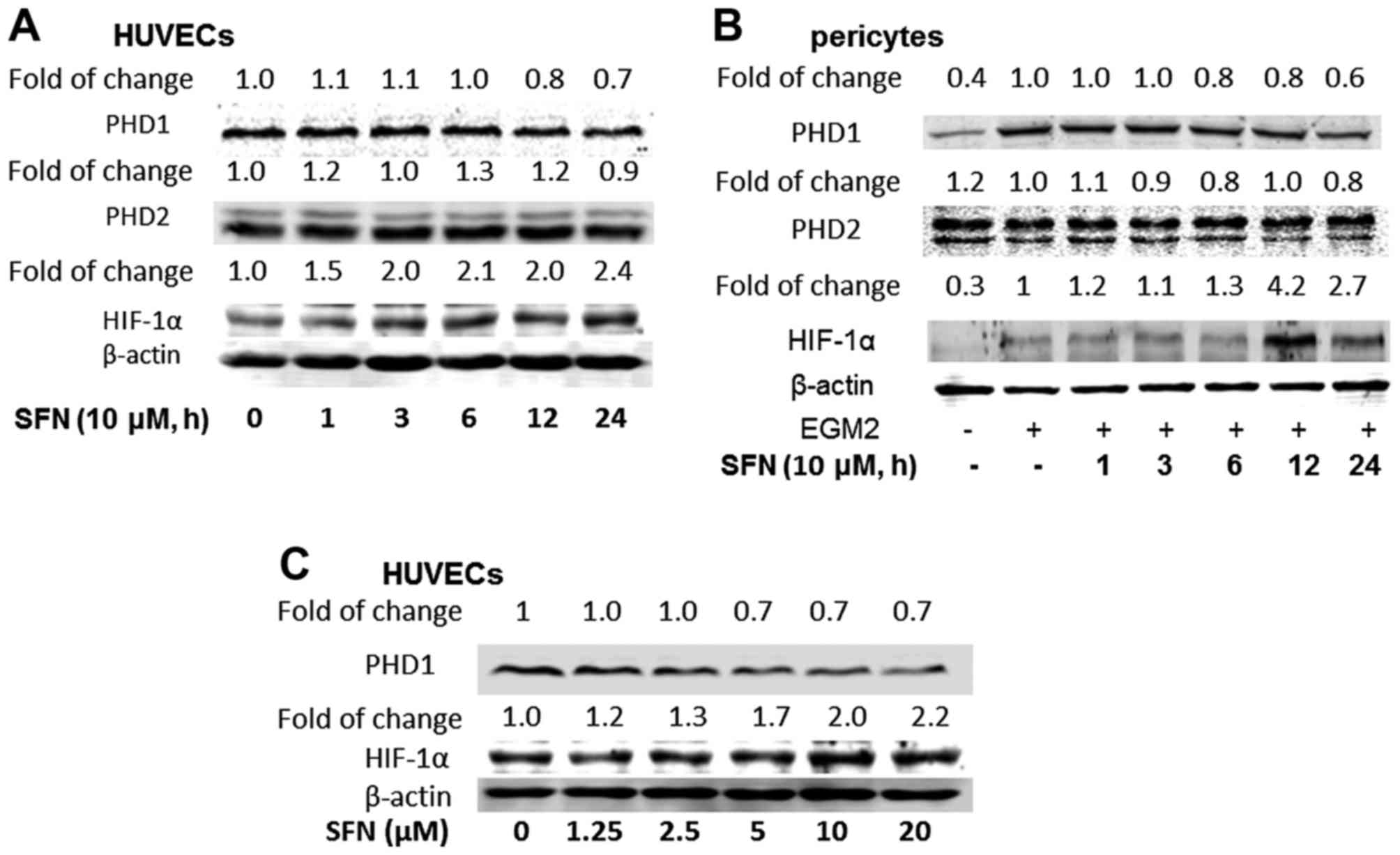
Differential regulation of HIF
signalling pathway after SFN exposure
HIF pathway is an important signaling pathway for
HIF secretion especially in tumour micro-environments. In the
present study, HIF-1α, PHD1 and PHD2 protein expression in both
HUVECs and PVC were quantified by western blot assay. Both cell
types were treated with 10 µM SFN for different time periods (1, 3,
6, 12 and 24 h) and HIF-1α and the relative expressions in both
types of cells were measured. SFN increased HIF-1α expression in
both cell types, i.e. 2.0- to 2.4-fold in HUVECs and 4.2–2.7-fold
in PVC at 12 and 24 h, respectively (Fig. 4A and B). As expected, the effect of
SFN on the expression of PHD1 was suppressed by SFN, 30% in HUVECs
and 40% in pericytes at 24 h of treatment. Moreover, increasing SFN
concentration in HUVECs from 1.25 to 20 µM for 24 h, had no
significant effect on PHD1 protein expression, but again increased
HIF-1α expression (Fig. 4C).
Activation of HIF-1α may associate with the low-dose promotion
effect of SFN on cell growth.
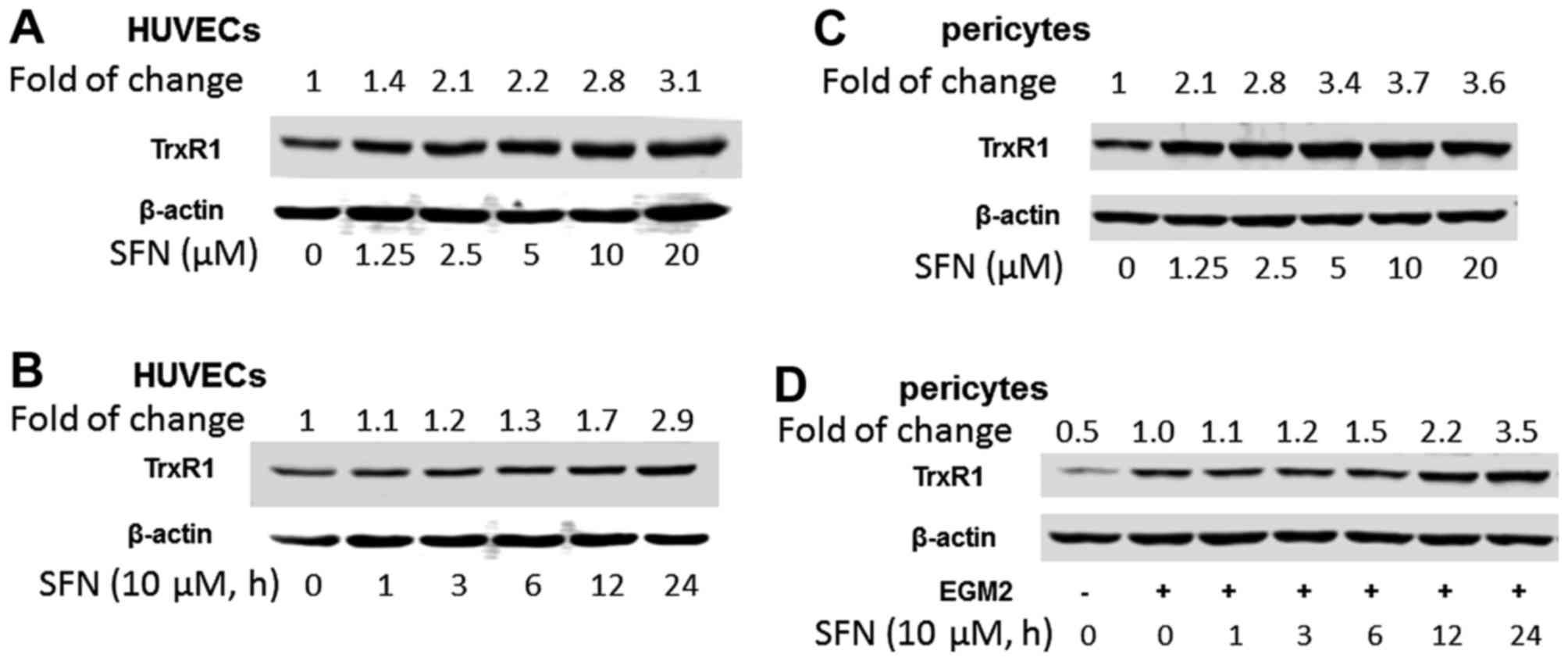
Effect of SFN on TrxR1 expression
Inhibition of thioredoxin reductase (TrxR1) has been
shown to regulate angiogenesis by increasing endothelial
cell-derived VEGF (39). SFN
increased TrxR1 expression in both HUVECs and PVC dose- and
time-dependently, i.e. SFN at 10 µM induced TrxR1 2.8- and 3.7-fold
in HUVECs (Fig. 5A and B) and PVC
(Fig. 5C and D), respectively.
TrxR1 plays an important role in SFN inhibition of angiogenesis.
Knockdown of TrxR-1 abolished the downregulation of PHD1 by SFN
treatment (10 µM, 24 h), suggesting a deficiency of TrxR-1 may
cause the accumulation of PHD1. However, knockdown of TrxR1 only
attenuated ~20% of the SFN-inhibited tube formation (data not
shown) suggesting that the inhibition of tube formation by SFN is
only partly TrxR1-dependent.
Discussion
In the present study, we demonstrated that SFN
exhibited differential effect on the regulation of angiogenesis in
ECs and pericytes which may interrupt the crosstalk mechanism
between them leading to reduced angiogenic capacity. Conventional
planar cultures fail to recreate the in vivo physiology of
the microvasculature with respect to 3D geometry (lumens and axial
branching points) and interactions of endothelium with perivascular
cells, extracellular tissue and blood flow (40,41).
There is strong evidence that the 3D in vitro model
consisting of multiple stromal cells is not only cheaper but also
provides quicker results than animal models. Additionally, 3D
models are more analogous to pathological progression of
angiogenesis than 2D cell culture models. Herein, we set up a 3D
collagen model of HUVECs and pericytes in co-culture with growth
factors to closely mimic the angiogenesis process in vivo
microenvironment, and to allow the detection of the effects of
dietary bioactives such as SFN, and the delineation of the
molecular mechanisms of SFN in the interactions between HUVEC and
PVCs.
SFN affected the network structure of
neovascularization, causing disruption at concentrations >5 µM
dose- and time-dependently. A marked increase in microvessel
formation at lower doses between 0.625–1.25 µM was also observed.
This bell-shaped effect (hormesis) is common in anticancer agents
(15,42) which suggests the importance of
maintaining the SFN at high concentrations (>5 µM) for cancer
chemoprevention or treatment. These tube formation results were
consistent with the cell viability data that showed that SFN
inhibited the growth of ECs at concentrations of 5–20 µM while at
low concentrations of 0.625–2.5 µM, SFN promoted cell growth. Our
findings suggest that the multi-targeted effect of SFN in
EC-pericyte coculture model and on proliferation of ECs,
demonstrates that its mechanism of action is complex. The biphasic
effects of SFN on angiogenesis indicate that lower concentrations
may provide benefit in conditions where the formation of an
inadequate number of new blood vessels prevent an adequate blood
supply, such as many cardiovascular diseases. In contrast, high
concentrations of SFN (in the present study, 5–20 µM) are needed
for the anti-angiogenic effects.
At the level of protein expression, SFN treatment
was found to inhibit VEGF secretion from pericytes but upregulate
VEGF receptor-2 expression in ECs. VEGF, one of the major
angiogenesis factors, is induced in growing tumours and stimulates
EC proliferation and migration primarily through the VEGFR2
(Flk1/KDR) pathway (43). HIF-1 is
overexpressed in many human cancers and it regulates the expression
of VEGF. Yao et al (23)
demonstrated the inhibitory effect of SFN on HIF-1α and VEGF
expression in human SCCs and prostate cancer cells via multiple
pathways. Bertl et al (31),
reached a similar conclusion in their study. The HIF pathway is
also responsible for acquisition of resistance against anti-VEGF
therapy. Our results contradict some other findings that SFN was
found to have unique character by activating HIF-1α pathway in both
HUVECs and PVC but supressing VEGF expression in PVC. The exact
mechanism is not clear but early research has revealed other
transcription factors are also involved in VEGF expression, such as
Sp1/Sp3, AP2, Egr-1 and STAT-2 (44). For example, SFN was found to be able
to inhibit STAT and SP1 pathways (45,46).
Our finding may suggest that SFN has a potential role in
attenuating anticancer resistance.
TrxR1, an Nrf2-driven selenoprotein, was upregulated
by SFN and may increase the anti-oxidant and redox regulation
capability. The increase in TrxR1 expression following SFN
treatment (10 µM) was found time- and dose-dependently (Fig. 4).
TrxR1 is involved in cell proliferation, redox
regulation of gene expression and signal transduction, protection
against oxidative stress, anti-apoptotic functions and regulation
of the redox state of the extracellular environment (47). Upregulation of TrxR1 plays a role in
protection against free-radical mediated cell death (48). In this study, knockdown of TrxR1
attenuated ~20% SFN inhibition of tube formation, and co-treatment
of HUVECs/PVC with SFN and N-acetylcysteine (NAC) at 2 mM abolished
the promoting effect of low dose SFN on tube formation (data not
shown). This indicates the involvement of reactive oxygen species
(ROS) (49,50). However, excessive ROS production may
play a role in microvascular instability (51).
It is well accepted that angiogenesis is a critical,
rate-limiting step in the development of cancers and its inhibition
suppresses tumour growth, progression and metastases.
Antiangiogenic therapy using dietary compounds represents a new
cost-effective approach to the early intervention and prevention of
cancer (52). There are many
natural and synthetic antioxidants such as curcumin, tea
polyphenols and vitamins E and C that possess anti-angiogenic
activities (53) and there is
significant potential in the further investigation of their
interactions in modulation of angiogenesis. Dietary isothiocynates
such as SFN, derived from glucoraphanin from cruciferous
vegetables, have attracted much attention for their potential to
prevent various types of cancers. SFN may protect endothelium from
oxidative stress by inducing TrxR1 expression and activity and also
by suppressing the activation of MAPKs (38,54).
When 5–20 µM SFN was used, the expression of TrxR1 was increased
and this is associated with resistance to inflammation (55). The downregulation of angiogenic
signalling pathways by SFN could offer a new therapeutic strategy
in suppressing malignant tumour progression, especially if multiple
molecules were targeted and suppressed in cells responsive to SFN
potentiated modulation of the angiogenic process. The results from
the present study strongly indicate a potential role of SFN at
higher doses in anti-angiogenesis cancer therapy. The action of SFN
in targeting HIF, PHD and VEGF in the interplay between ECs and
pericytes may shed insights into new treatment strategies.
Therefore, the therapeutic potential of SFN as a potential
alternative anti-VEGF agent in chemoprevention is worthy of further
exploration in animal models and small scale human trials.
Acknowledgements
The authors are grateful to Mr. Jim Bacon for
careful reading of the manuscript. The present study was supported,
in part, by an award from the Cancer Prevention Research Trust, UK
and an award from the National Natural Science Foundation of China
(NSFC no. 81372612).
Glossary
Abbreviations
Abbreviations:
|
DMSO
|
dimethyl sulfoxide
|
|
ECs
|
endothelial cells
|
|
EGM-2
|
endothelial growth medium-2
|
|
FBS
|
detal bovine serum
|
|
HIF-1α
|
hypoxia-inducible factor-1α
|
|
HUVECs
|
human umbilical vein endothelial
cells
|
|
ITCs
|
isothiocyanates
|
|
MTT
|
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium
bromide
|
|
Nfr2
|
NF-E2-related factor 2
|
|
PHD
|
prolyl hydroxylase
|
|
PVC
|
pericytes
|
|
ROS
|
reactive oxygen species
|
|
SFN
|
sulforaphane
|
|
TrxR1
|
thioredoxin reductase 1
|
|
VEGF
|
vascular endothelial growth factor
|
|
VEGFR
|
vascular endothelial growth factor
receptor
|
References
|
1
|
Folkman J: Role of angiogenesis in tumor
growth and metastasis. Semin Oncol. 29:(Suppl 16). 15–18. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sharma RA, Harris AL, Dalgleish AG,
Steward WP and OByrne KJ: Angiogenesis as a biomarker and target in
cancer chemoprevention. Lancet Oncol. 2:726–732. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hanahan D and Folkman J: Patterns and
emerging mechanisms of the angiogenic switch during tumorigenesis.
Cell. 86:353–364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carmeliet P and Jain RK: Molecular
mechanisms and clinical applications of angiogenesis. Nature.
473:298–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Armulik A, Abramsson A and Betsholtz C:
Endothelial/pericyte interactions. Circ Res. 97:512–523. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bergers G, Song S, Meyer-Morse N,
Bergsland E and Hanahan D: Benefits of targeting both pericytes and
endothelial cells in the tumor vasculature with kinase inhibitors.
J Clin Invest. 111:1287–1295. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Weis SM and Cheresh DA: Tumor
angiogenesis: Molecular pathways and therapeutic targets. Nat Med.
17:1359–1370. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Metzen E, Stiehl DP, Doege K, Marxsen JH,
Hellwig-Bürgel T and Jelkmann W: Regulation of the prolyl
hydroxylase domain protein 2 (phd2/egln-1) gene: Identification of
a functional hypoxia-responsive element. Biochem J. 387:711–717.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Berra E, Richard DE, Gothié E and
Pouysségur J: HIF-1-dependent transcriptional activity is required
for oxygen-mediated HIF-1alpha degradation. FEBS Lett. 491:85–90.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Olsson AK, Dimberg A, Kreuger J and
Claesson-Welsh L: VEGF receptor signalling - in control of vascular
function. Nat Rev Mol Cell Biol. 7:359–371. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Carmeliet P: Mechanisms of angiogenesis
and arteriogenesis. Nat Med. 6:389–395. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xie K, Wei D, Shi Q and Huang S:
Constitutive and inducible expression and regulation of vascular
endothelial growth factor. Cytokine Growth Factor Rev. 15:297–324.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang N, Wang L, Esko J, Giordano FJ, Huang
Y, Gerber HP, Ferrara N and Johnson RS: Loss of HIF-1alpha in
endothelial cells disrupts a hypoxia-driven VEGF autocrine loop
necessary for tumorigenesis. Cancer Cell. 6:485–495. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Reynolds AR: Potential relevance of
bell-shaped and u-shaped dose-responses for the therapeutic
targeting of angiogenesis in cancer. Dose Response. 8:253–284.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ferrara N and Kerbel RS: Angiogenesis as a
therapeutic target. Nature. 438:967–974. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ellis LM and Hicklin DJ: VEGF-targeted
therapy: Mechanisms of anti-tumour activity. Nat Rev Cancer.
8:579–591. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rapisarda A and Melillo G: Role of the
VEGF/VEGFR axis in cancer biology and therapy. Adv Cancer Res.
114:237–267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Terme M, Pernot S, Marcheteau E, Sandoval
F, Benhamouda N, Colussi O, Dubreuil O, Carpentier AF, Tartour E
and Taieb J: VEGFA-VEGFR pathway blockade inhibits tumor-induced
regulatory T-cell proliferation in colorectal cancer. Cancer Res.
73:539–549. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sessa C, Guibal A, Del Conte G and Rüegg
C: Biomarkers of angiogenesis for the development of antiangiogenic
therapies in oncology: Tools or decorations? Nat Clin Pract Oncol.
5:378–391. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Higdon JV, Delage B, Williams DE and
Dashwood RH: Cruciferous vegetables and human cancer risk:
epidemiologic evidence and mechanistic basis. Pharmacol Res.
55:224–236. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tse G and Eslick GD: Cruciferous
vegetables and risk of colorectal neoplasms: A systematic review
and meta-analysis. Nutr Cancer. 66:128–139. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yao H, Wang H, Zhang Z, Jiang BH, Luo J
and Shi X: Sulforaphane inhibited expression of hypoxia-inducible
factor-1alpha in human tongue squamous cancer cells and prostate
cancer cells. Int J Cancer. 123:1255–1261. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shan Y, Sun C, Zhao X, Wu K, Cassidy A and
Bao Y: Effect of sulforaphane on cell growth,
G0/G1 phase cell progression and apoptosis in
human bladder cancer T24 cells. Int J Oncol. 29:883–888.
2006.PubMed/NCBI
|
|
25
|
Shan Y, Wu K, Wang W, Wang S, Lin N, Zhao
R, Cassidy A and Bao Y: Sulforaphane down-regulates COX-2
expression by activating p38 and inhibiting NF-kappaB-DNA-binding
activity in human bladder T24 cells. Int J Oncol. 34:1129–1134.
2009.PubMed/NCBI
|
|
26
|
Wang Y, Dacosta C, Wang W, Zhou Z, Liu M
and Bao Y: Synergy between sulforaphane and selenium in protection
against oxidative damage in colonic CCD841 cells. Nutr Res.
35:610–617. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Asakage M, Tsuno NH, Kitayama J, Tsuchiya
T, Yoneyama S, Yamada J, Okaji Y, Kaisaki S, Osada T, Takahashi K,
et al: Sulforaphane induces inhibition of human umbilical vein
endothelial cells proliferation by apoptosis. Angiogenesis.
9:83–91. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nishikawa T, Tsuno NH, Tsuchiya T,
Yoneyama S, Yamada J, Shuno Y, Okaji Y, Tanaka J, Kitayama J,
Takahashi K, et al: Sulforaphane stimulates activation of
proapoptotic protein bax leading to apoptosis of endothelial
progenitor cells. Ann Surg Oncol. 16:534–543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nishikawa T, Tsuno NH, Okaji Y, Sunami E,
Shuno Y, Sasaki K, Hongo K, Kaneko M, Hiyoshi M, Kawai K, et al:
The inhibition of autophagy potentiates anti-angiogenic effects of
sulforaphane by inducing apoptosis. Angiogenesis. 13:227–238. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jackson SJ, Singletary KW and Venema RC:
Sulforaphane suppresses angiogenesis and disrupts endothelial
mitotic progression and microtubule polymerization. Vascul
Pharmacol. 46:77–84. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bertl E, Bartsch H and Gerhäuser C:
Inhibition of angiogenesis and endothelial cell functions are novel
sulforaphane-mediated mechanisms in chemoprevention. Mol Cancer
Ther. 5:575–585. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Davis R, Singh KP, Kurzrock R and Shankar
S: Sulforaphane inhibits angiogenesis through activation of FOXO
transcription factors. Oncol Rep. 22:1473–1478. 2009.PubMed/NCBI
|
|
33
|
Barrera LN, Cassidy A, Wang W, Wei T,
Belshaw NJ, Johnson IT, Brigelius-Flohé R and Bao Y: TrxR1 and GPx2
are potently induced by isothiocyanates and selenium, and mutually
cooperate to protect Caco-2 cells against free radical-mediated
cell death. Biochim Biophys Acta. 1823:1914–1924. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Whayne TF Jr, Parinandi N and Maulik N:
Thioredoxins in cardiovascular disease. Can J Physiol Pharmacol.
93:903–911. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cooley LS, Handsley MM, Zhou Z, Lafleur
MA, Pennington CJ, Thompson EW, Pöschl E and Edwards DR: Reversible
transdifferentiation of blood vascular endothelial cells to a
lymphatic-like phenotype in vitro. J Cell Sci. 123:3808–3816. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Brachvogel B, Pausch F, Farlie P, Gaipl U,
Etich J, Zhou Z, Cameron T, von der Mark K, Bateman JF and Pöschl
E: Isolated Anxa5+/Sca-1+ perivascular cells
from mouse meningeal vasculature retain their perivascular
phenotype in vitro and in vivo. Exp Cell Res. 313:2730–2743. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou Z, Pausch F, Schlötzer-Schrehardt U,
Brachvogel B and Pöschl E: Induction of initial steps of angiogenic
differentiation and maturation of endothelial cells by pericytes in
vitro and the role of collagen IV. Histochem Cell Biol.
145:511–525. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bader BL, Rayburn H, Crowley D and Hynes
RO: Extensive vasculogenesis, angiogenesis, and organogenesis
precede lethality in mice lacking all alpha v integrins. Cell.
95:507–519. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Streicher KL, Sylte MJ, Johnson SE and
Sordillo LM: Thioredoxin reductase regulates angiogenesis by
increasing endothelial cell-derived vascular endothelial growth
factor. Nutr Cancer. 50:221–231. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Griffith LG and Swartz MA: Capturing
complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol.
7:211–224. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fang C, Man YG, Cuttitta F,
Stetler-Stevenson W, Salomon D, Mazar A, Kulesza P, Rosen S, Avital
I, Stojadinovic A, et al: Novel phenotypic fluorescent
three-dimensional co-culture platforms for recapitulating tumor in
vivo progression and for personalized therapy. J Cancer. 4:755–763.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nascarella MA, Stanek EJ III, Hoffmann GR
and Calabrese EJ: Quantification of hormesis in anticancer-agent
dose-responses. Dose Response. 7:160–171. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ushio-Fukai M and Nakamura Y: Reactive
oxygen species and angiogenesis: NADPH oxidase as target for cancer
therapy. Cancer Lett. 266:37–52. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pagès G and Pouysségur J: Transcriptional
regulation of the vascular endothelial growth factor gene - a
concert of activating factors. Cardiovasc Res. 65:564–573. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Beaver LM, Buchanan A, Sokolowski EI,
Riscoe AN, Wong CP, Chang JH, Löhr CV, Williams DE, Dashwood RH and
Ho E: Transcriptome analysis reveals a dynamic and differential
transcriptional response to sulforaphane in normal and prostate
cancer cells and suggests a role for Sp1 in chemoprevention. Mol
Nutr Food Res. 58:2001–2013. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ji W, Yang M, Praggastis A, Li Y, Zhou HJ,
He Y, Ghazvinian R, Cincotta DJ, Rice KP and Min W: Carbamoylating
activity associated with the activation of the antitumor agent
laromustine inhibits angiogenesis by inducing ASK1-dependent
endothelial cell death. PLoS One. 9:e1032242014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lincoln DT, Emadi Ali EM, Tonissen KF and
Clarke FM: The thioredoxin-thioredoxin reductase system:
Over-expression in human cancer. Anticancer Res. 23:2425–2433.
2003.PubMed/NCBI
|
|
48
|
Li D, Wang W, Shan Y, Barrera LN, Howie
AF, Beckett GJ, Wu K and Bao Y: Synergy between sulforaphane and
selenium in the up-regulation of thioredoxin reductase and
protection against hydrogen peroxide-induced cell death in human
hepatocytes. Food Chem. 133:300–307. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kim YW and Byzova TV: Oxidative stress in
angiogenesis and vascular disease. Blood. 123:625–631. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bir SC, Shen X, Kavanagh TJ, Kevil CG and
Pattillo CB: Control of angiogenesis dictated by picomolar
superoxide levels. Free Radic Biol Med. 63:135–142. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
García-Quintans N, Sánchez-Ramos C, Prieto
I, Tierrez A, Arza E, Alfranca A, Redondo JM and Monsalve M:
Oxidative stress induces loss of pericyte coverage and vascular
instability in PGC-1α-deficient mice. Angiogenesis. 19:217–228.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Li WW, Li VW, Hutnik M and Chiou AS: Tumor
angiogenesis as a target for dietary cancer prevention. J Oncol.
2012:8796232012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Radomska-Leśniewska DM, Hevelke A,
Skopiński P, Bałan B, Jóźwiak J, Rokicki D, Skopińska-Różewska E
and Białoszewska A: Reactive oxygen species and synthetic
antioxidants as angiogenesis modulators: Clinical implications.
Pharmacol Rep. 68:462–471. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Campbell L, Howie F, Arthur JR, Nicol F
and Beckett G: Selenium and sulforaphane modify the expression of
selenoenzymes in the human endothelial cell line EAhy926 and
protect cells from oxidative damage. Nutrition. 23:138–144. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Shan Y, Zhao R, Geng W, Lin N, Wang X, Du
X and Wang S: Protective effect of sulforaphane on human vascular
endothelial cells against lipopolysaccharide-induced inflammatory
damage. Cardiovasc Toxicol. 10:139–145. 2010. View Article : Google Scholar : PubMed/NCBI
|