Introduction
Salinomycin, a carboxylic polyether ionopore
isolated from Streptomyces albus, has been used extensively
as an agricultural antibiotic to prevent coccidiosis in poultry.
Recent studies have shown that salinomycin displays potent
antitumor activities in different types of cancer cells, including
colorectal cancer (1),
hepatocellular carcinoma (2),
endometrial (3) and prostate cancer
(4), and osteosacoma cells
(5). However, there are few studies
on its effect on glioma cancer cells (6). Gliomas are the most primary prevalent
and aggressive form of intracranial tumors affecting adults 40–60
years of age (7,8). Despite multidisciplinary treatments
including surgery, chemotherapy and radiotherapy, it has a poor
prognosis with a median survival of less than 15 months (9). Moreover, although chemotherapy has
been used most extensively in glioma cancer patients and has
contributed to substantial improvement in the survival rate, it was
ultimately confirmed to be ineffective owing to severe toxicity,
the incapacity of many drugs to cross the blood-brain barrier and
high levels of drug resistance (10).
Reactive oxygen species (ROS) are normal byproducts
of numerous cellular processes, such as mitochondrial metabolism
and protein folding. The balance of redox homeostasis is regulated
by two major cellular antioxidant systems, including the
glutathione and the thioredoxin system, which plays a crucial role
in cellular viability and function (11,12).
In contrast, overproduction of ROS disrupts the intracellular redox
balance and exerts oxidative stress on cancer cells that can
ultimately cause cell senescence or death (13). ROS play an important role in the
determination of cell death or survival (14). Recent studies have shown that
endoplasmic reticulum (ER) stress has a dual function; either
promotion of cell survival or triggering of cell death depending on
an imbalance between ER protein folding load and capacity (15). ER has two key roles in eukaryotic
cells, namely protein processing and intracellular calcium storage.
ER stress is triggered under various physiological and pathological
conditions, such as exposure to chemotherapeutic agents and
accumulation of unfolded proteins (16). However, accumulation of misfolded
proteins in the ER lumen causes ER stress to initiate the
expression of chaperones and proteins and several folding enzymes.
While moderate ER stress promotes cell survival and enhances
chemotherapeutic resistance, severe stress leads to cell apoptosis
(17). Moreover, unfolded protein
response signaling may activate autophagy to clear the accumulated
misfolded proteins from the ER lumen (18). Autophagy is an intercellular process
for catabolic degradation to maintain cellular homeostasis during
metabolic stress and it is involved in the formation of
autophagosomes, which are further fused with lysosomes to form
acidic vesicular organelles including autolysosomes. Since cancer
cells often exhibit defective autophagic capacities, autophagic
cell death is considered as a tumor suppressor. However, emerging
evidence indicates that autophagy is not only a death pathway, but
also a survival pathway exploited by cancer cells to endure
metabolic stress (19). In
addition, the inhibition of autophagy leads to apoptotic cell death
as a result of the failure to adapt to stress. Therefore, autophagy
inhibitors are also considered as an attractive strategy to enhance
the sensitivity of cancer cells to anticancer drugs by manipulating
the autophagic process (20).
In the present study, we observed that salinomycin
induced autophagy and apoptosis in glioma U87MG cells. These
processes were regulated through a dual function of ER stress: cell
survival and cell death. In addition, ER stress responses were
regulated by upstream ROS. In addition, pharmacological inhibition
of autophagy enhanced salinomycin-mediated apoptosis, suggesting a
new approach for glioma cancer therapy.
Materials and methods
Reagents and antibodies
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltertrazolium bromide
(MTT), N-acetyl-L-cysteine (NAC), 3-methyladenine (3-MA),
4-phenylbutyric acid (4-PBA), 6-diamidino-2-phenylindole
dihydrochloride (DAPI), 2′-7′-dichlorodihydrofluoresceine diacetate
(DCFH-DA) and salinomycin were purchased from Sigma Chemical Co.
(St. Louis, MO, USA). Annexin V-FITC apoptosis detection kit was
purchased from BD Biosciences (San Jose, CA, USA). The
WesternBright ECL kit was purchased from Advansta, Inc. (Menlo
Park, CA, USA). Antibodies against Bip, pro-caspase-3, CHOP, Ire1α,
LC3B and β-actin were purchased from Cell Signaling Technology
(Beverly, MA, USA).
Cell lines and culture
Human glioma U87MG cells were obtained from the
American Type Culture Collection (ATCC; Manassas, VA, USA). The
U87MG cells were cultured in Dulbecco's modified Eagle's medium
minimal (DMEM) supplemented with 10% fetal bovine serum (FBS) and
100 U/ml of penicillin and 100 µg/ml of streptomycin (all from
WelGENE Inc., Daegu, Korea). Cells were cultured in a humidified
atmosphere with 5% CO2 at 37°C.
Cell viability
Cell viability was measured using the MTT assay.
Cells were seeded and treated with various concentrations of
salinomycin for 24 or 48 h. After salinomycin treatment, 1 mg/ml of
MTT was added to each well and incubated for 3 h at 37°C. Then, the
medium was removed and MTT-formazan complex was dissolved in
dimethyl sulfoxide. Absorbance was observed at 570 nm using the
VERSAmax microplate reader (Molecular Devices, Toronto,
Canada). Cell viability was determined as the relative percentage
of treated cells to the untreated cells by comparing optical
densities.
Morphological changes
Nuclear morphological changes were measured by
fluorescence microscopy. Cells were incubated in the absence or
presence of salinomycin for 48 h. The cells were fixed with 4%
paraformaldehyde, and then stained with 1 mg/ml of DAPI solution
for 10 min. After washing, the cells were observed under
fluorescence microscopy (Axio Imager; Zeiss, Jena, Germany).
Annexin V/PI double staining
Apoptotic cells were assessed by an Annexin V-FITC
staining kit. Briefly, the U87MG cells were treated with various
concentrations of salinomycin for 48 h and then were washed with
phosphate-buffered saline (PBS). Collected cells were mixed in 100
µl of 1X Annexin binding buffer. After Annexin V/PI double staining
for 20 min, cells were analyzed by flow cytometry (FACSCalibur;
Becton-Dickinson, Franklin Lakes, NJ, USA). The apoptotic cells
were calculated using Cell Quest Pro software on Mac® OS
9 (Becton-Dickinson).
ROS generation. ROS were measured
using DCFH-DA fluorescent dye
The cells were cultured in a 6-well plate at a
density of 2.5×104/well. After treatment with
salinomycin for 24 or 48 h, the cells were incubated with 10 µM of
DCFH-DA at 37°C for 30 min. After the cells were harvested, the
intensity of fluorescence was measured using flow cytometry and
calculated using Cell Quest Pro software on Mac® OS
9.
Acidic vesicular organelle
detection
To detect acidic vesicular organelles, the cells
were cultured in a 6-well plate at a density of
2.5×104/well. After treatment with 4 µM of salinomycin
for 48 h, cells were stained with 1 µM acridine orange for 30 min.
The stained cells were analyzed by flow cytometry and calculated
using Cell Quest Pro software on Mac® OS 9.
Western blotting
Whole extracts were prepared by incubating the cells
in lysis buffer [150 mM NaCl, 10 mM Tris (pH 7.4), 5 mM EDTA (pH
8.0), 1% Triton X-100, 1 mM PMSF, 20 µg/ml aprotinin, 50 µg/ml
leupeptin, 1 mM benzidine, 1 mg/ml pepstatin, 8 mM sodium
pyrophosphate and 20 mM β-glycerophosphate]. Forty micrograms of
proteins was electrophoretically separated using sodium dodecyl
sulfate-polyacrylamide gel electrophoresis on 8–15% gel and
transferred to a polyvinylidene fluoride membrane. After blocking
with TBS-T buffer [20 mM Tris (pH 7.4), 150 mM NaCl, 0.1% Tween-20]
containing 5% skim milk, the membranes were incubated with primary
or secondary antibodies. The membranes were then washed with TBS-T
buffer and visualized with enhanced chemiluminescence (ECL) western
blot analysis detection reagents. The density of each band was
determined using a fluorescence scanner (LAS 3000) and analyzed
with Multi Gauge V3.0 software (both from Fuji Film, Tokyo,
Japan).
Measurement of caspase-3 activity
For detection of caspase-3 activation, a caspase-3
colorimetric assay kit (R&D Systems Inc., Minneapolis, MN, USA)
was used according to the manufacturer's protocol. Equal amounts of
protein (220 µg) were resuspended in reaction buffer containing
substrate (Ac-DEVD-pNA), and then, incubated at 37°C for 4 h in the
dark. The absorbance of the released pNA was measured at 405 nm
using an ELISA reader.
Statistical analysis
All experiments were repeated at least three times.
Unless otherwise stated, data are expressed as the mean ± SD.
Comparison of the experimental groups to the control values was
carried out by ANOVA. Results were statistically significant at
p<0.05 or p<0.001 vs. the untreated group.
Results
Salinomycin induces apoptosis through
generation of ROS in U87MG cells
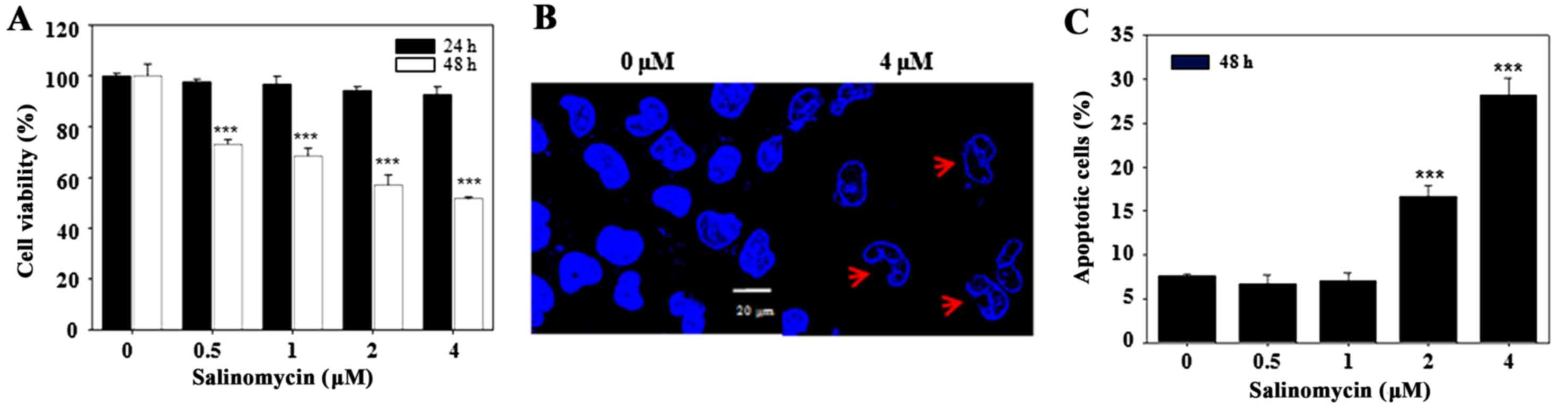
Salinomycin significantly decreased the cell
viability of U87MG cells in a dose- and time-dependent manner
(Fig. 1A). The 50% inhibitory
concentration after 48 h of treatment with salinomycin was ~4 µM.
Salinomycin caused a reduction in cell volume, nuclear condensation
and an increase in non-adherent cells (Fig. 1B). In order to quantify
salinomycin-induced apoptosis, Annexin V-PI double staining was
performed. The percentage of apoptotic cells was increased in the
salinomycin-treated cells, compared with the percentage in the
control group (Fig. 1C). These
results showed that salinomycin inhibited cell viability and
induced apoptotic cell death in the U87MG cells.
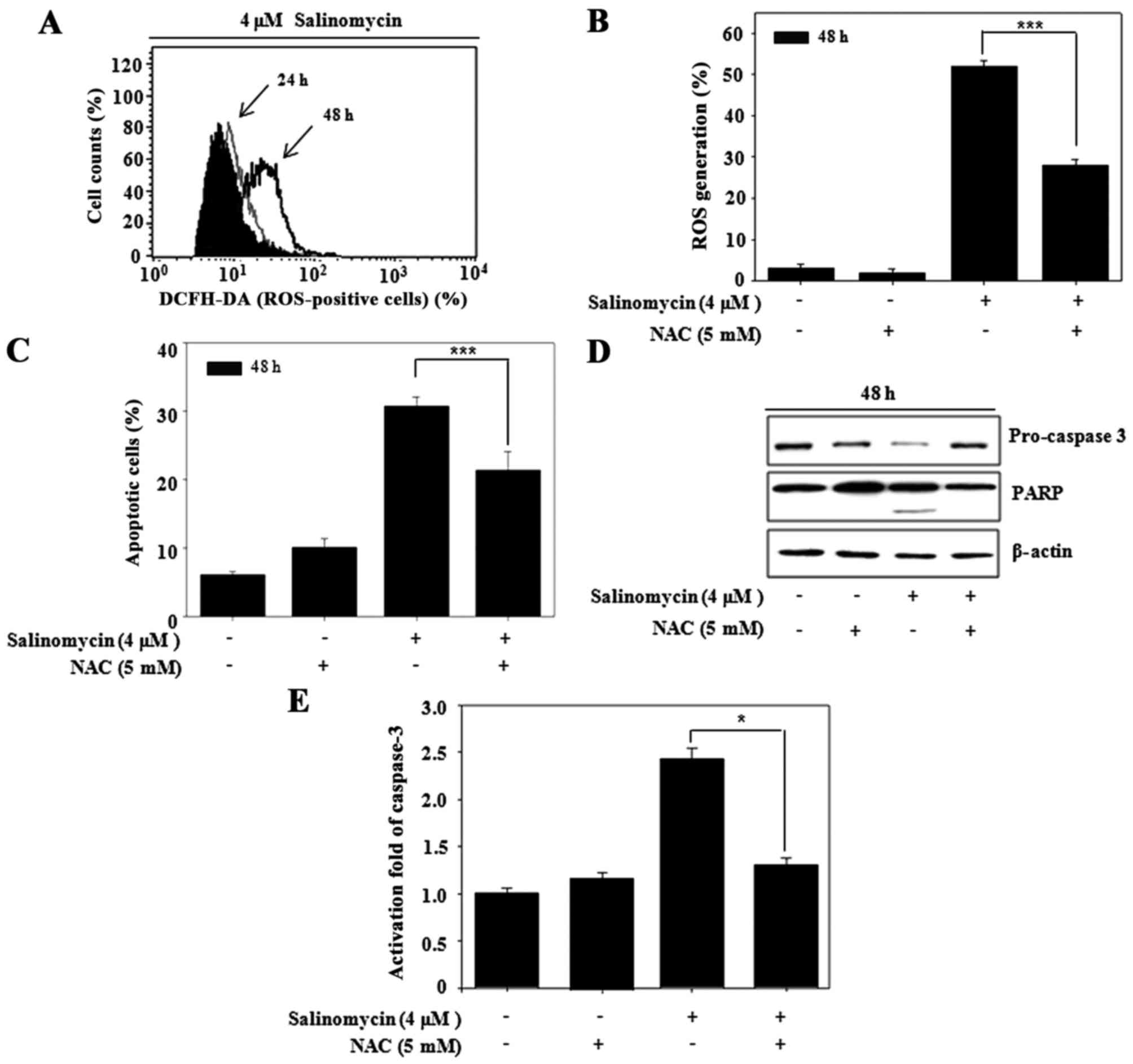
Recent research has shown that anticancer drugs
induce apoptosis, in part, by triggering ROS generation (4). To observe whether salinomycin produces
ROS, the intracellular ROS level was determined with the
fluorescent dye DCFH-DA. The ROS production was time-dependently
increased after salinomycin treatment (Fig. 2A). However, the salinomycin-induced
ROS production was reversed by the ROS scavenger NAC (Fig. 2B). Therefore, we observed whether
the salinomycin-induced apoptosis is associated with ROS
production. Pre-treatment with NAC recovered salinomycin-induced
apoptosis (Fig. 2C) and rescued
expression of apoptosis-related proteins, such as pro-caspase-3 and
PARP (Fig. 2D). Furthermore,
consistent with western blot analysis, salinomycin-induced
caspase-3 activation was reversed by NAC (Fig. 2E). Taken together, ROS induced by
salinomycin regulated apoptotic cell death in the U87MG cells.
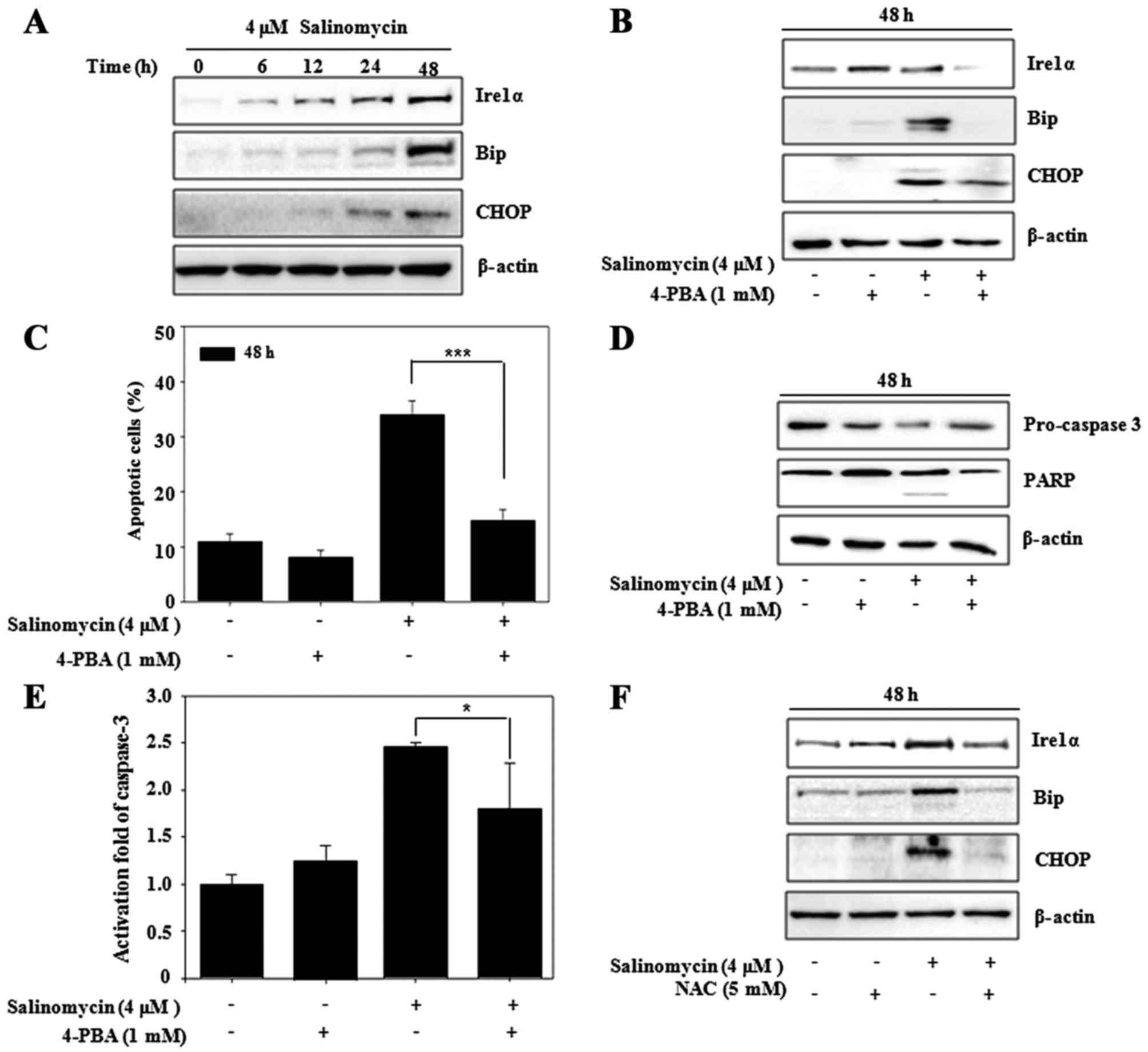
Salinomycin induces ER stress-mediated
apoptosis in U87MG cells
The misfolded proteins induced ER stress to restore
protein homeostasis and apoptotic cell death ensues when the stress
is prolonged. Recent research revealed that apoptosis is induced
via stimulation of ER stress in glioma cells (6,21). To
investigate the ER stress pathway involved in salinomycin-induced
apoptosis, we examined the expression levels of ER stress-related
proteins (Ire1α, Bip and CHOP) by western blot analysis.
Salinomycin increased the expression of Ire1α, Bip and CHOP in a
time-dependent manner (Fig. 3A).
However, addition of the ER stress inhibitor, 4-PBA, resulted in
suppression of these proteins (Fig.
3B). We observed the relationship between ER stress and
apoptosis using 4-PBA. As shown in Fig.
3C, salinomycin-induced apoptosis was also significantly
blocked by 4-PBA, which was confirmed by suppression of the
activation of caspase-3 and expression of PARP (Fig. 3D and E). In addition, we observed
the relationship between ROS production and ER stress responses in
regards to apoptosis. As shown in Fig.
3F, ER stress-related proteins were also suppressed by NAC.
These results indicated that ER stress plays a crucial role in the
upstream pathway of salinomycin-induced apoptosis and is regulated
by ROS generation in U87MG cells.
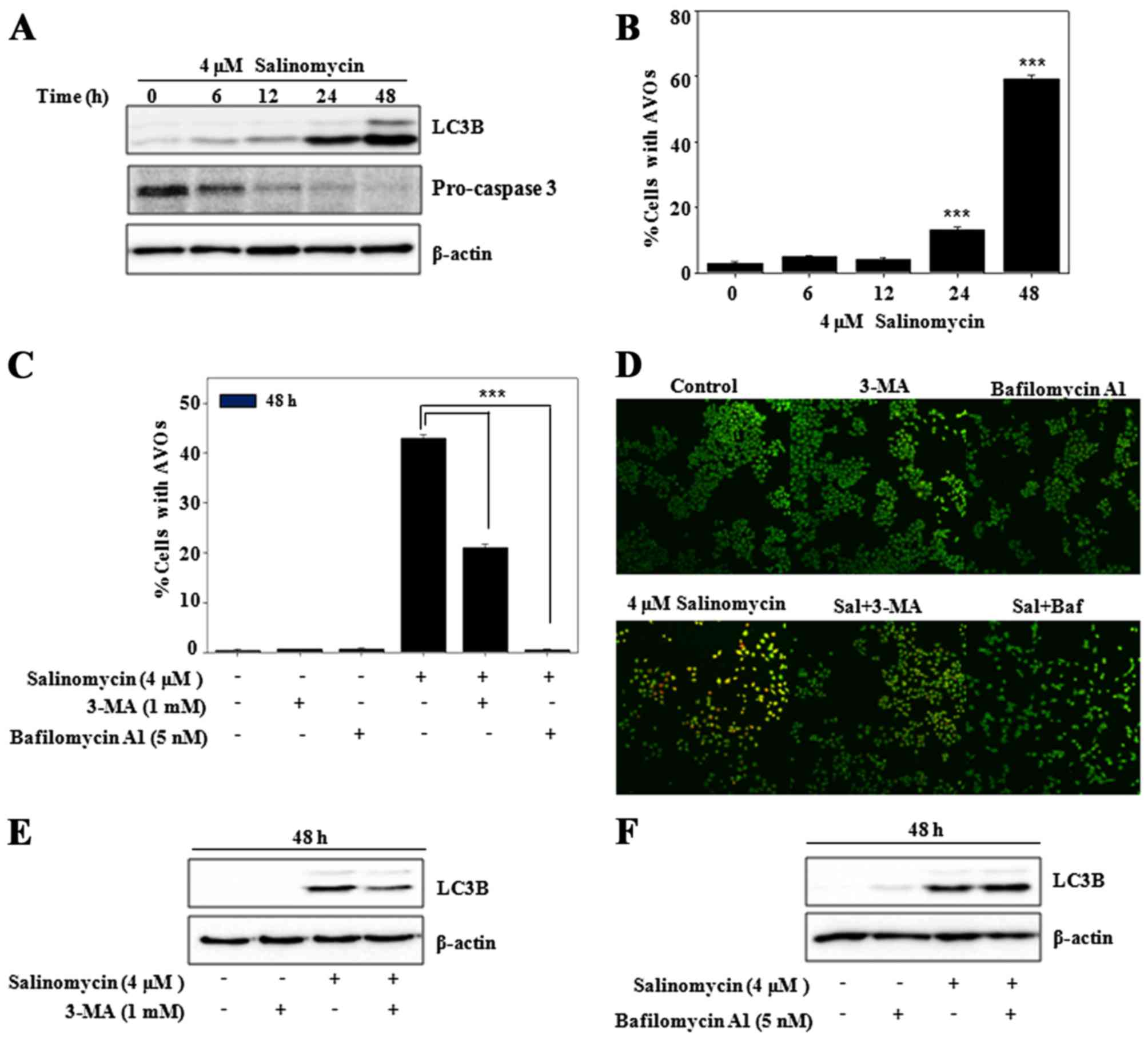
Salinomycin induces ER stress-mediated
autophagy in U87MG cells
Programmed cell death (apoptosis) is mainly
regulated by the autophagy pathway (22). However, autophagy can independently
act upon apoptotic signaling pathways, thus we determined the
autophagy level after salinomycin treatment in the U87MG cells.
Increased expression of autophagy marker protein LC3B and apoptosis
marker caspase-3 were observed in a time-dependent manner (Fig. 4A). The formation of acidic vesicular
organelles (AVOs), autophagy-related lysosomal structures, was also
increased, as determined using vital staining with acridine orange
(23) (Fig. 4B). However, the formation of AVOs
was suppressed by pre-treatment with 3-MA, an inhibitor of
autophagosome formation or bafilomycin A1, an inhibitor of lysosome
formation (Fig. 4C). These results
indicated that salinomycin induced autophagic flux in the U87MG
cells, as confirmed by acridin orange-stained cells with
co-treatment of 3-MA or bafilomycin Al (Fig. 4D). As shown in Fig. 4E, salinomycin also increased
autophagic marker protein LC3B, which was blocked in the presence
of 3-MA. However, addition of the lysosome inhibitor bafilomycin A1
resulted in further accumulation of LC3B as compared to cells
treated with the single agent (Fig.
4F). These results indicated that salinomycin induced
autophagic flux in the U87MG cells, which was recovered by
co-treatment of 3-MA and enhanced with bafilomycin A1.
Salinomycin regulates apoptosis
through autophagy in U87MG cells
To address the role of autophagy against
salinomycin-induced apoptosis, the percentage of apoptotic cells
and expression of apoptosis-related proteins were determined.
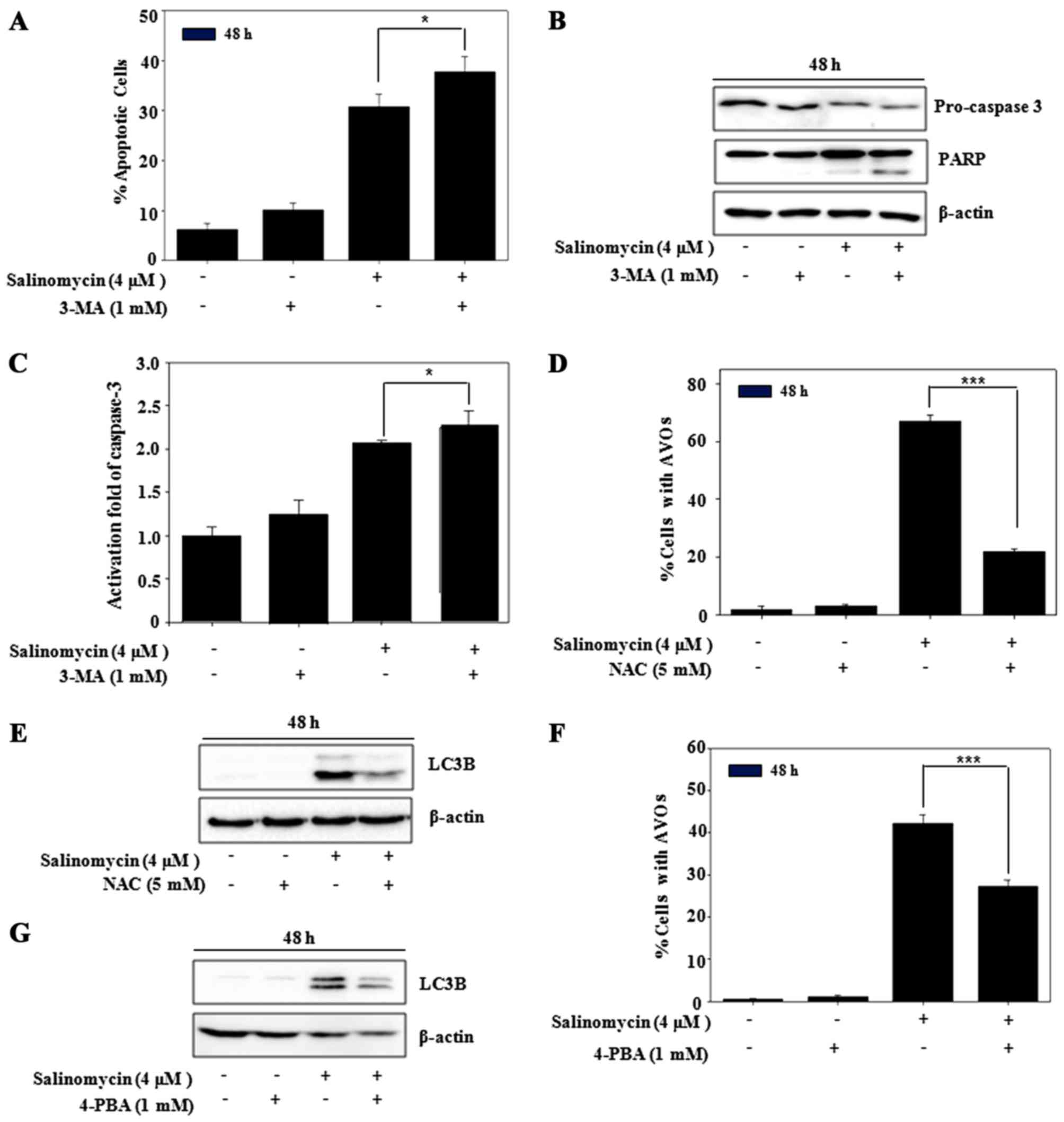
Pre-treatment with 3-MA enhanced the salinomycin-induced apoptosis
(Fig. 5A), resulting from reduced
pro-caspase-3 and accumulation of cleaved PARP in the U87MG cells
(Fig. 5B). Furthermore, salinomycin
induced caspase-3 activation enhanced by 3-MA (Fig. 5C). In addition, we also observed
that the formation of AVOs and expression of LC3B were suppressed
by the pre-treatment of NAC (Fig. 5D
and E), which indicated the regulation of autophagy by ROS.
Next, to observe the relationship between ER stress and autophagy,
4-PBA as an ER stress inhibitor was applied. As shown in Fig. 5F, the formation of AVOs was
significantly blocked by 4-PBA, leading to recovered expression of
LC3B (Fig. 5G). These results
demonstrated that autophagy flux caused a delay in
salinomycin-induced apoptosis in the U87MG cells, which was
regulated by ER stress responses mediated from upstream ROS.
Discussion
Autophagy is an intracellular metabolic system in
eukaryotic cells, in which autophagosomes fuse with lysosomes and
degrade intracellular materials to maintain cell homeostasis
(24). It function in a protective
role from drug-induced cell death (25). Autophagy inhibits apoptosis by
promoting cell survival or induces cell death by cooperating with
apoptosis signaling (26).
Apoptosis and autophagy are interrelated and undergo crosstalk. Due
to its pro-survival function, autophagy makes cancer cells
resistant to chemotherapy, radiotherapy or anti-angiogenic therapy.
It is tightly regulated by several conserved autophagy proteins.
Inhibition of autophagy has been widely recognized to improve the
efficacy of anticancer agents (27). Anticancer compounds may affect
cellular redox reactions through accumulation of intracellular ROS
(28,29), which in turn induce apoptosis and
autophagy (30). Accumulating
evidence indicates that apoptosis is also regulated by ER stress
(6,21). ER is a key organelle with protein
processing, intracellular calcium storage, as well as signaling
regulation functions in eukaryotic cells (31).
Although the anticancer effects of salinomycin have
been established in a variety of preclinical studies using many
different cancer types, there are few studies on the effects of
salinomycin on glioma cancer cells (6). In the present study, salinomycin
showed potent cytotoxic and apoptotic effects in human glioma U87MG
cells. We found that the level of intracellular ROS was increased
after salinomycin treatment and antioxidant NAC rescued the
apoptosis level. In addition, salinomycin stimulated the expression
of ER stress-related proteins, including Ire1α, Bip and CHOP.
Indeed, salinomycin-induced apoptosis was suppressed by ER stress
inhibitor, 4-PBA, in the U87MG cells. These results suggest that
apoptosis may be regulated via ER stress signaling. Moreover, NAC
suppressed the expression of Ire1α, Bip, CHOP and LC3B and the
formation of AVOs. Therefore, salinomycin induced ER stress and
autophagy by promoting ROS generation, resulting in cellular
apoptosis. In addition, salinomycin induced an increased autophagic
level by causing increased expression of LC3B and accumulation of
AVOs in the U87MG cells. These phenomena were recovered by
autophagy inhibitor 3-MA, which resulted in increased apoptosis. In
addition, suppression of ER stress using 4-PBA inhibited the
salinomycin-induced autophagy, as confirmed by reduction in LC3B
and AVO formation. This indicated that autophagy was regulated
through the ER stress responses induced by salinomycin.
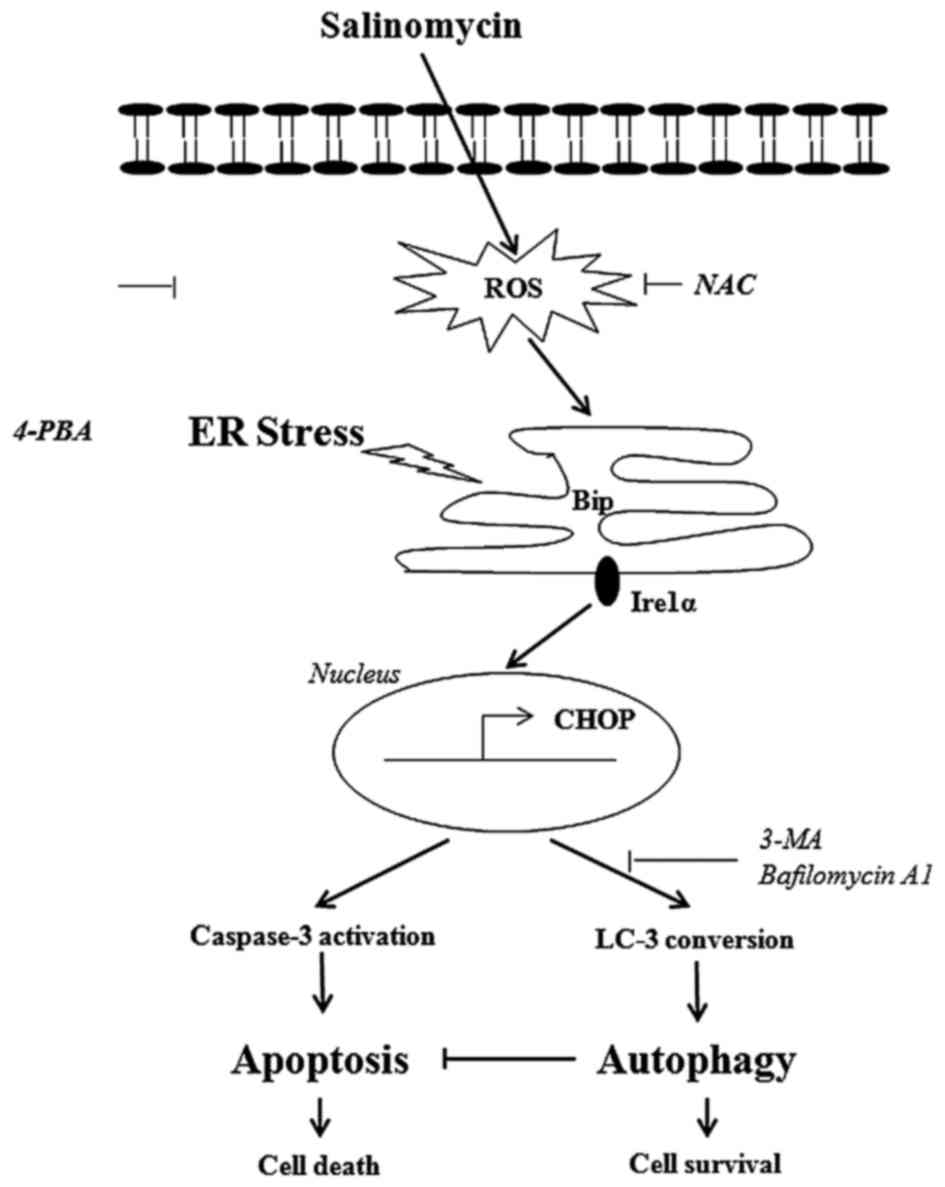
In conclusion, we demonstrated that salinomycin
induced apoptosis through the ROS-mediated ER stress signaling
pathway, which was protected by ER stress-mediated autophagy
(Fig. 6). As salinomycin has the
potential as a chemotherapeutic agent for human glioma cancer
cells, future pre-clinical studies are warranted to confirm its
usefulness as a clinical drug candidate for glioma cancer
treatment. In addition, blocking of ER stress responses could be a
useful strategy to target cancer cell resistance to
chemotherapies.
Acknowledgements
The present study was supported by the 2012
Specialization Project Research Grant funded by the Pusan National
University.
References
|
1
|
Dong TT, Zhou HM, Wang LL, Feng B, Lv B
and Zheng MH: Salinomycin selectively targets ‘CD133+’
cell subpopulations and decreases malignant traits in colorectal
cancer lines. Ann Surg Oncol. 18:1797–1804. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang F, He L, Dai WQ, Xu YP, Wu D, Lin CL,
Wu SM, Cheng P, Zhang Y, Shen M, et al: Salinomycin inhibits
proliferation and induces apoptosis of human hepatocellular
carcinoma cells in vitro and in vivo. PLoS One. 7:e506382012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kusunoki S, Kato K, Tabu K, Inagaki T,
Okabe H, Kaneda H, Suga S, Terao Y, Taga T and Takeda S: The
inhibitory effect of salinomycin on the proliferation, migration
and invasion of human endometrial cancer stem-like cells. Gynecol
Oncol. 129:598–605. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim KY, Yu SN, Lee SY, Chun SS, Choi YL,
Park YM, Song CS, Chatterjee B and Ahn SC: Salinomycin-induced
apoptosis of human prostate cancer cells due to accumulated
reactive oxygen species and mitochondrial membrane depolarization.
Biochem Biophys Res Commun. 413:80–86. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim SH, Choi YJ, Kim KY, Yu SN, Seo YK,
Chun SS, Noh KT, Suh JT and Ahn SC: Salinomycin simultaneously
induces apoptosis and autophagy through generation of reactive
oxygen species in osteosarcoma U2OS cells. Biochem Biophys Res
Commun. 473:607–613. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xipell E, Gonzalez-Huarriz M, de Irujo JJ
Martinez, García-Garzón A, Lang FF, Jiang H, Fueyo J, Gomez-Manzano
C and Alonso MM: Salinomycin induced ROS results in abortive
autophagy and leads to regulated necrosis in glioblastoma.
Oncotarget. 7:30626–30641. 2016.PubMed/NCBI
|
|
7
|
Chu SH, Feng DF, Ma YB, Zhang H, Zhu ZA,
Li ZQ and Jiang PC: Promoter methylation and downregulation of
SLC22A18 are associated with the development and progression of
human glioma. J Transl Med. 9:1562011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wei KC, Huang CY, Chen PY, Feng LY, Wu TW,
Chen SM, Tsai HC, Lu YJ, Tsang NM, Tseng CK, et al: Evaluation of
the prognostic value of CD44 in glioblastoma multiforme. Anticancer
Res. 30:253–259. 2010.PubMed/NCBI
|
|
9
|
Ohgaki H and Kleihues P: Epidemiology and
etiology of gliomas. Acta Neuropathol. 109:93–108. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Das A, Banik NL and Ray SK:
N-(4-Hydroxyphenyl) retinamide induced both differentiation and
apoptosis in human glioblastoma T98G and U87MG cells. Brain Res.
1227:207–215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Powis G, Gasdaska JR, Gasdaska PY,
Berggren M, Kirkpatrick DL, Engman L, Cotgreave IA, Angulo M and
Baker A: Selenium and the thioredoxin redox system: Effects on cell
growth and death. Oncol Res. 9:303–312. 1997.PubMed/NCBI
|
|
12
|
Sun Y and Rigas B: The thioredoxin system
mediates redox-induced cell death in human colon cancer cells:
Implications for the mechanism of action of anticancer agents.
Cancer Res. 68:8269–8277. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Simon HU, Haj-Yehia A and Levi-Schaffer F:
Role of reactive oxygen species (ROS) in apoptosis induction.
Apoptosis. 5:415–418. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rigoulet M, Yoboue ED and Devin A:
Mitochondrial ROS generation and its regulation: Mechanisms
involved in H2O2 signaling. Antioxid Redox
Signal. 14:459–468. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jang JH, Kim YJ, Kim H, Kim SC and Cho JH:
Buforin IIb induces endoplasmic reticulum stress-mediated apoptosis
in HeLa cells. Peptides. 69:144–149. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ron D and Hubbard SR: How IRE1 reacts to
ER stress. Cell. 132:24–26. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kouroku Y, Fujita E, Tanida I, Ueno T,
Isoai A, Kumagai H, Ogawa S, Kaufman RJ, Kominami E and Momoi T: ER
stress (PERK/eIF2alpha phosphorylation) mediates the
polyglutamine-induced LC3 conversion, an essential step for
autophagy formation. Cell Death Differ. 14:230–239. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Szegezdi E, Logue SE, Gorman AM and Samali
A: Mediators of endoplasmic reticulum stress-induced apoptosis.
EMBO Rep. 7:880–885. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
White E and DiPaola RS: The double-edged
sword of autophagy modulation in cancer. Clin Cancer Res.
15:5308–5316. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Amaravadi RK, Yu D, Lum JJ, Bui T,
Christophorou MA, Evan GI, Thomas-Tikhonenko A and Thompson CB:
Autophagy inhibition enhances therapy-induced apoptosis in a
Myc-induced model of lymphoma. J Clin Invest. 117:326–336. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yoon MJ, Kang YJ, Kim IY, Kim EH, Lee JA,
Lim JH, Kwon TK and Choi KS: Monensin, a polyether ionophore
antibiotic, overcomes TRAIL resistance in glioma cells via
endoplasmic reticulum stress, DR5 upregulation and c-FLIP
downregulation. Carcinogenesis. 34:1918–1928. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lockshin RA and Zakeri Z: Apoptosis,
autophagy, and more. Int J Biochem Cell Biol. 36:2405–2419. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Paglin S, Hollister T, Delohery T, Hackett
N, McMahill M, Sphicas E, Domingo D and Yahalom J: A novel response
of cancer cells to radiation involves autophagy and formation of
acidic vesicles. Cancer Res. 61:439–444. 2001.PubMed/NCBI
|
|
24
|
Liu M, Ma S, Liu M, Hou Y, Liang B, Su X
and Liu X: Synergistic killing of lung cancer cells by cisplatin
and radiation via autophagy and apoptosis. Oncol Lett. 7:1903–1910.
2014.PubMed/NCBI
|
|
25
|
Malhi H, Gores GJ and Lemasters JJ:
Apoptosis and necrosis in the liver: A tale of two deaths?
Hepatology. 43(Suppl 1): S31–S44. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Eisenberg-Lerner A, Bialik S, Simon HU and
Kimchi A: Life and death partners: Apoptosis, autophagy and the
cross-talk between them. Cell Death Differ. 16:966–975. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Maycotte P and Thorburn A: Autophagy and
cancer therapy. Cancer Biol Ther. 11:127–137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Delaunay-Moisan A and Appenzeller-Herzog
C: The antioxidant machinery of the endoplasmic reticulum:
Protection and signaling. Free Radic Biol Med. 83:341–351. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cheng Z and Ristow M: Mitochondria and
metabolic homeostasis. Antioxid Redox Signal. 19:240–242. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang CL, Liu C, Niu LL, Wang LR, Hou LH
and Cao XH: Surfactin-induced apoptosis through
ROS-ERS-Ca2+-ERK pathways in HepG2 cells. Cell Biochem
Biophys. 67:1433–1439. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Moir RD, Gross DA, Silver DL and Willis
IM: SCS3 and YFT2 link transcription of phospholipid biosynthetic
genes to ER stress and the UPR. PLoS Genet. 8:e10028902012.
View Article : Google Scholar : PubMed/NCBI
|