Introduction
Gastric cancer (GC) is one of the most common types
of cancer worldwide, and is the second leading cause of
cancer-related deaths (1). Despite
unremitting efforts to improve a variety of diagnostic and
therapeutic methods, tumor metastasis after surgery is still the
greatest threat to patients. Thus, elucidation of the process and
mechanism of GC metastases is required to improve the prognosis of
GC patients.
p21-activated kinase 1 (Pak1) is the best
characterized member of an evolutionarily conserved family of
serine/threonine kinases (2), which
plays a role in a variety of cellular functions such as
cytoskeletal reorganization, cell motility, apoptosis and
transformation (3–5). Pak1 has been identified as an effector
molecule for the small GTPases Rho, Rac1 and Cdc42 (6). Amplification of Pak1 has been found in
breast, renal, liver and colorectal cancer. Moreover, Pak1 has been
reported to induce proliferation, motility and invasion of these
cancer cells through its involvement in several cell signaling
pathways, such as mitogen-activated protein kinases (MAPKs) and
nuclear factor-κB (NF-κB) (5,7,8).
Matrix metalloproteinases (MMPs), a family of
secreted or transmembrane enzymes, can collectively digest almost
all extracellular matrix (ECM) and basement membrane components
(9–11). Upregulation of MMPs has been
reported to contribute to tumor cell invasion and metastasis, thus,
leading to the development of malignant tumors (9–12).
Both matrix metalloproteinase-2 and −9 (MMP-2 and MMP-9) which can
degrade collagen IV, the major ECM component of basement membranes,
have been found to be overexpressed in GC (13,14).
Studies have shown that high levels of MMP-2 and/or MMP-9 have a
significant correlation with GC invasion (14,15),
thus, resulting in poor prognosis and shortening disease-free
survival (15–17). MMP activity is closely controlled by
physiological inhibitors, TIMPs including TIMP-1, −2, −3 and −4
(18).
Our previous study indicated that Pak1 was
overexpressed in GC. Moreover, ectopic expression of Pak1 obviously
increased the invasion of GC cells through phosphorylation of c-Jun
N-terminal kinase (JNK) (19). In
the present study, we sought to further examine the exact mechanism
by which Pak1 stimulated the invasion of human GC cells. Our data
revealed that Pak1 markedly enhanced MMP-2 mRNA expression and
activity. Moreover, the JNK signaling pathway was involved in Pak1
upregulation of MMP-2 in MKN45 GC cells, which was important for
the invasion of MKN45 GC cells. We reported for the first time that
Pak1 induces the invasion of GC cells via the JNK-MMP-2 signaling
pathway.
Materials and methods
Tissue samples and cell culture
Fifty-seven samples of primary GC tissues were
obtained from the Shanghai Jiaotong University School of Medicine
Ruijin Hospital. Fresh samples were harvested, and then, were
immediately frozen in liquid nitrogen and kept at −70°C until use.
The study protocol was approved by the Medical Ethics and Human
Clinical Trial Committee at the Shanghai Jiaotong University School
of Medicine Ruijin Hospital. MKN45 human GC cells [American Type
Culture Collection (ATCC) Manassas, VA, USA] were maintained in
RPMI-1640 medium containing 10% fetal bovine serum (FBS).
Materials
Anti-Pak1 (N-20) (sc-882), anti-phospho-Pak1
(Thr423) (sc-12925), anti-DsRed (L-18) (sc-33353), anti-TIMP1
(sc-365905), anti-TIMP2 (sc-6835), anti-TIMP3 (sc-6836) and
anti-TIMP4 (sc-9375) were obtained from Santa Cruz Biotechnology
(Santa Cruz, CA, USA).
The anti-JNK and anti-phospho-JNK antibodies were
purchased from Cell Signaling Technology Inc., (Beverly, MA, USA).
The anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody
was purchased from KangChen Bio-tech (Shanghai, China).
Constructs and production of a stable
cell line
The Pak1 construct, pDs-Red2-Pak1, was a generous
gift from Dr Jonathan Chernoff (Fox Chase Cancer Centre,
Philadelphia, PA, USA). For constructing the stable transfectant,
pDs-Red2 and pDs-Red2-Pak1 were separately transfected into MKN45
human GC cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA,
USA) reagent according to the manufacturer's instructions. The
transfected cells were selected in growth medium containing 1,000
µg/ml Geneticin (G418; Life Technologies, Grand Island, NY, USA).
After 4–8 weeks, individual cell colonies were transferred for
clone expansion and maintained in culture medium supplemented with
600 µg/ml G418.
Pak1 siRNA
For RNA interference (RNAi) of Pak1, Pak1 small
interfering RNA and control siRNA were purchased from Santa Cruz
Biotechnology. Each one (100 pmol in 2 ml medium) was transfected
into MKN45 human GC cells using Lipofectamine 2000.
Reverse transcription-PCR
Total RNA was extracted from samples using TRIzol
reagent (Life Technologies), and 2 µg of each RNA sample was used
to prepare cDNA. The semi-quantitative PCR primer sequences for
MMP-2 were: 5′-TAC AGG ATC ATT GGC TAC ACA CC (forward) and 5′-GGT
CAC ATC GCT CCA GAC T (reverse). The semi-quantitative PCR primer
sequences for MMP-9 were: 5′-AGA CCT GGG CAG ATT CCA AAC (forward)
and 5′-CGG CAA GTC TTC CGA GTA GT (reverse). Quantitative real-time
PCR was carried out using the Applied Biosystems TaqMan system.
Cellular 18S mRNA was used as an internal control.
Western blot analysis
Cells were harvested into RIPA lysis buffer (Pierce,
Rockford, IL, USA) with a freshly added protease inhibitor cocktail
and a phosphatase inhibitor cocktail (both from Roche Diagnostics,
Mannheim, Germany). The cell lysate was cleared by centrifugation
at 4°C and the supernatant was stored in small aliquots at −80°C.
Normally, 20 µg of sample was loaded into each lane, separated by
SDS-polyacrylamide gel electrophoresis (SDS-PAGE), then transferred
to a polyvinylidene difluoride membrane, and probed with respective
antibodies. The density of blots was measured by PS software.
Gelatin zymography assay
For the zymography assay, cells (2.5×105)
were seeded into 12-well plates and incubated for 48 h.
Supernatants were collected and mixed with sample buffer followed
by electrophoresis on a 10% SDS-PAGE containing 5 mg/ml of gelatin.
The gel was washed with 2.5% Triton-X 100 solution for 2 h and
further incubated in the reaction buffer (50 mmol/l Tris-HCl, 5
mmol/l CaCl2, 1 µmol/l ZnCl2 and 1% Triton
X-100) for an additional 18 h at room temperature. The gel was then
stained with 0.5% Coomassie blue for 9 h, and subsequently immersed
with destaining buffer (30% methanol and 10% acetic acid) for 12 h.
Images were phtographed and the intensity of each band was
digitally quantified.
Cell invasion assay
The polycarbonate membranes, 6.5-mm in diameter with
8-µm pores (Corning Costar, New York, NY, USA), coated with
Matrigel (BD Biosciences, Bedford, MA, USA) were used for the
invasion assay. Following the addition of medium containing 10%
fetal calf serum (FCS) to the bottom chambers, single-cell
suspensions in medium containing 0.1% BSA were seeded onto the
filters (1×105 cells/each well) and incubated for 24 or
48 h at 37°C in 5% CO2. The filters were then washed and
the cells on the upper surface were removed with cotton swabs. The
cells that had invaded to the lower surface of the filter inserts
were fixed with 5% paraformaldehyde for 15 min and stained with
0.1% (w/v) crystal violet for 15 min. The number of invaded cells
was microscopically counted and 3 independent experiments were
carried out to get an average cell number at a high magnification
field.
Statistical analysis
Each experiment was duplicated at least 3 times.
Results are presented as the mean ± SE, and statistical comparisons
were made using the Student's t-test, Fisher's exact or
χ2 tests. Statistical SPSS version 15.0 was used to
analyze data. Significance was defined at P<0.05 levels.
Results
The effects of Pak1 on the expression
of MMP-2 and MMP-9 in human MKN45 GC cells
To elucidate the effects of Pak1 on the expression
of MMP-2 and MMP-9 in GC, MKN45 GC cells were used to establish a
stable cell line overexpressing the pDs-Red2 fusion form of Pak1.
The stable transfectant cell line MKN45-Pak1 was established and
confirmed with real-time quantitative reverse transcription PCR
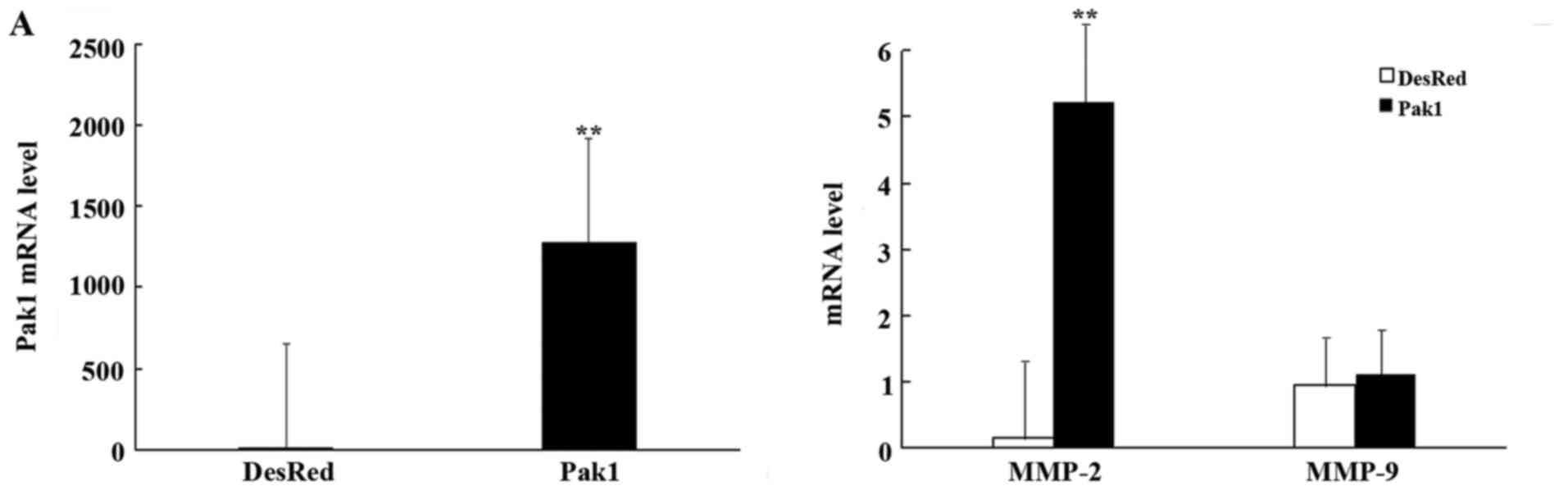
(qRT-PCR). The result revealed that Pak1 mRNA expression in the
MKN45-Pak1 stable cell line was markedly higher than in the mock
transfectant with pDsRed2 (named MKN45-DsRed2) (P<0.01; Fig. 1A, left panel). We then determined
the expression of matrix MMP-2 and −9 in the MKN45-DsRed2 and
MKN45-Pak1 cells by qRT-PCR. Compared with the MKN45-DsRed2 cells,
MMP-2 mRNA expression was significantly increased in the MKN45-Pak1
cells (P<0.01; Fig. 1A).
However, a significant difference in the mRNA level of MMP-9
between MKN45-Pak1 and the control cells was not found (P=0.241;
Fig. 1A, right panel). We also
performed gelatin zymography assay to assess the activities of
MMP-2 and MMP-9 in the MKN45-DsRed2 and MKN45-Pak1 cells.
Similarly, enhanced expression of Pak1 markedly increased MMP-2
activity (P<0.01; Fig. 1B), but
had no effect on MMP-9 activity (P=0.818; Fig. 1B). To further confirm the effects of
Pak1 on the expression of MMP-2 and MMP-9 in GC, we used RNAi to
specifically knock down endogenous Pak1 in MKN45 cells. The
specific knockdown of endogenous Pak1 in MKN45 cells was confirmed
with qRT-PCR (Fig. 1C). We observed
that MMP-2 mRNA expression and activity were sharply decreased in
the MKN45 cells transfected with Pak1 siRNA (P<0.01, Fig. 1C; P<0.01, Fig. 1D), but knockdown of endogenous Pak1
had no effects on MMP-9 mRNA expression and activity in the MKN45
cells (P=0.063, Fig. 1C; P=0.075,
Fig. 1D).
Effects of Pak1 on the expression of
TIMPs in human MKN45 GC cells
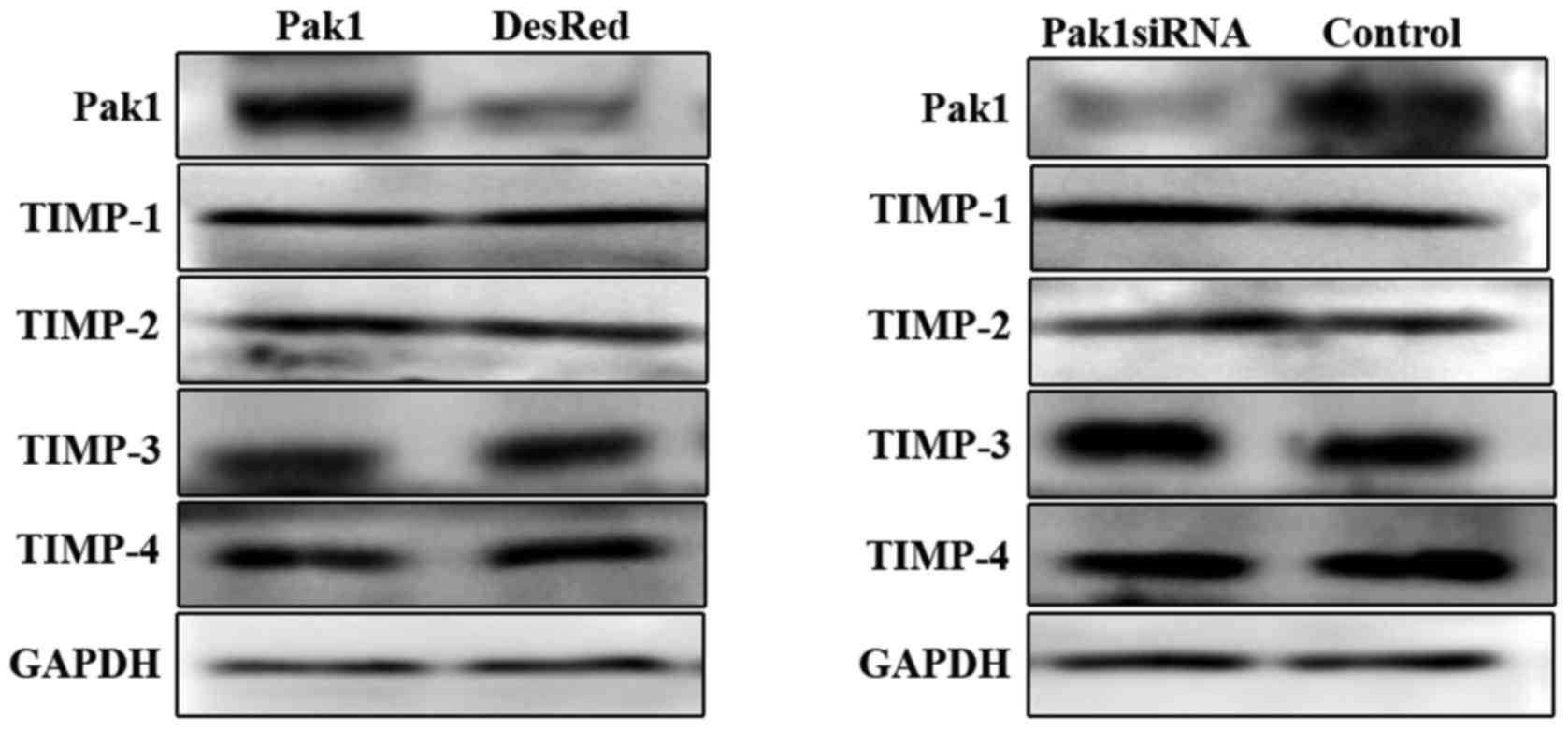
Activation of MMPs is inhibited by TIMPs (18), therefore, we further examined
whether the expression of TIMPs including TIMP-1, −2, −3 and −4,
was regulated by Pak1. Using western blot analysis, it was observed
that overexpression of Pak1 or knockdown of endogenous Pak1 in
MKN45 cells had no effects on the protein level of TIMPs (Fig. 2).
JNK mediates Pak1-induced upregulation
of MMP-2 in human MKN45 GC cells
Our previous study revealed that activation of JNK
was required for Pak1-mediated migration and invasion of GC cells
(19). We therefore assessed
whether JNK is involved in Pak1-upregulated MMP-2 in GC cells.
Endogenous Pak1 was activated in MKN45-DsRed2 cells with epidermal
growth factor (EGF) at 20 ng/ml at various times (0–40 min), and
then, lysates of these cells were subjected to western blotting
with respective antibodies. We observed that JNK phosphorylation
was increased in a time-dependent manner and a maximal increase was
achieved at 30 min, which was determined in our previous study
(19) (Fig. 3A). To investigate whether MMP-2
expression was regulated by JNK phosphorylation, we evaluated the
mRNA of MMP-2 and MMP-9 in MKN45-DsRed2 cells pretreated as in the
aforementioned method by qRT-PCR. The present study revealed that
MMP-2 mRNA expression was obviously increased in a time-dependent
manner and a maximal increase was achieved at 30 min, which was
consistent with the increase of JNK phosphorylation (Fig. 3B). We also performed gelatin
zymography assay to assess the activities of MMP-2 and MMP-9 in
these cells. In accordance with changes in the mRNA expression,
MMP-2 activity was observed to be enhanced in the same manner and a
maximal increase was also achieved at 30 min (Fig. 3C). However, MMP-9 mRNA expression
and activity were not found to be altered (Fig. 3B and C). In addition, we used JNK
special inhibitor SP600125 in MKN45-Pak1 cells at various
concentrations (0, 2.5, 5, 10 and 20 µM) for 1 h to specifically
inhibit the activity of JNK, and found that JNK phosphorylation was
decreased in a concentration-dependent fashion and a maximal
decrease was achieved at 10 µM (Fig.
3D). The qRT-PCR result revealed that MMP-2 mRNA expression was
sharply decreased in a concentration-dependent manner, and a
maximal decrease was achieved at 10 µM, which was consistent with
the downregulation of JNK phosphorylation (Fig. 3E). Gelatin zymography assay also
revealed that MMP-2 activity was downregulated in the same way
(Fig. 3F). MMP-9 mRNA expression
and activity were not observed to be modified due to downregulation
of JNK phosphorylation (Fig. 3E and
F).
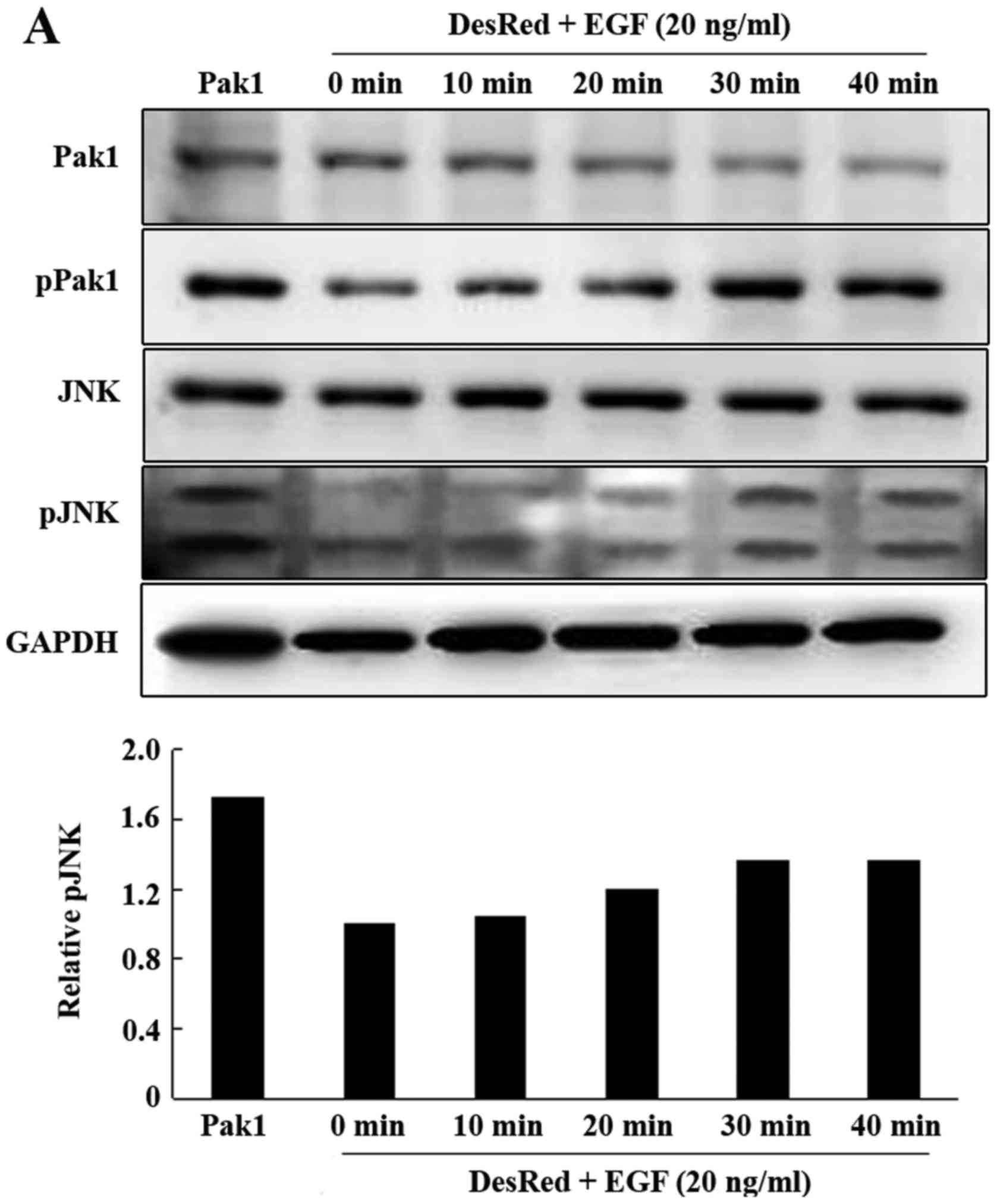 | Figure 3.JNK mediates Pak1-induced upregulation
of MMP-2 in human MKN45 gastric cancer cells. (A) MKN45-DsRed2
cells were serum starved and treated with EGF (20 ng/ml) for
various times as indicated and were subsequently lysed. Western
blot analysis of Pak1, phospho-Pak1, JNK and phospho-JNK (top
panel). Quantification of phospho-JNK at various times (bottom
panel). (B) MKN45-DsRed2 cells were serum starved and treated with
EGF (20 ng/ml) for various times as indicated, MMP-2 and MMP-9 mRNA
were subsequently evaluated using quantitative real-time RT-PCR.
(C) MKN45-DsRed2 cells were serum starved and treated with EGF (20
ng/ml) for various times as indicated. Gelatin zymography assay
results of MMP-2 and MMP-9 activities (top panel). Quantification
of MMP-2 and MMP-9 activities (bottom panel). (D) MKN45-Pak1 cells
were treated with increasing concentrations of SP600125 for 1 h and
were subsequently lysed. Western blot analysis of Pak1,
phospho-Pak1, JNK and phospho-JNK (top panel). Quantification of
phospho-JNK at various concentrations (bottom panel). (E)
MKN45-Pak1 cells were treated with increasing concentrations of
SP600125 for 1 h, MMP-2 and MMP-9 mRNA were subsequently evaluated
using quantitative real-time RT-PCR. (F) MKN45-Pak1 cells were
treated with increasing concentrations of SP600125 for 1 h. Gelatin
zymography assay results of MMP-2 and MMP-9 activities (top panel).
Quantification of MMP-2 and MMP-9 activities (bottom panel). Pak1,
p21-activated kinase 1; GC, gastric cancer; EGF, epidermal growth
factor; JNK, c-Jun N-terminal kinase; MMP, matrix
metalloproteinase. |
Upregulation of MMP-2 by Pak1 via the
JNK pathway is important for the invasion of human MKN45 GC
cells
To further investigate the regulatory role of MMP-2
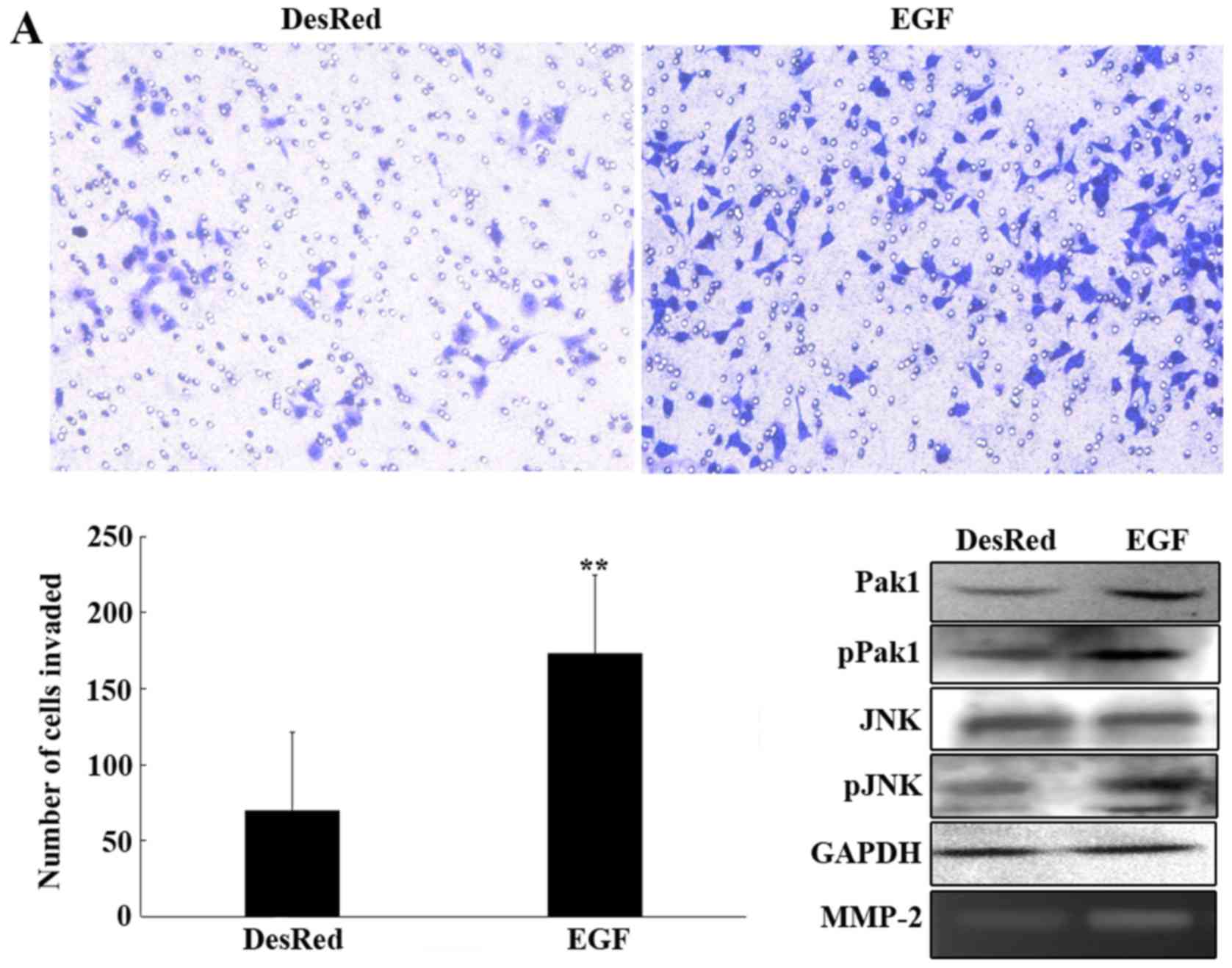
by the JNK pathway on GC cell invasion, an invasion assay was
performed with MKN45-DesRed2 cells incubated with EGF at 20 ng/ml
for 30 min, and the MKN45-DesRed2 cells were used as a control.
Compared with the control, upregulation of MMP-2 expression by JNK
enhanced cell invasion (173.25±6.82 vs. 69.75±7.78 P<0.01;
Fig. 4A). Activation of the JNK
pathway and increase of MMP-2 activity were confirmed by western
blotting and gelatin zymography assay, respectively (Fig. 4A). Another invasion assay was
performed with MKN45-Pak1 cells pretreated with the JNK inhibitor
SP600125 at 10 µM for 1 h, and the MKN45-Pak1 cells were used as a
control. We observed that the MKN45-Pak1 cells pretreated with
SP600125 invaded much more slowly than the control (73.50±5.68 vs.
226.25±10.15, P<0.01; Fig. 4B).
A decrease of JNK phosphorylation and MMP-2 activity were
determined using western blotting and gelatin zymography assay,
respectively (Fig. 4B).
Expression of MMP-2 correlates with
clinicopathological factors of human GC
To further confirm the biological functions of MMP-2
in GC cell invasion, MMP-2 mRNA was analyzed in 57 patient samples
of GC by RT-PCR. The results revealed that MMP-2 mRNA expression in
tumor tissue was significantly associated with the depth of
invasion (P<0.01), lymph node status (P<0.01), distant
metastasis (P<0.01) and tumor stage (P<0.01). However, the
mRNA level of MMP-2 was independent of Lauren classification, tumor
location, sex and age (Table
I).
 | Table I.Association of MMP-2 expression with
clinicopathological features of the GC cases. |
Table I.
Association of MMP-2 expression with
clinicopathological features of the GC cases.
| Clinicopathological
features | n | MMP-2 (mean ±
SD) | P-value |
|---|
| Age (years) |
|
| 0.881 |
|
>60 | 27 | 74.11±11.26 |
|
| ≤60 | 30 | 76.42±10.35 |
|
| Sex |
|
| 0.393 |
| Male | 37 | 80.09±9.56 |
|
|
Female | 20 | 66.39±12.43 |
|
| Tumor location |
|
| 0.699 |
|
Lower | 22 | 80.80±10.91 |
|
|
Middle | 26 | 67.49±12.25 |
|
|
Upper | 9 | 84.33±19.56 |
|
| Lauren
classification |
|
| 0.146 |
|
Intestinal | 35 | 66.53±9.50 |
|
|
Diffuse | 22 | 89.21±12.19 |
|
| Depth of
invasion |
|
| 0.000 |
| T1 | 4 | 4.61±1.94 |
|
| T2 | 18 | 43.43±12.96 |
|
| T3 | 13 | 95.60±13.16 |
|
| T4 | 22 | 102.20±9.87 |
|
| Lymph node
status |
|
| 0.000 |
| N0 | 18 | 35.25±10.59 |
|
| N1 | 21 | 74.65±12.10 |
|
| N2 | 14 | 111.75±10.32 |
|
| N3 | 4 | 131.19±25.49 |
|
| Distant
metastasis |
|
| 0.006 |
| M0 | 52 | 68.95±7.74 |
|
| M1 | 5 | 141.20±4.98 |
|
| Stage |
|
| 0.000 |
| I | 13 | 10.29±2.23 |
|
| II | 7 | 28.93±16.19 |
|
|
III | 23 | 102.71±7.33 |
|
| IV | 13 | 120.30±12.57 |
|
Discussion
Although, our previous study reported that Pak1 was
overexpressed in GC, and obviously induced the invasive potential
of GC cells (19), the exact
molecular mechanism remained unclear. Upregulation of MMP-2 and
MMP-9 is reported to degrade collagen IV, thus, leading to GC cell
invasion and metastasis (14,15).
Thus, we wondered whether Pak1-stimulated GC cell invasion resulted
from increased levels of MMP-2 and MMP-9. MMP-2 and MMP-9
expression are reported to be regulated at many levels, including
gene activation, mRNA stability, proenzyme activation, and
inactivation by endogenous inhibitors in a complex fashion by
numerous oncogene and tumor suppressor pathways and conditions of
hypoxia (20–23). In the present study, we demonstrated
that ectopic expression of Pak1 in MKN45 GC cells significantly
increased MMP-2 mRNA expression, whereas specific knockdown of
endogenous Pak1 in MKN45 cells sharply decreased the mRNA level of
MMP-2, which was not due to changes in mRNA stabilities. We further
revealed that upregulation of Pak1 expression directly induced
MMP-2 activity, whereas downregulation of Pak1 expression decreased
MMP-2 activity. However, Pak1 had no influence on MMP-9 mRNA
expression and activity in MKN45 cells.
Activation of MMPs is regulated by physiological
inhibitors, TIMPs (24). TIMPs not
only directly inhibit MMPs, but also form complexes with MMPs to
control activation and stability of MMPs (18,25).
Four different TIMP species have been identified as TIMP-1, TIMP-2,
TIMP-3 and TIMP-4. TIMP binds to MMP in a 1:1 stoichiometric ratio.
Injection of AdTIMP-2 into preestablished tumors resulted in the
significant decreased metastasis of LLC tumors by >90% (26), which emphasizes the importance of
endogenous regulation of MMP activity by TIMPs. In the present
study, we further detected the protein levels of TIMPs in the MKN45
cells with Pak1 overexpression and MKN45 cells with special
knockdown of Pak1, which surprisingly demonstrated that Pak1 had no
influence on the regulation of TIMPs. The results revealed that
activation of MMP-2 was not related to a decrease in the specific
inhibitor of TIMP-1, TIMP-2, TIMP-3 and TIMP-4 in MKN45 cells.
There was, however, an increase in MMP/TIMP ratios, which is also
in favor of ECM degradation.
Accumulating evidence has been dedicated to
exploring the molecular mechanisms involved in the upregulation of
cancer development. Mitogen-activated protein kinases (MAPKs)
include 3 major subfamilies: the extracellular signal-regulated
kinases (ERKs), the p38 MAPKs and the c-Jun N-terminal kinases
(JNKs) (27). JNK signaling was
revealed to regulate MMP-2 and MMP-9 production, which promoted
invasiveness in human gliomas, colon, ovarian and prostate cancer
(28–31). Our previous data revealed that
activation of JNK was involved in Pak1 regulation of GC cell
invasiveness (19). Thus, we
hypothesized that Pak1 may induce the invasion of GC cells through
the JNK-MMP-2 signaling pathway. Consistent with this hypothesis,
we offered the evidence for the first time, that Pak1 increased
MMP-2 mRNA expression and activity through a JNK-dependent pathway
in MKN45 GC cells. In addition, higher invasive properties were
also revealed in MKN45 cells with activation of JNK-MMP-2 by Pak1
overexpression. In the present study, we also confirmed that MMP-2
mRNA expression was significantly associated with aggressive
progression of GC (depth of invasion, lymph node status, distant
metastasis and tumor stage), mirroring previous studies (13–17).
One of the novel findings of the present study, is
the demonstration that Pak1 is a direct transcriptional activator
of MMP-2 synthesis. In the present study, we demonstrated that Pak1
increased MMP-2 mRNA expression and activity. This is the first
study to reveal that MMP-2 is a target gene of Pak1 activation.
Moreover, our results identified a JNK signaling pathway that
mediates Pak1-stimulated MMP-2 expression, activity and cell
invasion.
In summary, we demonstrated that Pak1 activates
JNK1/2 kinase, and then increases MMP-2 mRNA expression and
activity, which further promotes cellular invasion in human GC
cells.
Acknowledgements
The present study was supported by grants from the
Xiamen Science and Technology Projects (3502Z20124014).
References
|
1
|
Tsugane S and Sasazuki S: Diet and the
risk of gastric cancer: Review of epidemiological evidence. Gastric
Cancer. 10:75–83. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kumar R, Gururaj AE and Barnes CJ:
p21-activated kinases in cancer. Nat Rev Cancer. 6:459–471. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kumar R and Vadlamudi RK: Emerging
functions of p21-activated kinases in human cancer cells. J Cell
Physiol. 193:133–144. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schürmann A, Mooney AF, Sanders LC, Sells
MA, Wang HG, Reed JC and Bokoch GM: p21-activated kinase 1
phosphorylates the death agonist bad and protects cells from
apoptosis. Mol Cell Biol. 20:453–461. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vadlamudi RK, Adam L, Wang RA, Mandal M,
Nguyen D, Sahin A, Chernoff J, Hung MC and Kumar R: Regulatable
expression of p21-activated kinase-1 promotes anchorage-independent
growth and abnormal organization of mitotic spindles in human
epithelial breast cancer cells. J Biol Chem. 275:36238–36244. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Narumiya S, Ishizaki T and Watanabe N: Rho
effectors and reorganization of actin cytoskeleton. FEBS Lett.
410:68–72. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
O'Sullivan GC, Tangney M, Casey G, Ambrose
M, Houston A and Barry OP: Modulation of p21-activated kinase 1
alters the behavior of renal cell carcinoma. Int J Cancer.
121:1930–1940. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ching YP, Leong VY, Lee MF, Xu HT, Jin DY
and Ng IO: P21-activated protein kinase is overexpressed in
hepatocellular carcinoma and enhances cancer metastasis involving
c-Jun NH2-terminal kinase activation and paxillin phosphorylation.
Cancer Res. 67:3601–3608. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yoon WH, Jung YJ, Kim TD, Li G, Park BJ,
Kim JY, Lee YC, Kim JM, Park JI, Park HD, et al: Gabexate mesilate
inhibits colon cancer growth, invasion, and metastasis byreducing
matrix metalloproteinases and angiogenesis. Clin Cancer Res.
10:4517–4526. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Coussens LM, Fingleton B and Matrisian LM:
Matrix metalloproteinase inhibitors and cancer: Trials and
tribulations. Science. 295:2387–2392. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ellerbroek SM and Stack MS: Membrane
associated matrix metalloproteinases in metastasis. Bioessays.
21:940–949. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liotta LA and Stetler-Stevenson WG: Tumor
invasion and metastasis: An imbalance of positive and negative
regulation. Cancer Res. 51 Suppl 18:5054s–5059s. 1991.PubMed/NCBI
|
|
13
|
Nomura H, Fujimoto N, Seiki M, Mai M and
Okada Y: Enhanced production of matrix metalloproteinases and
activation of matrix metalloproteinase 2 (gelatinase A) in human
gastric carcinomas. Int J Cancer. 69:9–16. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Torii A, Kodera Y, Uesaka K, Hirai T,
Yasui K, Morimoto T, Yamamura Y, Kato T, Hayakawa T, Fujimoto N, et
al: Plasma concentration of matrix metalloproteinase 9 in gastric
cancer. Br J Surg. 84:133–136. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kubben FJ, Sier CF, van Duijn W, Griffioen
G, Hanemaaijer R, van de Velde CJ, van Krieken JH, Lamers CB and
Verspaget HW: Matrix metalloproteinase-2 is a consistent prognostic
factor in gastric cancer. Br J Cancer. 94:1035–1040. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sier CFM, Kubben FJGM, Ganesh S, Heerding
MM, Griffioen G, Hanemaaijer R, van Krieken JH, Lamers CB and
Verspaget HW: Tissue levels of matrix metalloproteinases MMP-2 and
MMP-9 are related to the overall survival of patients with gastric
carcinoma. Br J Cancer. 74:413–417. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shen W, Xi H, Wei B and Chen L: The
prognostic role of matrix metalloproteinase 2 in gastric cancer: A
systematic review with meta-analysis. J Cancer Res Clin Oncol.
140:1003–1009. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gomez DE, Alonso DF, Yoshiji H and
Thorgeirsson UP: Tissue inhibitors of metalloproteinases:
Structure, regulation and biological functions. Eur J Cell Biol.
74:111–122. 1997.PubMed/NCBI
|
|
19
|
Li LH, Luo Q, Zheng MH, Pan C, Wu GY, Lu
YZ, Feng B, Chen XH and Liu BY: P21-activated protein kinase 1 is
overexpressed in gastric cancer and induces cancer metastasis.
Oncol Rep. 27:1435–1442. 2012.PubMed/NCBI
|
|
20
|
Sehgal I and Thompson TC: Novel regulation
of type IV collagenase (matrix metalloproteinase-9 and −2)
activities by transforming growth factor-β1 in human prostate
cancer cell lines. Mol Biol Cell. 10:407–416. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Baruch RR, Melinscak H, Lo J, Liu Y, Yeung
O and Hurta RA: Altered matrix metalloproteinase expression
associated with oncogene-mediated cellular transformation and
metastasis formation. Cell Biol Int. 25:411–420. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nair RR and Boyd DD: Expression cloning of
novel regulators of 92 kDa type IV collagenase expression. Biochem
Soc Trans. 33:1135–1136. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Muñoz-Nájar UM, Neurath KM, Vumbaca F and
KP; MunozNajar UM Claffey: Hypoxia stimulates breast carcinoma cell
invasion through MT1-MMP and MMP-2 activation. Oncogene.
25:2379–2392. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li YY, McTiernan CF and Feldman AM:
Interplay of matrix metalloproteinases, tissue inhibitors of
metalloproteinases and their regulators in cardiac matrix
remodeling. Cardiovasc Res. 46:214–224. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Goldberg GI, Marmer BL, Grant GA, Eisen
AZ, Wilhelm S and He CS: Human 72-kilodalton type IV collagenase
forms a complex with a tissue inhibitor of metalloproteases
designated TIMP-2. Proc Natl Acad Sci USA. 86:8207–8211. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li H, Lindenmeyer F, Grenet C, Opolon P,
Menashi S, Soria C, Yeh P, Perricaudet M and Lu H: AdTIMP-2
inhibits tumor growth, angiogenesis, and metastasis, and prolongs
survival in mice. Hum Gene Ther. 12:515–526. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Marino M, Galluzzo P and Ascenzi P:
Estrogen signaling multiple pathways to impact gene transcription.
Curr Genomics. 7:497–508. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hu B, Jarzynka MJ, Guo P, Imanishi Y,
Schlaepfer DD and Cheng SY: Angiopoietin 2 induces glioma cell
invasion by stimulating matrix metalloprotease 2 expression through
the alphavbeta1 integrin and focal adhesion kinase signaling
pathway. Cancer Res. 66:775–783. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hsu HH, Hu WS, Lin YM, Kuo WW, Chen LM,
Chen WK, Hwang JM, Tsai FJ, Liu CJ and Huang CY: JNK suppression is
essential for 17β-Estradiol inhibits prostaglandin E2-Induced uPA
and MMP-9 expressions and cell migration in human LoVo colon cancer
cells. J Biomed Sci. 18:61–72. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cheung LW, Leung PC and Wong AS:
Gonadotropin-releasing hormone promotes ovarian cancer cell
invasiveness through c-Jun NH2-terminal kinase-mediated activation
of matrix metalloproteinase (MMP)-2 and MMP-9. Cancer Res.
66:10902–10910. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chien CS, Shen KH, Huang JS, Ko SC and
Shih YW: Antimetastatic potential of fisetin involves inactivation
of the PI3K/Akt and JNK signaling pathways with downregulation of
MMP-2/9 expressions in prostate cancer PC-3 cells. Mol Cell
Biochem. 333:169–180. 2010. View Article : Google Scholar : PubMed/NCBI
|