Introduction
Gastric cancer (GC) is a highly malignant digestive
tract tumor, and most GC patients have a poor prognosis due to lack
of an early diagnostic indicator (1). Thus, it is crucial to find early
effective biological indicators of GC to improve the survival rate
of GC patients. For the past few years, a large amount of reports
confirmed that miRNAs are closely related to GC genesis and
development (2–8).
miRNAs are short endogenous RNAs and they have no
function of protein coding. Numerous studies have shown that
dysregulated expression of miRNA participates in GC malignant
phenotype. Wu et al illuminated that high expression of
miR-15a in GC led to Bmi-1 protein inactivation and had a negative
effect on overall survival of GC patients (9). Seok et al proved that hypoxia
could activate the function of miR-382, whereafter, inhibited the
expression of PTEN gene and played a role of angiogenic oncogene in
GC (10). Previous studies also
suggested that miRNAs had a critical function of human GC
progression (11,12). However, the mechanism of aberrant
regulation of miRNA in GC remained largely unknown. Latest studies
found that the abnormal expression of miRNA might be ascribed to
promoter hypermethylation. For instance, Yin et al
considered that the inhibition of miR-33b was mediated by DNA
methylation in GC and the lower-expression of miR-33b contribute to
advanced GC clinical stage (13).
Li et al indicated that promoter hypermethylation was
responsible for the downregulation of miR-335 and overexpression of
miR-335 could inhibit GC cells invasion and metastasis by targeting
RASA1 (14). This evidence
suggested that miRNAs might serve as a novel biomarker and played
an important role in gastric cancer.
miR-497 was first reported in breast cancer and is
located on chromosome 17p13.1 (15). Numerous studies had reported that
miR-497 was closely related to various carcinomas. For example, Yan
et al demonstrated that miR-497 negatively regulated VEGFA
and inhibited hepatocellular carcinoma metastasis (16). Wang et al proved that miR-497
repressed angiogenesis of ovarian cancer by retrograde regulated
PI3K/AKT pathway (17). However,
Jiang et al found that miR-497 acted as an oncogene in
colorectal cancer (18). Wald et
al also proved that the expression of miR-497 was increased in
human head and neck carcinoma (19). In fact, there is some controversy
about the effect of miR-497 in different tumors and the unique
expression of miR-497 in different cancers might be
tissue-specific. Despite the many reports that miR-497 has an
important role in various human carcinomas, the regulatory
mechanism of miR-497 in GC was still unclear.
In this investigation, we validated that miR-497 was
reduced significantly in GC and repressed GC cell proliferation,
invasion and metastasis. Further research indicated that gene
promoter hypermethylation might contribute to dysregulation of
miR-497. We also revealed that miR-497 mediated GC progression by
retrograde targeting RAF1.
Materials and methods
Gastric cancer cell lines and clinical
samples
Human GC cell lines SGC-7901, MGC-803, MKN-45,
BGC-823 and a normal gastric mucomembrane cell line GES-1 were
obtained from Chinese Academy of Sciences, Shanghai Institutes for
Cell Resource Center. Cells were cultivated in RPMI-1640 medium
(Corning, Manassas, VA, USA) containing 10% FBS (Corning). All
cells were maintained in 5% CO2 at 37°C. Eighty-six
cases of GC tissues were collected from 2013 to 2015 at Cancer
Research Institute of China Medical University, tissues were
histologically confirmed and stored at −80°C after surgical removal
until further analysis. The study was approved by the Ethics
Committee of China Medical University.
Cell treatments
For cell transfection, cells were grown in 6-well
culture plates for 24 h, then, 50 µM miR-497 mimics or mimics
control (mimics-NC) (GenePharma, Shanghai, China) was added into
the culture medium using Lipofectamine 3000 reagent (Invitrogen,
Calsbad, CA, USA) following the manufacturer's instructions. For
5-Aza-dC (Sigma, St. Louis, MO, USA) and trichostatin A (TSA)
(Sigma) treatment assay, SGC-7901 and MGC-803 cells were first
incubated in 6-well culture dishes for 24 h. Then, for 5-Aza-dC
treatment group, 0, 0.5, 1 or 1.5 µmol/l 5-Aza-dC were mixed with
the cell culture medium for 72 h. For TSA treatment group, cells
were treated with 0.3 µmol/l TSA for 24 h. For combination of drugs
intervention group, 1 µmol/l 5-Aza-dC was first mixed with the
medium for 48 h and then 0.3 µmol/l 5-Aza-dC was added for another
24 h.
RNA extraction and qRT-PCR
RNA Extraction kit (Takara, Dalian, China) was used
to purify total RNA from GC cells and tissues. Reverse
transcription and qRT-PCR were performed using SYBR Green qRT-PCR
kit (GenePharma). U6 SnRNA was identified as an internal reference.
The U6 primers used were 5′-ATTGGAACGATACAGAGAAGATT-3′ and
5′-GGAACGCTTCACGAATTTG-3′, the miR-497 primers used were
5′-GACGACCTCAGCAGCACACT-3′ and 5′-CAGAGCAGGGTCCGAGGTA-3′. We
conducted the PCR assay according to our previous report (20) and the experiment was repeated in
triplicate.
DNA extraction and MSP
A GenElute™ Mammalian Genomic DNA Miniprep kit
(Sigma) was used to separate the genetic DNA from the paired GC
tissues. Then, purified DNA was conducted with a bisulfite
treatment using the EZ DNA Methylation-Gold kit™ (Zymo Research,
Irvine, CA, USA), this assay was carried out according to the
manufacturer's instructions. MethPrimer 1.0 was used to design MSP
primer for methylation-specific PCR (MSP). The methylated primers
used were 5′-TTTGTTTTTGTGTTAGAGAGGGTTC-3′ (forward) and
5′-TTTGTTTTTGTGTTAGAGAGGGTTC-3′ (reverse), the unmethylated primers
used were 5′-TGTTTTTGTGTTAGAGAGGGTTTG-3′ (forward) and
5′-ACTAACAATACAACTACATCCCATA-3′ (reverse). We conducted the PCR
assay according to Jia et al (21), this experiment was repeated in
triplicate.
Cell proliferation assay and cell
apoptosis assay
Cell Counting Kit-8 (Dojindo, Kumamato, Japan) was
used to detect the proliferation rates of GC cells. First, 3,000
SGC-7901 and MGC-803 cells were incubated into 96-well plates for
24 h. Then, 10 µl CCK-8 was added into each well, proliferation
rates of GC cells were surveyed at 0, 24, 48 and 72 h after
transfection. This experiment was repeated for three times. Annexin
V-PE/7AAD Apoptosis Detection kit (KeyGen, Jiangsu, China) was
applied to perform the cell apoptosis assay according to the
manufacturer's instructions and this experiment was repeated in
triplicate.
Cell migration and invasion
assays
Transfected cell lines were grown in 6-well plates
for 24 h and linear wounds were scratched on the confluent cells
using a 200-µl pipette tip. The serum-free medium was replaced by
cell culture and we used an inverted microscope to visualize
migrated cells and wound healings. For cell invasion assay,
Matrigel-coated membrane matrix (Dojindo) was smeared onto the
upper chamber for 24 h, then, 1×105 transfected cells
were grown in the upper chambers with serum-free medium and the
lower chambers was added with medium containing 10% FBS.
Twenty-four hours later, non-invasive cells were removed using a
cotton tip and the invasive cells were stained using crystal violet
(Tiangen, Beijing, China). Images were taken using an inverted
microscope. Each sample was repeated in triplicate.
Western blot analysis and
histology
Proteins were extracted from GC cells and clinical
samples using RIPA buffer (Beyotime, Shanghai, China) and BCA
Protein assay kit (Takara) was used to measure the protein
concentration. Then, 30 µg proteins were added into 10% SDS-PAGE
(Takara) and polyvinyl fluoride membranes (Beyotime) were conducted
as a transmembrane. Membranes were blocked for 2 h with BSA (Sigma)
and kept at 4°C overnight with anti-RAF1 antibody and we set an
internal control using β-actin. The dilution ratio was 1:1,000.
Thereafter, the membrances were incubated with secondary antibody
(Sigma) at room temperature for 2 h. At last, images were
determined using an enhanced chemiluminescence system. For
histology, first, buffered formalin was used to fix tissues and
then we embedded tissues in paraffin. Second, paraffin-embedded
blocks were cut into 3 µm slices and stained with hematoxylin and
eosin (H&E).
Luciferase reporter assay
miR-497 mimics or mimics control (mimics-NC),
reporter construct or control vector (GenePharma) were transfected
in GC cells for 24 h. Dual Luciferase assay kit (KeyGen) was used
to detect luciferase activity. The ratio of luciferase intensities
was calculated and normalized to controls. Each sample was carried
out in triplicate.
Statistical analysis
We used SPSS 19.0 to conduct statistical analyses
and Student's t-test, an ANOVA test and χ2 test was
utilized to analyze P-values. P<0.05 was considered to indicate
a statistically significant difference (two-sided).
Results
miR-497 is decreased in GC cell lines
and tissues
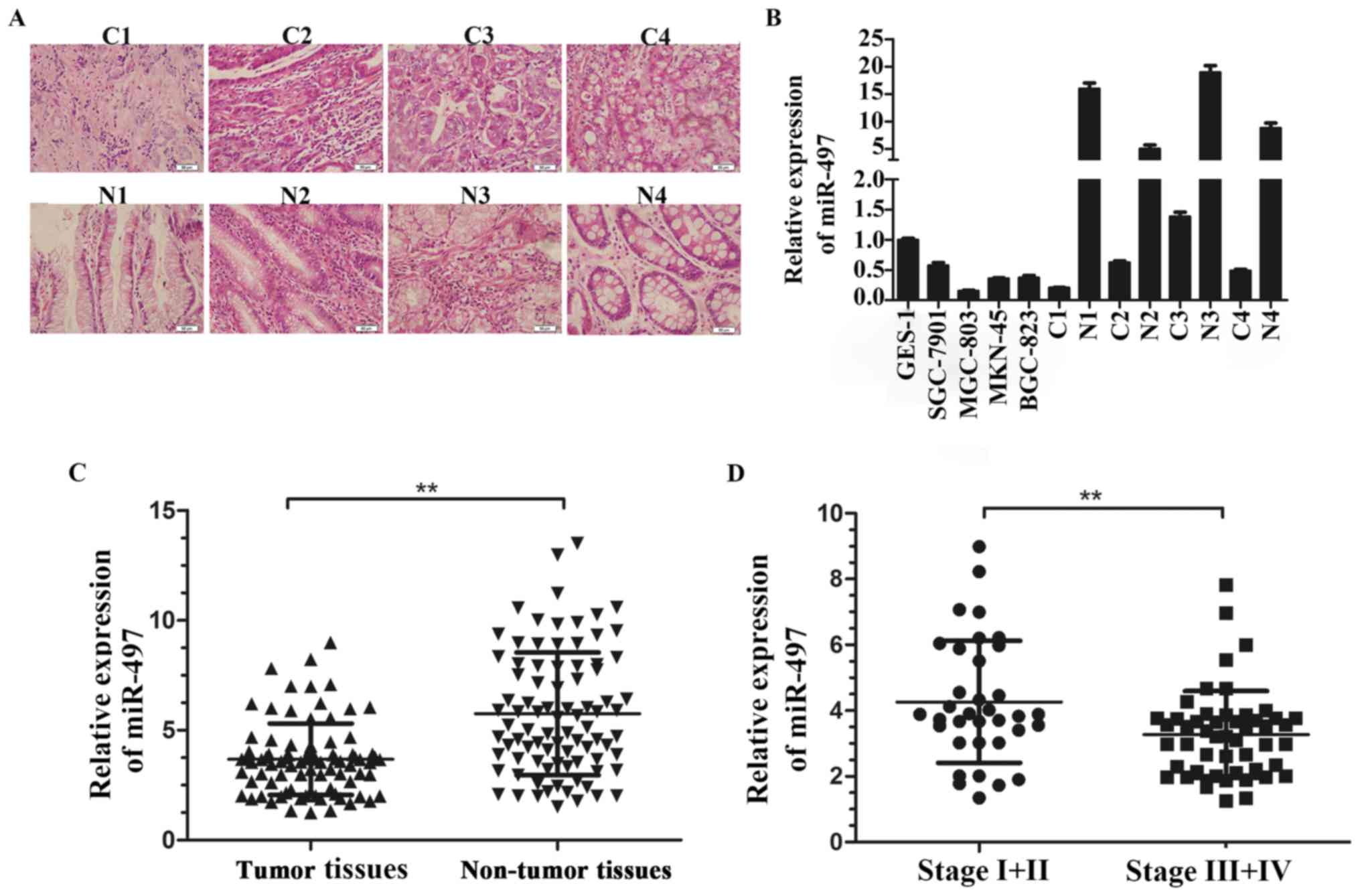
We first examined the expression level of miR-497 in
four GC cell lines (SGC-7901, MGC-803, MKN-45, BGC-823) and four
paired GC tissues (Fig. 1A) and
GES-1 was used as a standard control, the data suggested miR-497
was significantly downregulated in GC cell lines compared with
GES-1, similarly, GC tissues showed low expression of miR-497
compared to the corresponding normal tissues (Fig. 1B). To further assess miR-497
expression in GC, we measured the expression level of miR-497 in 86
pairs of GC tissues and corresponding normal tissues. The data
demonstrated that miR-497 was significantly downregulated in GC
tissues compared to adjacent normal tissues (Fig. 1C). The data proved that low
expression of miR-497 was frequent in gastric cancer and might be
relevant to GC genesis.
The low expression of miR-497 in GC
indicates advanced clinical stage
To illuminate the relevance between miR-497
expression and GC clinicopathological parameters, we separated the
patients into two groups according to the mean level of miR-497.
Our data showed low miR-497 expression was apt to have an advanced
GC TNM stage (P=0.014). However, no obvious relationship was found
between miR-497 expression and tumor size or location (Table I and Fig. 1D). The result indicated that miR-497
might be involved in GC malignant progression.
 | Table I.Clinicopathological characteristics
of patients with gastric cancer (GC). |
Table I.
Clinicopathological characteristics
of patients with gastric cancer (GC).
|
|
| miR-497
expression |
|
|---|
|
|
|
|
|
|---|
| Parameter | Total samples (n)
n=86 | Low expression
(%) | High expression
(5) | P-value |
|---|
| Age (years) |
| 48 | 38 | 0.681 |
|
≥60 | 23 | 12 (52.2) | 11 (47.8) |
|
|
<60 | 63 | 26 (57.1) | 27 (42.9) |
|
| Sex |
|
|
| 0.310 |
|
Male | 66 | 39 (59.1) | 27 (40.9) |
|
|
Female | 20 | 9
(45.0) | 11 (55.0) |
|
| Location |
|
|
| 0.557 |
|
Proximal | 12 | 5
(41.7) | 7
(58.3) |
|
|
Body | 20 | 12 (60.0) | 8
(40.0) |
|
|
Distal | 54 | 31 (57.4) | 23 (42.6) |
|
| Tumor size |
|
|
| 0.730 |
|
T1-T2 | 38 | 22 (57.9) | 16 (42.1) |
|
|
T3-T4 | 48 | 26 (54.2) | 22 (45.8) |
|
| Borrmann type |
|
|
| 0.459 |
|
Borrmann 1+2 | 11 | 5
(45.5) | 6
(54.5) |
|
|
Borrmann 3+4 | 75 | 43 (57.3) | 32 (42.7) |
|
| Grade |
|
|
| 0.298 |
| Well
and moderately differentiated | 31 | 15 (48.4) | 16 (51.6) |
|
| Poorly
differentiated | 55 | 33 (60.0) | 22 (40.0) |
|
| TNM stage |
|
|
| 0.014 |
|
I+II | 35 | 14 (66.7) | 21 (33.3) |
|
|
III+IV | 51 | 34 (55.8) | 17 (44.2) |
|
Effects of miR-497 on GC cell
proliferation and apoptosis
In order to analyze the role of miR-497 in GC
tumorigenesis and progression, miR-497 mimics were transfected in
SGC-7901 and MGC-803 cells using Lipo 3000. To measure the
transfection efficiency, qRT-PCR was applied to determine miR-497
expression level after cell transfection, the data exhibited that
miR-497 was upregulated 154.33±11.50 times and 96.74±6.82 times in
SGC-7901 and MGC-803 cells (Fig.
2A). As shown in CCK-8 growth assay, restoration of miR-497
obviously reduced the growth of SGC-7901 and MGC-803 cells
(Fig. 2B). Our data proved that
miR-497 obviously inhibted GC cell proliferation. To further
explore the effect of miR-497 on GC cell apoptosis, cell apoptosis
assay was carried out to count early apoptosis cells. The miR-497
mimics control group showed few early apoptotic cells (5.32±0.93%
in SGC-7901 and 4.24±0.82% in MGC-803), whereas mimics-transfected
group revealed a higher percentage of early apoptotic cells
(12.66±2.31% in SGC-7901 and 8.11±1.27% in MGC-803), Quadrant IV of
flow cytometry chart is early apoptotic cells (Fig. 2C). In general, we showed that
miR-497 might inhibit GC cell survival by promoting cell
apoptosis.
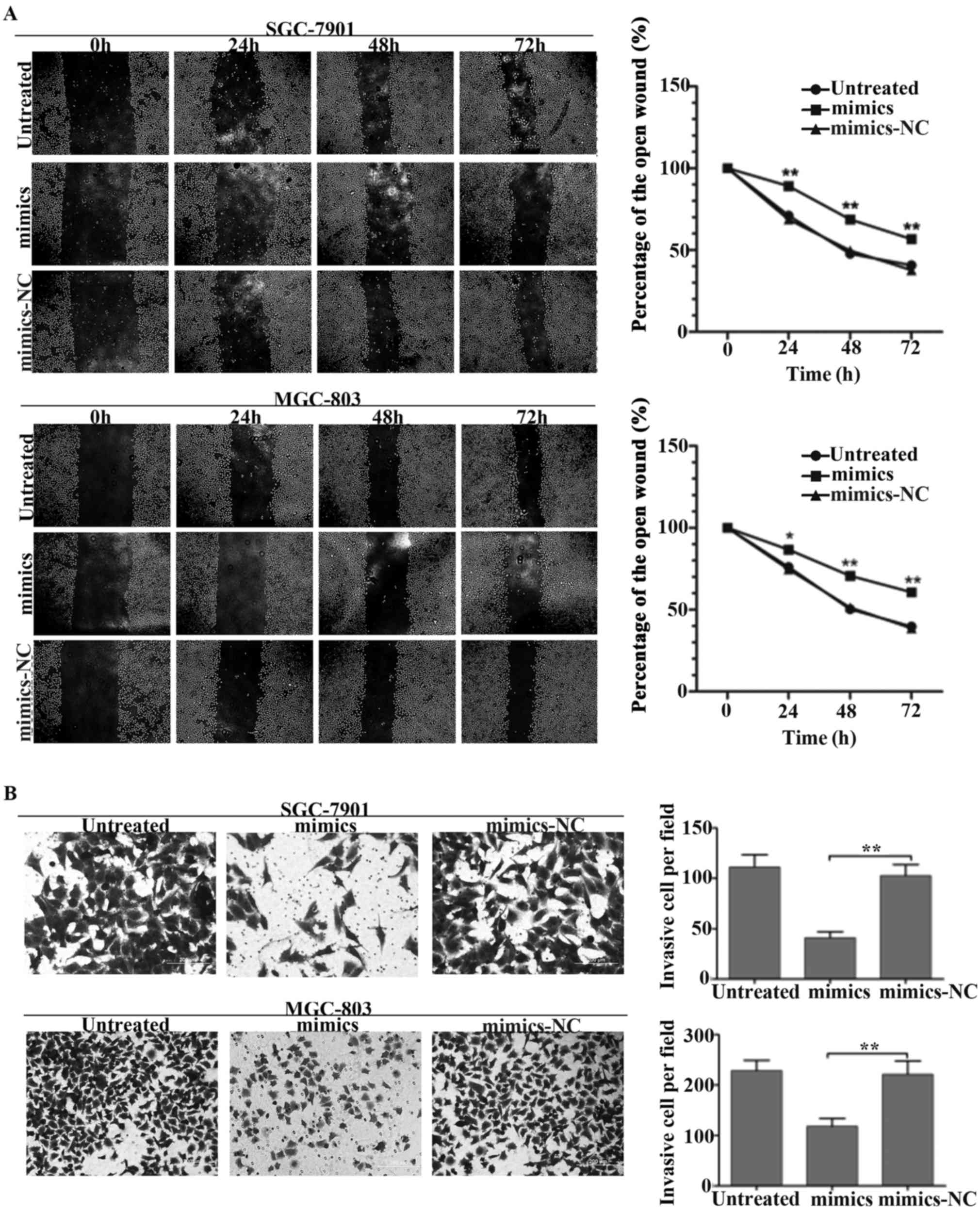
miR-497 inhibits GC cell migration and
invasion in vitro
To explore whether miR-497 could regulate the
metastasis ability of GC, a wound healing assay was performed to
measure the GC cell migration ability. As showed in Fig. 3A, a significant difference was
detected in a wound healing assay. Overexpression of miR-497 in
SGC-7901 and MGC-803 cells induced a great reduction of cell
migration. Moreover, transwell invasion assay also demonstrated
that upregulation of miR-497 dramatically inhibited cell migration
in GC cells (Fig. 3B). The result
was consistent with our viewpoint that the reduction of miR-497 in
GC might contribute to tumor metastasis in gastric
carcinogenesis.
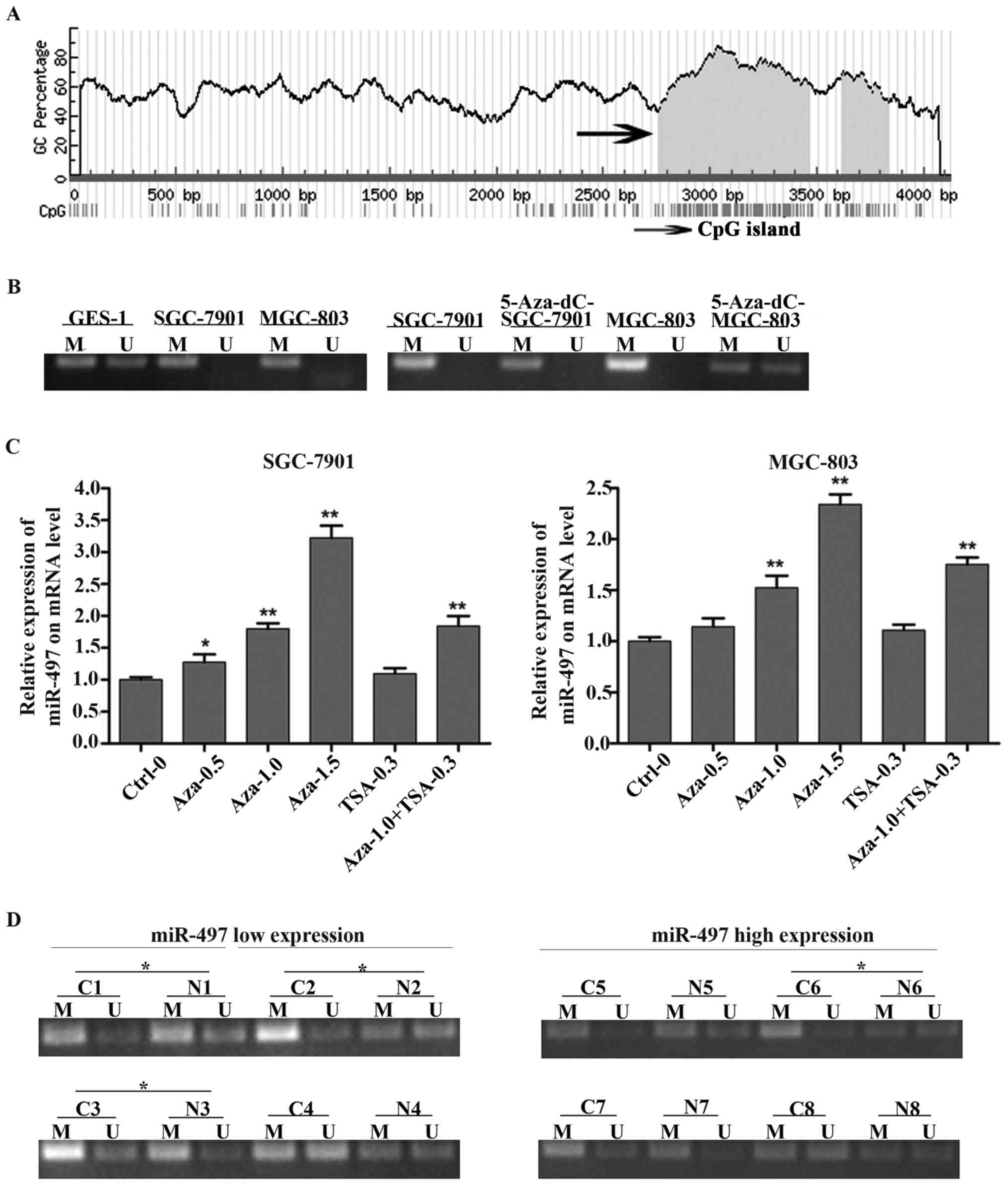
Downregulation of miR-497 is
associated with promoter hyper-methylation
In order to investigate the regulatory mechanism of
low expression of miR-497 in GC, human genome database was used to
find CpG islands around miR-497 (Fig.
4A) and MSP primers were conducted to analyze the methylation
status of the miR-497 gene promoter in GC cell lines (SGC-7901,
MGC-803), the result showed that SGC-7901 and MGC-803 cells
possessed a hypermethylation status of miR-497 gene promoter
compared with GES-1. We also analyzed the methylation status of
SGC-7901 and MGC-803 cells treated with 5-Aza-dC, the result showed
that 5-Aza-dC could change the hypermethylation status of miR-497
gene promoter in SGC-7901 and MGC-803 cells (Fig. 4B). To further confirm whether the
reduction of miR-497 in GC was due to DNA methylation, DNA
methylation inhibitor 5-Aza-dC and histone deacetylase inhibitor
TSA were used to treat GC cells and the expression level of miR-497
was detected immediately. The result proved that miR-497 expression
was increased after treatment with 5-Aza-dC and miR-497 expression
was restored the most when the 5-Aza-dC concentration was set to
1.5 µM. However, there was no obvious change of miR-497 expression
after treatment with TSA alone (Fig.
4C). To further illuminate the relationship between low
expression of miR-497 and gene promoter methylation in GC, we
measured the methylation status of the miR-497 promoter of 8 cases
of paired GC tissues. Eight cases of GC samples were divided into
two groups according to the expression level of miR-497. The result
showed that the low miR-497 expression group possessed a higher
hyper-methylation percentage (75%, 3/4) opposed to the high miR-497
expression group (25%, 1/4) (Fig.
4D). The result indicated that the low miR-497 expression in GC
might be due to gene promoter hyper-methylation.
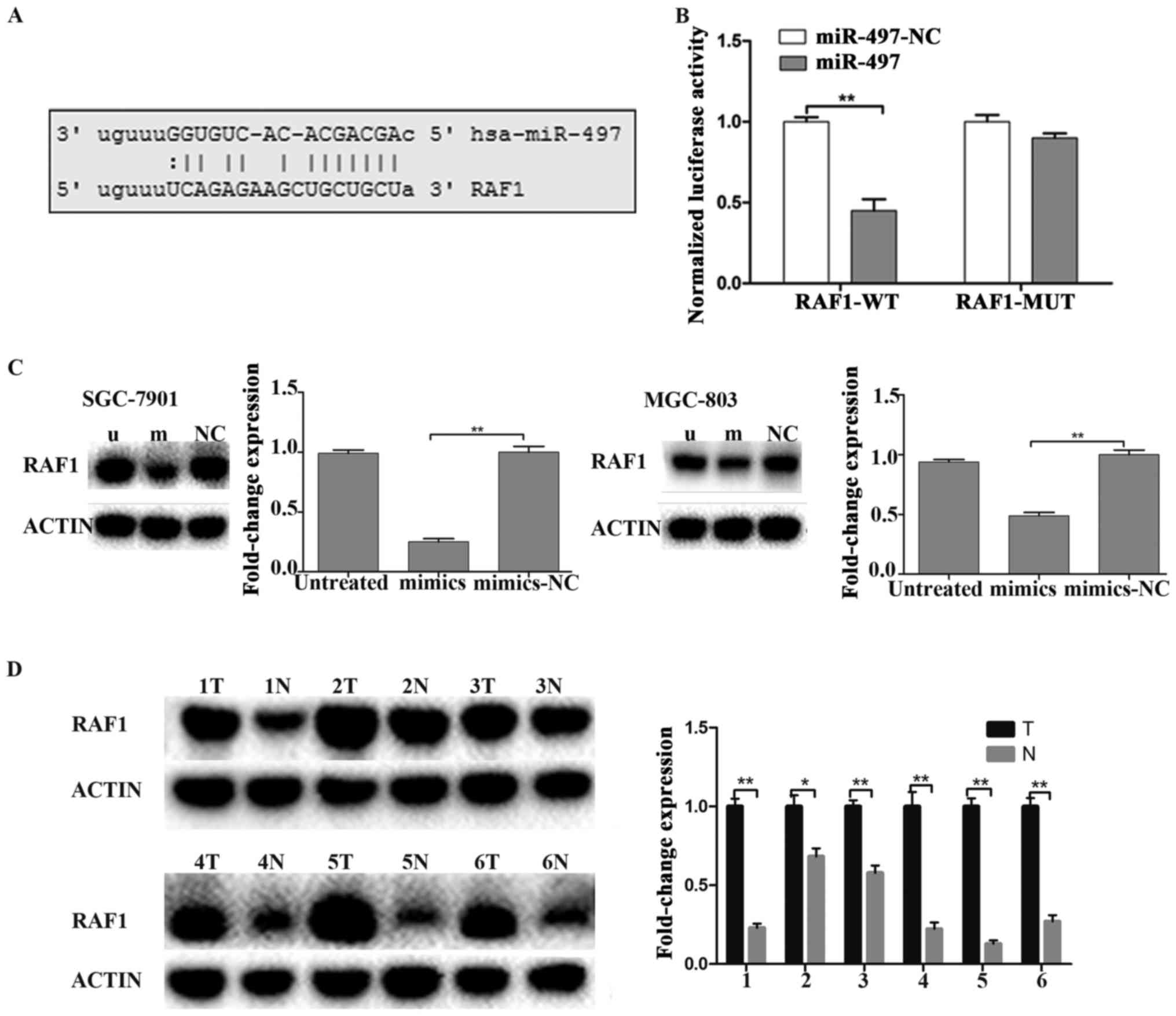
miR-497 targets RAF1 in GC
miRNAs play their biologic role through
downregulating their target genes. microRNA.org
was utilized for finding target genes of miR-497. The result showed
that miR-497 has a binding site on the RAF1 mRNA 3′-UTR (Fig. 5A). Futhermore, luciferase reporter
assay showed that miR-497 mimics could induce an obviously
reduction of the luciferase activity of wild-type RAF1-3′-UTR.
However, miR-497 mimics could not affect the luciferase activity of
mutant RAF1-3′-UTR (Fig. 5B). The
result further indicated that miR-497 has a binding site on the
RAF1 mRNA 3′-UTR. To confirm that miR-497 performs biological
functions in GC by targeting RAF1, western blotting was applied to
measure the protein level of RAF1 in SGC-7901 and MGC-803 cells. We
concluded that the protein level of RAF1 was obviously
downregulated in miR-497 mimics-treated cells compared to
scramble-treated and untreated cells (Fig. 5C). We further measured the
expression of RAF1 protein in low miR-497 expression GC patients
and we found that RAF1 was obviously increased in GC tissues
compared to the adjacent normal tissues (Fig. 5D), which was consistent with the
results in GC cells.
Discussion
Previous studies suggested that miRNAs functioned as
oncogenes or tumor suppressors in various carcinomas and miRNAs
played important roles in GC carcinogenesis and progression
(22–24). However, the possible molecular
mechanism of miRNAs in regulating GC occurrence and progression was
unknown. Here we proved that miR-497 was obviously downregulated in
GC cells and tissues and the most important was that reduction of
miR-497 was significantly associated with advanced clinical stage.
Moreover, we demonstrated that restoration of miR-497 induced GC
cell apoptosis and inhibited GC cell proliferation, migration, and
invasion. To further clarify the regulatory mechanism of miR-497 in
GC, we found that downregulation of miR-497 might due to DNA
methylation and RAF1 was regarded as a direct target of miR-497 in
GC.
Yan et al first indicated that miR-497 was
downregulated in human breast cancer (15), which was further confirmed by
Lehmann et al and Li et al (25,26).
Han et al showed that low expression of miR-497 in lung
cancer could inhibit lung cancer cell growth through negatively
regulating cyclin E1 expression (27). Xu et al found that miR-497
was decreased in ovarian cancer and might act as a potential
therapeutic target. However, Wald et al reported that
miR-497 was upregulated in human head and neck carcinoma (19). Jiang et al also found that
miR-497 was overexpressed in colorectal cancer (18). The aberrant expression of miR-497 in
different tumors might be ascribed to tissue specificity. A recent
study proved that miR-497 was downregulated in multidrug-resistant
SGC-7901 cell lines and a latest research indicated that miR-497
was downregulated in GC tissues (28,29).
However, the molecular mechanism and the possible regulatory
pathway of downregulation of miR-497 in GC remain unclear. For this
reason, we detected the expression of miR-497 in GC cell lines and
tissues. The data indicated that miR-497 was significantly
decreased in GC cell lines as well as in GC tissues compared to the
normal control. Furthermore, we explored the clinicopathological
correlations of miR-497 in GC tissues and the data proved that
patients with low miR-497 expression possessed an advanced clinical
stage. All the data suggested miR-497 might participate in GC
tumorigenesis and progression.
Accumulating studies illuminated that miRNAs were
closely related to tumorigenesis, however, the exact mechanism
involved were unknown. To demonstrated the effect of miR-497 on GC
carcinogenesis and progression, we conducted cellular function
tests including cell proliferation assay, cell invasion assay and
cell apoptosis assay. The result proved that restoration of miR-497
obviously inhibited GC cell proliferation, migration, and invasion,
the result also indicated that overexpression of miR-497
significantly induced GC cell apoptosis. Apoptosis can be seen as a
stage-dependent process from its induction to early, intermediate
and late-stage apoptotic events. Early apoptosis is represented by
changes to, and ultimate loss of, the mitochondrial membrane
potential. Our study indicated that low expression of miR-497 in GC
was a frequent event and might play crucial roles in GC
tumorigenesis. Recently, numerous studies proved that
downregulation of miRNAs was associated with epigenetic
modifications, including DNA methylation and histone modification
(30,31). Among them, DNA methylation was one
of the most frequent epigenetic changes in human cancers and might
be responsible for downregulation of miRNAs in various carcinomas
(14,32). In this study, we detected that
miR-497 was located in a CpG island on chromosome 17P13.1 and the
MSP analysis showed that GC cell lines possessed a
hyper-methylation status compared with the normal control.
Moreover, GC cells treated with 5-Aza-dC showed DNA promoter
demethylation, whereas cells treated with TSA demonstrated no
obvious alterations of DNA methylation status. Thus, we concluded
that downregulation of miR-497 might be inactivated by
hyper-methylation in GC.
Moreover, our study found RAF1 harboring miR-497
binding site and RAF1 was considered to be a potential target of
miR-497. Particularly, RAF1 gene was located in 3p25.2 chromosome
and its aberrant expression was reported to be associated with
tumor malignant biological properties (33–35).
Activation of the RAS-RAF pathway was responsible for poor
prognosis of colorectal cancer patients (36). Moreover, hyperactivation of RAF1
could induce cancer cell resistance to multidrugs through
RAF1-MEK1-ERK/AKT pathway and might have predictive value in
treatments of cancers (37). These
studies suggested that RAF1 could act as a critical parameter for
tumor genesis and progression. Furthermore, Wang et al
showed that miR-195 inhibited cell proliferation through blocking
the expression of RAF1 in thyroid cancer (38). Liu et al indicated that
miR-7-5p suppressed cell proliferation through directly targeting
RAF1 in glioblastoma (39).
Accumulating evidence has proved that RAF1 is upregulated in
various carcinomas, however, the relationship between miRNAs and
RAF1 in GC was still unclear. In this report, we found the protein
level of RAF1 in miR-497 mimics-transfected GC cells was
significantly lower than normal control. The result proved that
miR-497 might serve as a tumor suppressor gene in GC by targeting
RAF1.
In conclusion, our results indicated that miR-497
had a tumoral suppression function through targeting RAF1 in GC and
decreased expression of miR-497 might due to DNA hypermethylation.
GC patients who possessed low miR-497 level were apt to have an
advanced GC clinical stage. Restoration of miR-497 obviously
inhibited GC cell proliferation, migration and invasion. The result
proved that miR-497 could act as a novel biomarker and therapeutic
target in gastric cancer.
Acknowledgements
This study was supported in part by a grant from the
National Natural Science Foundation of China (no. 30572162), the
Liaoning Province Science and Technology Plan Project (no.
2013225021) and the Natural Science Foundation of Liaoning Province
(no. 201602817).
References
|
1
|
Li G, Wang Z, Ye J, Zhang X, Wu H, Peng J,
Song W, Chen C, Cai S, He Y, et al: Uncontrolled inflammation
induced by AEG-1 promotes gastric cancer and poor prognosis. Cancer
Res. 74:5541–5552. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jiang C, Chen X, Alattar M, Wei J and Liu
H: MicroRNAs in tumorigenesis, metastasis, diagnosis and prognosis
of gastric cancer. Cancer Gene Ther. 22:291–301. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang L, Liang H, Wang Y, Gao S, Yin K, Liu
Z, Zheng X, Lv Y, Wang L, Zhang CY, et al: MiRNA-203 suppresses
tumor cell proliferation, migration and invasion by targeting Slug
in gastric cancer. Protein Cell. 7:383–387. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhu M, Wang M, Yang F, Tian Y, Cai J, Yang
H, Fu H, Mao F, Zhu W, Qian H, et al: miR-155-5p inhibition
promotes the transition of bone marrow mesenchymal stem cells to
gastric cancer tissue derived MSC-like cells via NF-κB p65
activation. Oncotarget. 7:16567–16580. 2016.PubMed/NCBI
|
|
5
|
Zhang X, Ni Z, Duan Z, Xin Z, Wang H, Tan
J, Wang G and Li F: Overexpression of E2F mRNAs associated with
gastric cancer progression identified by the transcription factor
and miRNA co-regulatory network analysis. PLoS One.
10:e01169792015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang X, Tang J, Zhi X, Xie K, Wang W, Li
Z, Zhu Y, Yang L, Xu H and Xu Z: miR-874 functions as a tumor
suppressor by inhibiting angiogenesis through STAT3/VEGF-A pathway
in gastric cancer. Oncotarget. 6:1605–1617. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim JG, Kim TO, Bae JH, Shim JW, Kang MJ,
Yang K, Ting AH and Yi JM: Epigenetically regulated MIR941 and
MIR1247 target gastric cancer cell growth and migration.
Epigenetics. 9:1018–1030. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Matsuo M, Nakada C, Tsukamoto Y, Noguchi
T, Uchida T, Hijiya N, Matsuura K and Moriyama M: miR-29c is
downregulated in gastric carcinomas and regulates cell
proliferation by targeting RCC2. Mol Cancer. 12:152013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu C, Zheng X, Li X, Fesler A, Hu W, Chen
L, Xu B, Wang Q, Tong A, Burke S, et al: Reduction of gastric
cancer proliferation and invasion by miR-15a mediated suppression
of Bmi-1 translation. Oncotarget. 7:14522–14536. 2016.PubMed/NCBI
|
|
10
|
Seok JK, Lee SH, Kim MJ and Lee YM:
MicroRNA-382 induced by HIF-1α is an angiogenic miR targeting the
tumor suppressor phosphatase and tensin homolog. Nucleic Acids Res.
42:8062–8072. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu X, Wang W, Su N, Zhu X, Yao J, Gao W,
Hu Z and Sun Y: miR-374a promotes cell proliferation, migration and
invasion by targeting SRCIN1 in gastric cancer. FEBS Lett.
589:407–413. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sakamoto N, Naito Y, Oue N, Sentani K,
Uraoka N, Oo H Zarni, Yanagihara K, Aoyagi K, Sasaki H and Yasui W:
MicroRNA-148a is downregulated in gastric cancer, targets MMP7, and
indicates tumor invasiveness and poor prognosis. Cancer Sci.
105:236–243. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yin H, Song P, Su R, Yang G, Dong L, Luo
M, Wang B, Gong B, Liu C, Song W, et al: DNA methylation mediated
down-regulating of MicroRNA-33b and its role in gastric cancer. Sci
Rep. 6:188242016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Z, Li D, Zhang G, Xiong J, Jie Z, Cheng
H, Cao Y, Jiang M, Lin L, Le Z, et al: Methylation-associated
silencing of MicroRNA-335 contributes tumor cell invasion and
migration by interacting with RASA1 in gastric cancer. Am J Cancer
Res. 4:648–662. 2014.PubMed/NCBI
|
|
15
|
Yan LX, Huang XF, Shao Q, Huang MY, Deng
L, Wu QL, Zeng YX and Shao JY: MicroRNA miR-21 overexpression in
human breast cancer is associated with advanced clinical stage,
lymph node metastasis and patient poor prognosis. RNA.
14:2348–2360. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yan JJ, Zhang YN, Liao JZ, Ke KP, Chang Y,
Li PY, Wang M, Lin JS and He XX: miR-497 suppresses angiogenesis
and metastasis of hepatocellular carcinoma by inhibiting VEGFA and
AEG-1. Oncotarget. 6:29527–29542. 2015.PubMed/NCBI
|
|
17
|
Wang W, Ren F, Wu Q, Jiang D, Li H and Shi
H: MicroRNA-497 suppresses angiogenesis by targeting vascular
endothelial growth factor A through the PI3K/AKT and MAPK/ERK
pathways in ovarian cancer. Oncol Rep. 32:2127–2133.
2014.PubMed/NCBI
|
|
18
|
Jiang Y, Meng Q, Qi J, Shen H and Sun S:
miR-497 promotes metastasis of colorectal cancer cells through
Nrdp1 inhibition. Tumour Biol. 36:7641–7647. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wald AI, Hoskins EE, Wells SI, Ferris RL
and Khan SA: Alteration of microRNA profiles in squamous cell
carcinoma of the head and neck cell lines by human papillomavirus.
Head Neck. 33:504–512. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu J, Zhang J, Li Y, Wang L, Sui B and
Dai D: miR-455-5p acts as a novel tumor suppressor in gastric
cancer by down-regulating RAB18. Gene. 592:308–315. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jia H, Zhang Z, Zou D, Wang B, Yan Y, Luo
M, Dong L, Yin H, Gong B, Li Z, et al: MicroRNA-10a is
down-regulated by DNA methylation and functions as a tumor
suppressor in gastric cancer cells. PLoS One. 9:e880572014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lei H, Zou D, Li Z, Luo M, Dong L, Wang B,
Yin H, Ma Y, Liu C, Wang F, et al: MicroRNA-219-2-3p functions as a
tumor suppressor in gastric cancer and is regulated by DNA
methylation. PLoS One. 8:e603692013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsai MM, Wang CS, Tsai CY, Chen CY, Chi
HC, Tseng YH, Chung PJ, Lin YH, Chung IH, Chen CY, et al:
MicroRNA-196a/-196b promote cell metastasis via negative regulation
of radixin in human gastric cancer. Cancer Lett. 351:222–231. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou X, Xia Y, Li L and Zhang G: miR-101
inhibits cell growth and tumorigenesis of Helicobacter pylori
related gastric cancer by repression of SOCS2. Cancer Biol Ther.
16:160–169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lehmann U, Streichert T, Otto B, Albat C,
Hasemeier B, Christgen H, Schipper E, Hille U, Kreipe HH and Länger
F: Identification of differentially expressed microRNAs in human
male breast cancer. BMC Cancer. 10:1092010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li D, Zhao Y, Liu C, Chen X, Qi Y, Jiang
Y, Zou C, Zhang X, Liu S, Wang X, et al: Analysis of miR-195 and
miR-497 expression, regulation and role in breast cancer. Clin
Cancer Res. 17:1722–1730. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Han Z, Zhang Y, Yang Q, Liu B, Wu J, Zhang
Y, Yang C and Jiang Y: miR-497 and miR-34a retard lung cancer
growth by co-inhibiting cyclin E1 (CCNE1). Oncotarget.
6:13149–13163. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu W, Zhu D, Lu S, Wang T, Wang J, Jiang
B, Shu Y and Liu P: miR-497 modulates multidrug resistance of human
cancer cell lines by targeting BCL2. Med Oncol. 29:384–391. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li W, Jin X, Deng X, Zhang G, Zhang B and
Ma L: The putative tumor suppressor microRNA-497 modulates gastric
cancer cell proliferation and invasion by repressing eIF4E. Biochem
Biophys Res Commun. 449:235–240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ye Z, Shen N, Weng Y, Li K, Hu L, Liao H,
An J, Liu L, Lao S and Cai S: Low miR-145 silenced by DNA
methylation promotes NSCLC cell proliferation, migration and
invasion by targeting mucin 1. Cancer Biol Ther. 16:1071–1079.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hirata H, Hinoda Y, Shahryari V, Deng G,
Tanaka Y, Tabatabai ZL and Dahiya R: Genistein downregulates
onco-miR-1260b and upregulates sFRP1 and Smad4 via demethylation
and histone modification in prostate cancer cells. Br J Cancer.
110:1645–1654. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cao J, Song Y, Bi N, Shen J, Liu W, Fan J,
Sun G, Tong T, He J, Shi Y, et al: DNA methylation-mediated
repression of miR-886-3p predicts poor outcome of human small cell
lung cancer. Cancer Res. 73:3326–3335. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cianci P, Tono V, Sala A, Locatelli L,
Carta C, Rizzari C, Biondi A and Selicorni A: A boy with Burkitt
lymphoma associated with Noonan syndrome due to a mutation in RAF1.
Am J Med Genet A. 161A:1401–1404. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ren G, Liu X, Mao X, Zhang Y, Stankiewicz
E, Hylands L, Song R, Berney DM, Clark J, Cooper C, et al:
Identification of frequent BRAF copy number gain and alterations of
RAF genes in Chinese prostate cancer. Genes Chromosomes Cancer.
51:1014–1023. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Albers C, Illert AL, Miething C, Leischner
H, Thiede M, Peschel C and Duyster J: An RNAi-based system for
loss-of-function analysis identifies Raf1 as a crucial mediator of
BCR-ABL-driven leukemogenesis. Blood. 118:2200–2210. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li X, Stevens PD, Liu J, Yang H, Wang W,
Wang C, Zeng Z, Schmidt MD, Yang M, Lee EY, et al: PHLPP is a
negative regulator of RAF1, which reduces colorectal cancer cell
motility and prevents tumor progression in mice. Gastroenterology.
146:1301–12.e1, 10. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xu ZH, Hang JB, Hu JA and Gao BL:
RAF1-MEK1-ERK/AKT axis may confer NSCLC cell lines resistance to
erlotinib. Int J Clin Exp Pathol. 6:1493–1504. 2013.PubMed/NCBI
|
|
38
|
Wang F, Jiang C, Sun Q, Yan F, Wang L, Fu
Z, Liu T and Hu F: miR-195 is a key regulator of Raf1 in thyroid
cancer. Onco Targets Ther. 8:3021–3028. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu Z, Liu Y, Li L, Xu Z, Bi B, Wang Y and
Li JY: miR-7-5p is frequently downregulated in glioblastoma
microvasculature and inhibits vascular endothelial cell
proliferation by targeting RAF1. Tumour Biol. 35:10177–10184. 2014.
View Article : Google Scholar : PubMed/NCBI
|