Introduction
Lung cancer is by far the biggest cancer killer in
the world. Almost a million people die from lung cancer every year
worldwide, and nearly 600,000 in China (1). Though many advances have been achieved
in clinical and experimental oncology, lung cancer treatment is
still not satisfactory, with a 5-year overall survival rate of
barely 11% (2). Non-small cell lung
carcinoma (NSCLC) has the highest incidence in lung cancers,
accounting for approximately 85% of lung neoplasms (3,4).
Research into NSCLC is restricted, due to the complex cell
proliferation mechanism and delayed diagnosis (5). In general, chemotherapy combined with
surgical resection is the classic therapeutic strategy for NSCLC
(6). However, chemotherapy always
comes with some critical side-effects, such as leukopenia, hepatic
dysfunction and disorder in renal function (7,8).
Hence, more secure therapeutic strategies and more thorough studies
are needed to improve therapeutic actuality of NSCLC.
Barbaloin, also named as aloin
(C21H22O9), is a natural bioactive
anthracycline. Barbaloin is usually extracted from Aloe
barnadensis Miller leaves (9).
Considerable number of reports have shown the anti-inflammatory,
antimicrobial, antioxidant activities, anti-virus and anticancer
potentials of barbaloin (9).
Besides, previous studies identified the effective role of
barbaloin in regulating cell cycle arrest and apoptosis in human
cancers, such as colon (10),
breast (11), ovarian cancer
(12), uterine carcinoma (13). Aloe emodin has been demonstrated to
suppress the invasion and metastasis of breast cancer MDA-MB-231
cells (14) and human ovarian
cancer cell line HO-8910PM (15).
These investigations illustrated the potential of barbaloin in
suppressing the proliferation and metastasis of cancers.
In addition, other researchers suggested that
aloe-emodin inhibited the invasion of nasopharyngeal carcinoma
cells by downregulating the expression of MMP-2 via p38
mitogen-actived protein kinase (p38MAPK)-NF-κB-dependent pathway
(16). Some others pointed out that
p38 was an important determinant of apoptotic death induced by
aloe-emodin in NSCLC H460 cells (17). However, no study has indicated the
anticancer potential of barbaloin in NSCLC.
In the present study, we investigated the possible
anticancer effects of aloin on NSCLC in vitro and in
vivo and the underlying molecular mechanisms. The results
demonstrated that barbaloin inhibited the proliferation of A549
cells, promoted cell apoptosis and induced a G2/M cell cycle
arrest. Further research identified that barbaloin suppressed the
invasion and migration ability of A549 cells. The in vivo
experiment convinced that barbaloin restrained the growth and
hepatic metastases of A549 cells in nude mice, and involved
inactivation of the p38MAPK signaling pathway. Our research may
present new aspects in the treatment of NSCLC.
Materials and methods
Animal ethics
NOD/SCID mice were euthanized via the abdominal
injection a lethal dose of pentobarbital sodium. All of the animal
work was conducted according to relevant national and international
guidelines and was approved by the Animal Experimental Ethics
Committee of The First Affiliated Hospital of Xinxiang Medical
University.
Cell lines and cell cultures
Human NSCLC cell line A549 was obtained from the
American Type Culture Collection (ATCC; Manassas, VA, USA), and
cells were pre-covered with Matrigel (BD Biosciences, Shanghai,
China) at 5 µg/cm2. Then, cells were cultivated in
modified Eagle's medium (MEM; Invitrogen, Carlsbad, CA, USA),
supplemented with 20% fetal bovine serum (FBS; Invitrogen) and 1%
antibiotic-antimycotic (Invitrogen). The cells were cultured
according to the suppliers instruction, at 37°C, 5%
CO2.
Cell viability analysis
Barbaloin (HPLC ≥98%) was purchased from
Sigma-Aldrich (St. Louis, MO, USA). Barbaloin was dissolved in
dimethyl sulfoxide (DMSO) at concentration of 20 mmol/l and stored
at −20°C. To evaluate cell viability, 2×103 A549 cells
were seeded onto 96-well plates coated with 0.1% gelatin and
allowed to attach overnight. Human NSCLC cell lines A549 were
directly exposed to various concentrations of barbaloin (0, 10, 50
and 100 µM) for 72 h. Cell viability was identified using
methylthiazol tetrazolium (MTT) assay. Briefly, 20 µl of MTT dye
solution (5 mg/ml; Sigma-Aldrich) was added to each well and
incubated for 4 h. Then, the old medium was discarded and fresh
medium containing MTT (5 mg/ml MTT in PBS; Sangon Biotech, Co.
Ltd., Shanghai, China) was added and incubated further for 4 h.
Then, DMSO was used to dissolve the formazan. Finally, the OD was
determined with a microplate spectrophotometer (ELx800; BioTek
Instruments Inc., Winooski, VT, USA) at a wavelength of 470 nm.
Experiments were carried out in triplicate.
Western blotting
The expression of key proteins involved in the
biological functions of NSCLC cells was measured by western
blotting. Cells were treated with different concentrations of
barbaloin (0, 10, 50 and 100 µM). Total cell lysate preparation and
western blot analysis were performed according to a previous report
(18). Briefly, equal amounts of
protein (40 µg) were resolved on (6–12%) SDS-PAGE,
electrotransferred onto PVDF membranes (Millipore, Billerica, MA,
USA), probed with specific antibodies. The following primary
antibodies were used: anti-Ki-67, anti-proliferating cell nuclear
antigen (PCNA); anti-caspase-3; anti-caspase-8; anti-caspase-9;
anti-p27; anti-p53; anti-cyclin A; anti-matrix metalloproteinase
(MMP)-2; anti-MMP-9; anti-MMP-14; anti-vascular endothelial growth
factor (VEGF); anti-AKT; anti-nuclear factor kappa B (NF-κB);
anti-p38 mitogen-actived protein kinase (p38MAPK); anti-β-catenin;
anti-p-AKT; anti-p-NF-κB; anti-p-p38MAPK; anti-p-β-catenin and
anti-GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA, USA), which
was used as the internal reference. After incubation with the
appropriate horseradish peroxidase (HRP)-conjugated secondary
antibody, proteins were detected using a ChemiDoc XRS imaging
system and Quantity One analysis software (Bio-Rad Laboratories,
San Francisco, CA, USA).
Cell apoptosis analysis
Cell apoptosis of A549 cells was detected by the
Annexin V apoptosis detection kit (Beyotime Institute of
Biotechnology, Shanghai, China) following a previous study
(19). Briefly, the medium
containing the floating cells was collected, and the attached cells
were washed and trypsinized. The floating and trypsinized cells
were collected in the same centrifuge tubes. After centrifugation
and washing, the cell pellets were fixed in cold 70% ethanol. The
fixed cells were stained with propidium iodide (PI) and PE-Annexin
V. Cell apoptosis percentage was reflected by Annexin V/PI ratio,
detected by a flow cytometer (BD Biosciences, Shanghai, China).
Cell cycle analysis
The analysis was measured by flow cyto-metry (BD
Biosciences, San Jose, CA, USA). In short, A549 cells at
1×106 cells/well were treated with different
concentrations of barbaloin. The cells were then harvested and
fixed in 70% ethanol overnight at 4°C. Cell nuclei were stained
with PI for 30 min. A total of 104 nuclei were examined
in a FACSCalibur flow cytometer (Becton-Dickinson, Mountain View,
CA, USA) and DNA histograms were analyzed by CellQuest software
(Becton-Dickinson). Results are presented as the percentage of
cells in each phase.
Wound healing assay
The migratory activity of A549 cells was assessed
using the scratch assay according to standard methods. A narrow
area on the confluent A549 monolayers in 6-well plates were
scratched off with a p200-pipette tip. After washing, cells were
treated with indicated concentrations of barbaloin. Image
acquisition of wound fields was done after removal of inserts (0 h)
and wound closure documentation was done after 24 h with a
phase-contrast microscope (Leica DM IL; Leica Microsystems,
Wetzlar, Germany) equipped with a digital camera (Leica DFC300 FX).
Images were taken from the same areas as those recorded at zero
time and the numbers of the migrated cells were counted.
Transwell invasion assay
The in vitro cell invasion assay was
performed in the 24-well plates by a modified Boyden chamber coated
with Matrigel (Becton-Dickinson, Franklin Lakes, NJ, USA). A
single-cell suspension containing 2×104 A549 cells were
treated with barbaloin (0, 10, 50 and 100 µM) and loaded into the
upper chamber, whereas 20% FBS was added to the medium in the lower
chamber. After incubating for 24 h, non-invading cells were removed
from the top well with a cotton swab, while the bottom cells were
fixed in 95% ethanol, and stained with hematoxylin. Invasiveness
was determined by counting the cells that migrated through the
filter.
Immunofluorescence staining
Fluorescent cells were cultured on an 8-well chamber
CultureSlides (Becton-Dickinson, Bedford, MA, USA). After 8 h,
cells were fixed in 3% paraformaldehyde in phosphate-buffered
saline (PBS) at room temperature for 8 min, then permeabilized with
0.2% Triton X-100 for 15 min at room temperature. After washing in
PBS, the cells were incubated with primary mouse anti-p38
monoclonal antibody (1 mg/ml; Transduction Laboratories, Lexington,
KY, USA) at 4°C overnight. After washing, cells were incubated with
biotinylated goat anti-mouse IgG (Pierce, Rockford, IL, USA) at
room temperature for 1 h. The immunoreactivity was revealed using
Alexa568-conjugated streptavidin (Molecular Probes, Eugene, OR,
USA), and cells were counterstained with 10 mg/ml DAPI. The cells
were examined under a Nikon fluorescence microscope (Image Systems,
Columbia, MD, USA).
Subcutaneous NSCLC xenografts
All the animals involved in this study were
purchased from the the Institute of Zoology, Chinese Academy of
Medical Sciences (NOD/SCID mice, clean, 8-weeks old and weighing
20–22 g). A549 cells were digested by the pancreatic enzymes and
the final concentration was adjusted to 1×106/ml. On day
0, the mice (n=50) were administered with 200 l of 0.75% sodium
pentobarbital solution per mouse and then subcutaneous injection
was conducted of 5×106 A549 cells. The mice in treatment
group (n=25) were injected with barbaloin (40 mg/kg body weight)
every other day, while the mice (n=25) in model group received the
same volume injection of saline. After development of a palpable
tumor, the tumor volume was monitored every 5 days, briefly, tumor
was isolated from five mice in each group, the volume was assessed
by the formula: tumor volume (mm3) = maximal length (mm)
× perpendicular width (mm)2/2. All mice were assigned to
euthanasia at the end of the measurements. All animal experiments
were performed according to current guidelines and under a protocol
approved by the Institutional Animal Care and Use Committee.
Ex vivo fluorescence imaging of the
liver
Fluorescence in livers from NSCLC xenograft mice was
observed using the Xenogen IVIS spectrum imager (Caliper Life
Sciences, Inc., Hopkinton, MA, USA). The total signal intensities
were quantified by drawing the region of interest (ROI) using the
matching analysis software package supplied by the
manufacturer.
Statistical analysis
All results are presented as mean ± SD. The
statistical significance of the studies was analyzed using Students
t-test. The difference was considered statistically significant at
P<0.05.
Results
Barbaloin inhibits the growth of NSCLC
cells
To explore the effect of barbaloin on cell viability
of NSCLC cells, the NSCLC A549 cells were treated with barbaloin at
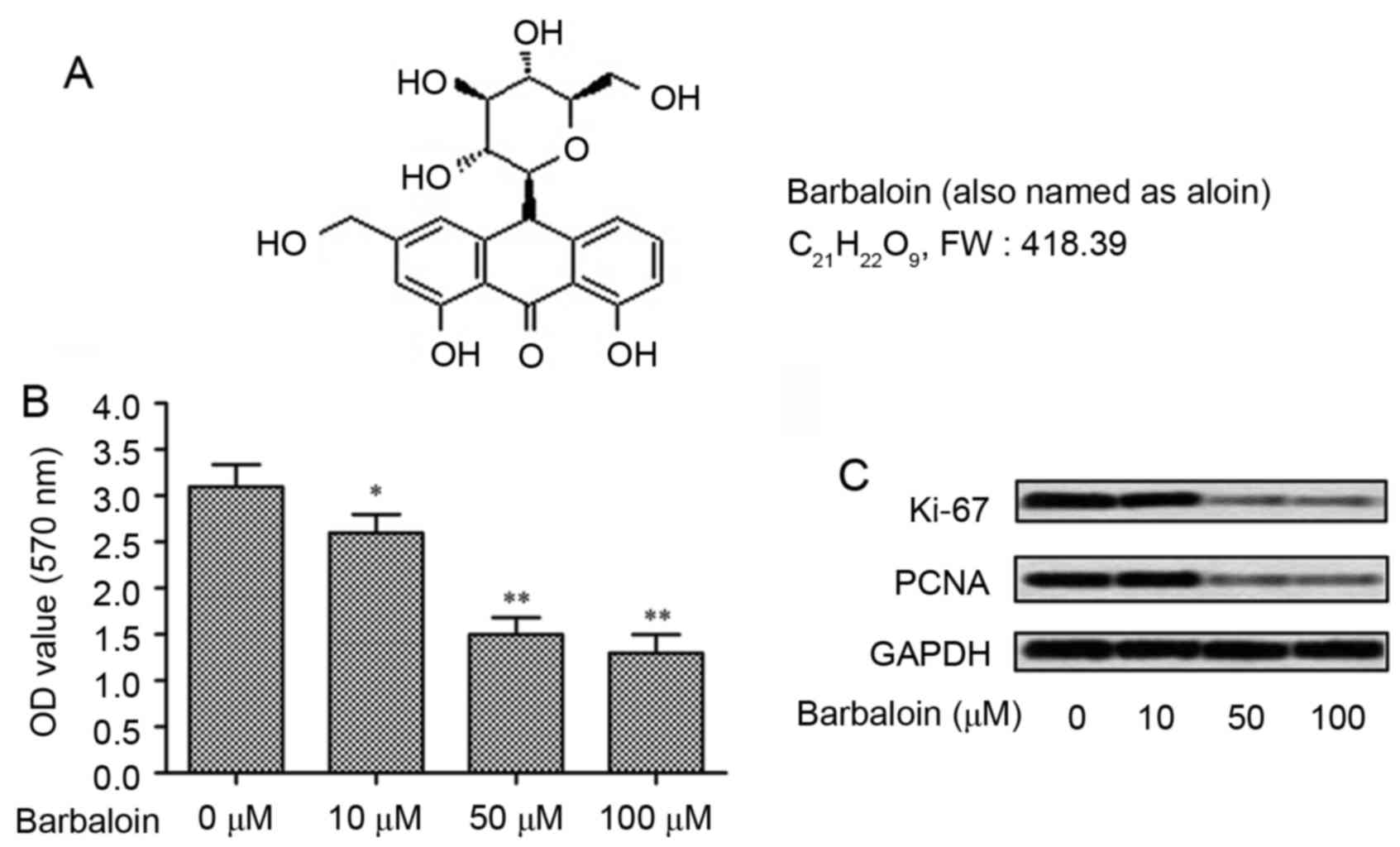
different concentrations (0, 10, 50 and 100 µM). The structure of
barbaloin is shown in Fig. 1A. The
anti-growth effect of barbaloin was measured even at low
concentrations (10 µM) (P<0.05; Fig.
1B), while higher concentrations (50 and 100 µM) of barbaloin
strongly decreased the OD values of A549 cells (P<0.001). To
confirm the results, the expression of proliferation markers (Ki-67
and PCNA) were measured by western blot analysis. Results displayed
that barbaloin reduced the level of Ki-67 and PCNA, especially at
higher concentrations (50 and 100 µM) (Fig. 1C). These results indicated that
barbaloin could effectively inhibit the viability of NSCLC
cells.
Barbaloin induces apoptosis and G2/M
cell cycle arrest in A549 cells
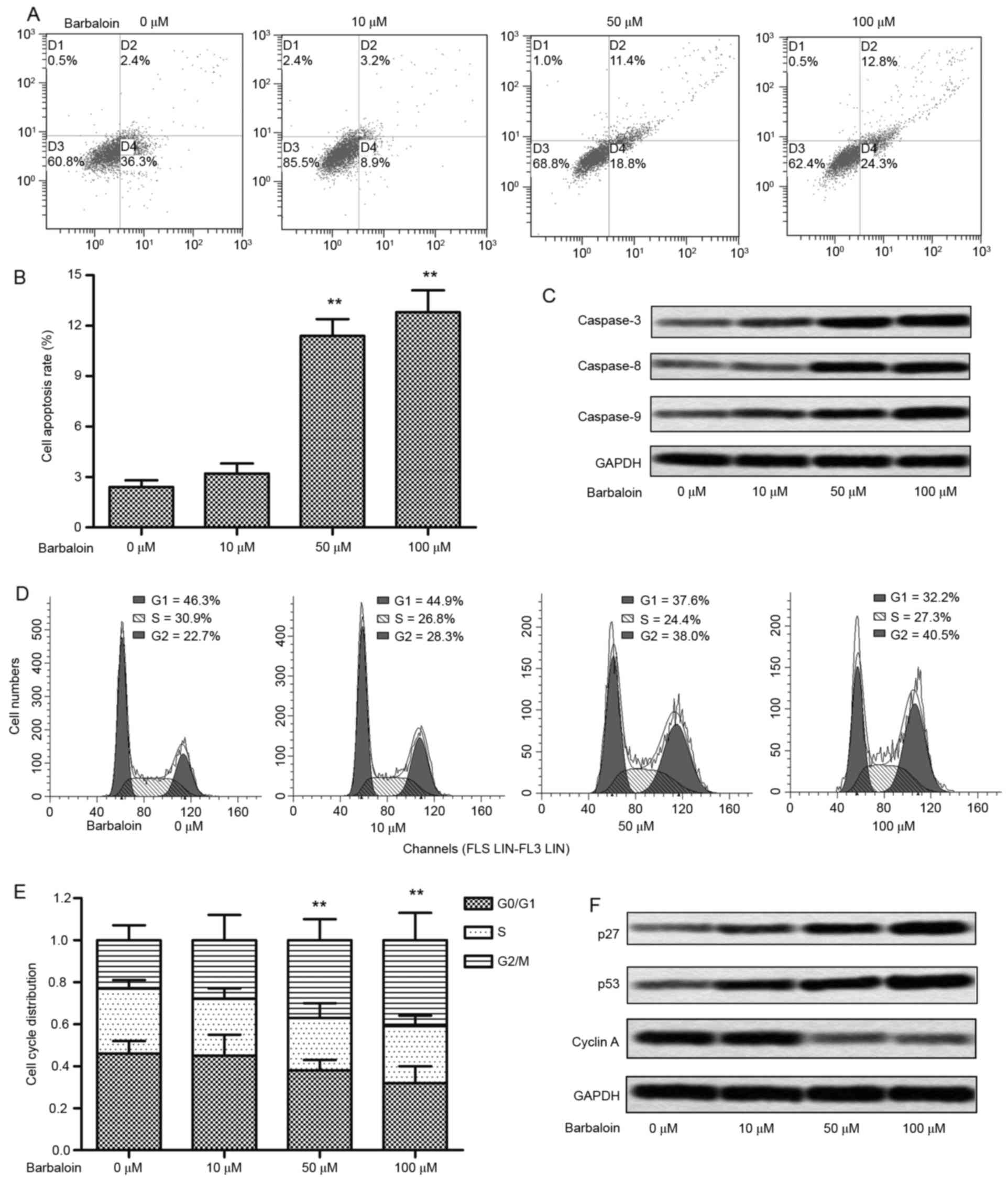
To further investigate the impact of barbaloin on
the viability of NSCLC cells, we studied whether barbaloin was
capable of affecting apoptosis and the cell cycle. Flow cytometric
analysis indicated that no significant difference of apoptosis was
detected in A549 cells treated with 10 µM barbaloin, whereas 50 and
100 µM barbaloin strongly induced cell apoptosis (P<0.01;
Fig. 2A and B). Similarly, the
expression of apoptosis-related proteins (caspase-3, −8 and −9) was
enhanced under treatment of barbaloin, especially at higher
concentrations (50 and 100 µM) (Fig.
2C). The analysis of cell cycle indicated that an accumulation
of G2/M phase was caused by 10 µM barbaloin (P<0.05), and the
G2/M cycle arrest was strongly induced by 50 and 100 µM barbaloin
(P<0.01; Fig. 2D and E). The
elevated level of cell cycle checkpoint proteins (p27 and p53) and
decreased level of cyclin A further convinced of the cycle arrest
induced by barbaloin (Fig. 2F).
These results indicated that barbaloin induced cell apoptosis and
cell arrest in NSCLC.
Barbaloin suppresses the invasion and
migration of A549 cells
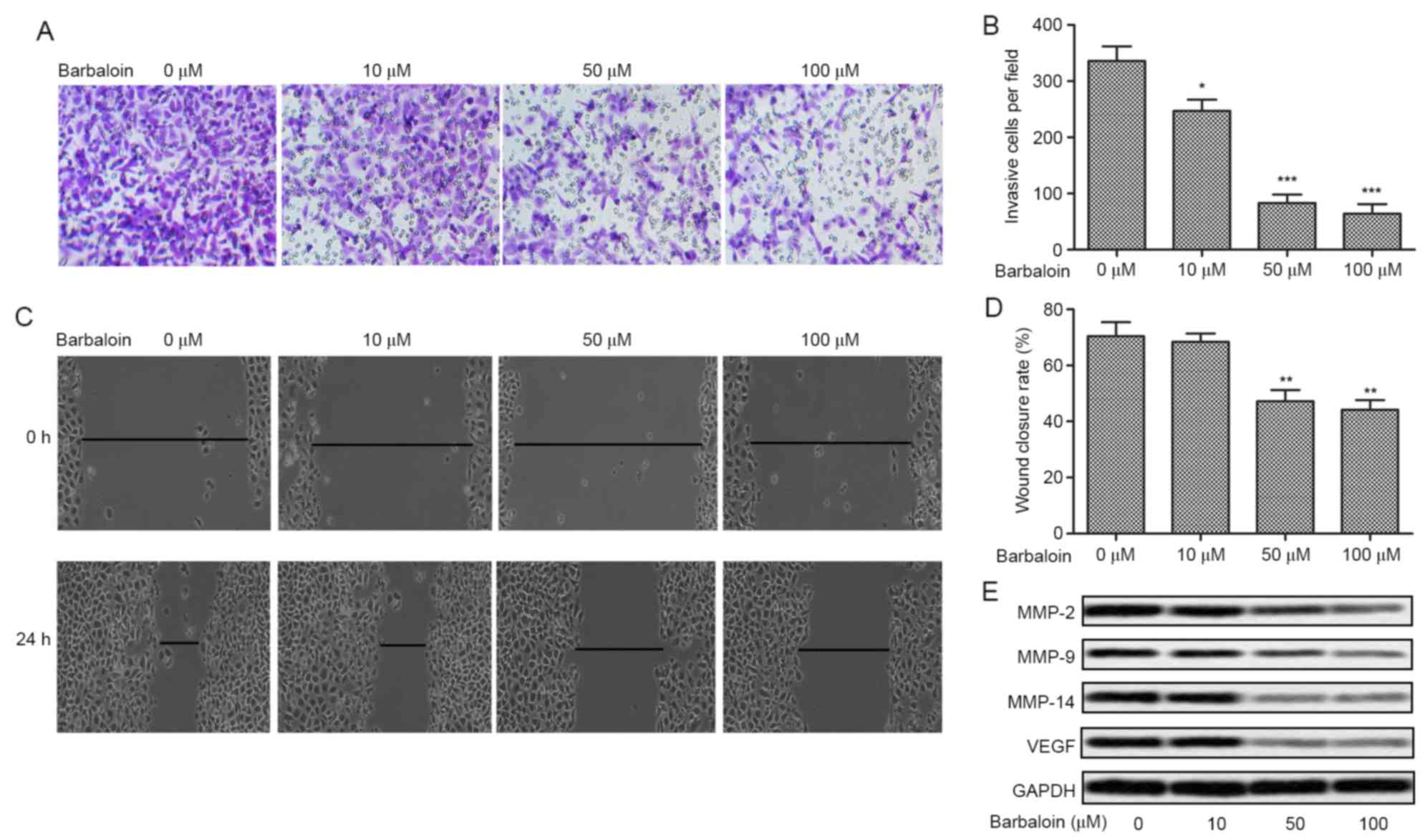
The former results revealed the anti-viability
effect of barbaloin, we next investigate the effect of barbaloin on
the motility of NSCLC cells (A549) by Transwell assays and scratch
assays. The number of invasive cells was decreased over 3-fold
adding barbaloin (50 and 100 µM) in A549 cells (P<0.001;
Fig. 3A and B). The results of
scratch assays were in agreement with the Transwell assays. The
A549 group showed a large closure of the gap, whereas barbaloin (50
and 100 µM) increased the gap by ~30% (P<0.01; Fig. 3C and D). Further western blot
analysis indicated that the levels of tumor metastasis-related
proteins (MMP-2, MMP-9, MMP-14 and VEGF) were decreased by
barbaloin at different concentrations (Fig. 3E). These results suggested the
inhibition effect of barbaloin on the motility of NSCLC.
Barbaloin inactivates the
p38MAPK/Cdc25B/Hsp27 pathway
Activation of pro-survival or pro-metastasis
signaling pathways, such as PI3K/AKT, NF-κB, MAPK and β-catenin,
has been shown to play a role in mediating oncogenic effects in
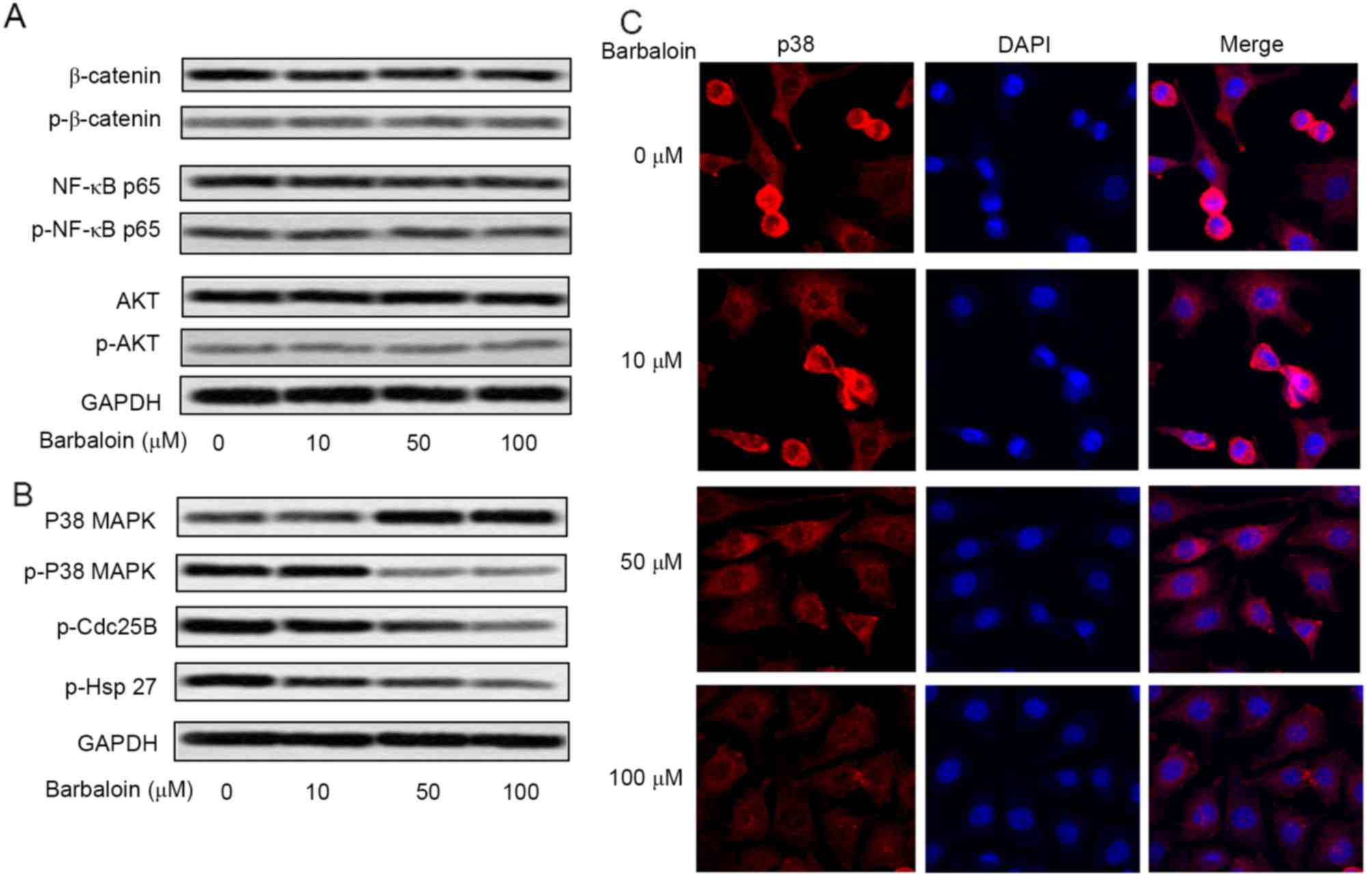
human NSCLC (20–23). To explore the mechanism of barbaloin
on the viability and motility of NSCLC cells, the level of AKT,
NF-κB, p38MAPK and β-catenin and their phosphorylated forms was
measured by western blot analysis (Fig.
4A). No difference was observed in the activated
(phosphorylated) forms of AKT, NF-κB and β-catenin in A549 cells
treated with barbaloin in various concentrations, but the level of
p-p38MAPK was significantly reduced by barbaloin, especially at 50
and 100 µM (Fig. 4B). To verify the
inactivating role of barbaloin in p38MAPK pathway, the expression
of downstream genes of p38MAPK (p-Cdc25B and p-Hsp27) was measured
by western blot analysis. The result revealed that the level of
p-Cdc25B and p-Hsp27 was strongly decreased by barbaloin. Moreover,
the subcellular localization of p38 was measured. Cells in control
group (0 µM) showed accumulated p38 staining in cytoplasm and
nucleus. Cells treated with 50 and 100 µM barbaloin displayed
reduced cytoplasmic and nuclear staining of p38 (Fig. 4C). These results indicated that
barbaloin restrained the activation of p38MAPK/Cdc25B/Hsp27 pathway
through inhibition of p38 nucleus translocation.
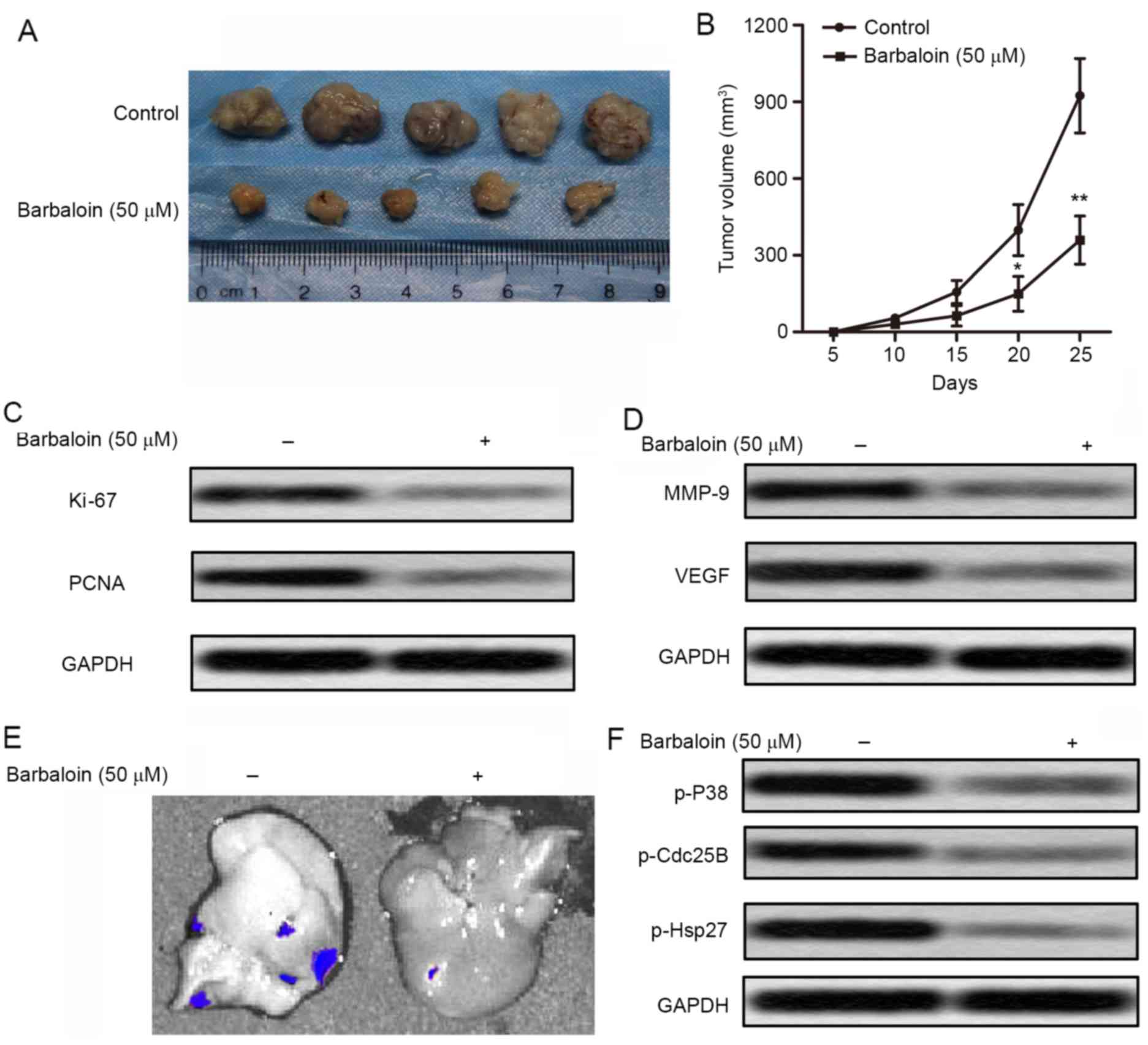
Barbaloin restrains the growth and
hepatic metastases of A549 cells in nude mice
To investigate the effects of barbaloin on NSCLC
cell migration and invasion in vivo, xenograft mouse model
was created by subcutaneous injection of A549 cells pretreated with
or without barbaloin (50 µM) into SPF nude mice. Barbaloin
effectively suppressed tumor formation and tumor volume compared
with the control group (Fig. 5A and
B). The level of proliferation markers (Ki-67 and PCNA) was
also decreased in barbaloin treated mice (Fig. 5C). These results suggested that
barbaloin restrained tumor growth of NSCLC in vivo. Besides,
the level of tumor metastasis-related proteins (MMP-9 and VEGF) was
also downregulated by barbaloin in NSCLC model mice (Fig. 5D). Fluorescent labeled recombinant
A549 cells were found to metastasize to the liver. Fluorescence
signal in liver was observed directly through IVIS Spectrum system.
We can see that fluorescence signal was significantly reduced by 50
µM barbaloin (Fig. 5E). The
expression level of p38MAPK (p-Cdc25B and p-Hsp27) was also
decreased by 50 µM barbaloin (Fig.
5F). These results indicated that barbaloin restrains the
growth and hepatic metastases of A549 cells in nude mice and may
involve inactivation of the p38MAPK pathway.
Discussion
Lung cancer is one of the most malignant cancers in
the world (24). Worst still, in
China, more and more patients younger than 45 years suffer from
lung cancer (25). NSCLC is the
most common type of lung cancers (4). At present, chemotherapy combined with
surgical resection is the most commonly used method in the
treatment of NSCLC. However, the treatment of lung cancer is still
not satisfactory with many side-effects and recurrence. Therefore,
it is critical to deeply understand the exact molecular mechanisms
related to NSCLC development and progression and develop new
therapeutic methods for medication and treatment.
In recent years, natural herbal products are applied
to the treatment of cancers due to their antitumor activities
including apoptosis induction and anti-proliferative activities.
For example, phloretin induced apoptosis of NSCLC A549 cells
(26). Matrine promoted cell
apoptosis in NSCLC by activating p38 (27). These natural compounds effectively
restricted the proliferation of cancers with little side-effects.
Barbaloin used in the present study is a natural compound extracted
from aloe, and has been identified effective in various types of
cancer (10–13). A previous research proved that
barbaloin significantly inhibited the proliferation, migration and
tube formation of HUVECs (human umbilical vein endothelial cells)
in vitro by suppressing the activation of VEGF (vascular
endothelial growth factor) receptor 2 and STAT3 phosphorylation
(10). Similarly, in this study,
barbaloin effectively suppressed the cell growth and expression of
proliferation markers (Ki-67 and PCNA), especially at higher
concentrations (50 and 100 µM). These results indicate that
barbaloin inhibits the viability of NSCLC cells.
Accumulated studies have reported the anti-viability
effect of barbaloin. Reports have suggested that the combination of
aloe with chemotherapy could increase the efficacy in both tumor
regression rate and survival time (28). Some research pointed out that Aloe
emodin promoted cell cycle arrest and apoptosis in human U87
malignant glioma cells via the mitochondrial membrane potential
disruption (29). In this study,
the cell viability was largely reduced in A549 cells treated with
barbaloin compared with the control group, the inhibition rates
were augmented with the increasing concentration of barbaloin.
Besides, barbaloin increased the apoptosis rate of A549 cells and
induced an accumulation of G2/M phase. Increased expression of
apoptosis-related proteins (caspase-3, −8 and −9) and the changed
levels of cell cycle checkpoint proteins (p27, p53 and cyclin A)
further convinced the anti-viability effect of barbaloin in A549
cells. The results above indicated that barbaloin inhibited cell
viability of NSCLC via suppressing proliferation, inducing cell
apoptosis and G2/M cell cycle arrest.
Barbaloin has also been indicated to possess
anti-metastasis effect. According to the research of He et
al (14), the invasion and
metastasis ability was largely reduced in breast cancer MDA-MB-231
cells treated with aloe emodin. Aloe-emodin has also been reported
to inhibit the metastasis of HO-8910PM cells by reducing the
expression of FAK (focal adhesion kinase) protein and mRNA
(15). Similar conclusion was drawn
in this study. The invasion and migration ability of A549 cells was
largely suppressed under barbaloin treatment. The levels of tumor
metastasis-related proteins (MMP-2, MMP-9, MMP-14 and VEGF) were
also decreased in A549 cells treated with barbaloin, especially at
higher concentrations (50 and 100 µM). These results suggest the
inhibition effect of barbaloin on the motility of NSCLC.
Activation of pro-survival or pro-metastasis
signaling pathways has been shown to play a role in mediating
oncogenic effects in human NSCLC. Research has proven that
aloe-emodin induced apoptosis in human H460 lung non-small
carcinoma cells by regulating the expression of protein kinase C,
Bcl-2, caspase-3 and p38 protein (17), and others indicated that aloe-emodin
induced chondrogenic differentiation of ATDC5 cells via MAP kinases
and BMP-2 signaling pathways (30).
In this study, the level of classical signaling pathway marker AKT,
NF-κB, p38MAPK and β-catenin and their phosphorylated forms was
measured in barbaloin-treated A549 cells by western blot analysis.
The obviously decreased level of p-p38MAPK indicated that p38MAPK
is inactivated in A549 cells under barbaloin treatment. The
decreased expression of p-Cdc25B and p-Hsp27 (downstream genes of
p38MAPK) was further verification. In addition, immunofluorescence
staining assay showed that barbaloin reduced cytoplasmic and
nuclear staining of p38 in A549 cells. The research above indicated
that barbaloin restrained the activation of p38MAPK/Cdc25B/Hsp27
pathway via inhibiting p38 nucleus translocation.
Aloe also exhibits tumor suppression effects in
vivo. Aloe-emodin has been reported to suppress the gowth of
prostate cancer in an athymic nude mouse model (31). In vivo study showed the
positive result of antitumor activity of aloe-emodin in nude mice
injected with human HER-2-overexpressing breast cancer cells
(32). The antineoplastic
properties of aloe-emodin were also observed in highly metastatic
B16-F10 melanoma murine cells (33). In this study, barbaloin effectively
suppressed tumor formation and tumor volume in the xenograft mouse
model. Besides, barbaloin suppressed expression level of
proliferation markers (Ki-67 and PCNA) and tumor metastasis-related
proteins (MMP-9 and VEGF) in vivo. The fluorescent labeled
A549 cells metastasized to liver and barbaloin largely reduced the
fluorescence intensity in the liver. The expression level of
p38MAPK and downstream genes was also significantly decreased by
barbaloin. These results indicated that barbaloin restrains the
growth and hepatic metastases of A549 cells in nude mice and may
involve inactivation of p38MAPK/Cdc25B/Hsp27 pathway.
In conclusion, the present study found that
barbaloin inhibited the cell viability by suppressing cell
proliferation, inducing cell apoptosis and G2/M cell cycle arrest
in A549 cells. Besides, barbaloin inhibited cell motility by
restraining the invasion and migration ability of A549 cells and
the level of tumor metastasis-related proteins. Moreover, barbaloin
inhibited tumor growth and hepatic metastases of A549 cells in nude
mice and this may include restraining the p38MAPK/Cdc25B/Hsp27
pathway. This study may provide new aspects into the mechanism of
NSCLC proliferation and an approach for the treatment of NSCLC.
Acknowledgements
The present study was funded by the Natural Science
Foundation of China (no. 81473775) and the Young Teacher Support
Project of Beijing University of Chinese Medicine (no.
2011-JYB22-JS122).
Glossary
Abbreviations
Abbreviations:
|
NSCLC
|
non-small cell lung cancer
|
|
PCNA
|
proliferating cell nuclear antigen
|
|
MMP
|
matrix metalloproteinase
|
|
VEGF
|
vascular endothelial growth factor
|
|
MAPK
|
mitogen-actived protein kinase
|
References
|
1
|
Zhang BY, Wang YM, Gong H, Zhao H, Lv XY,
Yuan GH and Han SR: Isorhamnetin flavonoid synergistically enhances
the anticancer activity and apoptosis induction by cis-platin and
carboplatin in non-small cell lung carcinoma (NSCLC). Int J Clin
Exp Pathol. 8:25–37. 2015.PubMed/NCBI
|
|
2
|
Verdecchia A, Francisci S, Brenner H,
Gatta G, Micheli A, Mangone L and Kunkler I: EUROCARE-4 Working
Group: Recent cancer survival in Europe: A 2000–02 period analysis
of EUROCARE-4 data. Lancet Oncol. 8:784–796. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
DArcangelo M and Hirsch FR: Clinical and
comparative utility of afatinib in non-small cell lung cancer.
Biologics. 8:183–192. 2014.PubMed/NCBI
|
|
4
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Asamura H, Chansky K, Crowley J, Goldstraw
P, Rusch VW, Vansteenkiste JF, Watanabe H, Wu YL, Zielinski M, Ball
D and Rami-Porta R: International Association for the Study of Lung
Cancer Staging and Prognostic Factors Committee, Advisory Board
Members, and Participating Institutions: The International
Association for the Study of Lung Cancer Lung Cancer Staging
Project: Proposals for the Revision of the N Descriptors in the
Forthcoming 8th Edition of the TNM Classification for Lung Cancer.
J Thorac Oncol. 10:1675–1684. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Avelino CU, Cardoso RM, Aguiar SS and
Silva MJ: Assessment of quality of life in patients with advanced
non-small cell lung carcinoma treated with a combination of
carboplatin and paclitaxel. J Bras Pneumol. 41:133–142. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Waller DA: Neoadjuvant chemotherapy in non
small cell lung cancer-the UK experience. Lung Cancer. 34:(Suppl
3). S31–S33. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Azim HA Jr and Ganti AK: Treatment options
for relapsed small-cell lung cancer. Anticancer Drugs. 18:255–261.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tabolacci C, Rossi S, Lentini A,
Provenzano B, Turcano L, Facchiano F and Beninati S: Aloin enhances
cisplatin antineoplastic activity in B16-F10 melanoma cells by
transglutaminase-induced differentiation. Amino Acids. 44:293–300.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pan Q, Pan H, Lou H, Xu Y and Tian L:
Inhibition of the angiogenesis and growth of Aloin in human
colorectal cancer in vitro and in vivo. Cancer Cell Int. 13:692013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Esmat AY, Tomasetto C and Rio MC:
Cytotoxicity of a natural anthraquinone (Aloin) against human
breast cancer cell lines with and without ErbB-2: Topoisomerase
IIalpha coamplification. Cancer Biol Ther. 5:97–103. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Esmat AY, El-Gerzawy SM and Rafaat A: DNA
ploidy and S phase fraction of breast and ovarian tumor cells
treated with a natural anthracycline analog (aloin). Cancer Biol
Ther. 4:108–112. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nićiforović A, Adzić M, Spasić SD and
Radojcić MB: Antitumor effects of a natural anthracycline analog
(Aloin) involve altered activity of antioxidant enzymes in HeLaS3
cells. Cancer Biol Ther. 6:1200–1205. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He ZH, Huang YQ, Weng SF, Tan YR, He TP,
Qin YM and Liang NC: Effect of Aloe emodin on invasion and
metastasis of high metastatic breast cancer MDA-MB-231 cells. Zhong
Yao Cai. 36:1481–1485. 2013.(In Chinese). PubMed/NCBI
|
|
15
|
He TP, Yan WH, Mo LE and Liang NC:
Inhibitory effect of aloe-emodin on metastasis potential in
HO-8910PM cell line. J Asian Nat Prod Res. 10:383–390. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin ML, Lu YC, Chung JG, Wang SG, Lin HT,
Kang SE, Tang CH, Ko JL and Chen SS: Down-regulation of MMP-2
through the p38 MAPK-NF-kappaB-dependent pathway by aloe-emodin
leads to inhibition of nasopharyngeal carcinoma cell invasion. Mol
Carcinog. 49:783–797. 2010.PubMed/NCBI
|
|
17
|
Yeh FT, Wu CH and Lee HZ: Signaling
pathway for aloe-emodin-induced apoptosis in human H460 lung
nonsmall carcinoma cell. Int J Cancer. 106:26–33. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He H, Zheng L, Sun YP, Zhang GW and Yue
ZG: Steroidal saponins from Paris polyphylla suppress adhesion,
migration and invasion of human lung cancer A549 cells via
down-regulating MMP-2 and MMP-9. Asian Pac J Cancer Prev.
15:10911–10916. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mou H, Zheng Y, Zhao P, Bao H, Fang W and
Xu N: Celastrol induces apoptosis in non-small-cell lung cancer
A549 cells through activation of mitochondria- and
Fas/FasL-mediated pathways. Toxicol In Vitro. 25:1027–1032. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fei F, Li X, Xu L, Li D, Zhang Z, Guo X,
Yang H, Chen Z and Xing J: CD147-CD98hc complex contributes to poor
prognosis of non-small cell lung cancer patients through promoting
cell proliferation via the PI3K/Akt signaling pathway. Ann Surg
Oncol. 21:4359–4368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gastonguay A, Berg T, Hauser AD, Schuld N,
Lorimer E and Williams CL: The role of Rac1 in the regulation of
NF-κB activity, cell proliferation, and cell migration in non-small
cell lung carcinoma. Cancer Biol Ther. 13:647–656. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chien ST, Lin SS, Wang CK, Lee YB, Chen
KS, Fong Y and Shih YW: Acacetin inhibits the invasion and
migration of human non-small cell lung cancer A549 cells by
suppressing the p38α MAPK signaling pathway. Mol Cell Biochem.
350:135–148. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miao Y, Wang L, Zhang X, Xu X, Jiang G,
Fan C, Liu Y, Lin X, Yu J, Zhang Y, et al: Promoter
methylation-mediated silencing of β-catenin enhances invasiveness
of non-small cell lung cancer and predicts adverse prognosis. PLoS
One. 9:e1122582014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McGuire S: World Cancer Report 2014.
Geneva, Switzerland: World Health Organization, International
Agency for Research on Cancer, WHO Press, 2015. Adv Nutr.
7:418–419. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang J, Chen SF, Zhen Y, Xiang J, Wu C,
Bao P, Luketich J, Hu H, Zhou X, Zhang J, et al: Multicenter
analysis of lung cancer patients younger than 45 years in Shanghai.
Cancer. 116:3656–3662. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Min J, Huang K, Tang H, Ding X, Qi C, Qin
X and Xu Z: Phloretin induces apoptosis of non-small cell lung
carcinoma A549 cells via JNK1/2 and p38 MAPK pathways. Oncol Rep.
34:2871–2879. 2015.PubMed/NCBI
|
|
27
|
Tan C, Qian X, Jia R, Wu M and Liang Z:
Matrine induction of reactive oxygen species activates p38 leading
to caspase-dependent cell apoptosis in non-small cell lung cancer
cells. Oncol Rep. 30:2529–2535. 2013.PubMed/NCBI
|
|
28
|
Lissoni P, Rovelli F, Brivio F, Zago R,
Colciago M, Messina G, Mora A and Porro G: A randomized study of
chemotherapy versus biochemotherapy with chemotherapy plus Aloe
arborescens in patients with metastatic cancer. In Vivo.
23:171–175. 2009.PubMed/NCBI
|
|
29
|
Ismail S, Haris K, Ghani Abdul AR,
Abdullah JM, Johan MF and Yusoff Mohamed AA: Enhanced induction of
cell cycle arrest and apoptosis via the mitochondrial membrane
potential disruption in human U87 malignant glioma cells by aloe
emodin. J Asian Nat Prod Res. 15:1003–1012. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang M, Li L, Heo SM and Soh Y:
Aloe-emodin induces chondrogenic differentiation of ATDC5 cells via
MAP kinases and BMP-2 signaling pathways. Biomol Ther (Seoul).
24:395–401. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu K, Park C, Li S, Lee KW, Liu H, He L,
Soung NK, Ahn JS, Bode AM, Dong Z, et al: Aloe-emodin suppresses
prostate cancer by targeting the mTOR complex 2. Carcinogenesis.
33:1406–1411. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ma JW, Hung CM, Lin YC, Ho CT, Kao JY and
Way TD: Aloe-emodin inhibits HER-2 expression through the
downregulation of Y-box binding protein-1 in HER-2-overexpressing
human breast cancer cells. Oncotarget. 7:58915–58930.
2016.PubMed/NCBI
|
|
33
|
Tabolacci C, Lentini A, Mattioli P,
Provenzano B, Oliverio S, Carlomosti F and Beninati S: Antitumor
properties of aloe-emodin and induction of transglutaminase 2
activity in B16-F10 melanoma cells. Life Sci. 87:316–324. 2010.
View Article : Google Scholar : PubMed/NCBI
|