Introduction
Renal cell carcinoma (RCC) is the most common kidney
cancer, and has become the 7th most common cancer in men in the
USA. Over the past two decades, the global incidence of RCC has
increased by 2% per year (1). The
incidence and mortality rates of RCC vary widely throughout the
world, which may be attributed to environmental and genetic
influences (2), but the
mortality-to-incidence ratio is highest in Africa and Asia
(3). Despite advances in early
detection of RCC, ~20–30% of patients still present with metastatic
disease at diagnosis, and around one third of patients undergoing
nephrectomy for localized tumor eventually develop metastases. RCC
is highly heterogeneous in patterns of growth, invasion and
metastasis as well as in treatment outcomes (4). Patients with localized RCC tend to
have a better prognosis. Despite new targeted therapies, the
prognosis for metastatic RCC remains poor, and the 5-year survival
rate is <20% (5–7). RCC has various histological subtypes.
The most common of these is clear cell RCC (CCRCC), which
represents 75–80% of RCC (8), and
has a worse outcome than other common types of RCC (4). It is therefore essential to identify
molecular markers for translational application in the diagnosis
and prognosis of CCRCC.
Kindlin-2 which is encoded by the FERMT2 gene is an
integrin-binding focal adhesion protein that is essential in
activation of integrin (9).
Kindlin-2 not only regulates cell-matrix adhesion and controls
cytoskeletal dynamics, but also plays an important role in
carcinogenesis (10). High
expression of Kindlin-2 can promote tumor progression in prostate
cancer, gastric cancer, bladder cancer, lung cancer and malignant
mesothelioma (11–15). Kindlin-2 also activates Wnt
signaling by forming a tripartite complex with β-catenin and TCF4
to promote breast cancer cell invasion (16). However, in serous ovarian carcinoma,
Kindlin-2 inhibits peritoneal dissemination and predicts a more
favorable outcome in these patients (17). Kindlin-2 has also been shown to
inhibit mesenchymal tumor cell invasion (18). These findings suggest that Kindlin-2
is a molecule that functions in a context-dependent manner.
However, little is known about the function of Kindlin-2 in RCC.
Here we report that Kindlin-2 expression is significantly
correlated with unfavorable prognosis in CCRCC patients and
promotes RCC cell migration, invasion and proliferation via
activation of Wnt signaling pathway.
Materials and methods
Patients and samples
Two CCRCC tissue microarrays (TMA) purchased from
Shanghai Outdo Biotech (Shanghai, China), which included 109 pairs
of tumors and matched peritumoral tissues were used for this study.
For each patient, comprehensive pathologic and clinical information
was recorded, and anonymity was maintained for all patient
information. Tumor grade was determined using the 2016 WHO/ISUP
grading system. Pathologic stage was reassigned according to the
2010 American Joint Committee on Cancer TNM Classification. Primary
tumors (Tx) in four patients and regional lymph nodes
(Nx) in two patients could not be assessed.
Kindlin-2 immunohistochemistry
(IHC)
The two TMAs were used for immunostaining analysis
of Kindlin-2 protein expression. Deparaffinization and hydration
were performed followed by abolition of endogenous peroxidase
activity using 3% hydrogen peroxide for 30 min and microwave for
antigen retrieval in 10 mM sodium citrate buffer (pH 6.0) for 20
min. Mouse monoclonal anti-Kindlin-2 antibody was applied
(Millipore, USA) at 2 µg/ml at 4°C overnight. The PV9000 2-step
plus Poly-HRP Antimouse/rabbit IgG Detection system (Zhongshan
Biotech, China) and DAB kit (Zhongshan Biotech) was applied at room
temperature. Hematoxylin was used for counterstaining. Non-immune
IgG was used to replace primary antibody as a negative control.
Kindlin-2 immunohistochemical staining was
independently assessed by two pathologists blinded to patient
outcomes and clinicopathologic parameters. As there was no
intrafocal and interfocal heterogeneity in Kindlin-2 protein
expression, evaluation of its expression level was based on the
intensity of cytoplasmic staining. By using cytoplasmic staining in
capillary network of tumor stroma as control, the expressional
intensity levels of Kindlin-2 in tumor cells were classified into 3
grades: negative (no brown staining), weak (faint brown staining)
and strong (dark brown staining).
Cell culture and establishment of
stable cell lines
Human RCC cell lines (ACHN and 769-P) were obtained
from the Basic Medical Institute Cell Center, Chinese Academy of
Medical Sciences (Beijing, China), and 769-P and ACHN cells were
maintained in RPMI-1640 (Hyclone, USA) and minimum essential medium
(MEM; Hyclone) respectively, supplemented with 10% FBS. All cells
were cultured in a sterile incubator maintained at 37°C with 5%
CO2. Growth media were changed every two days.
Kindlin-2 cDNA was amplified by PCR, inserted into
the pLenti6/V5 plasmid, and transfected into 293T cells to obtain a
virus. 769-P cells were then transduced with the virus. Two days
post-transduction, cells were cultured in selection medium with 5
µg/ml blasticidin (Invitrogen, USA) until cells which had not
successfully undergone transduction died. Establishment of
Kindlin-2 knockdown stable cell lines in ACHN was carried out as
described in Zhao et al (19). For transfection, cells were plated
in 6-well plates (1.5×105 cells/well) and transfected 24
h after plating with Lipofectamine 2000 (Roche Applied Science,
USA) according to the manufacturer's instructions. Two days
post-transfection, cells were cultured in selective medium with
addition of 800 µg/ml G418 (Invitrogen) for selection until cells
which were not transfected died.
For signaling pathway inhibitor assay, cells were
treated for 24 h with ILK inhibitor Cpd 22 (Calbiochem, USA) and
GSK3β inhibitor LiCl (Sigma, USA).
Cell growth analysis by MTS assay
To assess proliferation, ACHN stable cells
(6×103) and 769-P stable cells (3×103) were
seeded into 96-well plates in 100 µl of 10% FBS/culture medium and
cultured at 37°C in a 5% CO2 incubator. After culturing
for 1, 2, 3, 4, 5 or 6 days, 20 µl of CellTiter 96 Aqueous One
Solution (Promega, USA) was added to each well and followed by
incubation for 2 h at 37°C under 5% CO2. Absorbance was
measured at 490 nm using a microplate reader.
Cell migration and invasion by
Transwell assay
Assays were carried out using Transwell chambers (8
µm polycarbonate membrane, Costar, Corning Inc.) that were coated
with or without Matrigel (BD Biosciences, USA), and cells were
harvested and adjusted to 5×105/ml using adhesion buffer
or culture medium with 0.5% FBS for separate invasion and migration
assays. Cells in 100 µl 0.5% FBS culture medium were added to the
upper chamber. For migration assays, the lower chamber was coated
with 10 µg/ml collagen. After 8–10 h, cells attached to the filter
were fixed with methanol and stained with crystal violet (0.1%).
For the invasion assay, the lower chamber was loaded with culture
medium with 20% FBS. After 24–48 h of incubation, the non-invading
cells on the upper side of the chamber were removed. The membranes
were fixed with methanol, and stained with Crystal violet. Cells
demonstrating migration or invasion were quantified by counting the
number of cells in five random fields in each Transwell, and the
average cell number was analyzed with the Student's t-test. All
experiments were conducted in triplicate and repeated three
times.
Real-time PCR
Total cellular RNA was extracted with TRIzol
(Invitrogen), and 2 µg total RNA were used for reverse
transcription with MMLV Reverse Transcriptase (Promega, USA).
Real-time PCR was carried out using SYBR Green mix (Applied
Biosystems, USA) with PCR conditions as follows: 95°C 3 min; 95°C
20 sec, 60°C 1 min, for 40 cycles. Gene expression was determined
by the comparative CT method (2−∆∆ct). All genes were
normalized to actin levels. Quantitative PCR was analyzed in
triplicate and all experiments were repeated three times.
Tumor formation in vivo
ACHN control cells and Kindlin-2 depleted cells
(2×106) were counted and resuspended in 100 µl MEM
medium, then injected subcutaneously into 4-week-old male nude mice
(Center of Experimental Animals, Peking University, Beijing,
China), which were sacrificed 11 weeks after implantation. Tissues
from subcutaneous xenografts were used for histologic and
immunostaining examination. Mice were maintained according to the
Peking University Guidelines of Animal Experiments.
Western blot analyses and
reagents
Cells were washed with ice-cold PBS and lysed in a
PBS-TDS buffer [PBS with 1% Triton X-100, 0.5% sodium deoxycholate,
0.1% sodium dodecyl sulfate (SDS) 1 mM EDTA, 1 mM
phenylmethanesulfonyl fluoride (PMSF), 1 complete inhibitor
cocktail (Boehringer)], and centrifuged at 15,000 g for 20 min at
4°C to obtain a clear lysate. Samples were heated at 95°C for 5 min
and then separated on SDS-PAGE gels and blotted onto PVDF
membranes. Primary antibodies against Kindlin-2 (Millipore), active
β-catenin (Millipore), β-catenin (Santa Cruz Biotechnology, USA),
TCF4 (Millipore), Snail (Cell Signaling Technology, USA), β-actin
(Zhongshan Biotech) were incubated with these membranes separately
under rotation. After thorough washing, membranes were further
incubated with corresponding secondary antibodies recognizing
either rabbit or mouse Ig (Jackson ImmunoResearch, USA). Bands were
visualized with enhanced chemiluminescence (Pierce, USA).
Affymetrix GeneChip human Gene 1.0 ST
array
cDNA from ACHN control cells and Kindlin-2 depleted
cells were hybridized to Affymetrix GeneChip Human Gene 1.0 ST
arrays (Affymetrix, USA). These arrays analyze the expression level
for 28,869 transcripts and variants, including 28,132
well-identified human genes by Ensemble. Data were analyzed with
GeneChip Operating Software 1.4. GO and pathway analysis derived
from KEGG was carried out.
Statistical analyses
The Student's t-test was used for statistical
analysis for paired samples. Statistical analysis for patient
materials was performed with the Chi-square test, the
Kruskal-Wallis test and the Pearson's correlation coefficient.
Patient survival was calculated using Kaplan-Meier analysis and
comparisons were made using the log-rank test. Univariate and
multivariate survival data were analyzed using Cox's proportional
hazard model. Variables associated with overall survival at a
significance level of P<0.1 on univariate analysis were included
in multivariate modelling using backward conditional regression.
Overall survival was defined as time from the date of surgery to
the date of death from any cause. All statistical analysis employed
SAS version 9.1. Results were considered statistically significant
at P<0.05.
Results
Expression of Kindlin-2 correlates
with higher tumor grade, lymph node metastasis and poor prognosis
in CCRCC patients
To evaluate the involvement of Kindlin-2 in CCRCC
progression, IHC analysis of Kindlin-2 was performed on a cohort
which included 109 CCRCC patients. For a control, we found
Kindlin-2 is highly expressed in the glomerulus and distal
convoluted renal tubules, while its expression is generally reduced
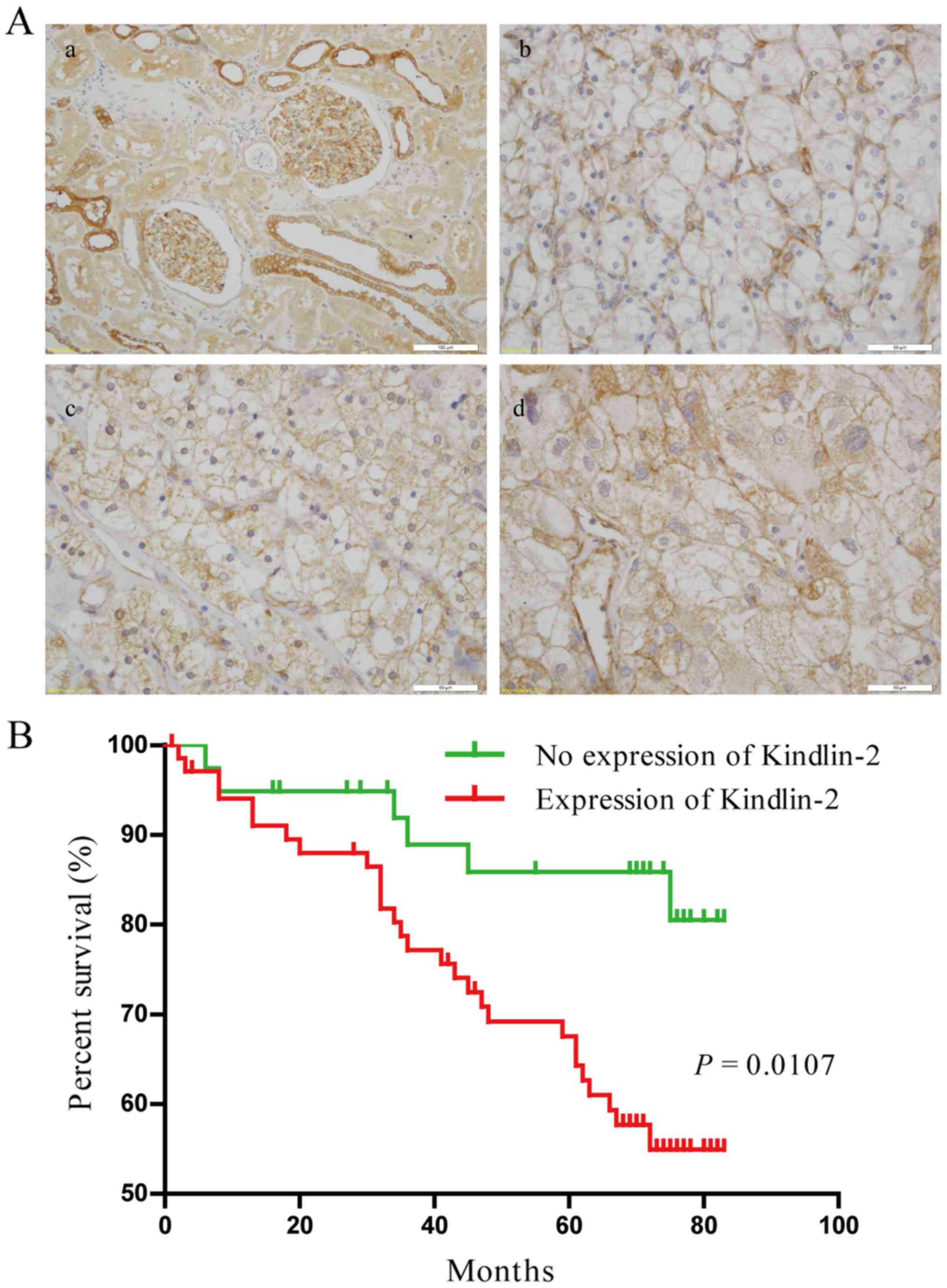
in proximal convoluted tubules as shown in Fig. 1A-a. In CCRCC, Kindlin-2 was highly
expressed in tumor stroma, especially in the capillary network.
Kindlin-2 expression rate (84.38%, 27/32) and intensity [weak
staining in 40.63% (13/32) and strong staining in 43.75% (14/32)]
were much higher in high-grade tumors (Fuhrman grade 3 and 4) than
in low-grade tumors (Fuhrman grade 1 and 2). Low grade tumors
showed an expression rate of 55.84% (43/77), and rates for weak
staining and strong staining were 38.96% (30/77) and 16.88% (13/77)
respectively (P=0.0101, Table I,
Fig. 1A-b-d). In addition, advanced
tumors with regional lymph node metastasis showed stronger staining
for Kindlin-2 than localized cases of CCRCC (P=0.0227, Table I). These results indicate the
involvement of Kindlin-2 in the process of CCRCC progression.
 | Table I.Relationship between Kindlin-2
expression and various clinicopathological features in CCRCC
patients. |
Table I.
Relationship between Kindlin-2
expression and various clinicopathological features in CCRCC
patients.
|
|
| Kindlin-2
expression |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
features | N=109 | Negative (%) | Weak (%) | Strong (%) | P-value |
|---|
| Sex |
|
Male | 67 | 21 (31.34) | 30 (44.78) | 16 (23.88) | 0.502 |
|
Female | 42 | 18 (42.86) | 13 (30.95) | 11 (26.19) |
|
| Age, years |
|
≤60 | 62 | 23 (37.10) | 22 (35.48) | 17 (27.42) | 0.875 |
|
>60 | 47 | 16 (34.04) | 21 (44.68) | 10 (21.28) |
|
| AJCC stage |
| X | 6 | 2
(33.33) | 3
(50.00) | 1
(16.67) |
|
| I | 64 | 26 (40.63) | 26 (40.63) | 12 (18.75) | 0.0538 |
| II | 20 | 6
(30.00) | 8
(40.00) | 6
(30.00) |
|
|
III-IV | 19 | 5
(26.32) | 6
(31.58) | 8
(42.10) |
|
| Nuclear grade
(Fuhrman) |
| 1 | 36 | 15 (41.67) | 14 (38.89) | 7
(19.44) | 0.0101* |
| 2 | 41 | 19 (46.34) | 16 (39.02) | 6
(14.63) |
|
| 3 and
4 | 32 | 5
(15.63) | 13 (40.63) | 14 (43.75) |
|
| pT stage |
|
Tx | 4 | 1
(25.00) | 3
(75.00) | 0
(0.00) |
|
|
T1 | 68 | 28 (41.18) | 26 (38.24) | 14 (20.59) | 0.0596 |
|
T2 | 20 | 6
(30.00) | 8
(40.00) | 6
(30.00) |
|
|
T3 and
T4 | 17 | 4
(23.53) | 6
(35.29) | 7
(41.18) |
|
| N stage |
|
Nx | 2 | 1
(50.00) | 0
(0.00) | 1
(50.00) |
|
|
N0 | 99 | 37 (37.37) | 41 (41.41) | 21 (21.21) | 0.0227* |
|
N1 | 8 | 1
(12.50) | 2
(25.00) | 5
(62.50) |
|
To determine whether Kindlin-2 expression is
correlated with patient prognosis, survival analysis was used to
evaluate patients with available follow-up data of up to 82 months.
Among 109 patients, 34 (31.19%) patients died during follow-up, 66
(60.55%) patients were alive, and 9 (8.26%) patients were lost to
follow-up. The median survival time was 69 months. Kaplan-Meier
plots and log-rank tests showed that CCRCC patients with Kindlin-2
expression had shorter overall survival than patients without
Kindlin-2 expression (P=0.0107, Fig.
1B). There was no apparent difference in overall survival among
patients with different intensities of Kindlin-2 staining
(P=0.5412, data not shown). Upon univariate analysis (Table II), Kindlin-2 (HR, 0.41; 95% CI,
0.20–0.81; P=0.0107), age (HR, 0.53; 95% CI, 0.27–1.05; P=0.0703),
Fuhrman grade (HR, 0.04; 95% CI, 0.02–0.10; P<0.0001), pT stage
(HR, 0.07; 95% CI, 0.02–0.28; P=0.0002), N stage (HR, 0.000029; 95%
CI, 0.000001–0.0008; P<0.0001), and AJCC stage (HR, 0.03; 95%
CI, 0.01–0.13; P<0.0001) were associated with overall survival
and these factors were included in multivariate analysis.
Multivariate analysis (Table II)
showed that Fuhrman grade (HR, 0.14; 95% CI, 0.06–0.30;
P<0.0001) and AJCC stage (HR, 0.07; 95% CI, 0.01–0.74; P=0.028)
were independently associated with overall survival. However,
Kindlin-2 had a P-value of 0.276 and was therefore not an
independent prognostic factor in CCRCC. These results indicate that
the presence of Kindlin-2 is a factor that predicts poor overall
survival in CCRCC patients.
 | Table II.The effect of clinicopathological
characteristics on overall survival by Cox regression analyses. |
Table II.
The effect of clinicopathological
characteristics on overall survival by Cox regression analyses.
|
| OS |
|---|
|
|
|
|---|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Kindlin-2 (negative
vs. positive) | 0.41
(0.20–0.81) |
0.0107 | 0.59
(0.23–1.52) | 0.276 |
| Age years (<60
vs. ≥ 60) | 0.53
(0.27–1.05) |
0.0703 | 0.51
(0.23–1.11) | 0.091 |
| Sex (male vs.
female) | 1.03
(0.52–2.03) |
0.943 | − | − |
| Fuhrman grade (1-2
vs. 3-4) | 0.04
(0.02–0.10) | <0.0001 | 0.14
(0.06–0.30) | 0.000a |
| pT stage (1~2 vs.
3~4) | 0.07
(0.02–0.28) |
0.0002 | 4.17
(0.53–32.68) | 0.174 |
| N stage (0 vs.
1) | 0.000029
(0.000001–0.0008) | <0.0001 | 0.68
(0.11–4.12) | 0.678 |
| AJCC stage (1-2 vs.
3-4) | 0.03
(0.01–0.13) | <0.0001 | 0.07
(0.01–0.74) | 0.028a |
Kindlin-2 promotes cell migration,
invasion and proliferation in CCRCC in vitro
Kindlin-2 expression showed significant correlation
with tumor grade and patient prognosis, which raised the
possibility it plays a role in CCRCC progression. To evaluate this
possibility, we chose two CCRCC cell lines, 769-P and ACHN for
further study. 769-P cells stably overexpressing Kindlin-2
following retroviral transduction, and ACHN cells with stable
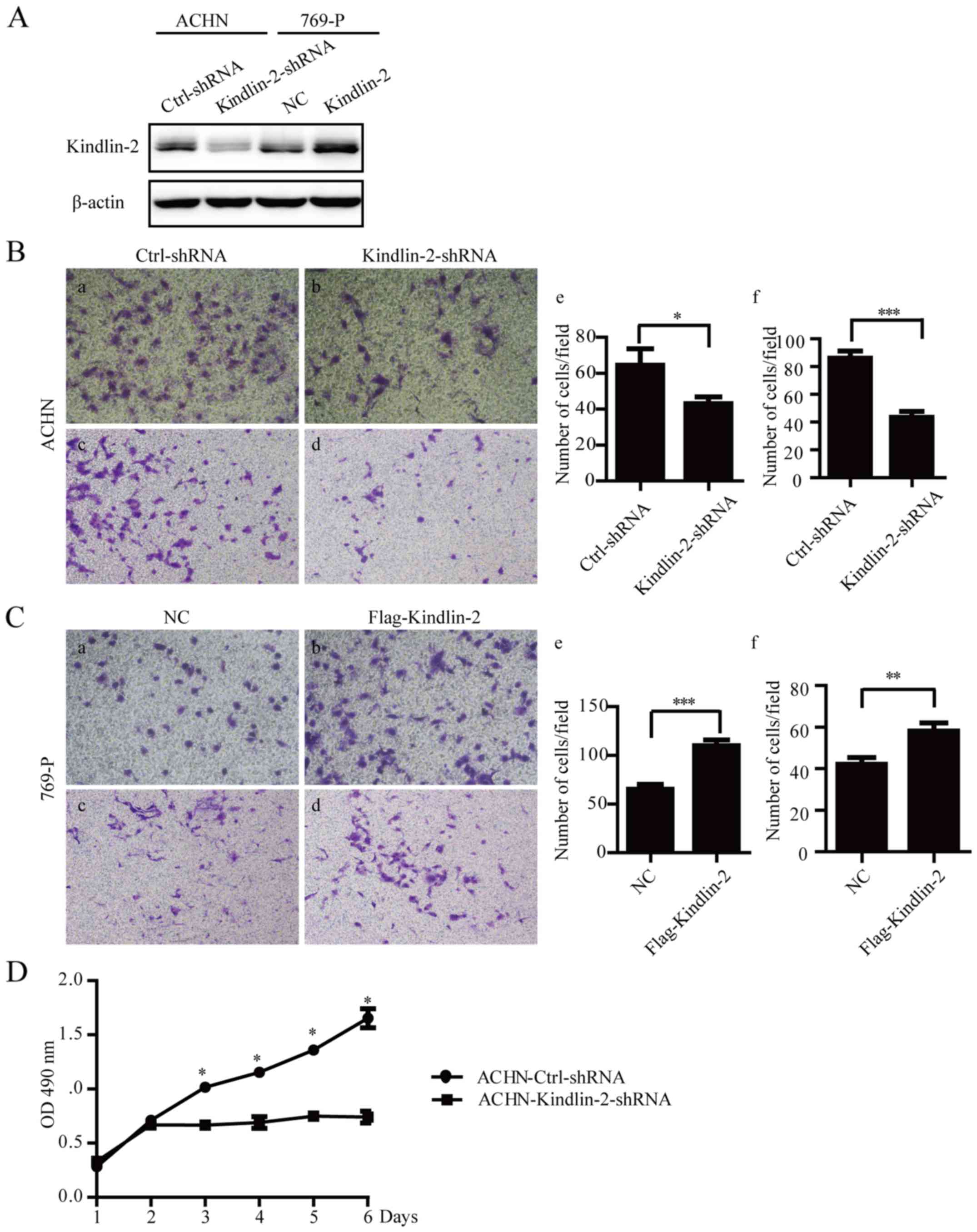
knockdown of Kindlin-2 were established (Fig. 2A). Stable depletion of Kindlin-2 by
shRNA inhibited the migratory (P=0.0352) and invasive (P<0.0001)
capacity of ACHN cells (Fig. 2B).
In comparison, overexpression of Kindlin-2 in 769-P cells
significantly promoted potential for cell migration (P<0.0001)
and invasion (P=0.0043) (Fig. 2C).
Depletion of Kindlin-2 in ACHN cells reduced cell proliferation
compared to the control group (P=0.0009 on the sixth day as shown
in Fig. 2D. However, there was no
significant difference in 769-P cell growth in the Kindlin-2
overexpression group versus the control group (data not shown).
Knockdown of Kindlin-2 inhibits CCRCC
cell proliferation in vivo
Observation of Kindlin-2 in RCC specimens and RCC
cell lines in vitro raised a question as to whether
Kindlin-2 plays a role in the regulation of tumor growth in
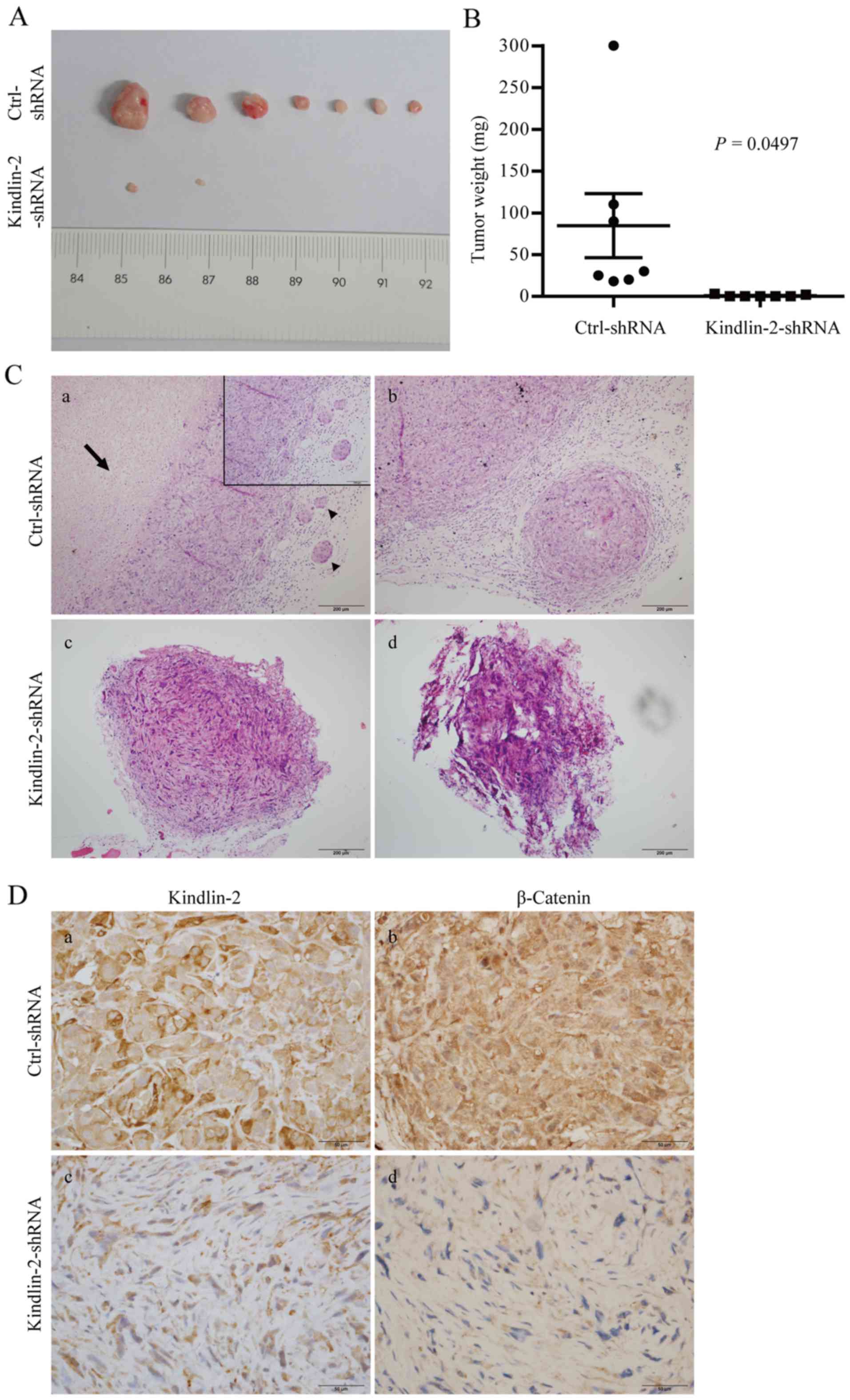
vivo. As expected, mouse tumor xenografts with Kindlin-2
knockdown grew significantly more slowly, and tumor size was
significantly smaller than that of the control group (Fig. 3A and B). In addition, massive
central tumor necrosis, intravenous tumor emboli and peritumoral
satellite nodules were found in the control group, but these
phenomena which reflect tumor aggressiveness were absent in the
ACHN-Kindlin-2-shRNA group (Fig.
3C). Lung and liver metastasis were not observed in either
group. We found that β-catenin immunohistochemical staining was
markedly reduced in Kindlin-2 depleted tumor xenografts compared
with counterpart controls (Fig.
3D), which stimulated consideration of the Kindlin-2
downstream.
Kindlin-2 activates the Wnt signaling
pathway to promote CCRCC progression
To evaluate the mechanism by which Kindlin-2
promotes CCRCC progression, ACHN control cells and Kindlin-2
depleted cells were analyzed with the Affymetrix GeneChip human
Gene 1.0 ST array. The detailed cDNA expression data can be
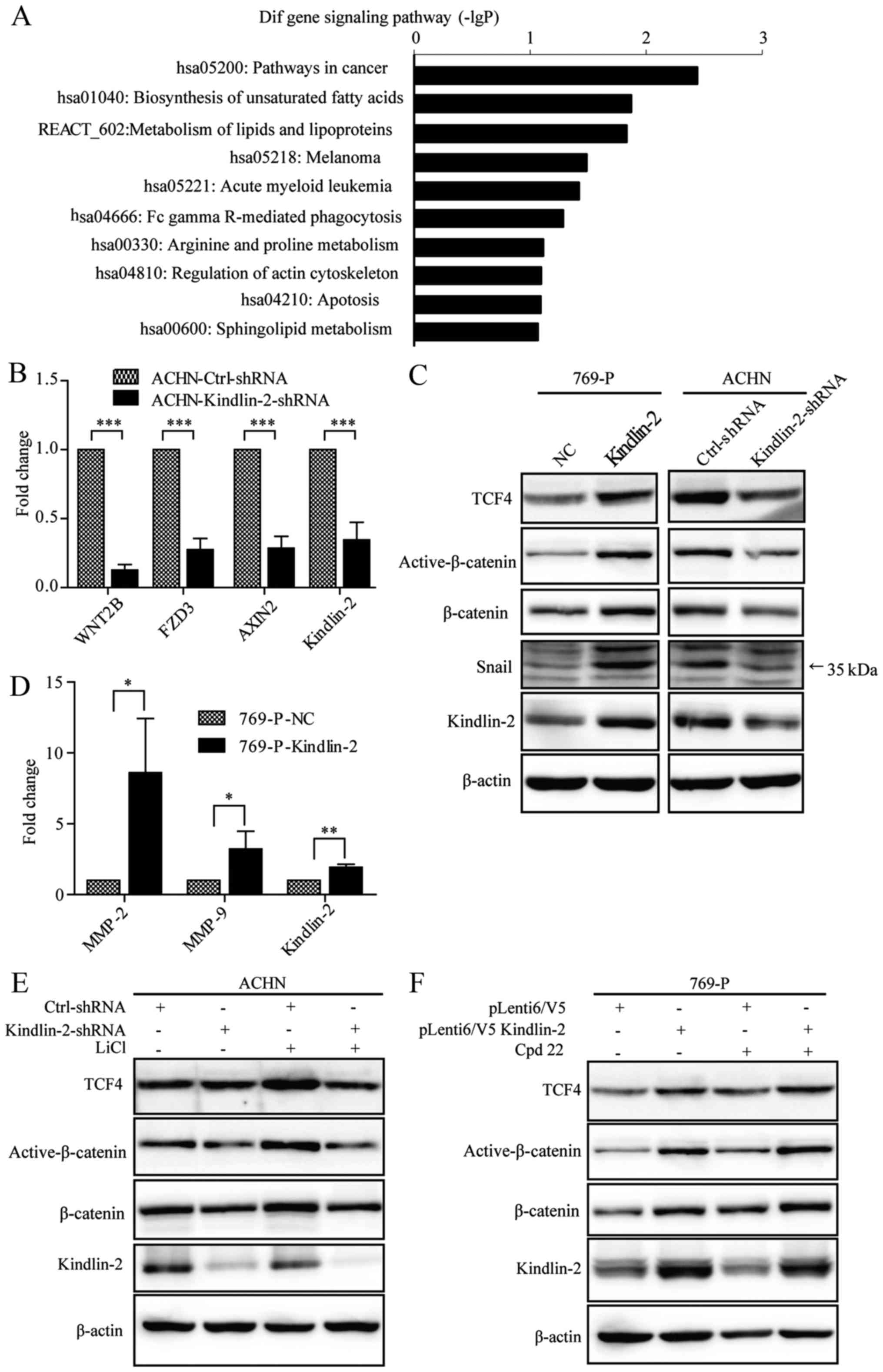
accessed on GEO GSE76020. Based on the KEGG database, pathways in
cancer was the most significantly downregulated pathway targeted by
Kindlin-2 depletion (Fig. 4A).
Among numerous tumor-associated genes, we found that expression of
genes involved in the Wnt signaling pathway were altered
significantly. To confirm these GeneChip results, the alteration of
expression of selected genes in the Wnt pathway (WNT2B, FZD3 and
Axin2) were validated with real-time PCR (Fig. 4B). Kindlin-2 combines with β-catenin
and TCF4 to activate the Wnt pathway (16), and expression of TCF4, β-catenin,
active β-catenin and Snail was found in both Kindlin-2
overexpressing 769-P cells and Kindlin-2 depleted ACHN cells.
Overexpression of Kindlin-2 elevated the expression of these four
genes, whereas knockdown of Kindlin-2 inhibited their expression as
shown in Fig. 4C. MMP-2 and MMP-9
are well known target genes of Wnt pathway, and overexpression of
Kindlin-2 increased the mRNA level of MMP-2 and MMP-9 in 769-P
cells (Fig. 4D).
To further determine whether Kindlin-2 is involved
in Wnt pathway activation, ACHN stable cells were treated for 24 h
with LiCl which is a specific GSK3β inhibitor. LiCl elevated
expression of TCF4, β-catenin and active β-catenin in
ACHN-Ctrl-shRNA cells (Fig. 4E,
compare lanes 1 with 3), showing that LiCl activates the Wnt
pathway in ACHN cells. However, treatment of ACHN stable Kindlin-2
depleted cells with LiCl did not result in a change of expression
of TCF4, β-catenin or active β-catenin (Fig. 4E, compare lanes 2 with 4). These
findings argue that knockdown of Kindlin-2 inhibits Wnt pathway
activation, and raises the possibility Kindlin-2 is required for
Wnt pathway activation. Kindlin-2 is known to bind integrin-linked
kinase (ILK) for regulation of integrin-mediated cell adhesion
(20,21), which raises a question as to whether
Kindlin-2 regulation of Wnt signaling is mediated by ILK. To
address this question, 769-P stable Kindlin-2 overexpressing cells
were treated with the ILK inhibitor Cpd 22 for 24 h, however,
expression of TCF4, β-catenin and active β-catenin did not change
significantly in 769-P stable cells treated with Cpd 22 (Fig. 4F). This finding indicates that
Kindlin-2 is not dependent on ILK binding for regulation of the Wnt
signaling pathway.
Discussion
Kindlin-2 is being known for the multiple roles it
plays in various cancers (11–18).
However, to date there has been no detailed study of Kindlin-2
function in RCC. In this study, we evaluated Kindlin-2 expression
and focused on its role in CCRCC. CCRCC is the major subtype of
RCC, and accounts for approximately 80% of renal carcinomas. The
prognosis for this subtype is poorer than the other two common RCC
subtypes including papillary RCC and chromophobe RCC (8).
Higher Kindlin-2 expression in CCRCC is associated
with higher nuclear grades and local lymph node metastasis.
Although CCRCC patients with Kindlin-2 tumor expression have poor
survival, Kindlin-2 expression is not an independent prognostic
factor. Kindlin-2 showed higher expression in CCRCC paraneoplastic
kidney tissues than that in the tumor itself, and this paradoxical
phenomenon raises the possibility Kindlin-2 is involved in CCRCC
progression, but not in tumor formation. Recently Yan et al
found that increased Kindlin-2 expression was associated with
advanced stage, hematogenous metastasis and short survival in CCRCC
patients (22). To our surprise,
all tumor cases showed Kindlin-2 expression of different intensity
in their study, with absent expression in adjacent renal contex
tissues. It is oppsite to our finding and earlier observation of
Kindlin-2 in adult kidney tissue (23) that Kindlin-2 has consistent strong
expression in renal contex. The different antibodies being used may
account for the discrepancy.
Several recent studies in cell models have shown
that Kindlin-2 is involved in cancer cell migration and invasion.
For example, upregulated Kindlin-2 expression promoted the
migration and invasion of breast cancer cells (16,19,24).
Kindlin-2 also promoted the invasion of gastric cancer cells
mediated by tumor-associated macrophages and phosphorylation of
integrin β1 and β3 (25,26). Inhibition of Kindlin-2 suppressed
migratory/invasive properties of esophageal squamous cell carcinoma
(27). In the present study,
Transwell assays showed that overexpression of Kindlin-2 in 796-P
cells promotes cell migration and invasion. In contrast, knockdown
of Kindlin-2 in ACHN cells inhibited cell migration and invasion.
Distant metastases were not found in the tumor xenograft model, but
absence of tumor emboli and satellite nodules in the Kindlin-2
depleted group as compared to the control group further supports
the concept that downregulation of Kindlin-2 expression suppresses
invasive and metastatic capacity in RCC cells in vivo.
Moreover, it was notable that knockdown of Kindlin-2 significantly
inhibited ACHN cell proliferation in vitro and tumorigenesis
in vivo. This is in accord with previous findings in breast
cancer and pancreatic cancer (19,28).
However, at the same time, there are several studies showing that
Kindlin-2 acts as a tumor suppressor in some cancers. It has been
shown to inhibit mesenchymal cell invasion, growth and migration of
colorectal cancer cells, and migration and invasion in ovarian
cancer cells (17,18,29).
In conclusion, it appears Kindlin-2 function is cellular
context-dependent.
We demonstrated that the genes involved in the Wnt
signaling pathway including WNT2B, FZD3 and Axin2 are indeed
downregulated by Kindlin-2 depletion through analyzing gene
expression profiles of Kindlin-2 depleted RCC cells with the Gene
1.0 ST array. The constitutive activation of Wnt signaling cascades
plays a critical role in the progression of RCC. Canonical Wnt
ligands bind to FZD (frizzled) family receptors and the LRP5/LRP6
co-receptor, which stabilizes β-catenin. After stabilization
β-catenin enters the nucleus and interacts with the members of the
LEF/TCF family, resulting in a functional transcription factor
complex with resultant expression of downstream target genes (Myc,
cyclin D1, MMP7, Axin2, and so on) (30). There are several lines of evidence
which implicate the Wnt signaling pathway in RCC. Wnt10A was shown
to promote RCC carcinogenesis and progression by activating the
Wnt/β-catenin pathway (31). In
addition, β-catenin is the key molecule in the pathogenesis of RCC.
Elevation of the β-catenin expression level induced renal tumors in
a mouse model (32). It is well
known that the defects in VHL tumor suppressor gene contribute to
most sporadic CCRCC. Peruzzi et al found that VHL
loss in CCRCC may activate oncogenic β-catenin signaling, leading
to promoted tumor cell motility and invasiveness (33). Targeting the Wnt signaling pathway
may have potential as a therapeutic modality for RCC. A very recent
report has shown that several drugs including ethacrynic acid,
ciclopirox olamine and piroctone olamine may induce suppression of
RCC in part due to inhibition of Wnt signaling (34).
In addition, Kindlin-2 has been shown to be involved
in tumor progression through regulation of the Wnt pathway. For
example, the Kindlin-2-β-catenin-TCF4 tripartite complex promotes
Wnt target gene expression and in turn regulates breast cancer cell
invasion (16). However, another
study showed that Kindlin-2 inhibited the growth and migration of
colorectal cancer cells by promoting the ubiquitination of
β-catenin (29)In the present study
we demonstrated Kindlin-2 is involved in Wnt signaling activation
in CCRCC by immunostaining of β-catenin, active β-catenin, TCF4 and
Snail. In addition, IHC analysis in xenograft tumors further
demonstrated that expression of β-catenin is markedly reduced by
Kindlin-2 knockdown. It is known that glycogen synthase kinase 3β
(GSK3β) which is a negative regulator of Wnt signaling, initiates
degradation of β-catenin. Wnt signaling can be activated using the
GSK3β inhibitor LiCl, whereas expression of β-catenin, active
β-catenin, and TCF4 was significantly inhibited by Kindlin-2
knockdown, even in the presence of LiCl. These findings argue that
Kindlin-2 is required for Wnt pathway activation. However, its role
in activating the Wnt signaling pathway is independent of the
Kindlin-2/ILK interaction. The underlying mechanism by which
Kindlin-2 regulates the Wnt signaling pathway thus warrants future
investigation.
In conclusion, in this study we demonstrate that
high expression of Kindlin-2 is associated with high-grade tumors
and lymph node metastasis of CCRCC; the presence of Kindlin-2
expression is associated with short survival in CCRCC patients,
although it is not an independent prognostic factor; Kindlin-2
promotes CCRCC cell migration, invasion and proliferation by
activating the Wnt signaling pathway.
Acknowledgements
This study was supported by grants from the ministry
of Science and Technology of China 2016YFC1302103, 2015CB553906,
2013CB910501, and the National Natural Science Foundation of China
grants 81230051, 81472734, 31170711, 81321003 and 30830048, Beijing
Natural Science Foundation grant 7120002, the 111 Project of the
Ministry of Education, Peking University grants BMU20120314 and
BMU20130364, and a Leading Academic Discipline Project of Beijing
Education Bureau to H.Z.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ye DW, Eto M, Chung JS, Kimura G, Chang
W-C, Chang Y-H, Pang S-T, Lee JL, Niu Y, Gurney H, et al: Use of
targeted therapies for advanced renal cell carcinoma in the
Asia-Pacific region: Opinion statement from China, Japan, Taiwan,
Korea, and Australia. Clin Genitourin Cancer. 12:225–233. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Patel AR, Prasad SM, Shih YC and Eggener
SE: The association of the human development index with global
kidney cancer incidence and mortality. J Urol. 187:1978–1983. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Delahunt B: Advances and controversies in
grading and staging of renal cell carcinoma. Mod Pathol. 22:(Suppl
2). S24–S36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Motzer RJ, Escudier B, Oudard S, Hutson
TE, Porta C, Bracarda S, Grünwald V, Thompson JA, Figlin RA,
Hollaender N, et al: Phase 3 trial of everolimus for metastatic
renal cell carcinoma: Final results and analysis of prognostic
factors. Cancer. 116:4256–4265. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sternberg CN, Davis ID, Mardiak J,
Szczylik C, Lee E, Wagstaff J, Barrios CH, Salman P, Gladkov OA,
Kavina A, et al: Pazopanib in locally advanced or metastatic renal
cell carcinoma: Results of a randomized phase III trial. J Clin
Oncol. 28:1061–1068. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rini BI, Escudier B, Tomczak P, Kaprin A,
Szczylik C, Hutson TE, Michaelson MD, Gorbunova VA, Gore ME,
Rusakov IG, et al: Comparative effectiveness of axitinib versus
sorafenib in advanced renal cell carcinoma (AXIS): A randomised
phase 3 trial. Lancet. 378:1931–1939. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mancini V, Battaglia M, Ditonno P, Palazzo
S, Lastilla G, Montironi R, Bettocchi C, Cavalcanti E, Ranieri E
and Selvaggi FP: Current insights in renal cell cancer pathology.
Urol Oncol. 26:225–238. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Larjava H, Plow EF and Wu C: Kindlins:
Essential regulators of integrin signalling and cell-matrix
adhesion. EMBO Rep. 9:1203–1208. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tu Y, Wu S, Shi X, Chen K and Wu C:
Migfilin and Mig-2 link focal adhesions to filamin and the actin
cytoskeleton and function in cell shape modulation. Cell.
113:37–47. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gong X, An Z, Wang Y, Guan L, Fang W,
Strömblad S, Jiang Y and Zhang H: Kindlin-2 controls sensitivity of
prostate cancer cells to cisplatin-induced cell death. Cancer Lett.
299:54–62. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shen Z, Ye Y, Dong L, Vainionpää S,
Mustonen H, Puolakkainen P and Wang S: Kindlin-2: A novel adhesion
protein related to tumor invasion, lymph node metastasis, and
patient outcome in gastric cancer. Am J Surg. 203:222–229. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Talaat S, Somji S, Toni C, Garrett SH,
Zhou XD, Sens MA and Sens DA: Kindlin-2 expression in arsenite- and
cadmium-transformed bladder cancer cell lines and in archival
specimens of human bladder cancer. Urology. 77:1507.e1–7. 2011.
View Article : Google Scholar
|
|
14
|
Zhan J, Zhu X, Guo Y, Wang Y, Wang Y,
Qiang G, Niu M, Hu J, Du J, Li Z, et al: Opposite role of Kindlin-1
and Kindlin-2 in lung cancers. PLoS One. 7:e503132012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
An Z, Dobra K, Lock JG, Stromblad S,
Hjerpe A and Zhang H: Kindlin-2 is expressed in malignant
mesothelioma and is required for tumor cell adhesion and migration.
Int J Cancer. 127:1999–2008. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu Y, Wu J, Wang Y, Zhao T, Ma B, Liu Y,
Fang W, Zhu W-G and Zhang H: Kindlin 2 forms a transcriptional
complex with beta-catenin and TCF4 to enhance Wnt signalling. EMBO
Rep. 13:750–758. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ren C, Du J, Xi C, Yu Y, Hu A, Zhan J, Guo
H, Fang W, Liu C and Zhang H: Kindlin-2 inhibits serous epithelial
ovarian cancer peritoneal dissemination and predicts patient
outcomes. Biochem Biophys Res Commun. 446:187–194. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shi X and Wu C: A suppressive role of
mitogen inducible gene-2 in mesenchymal cancer cell invasion. Mol
Cancer Res. 6:715–724. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao T, Guan L, Yu Y, Pei X, Zhan J, Han
L, Tang Y, Li F, Fang W and Zhang H: Kindlin-2 promotes genome
instability in breast cancer cells. Cancer Lett. 330:208–216. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fukuda K, Bledzka K, Yang J, Perera HD,
Plow EF and Qin J: Molecular basis of Kindlin-2 binding to
integrin-linked kinase pseudokinase for regulating cell adhesion. J
Biol Chem. 289:28363–28375. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huet-Calderwood C, Brahme NN, Kumar N,
Stiegler AL, Raghavan S, Boggon TJ and Calderwood DA: Differences
in binding to the ILK complex determines kindlin isoform adhesion
localization and integrin activation. J Cell Sci. 127:4308–4321.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yan M, Zhang L, Wu Y, Gao L, Yang W, Li J,
Chen Y and Jin X: Increased expression of Kindlin-2 is correlated
with hematogenous metastasis and poor prognosis in patients with
clear cell renal cell carcinoma. FEBS Open Bio. 6:660–665. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhan J, Yang M, Chi X, Zhang J, Pei XL,
Ren CX, Guo YQ, Liu W and Zhang HQ: Kindlin-2 expression in adult
tissues correlates with their embryonic origins. Sci China Life
Sci. 57:690–697. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu Y, Wu J, Guan L, Qi L, Tang Y, Ma B,
Zhan J, Wang Y, Fang W and Zhang H: Kindlin 2 promotes breast
cancer invasion via epigenetic silencing of the microRNA200 gene
family. Int J Cancer. 133:1368–1379. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shen Z, Ye Y, Kauttu T, Seppänen H,
Vainionpää S, Wang S, Mustonen H and Puolakkainen P: The novel
focal adhesion gene Kindlin-2 promotes the invasion of gastric
cancer cells mediated by tumor-associated macrophages. Oncol Rep.
29:791–797. 2013.PubMed/NCBI
|
|
26
|
Shen Z, Ye Y, Kauttu T, Seppänen H,
Vainionpää S, Wang S, Mustonen H and Puolakkainen P: Novel focal
adhesion protein Kindlin-2 promotes the invasion of gastric cancer
cells through phosphorylation of integrin beta1 and beta3. J Surg
Oncol. 108:106–112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang HF, Zhang K, Liao LD, Li L-Y, Du
Z-P, Wu B-L, Wu J-Y, Xu X-E, Zeng F-M, Chen B, et al: miR-200b
suppresses invasiveness and modulates the cytoskeletal and adhesive
machinery in esophageal squamous cell carcinoma cells via targeting
Kindlin-2. Carcinogenesis. 35:292–301. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhan J, Song J, Wang P, Chi X, Wang Y, Guo
Y, Fang W and Zhang H: Kindlin-2 induced by TGF-beta signaling
promotes pancreatic ductal adenocarcinoma progression through
downregulation of transcriptional factor HOXB9. Cancer Lett.
361:75–85. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ren Y, Jin H, Xue Z, Xu Q, Wang S, Zhao G,
Huang J and Huang H: Kindlin-2 inhibited the growth and migration
of colorectal cancer cells. Tumour Biol. 36:4107–4114. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Niehrs C: Function and biological roles of
the Dickkopf family of Wnt modulators. Oncogene. 25:7469–7481.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hsu RJ, Ho JY, Cha TL, Yu D-S, Wu C-L,
Huang W-P, Chu P, Chen Y-H, Chen J-T and Yu C-P: WNT10A plays an
oncogenic role in renal cell carcinoma by activating
WNT/beta-catenin pathway. PLoS One. 7:e476492012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Saadi-Kheddouci S, Berrebi D, Romagnolo B,
Cluzeaud F, Peuchmaur M, Kahn A, Vandewalle A and Perret C: Early
development of polycystic kidney disease in transgenic mice
expressing an activated mutant of the beta-catenin gene. Oncogene.
20:5972–5981. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Peruzzi B, Athauda G and Bottaro DP: The
von Hippel-Lindau tumor suppressor gene product represses oncogenic
beta-catenin signaling in renal carcinoma cells. Proc Natl Acad Sci
USA. 103:14531–14536. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Von Schulz-Hausmann SA, Schmeel LC,
Schmeel FC and Schmidt-Wolf IG: Targeting the Wnt/beta-catenin
pathway in renal cell carcinoma. Anticancer Res. 34:4101–4108.
2014.PubMed/NCBI
|