Introduction
Ovarian carcinoma is the most lethal gynecologic
malignancy (1). To date, there is
no effective early screening method, and more than one-half of
cases are diagnosed at an advanced stage (2). Unlike other gynecological
malignancies, extensive pelvic and peritoneal dissemination is the
most common path of metastasis in ovarian cancer (3). Therefore, better understanding of the
mechanisms of cell migration and invasion is urgently needed to
identify novel therapeutic strategies for ovarian cancer
treatment.
Human chorionic gonadotropin (hCG) has a
physiologically significant role during pregnancy. The hCG family
refers to a group of five molecules, each sharing a common amino
acid sequence but differing in multimeric structure and
carbohydrate side chain structure. Of these five molecules, β-hCG
and hyperglycosylated β-hCG were confirmed to be associated with
advanced malignancies (4).
Recently, it was shown that elevated levels of β-hCG in serum,
urine or tumor tissue correlates with poor prognosis,
aggressiveness and resistance to therapy in a variety of
nontrophoblastic tumors, such as bladder, colon, lung (5) and testicular cancer (6). Moreover, it is also ectopically
expressed in numerous gynecological malignancies, such as
endometrial carcinoma (7), ovarian
cancer (8) and cervical carcinoma
(9,10).
In our previous study, β-hCG was confirmed to
facilitate proliferation and cell cycle progression, attenuate
apoptosis and promote tumorigenesis in ovarian surface epithelial
cells (11). To test whether this
molecule plays a potential role in ovarian epithelial cancer
tumorigenesis, a series of experiments were performed. We found
that the β-hCG level was significantly elevated in metastatic
tissue compared to the level noted in the primary ovarian cancer
tissue, which indicated that β-hCG may play a role in ovarian
cancer metastasis. To further verify this hypothesis, a series of
in vitro and in vivo investigations were
performed.
Materials and methods
Patients and tissue specimens
The present study was approved by the Medical Ethics
Committee at The First Affiliated Hospital of the Medical College
of Shihezi University. Written consent was obtained from all
enrolled patients. Paraffin-embedded human ovarian cancer specimens
were obtained from 24 patients who underwent surgical resection
without prior adjuvant therapy from December 2006 to December 2013
at The First Affiliated Hospital of the Medical College of Shihezi
University, and 20 cases of normal ovarian tissue samples were used
as a control. All samples were pathologically diagnosed according
to the World Health Organization (WHO) classification guidelines
(2004). All procedures were performed in accordance with the
Declaration of Helsinki.
Cell culture
The human ovarian epithelial cancer cell lines ES-2
and SKOV3 used in the present study were obtained from the American
Type Culture Collection (ATCC; Manassas, VA, USA). Cells were
maintained in RPMI-1640 medium (HyClone, Logan, UT, USA),
containing 10% fetal bovine serum (FBS; Gibco, Grand Island, NY,
USA), 100 U/ml penicillin and 100 mg/ml streptomycin. Cells were
cultured at 37°C in a humidified 5% CO2 environment.
Immunohistochemistry (IHC)
Following formalin fixation and paraffin-embedding,
the 4-µm thick tissue sections were incubated with primary rabbit
polyclonal antibodies against β-hCG (1:50; ab53087; Abcam,
Cambridge, MA, USA) overnight at 4°C, washed with
phosphate-buffered saline (PBS), and then incubated with the
secondary antibody for 1 h at 37°C. Finally, the sections were
stained with 3,3-diaminobenzidine and then counterstained with
hematoxylin. Images were obtained with a Nikon Eclipse TE2000
fluorescence microscope (Nikon, Tokyo, Japan). Stained tissues were
classified according to staining intensity by two investigators.
The extent of β-hCG staining in tissue cores was quantified using a
four-tier grading system: 0, ≤5% positive staining; 1, 5–20%
positive staining; 2, 20–50% positive staining; and 3, ≥50%
positive staining. For statistical analysis, we divided cases into
two groups: negative expression (with scores of 0) and positive
expression (with scores of 1, 2 or 3) (11).
Establishment of β-hCG-overexpressing
cell lines
We established β-hCG-overexpressing ovarian cancer
cell lines in ES-2 and SKOV3 cells via lentivirus transfection. A
lentiviral vector encoding β-hCG (LV-β-hCG) and a negative control
vector (LV-vector) were purchased from Obio Technology (Shanghai,
China), carrying an enhanced green fluorescent protein reporter
gene, eGFP. For β-hCG exogenous overexpression, lentivirus
containing LV-β-hCG or the LV-vector were transfected into ES-2 and
SKOV3 cells using Polybrene (5.0 µg/ml) from Obio Technology,
following the manufacturer's instructions. Medium containing
puromycin (0.2 mg/ml) was used to select stably transduced cells.
The cells were photographed with a fluorescence microscope. β-hCG
upregulation efficiency was assessed using qPCR and a western blot
assay.
β-hCG-siRNA transfection in ovarian
cancer cells
ES-2 and SKOV3 cells were separately seeded in
plates, and then transfected with β-hCG-siRNAs or nc-siRNA (100 nM;
RiboBio, Guangzhou, China) using Lipofectamine 2000 (Invitrogen,
Carlsbad, CA, USA) transfection reagent, following the
manufacturer's protocol. Cells were harvested at 48–72 h
post-transfection for future experiments. β-hCG knockdown
efficiency was assessed using qPCR and a western blot assay.
Wound healing and Transwell
assays
Wound healing assay: cells were seeded into 24-well
plates and allowed to grow to 90–95% confluence. Similar sized
wounds were introduced to a monolayer of cells using a sterile
white pipette tip. The wounded monolayer of cells was washed three
times with PBS to remove cell debris and then cultured. The speed
of wound closure was monitored and photographed every 4 h until the
wound filled. Transwell assay: 1.0×105 cells in 100 µl
of RPMI-1640 with 2% FBS were seeded into Transwell upper chambers
(cat. 3422; Corning Inc., Corning, NY, USA) with or without
pre-coated Matrigel matrix (cat. 356234; BD Biosciences, Franklin
Lakes, NJ, USA), and 500 µl of RPMI-1640 containing 10% FBS was
added into the lower chamber to serve as the chemoattractant. After
16–48 h of incubation, the cells that did not migrate or invade
through the pores were carefully removed. Cells on the filters were
fixed in 100% methanol followed by hematoxylin staining (BA4025;
Baso Diagnostics, Inc., Zhuhai, China). The number of migrated
cells were counted with an inverted microscope (magnification,
×200; Nikon Eclipse), in 10 random fields/chamber. All experiments
were performed in triplicate.
Colony-formation assay
For colony-formation assays, cells were plated into
6-well plates at a concentration of 150 cells/well and incubated
for ~2 weeks. Then, colonies of cells were observed, fixed with
100% methanol and stained with hematoxylin.
Cell adhesion assay
Cells were seeded into 6-well plates until they
reached 90–95% confluence, and then, the cell culture medium was
removed, and cells were washed twice with PBS. After that, the
mixture of trypsin (cat. 25200072; Gibco) and EDTA (cat. E8008;
Sigma-Aldrich, St. Louis, MO, USA) in a ratio of 1:20 was added
into the plates, and cell images were captured at 0, 5, 10 and 20
min, separately. The pre-experimental adhesion assay was firstly
performed to determine the optimal time in ES-2 and SKOV3 cells,
and the remaining cells were captured and counted at different time
points. According to the results of pre-experiment (data not
shown), ES-2 and SKOV3 cells were captured at 5 and 20 min after
replacing the cell culture medium with the mixture of trypsin and
EDTA, respectively. Finally, the cells that remained adherent were
counted, the residual cell adhesion proportion was calculated, and
the residual cells were divided to initial cells in ES-2 and SKOV3
cells at 5 or 20 min, respectively.
Immunofluorescence staining
For immunofluorescence microscopy, the cells were
seeded on a culture dish (cat. 801001; Nest Biotechnology, Rahway,
NJ, USA) and incubated with a primary antibody against E-cadherin,
N-cadherin, Snail, vimentin, β-catenin (cat. 9782; Cell Signaling
Technology, Beverly, MA, USA), followed by incubation with Alexa
488-conjugated secondary antibody (Sigma, St. Louis, MO, USA).
Fluorescence staining for vimentin was visualized by confocal
laser-scanning microscopy (FluoView FV1000; Olympus, Japan); DAPI
(Sigma) was used to counterstain DNA.
RNA extraction and real-time
RT-PCR
Total RNA from ES-2 and SKOV3 cells was isolated by
TRIzol reagent (Invitrogen) according to the manufacturer's
protocol. Reverse transcription reactions were performed with
PrimeScript™ RT Master Mix kit (Takara Bio, Inc., Shiga, Japan)
according to the protocol. RT-PCR was performed on an Applied
Biosystems StepOnePlus™ Real-Time PCR System using a SuperReal
PreMix Plus (SYBR-Green) kit (Tiangen Biotech, Beijing, China). The
PCR cycling program was run with an initial predenaturation step at
95°C for 15 min, then with 45 cycles for amplification, at 95°C for
10 sec and 60°C for 32 sec.
GAPDH was used as an internal reference. Each test
was performed in triplicate, and the 2−ΔΔCt method was
used to calculate the expression of mRNA in each of the cell lines.
The primers used were: β-hCG forward, 5′-TCTGTGCCGGCTACTGCCCC-3′
and reverse, 5′-TTGGGACCCCCGCAGTCAGT-3′; GAPDH forward,
5′-ACAACTTTGGTATCGTGGAAGG-3′ and reverse,
5′-GCCATCACGCCACAGTTTC-3′.
Western blotting
Total protein from cells and tissues was lysed in
KeyGen Whole Cell Lysis Assay (cat. KGP250; KeyGen Biotech,
Nanjing, China). Then, 20–50 µg protein/sample from different cell
lines or treatments was separated by SDS-PAGE and blotted onto
polyvinylidene fluoride (PVDF) membranes that were blocked for 2 h
at room temperature with 5% BSA in TBS containing 0.05% Tween-20.
The membranes were then incubated overnight at 4°C in BSA in TBS
containing 0.05% Tween-20 and probed with mouse antibodies against
β-actin (1:4,000; cat. 20010; Abmart, Arlington, MA, USA) or rabbit
antibodies against β-hCG (1:1,000; cat. AP13036b, Abgent, San
Diego, CA, USA), EMT markers β-catenin, Slug, vimentin, Snail,
claudin, N-cadherin and E-cadherin (1;1,000; cat. 9782; Cell
Signaling Technology). Membranes were washed in Tris-buffered
saline with Tween-20 (TBST) and peroxidase-conjugated AffiniPure
goat anti-rabbit IgG (1:10,000; cat. KGAA35; KeyGen Biotech) and
peroxidase-conjugated goat anti-mouse IgG secondary antibody
(1:10,000; cat. L3032-2; Signalway Antibody, College Park, MD, USA)
were added and incubated at room temperature for 1 h. The membranes
were washed with PBST three times, and visualization of the protein
bands was achieved using an enhanced chemiluminescence Plus kit
(cat. WBKLS0500; Millipore, Billerica, MA, USA) as recommended by
the manufacturer.
Construction of a peritoneal xenograft
model in nude mice
All animal procedures were approved by the
Institutional Use and Care of Animals Committee. Female nude mice,
aged 4–6 weeks (weighing ~20 g) were housed and cared for at the
Animal Center of Tongji University (Shanghai, China).
Abdominopelvic cavity invasion is the most common form of tumor
dissemination in human ovarian cancer in the clinic; therefore,
direct intraperitoneal implantation in mice is a routine method to
simulate an ovarian cancer model to measure metastatic ability
in vivo. For xenografts, 1×107 cells transfected
with LV-vector or LV-β-hCG were intraperitoneally injected into
mice (6 mice/group) in the right flank. Xenograft growth was
monitored by NightOWL LB 983 In Vivo Imaging System (cat.
LB983 NC100; Berthold Technologies, Bad Wildbad, Germany) every two
days. Mice were sacrificed after 20 days of follow-up, or according
to tumor burden. Tumors were removed, fixed in 10% formalin, and
subjected to routine histological examination. The ovaries, uterus,
omentum, spleen, liver, intestines, colon and kidney were dissected
from the mice, fixed in 10% formalin and IHC stained to detect
β-hCG. All mouse experiments were conducted according to the
approved animal protocol of the Animal Center of Tongji
University.
Statistical analysis
All experiments were repeated at least three times
in duplicates. Data are presented as the mean values ± SEM. The
data were tested for significance employing Student's unpaired
t-test, Chi-square test and analysis of variance (ANOVA). The level
of significance was set at P<0.05.
Results
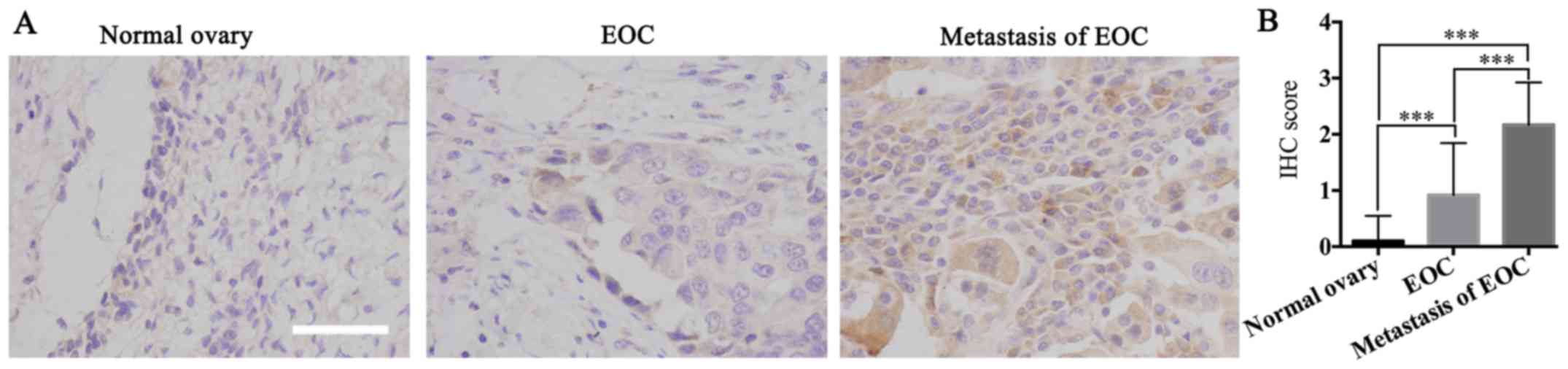
β-hCG is highly elevated in metastatic
tissue compared to tumor tissue of the ovary and normal tissue
To determine the biological function of β-hCG in
human ovarian cancer progression, IHC was performed to examine
β-hCG expression in 20 normal ovarian tissue samples, 24 human
ovarian cancer tissue samples and 24 metastatic tissues of ovarian
cancer samples. The location of the metastatic sample were mainly
from omentum and mesenterium, and all the tumor samples used in the
present study were epithelial ovarian tumors, including serous
ovarian and mucinous ovarian cancer, and clear cell ovarian
carcinoma, verified and analyzed by two gynecologic pathologists.
Results showed that 5.0% (1/20), 54.2% (13/24) and 83.3% (20/24) of
the cases were β-hCG-positive in normal ovarian tissue samples,
human ovarian cancer tissue samples and metastatic tissue of
ovarian cancer samples, respectively. β-hCG was expressed at
significant levels in the metastatic tissues of ovarian cancer and
at relatively insignificant levels in the primary ovarian cancer
tissues, and it was barely expressed in normal ovarian tissue
(P<0.05) (Fig. 1), which
suggested that β-hCG may play an important role in tumorigenesis
and metastasis of ovarian cancer.
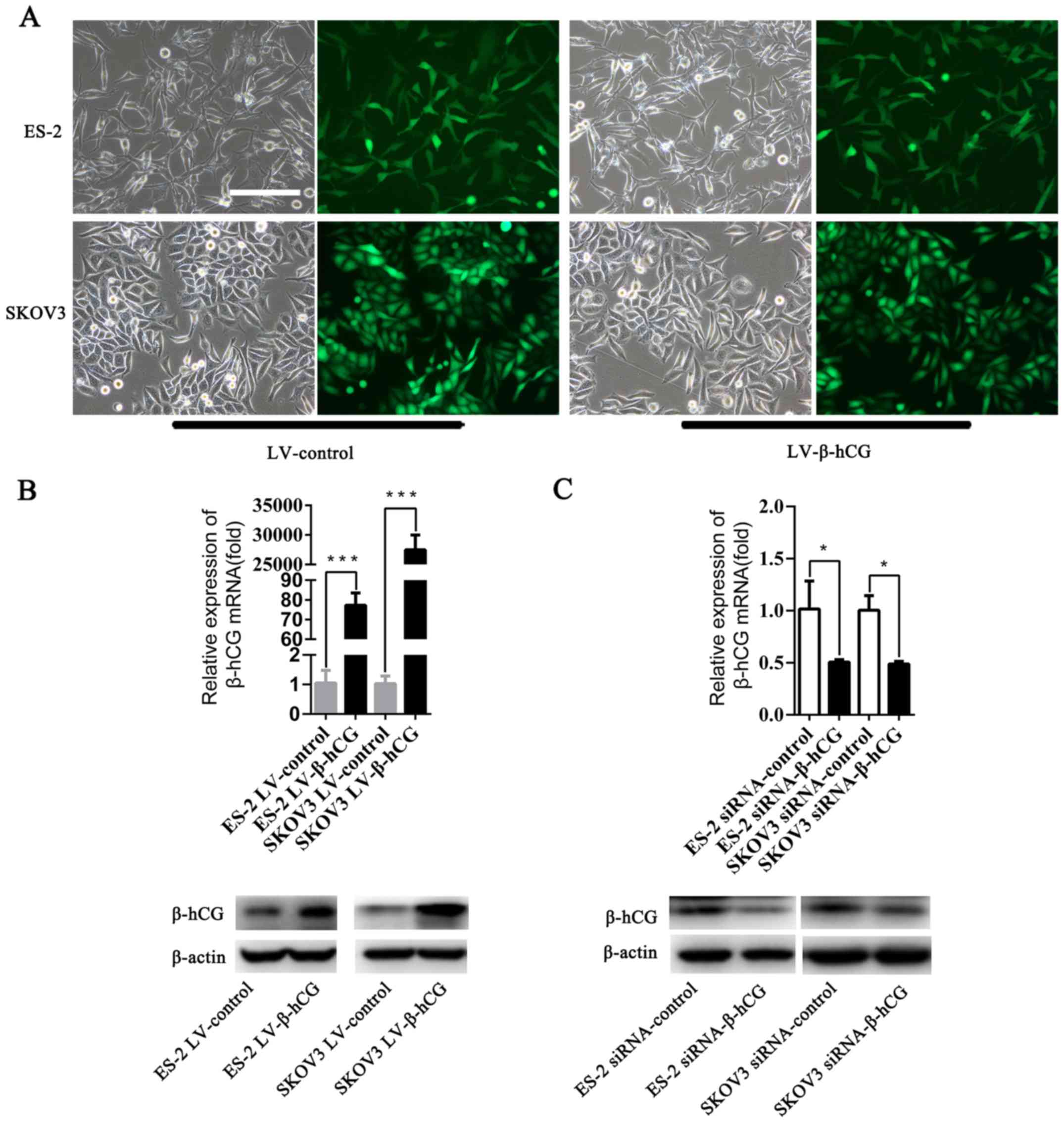
Successful construction of
β-hCG-overexpressing and silenced ovarian cancer cell lines
To obtain a β-hCG overexpression model in ovarian
cancer cells, LV-β-hCG or the LV-vector (both with a GFP gene) were
introduced into the ovarian cancer cell lines ES-2 and SKOV3 via a
lentiviral expression vector. Sequence information for the LV-β-hCG
and LV-vector plasmids were validated by sequencing (data not
shown). Stable β-hCG-overexpressing cell lines were selected with
puromycin. As shown in Fig. 2A, the
stable cell lines were successfully constructed, and nearly 90–95%
infection efficiency was determined by GFP assay in both ES-2 and
SKOV3 cell lines using fluorescence microscopy. Results showed that
β-hCG was obviously overexpressed at both the mRNA and protein
levels in the LV-β-hCG-transfected ES-2 and SKOV3 cells compared
with the control group by RT-PCR and western blotting (P<0.001)
(Fig. 2B). In addition to
construction of stable overexpressing β-hCG cell lines, depletion
of endogenous expression of β-hCG in ES-2 and SKOV3 cells was also
established through transient transfection with a small
interference RNA (siRNA) technique and was confirmed by RT-PCR and
western blotting (P<0.05) (Fig.
2C).
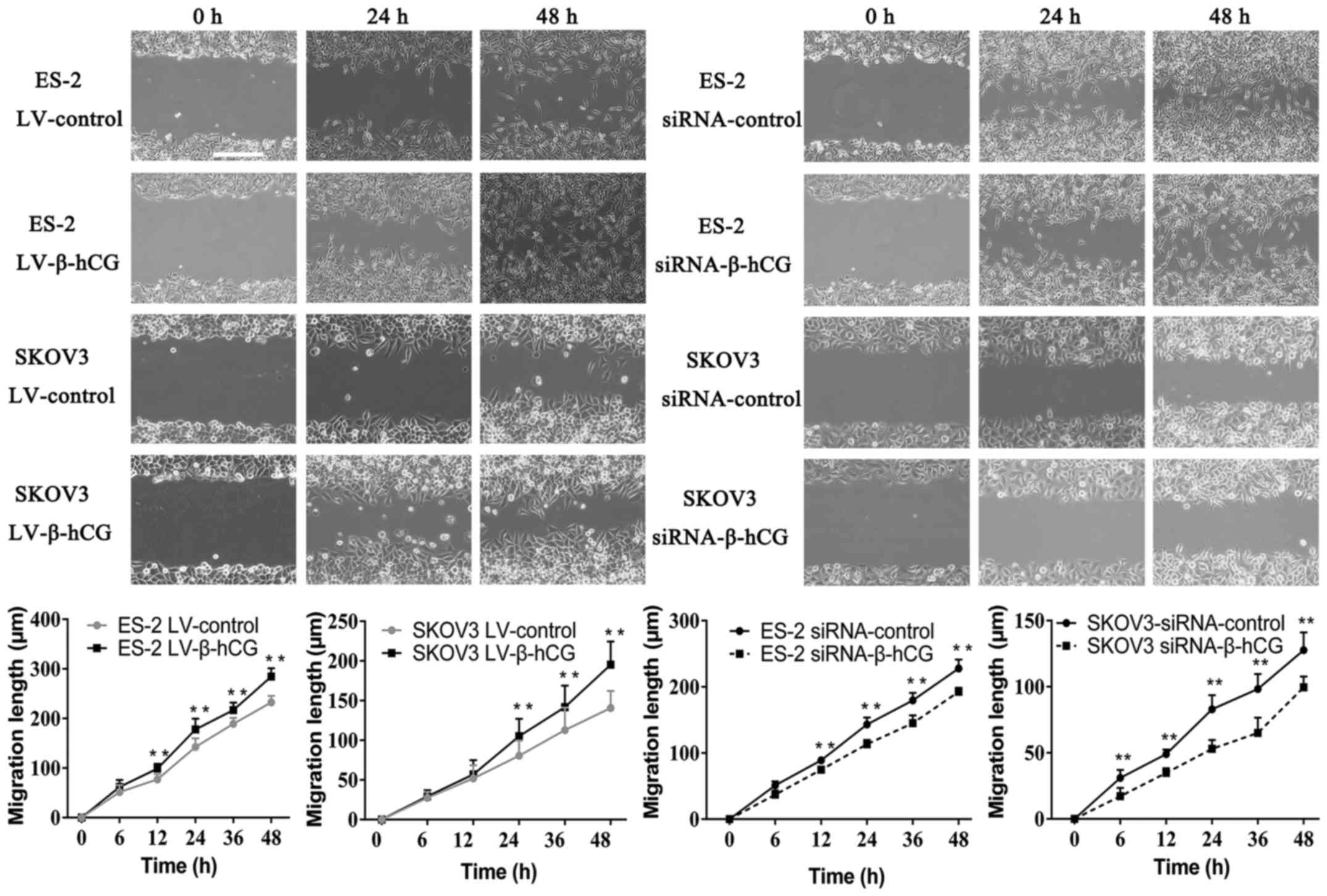
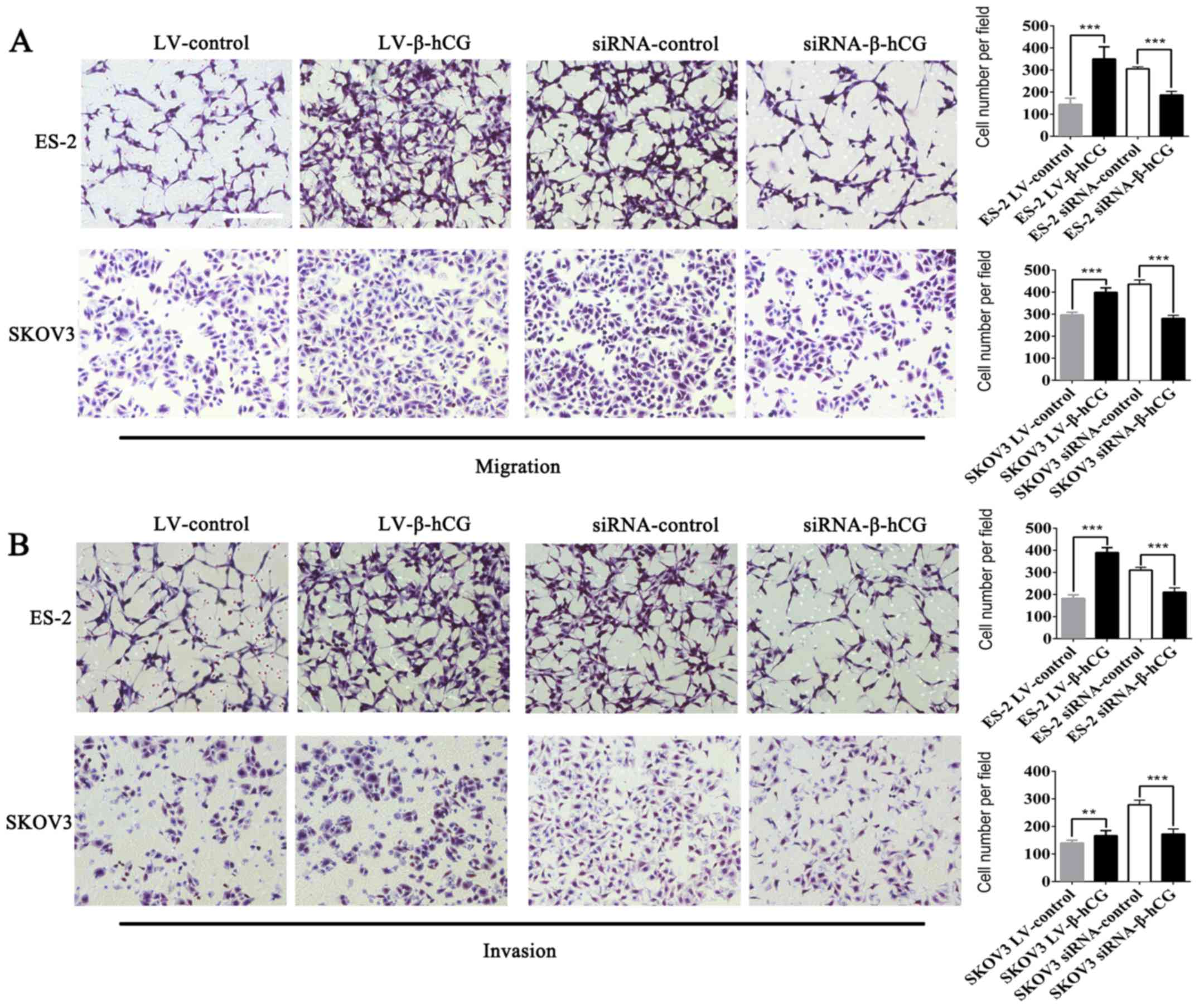
β-hCG regulates ovarian cancer cell
migration, morphology and attachment ability in vitro
Wound-healing and Transwell assays were performed to
validate the effect of β-hCG overexpressing and silencing of
ovarian cancer ES-2 and SKOV3 cells on migration and invasion
ability. As shown in Figs. 3 and
4 the overexpression of β-hCG
greatly increased cell migration and invasion abilities compared
with the control (P<0.001), while the inhibition of β-hCG
expression resulted in a slower rate to fill the gap in the
wound-healing assay, and the number of cells that penetrated
through the Matrigel matrix was decreased in the Transwell invasion
assay, which indicated that inhibition of β-hCG markedly decreased
migration and invasion abilities in the ES-2 and SKOV3 cells
(P<0.01). To investigate the biological changes induced by β-hCG
expression, a colony-formation assay was performed to assess
alterations in cell morphology. Under microscopic observation, the
stable β-hCG-overexpressing ES-2 and SKOV3 cells displayed an
elongated spindle-like mesenchymal morphology and became
dissociated from each other, whereas control cells exhibited a
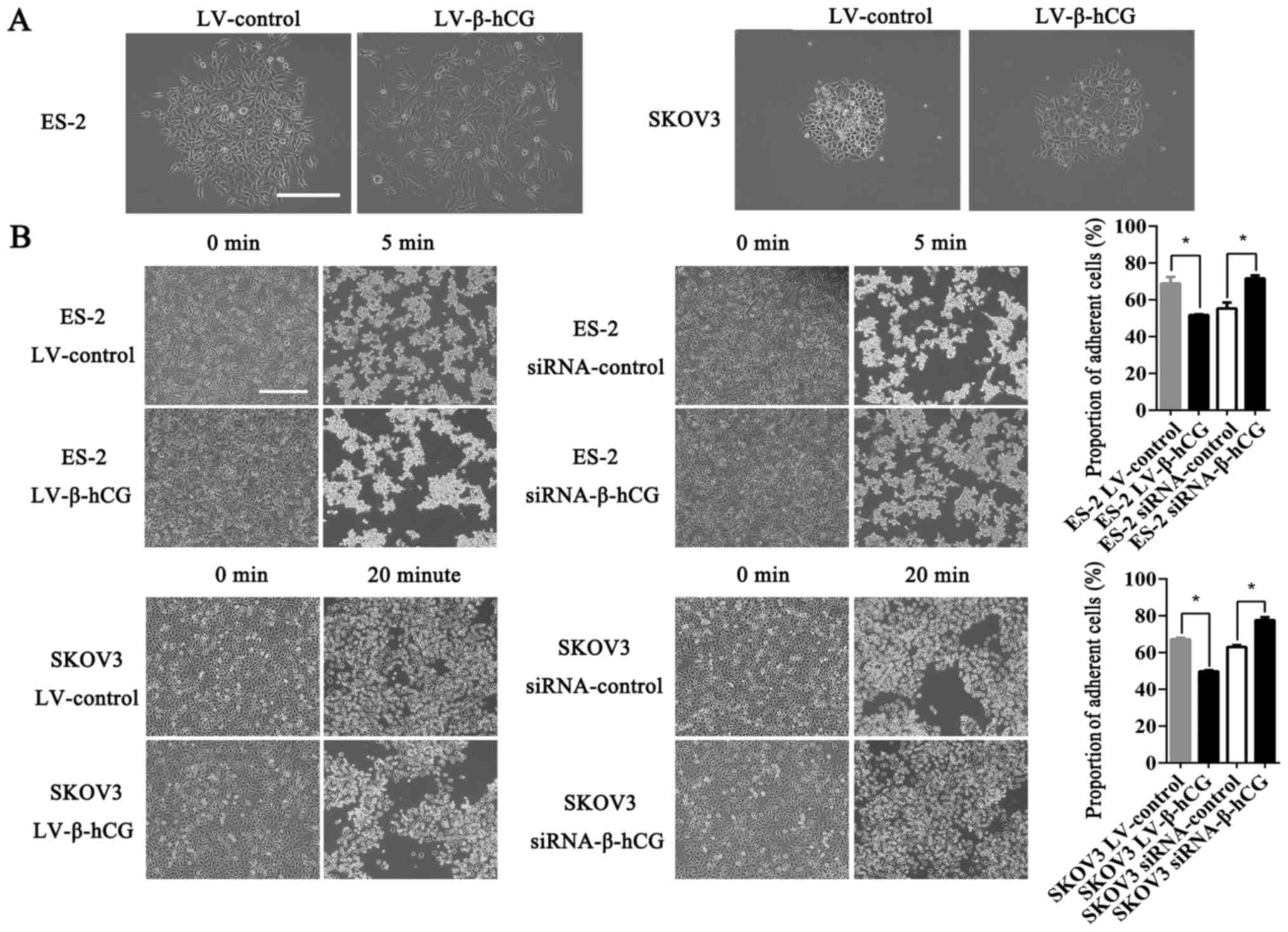
cobblestone-like epithelial phenotype (Fig. 5A). In addition, a cell adhesion
assay was performed to investigate the adhesive ability of ovarian
cancer cells mediated by β-hCG expression. The results showed that
the adhesion proportion was significantly decreased in the
β-hCG-overexpressing ES-2 and SKOV3 cells, while adhesion
proportion was highly increased in the β-hCG-seilenced cells, which
indicated that β-hCG markedly regulated ovarian cancer cell
attachment ability (P<0.05) (Fig.
5B).
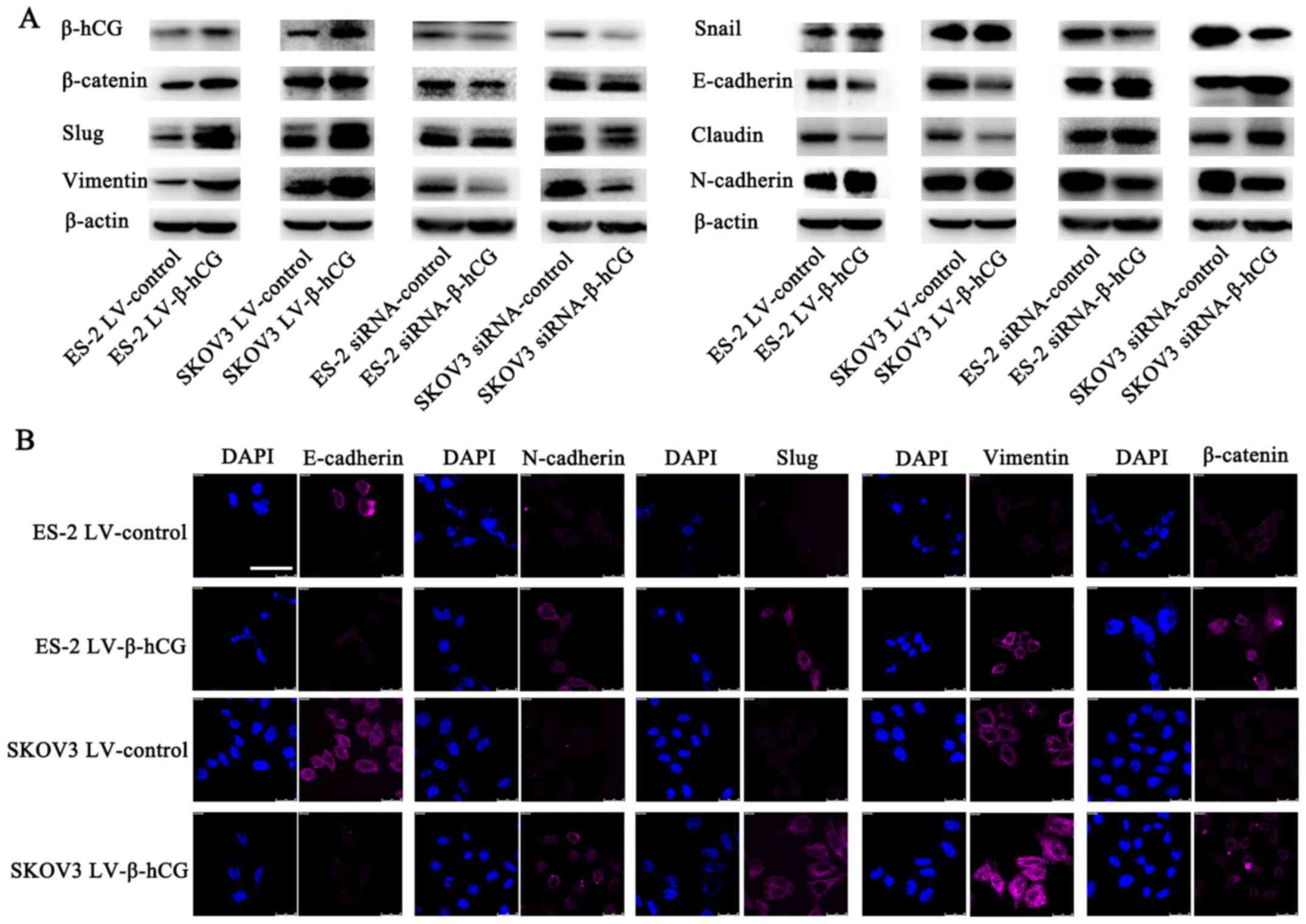
β-hCG regulates EMT in ovarian cancer
cells
To determine the potential molecular mechanisms of
β-hCG in EMT, western blot analysis was performed to detect the
expression of epithelial and mesenchymal protein markers. The
results showed that overexpression of β-hCG upregulated the
expression of mesenchymal markers: vimentin, N-cadherin, β-catenin,
Slug and Snail, while it downregulated the expression of the
epithelial markers E-cadherin and claudin (Fig. 6A). Conversely, the β-hCG-depleted
ES-2 and SKOV3 cells demonstrated increased E-cadherin and claudin
expression, but decreased vimentin, N-cadherin, β-catenin, Slug and
Snail expression. Furthermore, the immunofluorescence staining of
β-hCG-overexpressing cells pictured by laser scanning confocal
microscopy were consistent with the western blot results (Fig. 6B).
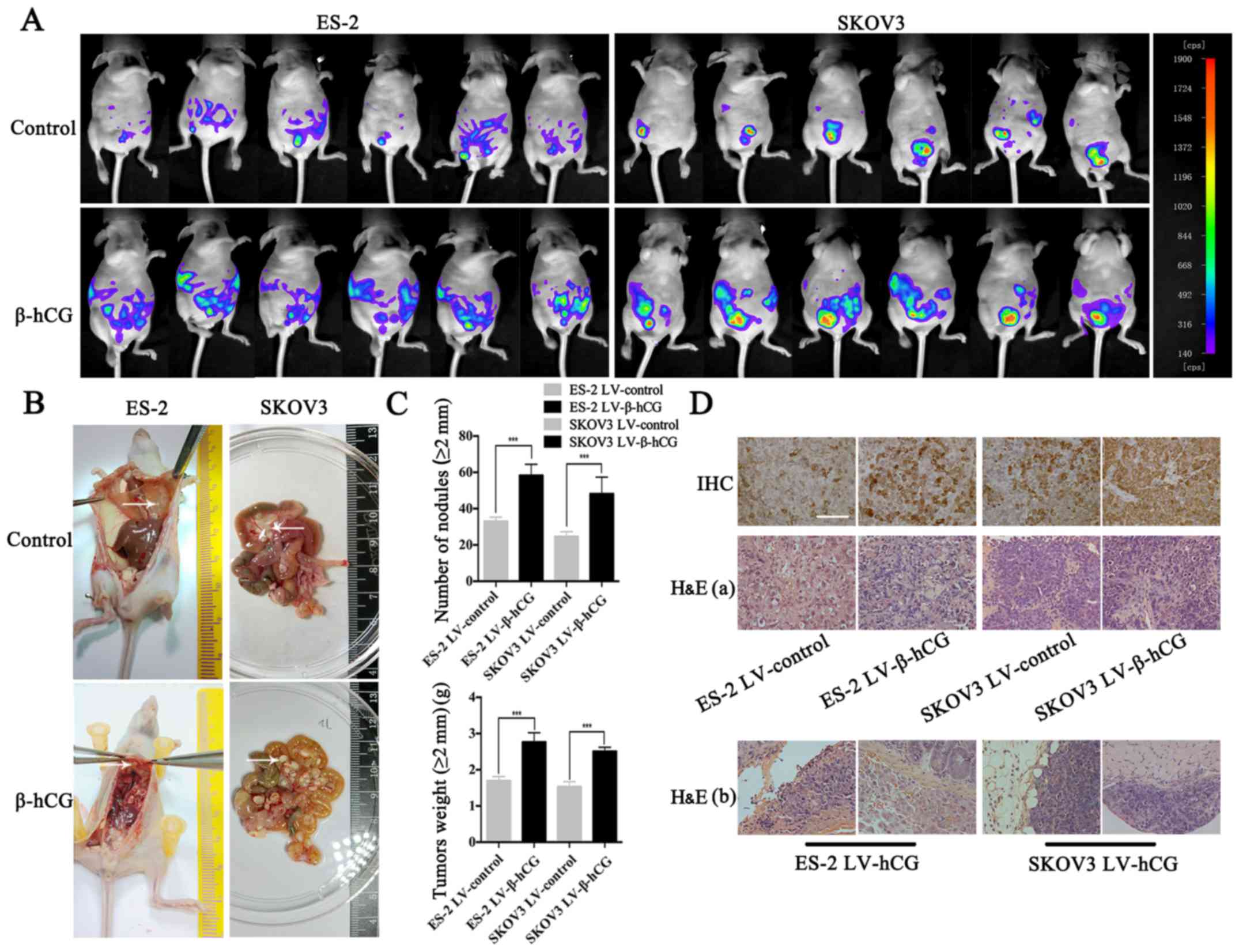
Overexpression of β-hCG promotes
metastasis in a nude mouse peritoneal xenograft tumor model
β-hCG-overexpressing and control cells were
intraperitoneally injected into nude mice, and the growth of
xenografts was continuously detected by NightOWL LB 983 In
Vivo Imaging System. Results showed that β-hCG upregulation
promoted tumor burden and spread range (Fig. 7A). On the 23rd and 26th day after
intraperitoneal inoculation, ES-2 and SKOV3 group nude mice were
sacrificed and dissected, respectively. Gross visualization showed
that overexpression of β-hCG induced more and larger metastatic
nodule formation in omentum, mesentery, peritoneum and the
diaphragm compared with the control group (Fig. 7B). In order to objectively evaluate
the metastasis ability regulated by β-hCG, all the metastatic
nodules, diameter >2 mm, were collected and weighted, and the
results showed that β-hCG significantly promoted tumor metastasis
(Fig. 7C). Pathology experts
confirmed nodular lesions observed with the naked eye to be ovarian
cancer tissue by microscopic examination of H&E-stained
tissues, and β-hCG was confirmed to be upregulated compared to the
control group by IHC (Fig. 7D).
Discussion
HCG is an accurate marker that is currently widely
used in clinical diagnosis and monitoring of pregnancy,
trophoblastic and ovarian germ cell tumors. Of course, the
involvement of hCG in the progression of malignancy is
intrinsically different from pregnancy-associated hCG. Among the
five family members of hCG, sulfated hCG and hCG are hormones
produced by placental syncytiotrophoblast cells and pituitary
gonadotrope cells, whereas hyperglycosylated hCG is an autocrine
factor produced by placental cytotrophoblast cells, which drives
malignancy in placental cancers and testicular and ovarian germ
cell malignancies. β-hCG and hyperglycosylated β-hCG are autocrine
factors produced by many advanced malignancies (4).
We found that β-hCG facilitated proliferation and
tumorigenesis in ovarian epithelial cells and was significantly
elevated in malignant ovarian tumors, compared with normal
epithelial expression in ovaries, fallopian tubes and endometrium
(11). In the present study, we
observed that the expression of β-hCG in metastases was obviously
higher compared to the tumor tissues from the ovary, which
indicates that β-hCG may play a role in promoting the spread of
ovarian cancer. To verify this speculation, β-hCG was upregulated
and downregulated in ovarian cancer cell lines by lentiviral
transfection and siRNA interference techniques, respectively, and
the modulated effect of β-hCG on migration and invasion of ovarian
cancer cells was confirmed by a series of experiments in
vitro and in vivo.
Some scholars have found that β-hCG may promote
ovarian cancer growth and vasculogenic mimicry formation via
activation of the luteinizing hormone receptor signal transduction
pathway (12). Various scholars
have demonstrated that β-hCG, Erk1/2 and MMP-2 are potential
targets with which to block glioblastoma invasion (13). There are other scholars that have
verified that β-hCG has a cystine knot structure, and this
structure happens to be similar to that of transforming growth
factor β (TGFβ), and thus, can seemingly antagonize a TGFβ
receptor, while the pregnancy-related hormone hCG does not appear
to expose these sequences and structures (14,15).
TGF-β, a ubiquitously expressed cytokine, is perhaps
the best-characterized promoter of EMT (16). EMT refers to a global cellular and
molecular transition by which polarized epithelial cells gain
mesenchymal properties allowing them to migrate, which plays a
vital role in local invasion and metastatic dissemination during
malignancy (17). Activating TGFβ
ligands initiate signaling, and closely related Smad-dependent
pathways, including phosphoinositide 3-kinase (PI3K)-Akt (18), focal adhesion kinase (FAK) (19), p38 mitogen-activated protein kinase
(p38 MAPK) (20), and extracellular
signal-regulated kinase (Erk) (21), have been identified as crucial for
EMT. During EMT, epithelial cells reorganize their cytoskeleton,
resolve cell-cell junctions and switch off the expression of
epithelial markers, turning on mesenchymal genes, such as,
E-cadherin, vimentin, N-cadherin, β-catenin, Snail, claudin, ZO-1
and others (22). Accordingly, we
found that overexpression of β-hCG induced morphological changes,
and the appearance of epithelial ovarian cancer cells changed from
closely arranged polygons into a fusiform morphology with a loose
arrangement, demonstrating that β-hCG has a role in regulating
EMT-associated gene expression.
In combination with the findings from other recent
studies, our results shed light on the molecular mechanisms of
β-hCG expression and its functional role in promoting ovarian
carcinoma metastasis and invasion, and thus may point to a new
target for therapeutic intervention.
Acknowledgements
The present study was supported by the National
Natural Science foundation of China (no. 81372305).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jelovac D and Armstrong DK: Recent
progress in the diagnosis and treatment of ovarian cancer. CA
Cancer J Clin. 61:183–203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bachmayr-Heyda A, Auer K, Sukhbaatar N,
Aust S, Deycmar S, Reiner AT, Polterauer S, Dekan S and Pils D:
Small RNAs and the competing endogenous RNA network in high grade
serous ovarian cancer tumor spread. Oncotarget. 7:39640–39653.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cole LA: HCG variants, the growth factors
which drive human malignancies. Am J Cancer Res. 2:22–35.
2012.PubMed/NCBI
|
|
5
|
Khare P, Bose A, Singh P, Singh S, Javed
S, Jain SK, Singh O and Pal R: Gonadotropin and tumorigenesis:
Direct and indirect effects on inflammatory and immunosuppressive
mediators and invasion. Mol Carcinog. 56:359–370. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lempiäinen A, Stenman UH, Blomqvist C and
Hotakainen K: Free beta-subunit of human chorionic gonadotropin in
serum is a diagnostically sensitive marker of seminomatous
testicular cancer. Clin Chem. 54:1840–1843. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jankowska AG, Andrusiewicz M, Fischer N
and Warchol PJ: Expression of hCG and GnRHs and their
receptors in endometrial carcinoma and hyperplasia. Int J Gynecol
Cancer. 20:92–101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Muller CY and Cole LA: The quagmire of hCG
and hCG testing in gynecologic oncology. Gynecol Oncol.
112:663–672. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hameed A, Miller DS, Muller CY, Coleman RL
and Albores-Saavedra J: Frequent expression of beta-human chorionic
gonadotropin (beta-hCG) in squamous cell carcinoma of the cervix.
Int J Gynecol Pathol. 18:381–386. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mustafa A, Bozdag Z, Tepe NB and Ozcan HC:
An unexpected reason for elevated human chorionic gonadotropin in a
young woman. Cervical squamous carcinoma. Saudi Med J. 37:905–907.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guo X, Liu G, Schauer IG, Yang G,
Mercado-Uribe I, Yang F, Zhang S, He Y and Liu J: Overexpression of
the β subunit of human chorionic gonadotropin promotes the
transformation of human ovarian epithelial cells and ovarian
tumorigenesis. Am J Pathol. 179:1385–1393. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao S, Fan C, Huang H, Zhu C, Su M and
Zhang Y: Effects of HCG on human epithelial ovarian cancer
vasculogenic mimicry formation in vivo. Oncol Lett.
12:459–466. 2016.PubMed/NCBI
|
|
13
|
Li Z, Du L, Li C and Wu W: Human chorionic
gonadotropin β induces cell motility via ERK1/2 and MMP-2
activation in human glioblastoma U87MG cells. J Neurooncol.
111:237–244. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tegoni M, Spinelli S, Verhoeyen M, Davis P
and Cambillau C: Crystal structure of a ternary complex between
human chorionic gonadotropin (hCG) and two Fv fragments specific
for the alpha and beta-subunits. J Mol Biol. 289:1375–1385. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cole LA and Butler S: Hyperglycosylated
hCG, hCGβ and Hyperglycosylated hCGβ: Interchangeable cancer
promoters. Mol Cell Endocrinol. 349:232–238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
O'Connor JW and Gomez EW: Biomechanics of
TGFβ-induced epithelial-mesenchymal transition: Implications for
fibrosis and cancer. Clin Transl Med. 3:23–35. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jung HY, Fattet L and Yang J: Molecular
pathways: Linking tumor microenvironment to epithelial-mesenchymal
transition in metastasis. Clin Cancer Res. 21:962–968. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bakin AV, Tomlinson AK, Bhowmick NA, Moses
HL and Arteaga CL: Phosphatidylinositol 3-kinase function is
required for transforming growth factor beta-mediated epithelial to
mesenchymal transition and cell migration. J Biol Chem.
275:36803–36810. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cicchini C, Laudadio I, Citarella F,
Corazzari M, Steindler C, Conigliaro A, Fantoni A, Amicone L and
Tripodi M: TGFbeta-induced EMT requires focal adhesion kinase (FAK)
signaling. Exp Cell Res. 314:143–152. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bhowmick NA, Zent R, Ghiassi M, McDonnell
M and Moses HL: Integrin beta 1 signaling is necessary for
transforming growth factor-beta activation of p38MAPK and
epithelial plasticity. J Biol Chem. 276:46707–46713. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie L, Law BK, Chytil AM, Brown KA, Aakre
ME and Moses HL: Activation of the Erk pathway is required for
TGF-beta1-induced EMT in vitro. Neoplasia. 6:603–610. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|