Introduction
Although long-term declines in colorectal cancer
(CRC) incidence and mortality rates have been reported in the
United States (1,2), these rates have increased rapidly in
several areas including Spain, Eastern Europe, and China (3–5).
Presently, CRC is the third most common malignant tumor in the
world (6). Although
chemotherapeutic drugs are used extensively for treating CRC, drug
resistance is a major obstacle to the success of cancer
chemotherapy. Therefore, it is important to identify a new drug
capable of overcoming chemotherapy resistance in CRC patients
(7).
Recent studies have highlighted that cancer stem
cells (CSCs) are responsible for chemotherapy resistance (8,9).
According to the currently accepted and putative definition, CSCs
have the capacity of self-renewal and differentiation, stress and
drug resistance, and enhanced migration (10,11).
Colorectal CSCs have been identified and isolated from CRCs
(12,13). The stemness of colorectal CSCs was
identified to be associated with specific properties such as high
expression of CD133, CD34, ALDH, NANOG, OCT4, and SOX2 (13–16).
The overexpression of these molecules is often related to the drug
resistance of tumors (17).
Considering the chemotherapy resistance role of the stemness of
CSCs, stemness markers have become new therapeutic targets in CRC
patients (7,18).
Bufalin, a traditional Chinese medicine monomer, is
a major active ingredient isolated from the traditional Chinese
medicine Chansu (19). In the past
decade, bufalin was shown to possess high anticancer ability in
various cancers (20–24). The anticancer mechanisms of bufalin
can be summarized as: inhibition of proliferation (20), promotion of apoptosis (24), inhibition of angiogenesis and
metastasis (21), reversal of drug
resistance (23), and induction of
autophagy (25). Recent studies
have suggested that bufalin inhibits differentiation,
proliferation, and drug resistance in cancers via the inhibition of
stemness (26–28). According to studies on bufalin and
colorectal CSCs, signal pathways regulated by bufalin such as
Wnt/β-catenin (29), PI3K/AKT
(30), Jak/STAT3 (31), Hedgehog (28), and Notch (27) are correlated with the stemness of
CRC (32). Therefore, we speculated
that bufalin reverses drug resistance via the inhibition of the
stemness of CRC.
In the present study, we investigated the effects of
bufalin on the stemness of CRC. We hypothesized that bufalin
inhibits the stemness induced by cisplatin and increases the
therapeutic effect of cisplatin in CRC.
Materials and methods
Cell culture
Human CRC cell lines, including HCT116 and LoVo,
were cultured in RPMI-1640 medium (Gibco Laboratories, Grand
Island, NY, USA) supplemented with 10% fetal calf serum (Gibco
Laboratories) at 37°C in a 5% CO2 humidified
atmosphere.
Reagents and antibodies
Cisplatin was purchased from Qilu Pharmaceutical
(Jinan, China). Bufalin was purchased from Sigma (St. Louis, MO,
USA). CD44 (60224-1-IG), CD133 (18470-1-IG), OCT4 (11263-1-AP),
SOX2 (11064-1-AP), and NANOG (14295-1-AP) primary antibodies were
purchased from Proteintech (Chicago, IL, USA). GAPDH (#2118) and
ABCG2 (#42078) were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA).
Cell viability assays
Cells were seeded in a 96-well plate at a density of
1×104 cells/well. Cell viability assays used the Cell
Counting Kit-8 (CCK-8, Dojindo Laboratories, Kumamoto, Japan). Cell
viability was evaluated by determining the absorbance of each well
at 450 nm using a plate reader (Bio-Rad, Hercules, CA, USA). Each
sample was analyzed in sextuplicate, and experiments were repeated
thrice.
Flow cytometry
The Annexin V-FITC/PI Apoptosis Detection kit
(Becton-Dickinson, Franklin Lakes, NJ, USA) was used to investigate
apoptosis. Tumorsphere cells were dissociated into single cells and
were then stained with Annexin and PI separately. The apoptosis
ratio was assessed by flow cytometry using the FACSCalibur system
(Becton-Dickinson).
The DNA-binding dye Hoechst 33342 was used to
evaluate the SP ratio. Dissociated sphere cells were stained with
Hoechst 33342 for 10 min and were tested through dual-wavelength
analysis using flow cytometry (Hoechst red 675/20; Hoechst blue
424/44). SP cells were shown to have a characteristic tail, which
differentiated them from other cells of the population.
The protein expression of stemness markers, such as
CD133 and CD44, was detected using flow cytometry. Dissociated
sphere cells were incubated with primary antibodies, including CD44
and CD133 antibodies, at 4°C for 1 h. They were then washed with
phosphate-buffered saline (PBS) twice and incubated with Alexa
Fluor 488 conjugated anti-rabbit secondary antibodies (R37116) and
Alexa Fluor 488 conjugated anti-mouse secondary antibodies
(A-21202) (Invitrogen, Carlsbad, CA, USA) at 4°C for 30 min in the
dark. The fluorescence values of CD133 and CD44 were determined
using flow cytometry and analyzed using the FlowJo 7.6 software
(Treestar, Inc., Ashland, OR, USA).
Immunofluorescence staining
Dissociated sphere cells were seeded on cover slips
pre-coated with 0.01% polylysine at a density of 1,000 cells per
well in a 48-well chamber. After 24 h, the cells were treated with
cisplatin and bufalin for 48 h. The cells were then treated in turn
with 4% paraformaldehyde for 20 min, 0.1% Triton X-100 for 10 min,
5% bovine serum albumin (BSA) for 60 min, and primary antibodies
overnight at 4°C. Next, the cells were washed thrice using PBS and
incubated with Alexa Fluor 488 conjugated anti-mouse secondary
antibodies, Alexa Fluor 488 conjugated anti-rabbit secondary
antibodies, and Alexa Fluor 555 conjugated anti-rabbit secondary
antibodies (A-31572) (Invitrogen) for 1 h. The cells were then
observed using a fluorescence microscope (Leica, Wetzlar,
Germany).
Western blotting
Secondary tumorspheres treated with cisplatin and
bufalin were collected through centrifugation and concentration.
Subsequently, tumorspheres were lysed with M-PER Mammalian Protein
Extraction reagent with protease inhibitor cocktail (100X) (Sangon
Biotech, China) and 1 mM PMSF. The lysate was centrifuged at 4°C at
12,000 g for 15 min, and the supernatant was used for western
blotting. The protein concentration was measured using the Bradford
Coomassie Blue G-250 method. Protein (40 µg) was mixed with 5X SDS
sample buffer and was denatured by boiling for 10 min. The
denatured protein was loaded onto 10% polyacrylamide SDS gels
(PAGE-SDS) and transferred onto PVDF membranes (Millipore,
Billerica, MA, USA). Membranes were blocked in 5% BSA for 2 h
followed by incubation with primary antibodies overnight at 4°C.
After washing thrice for 10 min in TBST, membranes were incubated
with HRP-conjugated secondary antibodies for 2 h at room
temperature (RT). Subsequently, the membranes were washed thrice
for 10 min in TBST and were visualized using the ECL Western
Blotting Detection system (Millipore). The ratio of the optical
densities of the bands was measured using a gel image analysis
system (Bio-Rad) and normalized to GAPDH.
Tumorsphere formation assays
HCT116 and LoVo cells were separately seeded in
ultra-low attachment 24-well plates (Corning, Corning, NY, USA)
with DMEM/F-12 (12660012, Gibco) culture media, B27 (17504044,
Gibco), 20 ng/ml EGF (PHG0311, Gibco), and 20 ng/ml bFGF (13256029,
Gibco) at a density of 200 cells/well. The medium was replaced by
half every 3 days. After 14 days, tumorspheres were counted and
photographs were obtained through microscopy.
Colony formation assays
Single cells were prepared and seeded into 96-well
plates at a density of 200 cells/well. The medium was replaced
every 2 days. After 10 days, cells were treated in turn with 4%
paraformaldehyde for 20 min and crystal for 20 min, and were washed
with PBS at least twice. The colonies were counted, and photographs
were obtained through microscopy.
In vivo tumor xenograft model
For the in vivo xenograft tumor growth assay,
male nude mice [BALB/c nu/nu, 5-week-old, purchased from SLAC
(Shanghai Laboratory Animal Center, Shanghai, China)] were used to
prepare the in vivo tumor xenograft model. Two million cells
in 0.1 ml of PBS were injected into the subcutaneous tissues of
each mouse. After 2 weeks, mice were injected intraperitoneally
with cisplatin (10 mg/kg body weight) and bufalin (1 mg/kg body
weight) every 3 days for 4 weeks. Finally, the tumor-bearing mice
were sacrificed and the tumors were excised and weighed.
Immunochemistry
All tumor xenograft bodies were formalin-fixed,
embedded in paraffin, serially sectioned (5-µm thickness), and
mounted on glass slides. The reagents in the subsequent process
were purchased from Maixin Bio (Fuzhou, China). Sections were
incubated for 10 min in peroxidase blocking agent, washed for 3 min
thrice with PBS, blocked with rabbit serum for 60 min at RT, and
incubated with antibodies at 4°C overnight. Subsequently, the
sections were washed thrice with PBS, incubated with HRP-conjugated
secondary antibodies for 10 min at RT, washed again with PBS,
developed with diaminobenzidine solution, and counterstained with
hematoxylin. Additionally, serial sections were stained with
hematoxylin and eosin.
Statistical analysis
Data are presented as mean ± SD. All analyses were
performed using the SPSS 17.0 software (IBM Corp., Armonk, NY,
USA). A p-value of <0.05 was considered statistically
significant.
Results
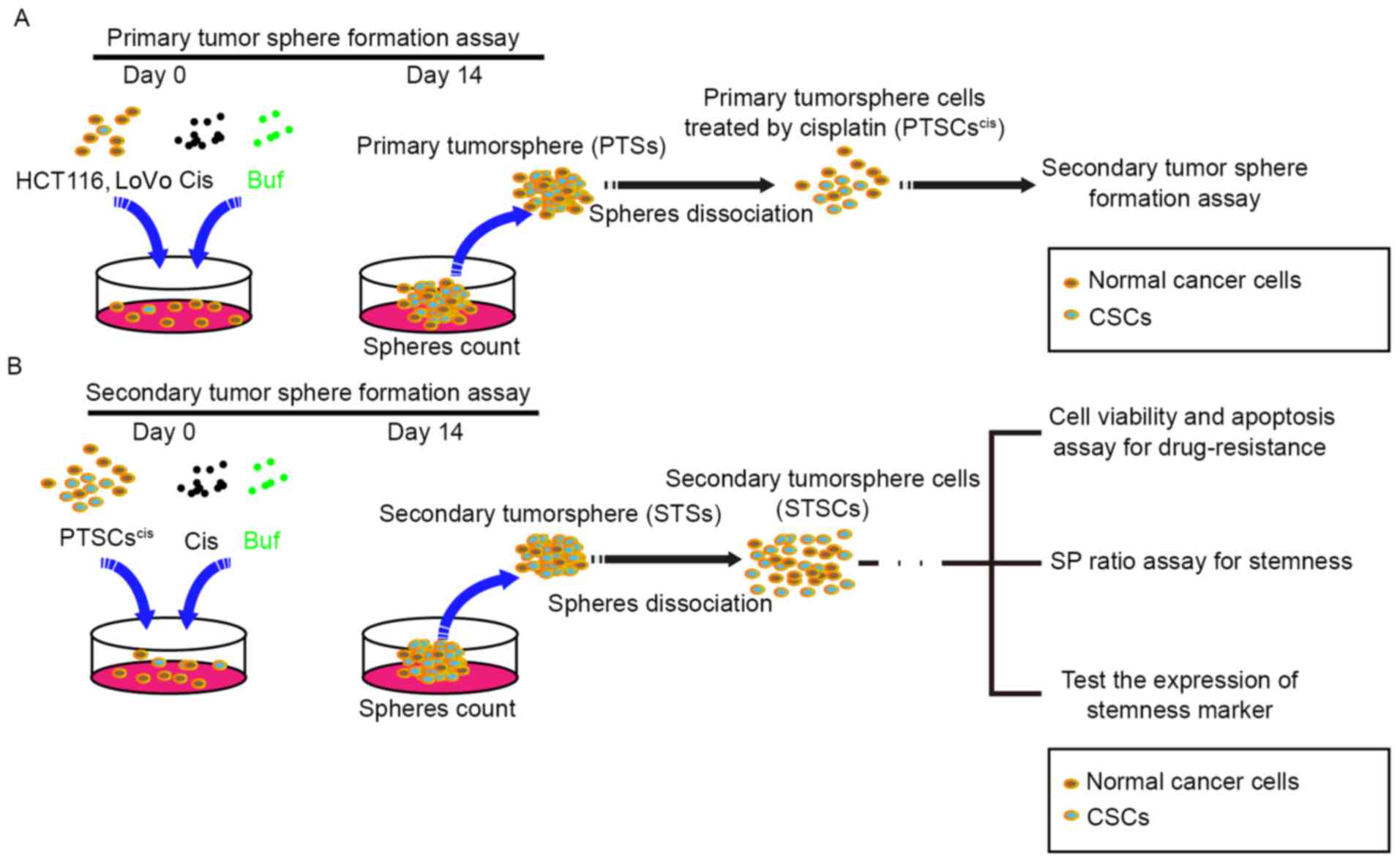
Previous studies suggested that the self-renewal
properties of CSCs could be judged by the formation of 3D spheroids
in a non-adhesive environment, which was called tumorsphere
formation assay (33,34). In this study, tumorsphere formation
assays were used to analyze the effects of cisplatin and bufalin on
stemness in two CRC cell lines (HCT116 and LoVo). Initially, HCT116
and LoVo cells were treated separately with cisplatin and bufalin
at different concentrations in a non-adhesive culture system for 14
days. Subsequently, the numbers and diameters of the spheres were
counted to analyze the effects of cisplatin and bufalin on the
stemness of CRC cells (Fig. 1A).
Then, primary tumorspheres (PTSs) treated with cisplatin (5 µM)
were dissociated into single cells (PTSCscis) for
secondary tumorsphere formation assay. The cells were treated
separately with cisplatin (5 µM), bufalin (5 nM), and their
combination for 14 days, and the numbers and diameters of the
tumorspheres were counted. Subsequently, secondary tumorspheres
(STSs) were dissociated into single cells (STSCs) for: i) cell
viability and apoptosis assay for drug resistance; ii) side
population (SP) ratio assay for stemness; and iii) assay for the
expression of stemness markers (Fig.
1B). Recent studies found that a small population of cells
differed from the main population of cancer cells on observing
staining with a DNA-binding dye using flow cytometry. The small
population of cells was called the SP, which was thought to be part
of CSCs with CSC-like phenotypic properties.
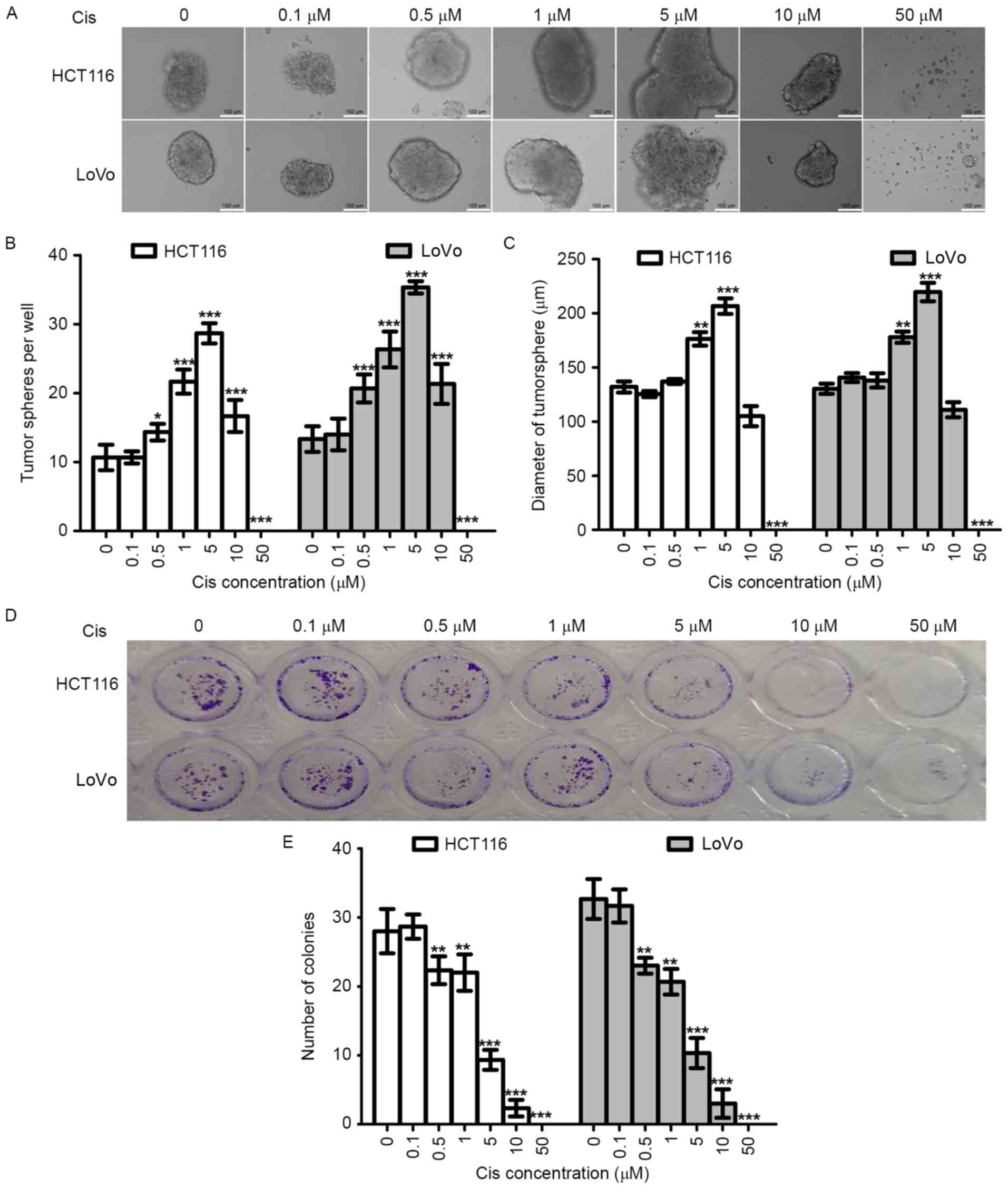
Cisplatin enhances the tumorsphere
formation capacity of colorectal cancer cells in vitro
Tumorsphere formation assay using a non-adhesive
culture system is an important method for the identification of
stemness in vitro (34,35).
To evaluate the effect of cisplatin on the stemness of CRC cells,
we tested the ability of tumorsphere formation of two CRC cell
lines (HCT116 and LoVo). At the same time, colony formation assay
was used to evaluate the effects of cisplatin on the proliferation
of these two cell lines. In order to analyze the results of the two
experiments, we used the same cisplatin concentrations and the same
experiment duration (14 days).
As shown in Fig.
2A-C, with increasing cisplatin concentration (0–5 µM), the
numbers and diameters of HCT116-PTSscis and
LoVo-PTSscis increased. Therefore, cisplatin could
increase tumorsphere formation of CRC cells in a dose-dependent
manner within a certain concentration range. However, the numbers
and diameters started to decrease when the cisplatin concentration
reached 10 µM, and tumorspheres were not found when the cisplatin
concentration reached 50 µM, which suggested that the
anti-proliferation effects of higher concentrations of cisplatin
(10–50 µM) were greater than the stemness effects.
Colony formation assay using the adhesive culture
system was used to analyze the anti-proliferation effects of
cisplatin in this study. We found that the efficiency of colony
formation decreased with cisplatin treatment in a dose-dependent
manner (Fig. 2D and E). When the
cisplatin concentrations were 5 µM and 10 µM, the number of
colonies decreased. The results of the colony formation assay and
tumorsphere formation assay were opposite with cisplatin treatment
at the same concentrations and experiment durations, which further
supported the increasing stemness effects of cisplatin on CRC
cells.
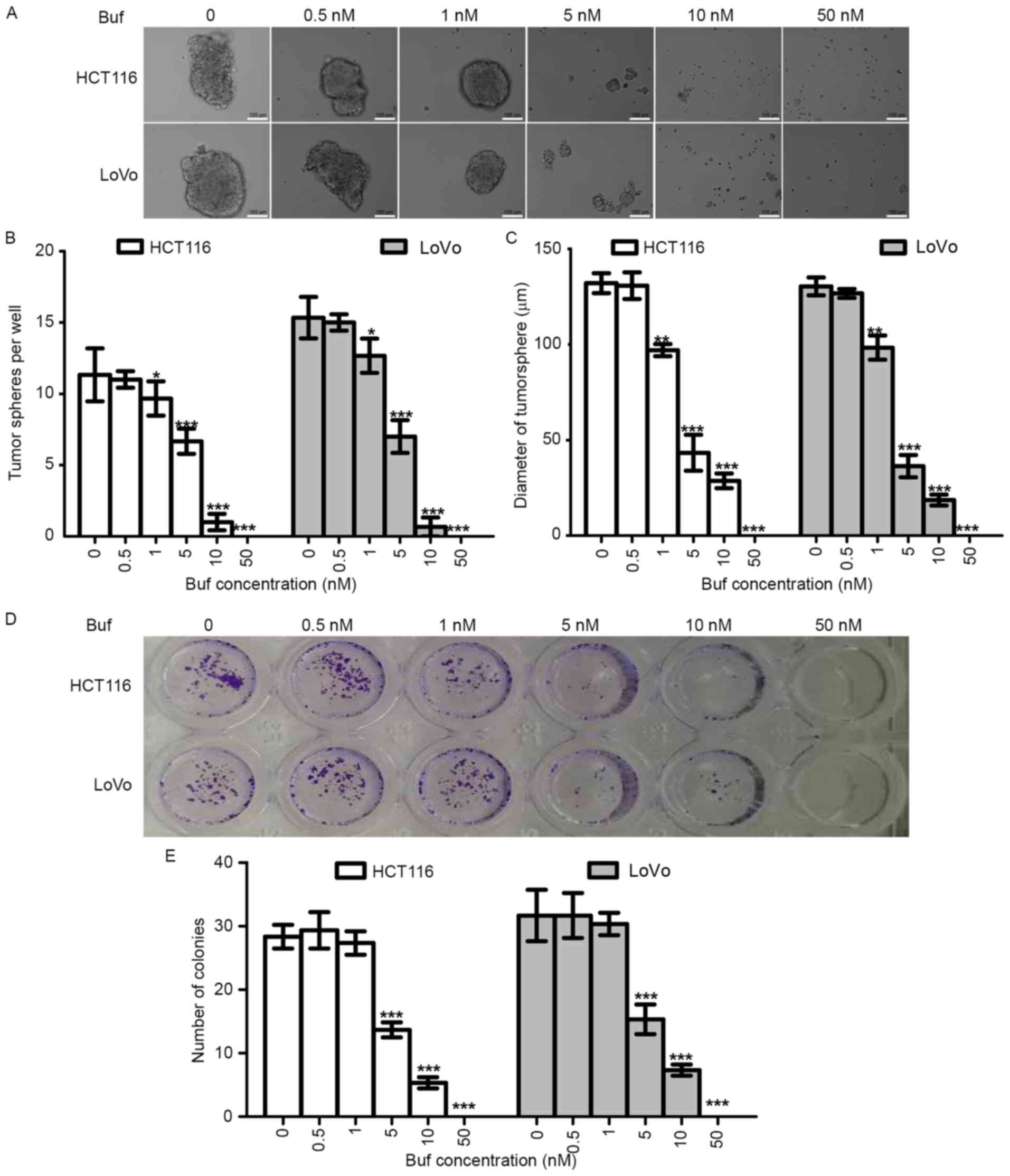
Bufalin decreased the tumorsphere
formation capacity of colorectal cancer cells in vitro
To determine the effects of bufalin on the stemness
of CRC cells, we first tested the tumorsphere formation capacity of
CRC cells treated with different concentrations of bufalin. We also
analyzed the effects of bufalin on killing and proliferation
inhibition using the colony formation assay. We found that bufalin
could inhibit tumorsphere formation of HCT116 and LoVo cells in a
dose-dependent manner (Fig. 3A-C).
The trend of the colony formation assay results was similar to that
of the tumorsphere formation assay results (Fig. 3D and E); however, 1 nM of bufalin
inhibited tumorsphere formation but not colony formation, which
suggested that the inhibition of tumorsphere formation effects of
bufalin relied not only on anti-proliferation but also on
anti-stemness.
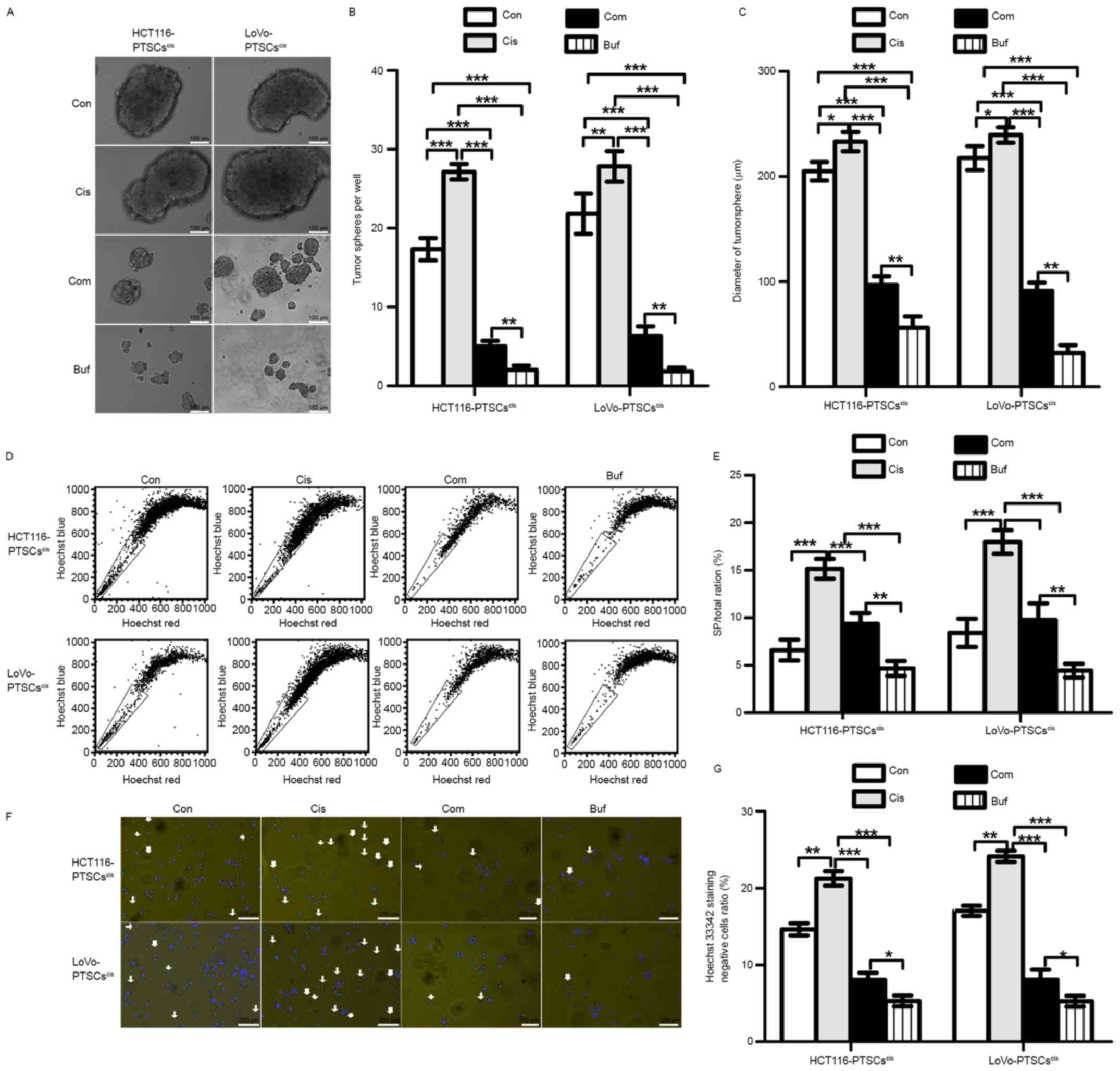
Bufalin is effective against cisplatin
with regard to the stemness of colorectal cancer cells
In view of the anti-stemness role of bufalin, we
speculated that it would be effective against cisplatin with regard
to the stemness of CRC cells. Primary tumorsphere cells treated by
cisplatin (5 µM), referred to as PTSCscis, were used for
the secondary tumorsphere formation assay (Fig. 4A-C). When compared with the control,
we found that cisplatin promoted the formation of secondary
tumorspheres, while bufalin alone decreased the formation of
secondary tumorspheres. On the other hand, the combination of
cisplatin and bufalin could inhibit the numbers and diameters of
secondary tumorspheres relative to the control and cisplatin
groups. These results suggested that bufalin works against
cisplatin with regard to the stemness of CRC cells.
After the secondary tumorsphere formation assay, the
ratios of SP cells were tested using flow cytometry through Hoechst
33342 staining. As shown in Fig. 4D and
E, the SP/total ratio increased in secondary tumorspheres
treated with cisplatin relative to that of the control and
decreased with the combination treatment or with bufalin alone.
Moreover, Hoechst 33342-stained cells were photographed using a
fluorescence microscope (Fig. 4F and
G). The results of photography and flow cytometry corresponded
with each other. These findings further confirmed the reversing
effects of bufalin on an increase in stemness induced by cisplatin
in CRC cells.
Bufalin antagonizes the effects of
cisplatin with regard to the expression of stemness markers
Drug-treated cancer cells in the tumorsphere
formation assay showed higher expression of stemness markers such
as CD133, CD44, NANOG, OCT4, SOX2, and ABCG2 (36–39).
Therefore, we tested the expression of these stemness markers in
secondary tumorsphere cells using immunofluorescence, flow
cytometry, and western blotting.
Initially, secondary tumorspheres were dissociated
into single cells and were seeded in a 24-well plate with slides.
When most cells adhered to the slides, immunofluorescence assay was
used to detect the expression and locations of the stemness markers
in the cells. As shown in Fig. 5A,
the expression of CD133, CD44, NANOG, OCT4, SOX2, and ABCG2
increased in the secondary tumorsphere cells treated with cisplatin
alone. However, bufalin and combination treatment inhibited their
expression.
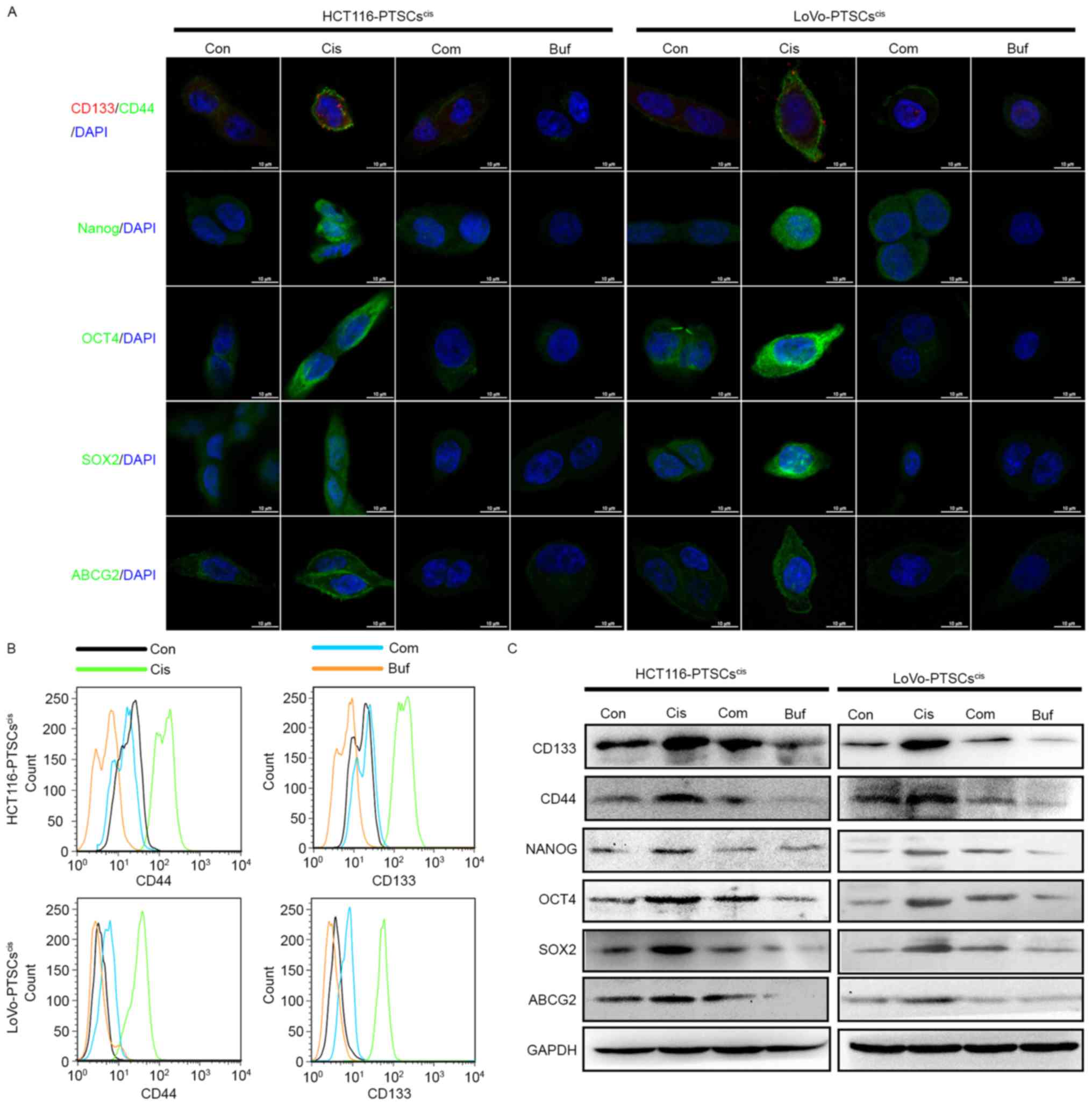 | Figure 5.Bufalin antagonizes cisplatin with
regard to the expression of stemness markers in colorectal cancer
cells in vitro. (A) All secondary tumorspheres are
dissociated into single cells and are seeded on cover slips in a
48-well plate. The protein expression of CD133, CD44, OCT4, SOX2,
NANOG, and ABCG2 are evaluated by immunofluorescence. After 14
days, images of tumorspheres were obtained using microscopy. (B)
The protein expression of CD133 and CD44 was evaluated using a flow
cytometry histogram plot. (C) The protein expression of CD133,
CD44, OCT4, SOX2, NANOG, and ABCG2 was evaluated and normalized
with GAPDH using western blotting. |
At the same time, the two colorectal CSC markers
CD133 and CD44 of secondary tumorsphere cells were assessed using
flow cytometry (Fig. 5B).
Consistent with the immunofluorescence results, bufalin antagonized
the effects of cisplatin with regard to the expression of CD133 and
CD44.
Finally, secondary tumorspheres underwent protein
extraction to test the expression of stemness markers using western
blotting. As shown in Fig. 5C,
secondary tumorsphere cells treated with cisplatin (both HCT116 and
LoVo cell lines) displayed higher expression of CD133, CD44, NANOG,
OCT4, SOX2, and ABCG2 proteins compared to the control. Bufalin
decreased their protein expression alone. In addition, high
expression of these proteins induced by cisplatin could be reversed
by bufalin. These data further supported the effect of bufalin
against cisplatin-induced stemness.
Bufalin reverses acquired drug
resistance in colorectal cancer cells induced by cisplatin in
vitro
Studies have shown that acquired drug resistance is
associated with increased expression of stemness markers induced by
chemotherapeutic drugs (38,40,41).
The results of this study also suggested that cisplatin increases
the expression of stemness markers, while the effects of bufalin
were the opposite. Therefore, we speculated that
STSCscis had drug-resistant properties, while bufalin
could inhibit this kind of acquired drug-resistance. To verify
these speculations, we compared the sensitivity of
STSCscis and parent cells to cisplatin. At the same
time, we tested the synergistic effects of bufalin on the
sensitivity of STSCscis to cisplatin. The
STSCscis and their parent cells were seeded in 10% FBS
RPMI-1640 medium at a density of 1×104 cells/well, in a
96-well plate. Then, 5 nM bufalin and different concentrations of
cisplatin were added for 48 h. The results of the cell viability
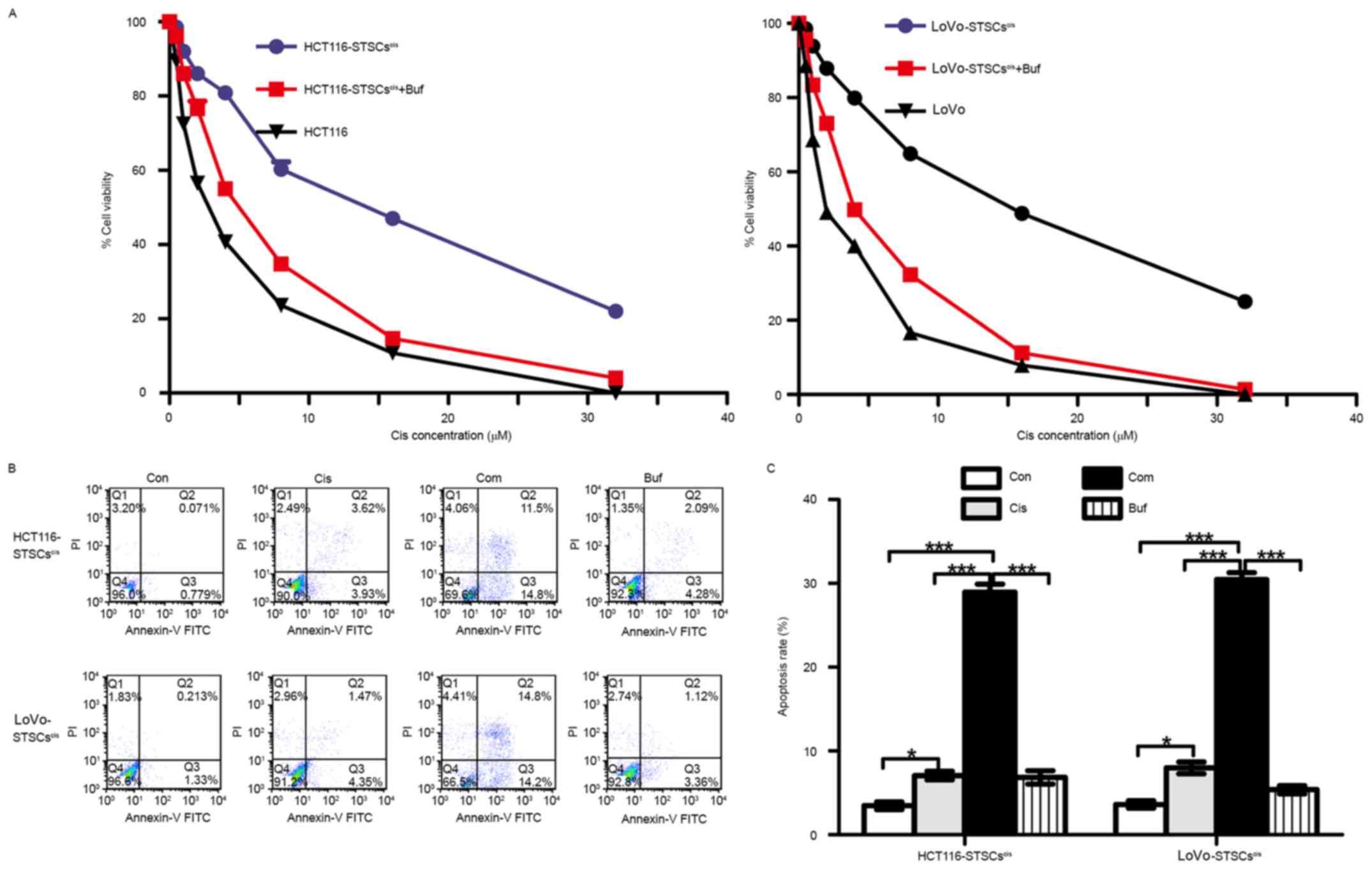
assay using CCK8 are shown in Fig.
6A. The IC50 (concentration that produces 50%
inhibition) of cisplatin in HCT116-STSCscis was
18.06±1.43 µM, which was higher than that of HCT116 cells
(IC50=2.13±0.12 µM). Bufalin decreased the
IC50 of HCT116-STSCscis to 5.61±0.42 µM.
Similarly, the IC50 values of LoVo cells,
LoVo-STSCscis, and LoVo-STSCscis treated with
bufalin were 20.81±1.15, 2.13±0.19 and 4.9±0.23 µM,
respectively.
Apoptosis assay involving flow cytometry was used to
verify the results of the cell viability assay (Fig. 6B). STSCscis were treated
with 5 mM cisplatin, 5 nM bufalin, and their combination for 48 h,
and the apoptosis rate was calculated. We found that the apoptosis
rate with the combination was much higher than the rates with
cisplatin and bufalin alone. The results further suggested that
bufalin reverses the acquired drug-resistance induced by cisplatin
in CRC cells.
Effect of bufalin on stemness marker
expression induced by cisplatin in vivo
In vitro studies have shown that bufalin
could inhibit stemness and increase the sensitivity of cisplatin in
CRC cells. To investigate the anti-stemness effect of bufalin in
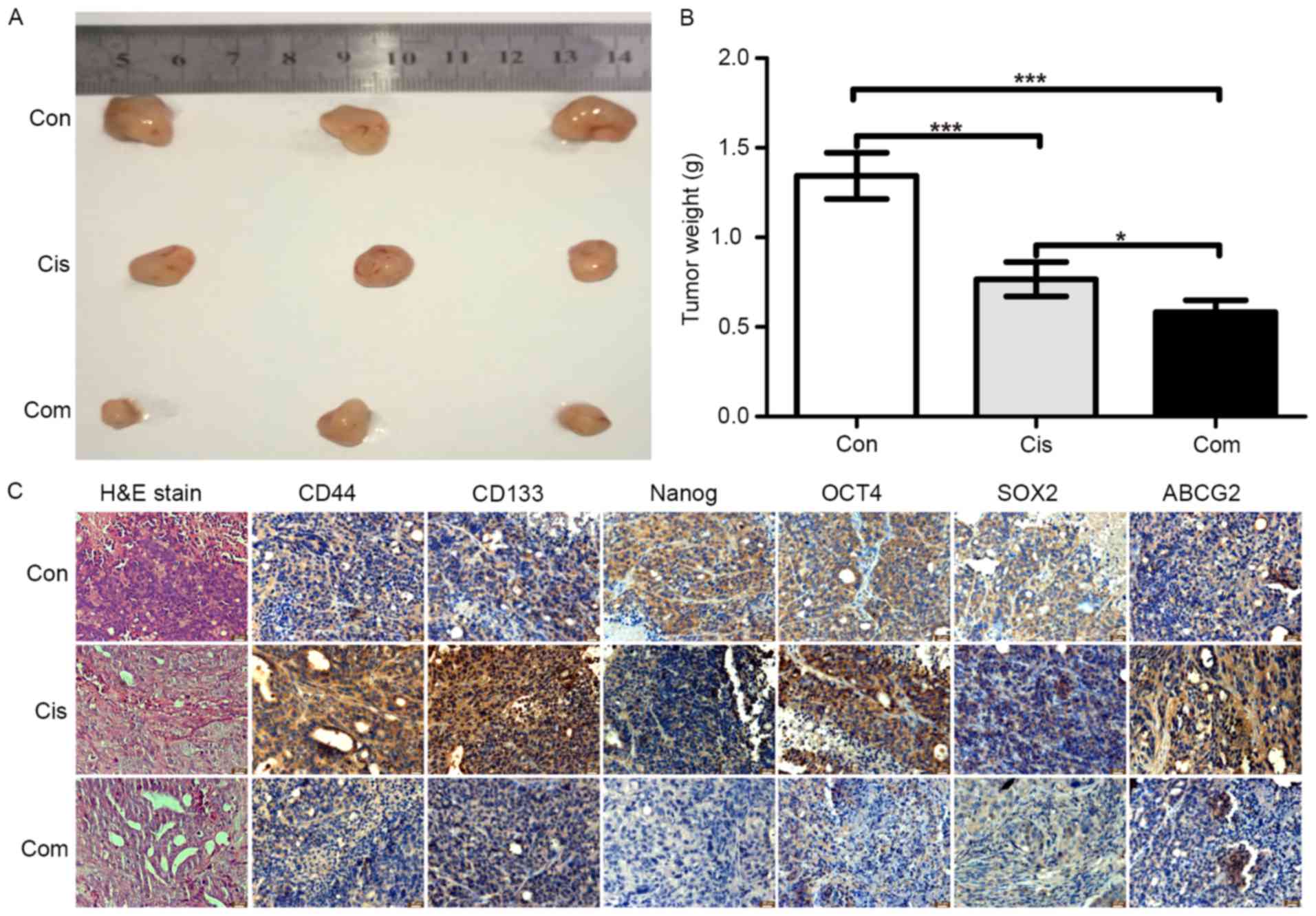
vivo, a subcutaneous xenograft model of HCT116 cells in nude
mice was used. HCT116 cells were subcutaneously injected into nude
mice for 2 weeks, and then, the mice were treated with cisplatin
alone or cisplatin + bufalin for 3 weeks. After sacrifice, the
tumor tissues were weighed and paraffin-embedded tissue blocks were
created for immunohistochemistry and H&E staining. As shown in
Fig. 7A and B, inhibition of tumor
growth was greater with the combination of cisplatin and bufalin
than with cisplatin alone. The tumor tissue weights also showed the
synergistic effects of bufalin on cisplatin. According to the
results of H&E staining (Fig.
7C), tumors treated with the combination of cisplatin and
bufalin showed more cell vacuolization and nuclear shrinkage than
with cisplatin alone.
The expression of CD133, CD44, NANOG, OCT4, SOX2,
and ABCG2 was assessed using immunohistochemistry to evaluate the
effect of bufalin on stemness in vivo. Similar to the in
vitro results, immunohistochemistry showed that cisplatin alone
increased the protein expression of stemness markers (Fig. 7C), while the combination of
cisplatin and bufalin decreased the protein expression of stemness
markers. These results suggested that bufalin increased the
sensitive of cisplatin in CRC cells through a reduction in
stemness.
Discussion
Chemotherapy is a necessary treatment method after
surgery in many advanced cancers. However, drug resistance has
become a major obstacle to the successful treatment of cancer
patients. Recent studies on molecular and cellular mechanisms have
suggested that high stemness induced by chemotherapeutic drugs was
an important reason for acquired drug-resistance. Therefore, many
researchers have attempted to identify adjuvant chemotherapeutic
drugs or new combinations of chemotherapeutic drugs that target
tumor cell stemness.
Many chemotherapeutic drugs have been found to
increase the stemness of cancer cells (42–46).
In these studies, cisplatin, a type of platinum-based drug, was
often used to investigate the relationship between acquired drug
resistance and stemness (42,47–52).
The tumorsphere formation assay is an important method for
verifying the stemness of cancer cells (34). In our study, we assessed the effects
of cisplatin on tumorsphere formation in two CRC cell lines (HCT116
and LoVo). We found that cisplatin promoted tumorsphere formation.
At the same time, the colony formation assay was used to analyze
the effects of cisplatin on proliferation and apoptosis. However,
we noted a reverse trend to that in the tumorsphere formation
assay. The opposite results further supported the stemness-inducing
effect of cisplatin in CRC cells. At present, traditional monolayer
cultured cells show great differences from natural growth body
cells in morphology, structure, function, and other aspects, which
cannot really reflect the three-dimensional (3D) state of tumor
growth in vivo. Therefore, a 3D cell culture system, such as
the tumorsphere formation assay, is better suited for tumor
invasion, metastasis, and drug-resistance research in vitro.
In this study, low cisplatin concentrations (0.1–5 µM) were found
to increase the tumorsphere effects of CRC cells, consistent with
other cancer cells (40,42,48,50).
Previous studies have shown the effects of bufalin on the
inhibition of CSCs or stemness in pancreatic cancer cells and
osteosarcoma CSCs (26–28). In our study, we also showed the
effects of bufalin on the inhibition of stemness in CRC cells.
Taking the same experiment, when treated by bufalin showed
different results than cisplatin. Therefore, we speculated that
bufalin could antagonize the increasing stemness induced by
cisplatin in CRC cells. We used the secondary tumorsphere formation
assay to test the inhibiting stemness effects of bufalin in CTSCs.
We found that the combination of bufalin and cisplatin could
inhibit tumorsphere formation, although the effect of bufalin alone
was better.
The stemness of cancer cells can be represented with
the CSC ratio, which could be evaluated with the SP ratio. Using
the flow cytometry assay and imaging with microscopy, we assessed
the Hoechst-negative SP ratio in the secondary tumorsphere assay.
We found that cisplatin could increase the SP ratio, while bufalin
inhibited the SP ratio. The SP cells can efflux out fluorescent
dyes, such as the DNA-binding dye Hoechst 33342, which will cause
the cells not to show staining under a fluorescence microscope or
flow cytometry (53). Therefore, a
high SP ratio induced by cisplatin represents a high CSC ratio or
high stemness of CRC cells.
High stemness was often accompanied by drug
resistance in cancer cells. We assessed the drug resistance of
STSCscis. We found that drug resistance was higher in
STSCscis than in their parent cells, which proved that
cisplatin could induce acquired drug resistance. In view of the
inhibiting stemness and acquired drug-resistance effects (23), we speculated that bufalin could
inhibit acquired cisplatin resistance in CRC cells via the
inhibition of stemness. We found that the combination of bufalin
and cisplatin could inhibit proliferation and induce apoptosis in
STSCscis in vitro. The combination of bufalin and
cisplatin showed higher effects than cisplatin alone in
vivo. These results verified our speculation that the reversion
effects of bufalin on acquired cisplatin resistance relied on the
inhibition of stemness in CRC.
Recent studies suggested that cisplatin induces high
expression of stemness markers such as CD133 (49), CD44 (47), NANOG (50), SOX2 (50), OCT4 (50), and ABCG2 (49). Therefore, we assessed the expression
of stemness markers in secondary tumorsphere cells. We found that
cisplatin could promote high expression of these markers of CRC
cells in vitro and in vivo, while bufalin could
antagonize the effect of cisplatin on the expression of these
markers. These results further supported our initial hypothesis
that bufalin could reverse acquired cisplatin resistance via the
inhibition of stemness in CRC cells.
In this study, we verified that the ability of
bufalin to reverse acquired cisplatin resistance relied on the
inhibition of stemness in CRC cells. These findings provide
information for new chemotherapy strategies for the clinical
treatment of CRC. In addition, these findings remind oncologists to
include agents than can inhibit the stemness effect to prevent
acquired drug resistance in tumor chemotherapy. The specific
molecular mechanisms are not very clear and require further
research.
In conclusion, bufalin can reverse acquired
cisplatin resistance both in vitro and in vivo by
inhibiting the stemness of CRC and decreasing the expression of
stemness markers, such as CD133, CD44, OCT4, SOX2, and NANOG, and
the drug-resistant protein ABCG2. These findings suggest that
bufalin plays an adjuvant role in CRC chemotherapy and may help
reverse acquired drug resistance.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (nos. 81473482 and 81503434).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Edwards BK, Ward E, Kohler BA, Eheman C,
Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I,
Seeff LC, et al: Annual report to the nation on the status of
cancer, 1975–2006, featuring colorectal cancer trends and impact of
interventions (risk factors, screening, and treatment) to reduce
future rates. Cancer. 116:544–573. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Center MM, Jemal A and Ward E:
International trends in colorectal cancer incidence rates. Cancer
Epidemiol Biomarkers Prev. 18:1688–1694. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Center MM, Jemal A, Smith RA and Ward E:
Worldwide variations in colorectal cancer. CA Cancer J Clin.
59:366–378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ramasamy TS, Ayob AZ, Myint HHL,
Thiagarajah S and Amini F: Targeting colorectal cancer stem cells
using curcumin and curcumin analogues: Insights into the mechanism
of the therapeutic efficacy. Cancer Cell Int. 15:962015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Donnenberg VS and Donnenberg AD: Multiple
drug resistance in cancer revisited: The cancer stem cell
hypothesis. J Clin Pharmacol. 45:872–877. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dean M: ABC transporters, drug resistance,
and cancer stem cells. J Mammary Gland Biol Neoplasia. 14:3–9.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Clarke MF, Dick JE, Dirks PB, Eaves CJ,
Jamieson CH, Jones DL, Visvader J, Weissman IL and Wahl GM: Cancer
stem cells - perspectives on current status and future directions:
AACR Workshop on cancer stem cells. Cancer Res. 66:9339–9344. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: Accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dalerba P, Dylla SJ, Park I-K, Liu R, Wang
X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, et al:
Phenotypic characterization of human colorectal cancer stem cells.
Proc Natl Acad Sci USA. 104:10158–10163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:111–115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lugli A, Iezzi G, Hostettler I, Muraro MG,
Mele V, Tornillo L, Carafa V, Spagnoli G, Terracciano L and Zlobec
I: Prognostic impact of the expression of putative cancer stem cell
markers CD133, CD166, CD44s, EpCAM, and ALDH1 in colorectal cancer.
Br J Cancer. 103:382–390. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meng H-M, Zheng P, Wang X-Y, Liu C, Sui
H-M, Wu S-J, Zhou J, Ding Y-Q and Li J: Over-expression of Nanog
predicts tumor progression and poor prognosis in colorectal cancer.
Cancer Biol Ther. 9:295–302. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saigusa S, Tanaka K, Toiyama Y, Yokoe T,
Okugawa Y, Ioue Y, Miki C and Kusunoki M: Correlation of CD133,
OCT4, and SOX2 in rectal cancer and their association with distant
recurrence after chemoradiotherapy. Ann Surg Oncol. 16:3488–3498.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jeter CR, Liu B, Liu X, Chen X, Liu C,
Calhoun-Davis T, Repass J, Zaehres H, Shen JJ and Tang DG: NANOG
promotes cancer stem cell characteristics and prostate cancer
resistance to androgen deprivation. Oncogene. 30:3833–3845. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kodach LL, Jacobs RJ, Voorneveld PW,
Wildenberg ME, Verspaget HW, van Wezel T, Morreau H, Hommes DW,
Peppelenbosch MP, van den Brink GR, et al: Statins augment the
chemosensitivity of colorectal cancer cells inducing epigenetic
reprogramming and reducing colorectal cancer cell ‘stemness’ via
the bone morphogenetic protein pathway. Gut. 60:1544–1553. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yin PH, Liu X, Qiu YY, Cai JF, Qin JM, Zhu
HR and Li Q: Anti-tumor activity and apoptosis-regulation
mechanisms of bufalin in various cancers: New hope for cancer
patients. Asian Pac J Cancer Prev. 13:5339–5343. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang J, Chen C, Wang S, Zhang Y, Yin P,
Gao Z, Xu J, Feng D, Zuo Q, Zhao R, et al: Bufalin inhibits HCT116
colon cancer cells and its orthotopic xenograft tumor in mice model
through genes related to apoptotic and PTEN/AKT pathways.
Gastroenterol Res Pract. 2015:4571932015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qiu YY, Hu Q, Tang QF, Feng W, Hu SJ,
Liang B, Peng W and Yin PH: MicroRNA-497 and bufalin act
synergistically to inhibit colorectal cancer metastasis. Tumour
Biol. 35:2599–2606. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu T, Jia T, Yuan X, Liu C, Sun J, Ni Z,
Xu J, Wang X and Yuan Y: Development of octreotide-conjugated
polymeric prodrug of bufalin for targeted delivery to somatostatin
receptor 2 overexpressing breast cancer in vitro and in vivo. Int J
Nanomed. 11:2235–2250. 2016.
|
|
23
|
Zhao H, Zhao D, Jin H, Li H, Yang X,
Zhuang L and Liu T: Bufalin reverses intrinsic and acquired drug
resistance to cisplatin through the AKT signaling pathway in
gastric cancer cells. Mol Med Rep. 14:1817–1822. 2016.PubMed/NCBI
|
|
24
|
Wu SH, Bau DT, Hsiao YT, Lu KW, Hsia TC,
Lien JC, Ko YC, Hsu WH, Yang ST, Huang YP, et al: Bufalin induces
apoptosis in vitro and has Antitumor activity against human lung
cancer xenografts in vivo. Environ Toxicol. 32:1305–1317. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xie CM, Chan WY, Yu S, Zhao J and Cheng
CH: Bufalin induces autophagy-mediated cell death in human colon
cancer cells through reactive oxygen species generation and JNK
activation. Free Radic Biol Med. 51:1365–1375. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chang Y, Zhao Y, Gu W, Cao Y, Wang S, Pang
J and Shi Y: Bufalin inhibits the differentiation and proliferation
of cancer stem cells derived from primary osteosarcoma cells
through mir-148a. Cell Physiol Biochem. 36:1186–1196. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chang Y, Zhao Y, Zhan H, Wei X, Liu T and
Zheng B: Bufalin inhibits the differentiation and proliferation of
human osteosarcoma cell line hMG63-derived cancer stem cells.
Tumour Biol. 35:1075–1082. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang H, Ning Z, Li Y, Zhu X and Meng Z:
Bufalin suppresses cancer stem-like cells in gemcitabine-resistant
pancreatic cancer cells via Hedgehog signaling. Mol Med Rep.
14:1907–1914. 2016.PubMed/NCBI
|
|
29
|
Gai JQ, Sheng X, Qin JM, Sun K, Zhao W and
Ni L: The effect and mechanism of bufalin on regulating
hepatocellular carcinoma cell invasion and metastasis via
Wnt/β-catenin signaling pathway. Int J Oncol. 48:338–348.
2016.PubMed/NCBI
|
|
30
|
Zhu Z, Sun H, Ma G, Wang Z, Li E and Liu Y
and Liu Y: Bufalin induces lung cancer cell apoptosis via the
inhibition of PI3K/Akt pathway. Int J Mol Sci. 13:2025–2035. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu Z, Li E, Liu Y, Gao Y, Sun H, Ma G,
Wang Z, Liu X, Wang Q, Qu X, et al: Inhibition of Jak-STAT3 pathway
enhances bufalin-induced apoptosis in colon cancer SW620 cells.
World J Surg Oncol. 10:2282012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bertrand FE, Angus CW, Partis WJ and
Sigounas G: Developmental pathways in colon cancer: Crosstalk
between WNT, BMP, Hedgehog and Notch. Cell Cycle. 11:4344–4351.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cao L, Zhou Y, Zhai B, Liao J, Xu W, Zhang
R, Li J, Zhang Y, Chen L, Qian H, et al: Sphere-forming cell
subpopulations with cancer stem cell properties in human hepatoma
cell lines. BMC Gastroenterol. 11:712011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen S-F, Chang Y-C, Nieh S, Liu C-L, Yang
C-Y and Lin Y-S: Nonadhesive culture system as a model of rapid
sphere formation with cancer stem cell properties. PLoS One.
7:e318642012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jung MJ, Rho JK, Kim YM, Jung JE, Jin YB,
Ko YG, Lee JS, Lee SJ, Lee JC and Park MJ: Upregulation of CXCR4 is
functionally crucial for maintenance of stemness in drug-resistant
non-small cell lung cancer cells. Oncogene. 32:209–221. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Levina V, Marrangoni AM, DeMarco R,
Gorelik E and Lokshin AE: Drug-selected human lung cancer stem
cells: Cytokine network, tumorigenic and metastatic properties.
PLoS One. 3:e30772008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hamilton G and Olszewski U:
Chemotherapy-induced enrichment of cancer stem cells in lung
cancer. J Bioanal Biomed. S9:2013.doi:10.4172/1948-593X.S9-003.
|
|
38
|
Sun FF, Hu YH, Xiong LP, Tu XY, Zhao JH,
Chen SS, Song J and Ye XQ: Enhanced expression of stem cell markers
and drug resistance in sphere-forming non-small cell lung cancer
cells. Int J Clin Exp Pathol. 8:6287–6300. 2015.PubMed/NCBI
|
|
39
|
Liu J, Wang L, Ma L, Xu J, Liu C, Zhang J,
Liu J and Chen R: Significantly increased expression of OCT4 and
ABCG2 in spheroid body-forming cells of the human gastric cancer
MKN-45 cell line. Oncol Lett. 6:891–896. 2013.PubMed/NCBI
|
|
40
|
Abubaker K, Latifi A, Luwor R, Nazaretian
S, Zhu H, Quinn MA, Thompson EW, Findlay JK and Ahmed N: Short-term
single treatment of chemotherapy results in the enrichment of
ovarian cancer stem cell-like cells leading to an increased tumor
burden. Mol Cancer. 12:242013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vidal SJ, Rodriguez-Bravo V, Galsky M,
Cordon-Cardo C and Domingo-Domenech J: Targeting cancer stem cells
to suppress acquired chemotherapy resistance. Oncogene.
33:4451–4463. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wiechert A, Saygin C, Thiagarajan PS, Rao
VS, Hale JS, Gupta N, Hitomi M, Nagaraj AB, DiFeo A, Lathia JD, et
al: Cisplatin induces stemness in ovarian cancer. Oncotarget.
7:30511–30522. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bu Y, Jia Q-A, Ren Z-G, Zhang J-B, Jiang
X-M, Liang L, Xue T-C, Zhang Q-B, Wang Y-H, Zhang L, et al:
Maintenance of stemness in oxaliplatin-resistant hepatocellular
carcinoma is associated with increased autocrine of IGF1. PLoS One.
9:e896862014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ress AL, Stiegelbauer V, Schwarzenbacher
D, Deutsch A, Perakis S, Ling H, Ivan C, Calin GA, Rinner B, Gerger
A, et al: Spinophilin expression determines cellular growth, cancer
stemness and 5-flourouracil resistance in colorectal cancer.
Oncotarget. 5:8492–8502. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang Z, Duan Q, Zhao H, Liu T, Wu H, Shen
Q, Wang C and Yin T: Gemcitabine treatment promotes pancreatic
cancer stemness through the Nox/ROS/NF-κB/STAT3 signaling cascade.
Cancer Lett. 382:53–63. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ayadi M, Bouygues A, Ouaret D, Ferrand N,
Chouaib S, Thiery J-P, Muchardt C, Sabbah M and Larsen AK: Chronic
chemotherapeutic stress promotes evolution of stemness and
WNT/beta-catenin signaling in colorectal cancer cells: Implications
for clinical use of WNT-signaling inhibitors. Oncotarget.
6:18518–18533. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nör C, Zhang Z, Warner KA, Bernardi L,
Visioli F, Helman JI, Roesler R and Nör JE: Cisplatin induces Bmi-1
and enhances the stem cell fraction in head and neck cancer.
Neoplasia. 16:137–146. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chowanadisai W, Messerli SM, Miller DH,
Medina JE, Hamilton JW, Messerli MA and Brodsky AS: Cisplatin
resistant spheroids model clinically relevant survival mechanisms
in ovarian tumors. PLoS One. 11:e01510892016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu YP, Yang CJ, Huang MS, Yeh CT, Wu AT,
Lee YC, Lai TC, Lee CH, Hsiao YW, Lu J, et al: Cisplatin selects
for multidrug-resistant CD133+ cells in lung
adenocarcinoma by activating Notch signaling. Cancer Res.
73:406–416. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang F, Duan S, Tsai Y, Keng PC and Chen
Y, Lee SO and Chen Y: Cisplatin treatment increases stemness
through upregulation of hypoxia-inducible factors by interleukin-6
in non-small cell lung cancer. Cancer Sci. 107:746–754. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yang J, Guo W, Wang L, Yu L, Mei H, Fang
S, Ji P, Liu Y, Liu G and Song Q: Cisplatin-resistant osteosarcoma
cells possess cancer stem cell properties in a mouse model. Oncol
Lett. 12:2599–2605. 2016.PubMed/NCBI
|
|
52
|
Tsai LL, Yu CC, Chang YC, Yu CH and Chou
MY: Markedly increased Oct4 and Nanog expression correlates with
cisplatin resistance in oral squamous cell carcinoma. J Oral Pathol
Med. 40:621–628. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
She JJ, Zhang PG, Wang X, Che XM and Wang
ZM: Side population cells isolated from KATO III human gastric
cancer cell line have cancer stem cell-like characteristics. World
J Gastroenterol. 18:4610–4617. 2012. View Article : Google Scholar : PubMed/NCBI
|