Introduction
Ionizing radiation has provided numerous advantages
since the discovery of X-rays in the 1890s with industrial,
military, and medical uses, but is associated with problems as
well. High-dose ionizing radiation has destroyed countless lives
and caused untold suffering among the victims of huge nuclear
accidents and terrorist activity (1,2). For
years, scientists have grappled with finding novel radioprotective
agents. Recently, an effective radioprotectant called amifostine
(WR-2721), has been clinically approved by the US FDA for patients
undergoing radiotherapy. However, in addition to its expensive
price tag, this drug has serious side-effects, which has limited
its effectiveness (3,4).
In view of this grim situation, radiation protection
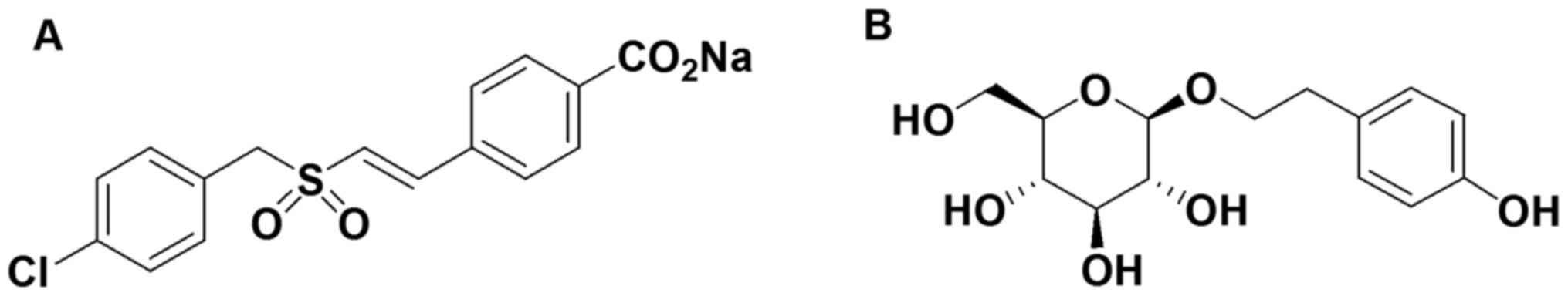
research has received intense focus. ON 01210.Na
(Ex-RAD®; Fig. 1A),
4-carboxystyryl-4-chlorobenzylsulfone, was developed for modifying
cell cycle distribution patterns in cancer cells subjected to
radiation therapy. It has also been developed as a novel
radioprotectant by Onconova Therapeutics Inc. (Newtown, PA, USA).
Pre-clinical pharmacokinetic studies have shown that Ex-RAD is
rapidly cleared from plasma and has no observable toxicity.
Radiation protection was observed with dose escalation to 9.8 Gy.
More than 78% of animals treated with Ex-RAD survived a dose of 8
Gy as compared to <20% in an untreated group (5). Ex-RAD received FDA approval as an
investigational new drug in December 2008 due to its significant
survival advantage and low toxicity. A previous study showed that
Ex-RAD alleviated radiation-induced DNA damage and may be an
inhibitor of p53-dependent apoptosis as demonstrated in in
vitro models (6). A similar
mechanism also occurred in vivo. Ex-RAD-treated mice
exhibited lower phospho-ATM and p53, along with a higher Bcl-2/Bax
ratio in the spleen. In addition, Ex-RAD treatment significantly
mitigated the hematopoietic and gastrointestinal toxicity before
and after exposure to ionizing radiation by accelerating recovery
of peripheral blood cells, protecting the granulocyte macrophage
colony forming units (GM-CFU), and enhancing intestinal crypt
survival in mice (7,8).
Compared to the majority of synthetic
radioprotectors, natural radioprotective agents are less toxic and
have less undesirable side-effects. Rhodiola rosea L. is a
traditional Chinese herb, which mainly grows at an altitude of
1,600–4,000 meters in the alpine zone characterized by hypoxia and
strong ultraviolet radiation. Salidroside (Sal; Fig. 1B) is the major water-soluble
pharmacological ingredient of Rhodiola rosea L., which is
known for its multiple pharmacological properties and in particular
its antioxidative effects. Sal was found to inhibit the UVB-induced
apoptosis of HaCaT cells via modulating the expression of NF-κB,
BCL-2 and CDK6 (9). It also
increased SOD activity and decreased the malondialdehyde (MDA)
level in myocardial tissues to protect against myocardial ischemia
injury (10). Moreover, Sal was
found to scavenge free radicals, reverse DNA damage and alter
expression of cytokines and antioxidative enzymes induced by
reactive oxygen species (ROS) (11,12).
Additionally, Sal was reported to protect endothelial progenitor
cells from γ-radiation-induced apoptosis and promote hematopoietic
recovery in mice (13).
Ionizing radiation directly causes DNA strand
breakage, or acts on water molecules, generating free radicals to
damage DNA indirectly. Potential radioprotectors may help to
alleviate radiation damage through scavenging free radicals,
enhancing DNA repair and inhibiting death signaling pathways
(14). In the early 1980s, scholars
realized that not all radioprotectants mitigate damage through
similar mechanisms; the use of multiple agents may in some
instances provide significantly better protection than single
agents (15). Currently, attention
has been paid to identify highly efficient and low toxic
traditional Chinese medicine for the combined treatment of
radiation damage. The structure of Sal contains phenolic hydroxyl
groups and unsaturated bonds. It is a natural powerful antioxidant,
which has the function of antioxidation and scavenging action. In
addition, Ex-RAD functions as a p53 inhibitor to block the
apoptotic pathway, protecting mice from whole body lethal
irradiation. In the present study, based on these clues, we
investigated the hypothesis that Sal may enhance the anti-radiation
effect of Ex-RAD in vivo by accelerating hematopoietic
recovery, scavenging ROS and increasing antioxidant capacity.
Further exploration in vitro also revealed that Sal
influenced DNA damage and enhanced the anti-radiation effect of
Ex-RAD via a p53-dependent apoptotic pathway.
Materials and methods
Chemicals and reagents
Ex-RAD was synthesized in our laboratory with purity
>99%. Sal was purchased from Xi'an Plants of Grass Technology
Co. Ltd. (Shaanxi, China) with a purity >99%. Fetal bovine serum
(FBS) and Dulbeccos modified Eagles medium (DMEM) were purchased
from Solarbio Science & Technology Co. (Beijing, China).
Chemicals for electrophoresis and immunoblots were purchased from
Abbkine, Inc. (Redlands, CA, USA). The SOD/CAT/MDA/GSH assay kits
were purchased from the Nanjing Jiancheng Institute of
Biotechnology (Xi'an, China). The cell apoptosis detection kit with
Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI)
and intracellular ROS detection kits were purchased from the
Beyotime Institute of Biotechnology (Jiangsu, China). Antibodies
for p53, Bax, Bcl-2 and γ-H2AX were purchased from Abcam
(Cambridge, UK).
Radioprotection activity in vivo
Six- to eight-week-old male BALB/c mice were
provided by the Animal Center of the Fourth Military Medical
University (Xi'an, China) and acclimated in the laboratory for 1
week prior to the experiments, and housed (10/cage) in an air
conditioned facility under standard maintenance conditions (12:12 h
light-dark cycle; 21–23°C; 55–65% relative humidity). All animals
were provided with sufficient food and clean water. The
experimental protocol involving animals was reviewed and approved
by the Institutional Animal Care and Use Committee of the Fourth
Military Medical University. All the studies carried out on animals
strictly complied with the Guidelines for the Care and Use of
Laboratory Animals. For irradiation, mice were placed in a
rectangular, well-ventilated plastic mouse holder, exposed to
general irradiation from a 60Co γ-ray irradiator (cobalt
tank, irradiation rate 200 cGy/min; Fourth Military Medical
University).
Ex-RAD was suspended in vehicle as reported in a
previous study (6). Ex-RAD (500
mg/kg) was subcutaneously (s.c.) administered using a 1-ml sterile
syringe with a 25G needle at 24 h and 15 min (two doses) before
irradiation (16). Sal (100 mg/kg)
was dissolved in normal saline (NS) and was chronically
administered to animals for 7 days before general irradiation.
To determine whether Sal enhances the anti-radiation
effect of Ex-RAD in vivo, a survival assay of mice was
investigated. In the present study, 75 mice were randomly divided
into five groups (n=15/group) as follows: CON (control), VEH
(irradiation + vehicle), SAL (irradiation + Sal 100 mg/kg), EX
(irradiation + Ex-RAD 500 mg/kg) and SAL + EX (irradiation + Sal
100 mg/kg + Ex-RAD 500 mg/kg). Mice in the irradiation groups were
exposed to 8 Gy of whole body γ-radiation. Survival was monitored
for 30 days post-radiation, and the data are expressed as the
survival rate.
A peripheral blood study was investigated to
evaluate the mitigation of hematopoietic toxicity of Sal on whole
body radiation mice. Animals (n=10) were pretreated with Ex-RAD and
Sal prior to sub-lethal total body irradiation of 5 Gy and the
method of administration and dosage were mentioned above. Mice were
humanely anesthetized with pentobarbital sodium (50 mg/kg i.p.).
Blood (0.6–1.0 ml) was collected from the posterior vena cava using
a 23G needle on day 7. Blood was quickly collected in EDTA tubes
directly from the heart. Total white blood cells (WBC), red blood
cells (RBC), hemoglobin (HGB) and platelets (PLT) were counted
using automated hematologic analyzer, Cell-Dyn 3000 (Unipath Corp.,
Mountain View, CA, USA).
After 7 days following radiation, liver tissue
samples were homogenized with ice cold 150 mM NaCl and centrifuged
at 15,000 rpm at 4°C for 15 min. The supernatants were used for the
determination of superoxide dismutase (SOD), catalase (CAT),
reduced glutathione (GSH) and MDA. The assay procedure was
performed according to the kits instructions.
Cell cultures and treatment
Human umbilical vein endothelial cells (HUVECs) were
purchased from the Chinese Academy of Sciences and incubated at
37°C in 5% CO2 in high-glucose DMEM supplemented with
10% FBS serum and antibiotics. HUVECs were divided into six groups:
CON, VEH (irradiation + vehicle), SAL (irradiation + Sal 40 µmol/l,
EX (irradiation + Ex-RAD 20 µmol/l) and SAL + EX (irradiation + Sal
40 µmol/l + Ex-RAD 20 µmol/l). Ex-RAD and Sal were dissolved in
fresh DMEM, and their combination was dissolved in Ex-RAD medium.
Cells were serum-starved for 4 h and then treated with the
indicated concentrations of drugs 2 h before radiation
exposure.
Colony survival assay
HUVCEs were seeded into 12-well plates at a density
of 1×103 cells/well. Cells were treated with the drugs
as mentioned above for 2 h before γ-radiation (5 Gy). After 14 days
post-radiation, cell colonies were stained with crystal violet and
counted. The experiment was performed at least three times.
Annexin V/PI staining
The radiation-induced apoptosis of HUVCEs was
assessed using a flow cytometry method that measures Annexin
V-FITC/PI staining. Briefly, cells were pretreated with the drugs
for 2 h before 5 Gy γ-radiation. After being cultured for another
48 h, the cells were collected, washed twice with cold
phosphate-buffered saline (PBS), and then stained with Annexin V
and PI for 15 min in the dark. Finally, the cells were analyzed by
a flow cytometer. In regards to the analysis, Annexin V-positive
and PI-negative cells represent early apoptotic populations,
Annexin V-positive and PI-positive cells represent late apoptotic
or dead proportions.
Measurement of radiation-generated
intracellular ROS
2′,7′-Dichlorofluorescein diacetate (DCFH-DA) is a
highly sensitive probe which is used for detection of intracellular
ROS. This non-fluorescent dye diffuses readily into cells and
yields DCFH which cannot pass out of cells. In the presence of
peroxidase, DCFH can generate the fluorescent compound
dichlorofluorescein (DCF), which can be analyzed by flow cytometry
using a laser excitation and emission wavelength of 492–495 and
517–527 nm, respectively. Cells were treated with different
concentrations of the drugs at 37°C for 2 h before 5 Gy
γ-radiation. After 48 h, the cells were washed with cold PBS and 10
µM DCFH-DA was added for 30 min to measure the intracellular ROS by
flow cytometry.
Single-cell alkaline gel
electrophoresis (COMET assay)
The single cell gel electrophoresis or Comet assay
is a sensitive, reliable, and rapid method for DNA double- and
single-strand breaks, alkali-labile sites and delayed repair site
detection, in eukariotic individual cells (17). Cells were treated with drugs at 37°C
for 2 h before 8 Gy γ-radiation, and then harvested and suspended
in cold PBS for COMET assay after 2 h of radiation. Specific
procedures are outlined in a previous study (18).
γ-H2AX immunofluorescence
The number of γ-H2AX foci was used as a biomarker of
radiation-induced DNA double-strand breaks (DSBs) (19). HUVECs were seeded in a glass bottom
cell dish (NEST Instruments), and allowed to attach overnight.
Cells were treated with the different drugs for 2 h before 8 Gy
γ-radiation on ice, and 1 h after radiation the cells were washed
with cold PBS, and fixed in 4% paraformaldehyde for 15 min. We used
5% serum to block the non-specific protein interactions and then
the cells were incubated with the primary antibody (phosphorylated
H2AX) at 4°C, overnight. Cell dishes were washed in PBS before
addition of the secondary antibody [Cy3-goat anti-rabbit IgG (H +
L)] for 1 h at 37°C, and then incubated with DAPI (5 µg/ml) for 10
min. We used a laser scanning confocal microscope to acquire the
digital images. The γ-H2AX foci counts were calculated using ImageJ
foci counter.
Western blotting
Cultured cells were exposed to 5 Gy γ-radiation and
cultured for 24 h. Then, the cells were collected and lysed in RIPA
lysis buffer on ice for 1 h. Cell lysates were centrifuged at
12,000 rpm for 5 min at 4°C, and cell supernatants were collected.
The protein concentration was confirmed using a BCA protein assay
kit (Beyotime Institute of Biotechnology). Proteins (20 µg) from
each group were separated on 10% SDS-polyacrylamide gels and
subsequently transferred onto a polyvinylidene difluoride (PVDF)
membrane (Amersham, Braunschweig, Germany). The membranes were
blocked in 5% skimmed milk dissolved in Tris-buffered saline and
Tween-20 (TBST) (20 mM Tris, pH 7.5) for 2 h and then incubated
with primary antibodies (p53, Bax, Bcl-2, γ-H2AX) at 4°C overnight.
After three washings in TBST, the membranes were incubated with the
secondary antibody conjugated with anti-rabbit IgG peroxidase for 2
h at room temperature. Bands were monitored with chemiluminescence
using an ECL detection system (Amersham Pharmacia Biotech,
Piscataway, NJ, USA).
Statistical analysis
Values are expressed as mean ± standard error (SE)
or as a percentage. Analysis of variance (ANOVA) was used to
determine whether there was a significant difference among the
groups. If the difference was significant, a pairwise comparison by
Tukey-Kramer was used to identify which group was different from
the other. Log-rank (Mantel-Cox) test was used to compare survival
rates among groups. Values of P<0.05 were considered as
statistically significant.
Results
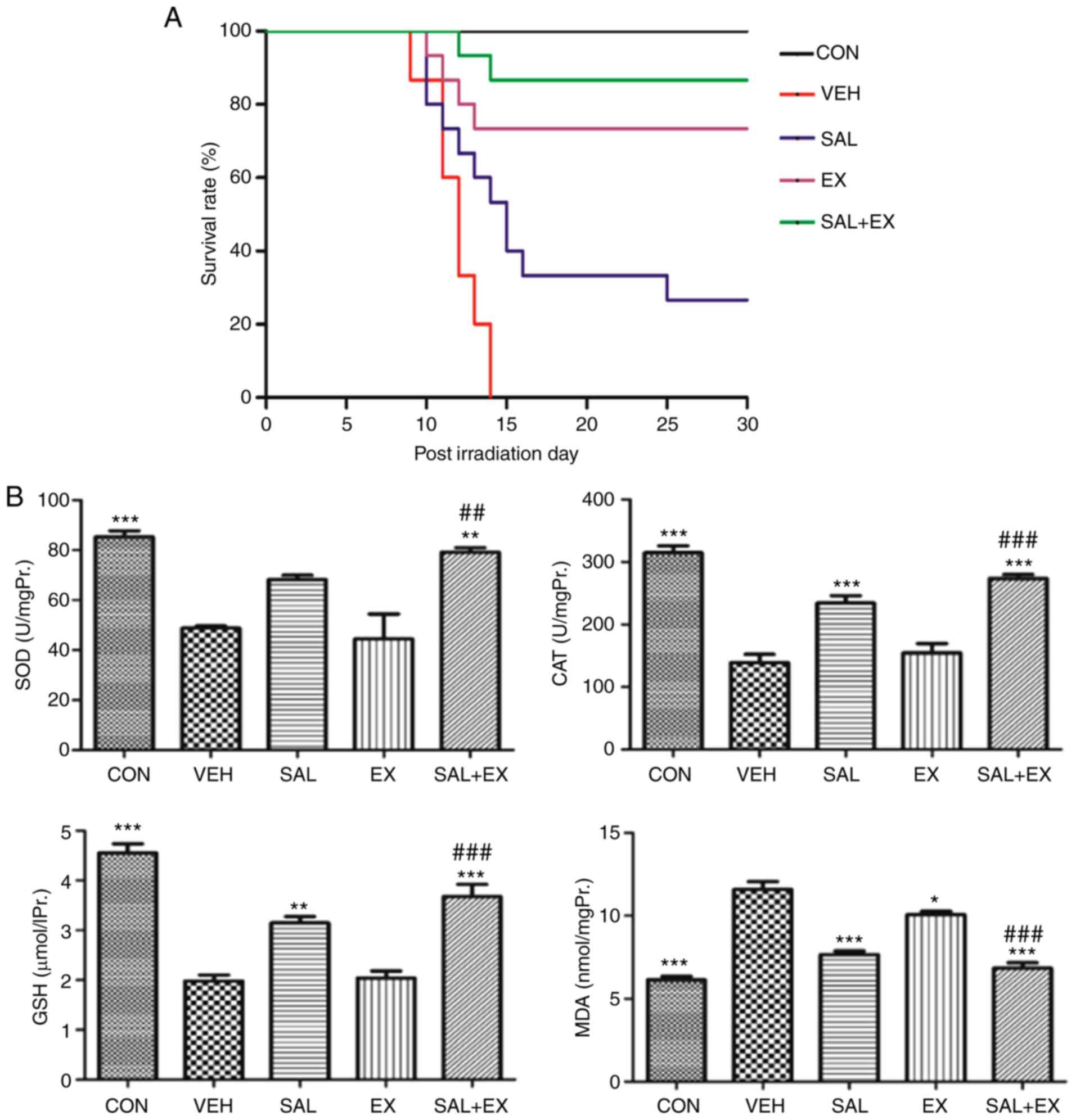
Radioprotection of Sal in vivo
The mice in the irradiation group exhibited a
reduction in food and water intake, epilation, weight loss,
emaciation, diarrhea and hemafecia within 3 to 5 days after
exposure to 6 Gy radiation (18).
The results of the survival studies are expressed as survival rate
(Fig. 2A). In the VEH group, no
mouse survived to day 30. Although only 4 mice in the SAL group
survived to day 30, the mean survival time was significantly higher
from 12 to 15 days when compared to the VEH group (P=0.0055;
log-rank test). The EX group (500 mg/kg) administered s.c. at 24 h
and 15 min before irradiation showed a significant increase in
survival, with 11 of 15 mice still alive on day 30 (P<0.0001).
The SAL + EX pretreatment group showed an increased survival rate
(from 73 to 86%; P<0.05, significant difference) compared to the
EX group. Thirteen of 15 mice remained alive on day 30. These
results collectively indicated that Sal administration
significantly enhanced the radioprotection activity of Ex-RAD.
To evaluate the antioxidant activity of Sal on
irradiated mice, we measured SOD, GSH, CAT activity and MDA levels
in liver homogenates. The average test results are presented in
Fig. 2B. γ-radiation significantly
reduced the level of antioxidant enzymes involving SOD and CAT
(P<0.001). However, Sal (100 mg/kg) significantly (P<0.001)
increased the antioxidant enzyme level. Compared with the VEH
group, SOD and CAT levels in the EX group had no difference. The
Sal combination group had obviously increased SOD and CAT activity
compared with the EX group (P<0.01; P<0.001, respectively).
As to the content of GSH, the same change was noted in the
combination group. Sal increased the GSH level in the
Ex-RAD-treated group (P<0.001). Conversely, the MDA levels in
the drug treatment groups were significantly decreased compared
with the VEH group (P<0.05), and the SAL + EX pretreatment group
was the most obvious group. These results indicated that Sal
protects mice against oxidative damage, and Sal enhances the
radioprotective activity of Ex-RAD by improving the antioxidant
capability.
The peripheral blood data are shown in Table I. Total WBC and PLT counts began to
decline shortly after radiation. However, recovery was accelerated
in the Sal and Ex-RAD treated mice after 7 days total body
irradiation. WBC and PLT counts were significantly increased in the
drug-treated mice compared with the VEH group (P<0.05). In
addition, the combination group was the fastest recovery group
compared with the Ex-RAD and Sal single use groups (P<0.05).
There was no statistical significance between these groups in
regards to HGB and RBC counts.
 | Table I.Effects of the drugs on red blood
cells, white blood cells, hemoglobin and platelets in the
peripheral blood of mice after 7 days following whole body
irradiation at a dose of 5 Gy. |
Table I.
Effects of the drugs on red blood
cells, white blood cells, hemoglobin and platelets in the
peripheral blood of mice after 7 days following whole body
irradiation at a dose of 5 Gy.
| Groups | WBC (×10E9/l) | RBC (×10E12/l) | HGB (g/l) | PLT (×10E9/l) |
|---|
| CON |
8.20±0.62b | 10.95±0.67 | 160.75±3.59 |
522±25.13b |
| VEH | 1.79±0.29 | 10.22±0.23 | 150.75±4.11 | 84.25±5.12 |
| SAL |
3.42±0.63a | 10.68±0.15 | 147±5.94 |
191.25±7.18b |
| EX |
4.17±0.27b | 11.04±0.62 | 153±8.64 |
332.25±29.85b |
| SAL + EX |
6.28±0.87b,c | 10.24±0.32 | 157.5±3.70 |
483.5±43.71b,d |
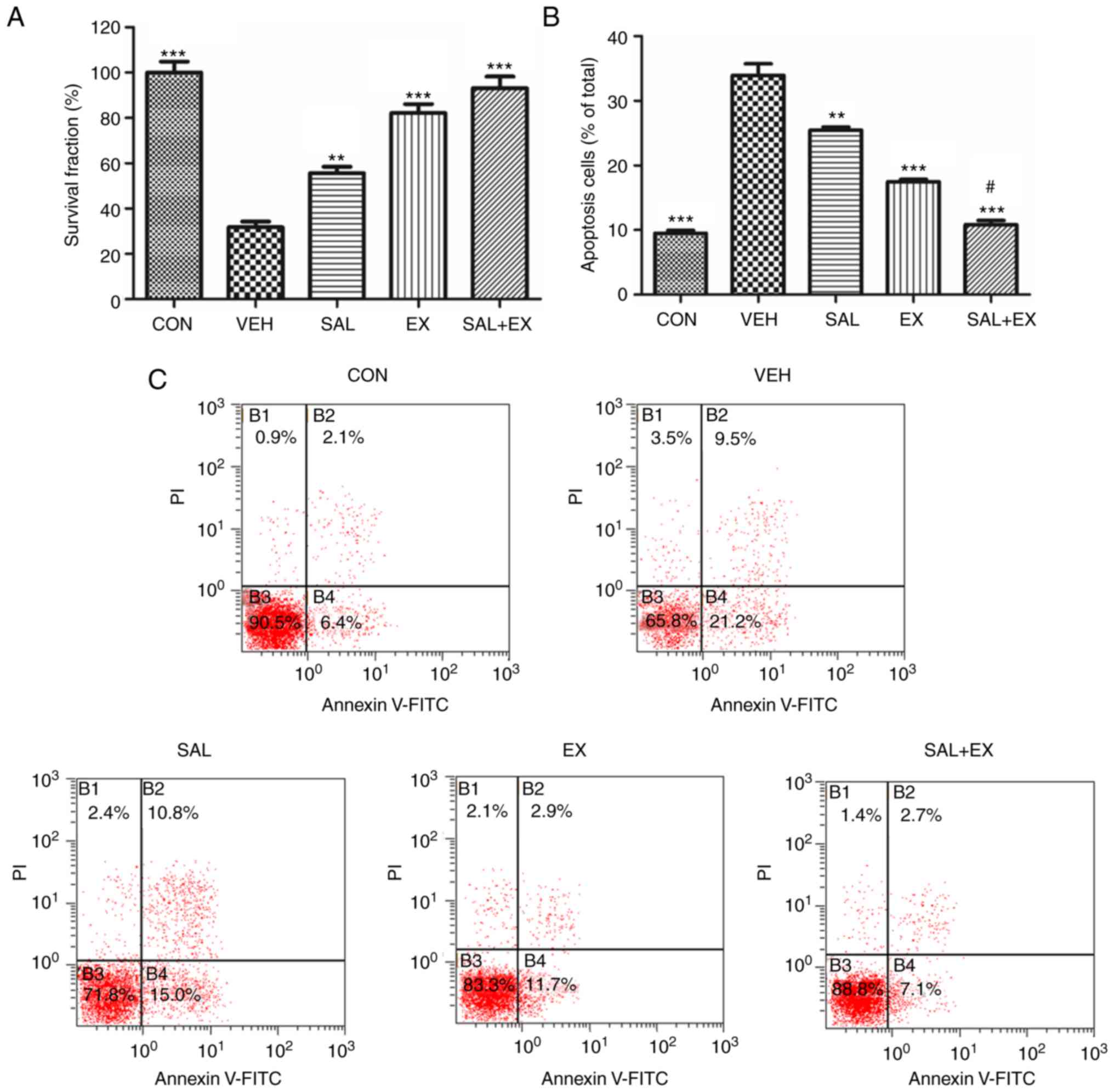
Clonogenic protection and
anti-apoptotic effects of Sal in HUVECs
HUVECs are sensitive to γ-radiation (6,20). We
used a clonogenic survival assay to examine the viability of
irradiated HUVECs. Pretreatment of Sal and Ex-RAD before radiation
provided excellent protection compared with the VEH group (Fig. 3A; P<0.01). However, there was no
significant difference between the EX and SAL + EX group.
In the apoptosis assay, radiation-induced apoptosis
in the HUVECs was significantly decreased in the SAL and EX groups
compared to the VEH group (Fig. 3B and
C; P<0.001). Meanwhile, the Sal combination group
demonstrated a better protective effect compared with the
Ex-RAD-treated group (P<0.05). These results indicated that Sal
enhanced the radioprotective effect of Ex-RAD due to the
attenuation of radiation-induced apoptosis.
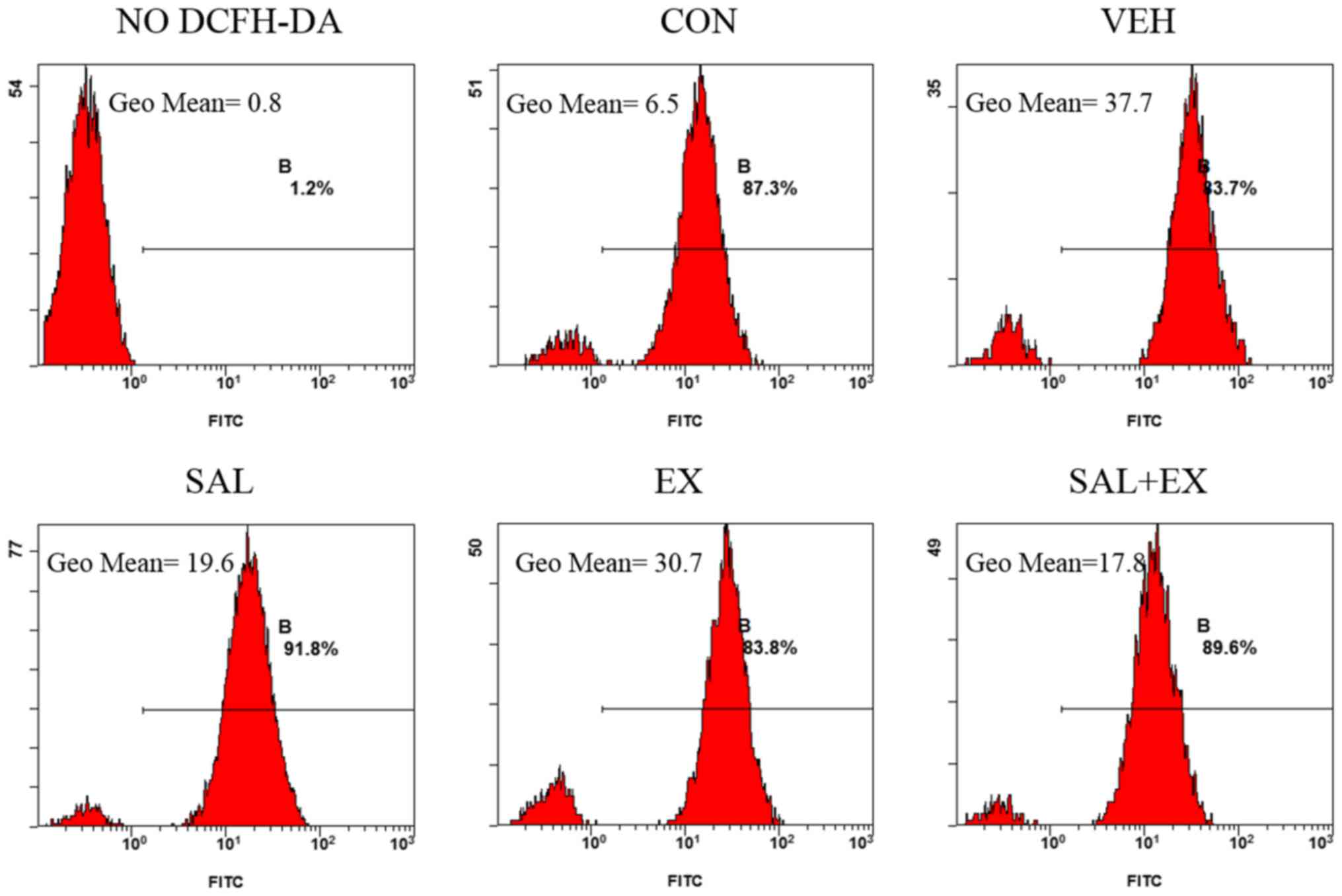
Effects of Sal on intracellular ROS
generation in HUVECs
The fluorescence of DCF was used to evaluate the
intracellular ROS in HUVECs (Fig.
4). Radiation (5 Gy) significantly increased the level of ROS
(5.8-fold) compared with the VEH group. A slight difference was
found between the EX and VEH group of cells, whereas the
fluorescence intensity of the SAL + EX and SAL group was
significantly (almost a half) lower than that noted in the EX
group. This indicated that pre-treatment with Sal significantly
blocked radiation-induced ROS generation. Sal enhanced the
radioprotective effect of Ex-RAD by scavenging intracellular
ROS.
Synergetic effects of the Sal and
Ex-RAD combination treatment on radiation-induced DNA damage in
HUVECs
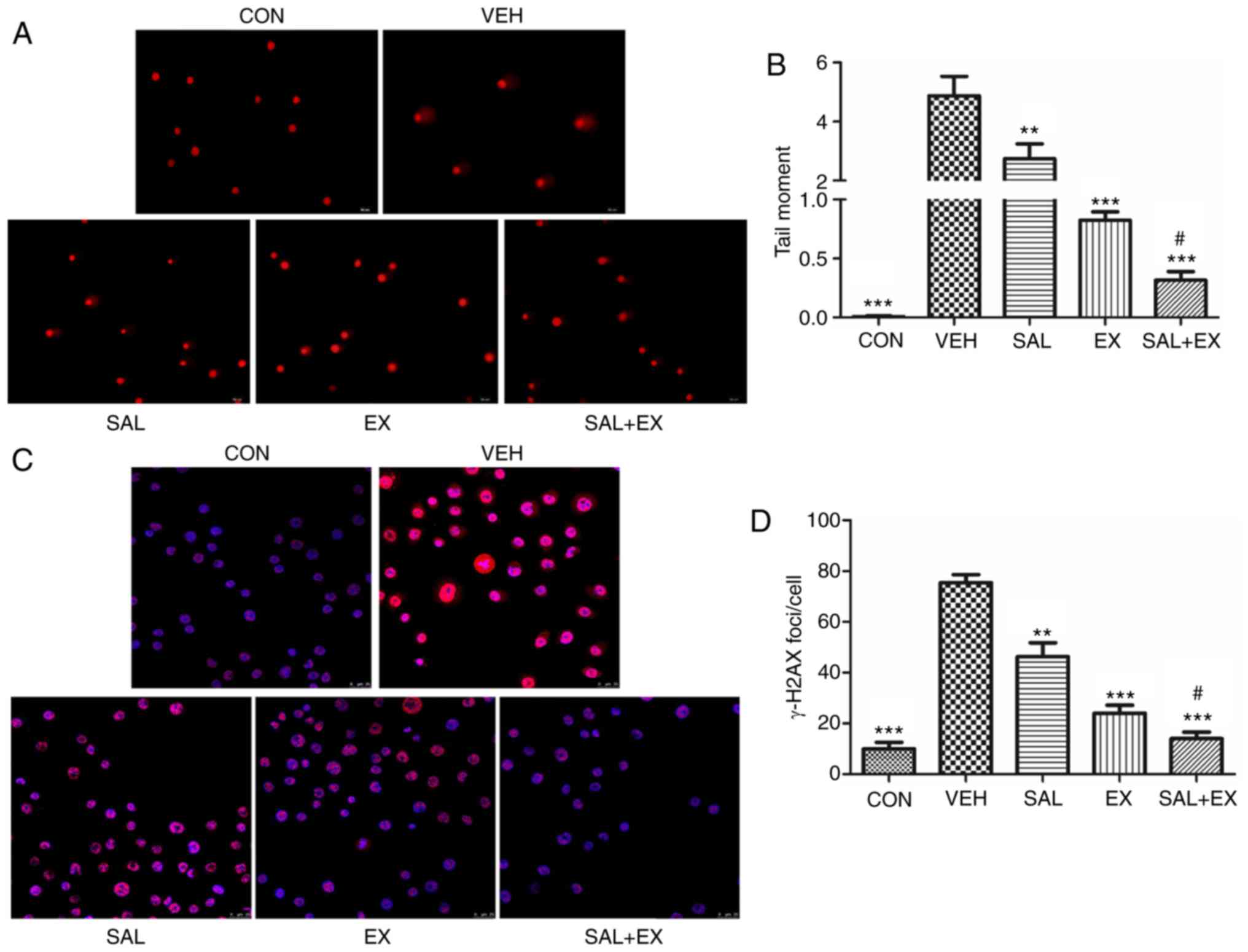
We used an alkaline comet assay to detect radiation
damage. The results showed a faster resolution of fragmented tail
DNA in the cells treated with Sal and Ex-RAD compared to the
untreated cells (Fig. 5A). One-way
ANOVA test showed a significant decrease in tail moment
(P<0.01), and there was also a significant difference between
tail moments of EX and SAL + EX treated cells before irradiation
(Fig. 5B; P<0.05).
The kinetics of γ-H2AX foci resolution was used as a
biomarker to ascertain the effect of drugs on the repair of
double-strand breaks. Immunofluorescence staining of γ-H2AX was
assessed 1 h after radiation (Fig.
5C). Both Sal and Ex-RAD obviously reduced the number of γ-H2AX
foci in cells compared to the VEH group (P<0.01). Moreover, the
combination group showed a markedly decreased γ-H2AX foci number
compared with the EX group (Fig.
5D; P<0.05) The results indicated a positive effect of Sal
in facilitating repair of DNA damage, and improving the curative
effect of Ex-RAD.
Effects of Sal on the p53 signaling
pathway in HUVECs
In order to elucidate the molecular mechanisms
involved in the cell apoptosis induced by γ-radiation in HUVECs,
western blotting was used to measure the expression of several
apoptosis-related proteins. As shown in Fig. 6A, we found that the expression of
the pro-apoptotic protein p53 and Bax was markedly increased after
irradiation. As shown in Fig. 6B and
C, Sal significantly inhibited the upregulation of p53
(P<0.001), and promoted the increase in the Bcl-2/Bax ratio
(P<0.01). The results also showed that combined treatment with
Ex-RAD and Sal significantly increased the Bcl-2/Bax ratio
(P<0.001), accompanied by a decrease in the p53 level
(P<0.01), compared to the levels following treatment with either
drug alone. γ-H2AX is a marker to assess the effectiveness of DNA
repair by nonhomologous end joining (21). Both Sal and Ex-RAD increased the
expression of γ-H2AX (Fig. 6D,
P<0.001, P<0.01). There was no significant difference between
the EX and SAL + EX group.
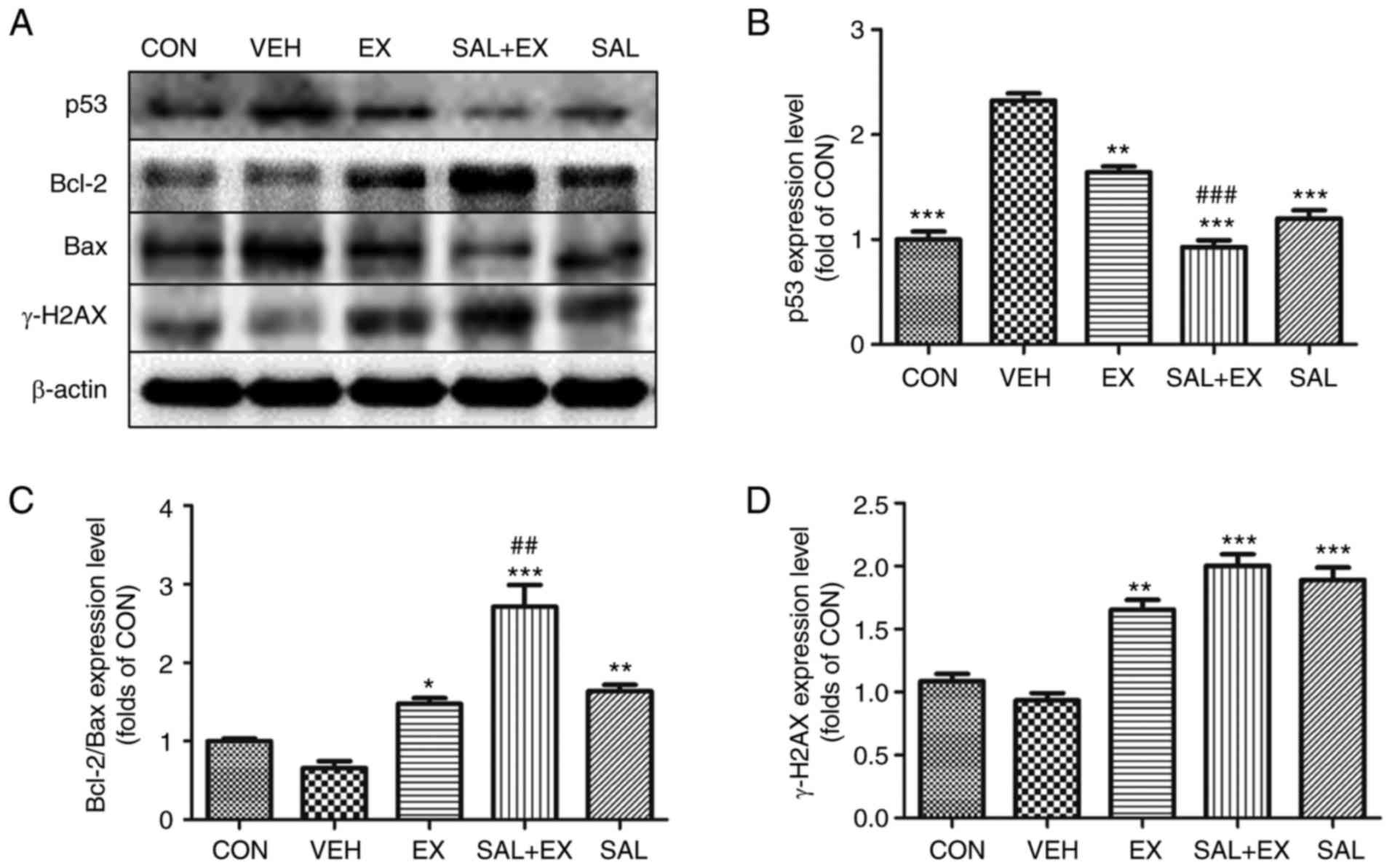 | Figure 6.Western blot analysis of DNA damage
response in HUVECs. (A) Scanned images showing western blotting of
p53, Bcl-2, Bax and γ-H2AX. (B-D) The expression levels of p53,
Bcl-2/Bax ratio, and γ-H2AX were quantified by densitometry. The
data values are indicated as the relative densitometry of the
control. Results are represented as mean ± SE of three independent
experiments; *P<0.05, **P<0.01, ***P<0.001 vs. VEH;
#P<0.05, ##P<0.01,
###P<0.001 vs. EX. Groups: CON (control), VEH
(irradiation + vehicle), SAL (irradiation + Sal 100 mg/kg), EX
(irradiation + Ex-RAD 500 mg/kg) and SAL + EX (irradiation + Sal
100 mg/kg + Ex-RAD 500 mg/kg). |
Discussion
In the present study, the results confirmed that
salidroside (Sal) significantly enhanced the radioprotective effect
of Ex-RAD. The 30-day survival assay showed that the combined
administration of Sal and Ex-RAD provided significant protection to
mice after 8 Gy irradiation. Acute radiation exposure leads to a
drop in circulating blood cells and hemorrhage due to
radiation-induced loss of platelets remains a life-threatening
issue (22,23). In this initial investigation, we
demonstrated that Sal and Ex-RAD significantly increased the
peripheral WBC and platelet counts, mitigating radiation-induced
hematopoietic toxicity. In addition, the results demonstrated that
hematopoietic recovery was also significantly accelerated in mice
treated with the combined agents when compared to mice receiving
single-agent treatments.
In the healthy physiological status, ROS remain at
low levels and play a normal function. Once the level is enhanced
due to radiation, the high levels of ROS may cause severe damage to
DNA, which eventually leads to cell death via either apoptotic or
other mechanisms (24). Sal, a
powerful natural antioxidant, was widely reported for its
inhibition of ROS generation in different diseases (25–27).
In the present study, intracellular ROS significantly increased
after radiation, whereas the level of ROS was obviously decreased
in the Sal-treated cells. Moreover, we found that radiation-induced
apoptosis was greatly reduced in the Sal and Ex-RAD combined group.
Results indicated that Sal promoted the radioprotection of Ex-RAD
probably by decreasing ROS generation and protecting against cell
death.
Organisms develop various defense mechanisms to
protect themselves against ROS, such as antioxidant enzymes. The
antioxidant enzymes include SOD, GSH and CAT. SOD catalyzes the
dismutation of superoxide (O2−) to hydrogen peroxide
(H2O2), while CAT and GSH convert
H2O2 to H2O. MDA is considered to
reflect the degree of lipid peroxidation (28–30).
In the present study, Sal significantly decreased the MDA level,
and significantly increased the activity of SOD, CAT and GSH
enzymes in the mouse liver. This indicated that Sal was able to
protect against the damage produced by radiation with upregulation
of antioxidant enzymes and a decrease in lipid peroxidation.
Radiation-induced damage to DNA is the primary
determinant of radiation-induced mutations, carcinogenesis and
lethality (31). We used COMET
assay and γ-H2AX foci formation to investigate DNA single- and
double-strand breaks (DSB), respectively. The intensity of the
comet tail relative to the head reflected the number of DNA breaks
(17). γ-H2AX molecules appeared in
discrete nuclear foci and each γ-H2AX focus represented one DNA DSB
(19,32). It was noted that pretreatment of Sal
and Ex-RAD significantly reduced the comet parameters compared with
the Ex-RAD alone group. γ-H2AX immunofluorescence assay showed that
the Sal combination group exhibited significant protection against
radiation-induced DNA damage. The results confirmed that the
radioprotective effect of Ex-RAD was largely increased by Sal.
To further investigate the molecular radiation
protection mechanism of Sal and Ex-RAD, western blot assay was
performed to measure the expression of several apoptosis-related
and DNA repair proteins. It is well known that pro-apoptosis
signaling pathways include activation and stabilization of p53,
Bcl-2, Bax, c-Abl, p21 and many more signaling molecules (33–35).
Bcl-2 family proteins include pro- and anti-apoptotic molecules.
Bcl-2 is an anti-apoptotic protein and Bax is a pro-apoptotic
protein. They have been identified as major regulators and the
ratio of Bcl-2/Bax plays an important role in cell apoptosis or
survival (34,36,37).
Both Bcl-2 and Bax are transcriptional targets for the
tumor-suppressor protein, p53, which induces cell cycle arrest or
apoptosis in response to DNA damage (33,37).
Sal downregulated the expression of Bax, p53, and increased the
ratio of Bcl-2/Bax. The radiation protection offered by Sal
probably involves inhibition of apoptosis through p53-dependent
pathways. DNA DSB induced by γ-radiation leads to rapid
phosphorylation of H2AX at Ser139 by ATM, ATR and DNA-PKcs,
resulting in γ-H2AX. The phosphorylation and dephosphorylation of
H2AX are necessary for the DNA damage repair process (38). Our results demonstrated that Ex-RAD
and Sal increased the expression of γ-H2AX, Sal is a beneficial
natural drug that aids Ex-RAD in DNA repair.
Furthermore, previous investigations have also shown
that Sal can inhibit the proliferation of a variety of cancer cells
(39–43). We suspected that Sal may have
different biological effects in normal cells and tumor cells. We
demonstrated that Sal has radioprotective effects on normal cells.
However, studies are still needed to further evaluate whether Sal
has an inhibitory effect on cancer cells and the different
mechanisms of these functions.
In conclusion, the present study indicated that Sal
enhanced the radioprotective effect of Ex-RAD. Sal exerted its
radioprotective action, largely via scavenging intracellular ROS,
reducing DNA damage and upregulating antioxidant enzymes against
γ-radiation in HUVECs. The molecular mechanism underlying its
radioprotective properties may result from alleviation of
radiation-induced apoptosis by inhibiting p53-dependent apoptosis.
These data suggest that Sal may be a potential choice for
combination treatment against radiation-related damage. However,
the detailed in vivo radioprotective activities, clinical
application, molecular mechanisms and drug targets of Sal warrant
further investigation.
Acknowledgements
We thank the National Natural Science Foundation of
China (nos. 81573343 and 21503272) and the Major Science and
Technology Projects in Shanxi Province (Xianyang China)
(2015SF2-08-01 and 2017ZDJC-05).
References
|
1
|
Won EJ and Lee JS: Gamma radiation induces
growth retardation, impaired egg production, and oxidative stress
in the marine copepod Paracyclopina nana. Aquat Toxicol. 150:17–26.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Coeytaux K, Bey E, Christensen D, Glassman
ES, Murdock B and Doucet C: Reported radiation overexposure
accidents worldwide, 1980–2013: A systematic review. PLoS One.
10:e01187092015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Demiral AN, Yerebakan O, Simşir V and
Alpsoy E: Amifostine-induced toxic epidermal necrolysis during
radiotherapy: A case report. Jpn J Clin Oncol. 32:477–479. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kouvaris JR, Kouloulias VE and Vlahos LJ:
Amifostine: The first selective-target and broad-spectrum
radioprotector. Oncologist. 12:738–747. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chun AW, Freshwater RE, Taft DR, Gillum AM
and Maniar M: Effects of formulation and route of administration on
the systemic availability of Ex-RAD®, a new
radioprotectant, in preclinical species. Biopharm Drug Dispos.
32:99–111. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ghosh SP, Perkins MW, Hieber K, Kulkarni
S, Kao TC, Reddy EP, Reddy MV, Maniar M, Seed T and Kumar KS:
Radiation protection by a new chemical entity, Ex-Rad: Efficacy and
mechanisms. Radiat Res. 171:173–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Suman S, Maniar M, Fornace AJ Jr and Datta
K: Administration of ON 01210. Na after exposure to ionizing
radiation protects bone marrow cells by attenuating DNA damage
response. Radiat Oncol. 7:6. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ghosh SP, Kulkarni S, Perkins MW, Hieber
K, Pessu RL, Gambles K, Maniar M, Kao TC, Seed TM and Kumar KS:
Amelioration of radiation-induced hematopoietic and
gastrointestinal damage by Ex-RAD® in mice. J Radiat
Res. 53:526–536. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao XT, Feng JB, Li YW, Luo Q, Yang XC,
Lu X, Chen DQ and Liu QJ: Identification of two novel mitochondrial
DNA deletions induced by ionizing radiation. Biomed Environ Sci.
25:533–541. 2012.PubMed/NCBI
|
|
10
|
Zhu L, Wei T, Chang X, He H, Gao J, Wen Z
and Yan T: Effects of salidroside on myocardial injury in vivo in
vitro via regulation of Nox/NF-κB/AP1 pathway. Inflammation.
38:1589–1598. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Y, Wu J, Shi R, Li N, Xu Z and Sun M:
Antioxidative effects of Rhodiola genus: Phytochemistry and
pharmacological mechanisms against the diseases. Curr Top Med Chem.
17:1692–1708. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li X, Sipple J, Pang Q and Du W:
Salidroside stimulates DNA repair enzyme Parp-1 activity in mouse
HSC maintenance. Blood. 119:4162–4173. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu S, Zhu J, Chen X and Liu G: Protective
effects of salidroside on endothelial progenitor cells damaged by
radiation. Chin J Pathophysiol. 32:240–244. 2016.
|
|
14
|
Kamran MZ, Ranjan A, Kaur N, Sur S and
Tandon V: Radio-protective agents: Strategies and translational
advances. Med Res Rev. 36:461–493. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Patchen ML, MacVittie TJ and Weiss JF:
Combined modality radioprotection: The use of glucan and selenium
with WR-2721. Int J Radiat Oncol Biol Phys. 18:1069–1075. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suman S, Datta K, Doiron K, Ren C, Kumar
R, Taft DR, Fornace AJ Jr and Maniar M: Radioprotective effects of
ON 01210. Na upon oral administration. J Radiat Res. 53:368–376.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rojas E, Lopez MC and Valverde M: Single
cell gel electrophoresis assay: Methodology and applications. J
Chromatogr B Biomed Sci Appl. 722:225–254. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo J, Zhang Y, Zeng L, Liu J, Liang J and
Guo G: Salvianic acid A protects L-02 cells against
γ-irradiation-induced apoptosis via the scavenging of reactive
oxygen species. Environ Toxicol Pharmacol. 35:117–130. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Redon CE, Dickey JS, Bonner WM and
Sedelnikova OA: γ-H2AX as a biomarker of DNA damage induced by
ionizing radiation in human peripheral blood lymphocytes and
artificial skin. Adv Space Res. 43:1171–1178. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kang AD, Cosenza SC, Bonagura M, Manair M,
Reddy MV and Reddy EP: ON01210. Na (Ex-RAD®) mitigates
radiation damage through activation of the AKT pathway. PLoS One.
8:e583552013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lukas J, Lukas C and Bartek J: More than
just a focus: The chromatin response to DNA damage and its role in
genome integrity maintenance. Nat Cell Biol. 13:1161–1169. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Patchen ML, MacVittie TJ, Williams JL,
Schwartz GN and Souza LM: Administration of interleukin-6
stimulates multilineage hematopoiesis and accelerates recovery from
radiation-induced hematopoietic depression. Blood. 77:472–480.
1991.PubMed/NCBI
|
|
23
|
Fliedner TM, Friesecke I, Graessle D,
Paulsen C and Weiss M: Hematopoietic cell renewal as the limiting
factor in low-level radiation exposure: Diagnostic implications and
therapeutic options. Mil Med. 167 Suppl 2:S46–S48. 2002.
|
|
24
|
Yu Y, Fan SM, Song JK, Tashiro S, Onodera
S and Ikejima T: Hydroxyl radical (·OH) played a pivotal role in
oridonin-induced apoptosis and autophagy in human epidermoid
carcinoma A431 cells. Biol Pharm Bull. 35:2148–2159. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang S, He H, Chen L, Zhang W, Zhang X and
Chen J: Protective effects of salidroside in the
MPTP/MPP+-induced model of Parkinson's disease through
ROS-NO-related mitochondrion pathway. Mol Neurobiol. 51:718–728.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee SY, Shi LS, Chu H, Li MH, Ho CW, Lai
FY, Huang CY and Chang TC: Rhodiola crenulata and its bioactive
components, salidroside and tyrosol, reverse the hypoxia-induced
reduction of plasma-membrane-associated Na,K-ATPase expression via
inhibition of ROS-AMPK-PKC ξ pathway. Evid Based Complement
Alternat Med. 2013:2841502013.PubMed/NCBI
|
|
27
|
Tan CB, Gao M, Xu WR, Yang XY, Zhu XM and
Du GH: Protective effects of salidroside on endothelial cell
apoptosis induced by cobalt chloride. Biol Pharm Bull.
32:1359–1363. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Karslioglu I, Ertekin MV, Koçer I, Taysi
S, Sezen O, Gepdiremen A and Balci E: Protective role of
intramuscularly administered vitamin E on the levels of lipid
peroxidation and the activities of antioxidant enzymes in the lens
of rats made cataractous with gamma-irradiation. Eur J Ophthalmol.
14:478–485. 2004.
|
|
29
|
Karslioğlu I, Ertekin MV, Taysi S, Koçer
I, Sezen O, Gepdiremen A, Koç M and Bakan N: Radioprotective
effects of melatonin on radiation-induced cataract. J Radiat Res.
46:277–282. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bardak Y, Ozertürk Y, Ozgüner F, Durmuş M
and Delibaş N: Effect of melatonin against oxidative stress in
ultraviolet-B exposed rat lens. Curr Eye Res. 20:225–230. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Téoule R: Radiation-induced DNA damage and
its repair. Int J Radiat Biol Relat Stud Phys Chem Med. 51:573–589.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sedelnikova OA and Bonner WM: GammaH2AX in
cancer cells: A potential biomarker for cancer diagnostics,
prediction and recurrence. Cell Cycle. 5:2909–2913. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Basu A and Haldar S: The relationship
between BcI2, Bax and p53: Consequences for cell cycle progression
and cell death. Mol Hum Reprod. 4:1099–1109. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Adams JM and Cory S: The Bcl-2 protein
family: Arbiters of cell survival. Science. 281:1322–1326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Reed JC: Proapoptotic multidomain
Bcl-2/Bax-family proteins: Mechanisms, physiological roles, and
therapeutic opportunities. Cell Death Differ. 13:1378–1386. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gabriel B, Sureau F, Casselyn M, Teissié J
and Petit PX: Retroactive pathway involving mitochondria in
electroloaded cytochrome c-induced apoptosis. Protective properties
of Bcl-2 and Bcl-XL. Exp Cell Res. 289:195–210. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gross A, McDonnell JM and Korsmeyer SJ:
BCL-2 family members and the mitochondria in apoptosis. Genes Dev.
13:1899–1911. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
An J, Huang YC, Xu QZ, Zhou LJ, Shang ZF,
Huang B, Wang Y, Liu XD, Wu DC and Zhou PK: DNA-PKcs plays a
dominant role in the regulation of H2AX phosphorylation in response
to DNA damage and cell cycle progression. BMC Mol Biol. 11:182010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang J, Li JZ, Lu AX, Zhang KF and Li BJ:
Anticancer effect of salidroside on A549 lung cancer cells through
inhibition of oxidative stress and phospho-p38 expression. Oncol
Lett. 7:1159–1164. 2014.PubMed/NCBI
|
|
40
|
Sun KX, Xia HW and Xia RL: Anticancer
effect of salidroside on colon cancer through inhibiting JAK2/STAT3
signaling pathway. Int J Clin Exp Pathol. 8:615–621.
2015.PubMed/NCBI
|
|
41
|
Zhao G, Shi A, Fan Z and Du Y: Salidroside
inhibits the growth of human breast cancer in vitro and in vivo.
Oncol Rep. 33:2553–2560. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Fan XJ, Wang Y, Wang L and Zhu M:
Salidroside induces apoptosis and autophagy in human colorectal
cancer cells through inhibition of PI3K/Akt/mTOR pathway. Oncol
Rep. 36:3559–3567. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lv C, Huang Y, Liu ZX, Yu D and Bai ZM:
Salidroside reduces renal cell carcinoma proliferation by
inhibiting JAK2/STAT3 signaling. Cancer Biomark. 17:41–47. 2016.
View Article : Google Scholar : PubMed/NCBI
|