Introduction
Fatty acid synthase (FASN) is a multifunctional
enzyme involved in the synthesis of palmitate from acetyl-CoA and
malonyl-CoA (1). While FASN is
minimally expressed in normal human tissues (2), it exhibits markedly increased
expression in several human cancers, and its overexpression in
tumors is associated with poor prognosis (3–6). FASN
overexpression induced invasive adenocarcinomas in human prostate
epithelial cells. It also protected cells from apoptosis, with FASN
expression being inversely associated with the apoptotic rate in
human prostate cancer specimens (7). Increased FASN expression was also
linked to short-term survival in cases of colorectal and ovarian
cancer (3). Increased FASN
expression and activity was an early event in the development and
progression of lung squamous cell (8) and prostate cancer (9), and melanoma (10). Furthermore, high FASN expression was
associated with an overall high proliferative index in prostate
cancer (9), and FASN expression
intensity was related to prognosis in melanoma (10).
Cerulenin, a natural antibiotic product of the
fungus Cephalosporium caerulens (11), inhibited FASN activity and caused
apoptotic death of cancer cells in vitro (12,13).
The synthetic cerulenin analog C75, a potent FASN inhibitor
(14), was also cytotoxic to cancer
cells in vitro, and exhibited substantial in vivo
antitumor activity against some human cancer xenografts (15,16).
Pharmacological inhibition of fatty acid synthesis reportedly
caused selective toxicity to cancer cells, and delayed tumor growth
induction in vivo and in vitro (17–19).
Moreover, specific inhibition of the FASN gene by siRNA led to
apoptosis of prostate tumor cells (20). Collectively, these data strongly
indicated that de novo lipogenesis plays a substantial role
in tumor pathogenesis (21).
However, the molecular mechanism underlying the increased FASN
expression in cancer is not fully understood.
Peroxisome proliferator-activated receptor γ
coactivator (PGC)-1α is a coactivator that regulates multiple
metabolic processes through interactions with various transcription
factors (22). Recent studies of
the PGC-1α roles in cancer and in metabolic regulation have
produced controversial results. Various studies have reported
decreased PGC-1α expression in breast and colon cancer (23–25).
Another investigation demonstrated that PGC-1α overexpression
induced apoptosis in ovarian cancer (26). Although PGC-1α reportedly acts as a
tumor suppressor, it can also promote cell growth in prostate
cancer (27). Thus, there remains a
need for additional studies of PGC-1α. We previously demonstrated
that PGC-1α enhanced cell proliferation and tumorigenesis by
upregulation of specificity protein 1 (Sp1) and acyl-CoA-binding
protein (ACBP) (28). We further
found that PGC-1α may promote increased production of antioxidant
enzymes, including catalase and superoxide dismutase, contributing
to apoptosis resistance (28). Vock
et al reported that FASN is a target molecule of ACBP, based
on the downregulation of FASN in ACBP-knockdown cells (29).
Although both FASN and PGC-1α play roles in several
types of cancer cells, the precise molecular mechanism for the
interaction between FASN and PGC-1α has not been clearly
elucidated. In the present study, we investigated whether FASN
expression was regulated by PGC-1α and contributed to increased
cell proliferation.
Materials and methods
Cell preparations
We obtained human colorectal cancer (HT-29 and
SNU-C4) cells from the Korean Cell Line Bank (Seoul National
University, Seoul, Korea). These cell lines were cultured in
Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
fetal bovine serum (FBS), 100 U/ml penicillin and 100 µg/ml
streptomycin (Gibco, Carlsbad, CA, USA). Cultures were maintained
at 37°C in a humidified atmosphere of 95% air/5%
CO2.
Materials
We purchased 2′,7′-dichlorofluorescein diacetate
(DCFDA) and carboxyfluorescein succinimidyl ester (CFSE) from
Molecular Probes (Carlsbad, CA, USA). The Annexin V-fluorescein
isothiocyanate (FITC) apoptosis detection kit was obtained from BD
Biosciences (San Jose, CA, USA). We purchased the following primary
antibodies: anti-PGC-1α (sc-13067), anti-ACBP (sc-30190),
anti-superoxide dismutase (SOD)-2 (sc-30080), anti-Sp1 (sc-59) (all
from Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-catalase
(ab1877) (Abcam, Cambridge, UK) and anti-FASN (6910962) (BD
Biosciences). The anti-β-actin (A1978), anti-rabbit IgG (A0545) and
anti-mouse IgG secondary antibodies (A9044) were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Unless otherwise stated, all
other chemicals were purchased from Sigma (St. Louis, MO, USA).
Western blot analysis
Cell lysis and western blot analysis were performed
as previously described (28). We
used 30 µg protein for the immunoblotting, and β-actin was used as
the loading control.
Immunofluorescence staining
Cells were cultured on a Lab-Tek® Chamber
Slide™ (Nalge Nunc, Inc., Rochester, NY, USA), and then fixed with
3% formaldehyde, permeabilized using 0.01% Triton X-100 and blocked
for 30 min with 3% FBS. Next, the cells were incubated with a
primary antibody for 1 h, and then with a fluorescence-labeled
secondary antibody (Sigma) for 30 min. The cells were subsequently
washed, mounted using glycerol, and analyzed using a Zeiss LSM 510
confocal microscope (Carl Zeiss Co., Ltd., Jena, Germany) with a
40× C-Apochromat objective. Negative control staining was performed
with only secondary antibodies.
Promoter reporter constructs and
luciferase assays
The FASN promoter construct (30) was provided by Professor Kim
Kyung-Sup (Department of Biochemistry and Molecular Biology, Yonsei
University, Seoul, Republic of Korea). For the luciferase assay,
SNU-C4, HT-29 and FASN shRNA-silenced cells were seeded into a
6-well plate (1×106 cells/well) in DMEM containing 10%
FBS. After two days, the cells were co-transfected with 1 µg FASN
promoter-driven luciferase reporter vector and 0.1 µg
Renilla luciferase control vector, with or without pcDNA3.1
or PGC-1α expression vector, using Lipofectamine 2000 (Invitrogen,
Carlsbad, CA, USA) following the manufacturer's instructions. After
a 6-h incubation, the cell medium was replaced with fresh DMEM
containing 10% FBS. After a 24-h incubation, the cells were washed
with phosphate-buffered saline (PBS) and harvested in 200 µl
reporter lysis buffer (Promega, Madison, WI, USA). The cells were
vigorously mixed for 15 min, and then centrifuged at 12,000 × g for
10 min at 4°C. The supernatants were transferred into fresh tubes,
and 10 µg/ml aliquots of the cleared whole cell lysate were assayed
for luciferase activity. Luciferase assays were performed using a
Dual-Luciferase Assay kit (Promega) and quantitation was performed
with a Lumat LB9501 luminometer. Luciferase activity was normalized
by quantitating the protein and adjusting the amount of extract to
a fixed amount of protein.
For the siRNA transfection experiment, the cells
were seeded into 6-well culture plates and cultured overnight. The
cells were first transfected with non-silencing control (NC) siRNA,
PGC-1α siRNA, Sp1 siRNA, SREBP-1c siRNA or both Sp1 siRNA and
SREBP-1c siRNA using Lipofectamine 2000. After a 24-h incubation,
the cells were co-transfected with FASN promoter-driven reporter
constructs and Renilla luciferase vector, with or without
pcDNA3.1 or PGC-1α expression vector, for 24 h. Finally, these
cells were collected for use in luciferase assays. All
transfections were performed in triplicate, and repeated at least
thrice in independent experiments.
siRNA transfection
The siRNA sequence used for targeted silencing of
PGC-1α was designed by Qiagen (GS10891; Valencia, CA, USA). The Sp1
siRNA (SC-29487) and SREBP-1c siRNA (SC-36557) were purchased from
Santa Cruz Biotechnology. The cells were resuspended in PBS at a
density of 1.3×107 cells/0.5 ml, and transfected with 4
nM PGC-1α (4 nM Sp1 or 4 nM SREBP-1c, both Sp1 and SREBP-1c) or NC
siRNA using Lipofectamine 2000 following the manufacturer's
procedure. After transfection, the cells were cultured in DMEM with
10% FBS for 48 h. These cells were then used for luciferase assays,
immunofluorescence staining, and western blot analysis.
Cell counting
SNU-C4 and HT-29 cells, transfected with shRNA for
FASN or with NC shRNA, were seeded into a 6-well plate
(1×105 cells/well). After 24, 48 or 72 h of incubation,
the cells were harvested by trypsinization using trypsin/EDTA, and
stained with trypan blue. The vital cells (those not stained with
trypan blue) were counted under a Nikon Eclipse TS100 microscope
(Nikon, Tokyo, Japan). Three independent experiments were
conducted.
Cell proliferation assay
Cell proliferation was assessed using the CFSE
labeling assay as previously described (31). Briefly, the cells were washed three
times with PBS, and incubated for 15 min with 1 µM CFSE dye
(Molecular Probes). The cells were then washed again, incubated
with fresh medium containing 10% FBS, and seeded in 6-well plates
(1×105 cells/well). Cells were incubated for 24, 48 or
72 h, and then analyzed using flow cytometry (FACSCalibur; BD
Biosciences). Each condition was tested in triplicate.
Generation of a FASN-silenced cell
line
An shRNA construct containing FASN shRNA
(MISSION® shRNA plasmid DNA; FASN-pLKO.1-puro) and a
non-targeting control construct (NC-pLKO.1-puro) were provided by
Sigma. The sequences were as follows: FASN shRNA,
5′-CCGGCATGGAGCGTATCTGTGAGAACTCGAGTTCTCACAGATACGCTCCATGTTTTT-3′; NC
shRNA, 5′-GCGCGATAGCGCTAATAATTT-3′. SNU-C4, and HT-29 cells
(1×106) were transfected with 2 µg of FASN-pLKO.1-puro
or NC-pLKO.1-puro using Lipofectamine 2000, following the
manufacturer's procedure. At 48 h post-transfection, the cells were
incubated with 2 µg/ml puromycin for 14 days to select stable
clones. Positive clones were chosen for identification, and
cultured in DMEM supplemented with 10% FBS, 2 µg/ml puromycin, 100
U/ml penicillin and 100 µg/ml streptomycin (Gibco). Cultures were
maintained at 37°C in a humidified atmosphere of 95% air/5%
CO2.
Annexin V-PI staining assay
The extent of apoptosis was evaluated using Annexin
V-FITC and flow cytometry as previously described (32).
Assessment of ROS production
ROS production was monitored by flow cytometry using
carboxy-H2DCFDA (Molecular Probes). FASN-silenced SNU-C4 and HT-29
cells were washed twice with PBS to remove extracellular compounds.
FASN-silenced SNU-C4 and HT-29 cells were transfected with
pReceiver-M13 (EX-M13) or FASN expression vector
(pReceiver-M13-FASN; EX-T3050-M13) (both from GeneCopoeia,
Rockville, MD, USA) and treated with PBS or
H2O2 and then washed twice with PBS to remove
extracellular compounds. Next, the cells were incubated for an hour
with H2DCFDA (100 µmol/l). Finally, for flow cytometric analysis,
green fluorescence was excited using an argon laser and detected
using a 525-nm band-pass filter.
Statistical analysis
Statistical analyses were performed using the SPSS
22.0 statistical package for Windows (SPSS, Inc., Chicago, IL,
USA). Data are expressed as mean ± standard deviation (SD). One-way
analysis of variance (ANOVA) was used to evaluate whether cell
viability significantly differed between FASN-silenced and control
cells. Statistical significance was defined as P<0.05.
Results
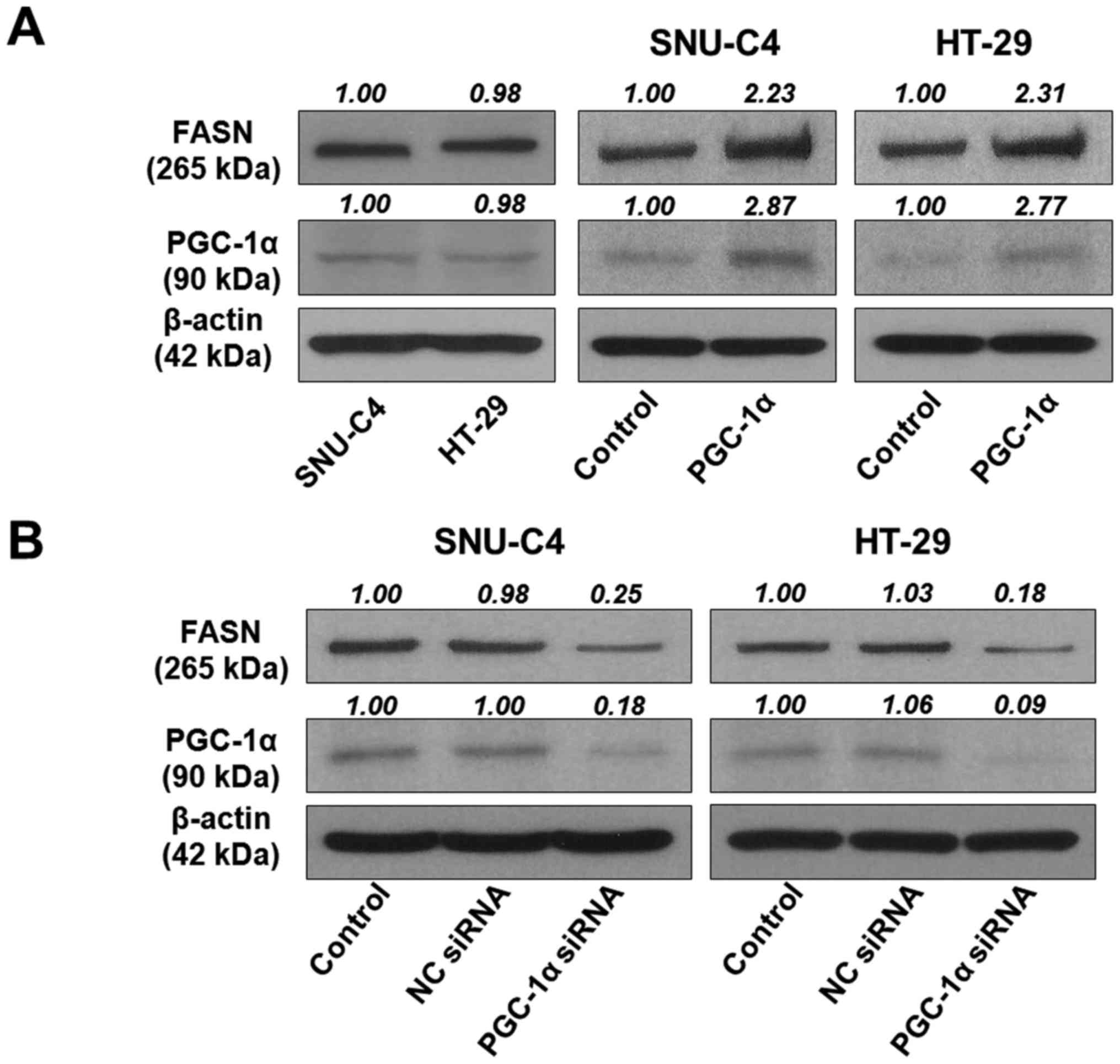
FASN is upregulated in colorectal
cancer cell lines that overexpress PGC-1α
Prior studies have demonstrated an association
between FASN overexpression in tumors and proliferation (33–35),
and we previously revealed that PGC-1α overexpression enhanced cell
proliferation (28). To investigate
the relationship between FASN and PGC-1α expression, we used
western blotting and immunofluorescence staining to examine FASN
and PGC-1α expression in HT-29 and SNU-C4 cells. The FASN and
PGC-1α proteins were expressed at slightly lower levels in HT-29
cells compared to SNU-C4 cells (Fig. 1A
and C). We previously reported that knockdown of PGC-1α
expression in human colorectal cancer cells led to decreased cell
proliferation (28). To determine
whether FASN contributed to the PGC-1α role in cell proliferation,
we used western blotting and immunofluorescence staining to observe
FASN expression in PGC-1α siRNA-transfected cells. We found that
PGC-1α siRNA-transfected SNU-C4 and HT-29 cells showed decreased
FASN expression (Fig. 1B and D),
suggesting that FASN expression was related to PGC-1α
expression.
FASN expression is regulated through
upregulation of Sp1 and SREBP-1c by PGC-1α
To investigate whether FASN expression was regulated
by PGC-1α, we performed a luciferase assay in SNU-C4 and HT-29
cells that were co-transfected with the PGC-1α expression vector
and a FASN promoter-driven luciferase plasmid. As shown in Fig. 2A, FASN promoter activity was
significantly increased by PGC-1α expression in SNU-C4 and HT-29
cells, by 3.03- and 2.72-fold, respectively (Fig. 2A). We further confirmed that
PGC-1α-siRNA transfection reduced the PGC-1α-induced increase in
FASN promoter activity (Fig.
2B).
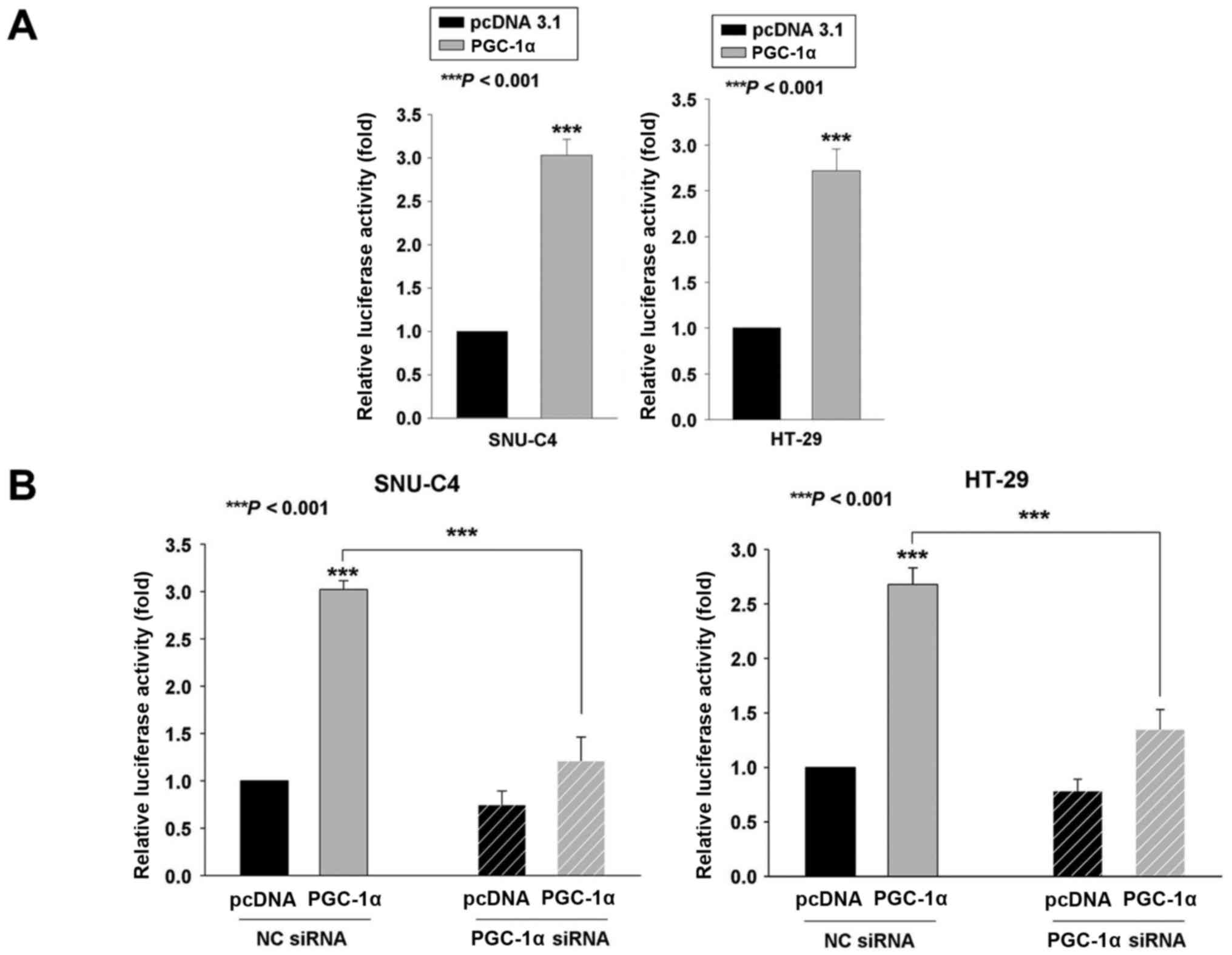 | Figure 2.FASN promoter activity is regulated
through Sp1 and SREBP-1c by PGC-1α. (A) PGC-1α enhanced FASN
promoter activity in SNU-C4 and HT-29 cells. All cell lines were
co-transfected for 24 h with the FASN promoter-driven luciferase
reporter plasmid and Renilla luciferase control vector, as
well as the pcDNA3.1 or PGC-1α expression vector. Results are
presented as normalized relative luciferase activity (n=3). (B)
Enhanced FASN promoter activity was reversed by PGC-1α siRNA
transfection. SNU-C4 and HT-29 cells were transfected for 24 h with
NC siRNA or PGC-1α siRNA. Next, the cells were co-transfected for
24 h with the FASN promoter-driven luciferase reporter plasmid and
Renilla luciferase control vector, as well as the pcDNA3.1
or PGC-1α expression vector. Results are presented as normalized
relative luciferase activity (n=3). Results are the average of
three independent experiments, with statistical significance
measured using a t-test (***P<0.001). (C, left panel) SNU-C4 and
HT-29 cells were transfected with the empty vector (pcDNA 3.1) or
PGC-1α expression vector. (C, right panel) SNU-C4 and HT-29 cells
were transfected with non-specific control (NC) or PGC-1α-specific
siRNAs. At 72 h post-transfection, Sp1 and SREBP-1c protein levels
were analyzed by western blotting using anti-Sp1 or anti-SREBP-1c
antibodies. β-actin was probed for equal protein loading.
Densitometry results are expressed above the bands. (D) Enhanced
FASN promoter activity was reversed by Sp1, SREBP-1c or both Sp1
and SREBP-1c siRNA transfection. SNU-C4 and HT-29 cells were
transfected for 24 h with NC siRNA, Sp1, SREBP-1c or both Sp1 and
SREBP-1c siRNA. Next, the cells were co-transfected for 24 h with
the FASN promoter-driven luciferase reporter plasmid and
Renilla luciferase control vector, as well as the pcDNA3.1
or PGC-1α expression vector. Results are presented as normalized
relative luciferase activity (n=3). Results are the average of
three independent experiments, with statistical significance
measured using a t-test (***P<0.001). |
Previous studies revealed that the promoter activity
of FASN was regulated by binding of Sp1 and SREBP-1c to the FASN
promoter (36). We previously have
shown that the expression of Sp1 was enhanced by PGC-1α expression
(28). In the present study we
observed that PGC-1α expression increased Sp1 and SREBP-1c
expression (Fig. 2C). We also
observed that PGC-1α-siRNA transfection decreased the Sp-1 and
SREBP-1c expression (Fig. 2C). We
evaluated whether the enhanced FASN promoter activity by PGC-1α was
mediated through Sp1 and SREBP-1c using siRNA transfection with Sp1
siRNA, SREBP-1c siRNA or both Sp1 and SREBP-1c siRNA. As shown in
Fig. 2D, the increased FASN
promoter activity was decreased by Sp1 siRNA, SREBP-1c siRNA or
both Sp1 siRNA and SREBP-1c siRNA even with the presence of PGC-1α.
These data revealed that the promoter activity of FASN may be
regulated indirectly through upregulation of Sp1 and SREBP-1c.
FASN knockdown reduces cell
proliferation in SNU-C4 and HT-29 cells
We next evaluated the functional significance of
FASN expression in the growth of SNU-C4 and HT-29 cells. These cell
lines were transfected with FASN shRNA or with NC shRNA, and then
cultured with G418 for 14 days. Next, several colonies were chosen
and amplified. FASN knockdown was detected by western blot analysis
with an antibody against FASN. Compared to the corresponding
control cells, FASN protein levels were, respectively, ~72 and 78%
(SNU-C4) lower in the SNU-C4-FASN shRNA-1 and −3 cells, and 78 and
80% (HT-29) lower in the HT-29-FASN shRNA-2 and −3 cells,
respectively (Fig. 3A).
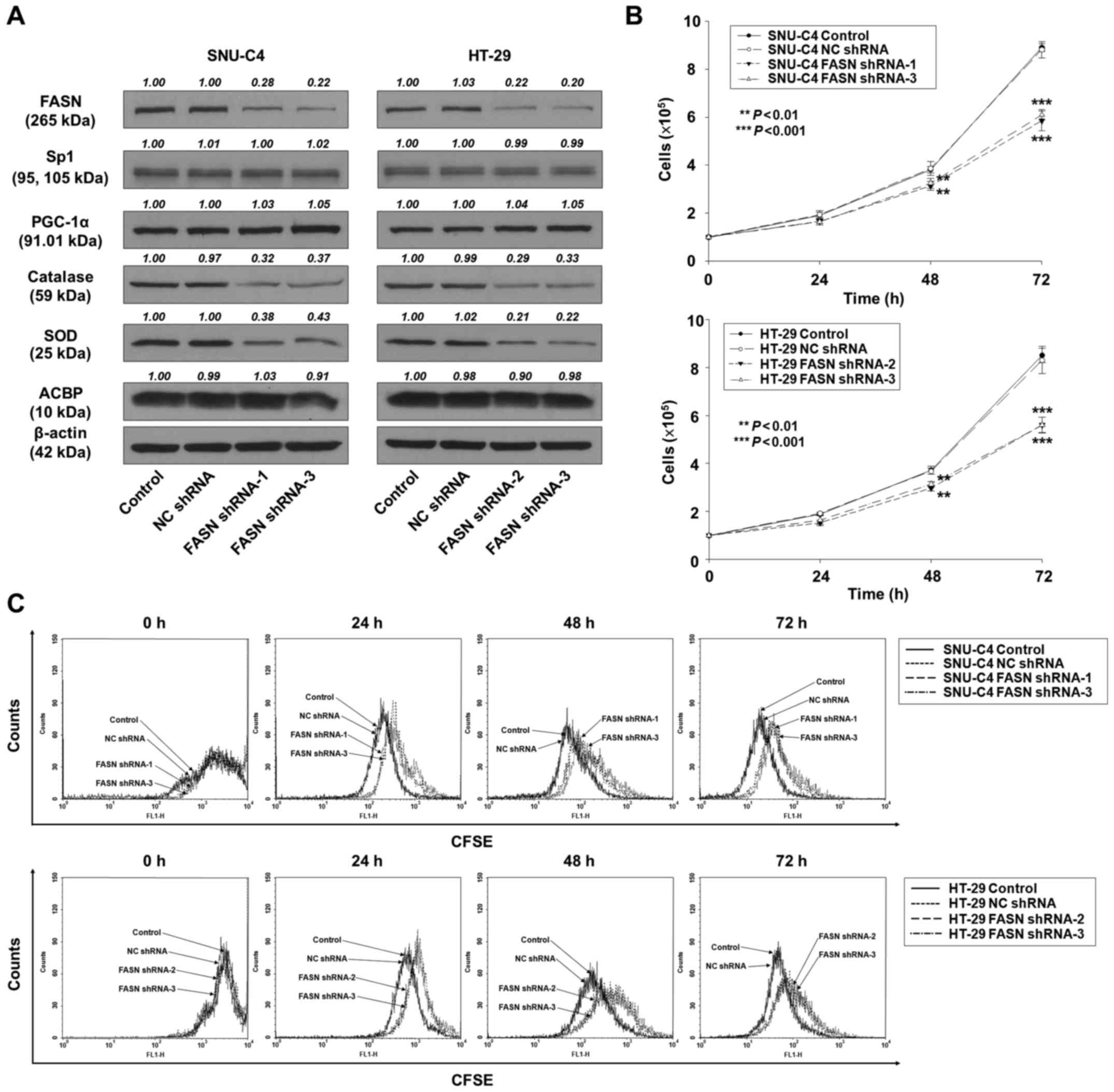 | Figure 3.FASN knockdown significantly
decreases the proliferation of SNU-C4 and HT-29 cells. Using
Lipofectamine, SNU-C4 and HT-29 cells were stably transfected with
no shRNA (control), non-specific control (NC) shRNA, or shRNA for
FASN. (A) Whole-cell lysates were prepared from SNU-C4 and HT-29
cells, and used for western blot analysis to determine FASN, Sp1,
PGC-1α, catalase, SOD and ACBP protein expression. Band densities
were assessed by densitometric analysis. Data are expressed as the
fold change in protein expression normalized to β-actin expression,
with respect to the control and NC shRNA. (B) SNU-C4 and HT-29
cells were stably transfected with FASN shRNA or NC shRNA, and then
studied at 24-h intervals for 72 h after replating. Cell
proliferation was determined by cell counting. **P<0.01 and
***P<0.001, vs. the control or NC shRNA-transfected cells. (C)
CFSE-labeled cells (1×105 cells/well) were incubated
with fresh medium containing 10% FBS for the indicated times. The
samples were analyzed by flow cytometry using a FACScan flow
cytometer. Data were analyzed using CellQuest software (BD
Biosciences). |
To investigate whether FASN knockdown affected cell
growth in human SNU-C4 and HT-29 cells, we assessed cell
proliferation by cell counting and CFSE labeling assays. At 72 h,
the SNU-C4-FASN shRNA-1 and −3 cells exhibited cell numbers that
were, respectively, 32 and 34% lower than that of the
SNU-C4-control cells (Fig. 3B;
P<0.001), and the HT-29-FASN shRNA-2 and −3 cell numbers were,
respectively, 34 and 37% lower than that of the HT-29-control cells
(Fig. 3B; P<0.001). As
aforementioned, CFSE labeling analysis confirmed that FASN
knockdown also decreased cell proliferation in SNU-C4 and HT-29
cells (Fig. 3C). However, the
protein levels of PGC-1α were not downregulated in FASN
shRNA-transfected SNU-C4 and HT-29 cells (Fig. 3A). These data indicated that FASN
acts downstream of PGC-1α in the regulation of cell
proliferation.
FASN downregulation increases
sensitivity to H2O2-induced apoptosis
We previously demonstrated that PGC-1α induced
catalase and SOD, and decreased ROS production, resulting in
decreased sensitivity to H2O2-induced
apoptosis and enhanced cell proliferation (28). In the present study, we evaluated
the role of FASN in regulating sensitivity to
H2O2-induced apoptosis, by assessing the
extent of H2O2-induced apoptosis and ROS
levels in SNU-C4 and HT-29 cells transfected with FASN shRNA or NC
control shRNA. The FASN shRNA-transfected SNU-C4 and HT-29 cells
exhibited a significantly greater extent of
H2O2-induced apoptosis and ROS level compared
to control SNU-C4 and HT-29 cells (Fig.
4B and C). Moreover, the expressions of SOD and catalase
(except PGC-1α, Sp1 and ACBP) were decreased by FASN shRNA
knockdown in SNU-C4 and HT-29 cells (Fig. 4A).
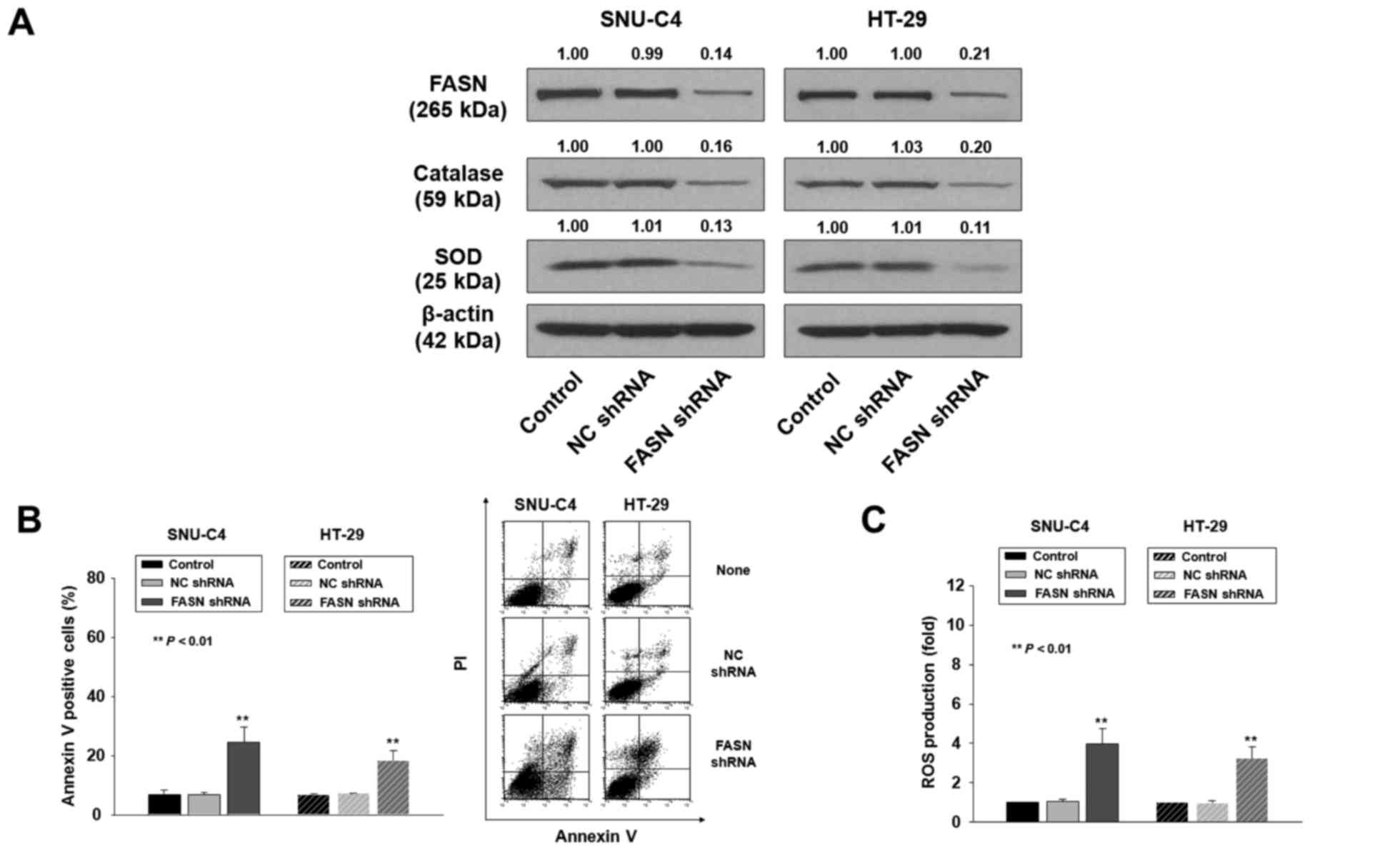 | Figure 4.FASN knockdown increases sensitivity
to H2O2-induced apoptosis. (A) SNU-C4 and
HT-29 cells were stably transfected with no shRNA (control),
non-specific control (NC) shRNA, or FASN shRNA. Whole-cell lysates
were prepared from SNU-C4 and HT-29 cells, and used for western
blot analysis to determine FASN, catalase and SOD expression. Band
densities were assessed by densitometric analysis. Data are
expressed as the fold change in protein expression normalized to
β-actin expression, with respect to the control and NC shRNA. (B,
left panel) Control, NC shRNA-silenced and FASN shRNA-silenced
SNU-C4 cells and control, NC shRNA-silenced, and FASN
shRNA-silenced HT-29 cells were treated for 24 h with 0.5 mM
H2O2, stained with FITC-Annexin V/PI, and
analyzed by flow cytometry. Annexin V-positive cells were
considered apoptotic. (B, right panel) Representative flow
cytometric data of three independent experiments. (C) Control, NC
shRNA-silenced, and FASN shRNA-silenced SNU-C4 cells and control,
NC shRNA-silenced, and FASN shRNA-silenced HT-29 cells were treated
for 24 h with 0.5 mM H2O2, and labeled with
carboxy-H2DCFDA. Then, ROS levels were quantified by flow
cytometry. Data represent the mean ± SD of three independent
experiments; **P<0.01, vs. the control or NC shRNA-transfected
cells. (D-F) SNU-C4 and HT-29 cells were stably transfected with no
shRNA (control), non-specific control (NC) shRNA, or FASN shRNA,
and transfected with pcDNA or FASN cDNA expression vector. (D)
Whole-cell lysates were used for western blot analysis to determine
FASN, catalase and SOD expression. Band densities were assessed by
densitometric analysis. Data are expressed as the fold change in
protein expression normalized to β-actin expression, with respect
to the control and NC shRNA. (E, left panel) Control, NC
shRNA-silenced and FASN shRNA-silenced cells (SNU-C4 and HT-29) and
pcDNA-transfected control, FASN cDNA-transfected and FASN
cDNA-transfected FASN shRNA-silenced cells (SNU-C4 and HT-29) were
treated for 24 h with 0.5 mM H2O2, stained
with FITC-Annexin V/PI, and analyzed by flow cytometry. Annexin
V-positive cells were considered apoptotic. **P<0.01 and
***P<0.001, vs. the control or NC shRNA-transfected cells. (E,
right panel) Representative flow cytometric data of three
independent experiments. (F) Control, NC shRNA-silenced, and FASN
shRNA-silenced cells (SNU-C4 and HT-29) and pcDNA-transfected
control, FASN cDNA-transfected and FASN cDNA-transfected FASN
shRNA-silenced cells (SNU-C4 and HT-29) were treated for 24 h with
0.5 mM H2O2, and labeled with
carboxy-H2DCFDA. Then, the ROS levels were quantified by flow
cytometry. Data represent the mean ± SD of three independent
experiments; ***P<0.001, vs. the control or NC shRNA-transfected
cells. |
These data revealed that FASN acts downstream of
PGC-1α, Sp1 and ACBP in the regulation of ROS-induced apoptosis and
ROS production. Additionally, our results indicated that FASN
protected SNU-C4 and HT-29 cells from oxidative stress, such as
H2O2. Increased susceptibility to ROS-induced
apoptosis may contribute to the decreased cell proliferation
observed following FASN shRNA knockdown in SNU-C4 and HT-29 cells.
To confirm whether FASN expression regulate the expression of
catalase and SOD and ROS-induced apoptosis, FASN shRNA-silenced
SNU-C4 and HT-29 cells were transfected with pReceiver-M13 or
pReceiver-M13-FASN expression vector, and then treated with
H2O2. The results revealed the decreased
expression of catalase and SOD by FASN knockdown was reversed by
FASN expression (Fig. 4D).
Increased ROS production and H2O2-induced
apoptosis by FASN knockdown were also reversed by FASN expression
(Fig. 4E and F). Overall, these
results revealed that FASN enhanced cell proliferation and
decreased H2O2-induced apoptosis through
upregulation of catalase and SOD in SNU-C4 and HT-29 cells.
Discussion
In the present study, we found that FASN expression
and promoter activity were increased by PGC-1α expression in SNU-C4
and HT-29 cells. To confirm that this increased FASN promoter
activity was caused by PGC-1α itself, we performed a knockdown
experiment using PGC-1α siRNA. FASN promoter activity was
significantly decreased by PGC-1α knockdown experiments in SNU-C4
and HT-29 cells. These results provide the first evidence that FASN
promoter activity was enhanced by PGC-1α expression in colorectal
cancer cells. To evaluate the molecular mechanism for increased
FASN promoter activity by PGC-1α, the Sp1 siRNA, SREBP-1c siRNA or
both Sp1 and SREBP-1c siRNA knockdown experiments were performed.
The results revealed that the increased FASN promoter activity by
PGC-1α was decreased by Sp1 siRNA, SREBP-1c siRNA or both Sp1 siRNA
and SREBP-1c siRNA. These data indicated that the enhanced FASN
promoter activity by PGC-1α may be regulated indirectly through Sp1
and SREBP-1c. Despite differences in the utilized cell lines, our
data were similar to previous results demonstrating that PGC-1α
enhances lipogenesis in skeletal muscle through liver X receptor α
(LXRα)-dependent activation of the FASN promoter and by increasing
FASN activity (37). However, we
did not examine whether LXRα was involved in the PGC-1α-mediated
regulation of the FASN promoter. Further studies are required to
evaluate the possible involvement of LXRα in the PGC-1α-mediated
regulation of FASN promoter activity in colorectal cancer
cells.
PGC-1α overexpression enhanced cell proliferation
and tumorigenesis through the increased expression of Sp1 and ACBP
in HEK293 cells, and FASN overexpression reportedly promoted cancer
growth and metastasis (38–41). Increased FASN may confer a cell
survival advantage due to apoptosis resistance, with associated
tumor aggressiveness, increased metastasis and poor prognosis
(7). Previous data revealed that
specific knockdown of either acetyl-CoA carboxylase α (a key enzyme
in fatty acid synthesis) or FASN genes in cancer cells led to
substantially decreased palmitic acid synthesis. Palmitic acid
depletion was associated with apoptosis induction concomitant with
ROS formation and mitochondrial impairment (42). Our present results demonstrated that
FASN expression was decreased by PGC-1α siRNA transfection, and
that FASN knockdown significantly inhibited cell proliferation and
decreased the expression of catalase and SOD, resulting in
increased ROS and H2O2-induced apoptosis in
SNU-C4 and HT-29 cells. These findings revealed that PGC-1α loss
was protective against carcinogenesis, and that PGC-1α coordinately
regulated mitochondrial and fatty acid metabolism to promote tumor
growth (43).
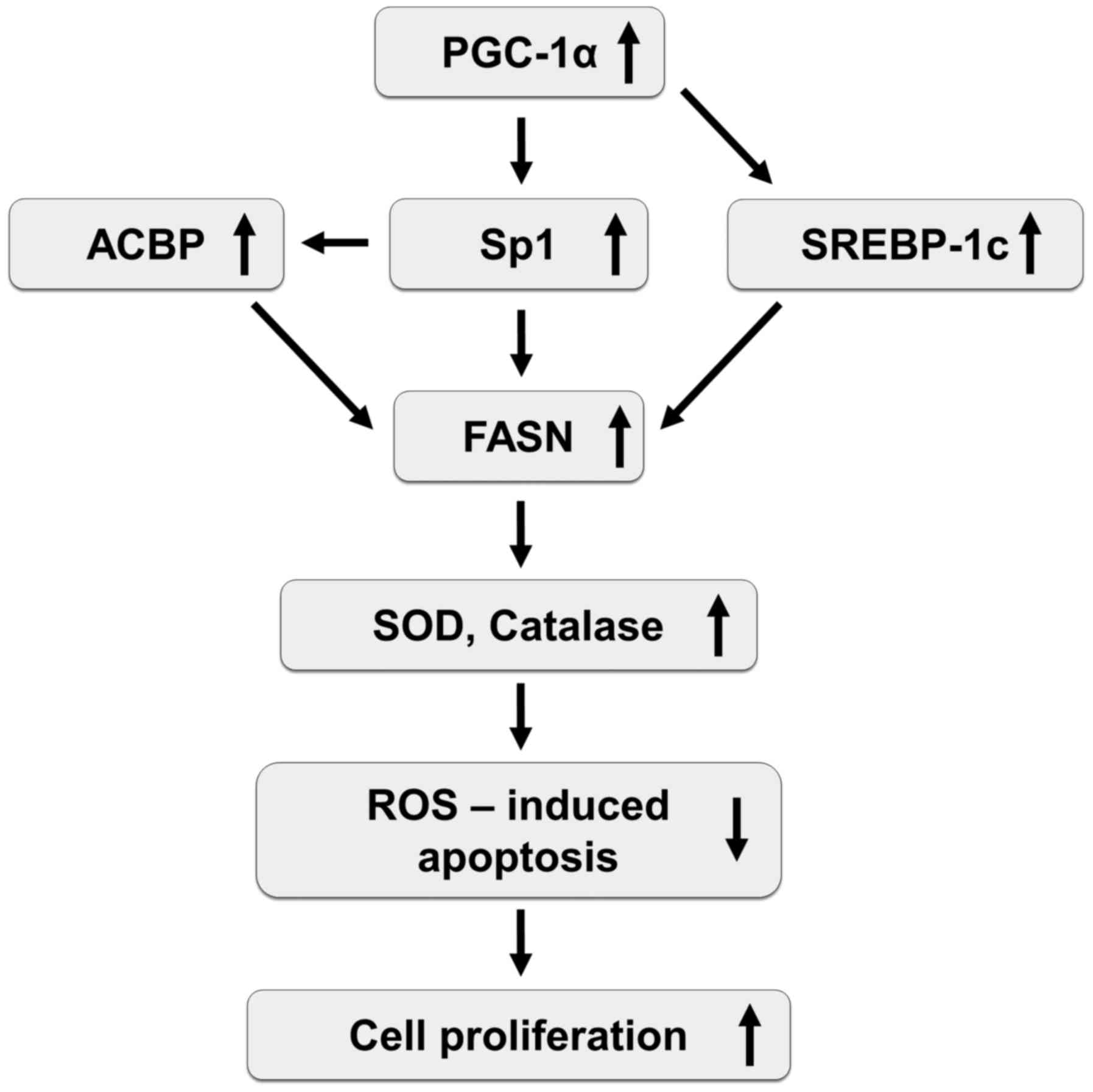
In conclusion, the present study revealed that FASN
expression was indirectly regulated through upregulation of Sp1 and
SREBP-1c by PGC-1α, and may contribute to enhanced cell
proliferation due to increased antioxidant enzyme expression and
resistance to ROS-induced apoptosis. Based on our results, we
suggest hypothetical molecular mechanisms behind the enhancement of
cell proliferation by FASN and PGC-1α (Fig. 5). However, further studies, in
greater detail are needed to clarify the mechanisms involved in the
regulation of cell proliferation and tumorigenesis. Our results
revealed that PGC-1α and FASN may be useful targets for colorectal
cancer treatment. To determine the clinical relevance of the
PGC-1α-mediated regulation of FASN expression in these cell lines,
it is necessary to investigate the correlation between PGC-1α and
FASN expression in specimens from human colorectal cancer
patients.
Acknowledgements
The present study was supported by the National
Research Foundation of Korea (NRF), funded by the Korean Government
(MSIP) (no. 2016R1A5A2007009), and by the Basic Science Research
Program through the National Research Foundation of Korea (NRF)
funded by the Ministry of Science, ICT and Future Planning
(2017R1A2B4011428).
References
|
1
|
Little JL and Kridel SJ: Fatty acid
synthase activity in tumor cells. Subcell Biochem. 49:169–194.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kusakabe T, Maeda M, Hoshi N, Sugino T,
Watanabe K, Fukuda T and Suzuki T: Fatty acid synthase is expressed
mainly in adult hormone-sensitive cells or cells with high lipid
metabolism and in proliferating fetal cells. J Histochem Cytochem.
48:613–622. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gansler TS, Hardman W III, Hunt DA,
Schaffel S and Hennigar RA: Increased expression of fatty acid
synthase (OA-519) in ovarian neoplasms predicts shorter survival.
Hum Pathol. 28:686–692. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takahiro T, Shinichi K and Toshimitsu S:
Expression of fatty acid synthase as a prognostic indicator in soft
tissue sarcomas. Clin Cancer Res. 9:2204–2212. 2003.PubMed/NCBI
|
|
5
|
Rossi S, Graner E, Febbo P, Weinstein L,
Bhattacharya N, Onody T, Bubley G, Balk S and Loda M: Fatty acid
synthase expression defines distinct molecular signatures in
prostate cancer. Mol Cancer Res. 1:707–715. 2003.PubMed/NCBI
|
|
6
|
Menendez JA and Lupu R: Fatty acid
synthase and the lipogenic phenotype in cancer pathogenesis. Nat
Rev Cancer. 7:763–777. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Migita T, Ruiz S, Fornari A, Fiorentino M,
Priolo C, Zadra G, Inazuka F, Grisanzio C, Palescandolo E, Shin E,
et al: Fatty acid synthase: A metabolic enzyme and candidate
oncogene in prostate cancer. J Natl Cancer Inst. 101:519–532. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Piyathilake CJ, Frost AR, Manne U, Bell
WC, Weiss H, Heimburger DC and Grizzle WE: The expression of fatty
acid synthase (FASE) is an early event in the development and
progression of squamous cell carcinoma of the lung. Hum Pathol.
31:1068–1073. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Swinnen JV, Roskams T, Joniau S, Van
Poppel H, Oyen R, Baert L, Heyns W and Verhoeven G: Overexpression
of fatty acid synthase is an early and common event in the
development of prostate cancer. Int J Cancer. 98:19–22. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Innocenzi D, Alò PL, Balzani A, Sebastiani
V, Silipo V, La Torre G, Ricciardi G, Bosman C and Calvieri S:
Fatty acid synthase expression in melanoma. J Cutan Pathol.
30:23–28. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Omura S: The antibiotic cerulenin, a novel
tool for biochemistry as an inhibitor of fatty acid synthesis.
Bacteriol Rev. 40:681–697. 1976.PubMed/NCBI
|
|
12
|
Pizer ES, Jackisch C, Wood FD, Pasternack
GR, Davidson NE and Kuhajda FP: Inhibition of fatty acid synthesis
induces programmed cell death in human breast cancer cells. Cancer
Res. 56:2745–2747. 1996.PubMed/NCBI
|
|
13
|
Zhao W, Kridel S, Thorburn A, Kooshki M,
Little J, Hebbar S and Robbins M: Fatty acid synthase: A novel
target for antiglioma therapy. Br J Cancer. 95:869–878. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kuhajda FP, Pizer ES, Li JN, Mani NS,
Frehywot GL and Townsend CA: Synthesis and antitumor activity of an
inhibitor of fatty acid synthase. Proc Natl Acad Sci USA.
97:3450–3454. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Alli PM, Pinn ML, Jaffee EM, McFadden JM
and Kuhajda FP: Fatty acid synthase inhibitors are chemopreventive
for mammary cancer in neu-N transgenic mice. Oncogene.
24:39–46. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang HQ, Altomare DA, Skele KL, Poulikakos
PI, Kuhajda FP, Di Cristofano A and Testa JR: Positive feedback
regulation between AKT activation and fatty acid synthase
expression in ovarian carcinoma cells. Oncogene. 24:3574–3582.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thupari JN, Pinn ML and Kuhajda FP: Fatty
acid synthase inhibition in human breast cancer cells leads to
malonyl-CoA-induced inhibition of fatty acid oxidation and
cytotoxicity. Biochem Biophys Res Commun. 285:217–223. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Barger JF and Plas DR: Balancing
biosynthesis and bioenergetics: Metabolic programs in oncogenesis.
Endocr Relat Cancer. 17:R287–R304. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Patel AV, Johansson G, Colbert MC,
Dasgupta B and Ratner N: Fatty acid synthase is a metabolic
oncogene targetable in malignant peripheral nerve sheath tumors.
Neuro Oncol. 17:1599–1608. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bandyopadhyay S, Pai SK, Watabe M, Gross
SC, Hirota S, Hosobe S, Tsukada T, Miura K, Saito K, Markwell SJ,
et al: FAS expression inversely correlates with PTEN level in
prostate cancer and a PI 3-kinase inhibitor synergizes with FAS
siRNA to induce apoptosis. Oncogene. 24:5389–5395. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang F and Du G: Dysregulated lipid
metabolism in cancer. World J Biol Chem. 3:167–174. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Finck BN and Kelly DP: PGC-1 coactivators:
Inducible regulators of energy metabolism in health and disease. J
Clin Invest. 116:615–622. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang WG, Douglas-Jones A and Mansel RE:
Expression of peroxisome-proliferator activated receptor-γ
(PPARgamma) and the PPARgamma co-activator, PGC-1, in human breast
cancer correlates with clinical outcomes. Int J Cancer.
106:752–757. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Watkins G, Douglas-Jones A, Mansel RE and
Jiang WG: The localisation and reduction of nuclear staining of
PPARγ and PGC-1 in human breast cancer. Oncol Rep. 12:483–488.
2004.PubMed/NCBI
|
|
25
|
Feilchenfeldt J, Bründler MA, Soravia C,
Tötsch M and Meier CA: Peroxisome proliferator-activated receptors
(PPARs) and associated transcription factors in colon cancer:
Reduced expression of PPARgamma-coactivator 1 (PGC-1). Cancer Lett.
203:25–33. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y, Ba Y, Liu C, Sun G, Ding L, Gao
S, Hao J, Yu Z, Zhang J, Zen K, et al: PGC-1α induces apoptosis in
human epithelial ovarian cancer cells through a PPARγ-dependent
pathway. Cell Res. 17:363–373. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shiota M, Yokomizo A, Tada Y, Inokuchi J,
Tatsugami K, Kuroiwa K, Uchiumi T, Fujimoto N, Seki N and Naito S:
Peroxisome proliferator-activated receptor γ coactivator-1α
interacts with the androgen receptor (AR) and promotes prostate
cancer cell growth by activating the AR. Mol Endocrinol.
24:114–127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shin SW, Yun SH, Park ES, Jeong JS, Kwak
JY and Park JI: Overexpression of PGC-1α enhances cell
proliferation and tumorigenesis of HEK293 cells through the
upregulation of Sp1 and Acyl-CoA binding protein. Int J Oncol.
46:1328–1342. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vock C, Biedasek K, Boomgaarden I, Heins
A, Nitz I and Döring F: ACBP knockdown leads to down-regulation of
genes encoding rate-limiting enzymes in cholesterol and fatty acid
metabolism. Cell Physiol Biochem. 25:675–686. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee MY, Moon JS, Park SW, Koh YK, Ahn YH
and Kim KS: KLF5 enhances SREBP-1 action in androgen-dependent
induction of fatty acid synthase in prostate cancer cells. Biochem
J. 417:313–322. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Quah BJ, Warren HS and Parish CR:
Monitoring lymphocyte proliferation in vitro and in vivo with the
intracellular fluorescent dye carboxyfluorescein diacetate
succinimidyl ester. Nat Protoc. 2:2049–2056. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shin SW, Seo CY, Han H, Han JY, Jeong JS,
Kwak JY and Park JI: 15d-PGJ2 induces apoptosis by reactive oxygen
species-mediated inactivation of Akt in leukemia and colorectal
cancer cells and shows in vivo antitumor activity. Clin Cancer Res.
15:5414–5425. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ishimura N, Amano Y, Sanchez-Siles AA,
Fukuhara H, Takahashi Y, Uno G, Tamagawa Y, Mishima Y, Yuki T,
Ishihara S, et al: Fatty acid synthase expression in Barrett's
esophagus: Implications for carcinogenesis. J Clin Gastroenterol.
45:665–672. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wellberg EA, Rudolph MC, Lewis AS,
Padilla-Just N, Jedlicka P and Anderson SM: Modulation of tumor
fatty acids, through overexpression or loss of thyroid hormone
responsive protein spot 14 is associated with altered growth and
metastasis. Breast Cancer Res. 16:4812014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yellen P and Foster DA: Inhibition of
fatty acid synthase induces pro-survival Akt and ERK signaling in
K-Ras-driven cancer cells. Cancer Lett. 353:258–263. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xiong S, Chirala SS and Wakil SJ: Sterol
regulation of human fatty acid synthase promoter I requires nuclear
factor-Y- and Sp-1-binding sites. Proc Natl Acad Sci USA.
97:3948–3953. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Summermatter S, Baum O, Santos G, Hoppeler
H and Handschin C: Peroxisome proliferator-activated receptor
{gamma} coactivator 1{alpha} (PGC-1{alpha}) promotes skeletal
muscle lipid refueling in vivo by activating de novo lipogenesis
and the pentose phosphate pathway. J Biol Chem. 285:32793–32800.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zaytseva YY, Elliott VA, Rychahou P,
Mustain WC, Kim JT, Valentino J, Gao T, O'Connor KL, Neltner JM,
Lee EY, et al: Cancer cell-associated fatty acid synthase activates
endothelial cells and promotes angiogenesis in colorectal cancer.
Carcinogenesis. 35:1341–1351. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zaytseva YY, Rychahou PG, Gulhati P,
Elliott VA, Mustain WC, O'Connor K, Morris AJ, Sunkara M, Weiss HL,
Lee EY, et al: Inhibition of fatty acid synthase attenuates
CD44-associated signaling and reduces metastasis in colorectal
cancer. Cancer Res. 72:1504–1517. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sounni NE, Cimino J, Blacher S, Primac I,
Truong A, Mazzucchelli G, Paye A, Calligaris D, Debois D, De Tullio
P, et al: Blocking lipid synthesis overcomes tumor regrowth and
metastasis after antiangiogenic therapy withdrawal. Cell Metab.
20:280–294. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Seguin F, Carvalho MA, Bastos DC, Agostini
M, Zecchin KG, Alvarez-Flores MP, Chudzinski-Tavassi AM, Coletta RD
and Graner E: The fatty acid synthase inhibitor orlistat reduces
experimental metastases and angiogenesis in B16-F10 melanomas. Br J
Cancer. 107:977–987. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chajès V, Cambot M, Moreau K, Lenoir GM
and Joulin V: Acetyl-CoA carboxylase alpha is essential to breast
cancer cell survival. Cancer Res. 66:5287–5294. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bhalla K, Hwang BJ, Dewi RE, Ou L, Twaddel
W, Fang HB, Vafai SB, Vazquez F, Puigserver P, Boros L, et al:
PGC1α promotes tumor growth by inducing gene expression programs
supporting lipogenesis. Cancer Res. 71:6888–6898. 2011. View Article : Google Scholar : PubMed/NCBI
|