Introduction
Glucose is the energy source and the important
metabolic intermediate in mammalian cells. Under a condition of
sufficient oxygen, glucose is metabolized into H2O and
CO2 via the Krebs cycle to generate abundant ATP to
satisfy energy requirements. Different from normal cells, in tumor
cells glucose is converted to pyruvate and lactate even in the
presence of oxygen, which is termed as aerobic glycolysis or the
Warburg effect (1). Increased
glucose consumption and glycolytic activity are important hallmarks
of cancer cells (2). Although tumor
glycolysis is not efficient in terms of generating ATP, it supplies
a large number of intermediate products for the synthesis of
nucleotides, amino acids and lipids which are required for cell
proliferation. Moreover, the accumulation of lactate in tumor
tissues results in an acidic microenvironment which enhances
chemotherapy resistance, tumor migration and metastasis (3). Owing to the importance of tumor
glycolysis, increasing attention has been given on how to interfere
with this process of cancer cells to provide a promising
therapeutic strategy. The first step of glucose metabolism is the
conversion to glucose-6-phosphate (G-6P), which is irreversible and
is mainly mediated by hexokinases (HKs). To date, four isoforms of
HK (HK1-4) have been identified in mammalian tissue. Among all HKs,
hexokinase 2 (HK2) upregulation is a major contributor to the
elevation of glycolysis. It has been found that HK2 is abnormally
expressed in various types of cancers, for instance gastric
(4), breast (5) and colorectal cancer (6) and hepatocellular carcinoma (7), and its expression levels are closely
associated with tumor grade, prognosis and mortality (6,8,9).
In contrast with small molecules developed through
rational chemical design, natural products have more potential to
be applied to tumor chemotherapy owing to their structural
diversity. Recently, numerous natural compounds have been
demonstrated to exert antitumor effects by inhibiting cell growth,
promoting cell death and suppressing angiogenesis (10–12).
Licochalcone A (LicA) (Fig. 1A) is
a characteristic chalcone derived from liquorice, which is also
named as ‘Gancao’ and used as a Traditional Chinese Medicine for
many generations. LicA was reported to possess different biological
activities, including anti-oxidative (13), anti-inflammatory (14), antiviral and antimicrobial
activities (15). Recently,
increasing evidence has demonstrated that LicA exhibits profound
antitumor activities in various types of cancers, such as breast
(16), non-small cell lung
(17) and cervical cancer.
Induction of cell apoptosis, cell cycle arrest and autophagy
(16,18,19),
inhibition of migration and metastasis (20), and blockade of angiogenesis in tumor
tissues (21) have been identified
to be the underlying mechanisms.
Although antitumor activities of LicA have been
verified, its effect on tumor glycolysis remains largely unknown.
In the present study, we investigated the effect of LicA on tumor
glycolysis in gastric cancer cells as well as the underlying
mechanisms. The results demonstrated that LicA exhibited
substantial activities against tumor glycolysis by reducing HK2
expression. Further investigation illustrated that the decrease in
HK2 by LicA was due to the blockade of the Akt signaling
pathway.
Materials and methods
Cell lines and reagents
Gastric cancer cell lines MKN45 and SGC7901 and the
gastric epithelial cell GES-1 were purchased from the Chinese
Committee of Type Culture Collection Cell Bank, Chinese Academy of
Sciences (Shanghai, China). MKN45 and SGC7901 cells were cultured
in Dulbecco's modified Eagle's medium (DMEM) containing 10% FBS and
1% antibiotics. The GES-1 cells were cultured in RPMI-1640 medium
containing 10% FBS and 1% antibiotics. LicA and specific signaling
pathway inhibitors including LY294002, PD98059, Bay11-7082 and
parthenolide were purchased from Selleck (Shanghai, China). Primary
anti-HK2 (#2867), Glut1 (#12939), PKM2 (#4053), LDHA (#3582), p-Akt
(#4060), p-ERK1/2 (#4370), p-p65 (#3031), p-IκBα (#9246), p-S6
(#4858), p-GSK3β (#12456), Akt1 (#75692), MCL-1 (#5453), BCL2
(#2870), BCL-XL (#2764), cleaved caspase-3 (#9664), cleaved PARP
(#5625) antibodies and secondary antibodies anti-rabbit IgG HRP
(#7074) and anti-mouse IgG HRP (#7076) were products of Cell
Signaling Technology Inc. (Beverly, MA, USA). Anti-β-actin antibody
(A5316) was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Myr-Akt1 plasmid was purchased from Addgene (Cambridge, MA, USA).
HK2 (ORF004940) construct was a product of Applied Biological
Materials (ABM) Inc. (Richmond, BC, Canada).
Lipofectamine® 2000 was purchased from Invitrogen
(Carlsbad, CA, USA). Annexin V-FITC and propidium iodide were
products of Biolegend (San Diego, CA, USA).
Cell proliferation assay
MKN45 or SGC7901 cells in logarithmic growth phase
(3×103/well) were seeded into 96-well plate. Twenty-four
hours later, the cells were treated with various concentrations of
LicA. At different time points (24, 48 or 72 h), CellTiter96
Aqueous One Solution (Promega Corp., Madison, WI, USA) (20 µl/well)
was added to a 96-well plate and the cell viability was assessed
according to the manufacturer's protocol.
Clonogenic survival assay
Cells cultured in 10-cm plastic dishes were treated
with different concentrations of LicA for 24 h, and the cells were
harvested and reseeded into a 6-well plate in duplicate at the
appropriate density. The cells were cultured for 1–3 weeks until
the colonies with substantially good size (minimum 50 cells per
colony) were formed in the control group. After washing with PBS,
the colonies were fixed with the fixation solution (methanol:acetic
acid 3:1) and then stained with 0.5% crystal violet for 2 h at room
temperature. The crystal violet was carefully removed by immersing
the plates in tap water repeatedly and the plates were air-dried.
The number of colonies was photographed and counted.
Western blot analysis
After the treatment of LicA, gastric cancer cells
were lysed with RIPA lysis buffer containing protease cocktail
(Roche, Mannheim, Germany) on ice for 30 min. The cell lysate was
harvested and centrifugated at 12,000 × g for 5 min, and the
supernatant was collected. The protein concentrations were
determined by the Bradford assay (Bio-Rad, Philadelphia, PA, USA).
Twenty micrograms per sample was subjected to SDS-PAGE and then
transferred onto polyvinylidene difluoride membranes (Millipore,
Billerica, MA, USA). After blocking the non-specific binding site
on the membrane with 5% non-fat milk solution, the membranes were
incubated with specific primary antibodies at a dilution of 1:1,000
at 4°C overnight. After washing three times with TBS-Tween-20, the
membranes were incubated with HRP-conjugated secondary antibody at
a dilution of 1:2,000 at room temperature for 1 h, and then the
bands on the membrane were visualized using an enhanced
chemiluminescence reagent (Thermo Fisher Scientific, Inc., Waltham,
MA, USA).
Glucose uptake and lactate production
measurement
Gastric cancer cells (5×105/well) were
plated in 6-well tissue culture plate and cultured overnight. Then,
the culture medium was discarded and cells were incubated with
fresh medium containing different concentrations of LicA for 12 h.
The Automatic Biochemical Analyzer (7170A, Hitachi, Tokyo, Japan)
was used for the measurement of the levels of glucose and lactate
in the culture medium. Protein concentration per sample acted as
the control to normalize the relative glucose consumption and
lactate production rate.
Flow cytometry
After LicA treatment, MKN45 cells and the cell
culture medium were collected and centrifugated. After washing with
PBS, the cell pellets were resuspended and stained with Annexin
V-FITC and propidium iodide according to the manufacturer's
instructions, and then the stained cells were subjected to flow
cytometry. The results were analyzed and quantified by a flow
cytometer (BD Biosciences, San Jose, CA, USA).
Cell transfection
For Myr-Akt1 or pORF-HK2 transfection, HCC cells
were seeded into a 6-well culture plate and grown to 70–90%
confluence. Before transfection, the culture medium was replaced
with 1.5 ml fresh medium without fetal bovine serum. Plasmid DNA
(2.5 µg) and Lipofectamine 2000 reagent (7.5 µl) were diluted with
150 µl Opti-MEM medium respectively and incubated at room
temperature for 5 min. The diluted DNA and transfection reagent
were mixed and incubated for 10 mins avoiding light, then 250 µl
mixture was added per well and the culture medium was replaced with
fresh medium with fetal bovine serum after 4–6 h. Forty-eight hours
later, the transfected cells were used for further studies.
Xenograft mouse model
In accordance with the protocol approved by the
Institutional Animal Care and Use Committee, BALB/ca nude mice
maintained under specific pathogen-free (SPF) conditions were
subcutaneously injected with MKN45 cells (2×106
cells/mice). Once the tumor was formed and the volume was ~50
mm3, the mice were randomly assigned into a vehicle and
experimental group. The vehicle group received 0.2 ml sterile PBS
solution, and the experimental group was treated with 10 mg/kg LicA
by i.p. injection daily. The tumor volume (V) was measured twice
per week with microcalipers and was calculated as V= (length ×
width2)/2. At the end of the experiment, the mice were
sacrificed and the tumors were removed.
Immunohistochemical staining
Tumor tissues isolated from the mice were embedded
in paraffin and cut into 5-µm sections. After dewaxing in xylene
and hydration in serial ethanol, the slides were boiled in sodium
citrate buffer (10 mmol/l, pH 6.0) for 10 min to expose antigens.
To block endogenous peroxidase, the slides were treated with 3%
H2O2 for 10 min. After incubation with goat
serum at room temperature for 1 h, the slides were incubated with
the anti-HK2 (1:100) or anti-Ki-67 (1:200) antibody respectively at
4°C in a humidified chamber overnight. Following washing with PBS
three times, the slides were hybridized with biotinylated goat
anti-rabbit secondary antibody at room temperature for 30 min.
After washing with PBS, the sections were probed with
HRP-conjugated streptavidin and then visualized with DAB solution.
After counterstaining with Harris' hematoxylin, the slides were
dehydrated and mounted. The intensity was analyzed by Image-Pro
PLUS (v.6) and ImageJ (NIH) software programs.
Statistical analysis
SPSS software (version 13.0; IBM SPSS, Armonk, NY,
USA) was used for statistical analysis. Student's t-test or one-way
ANOVA was adopted to evaluate statistical significance. P<0.05
indicated a statistically significant difference.
Results
LicA suppresses gastric cancer cell
proliferation in vitro
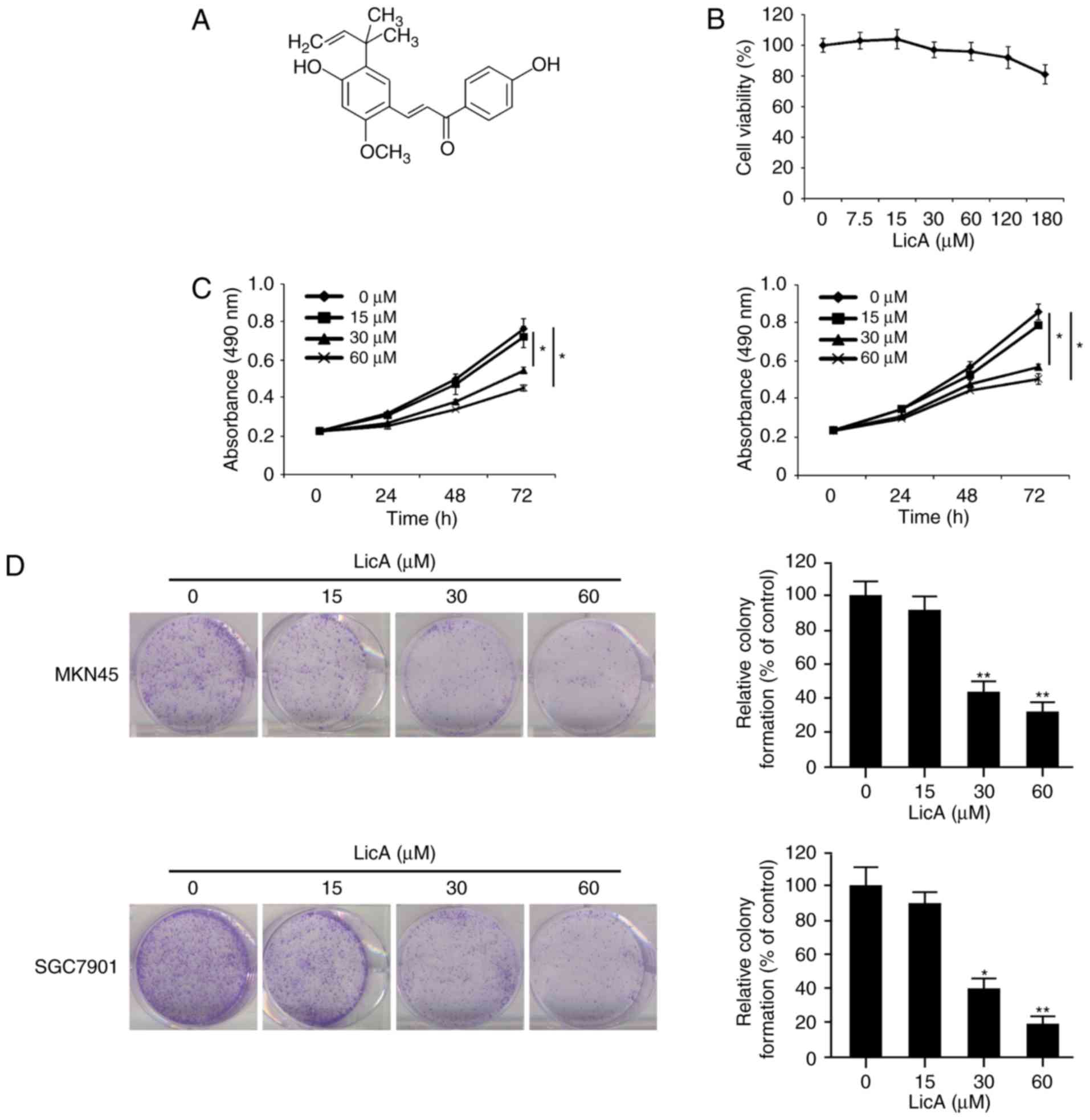
Firstly, we tested the effect of LicA on normal
gastric epithelium cell line GES-1. The results showed that LicA
had no obvious cytotoxicity at concentrations ≤180 µM (Fig. 1B). We next examined the
anti-proliferative activity of LicA in gastric cancer cells in
vitro. As shown in Fig. 1C, at
the low concentration (15 µM), no obvious inhibitory effect was
observed. With the increase in concentration (30–60 µM) and the
extension of treatment time (48–72 h), cell proliferation in the
MKN45 and SGC7901 cells was significantly inhibited. The
IC50 of LicA in MKN45 and SGC7901 cells was 63.57 and
55.56 µM respectively (data not shown). Beyond that, the effect of
LicA on clonogenic survival was also investigated. The incubation
of LicA resulted in the decrease in colonies formed in the agar,
and the number of clones was substantially decreased in a
dose-dependent manner. At 60 µM, little colonies were formed after
exposure to LicA (Fig. 1D). All
these data demonstrated that LicA exerted a profound antitumor
activity in gastric cancer cells in vitro.
LicA inhibits tumor glycolysis in
gastric cancer cells via reducing HK2
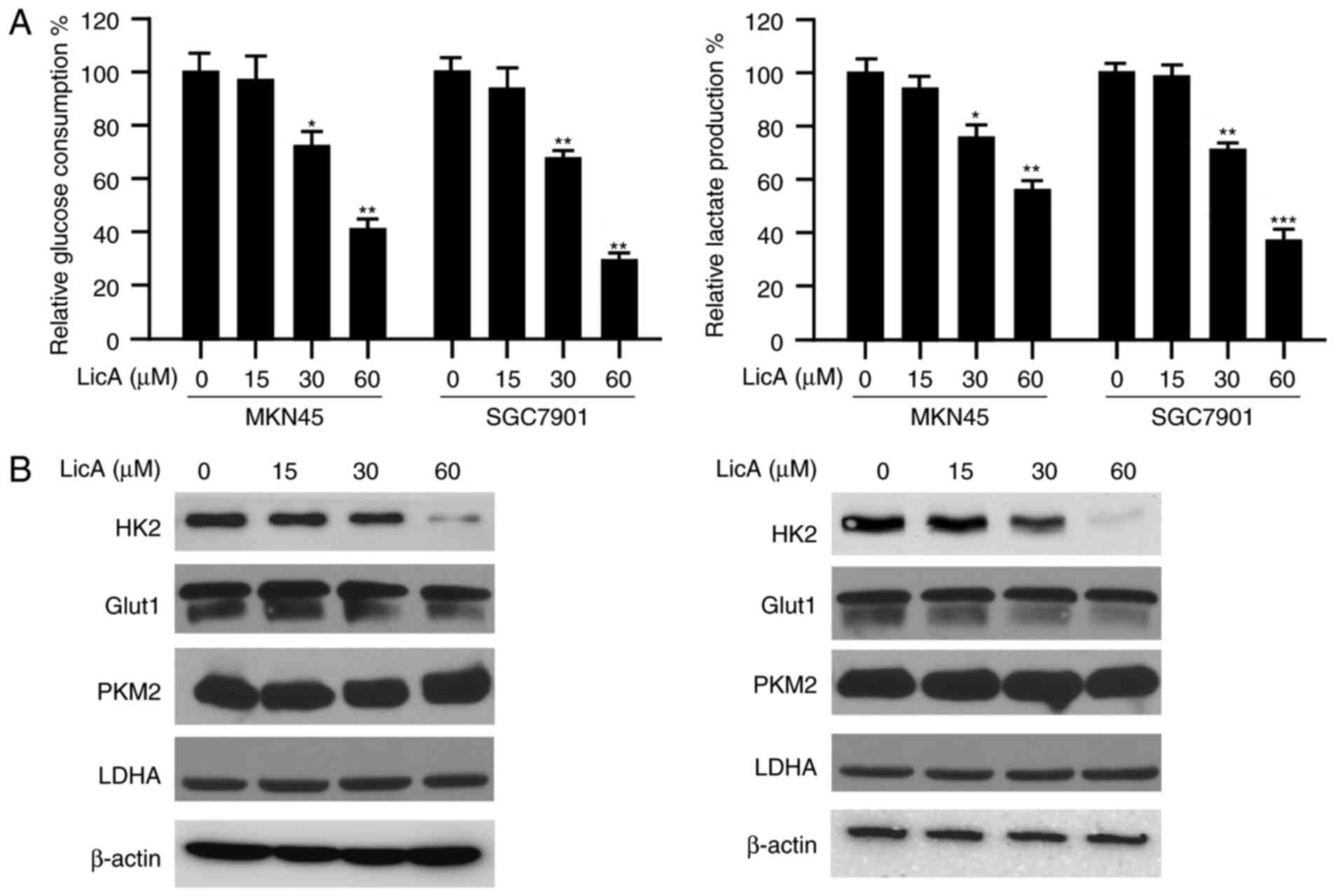
In order to study the effect of LicA on tumor
glycolysis, the amount of glucose consumption in gastric cancer
cells was determined. As shown in Fig.
2A, after LicA treatment, the glucose consumed by MKN45 and
SGC7901 cells was significantly decreased dose-dependently. At the
high concentration of 60 µM, ~50% inhibition was observed.
Accompanied by the reduction of glucose absorption, the amount of
lactate secreted by gastric cancer cells was also decreased
significantly. Furthermore, we tested the effect of LicA on key
glycolytic enzymes and the results demonstrated that the expression
of HK2 was decreased by LicA. The expression of other glycolytic
enzymes, such as GLUT1, PKM2 and LDHA, had no obvious change
(Fig. 2B). All these data imply
that HK2 is involved in the suppression of glycolysis mediated by
LicA.
Akt signaling pathway is involved in
the regulation of HK2 expression by LicA
To confirm which signaling pathway is engaged in the
regulation of HK2 expression, the effect of LicA on the main
signaling pathways in gastric cancer cells was investigated. As
demonstrated, several signaling pathways in gastric cancer, such as
ERK, Akt and NF-κB were blocked by LicA dose-dependently (Fig. 3A). To further validate the
inhibition of these pathways, we examined the effect of LicA on
EGF-induced activation of ERK and Akt, as well as TNF-α-induced
NF-κB activation. As shown in Fig. 3B
and C, the activation of ERK, Akt and NF-κB signaling was
suppressed by LicA dose and time-dependently. By using different
specific signaling pathway inhibitors to treat gastric cancer cells
and observe the change in HK2 expression, we identified that
LY294002, a specific PI3-K inhibitor, demonstrated a similar effect
on HK2 as LicA, implying that the Akt signaling pathway may be
involved in the mediation of HK2 expression (Fig. 3D).
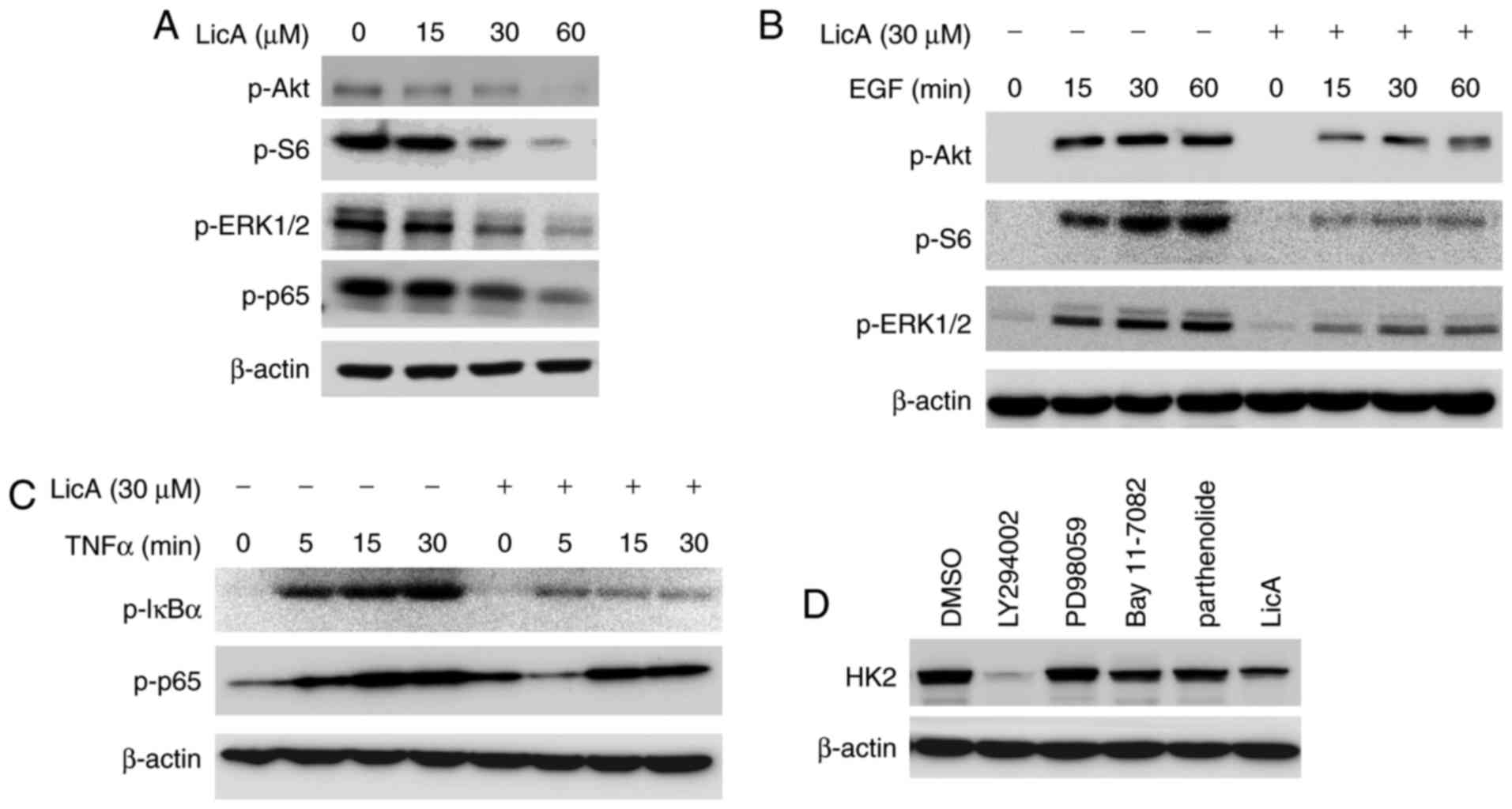 | Figure 3.LicA decreases HK2 expression by
blocking the Akt signaling pathway. (A) LicA inhibited the
signaling pathways in gastric cancer cells. MKN45 cells were
incubated with different concentrations of LicA for 24 h and the
effect on phosphorylation of the indicated proteins was examined.
(B and C) LicA blocked EGF-induced activation of ERK, Akt and S6
(B), and TNF-α-induced activation of the NF-κB signaling pathway
(C). MKN45 cells were starved overnight and then treated with 30 µM
LicA for 2 h. After stimulation with 100 ng/ml EGF or 30 ng/ml
TNF-α for the indicated times, cell lysates were collected and
western blotting was used to examine the indicated protein. (D)
MKN45 cells were treated with specific PI3-K inhibitor (LY294002),
MEK inhibitor (PD98059), NF-κB inhibitor (Bay11-7082) and
parthenolide, respectively, for 12 h, and cell lysates were probed
with anti-HK2 antibodies. LicA, licochalcone. HK2, hexokinase
2. |
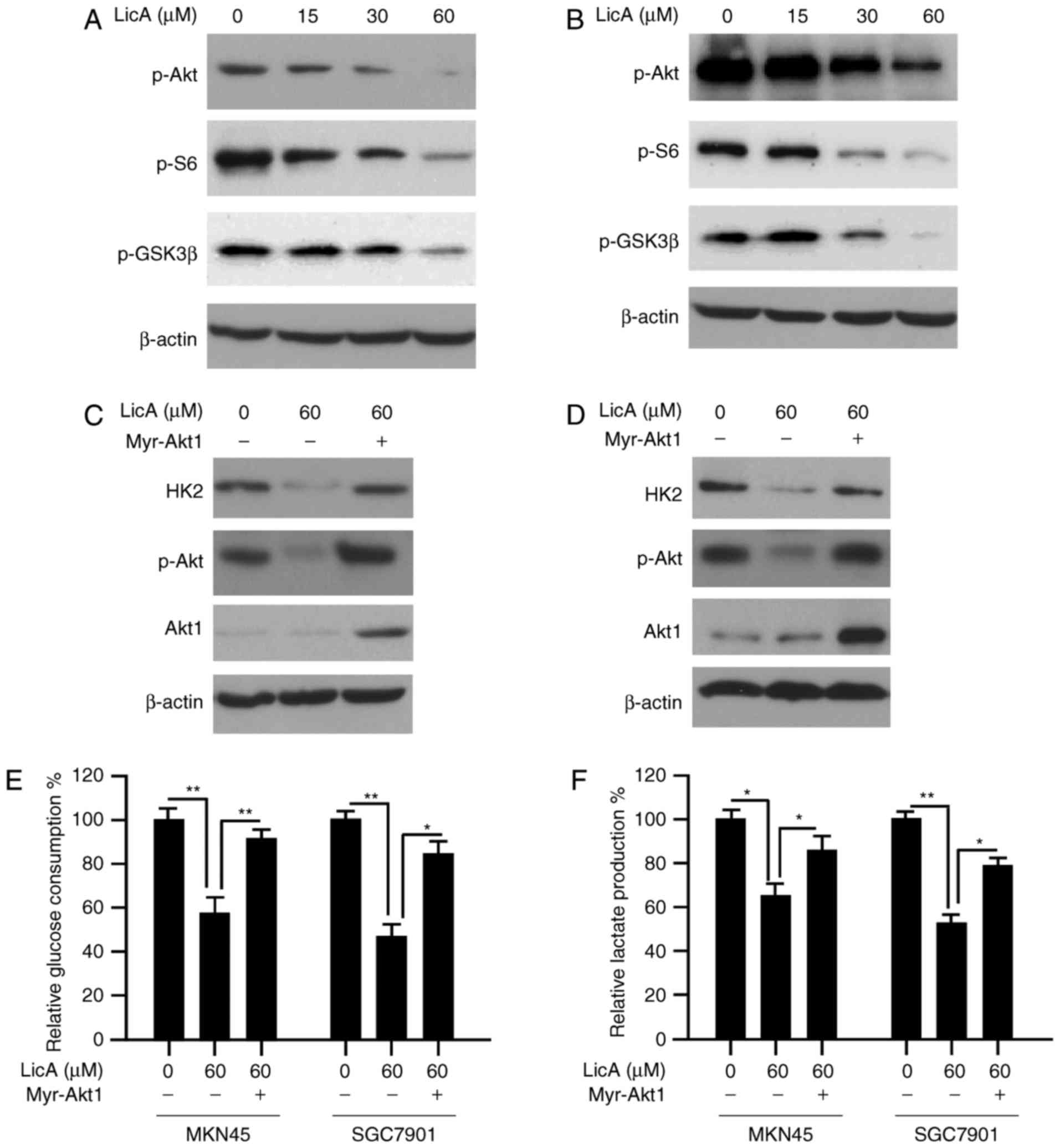
Hyperactivation of Akt in gastric
cancer cells attenuates LicA-mediated glycolysis suppression
Based on the results shown above, the HK2 decrease
caused by LicA may be attributed to the blockade of Akt activation,
thus, we tested the effect of LicA on the Akt signaling pathway.
After LicA treatment, along with the inactivation of Akt, the
phosphorylation of S6 and GSK3β, which are the main downstream
signaling of Akt, were inhibited dose-dependently (Fig. 4A and B). To illustrate the
importance of Akt in the regulation of HK2 expression,
constitutively activated Akt1 (myr-Akt1) was transfected into MKN45
and SGC7901 cells. As expected, with the recovery of Akt activity
in gastric cancer cells, HK2 reduction caused by LicA was notably
reversed (Fig. 4C and D). Moreover,
glucose consumption and lactate production in myr-Akt1 transfected
cells were also significantly increased (Fig. 4E and F). These results demonstrated
that Akt-mediated HK2 expression plays a pivotal role in the
effectd of LicA on tumor glycolysis.
Overexpression of HK2 impairs
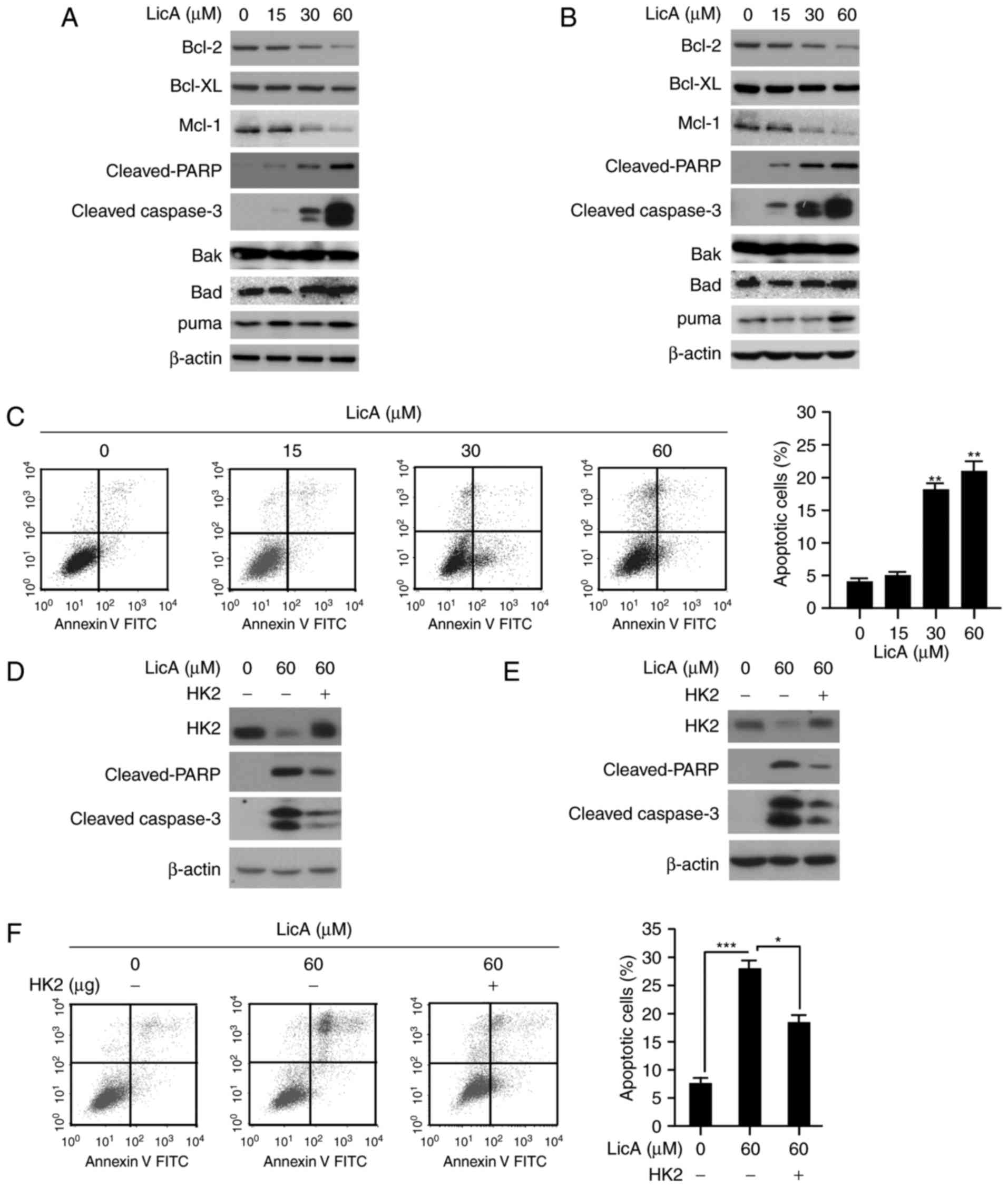
LicA-induced cell apoptosis
Except glycolysis suppression, LicA also induced
apoptosis in gastric cancer cells. The expression of
cleaved-caspase-3 and PARP, which are signals of cells undergoing
apoptosis, were significantly increased (Fig. 5A and B). The results of FACS
analysis also confirmed that MKN45 cells exhibited induced
apoptosis dose-dependently; 25% of tumor cells were subjected to
apoptosis at 60 µM (Fig. 5C).
Furthermore, the anti-apoptotic proteins such as Bcl-2, and Mcl-1
were found to be decreased after LicA treatment, whereas the
expression of pro-apoptotic proteins including Bad and Bak had no
significant change. In addition to mediating tumor glycolysis, HK2
is also involved in apoptosis regulation. Given the decrease in HK2
expression by LicA, we speculated that HK2 was also involved in
LicA-induced cell apoptosis. After exogenous HK2 overexpression in
gastric cancer, the apoptosis induced by LicA was substantially
attenuated and the expression of cleaved-PARP and caspase-3 was
significantly decreased in contrast with the control group,
suggesting that HK2 was involved in LicA-induced apoptosis
(Fig. 5D-F).
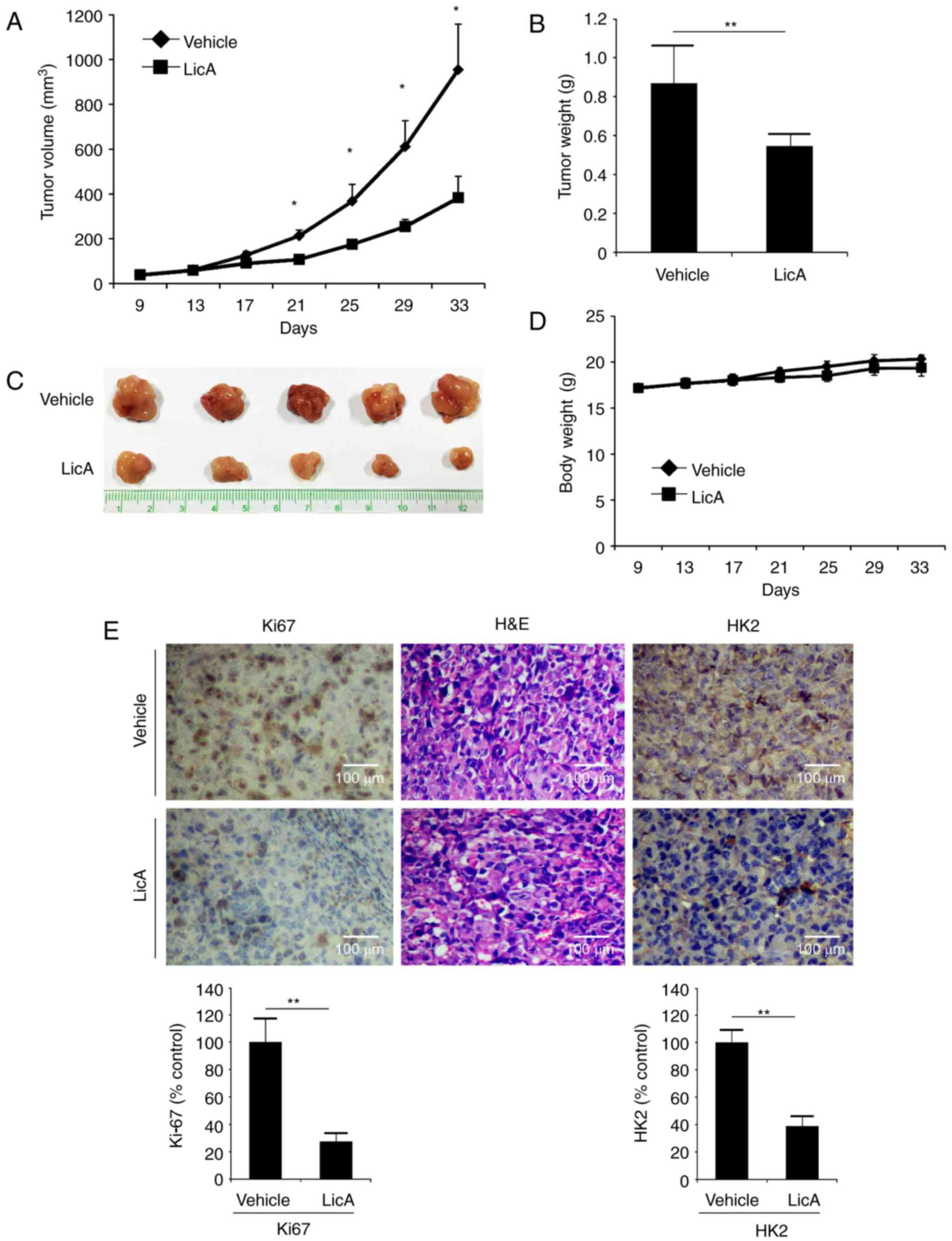
LicA restrains tumor growth in a
gastric xenograft model
The in vivo antitumor activity of LicA was
investigated in an MKN45 xenograft model. In contrast with the
vehicle group, tumor growth in the LicA-treated group was
significantly inhibited (Fig.
6A-C). The tumor volume of the vehicle group had reached about
900 mm3, whereas the average volume of the LicA-treated
group was ~400 mm3. The tumor weight of the LicA-treated
group was also significantly smaller than the vehicle group (0.52
vs. 0.85 g). Based on the change in body weight, no obvious
toxicity was observed (Fig. 6D). As
shown in Fig. 6E,
immunohistochemistry staining of LicA-treated tumor tissues
demonstrated that the expression of HK2 was substantially
decreased. Meanwhile, the expression of Ki-67, a marker of cell
proliferative potential, was also decreased, indicating that LicA
inhibited tumor growth in vivo by suppressing tumor
glycolysis.
Discussion
Gastric cancer ranks fourth as the most most common
malignancy and the second leading cause of cancer-associated
mortality. In China, it was estimated the mortality reached
~498,000 in 2015 (22). Despite the
advance in targeted therapy and early diagnosis of gastric cancer,
the progress in clinical therapy remains limited. Apart from small
molecules synthesized by medical chemistry, natural products have
drawn increasing attention in cancer chemopreventive therapies
(23). The results of the present
study demonstrated that LicA had profound potency against gastric
cancer in vitro and in vivo by suppressing tumor
glycolysis. Through reducing HK2 expression, the glycolysis in
gastric cancer cells was markedly inhibited by LicA. Meanwhile,
owing to the decrease in HK2, gastric cancer cells were subjected
to induction of apoptosis. Further studies identified that the
inhibition of the Akt signaling pathway was involved in the
regulation of HK2 activity.
The potency of LicA in different tumor types is
highly different. As reported, breast cancer cells demonstrated
high sensitivity to LicA with an IC50 of 20 µM (16), and the IC50 of LicA in
cervical cancer and head and neck cancer was 40 and 100 µM,
respectively (18,19). In our study, the IC50 of
LicA in gastric cancer was ~60 µM, which was in accordance with the
results reported by Hao et al (24). In cervical cancer, an i.p injection
of 10 mg/kg LicA substantially inhibited the xenograft growth
(19). Although the sensitivity of
head and neck cancer to LicA was relatively low, intravenous
injection of 10 mg/kg LicA also significantly delayed the tumor
growth (18). Therefore, in our
study, we also chose 10 mg/kg for the in vivo experiments.
As expected, the growth of MKN45 xenografts was substantially
attenuated by daily treatment of 10 mg/kg LicA.
Tumor glycolysis is an important hallmark of cancer
cells. Clinical observations verified the 18F-FDG uptake
is an independent and significant prognostic indicator of tumor
recurrence in gastric cancer (25,26).
The level of glucose uptake was closely correlated with the
overexpression of glycolytic enzymes, such as GLUT, HK2, PKM2,
which were found to be overexpressed in gastric cancer tissue and
were significantly correlated with disease progression (27–30).
After LicA treatment, the expression of HK2 was substantially
decreased, and both glucose absorption and lactate secretion in
gastric cancer cells were significantly inhibited. Meanwhile, the
western blotting results demonstrated that, except HK2, other key
enzymes involved in glycolysis regulation had no obvious change.
Additionally, Akt exogenous overexpression, which led to the
recovery of HK2 expression, significantly rescued glycolysis
suppression by LicA, suggesting that HK2 plays an important role in
LicA-mediated suppression of tumor glycolysis.
The regulation of HK2 expression in tumor cells is
complex. Like other glycolytic enzymes, its expression is primarily
mediated by altered oncogenic pathways, such as PI3K-Akt, NF-κB,
c-myc, HIF-1α and p53 (31). After
LicA treatment, the signaling pathways in gastric cancer cells
including Akt, ERK and NF-κB were blocked. Further investigation
clarified that HK2 downregulation was mainly attributed to the
effect of LicA on the Akt signaling pathway. Except LY294002, other
selective signaling pathway inhibitors had no effect on HK2
expression. Beyond that, HK2 reduction caused by LicA was
dramatically attenuated after Akt overexpression, further
confirming that the Akt signaling pathway is involved in the
regulation of HK2 by LicA. In other cancers, such as colorectal
(32), non-small cell lung cancer
(33,34), pediatric osteosarcoma (35), HK2 expression was also found to be
regulated by Akt activity. However, further investigation is needed
to elaborate the detailed mechanism of how HK2 is mediated by
Akt.
Numerous studies had reported that LicA induced
apoptosis in cancer cells via various mechanisms. In our study, the
results demonstrated that HK2 plays a pivotal role in LicA-induced
cell apoptosis in gastric cancer cells. Except the involvement of
tumor glycolysis regulation, HK2 interacts with VDAC-1 on the outer
mitochondria membrane to maintain membrane integrity under stressed
condition and prevents cancer cells from apoptosis (36). With the decrease of HK2, gastric
cancer cells underwent induced apoptosis by LicA, as evidenced by
the increase in cleaved caspase-3 and PARP and the results of
Annexin V-PI double staining. LicA-induced apoptosis was
significantly declined after exogenous introduction of HK2,
verifying that the decrease of HK2 was an important attributor to
the apoptosis induction by LicA. Recent studies have reported that
HK2 overexpression is closely correlated with chemotherapy
resistance in various cancers, such as in epithelial ovarian,
prostate and breast cancer (37–39).
Given the effect of LicA on HK2, we speculate that LicA may have
the potential to promote the efficacy of other chemotherapies in
combinational usage.
Taken together, to the best of our knowledge, the
present study is the first to report the effect of LicA on tumor
glycolysis. We found that the reduction in HK2 was an important
underlying mechanism for LicA to display its effects on glycolysis
suppression and apoptosis induction. Moreover, we also disclosed
that the decrease in HK2 caused by LicA was mainly attributed to
the inhibition of the Akt signaling pathway. Our studies provide a
preclinical rationale for LicA, or its derivatives to be
administered for gastric cancer therapy.
Acknowledgements
The present study was supported by the Foundation of
the Priority Academic Program Development of Jiangsu Higher
Education Institutions (PAPD), the Jiangsu Provincial Special
Program of Medical Science (BL2014100), the Jiangsu Hospital of TCM
(Y14074) and the National Natural Science Foundation of China (nos.
81202954 and 81473605).
References
|
1
|
Lee N and Kim D: Cancer Metabolism:
Fueling more than just growth. Mol Cells. 39:847–854. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Doherty JR and Cleveland JL: Targeting
lactate metabolism for cancer therapeutics. J Clin Invest.
123:3685–3692. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rho M, Kim J, Jee CD, Lee YM, Lee HE, Kim
MA, Lee HS and Kim WH: Expression of type 2 hexokinase and
mitochondria-related genes in gastric carcinoma tissues and cell
lines. Anticancer Res. 27A:1–258. 2007.
|
|
5
|
Palmieri D, Fitzgerald D, Shreeve SM, Hua
E, Bronder JL, Weil RJ, Davis S, Stark AM, Merino MJ, Kurek R, et
al: Analyses of resected human brain metastases of breast cancer
reveal the association between up-regulation of hexokinase 2 and
poor prognosis. Mol Cancer Res. 7:1438–1445. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu Y, Wu K, Shi L, Xiang F, Tao K and
Wang G: Prognostic significance of the metabolic marker
hexokinase-2 in various solid tumors: A meta-analysis. PLoS One.
11:e01662302016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang ZF, Feng XS, Chen H, Duan ZJ, Wang
LX, Yang D, Liu PX, Zhang QP, Jin YL, Sun ZG, et al: Prognostic
significance of synergistic hexokinase-2 and beta2-adrenergic
receptor expression in human hepatocelluar carcinoma after curative
resection. BMC Gastroenterol. 16:572016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kwee SA, Hernandez B, Chan O and Wong L:
Choline kinase alpha and hexokinase-2 protein expression in
hepatocellular carcinoma: Association with survival. PLoS One.
7:e465912012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hamabe A, Yamamoto H, Konno M, Uemura M,
Nishimura J, Hata T, Takemasa I, Mizushima T, Nishida N, Kawamoto
K, et al: Combined evaluation of hexokinase 2 and phosphorylated
pyruvate dehydrogenase-E1α in invasive front lesions of colorectal
tumors predicts cancer metabolism and patient prognosis. Cancer
Sci. 105:1100–1108. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu X, Deng Q, Li W, Xiao L, Luo X, Liu X,
Yang L, Peng S, Ding Z, Feng T, et al: Neoalbaconol induces cell
death through necroptosis by regulating RIPK-dependent autocrine
TNFα and ROS production. Oncotarget. 6:1995–2008. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu X, Li W, Deng Q, You S, Liu H, Peng S,
Liu X, Lu J, Luo X, Yang L, et al: Neoalbaconol inhibits
angiogenesis and tumor growth by suppressing EGFR-mediated VEGF
production. Mol Carcinog. 56:1414–1426. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee KW, Bode AM and Dong Z: Molecular
targets of phytochemicals for cancer prevention. Nat Rev Cancer.
11:211–218. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen X, Liu Z, Meng R, Shi C and Guo N:
Antioxidative and anticancer properties of Licochalcone A from
licorice. J Ethnopharmacol. 198:331–337. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu J and Liu J: Licochalcone A attenuates
kipopolysaccharide-induced acute kidney injury by inhibiting NF-κB
activation. Inflammation. 39:569–574. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang L, Yang R, Yuan B, Liu Y and Liu C:
The antiviral and antimicrobial activities of licorice, a
widely-used Chinese herb. Acta Pharm Sin B. 5:310–315. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bortolotto LF, Barbosa FR, Silva G,
Bitencourt TA, Beleboni RO, Baek SJ, Marins M and Fachin AL:
Cytotoxicity of trans-chalcone and licochalcone A against breast
cancer cells is due to apoptosis induction and cell cycle arrest.
Biomed Pharmacother. 85:425–433. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tang ZH, Chen X, Wang ZY, Chai K, Wang YF,
Xu XH, Wang XW, Lu JH, Wang YT, Chen XP, et al: Induction of C/EBP
homologous protein-mediated apoptosis and autophagy by licochalcone
A in non-small cell lung cancer cells. Sci Rep. 6:262412016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park MR, Kim SG, Cho IA, Oh D, Kang KR,
Lee SY, Moon SM, Cho SS, Yoon G, Kim CS, et al: Licochalcone-A
induces intrinsic and extrinsic apoptosis via ERK1/2 and p38
phosphorylation-mediated TRAIL expression in head and neck squamous
carcinoma FaDu cells. Food Chem Toxicol. 77:34–43. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsai JP, Lee CH, Ying TH, Lin CL, Lin CL,
Hsueh JT and Hsieh YH: Licochalcone A induces autophagy through
PI3K/Akt/mTOR inactivation and autophagy suppression enhances
Licochalcone A-induced apoptosis of human cervical cancer cells.
Oncotarget. 6:28851–28866. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang HC, Tsai LL, Tsai JP, Hsieh SC, Yang
SF, Hsueh JT and Hsieh YH: Licochalcone A inhibits the migration
and invasion of human lung cancer cells via inactivation of the Akt
signaling pathway with downregulation of MMP-1/-3 expression.
Tumour Biol. 35:12139–12149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim YH, Shin EK, Kim DH, Lee HH, Park JH
and Kim JK: Antiangiogenic effect of licochalcone A. Biochem
Pharmacol. 80:1152–1159. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kinghorn AD, DE Blanco EJ, Lucas DM,
Rakotondraibe HL, Orjala J, Soejarto DD, Oberlies NH, Pearce CJ,
Wani MC, Stockwell BR, et al: Discovery of anticancer agents of
diverse natural origin. Anticancer Res. 36:5623–5637. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hao W, Yuan X, Yu L, Gao C, Sun X, Wang D
and Zheng Q: Licochalcone A-induced human gastric cancer BGC-823
cells apoptosis by regulating ROS-mediated MAPKs and PI3K/AKT
signaling pathways. Sci Rep. 5:103362015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee JW, Lee SM, Lee MS and Shin HC: Role
of ¹8F-FDG PET/CT in the prediction of gastric cancer
recurrence after curative surgical resection. Eur J Nucl Med Mol
Imaging. 39:1425–1434. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Coupe NA, Karikios D, Chong S, Yap J, Ng
W, Merrett N and Lin M: Metabolic information on staging FDG-PET-CT
as a prognostic tool in the evaluation of 97 patients with gastric
cancer. Ann Nucl Med. 28:128–135. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Watanabe Y, Suefuji H, Hirose Y, Kaida H,
Suzuki G, Uozumi J, Ogo E, Miura M, Takasu K, Miyazaki K, et al:
18F-FDG uptake in primary gastric malignant lymphoma
correlates with glucose transporter 1 expression and histologic
malignant potential. Int J Hematol. 97:43–49. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qiu MZ, Han B, Luo HY, Zhou ZW, Wang ZQ,
Wang FH, Li YH and Xu RH: Expressions of hypoxia-inducible
factor-1α and hexokinase-II in gastric adenocarcinoma: The impact
on prognosis and correlation to clinicopathologic features. Tumour
Biol. 32:159–166. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yin L, Wang X, Luo C, Liu H, Zhang L,
Zhang H and Zhang Y: The value of expression of M2-PK and VEGF in
patients with advanced gastric cancer. Cell Biochem Biophys.
67:1033–1039. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lim JY, Yoon SO, Seol SY, Hong SW, Kim JW,
Choi SH and Cho JY: Overexpression of the M2 isoform of pyruvate
kinase is an adverse prognostic factor for signet ring cell gastric
cancer. World J Gastroenterol. 18:4037–4043. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mathupala SP, Ko YH and Pedersen PL:
Hexokinase II: Cancer's double-edged sword acting as both
facilitator and gatekeeper of malignancy when bound to
mitochondria. Oncogene. 25:4777–4786. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen GQ, Tang CF, Shi XK, Lin CY, Fatima
S, Pan XH, Yang DJ, Zhang G, Lu AP, Lin SH, et al: Halofuginone
inhibits colorectal cancer growth through suppression of Akt/mTORC1
signaling and glucose metabolism. Oncotarget. 6:24148–24162.
2015.PubMed/NCBI
|
|
33
|
Li W, Ma X, Li N, Liu H, Dong Q, Zhang J,
Yang C, Liu Y, Liang Q, Zhang S, et al: Resveratrol inhibits
Hexokinases II mediated glycolysis in non-small cell lung cancer
via targeting Akt signaling pathway. Exp Cell Res. 349:320–327.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li W, Gao F, Ma X, Wang R, Dong X and Wang
W: Deguelin inhibits non-small cell lung cancer via down-regulating
Hexokinases II-mediated glycolysis. Oncotarget. 8:32586–32599.
2017.PubMed/NCBI
|
|
35
|
Zhuo B, Li Y, Li Z, Qin H, Sun Q, Zhang F,
Shen Y, Shi Y and Wang R: PI3K/Akt signaling mediated Hexokinase-2
expression inhibits cell apoptosis and promotes tumor growth in
pediatric osteosarcoma. Biochem Biophys Res Commun. 464:401–406.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou Y, Lu N, Qiao C, Ni T, Li Z, Yu B,
Guo Q and Wei L: FV-429 induces apoptosis and inhibits glycolysis
by inhibiting Akt-mediated phosphorylation of hexokinase II in
MDA-MB-231 cells. Mol Carcinog. 55:1317–1328. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Suh DH, Kim MA, Kim H, Kim MK, Kim HS,
Chung HH, Kim YB and Song YS: Association of overexpression of
hexokinase II with chemoresistance in epithelial ovarian cancer.
Clin Exp Med. 14:345–353. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang L, Wang J, Xiong H, Wu F, Lan T,
Zhang Y, Guo X, Wang H, Saleem M, Jiang C, et al: Co-targeting
hexokinase 2-mediated Warburg effect and ULK1-dependent autophagy
suppresses tumor growth of PTEN- and TP53-deficiency-driven
castration-resistant prostate cancer. EBioMedicine. 7:50–61. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang J, Duan Z, Nugent Z, Zou JX, Borowsky
AD, Zhang Y, Tepper CG, Li JJ, Fiehn O, Xu J, et al: Reprogramming
metabolism by histone methyltransferase NSD2 drives endocrine
resistance via coordinated activation of pentose phosphate pathway
enzymes. Cancer Lett. 378:69–79. 2016. View Article : Google Scholar : PubMed/NCBI
|