Introduction
Traditionally, patients with type 2 diabetes
mellitus and cardiovascular diseases are treated with aspirin and
metformin (1–3). In recent years, both drugs have been
reported to decrease the risk of several types of cancers,
including breast cancer (4–10).
Aspirin, acetylsalicylic acid, has a wide range of
uses, such as an analgesic, antipyretic and anti-inflammatory agent
(11). As a nonsteroidal
anti-inflammatory drug and cyclooxygenase (COX) inhibitor, aspirin
prevents breast tumorigenesis in humans (12). The COX pathway plays an important
role in cellular proliferation, migration and invasiveness
(13,14). However, the precise mechanism
accounting for a possible anti-neoplastic action of aspirin is not
clear. A recent study showed that small interfering RNA-mediated
inhibition of the Smad signaling pathways decreases transforming
growth factor (TGF)-β1-induced COX-2 expression (15). Thus, there may be connection between
aspirin and TGF-β.
Metformin (1,1-dimethylbiguanide) is the most widely
prescribed drug to treat type 2 diabetes mellitus, notably in
overweight or obese individuals (16,17).
Recently, metformin was reported to limit proliferation of breast
cancer cells by acting upon specific micro (mi)RNAs (18–20).
Treatment with metformin inhibits growth by enhancing the tumor
suppressive function of TGF-β. This occurs as a result of metformin
disrupting the TGF-β/miRNA-181 signaling axis in cancer cells
(21–23).
TGF-β plays a central role in tumor inhibition by
both aspirin and metformin. Not surprisingly, metformin and aspirin
have synergistic effects and share several underlying mechanisms
for controlling cancer (24–26).
In the present study, we investigated the antitumor activity of
aspirin and metformin mediated by the TGF-β signaling pathway.
TGF-β is a versatile cytokine intimately involved in
cell growth (27–29). Depending on the tumor type and
tissue context, it may act both as a tumor suppressor or a promoter
of migration, invasion and tumor survival (30). Furthermore, TGF-β can be regulated
by estrogen (mainly estradiol) in vivo (31). Estrogen contributes to the
inhibition of TGF-β/Smad signaling by promoting R-Smad (Smad2 and
Smad3) degradation (31–34).
4T1 is a p53-deficient breast cancer cell line
(35,36). Triple-negative breast cancer cell
lines [i.e., lacking the estrogen receptor (ER), progesterone
receptor, and human epidermal growth factor receptor 2 (HER2)] are
less affected by estrogen than traditional cell lines (37,38).
Accordingly, estrogen suppression treatment is usually not
recommended in such cases, including in 4T1 cells. In the present
study, we hypothesized that the lack of estrogen inhibition in
triple-negative breast cancer cells may change the effect of
aspirin and metformin on tumor growth inhibition in vivo by
regulating TGF-β activity. We also discuss the link between
aspirin, metformin, TGF-β1 and estradiol in murine breast cancer
inhibition.
Materials and methods
Cell culture and treatment
The mouse breast carcinoma cell line 4T1 was
obtained from the American Type Culture Collection (ATCC; Manassas,
VA, USA), and maintained at 37°C in a humidified condition of 95%
air and 5% CO2. Cells were cultured in 75 cm2
flasks or 6-well plates with Dulbecco's modified Eagle's medium
(DMEM) (Life Technologies, Bedford, MA, USA) supplemented with 10%
heat-inactivated fetal bovine serum (FBS), 100 U/ml penicillin, and
100 U/ml streptomycin. Before addition of aspirin, metformin,
estradiol or LY364947, which is one of inhibitors of TGFβ R-I,
cells are allowed to attach to the substrate for 24 h. Aspirin,
metformin, estradiol and LY364947 were purchased from Sigma-Aldrich
(St. Louis, MO, USA).
For TGF-β1 treatment, 0.01 µg/ml to 1×104
4T1 cells of human recombinant TGF-β1 (BioLegend, Inc., San Diego,
CA, USA) were used, and 0.01 µg/ml to 1×104 4T1 cells
PBS as for the control group. For drug treatment group, nine groups
were divided: i) 5 µM aspirin; ii) 10 µM metformin; iii) 5 µM
aspirin plus 10 µM metformin; iv) 5 µM aspirin with 1 µM LY364947;
v) 10 µM metformin with 1 µM LY364947; vi) combination of 5 µM
aspirin and 10 µM metformin with 1 µM LY364947; vii) 5 µM aspirin
plus 10 nM estradiol; viii) 10 µM metformin plus 10 nM estradiol;
and ix) combination of 5 µM aspirin and 10 µM metformin plus 10 nM
estradiol, the same amount of dimethyl sulfoxide (DMSO) as control
group. Each group (n=5) was treated for 24, 36 and 48 h before
harvested for further study. The N-values of cell experiments are
three, respectively. The dose was based on literature (26,39)
and our earlier study, then we identified a roughly dose range.
From the range in our results from the MTT assay, we chose the
final dose.
The in vivo model
Five-week-old female BALB/c mice (Beijing HFK
Bioscience Co., Ltd., Beijing, China) were used for the in
vivo animal experiments. The animals were housed in constant
laboratory conditions with a 12-h light/dark cycle and fed with
water and food ad libitum. All animal care followed
institutional guidelines under a protocol approved by the
Institutional Animal Care and Use Committee of Sichuan University.
Mice were subcutaneously inoculated into the right-back with
1×106 4T1 cells in 100 µl PBS. For the treatment, the
tumor-bearing mice were divided into groups, respectively: i) 100
µl normal saline; ii) tamoxifen (100 mg/kg/24 h); iii) aspirin (60
mg/kg/24 h); iv) metformin (160 mg/kg/24 h); v) aspirin and
metformin; vi) aspirin with tamoxifen; vii) metformin with
tamoxifen; viii) aspirin and metformin with tamoxifen. Each group
had 5 mice. Metformin and tamoxifen were dissolved in normal saline
through intragastric administration for 15 days. Aspirin was
dissolved in ultrapure water with 4% ethanol as cosolvent through
intraperitoneal injection for the same days. The mice with a tumor
(0.5–1.0 cm wide, and 0.5–1.0 cm long) were randomized and injected
(n=5/group). The tumor volume [0.5 × [major axis] × [minor
axis]2] of every mouse was monitored every 2 days. Mice
were sacrificed at day 15, and samples were analyzed as previously
described (40). All procedures
regarding the care and use of animals followed the guidelines of
and were approved by the Animal Ethics Committee of Sichuan
University.
Cell proliferation assay
Cell proliferation was assessed using
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. Cells were seeded into 96-well plates and cultured for 24,
36 and 48 h following by addition of MTT solution to the cells for
4 h. After removing the medium, the remaining MTT formazan crystals
were solubilized in DMSO and measured at 560 nm using a microplate
reader (Benchmark Electronics, Angleton, TX, USA).
ELISA
4T1 tumors were collected and then homogenized in
radioimmunoprecipitation assay (RIPA) buffer (0.1% SDS, 0.5%
deoxycholate, 1% Triton X-100, 150 mM NaCl and 50 mM Tris-HCl),
followed by centrifugation at 13,300 rpm for 30 min at 4°C. DEAB
assay was used to test the protein concentration of samples. The
prepared samples were stored at −80°C until used. Levels of TGF-β1
in the samples were assessed by mouse ELISA kits (eBioscience or
R&D Systems, Minneapolis, MN, USA) according to the
manufacturer's instructions, and the colorimetric reaction was
measured at 450 nm, the color absorbance was recorded at 450 nm
using a Spectra MAX M5 microplate spectrophotometer (Molecular
Devices, Sunnyvale, CA, USA). The amount of TGF-β1 secreted into
the supernatant of 4T1 cells was quantified using the same ELISA
kits.
Blood samples were collected from the eye socket and
placed at room temperature for 3 h to obtain the serum. The serum
levels of estradiol detected using ELISA kits (Yan Hui Biological
Technology, Shanghai, China).
Flow cytometric analysis of
apoptosis
4T1 cells were treated as described above and then
harvested, washed in cold phosphate-buffered saline (PBS),
double-stained with fluorescein isothiocyanate (FITC)-conjugated
Annexin V and propidium iodide (PI) (BD Biosciences, San Jose, CA,
USA) and analyzed by flow cytometry (FACSAria SORP; BD Biosciences,
Erembodegem, Belgium). Apoptosis assays were performed with FITC/PI
as FITC+/PI− and
FITC+/PI+ to measure early and late
apoptosis, respectively. PI is a cell viability marker and FITC an
apoptosis marker.
Western blot analysis
4T1 cells were harvested, lysed and total protein
was quantified with Micro BCA Protein Assay kit (Pierce, Rockford,
IL, USA). Total protein (10 µg) from each sample was separated by
electrophoresis using 12% SDS-PAGE gels, transferred onto
polyvinylidene fluoride membranes (Merck Millipore, Billerica, MA,
USA), blocked with 5% skim milk, and incubated using the primary
antibodies (1:1,000) against Mcl-1, Bax, Bcl-2, caspase-8, TGF-β1,
Smad2/3, pSmad2, pSmad3, Smad4 and β-actin overnight (16 h) at 4°C.
β-actin was used as a loading control. All primary antibodies were
from Abcam Science Company (Cambridge, UK). Blots were then
incubated with the corresponding secondary antibodies (1:10,000;
Cell Signaling Technology, Danvers, MA, USA) for 1 h at room
temperature. After exposed to ECL reagent (Merck Millipore)
advanced luminescence, signals were developed on X-ray film (Kodak,
Rochester, NY, USA), and performed as previously described
(41).
Terminal deoxynucleotidyl
transferase-mediated dUTP nick end-labeling (TUNEL) assay
For each sample, 5-mm sections of root tips were
fixed using 4% paraformaldehyde (#18814; Polysciences, Inc.,
Warminster, PA, USA) in PBS for 45 min at room temperature. The
fixation step was followed by a permeabilization step with 0.25%
Triton X-100 in PBS for 20 min at room temperature. Next, terminal
deoxynucleotidyl transferase-mediated dUTP (2′-deoxyuridine,
5′-triphosphate) nick end-labeling (TUNEL) assay was performed
following the manufacturer's instructions (Click-iT®
TUNEL Alexa Fluor® Imaging Assay Protocol) and the
nuclei were stained for 3 min with 0.3 mg/ml
4′,6-diamidino-2-phenylindole (DAPI). Finally, the cells were
mounted in Vectashield embedding medium (Vector Laboratories,
Burlingame, CA, USA). All images were recorded at exactly the same
time of integration using an AxioCam ERc5s CCD camera and
AxioVision 4.8 software (both from Zeiss, Jena, Germany). Image
processing was carried out in Adobe Photoshop 7.0 Adobe Systems,
Inc., San Jose, CA, USA). Three samples worked each mouse and 10
visual fields were analyzed for each sample.
Statistical analysis
Data are expressed as mean ± standard deviation
(SD). Analysis of variation (ANOVA) were used for the statistical
analysis, and P<0.05 was considered statistically significant.
All statistics were performed using SAS 9.2.
Results
Aspirin combined with metformin
increases secretion of TGF-β1 by 4T1 cells
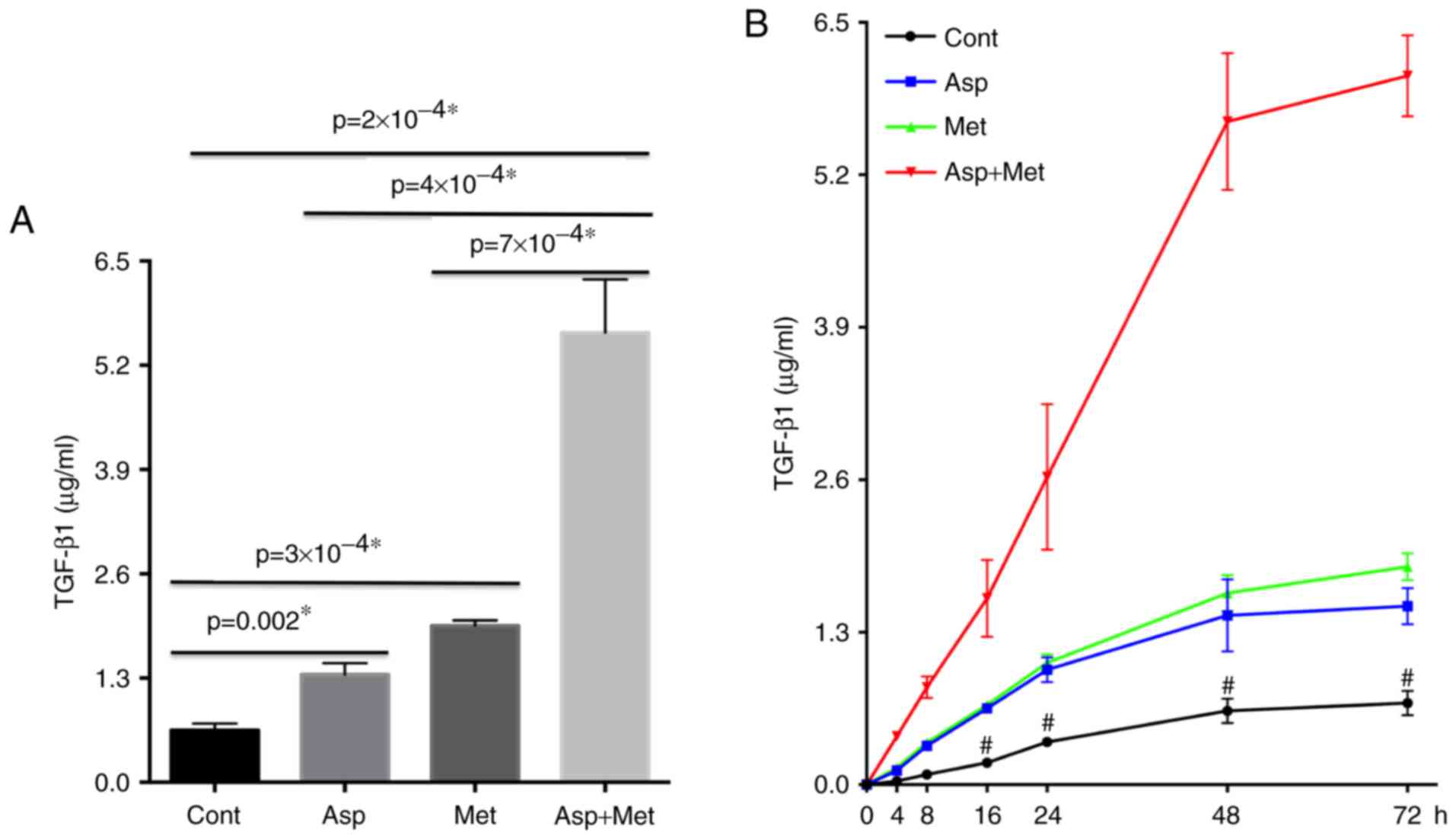
Following treatment with aspirin and metformin for
at least 48 h, we detected increased TGF-β1 secretion and
consequent tumor growth inhibition in 4T1 cells. Next, we used
ELISA to measure the amount of TGF-β1 in the supernatant of 4T1
cells after the different treatments (Fig. 1A). TGF-β1 levels were maximal
following combined aspirin and metformin treatment. Additionally,
TGF-β1 secretion was proportional to the length of the treatment,
in spite of slower growth after 48 h (Fig. 1B). This led us to conclude that
aspirin and metformin stimulated the secretion of TGF-β1. More
importantly, the combination of aspirin and metformin had a
synergistic effect on TGF-β1 secretion.
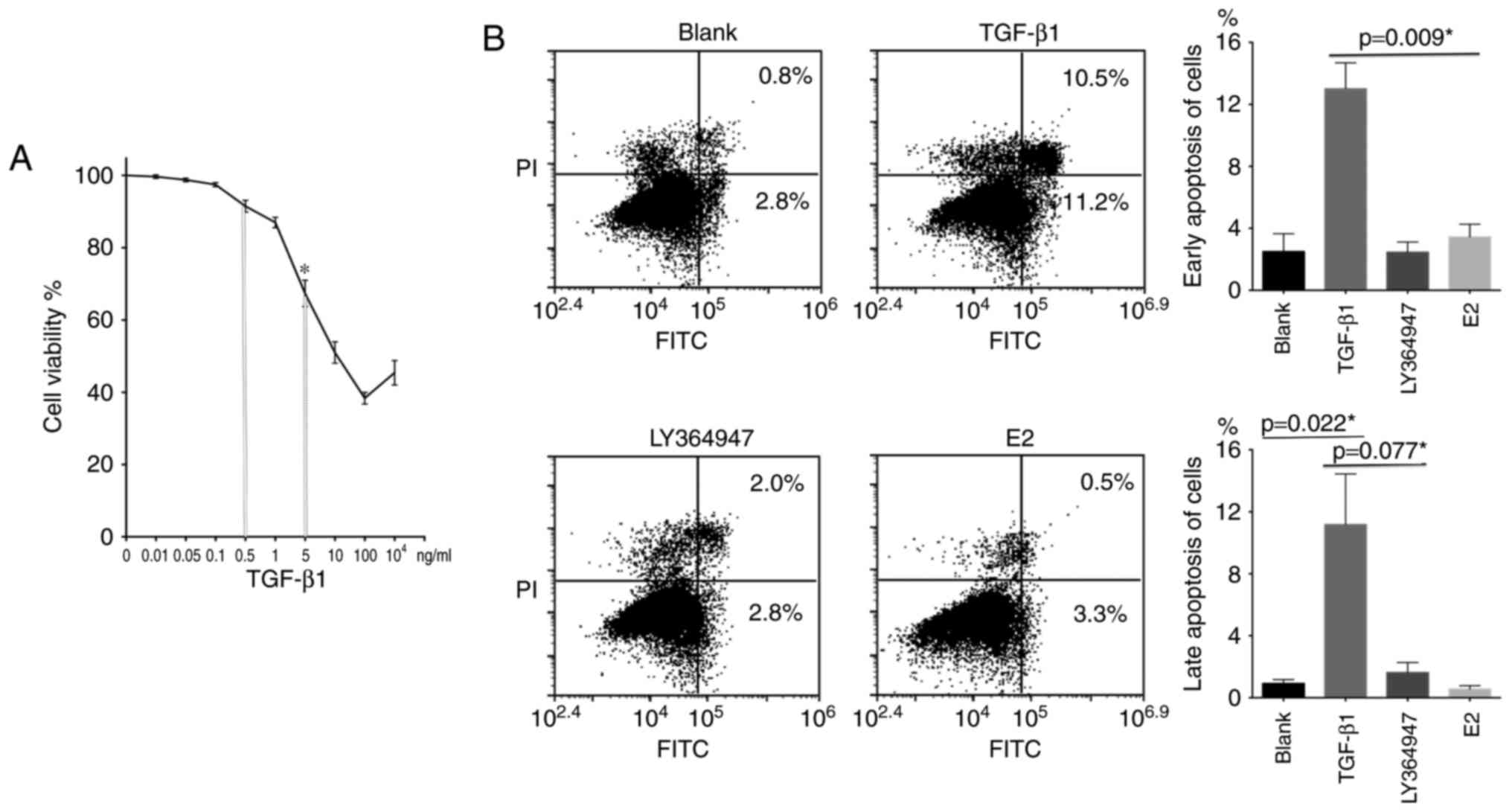
TGF-β1 reduces cell viability and
induces apoptosis in 4T1 cells
To evaluate the effect of TGF-β1 on proliferation
and apoptosis in 4T1 cells, we used the MTT assay with different
concentrations of TGF-β1 (Fig. 2A).
We observed that, within a certain range, TGF-β1 reduced 4T1 cell
viability. We then used flow cytometry to assess apoptosis of 4T1
cells following treatment with 100 ng/ml TGF-β1, 1 µM LY364947 (a
TGF-β type I receptor inhibitor), or 10 nM estradiol (Fig. 2B). The results indicated that,
depending on the concentration, TGF-β1 induced both early and late
apoptosis in 4T1 cells. PI is a cell viability marker and FITC an
apoptosis marker, of early apoptosis is
PI−/FITC+ and late apoptosis
PI+/FITC+.
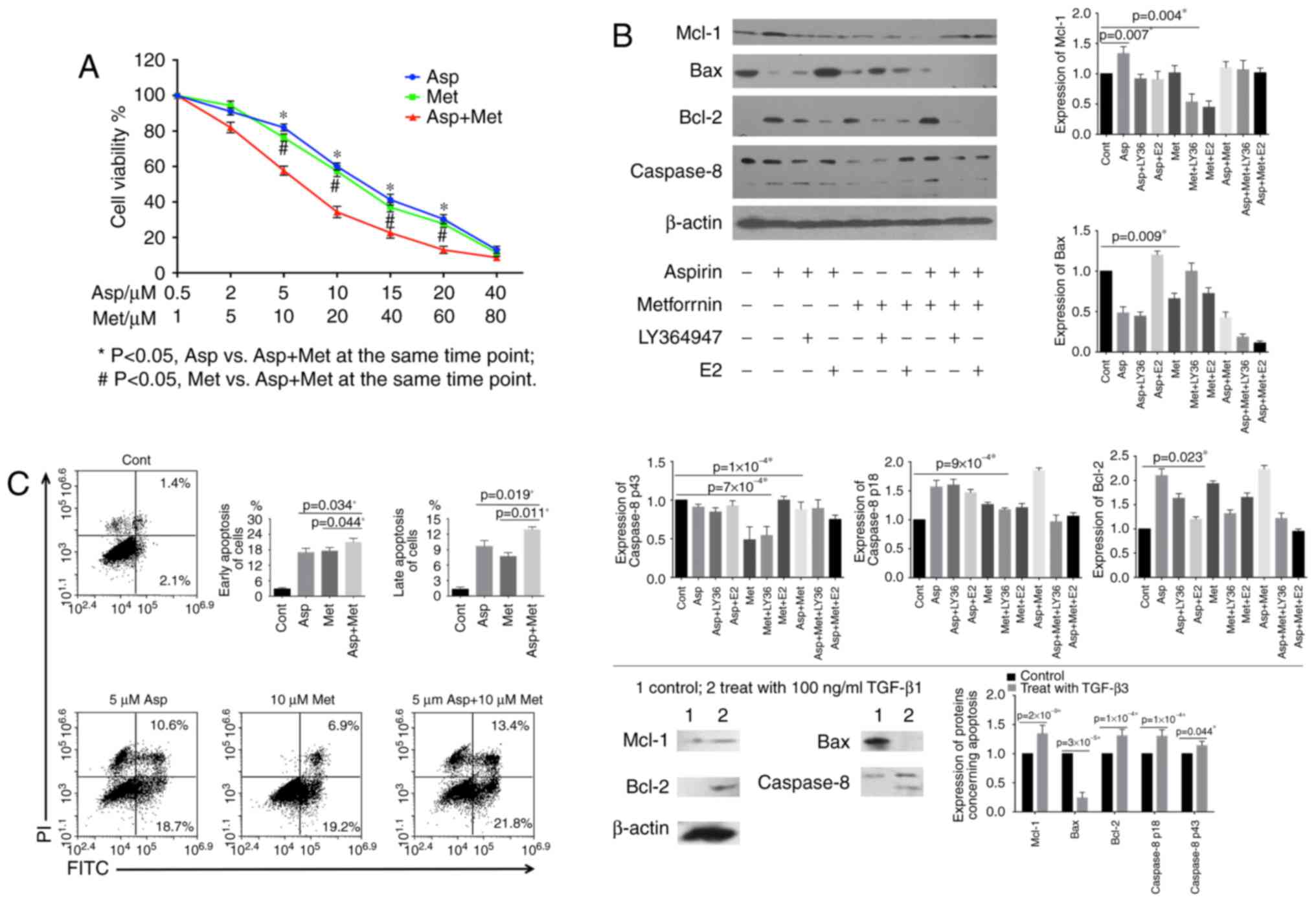
Aspirin and metformin reduce cell
viability and induce apoptosis in 4T1 cells
To evaluate whether different concentrations of
aspirin and metformin had a synergistic effect on the proliferation
of 4T1 cells, we performed the MTT assay. Combined treatment with
these drugs led to a synergistic inhibition of cell viability,
notably at 5 µM aspirin and 10 µM metformin (P=0.002) (Fig. 3A). Next, we assessed the expression
of apoptosis-related proteins. Western blotting revealed increased
levels of Bcl-2 and caspase-8 (p18), and decreased levels of Bax
and Mcl-1 following a 48-h treatment with aspirin and/or metformin.
Changes were notable after combined treatment (Fig. 3B). To determine whether apoptotic
cell death occurred, we evaluated cells by flow cytometry using
co-staining with FITC and PI (Fig.
3C). The results showed that aspirin and metformin decreased
cell viability and induced apoptosis in 4T1 cells, with the
combined treatment having the strongest effect.
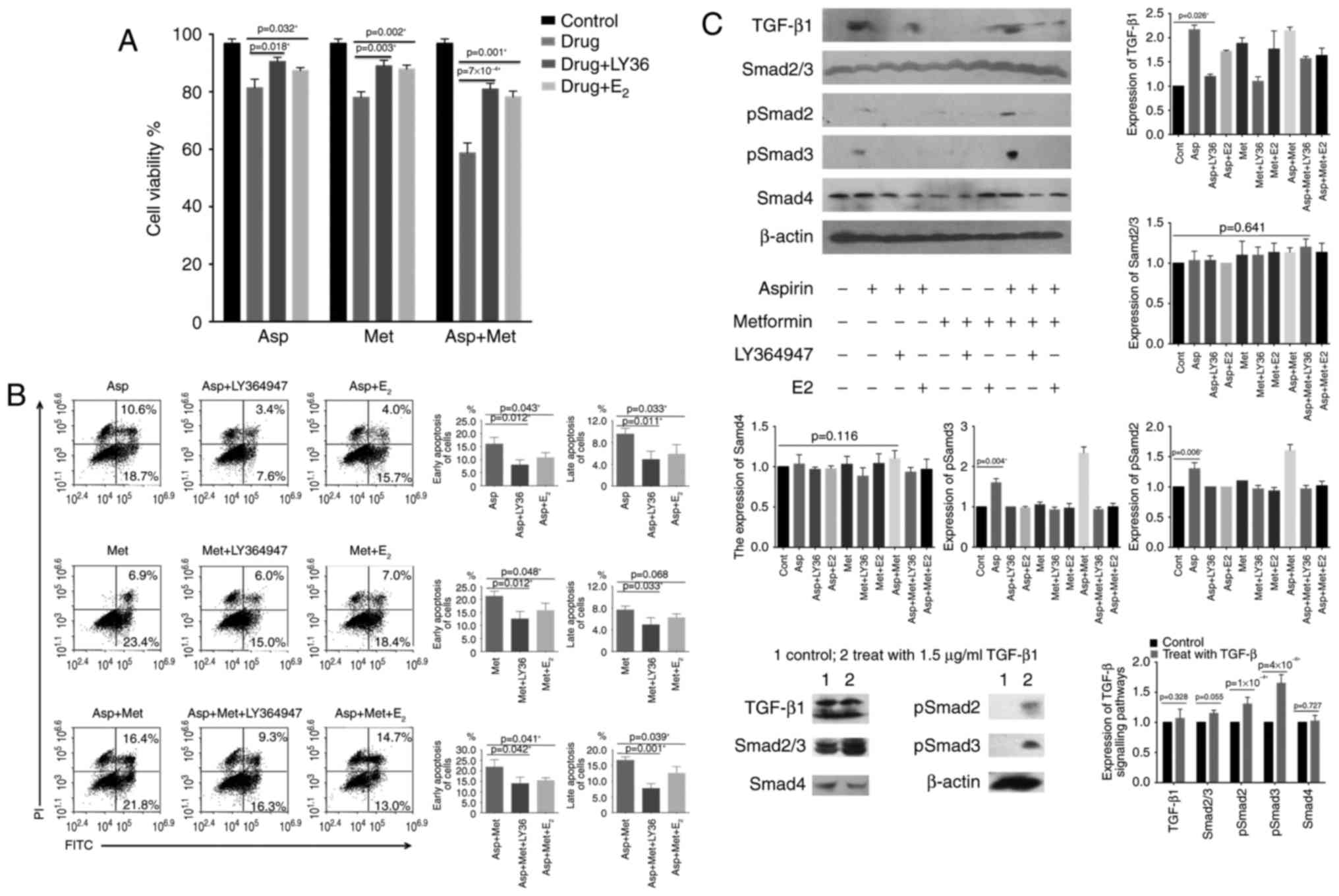
Aspirin and metformin enhance the
TGF-β-dependent pathway to promote suppression of 4T1 cells,
whereas estradiol weakens the effect
Given that secretion of TGF-β1 by 4T1 cells was a
major finding of the present study, we decided to design an
appropriate treatment. Results from the MTT assay (Fig. 3A) indicated that both drugs led to
growth inhibition, as determined by a decrease in optical
absorbance with 5 µM aspirin and 10 µM metformin. We used LY364947
to block the TGF-β1 receptor and observed an increase in optical
density. A similar effect was seen with estradiol (Fig. 4A). Expression of apoptosis-related
proteins decreased in 4T1 cells following addition of LY364947 and
estradiol, in contrast to the pro-apoptotic effect of aspirin and
metformin (Fig. 3B). These findings
were confirmed by flow cytometry (Fig.
4B).
Once cells were no longer stimulated by TGF-β1,
growth inhibition was relieved, suggesting that the inhibition
caused by aspirin was indeed mediated by TGF-β1. To further
determine whether the TGF-β-dependent pathway was involved in the
induction of apoptosis by metformin and aspirin, we examined the
effect of the two drugs on downstream targets of TGF-β1 (Smad2,
Smad3 and Smad4). Treatment with 100 ng/ml TGF-β1 (Fig. 4C) was used for comparison. The
phosphorylation of Smad2 and Smad3 was significantly stronger in
cells treated with a combination of metformin and aspirin whereas
treatment with aspirin or metformin alone had only a moderate or
small effect, respectively. Accordingly, specific bands
corresponding to phosphorylated Smad2 or Smad3 were barely
detectable once TGF-β1 induction was suspended.
Aspirin and metformin inhibit growth
of 4T1 tumors in BALB/c mice
A combination of metformin and aspirin caused a
strong inhibitory effect on tumor growth in vivo, whereas
aspirin or metformin alone had only a mild inhibitory effect. It
should be noted that administration of tamoxifen with aspirin and
metformin also had a significant inhibitory effect on tumor growth
in vivo (Fig. 5A); tumor
size in the group treated with all three agents was the smallest
while the control group showed the largest tumor size. Tumor size
in the aspirin plus metformin group was smaller than that in the
aspirin or metformin alone groups.
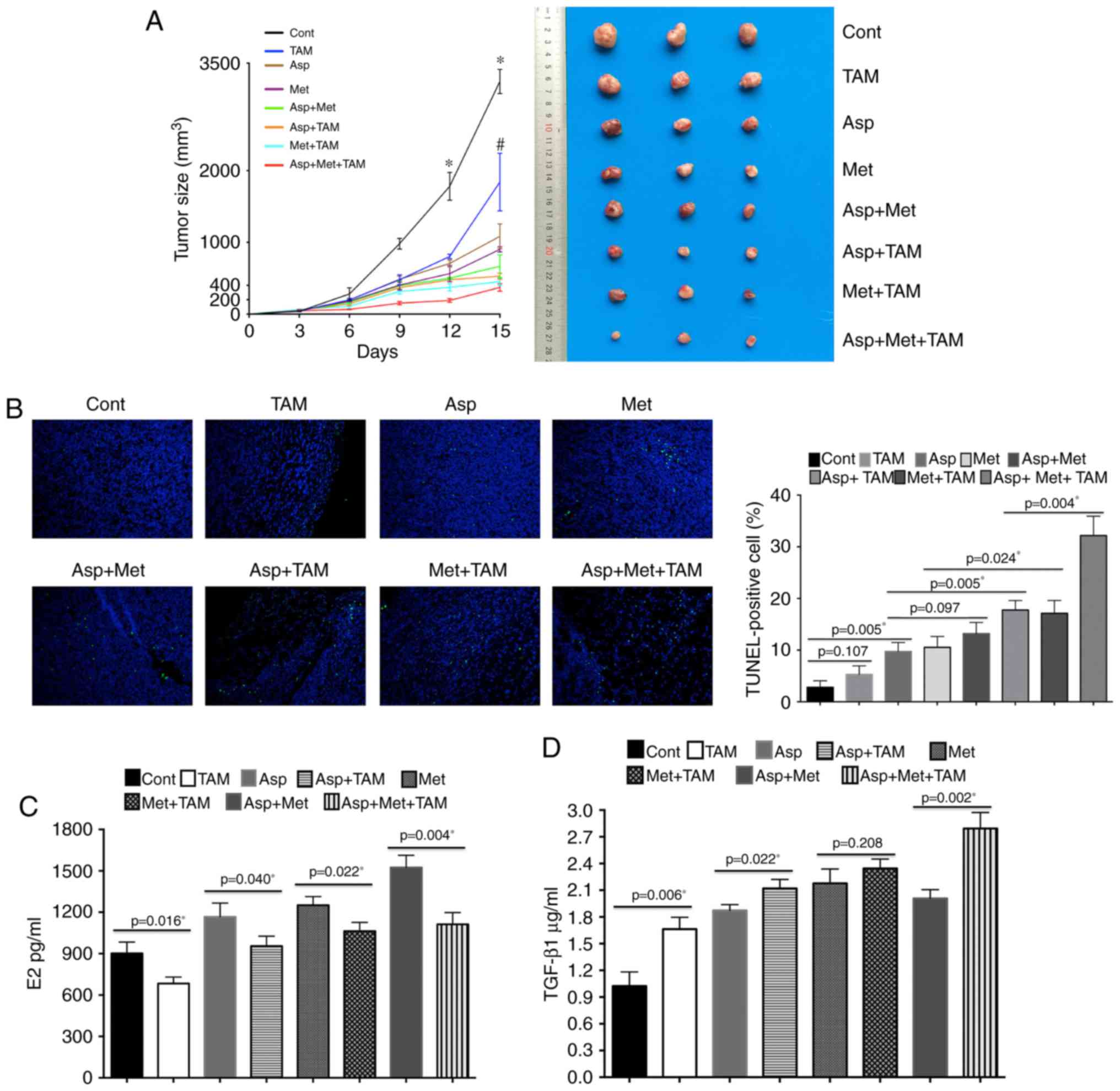 | Figure 5.Tumor sizes after treatment of BALB/c
mice with aspirin (Asp), metformin (Met) and tamoxifen (TAM). (A)
The administration of TAM with Asp and Met significantly inhibited
4T1 tumor growth. Tumor size in the Asp + Met + TAM group is the
smallest while that in control animals is the largest. Tumor size
in the Asp + Met group is smaller than in the Asp and Met alone
groups. The left panel shows changes of tumor volume in each group,
and the right panel shows representative images of the tumors.
Values are expressed as means ± SE, n=5; *P<0.05 vs. the TAM,
Asp, Met, Asp + Met, Asp + TAM, Met + TAM and Asp + Met + TAM
groups at the same time point; #P<0.05 vs. the Asp,
Met, Asp + Met, Asp + TAM, Met + TAM and Asp + Met + TAM groups at
the same time point. (B) Apoptosis assayed with TUNEL staining
(magnification, ×200). TUNEL (green) and DAPI (blue) merge. The
control shows the least apoptosis while the Asp + Met + TAM group
shows the most. (C) Serum levels of estradiol in 4T1 tumor-bearing
mice. After 15 days of treatment, blood samples were collected from
the eye socket and placed at room temperature for 3 h to obtain
serum. Estradiol was detected using ELISA kits. (D) Asp + Met + TAM
treatment increased transforming growth factor (TGF-β1) levels in
tumors. The DEAB assay was used to measure the protein
concentration of samples. Levels of TGF-β1 in the samples were
assessed by ELISA kits. Cont, control. |
The TUNEL assay was used to detect apoptosis in
subcutaneously transplanted tumors in mice. This experiment
revealed that the triple drug combination caused the most
significant increase in apoptosis (Fig.
5B). After euthanasia, the amount of estradiol in the blood was
measured (Fig. 5C). Based on these
results, a lower estradiol content could mediate the strong
inhibitory effect caused by a combination of aspirin and metformin.
The amount of TGF-β1 was greatest in subcutaneously transplanted
tumors of mice subjected to a combined drug treatment (Fig. 5D).
Discussion
In the present study, we evaluated the effect of
combining low doses of aspirin and metformin on the growth of 4T1
breast cancer cells in vitro and in vivo. We also
highlighted the link between TGF-β and estradiol in tumor
apoptosis. A combination of aspirin and metformin showed
synergistic cytotoxicity in 4T1 cells and a significant inhibitory
effect on in vivo tumor growth through regulation of
important apoptosis-related proteins, such as Bcl-2, Mcl-1, Bax,
and caspase-8, and consequent cell death. A combination of the two
drugs was notably effective at increasing TGF-β1 levels in the
supernatant fluid of 4T1 cells and in the blood of 4T1
tumor-bearing mice. Estradiol in 4T1 tumor-bearing mice weakened
the antitumor effect of aspirin and metformin by downregulating
TGF-β1 and promoting Smad2 and Smad3 degradation in
vivo.
To the best of our knowledge, this is the first
study to show that the TGF-β signaling pathway mediates the
inhibitory effect of combined aspirin and metformin treatment on
tumors. We reported previously that apoptosis of tumor tissue was
induced and micro-vessel density was decreased after high-dose
aspirin treatment, without any severe damage to the stomach, small
intestine, liver and spleen (40).
Epidemiological evidence has shown a consistent prophylactic effect
of aspirin on breast cancer (42,43).
After 20 years of follow-up, overall cancer mortality has been
shown to be decreased by ~20% in people taking aspirin, with the
greatest benefit for adenocarcinomas (36% reduction) in randomized
prevention trials (44,45). In particular, aspirin has a
significant effect on preventing colorectal cancer (46) and risk reduction (47).
Metformin therapy weakens the risk of
glioma-initiating cells (48), and
inhibits ovarian cancer by increasing sensitivity to cisplatin
(49,50), endometrial cancer through changes in
Ki-67 proliferation (51–53), and breast cancer (54,55)
and non-small cell lung carcinomas (56,57).
Laboratory studies on breast cancer have shown that metformin
increases the mean life span by 8% and mammary adenocarcinoma
latency by 13.2% (P<0.05) in HER2/neu mice (58). In retrospective studies, long-term
use of ≥40 prescriptions (>5 years) of metformin is associated
with an improved adjusted odds ratio of developing breast cancer
compared with no use (59).
In the present study, we observed that a combination
of aspirin and metformin had a stronger inhibitory effect on 4T1
cell proliferation than either drug alone. This effect depend on
TGF-β1, in which levels increased following aspirin and metformin
treatment. Western blot results showed markedly increased pSmad2
and pSmad3 levels in the recombinant TGF-β1 group, and the combined
aspirin and metformin group, but only a marginal increase in the
aspirin alone group. Moreover, we showed that metformin combined
with aspirin regulated apoptosis-related proteins, mimicking the
effect of recombinant TGF-β1 treatment. This resulted in decreased
Mcl-1 and Bax, and increased Bcl-2 and caspase-8 levels. No obvious
increase in caspase-8 (p18) was detected following metformin
treatment alone. Moreover, an increase in early and late apoptosis
following TGF-β addition was consistent with the above data.
4T1 cells were injected subcutaneously into BALB/c
mice in vivo. Compared to aspirin or metformin alone, mice
subjected to a combination of the two drugs showed the highest
TGF-β1 content, smallest tumor size, and highest degree of tumor
cell apoptosis. These results suggested that a combination of
aspirin and metformin could significantly inhibit 4T1 cell growth
in vitro and in vivo by promoting autocrine/paracrine
TGF-β1 to regulate apoptosis-related proteins.
TGF-β shows suppressive effects at the early stage
of tumorigenesis, whereas tumor cells in advanced stages can avoid
the antiproliferative effect and undergo tumorigenic progression in
response to TGF-β (60,61). In the present study, we report that
aspirin and/or metformin stimulated TGF-β1, which could then
suppress survival of breast cancer cells and phosphorylation of
Smad2 and Smad3. In vivo experiments revealed that, in
tumor-bearing mice treated for a maximum of 72 h with aspirin
and/or metformin, and sacrificed 15 days later when the tumor did
not develop to an advanced stage, smaller sized tumors contained
the most TGF-β1. Whether TGF-β secretion can also be induced by
aspirin and/or metformin in advanced cancer, and what the ensuing
effect may be, will be addressed in future studies.
It was reported earlier that treatment of an adenoma
cell line with TGF-β triggered an increase in COX-2, which led to
growth inhibition and apoptosis-mediated cell death (62–64).
As a nonsteroidal anti-inflammatory drug and COX inhibitor, aspirin
prevents breast tumorigenesis in humans (12). Inhibition of the Smad signaling
pathways attenuates TGF-β1-induced COX-2 expression (15). Thus, it is possible that the Smad
signaling pathway mediates TGF-β-induced COX-2 by producing
feedback inhibitory effects. There are some reports that metformin
weakens the effect of TGF-β1 or inhibits the TGF-β pathway in
normal cells (65,66) or some metastatic tumor cells
(67,68) that differ from 4T1 cells. TGF-β
plays different roles in different tumor stages (27–30).
Thus, the different states of experimental cells may cause these
different findings. It also has been shown that metformin increases
nuclear p53 and TGF-β1 levels in human breast cancer cell lines
(69) and the metformin-mediated
stimulation of TGF-β1 secretion by mesangial cells is
dose-dependent (70).
Tamoxifen, the mainstay of endocrine therapy for
breast cancer, acts as a competitive inhibitor of the ER (71). It blocks the feedback loop of TGF-β
signaling (72,73) by recruiting the N-CoR-histone
deacetylase complex to the promoter (74–76).
Binding of estrogen to the ER promotes formation of a multiprotein
complex including N-CoR-histone deacetylase that removes acetyl
groups and turns off transcription (77–79).
Estrogen can reduce the expression of more than two-thirds of the
genes induced by TGF-β treatment (33). Consistent with this, we believe that
estradiol from female BALB/c mice could attenuate the growth
inhibition induced by aspirin and metformin by downregulating
TGF-β1. In vitro, we observed that, during early and late
apoptosis, the levels of Bcl-2 and caspase-8 were further decreased
by simultaneous treatment with estradiol and aspirin or metformin,
compared to aspirin or metformin alone. Inhibitors of the TGF-β
receptor produced a similar outcome. In vivo, the
combination of aspirin, metformin, and tamoxifen led to the highest
level of TGF-β1, smallest tumor size, greatest degree of apoptosis
in tumors, and the least estradiol in peripheral blood.
In conclusion, a combination of aspirin and
metformin exhibits a synergetic cytotoxic effect in 4T1 breast
cancer cells in vitro, and a significant inhibitory effect
on 4T1 tumor growth in vivo. In addition, inhibition of
estrogen further maximizes antitumor activity of the combined drug
treatment in vivo. However, according to the National
Comprehensive Cancer Network Clinical Practice Guidelines in
Oncology (80), patients with
triple-negative breast cancer are not recommended for estrogen
suppression treatment. The present study provides a rationale for
clinical trials that combine aspirin with metformin, and brings
attention to the link between estrogen levels and the outcome in
triple-negative breast cancer patients.
Glossary
Abbreviations
Abbreviations:
|
COX
|
cyclooxygenase
|
|
E2
|
estradiol
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
ER
|
estrogen receptor
|
|
HER-2
|
human epidermal growth factor receptor
2, ErbB-2
|
|
PI
|
propidium iodide
|
|
pSmad2
|
phosphorylated Smad2
|
|
pSmad3
|
phosphorylated Smad3
|
|
miR
|
microRNA
|
|
TGF-β
|
transforming growth factor-β
|
References
|
1
|
Berstein LM: Metformin in obesity, cancer
and aging: Addressing controversies. Aging). 4:320–329. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Menendez JA, Oliveras-Ferraros C, Cufi S,
Corominas-Faja B, Joven J, Martin-Castillo B and Vazquez-Martin A:
Metformin is synthetically lethal with glucose withdrawal in cancer
cells. Cell Cycle. 11:2782–2792. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ma J, Guo Y, Chen S, Zhong C, Xue Y, Zhang
Y, Lai X, Wei Y, Yu S, Zhang J and Liu W: Metformin enhances
tamoxifen-mediated tumor growth inhibition in ER-positive breast
carcinoma. BMC Cancer. 14:1722014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Currie CJ, Poole CD and Gale EA: The
influence of glucose-lowering therapies on cancer risk in type 2
diabetes. Diabetologia. 52:1766–1777. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee MS, Hsu CC, Wahlqvist ML, Tsai HN,
Chang YH and Huang YC: Type 2 diabetes increases and metformin
reduces total, colorectal, liver and pancreatic cancer incidences
in Taiwanese: A representative population prospective cohort study
of 800,000 individuals. BMC Cancer. 11:202011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bodmer M, Becker C, Meier C, Jick SS and
Meier CR: Use of antidiabetic agents and the risk of pancreatic
cancer: A case-control analysis. Am J Gastroenterol. 107:620–626.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Niraula S, Dowling RJ, Ennis M, Chang MC,
Done SJ, Hood N, Escallon J, Leong WL, McCready DR, Reedijk M, et
al: Metformin in early breast cancer: A prospective window of
opportunity neoadjuvant study. Breast Cancer Res Treat.
135:821–830. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cazzaniga M, DeCensi A, Pruneri G, Puntoni
M, Bottiglieri L, Varricchio C, Guerrieri-Gonzaga A, Gentilini OD,
Pagani G, Dell'Orto P, et al: The effect of metformin on apoptosis
in a breast cancer presurgical trial. Br J Cancer. 109:2792–2797.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gronich N and Rennert G: Beyond
aspirin-cancer prevention with statins, metformin and
bisphosphonates. Nat Rev Clin Oncol. 10:625–642. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zannella VE, Dal Pra A, Muaddi H, McKee
TD, Stapleton S, Sykes J, Glicksman R, Chaib S, Zamiara P,
Milosevic M, et al: Reprogramming metabolism with metformin
improves tumor oxygenation and radiotherapy response. Clin Cancer
Res. 19:6741–6750. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ugurlucan M, Caglar IM, Caglar FN, Ziyade
S, Karatepe O, Yildiz Y, Zencirci E, Ugurlucan FG, Arslan AH,
Korkmaz S, et al: Aspirin: From a historical perspective. Recent
Pat Cardiovasc Drug Discov. 7:71–76. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Half E, Tang XM, Gwyn K, Sahin A, Wathen K
and Sinicrope FA: Cyclooxygenase-2 expression in human breast
cancers and adjacent ductal carcinoma in situ. Cancer Res.
62:1676–1681. 2002.PubMed/NCBI
|
|
13
|
Williams CS, Mann M and DuBois RN: The
role of cyclooxygenases in inflammation, cancer, and development.
Oncogene. 18:7908–7916. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ulrich CM, Bigler J and Potter JD:
Non-steroidal anti-inflammatory drugs for cancer prevention:
Promise, perils and pharmacogenetics. Nat Rev Cancer. 6:130–140.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fang L, Chang HM, Cheng JC, Leung PC and
Sun YP: TGF-β1 induces COX-2 expression and PGE2 production in
human granulosa cells through smad signaling pathways. J Clin
Endocrinol Metab. 99:E1217–1226. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Adler AI, Shaw EJ, Stokes T and Ruiz F;
Guideline Development Group, : Newer agents for blood glucose
control in type 2 diabetes: Summary of NICE guidance. BMJ.
338:b16682009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nathan DM, Buse JB, Davidson MB,
Ferrannini E, Holman RR, Sherwin R and Zinman B; American
DiabetesAssociation, ; European Association for Study of Diabetes,
: Medical management of hyperglycemia in type 2 diabetes: A
consensus algorithm for the initiation and adjustment of therapy: A
consensus statement of the American diabetes association and the
European association for the study of diabetes. Diabetes Care.
32:193–203. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takahashi RU, Miyazaki H, Takeshita F,
Yamamoto Y, Minoura K, Ono M, Kodaira M, Tamura K, Mori M and
Ochiya T: Loss of microRNA-27b contributes to breast cancer stem
cell generation by activating ENPP1. Nat Commun. 6:73182015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bacci M, Giannoni E, Fearns A, Ribas R,
Gao Q, Taddei ML, Pintus G, Dowsett M, Isacke CM, Martin LA, et al:
miR-155 drives metabolic reprogramming of ER+ breast
cancer cells following long-term estrogen deprivation and predicts
clinical response to aromatase inhibitors. Cancer Res.
76:1615–1626. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cabello P, Pineda B, Tormo E, Lluch A and
Eroles P: The antitumor effect of metformin is mediated by miR-26a
in breast cancer. Int J Mol Sci. 17:E12982016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dontu G, Abdallah WM, Foley JM, Jackson
KW, Clarke MF, Kawamura MJ and Wicha MS: In vitro propagation and
transcriptional profiling of human mammary stem/progenitor cells.
Genes Dev. 17:1253–1270. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Charafe-Jauffret E, Ginestier C, Iovino F,
Wicinski J, Cervera N, Finetti P, Hur MH, Diebel ME, Monville F,
Dutcher J, et al: Breast cancer cell lines contain functional
cancer stem cells with metastatic capacity and a distinct molecular
signature. Cancer Res. 69:1302–1313. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oliveras-Ferraros C, Cufi S,
Vazquez-Martin A, Torres-Garcia VZ, Del Barco S, Martin-Castillo B
and Menendez JA: Micro(mi)RNA expression profile of breast cancer
epithelial cells treated with the anti-diabetic drug metformin:
Induction of the tumor suppressor miRNA let-7a and suppression of
the TGFβ-induced oncomiR miRNA-181a. Cell Cycle. 10:1144–1151.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dowling RJ, Zakikhani M, Fantus IG, Pollak
M and Sonenberg N: Metformin inhibits mammalian target of
rapamycin-dependent translation initiation in breast cancer cells.
Cancer Res. 67:10804–10812. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Din FV, Valanciute A, Houde VP, Zibrova D,
Green KA, Sakamoto K, Alessi DR and Dunlop MG: Aspirin inhibits
mTOR signaling, activates AMP-activated protein kinase, and induces
autophagy in colorectal cancer cells. Gastroenterology.
142:1504–1515.e3. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yue W, Zheng X, Lin Y, Yang CS, Xu Q,
Carpizo D, Huang H, DiPaola RS and Tan XL: Metformin combined with
aspirin significantly inhibit pancreatic cancer cell growth in
vitro and in vivo by suppressing anti-apoptotic proteins Mcl-1 and
Bcl-2. Oncotarget. 6:21208–21224. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Blobe GC, Schiemann WP and Lodish HF: Role
of transforming growth factor beta in human disease. N Engl J Med.
342:1350–1358. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Govinden R and Bhoola KD: Genealogy,
expression, and cellular function of transforming growth
factor-beta. Pharmacol Ther. 98:257–265. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Reddi AH: BMPs: From bone morphogenetic
proteins to body morphogenetic proteins. Cytokine Growth Factor
Rev. 16:249–250. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Joshi A and Cao D: TGF-beta signaling,
tumor microenvironment and tumor progression: The butterfly effect.
Front Biosci. 15:180–194. 2010. View
Article : Google Scholar
|
|
31
|
Maurya VK, Jha RK, Kumar V, Joshi A,
Chadchan S, Mohan JJ and Laloraya M: Transforming growth
factor-beta 1 (TGF-B1) liberation from its latent complex during
embryo implantation and its regulation by estradiol in mouse. Biol
Reprod. 89:842013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dixon A and Maric C: 17beta-Estradiol
attenuates diabetic kidney disease by regulating extracellular
matrix and transforming growth factor-beta protein expression and
signaling. Am J Physiol Renal Physiol. 293:F1678–F1690. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ito I, Hanyu A, Wayama M, Goto N, Katsuno
Y, Kawasaki S, Nakajima Y, Kajiro M, Komatsu Y, Fujimura A, et al:
Estrogen inhibits transforming growth factor beta signaling by
promoting Smad2/3 degradation. J Biol Chem. 285:14747–14755. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li YC, Ding XS, Li HM, Zhang Y and Bao J:
Role of G protein-coupled estrogen receptor 1 in modulating
transforming growth factor-β stimulated mesangial cell
extracellular matrix synthesis and migration. Mol Cell Endocrinol.
391:50–59. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Garijo R, Hernández-Alonso P, Rivas C,
Diallo JS and Sanjuán R: Experimental evolution of an oncolytic
vesicular stomatitis virus with increased selectivity for
p53-deficient cells. PLoS One. 9:e1023652014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yerlikaya A, Okur E, Baykal AT, Acilan C,
Boyaci I and Ulukaya E: A proteomic analysis of p53-independent
induction of apoptosis by bortezomib in 4T1 breast cancer cell
line. J Proteomics. 113:315–325. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Saji S, Honma N, Hirose M, Hayashi S and
Kuroi K: Translational cell study exploring the role of estrogen
receptor beta expression as a predictive and/or prognostic factor
in breast cancer patients. J Clin Oncol. 27:e221852009.
|
|
38
|
Wiggins AK, Kharotia S, Mason JK and
Thompson LU: α-Linolenic acid reduces growth of both triple
negative and luminal breast cancer cells in high and low estrogen
environments. Nutr Cancer. 67:1001–1009. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
O'Brien AJ, Villani LA, Broadfield LA,
Houde VP, Galic S, Blandino G, Kemp BE, Tsakiridis T, Muti P and
Steinberg GR: Salicylate activates AMPK and synergizes with
metformin to reduce the survival of prostate and lung cancer cells
ex vivo through inhibition of de novo lipogenesis. Biochem J.
469:177–187. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang Y, Du C, Zhao M, Li M, Zhang N, Liu
Y, Wang J and Luo F: Treatment of colonic transplantation
tumor-bearing mice with a high-dose aspirin in a short period of
time. Int J Colorectal Dis. 31:1099–1100. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang Y, Jiang M, Li Z, Wang J, Du C,
Yanyang L, Yu Y, Wang X, Zhang N, Zhao M, et al: Hypoxia and TGF-β1
lead to endostatin resistance by cooperatively increasing cancer
stem cells in A549 transplantation tumors. Cell Biosci. 5:722015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cuzick J, Otto F, Baron JA, Brown PH, Burn
J, Greenwald P, Jankowski J, La Vecchia C, Meyskens F, Senn HJ and
Thun M: Aspirin and non-steroidal anti-inflammatory drugs for
cancer prevention: An international consensus statement. Lancet
Oncol. 10:501–507. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhao YS, Zhu S, Li XW, Wang F, Hu FL, Li
DD, Zhang WC and Li X: Association between NSAIDs use and breast
cancer risk: A systematic review and meta-analysis. Breast Cancer
Res Treat. 117:141–150. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rothwell PM, Fowkes FG, Belch JF, Ogawa H,
Warlow CP and Meade TW: Effect of daily aspirin on long-term risk
of death due to cancer: Analysis of individual patient data from
randomised trials. Lancet. 377:31–41. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rothwell PM, Wilson M, Price JF, Belch JF,
Meade TW and Mehta Z: Effect of daily aspirin on risk of cancer
metastasis: A study of incident cancers during randomised
controlled trials. Lancet. 379:1591–1601. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chan AT, Arber N, Burn J, Chia WK, Elwood
P, Hull MA, Logan RF, Rothwell PM, Schrör K and Baron JA: Aspirin
in the chemoprevention of colorectal neoplasia: An overview. Cancer
Prev Res. 5:164–178. 2012. View Article : Google Scholar
|
|
47
|
Algra AM and Rothwell PM: Effects of
regular aspirin on long-term cancer incidence and metastasis: A
systematic comparison of evidence from observational studies versus
randomised trials. Lancet Oncol. 13:518–527. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sato A, Sunayama J, Okada M, Watanabe E,
Seino S, Shibuya K, Suzuki K, Narita Y, Shibui S, Kayama T and
Kitanaka C: Glioma-initiating cell elimination by metformin
activation of FOXO3 via AMPK. Stem Cells Transl Med. 1:811–824.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Shank JJ, Yang K, Ghannam J, Cabrera L,
Johnston CJ, Reynolds RK and Buckanovich RJ: Metformin targets
ovarian cancer stem cells in vitro and in vivo. Gynecol Oncol.
127:390–397. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lengyel E, Litchfield LM, Mitra AK, Nieman
KM, Mukherjee A, Zhang Y, Johnson A, Bradaric M, Lee W and Romero
IL: Metformin inhibits ovarian cancer growth and increases
sensitivity to paclitaxel in mouse models. Am J Obstet Gynecol.
212:479.e471–479.e10. 2015. View Article : Google Scholar
|
|
51
|
Cantrell LA, Zhou C, Mendivil A, Malloy
KM, Gehrig PA and Bae-Jump VL: Metformin is a potent inhibitor of
endometrial cancer cell proliferation-implications for a novel
treatment strategy. Gynecol Oncol. 116:92–98. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mitsuhashi A, Kiyokawa T, Sato Y and Shozu
M: Effects of metformin on endometrial cancer cell growth in vivo:
A preoperative prospective trial. Cancer. 120:2986–2995. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sivalingam VN, Kitson S, McVey R, Roberts
C, Pemberton P, Gilmour K, Ali S, Renehan AG, Kitchener HC and
Crosbie EJ: Measuring the biological effect of presurgical
metformin treatment in endometrial cancer. Br J Cancer.
114:281–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Bowker SL, Lin M, Eurich DT and Johnson
JA: Time-varying risk for breast cancer following initiation of
glucose-lowering therapy in women with type 2 diabetes: Exploring
detection bias. Can J Diabetes. 41:204–210. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Rico M, Baglioni M, Bondarenko M, Laluce
NC, Rozados V, André N, Carré M, Scharovsky OG and Menacho Márquez
M: Metformin and propranolol combination prevents cancer
progression and metastasis in different breast cancer models.
Oncotarget. 8:2874–2889. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Gagnon B, Roseman M, Kasymjanova G,
MacDonald N, Kreisman H and Small D: Protective effect of metformin
in lung cancer patients. J Clin Oncol. 27:e220632009.
|
|
57
|
Storozhuk Y, Hopmans SN, Sanli T, Barron
C, Tsiani E, Cutz JC, Pond G, Wright J, Singh G and Tsakiridis T:
Metformin inhibits growth and enhances radiation response of
non-small cell lung cancer (NSCLC) through ATM and AMPK. Br J
Cancer. 108:2021–2032. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Anisimov VN, Egormin PA, Piskunova TS,
Popovich IG, Tyndyk ML, Yurova MN, Zabezhinski MA, Anikin IV,
Karkach AS and Romanyukha AA: Metformin extends life span of
HER-2/neu transgenic mice and in combination with melatonin
inhibits growth of transplantable tumors in vivo. Cell Cycle.
9:188–197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Bodmer M, Meier C, Krähenbühl S, Jick SS
and Meier CR: Long-term metformin use is associated with decreased
risk of breast cancer. Diabetes Care. 33:1304–1308. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Inman GJ: Switching TGFβ from a tumor
suppressor to a tumor promoter. Curr Opin Genet Dev. 21:93–99.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Meulmeester E and Ten Dijke P: The dynamic
roles of TGF-β in cancer. J Pathol. 223:205–218. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Sheng H, Shao J, Hooton EB, Tsujii M,
DuBois RN and Beauchamp RD: Cyclooxygenase-2 induction and
transforming growth factor beta growth inhibition in rat intestinal
epithelial cells. Cell Growth Differ. 8:463–470. 1997.PubMed/NCBI
|
|
63
|
Sheng H, Shao J, O'Mahony CA, Lamps L,
Albo D, Isakson PC, Berger DH, DuBois RN and Beauchamp RD:
Transformation of intestinal epithelial cells by chronic TGF-beta1
treatment results in downregulation of the type II TGF-beta
receptor and induction of cyclooxygenase-2. Oncogene. 18:855–867.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Crew TE, Elder DJ and Paraskeva C: A
cyclooxygenase-2 (COX-2) selective non-steroidal anti-inflammatory
drug enhances the growth inhibitory effect of butyrate in
colorectal carcinoma cells expressing COX-2 protein: Regulation of
COX-2 by butyrate. Carcinogenesis. 21:69–77. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Xiao H, Ma X, Feng W, Fu Y, Lu Z, Xu M,
Shen Q, Zhu Y and Zhang Y: Metformin attenuates cardiac fibrosis by
inhibiting the TGFbeta1-Smad3 signalling pathway. Cardiovasc Res.
87:504–513. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Fan K, Wu K, Lin L, Ge P, Dai J, He X, Hu
K and Zhang L: Metformin mitigates carbon tetrachloride-induced
TGF-β1/Smad3 signaling and liver fibrosis in mice. Biomed
Pharmacother. 90:421–426. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Cheng K and Hao M: Metformin inhibits
TGF-β1-induced epithelial-to-mesenchymal transition via PKM2
relative-mTOR/p70s6k signaling pathway in cervical carcinoma cells.
Int J Mol Sci. 17:E20002016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Leonel C, Borin TF, de Carvalho Ferreira
L, Moschetta MG, Bajgelman MC, Viloria-Petit AM and de Campos
Zuccari DA: Inhibition of epithelial-mesenchymal transition and
metastasis by combined TGFbeta knockdown and metformin treatment in
a canine mammary cancer xenograft model. J Mammary Gland Biol
Neoplasia. 22:27–41. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Marinello PC, da Silva TN, Panis C, Neves
AF, Machado KL, Borges FH, Guarnier FA, Bernardes SS,
de-Freitas-Junior JC, Morgado-Díaz JA, et al: Mechanism of
metformin action in MCF-7 and MDA-MB-231 human breast cancer cells
involves oxidative stress generation, DNA damage, and transforming
growth factor β1 induction. Tumour Biol. 37:5337–5346. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Cortes P, Riser BL, Asano K,
Rodríguez-Barbero A, Narins RG and Yee J: Effects of oral
antihyperglycemic agents on extracellular matrix synthesis by
mesangial cells. Kidney Int. 54:1985–1998. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kuiper GG, Enmark E, Pelto-Huikko M,
Nilsson S and Gustafsson JA: Cloning of a novel receptor expressed
in rat prostate and ovary. Proc Natl Acad Sci USA. 93:pp.
5925–5930. 1996; View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Schmierer B and Hill CS: TGFbeta-SMAD
signal transduction: Molecular specificity and functional
flexibility. Nat Rev Mol Cell Biol. 8:970–982. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Lönn P, Morén A, Raja E, Dahl M and
Moustakas A: Regulating the stability of TGFbeta receptors and
Smads. Cell Res. 19:21–35. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Luo K, Stroschein SL, Wang W, Chen D,
Martens E, Zhou S and Zhou Q: The Ski oncoprotein interacts with
the Smad proteins to repress TGFbeta signaling. Genes Dev.
13:2196–2206. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Nomura T, Khan MM, Kaul SC, Dong HD,
Wadhwa R, Colmenares C, Kohno I and Ishi S: Ski is a component of
the histone deacetylase complex required for transcriptional
repression by Mad and thyroid hormone receptor. Genes Dev.
13:412–423. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Stroschein SL, Wang W, Zhou S, Zhou Q and
Luo K: Negative feedback regulation of TGF-beta signaling by the
SnoN oncoprotein. Science. 286:771–774. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Heery DM, Kalkhoven E, Hoare S and Parker
MG: A signature motif in transcriptional co-activators mediates
binding to nuclear receptors. Nature. 387:733–736. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Torchia J, Rose DW, Inostroza J, Kamei Y,
Westin S, Glass CK and Rosenfeld MG: The transcriptional
co-activator p/CIP binds CBP and mediates nuclear-receptor
function. Nature. 387:677–684. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
79
|
Cheskis BJ, Greger JG, Nagpal S and
Freedman LP: Signaling by estrogens. J Cell Physiol. 213:610–617.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Gradishar WJ, Anderson BO, Balassanian R,
Blair SL, Burstein HJ, Cyr A, Elias AD, Farrar WB, Forero A,
Giordano SH, et al: NCCN guidelines insights breast cancer, Version
1.2016. J Natl Compr Canc Netw. 13:1475–1485. 2015. View Article : Google Scholar : PubMed/NCBI
|