Introduction
Thyroid cancer (TC) is the major endocrine
malignancy with estimated 298,000 new cases and 40,000 deaths
worldwide in 2012 (1). Thyroid
carcinoma is the fastest-growing cancer with the increasing
morbidity rate >5% due to the advanced diagnostic techniques as
well as multiple risk factors. Although the 5-year survival rate
for patients with thyroid carcinoma is relatively higher than other
malignant neoplasms, the mortality rate of TC has remained
ever-increasing over the past decade (2,3).
Thyroid cancer is a heterogeneous disease of 3 histological
subtypes: papillary TC (PTC), follicular TC (FTC) and anaplastic TC
(ATC) (4). As the most primary
malignant thyroid tumor, PTC accounts for up to 80% of total cases.
Although PTC is generally effectively treated with thyroidectomy
and hormone therapy, large numbers of unresponsive patients suffer
from progressive disease and metastases (5). Thus, it is urgent to explore the
molecular mechanisms underlying PTC for developing effective
diagnostic and therapeutic targets.
Non-coding RNAs (ncRNAs) are generally defined as a
wide class of RNAs without protein-coding function, but with
universal expression in organisms. ncRNAs are loosely split into
small ncRNAs and long non-coding RNAs (lncRNAs), both of which have
regulatory functions in the various biological processes (6). The well-documented microRNAs (miRNAs;
~22 nucleotides long) belong to small ncRNAs and are regarded as
vital regulators of cellular gene expression network (7). lncRNAs are transcripts >200
nucleotides with tissue-specific expression, but neither has
significant open reading frames nor translate into proteins
(8). Accumulating studies over the
past decades have altered the understanding of lncRNA from ‘dark
matter’ to important transcriptional and post-transcriptional
regulators. Abnormally expressed lncRNAs participate in
tumorigenesis by interrupting biological processes of both
oncogenic and tumor suppressive pathways (9). In 2011, Pandolfi et al put
forward the competitive endogenous RNA (ceRNA) hypothesis that
specific RNAs (including lncRNA, mRNA, pseudogene and circular RNA)
can impair miRNA activity by sponging miRNA via common miRNA
response elements (MREs) and upregulate target RNA expression
subsequently (10). This hypothesis
introduced a new RNA-RNA crosstalk theory that can generally
regulate gene expression. In addition, the promising area of
disclosing the landscape of the ceRNA mechanism has evoked much
interest. Therefore, the lncRNA-miRNA-mRNA networks that may lead
to tumor development and progression have already been constructed
in many malignancies, including gastric cancer (11), hepatocellular carcinoma (12), glioblastoma (13) and lung adenocarcinoma (14).
Recent studies have preliminarily revealed several
ceRNA regulatory interactions and corresponding mechanisms in
thyroid carcinoma. lncRNA H19 was certified to act as ceRNA by
sponging miR-17-5p to upregulate the target gene YES1,
demonstrating a potential ceRNA crosstalk including H19, miR-17-5p,
and YES1 in thyroid cancer pathogenesis (15). A bioinformatic ceRNA analysis
revealed that stem cell factor SOX2 could be functionally
co-regulated with other genes by crosstalk mediated by several
miRNAs in anaplastic thyroid carcinoma (16). Furthermore, both a ceRNA network
built by microarray analysis of 5-paired clinical samples and a
protein-coding modulatory network that regulated immune responses
have been reported in PTC (17,18).
However, a whole genome-wide analysis of the lncRNA-miRNA-mRNA
regulatory network of papillary thyroid cancer with large-scale
sample size is still lacking.
In the present study, we comprehensively
investigated both RNA and miRNA sequencing data of 348 PTC primary
tumor tissues and 58 non-cancerous thyroid samples from The Cancer
Genome Atlas (TCGA) data matrix to obtain the aberrantly expressed
lncRNAs, miRNAs, and mRNAs. Next, an lncRNA-associated ceRNA
network of papillary thyroid cancer was built based on ceRNA
theory. The expression levels of the important lncRNAs involved in
ceRNA crosstalk were also assessed for their prognostic values in
patients with papillary thyroid cancer.
Materials and methods
Date collection
PTC patients (502) were obtained from the TCGA
consortium. Inclusion criteria included: i) patients diagnosed with
PTC but with no other malignancies; ii) patients with complete
clinical data, including age, sex, race, the American Joint
Committee on Cancer (AJCC) TNM and pathologic stage; iii) patients
with overall survival time <2,000 days; and iv) patients with
complete lncRNA, mRNA and miRNA expression profiles. In total, 348
patients (cohort T) who were pathologically diagnosed as PTC and 58
normal samples (cohort N) were enrolled in downstream analysis. The
RNAseq and miRNAseq data (level 3) generated from
IlluminaHiseq_RNASeq and IlluminaHiseq_miRNASeq sequencing platform
was downloaded from TCGA data portal. The present study followed
the publication guidelines of TCGA Research Network (http://cancergenome.nih.gov/publications/publicationguidelines).
Thus, no further ethical approvals were required.
Analysis of differentially expressed
genes
Differential expression analysis was carried out to
identify differentially expressed lncRNAs, mRNAs and miRNAs
(DElncRNAs, DEmRNAs, DEmiRNAs) between cohort T and N, by the
R/Bioconductor package of edgeR (19) with the cut-off value of
|log2FC|>1 and FDR <0.05 (FC, fold change; FDR,
false discovery rate).
CeRNA network construction
lncRNA-miRNA interactions were predicted by miRcode
(http://www.mircode.org/) (20). TargetScan (http://www.TargetScan.org/mamm_31/), miRTarBase
(http://mirtarbase.mbc.nctu.edu.tw/)
and miRanda (http://www.microrna.org/microrna/home.do) were
cooperatively utilized to predict the mRNAs targeted by miRNAs
(21,22). According to the above lncRNA/miRNA
and miRNA/mRNA interactions, the Cytoscape (version 3.5.1) was
utilized to build and visualize the lncRNA-miRNA-mRNA network
(23).
Functional annotation
The Database for Annotation Visualization and
Integrated Discovery (DAVID) online tool (https://david.ncifcrf.gov/) was used to conduct the
functional and pathway enrichment analyses. Gene Ontology (GO) and
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment
analyses were performed to detect the potential biological
functions and pathways of the DEmRNAs involved in the network
(P-value <0.05).
Statistical analysis
Unpaired t-test was applied to identify
differentially expressed genes between cohort T and N, and to find
out DElncRNAs between different pathological subgroups of cohort T.
The univariate Cox proportional hazards regression was used to
estimate the expression of DElncRNAs in ceRNA network with overall
survival. Kaplan-Meier survival analysis was used to analyze the
correlation between lncRNA expression and PTC patient
prognosis.
Results
Patient characteristics
The clinical and pathological information of
patients in cohort T was summarized in Table I. The median age of all patients in
cohort T was ~48 years. The distributions of sex and race showed
that white female patients accounted for the majority in the
male/female ratio of 2.8/1 and white race ratio of 80.2%. The 3
histopathological variants, classical PTC (CPTC), follicular PTC
(FPTC) and tall-cell PTC (TCPTC) represented 75.9, 14.3 and 9.8%,
respectively. The proportions and distributions of these patients
clinical characteristics were consistent with previous studies
(24,25).
 | Table I.Demographic and clinical
characteristics of 348 patients with papillary thyroid cancer in
cohort T. |
Table I.
Demographic and clinical
characteristics of 348 patients with papillary thyroid cancer in
cohort T.
|
Parameter/feature | Cohort T (n=348)
(%) |
|---|
| Age, years (mean ±
SD) | 48.1±15.9 |
| Sex |
|
|
Male | 92
(26.4) |
|
Female | 256 (73.6) |
| Race |
|
|
Asian | 46
(13.2) |
| Black
or African American | 22 (6.3) |
|
American Indian or | 1
(0.3) |
| Alaska
native |
|
|
White | 279 (80.2) |
| Subtype |
|
|
CPTC | 264 (75.9) |
|
FPTC | 50
(14.3) |
|
TCPTC | 34 (9.8) |
| Pathologic
stage |
|
| I | 192 (55.2) |
| II | 28 (8.0) |
|
III | 88
(25.3) |
| IV | 40
(11.5) |
| Tumor size |
|
| T1 | 105 (30.2) |
| T2 | 108 (31.0) |
| T3 | 116 (33.3) |
| T4 | 17 (4.9) |
| TX | 2
(0.6) |
| Lymph node |
|
| N0 | 164 (47.2) |
| N1 | 157 (45.2) |
| NX | 27 (7.6) |
| Metastasis
status |
|
| M0 | 231 (66.4) |
| M1 | 6
(1.7) |
| MX | 111 (31.9) |
Differentially expressed lncRNA, mRNA
and miRNA
Considering the dissimilarity among the 3 PTC
variants, RNA expression levels in the 3 PTC variants (cohort T)
vs. normal tissues (cohort N) were investigated, respectively.
Genes with an absolute fold change >2 and FDR value <0.05
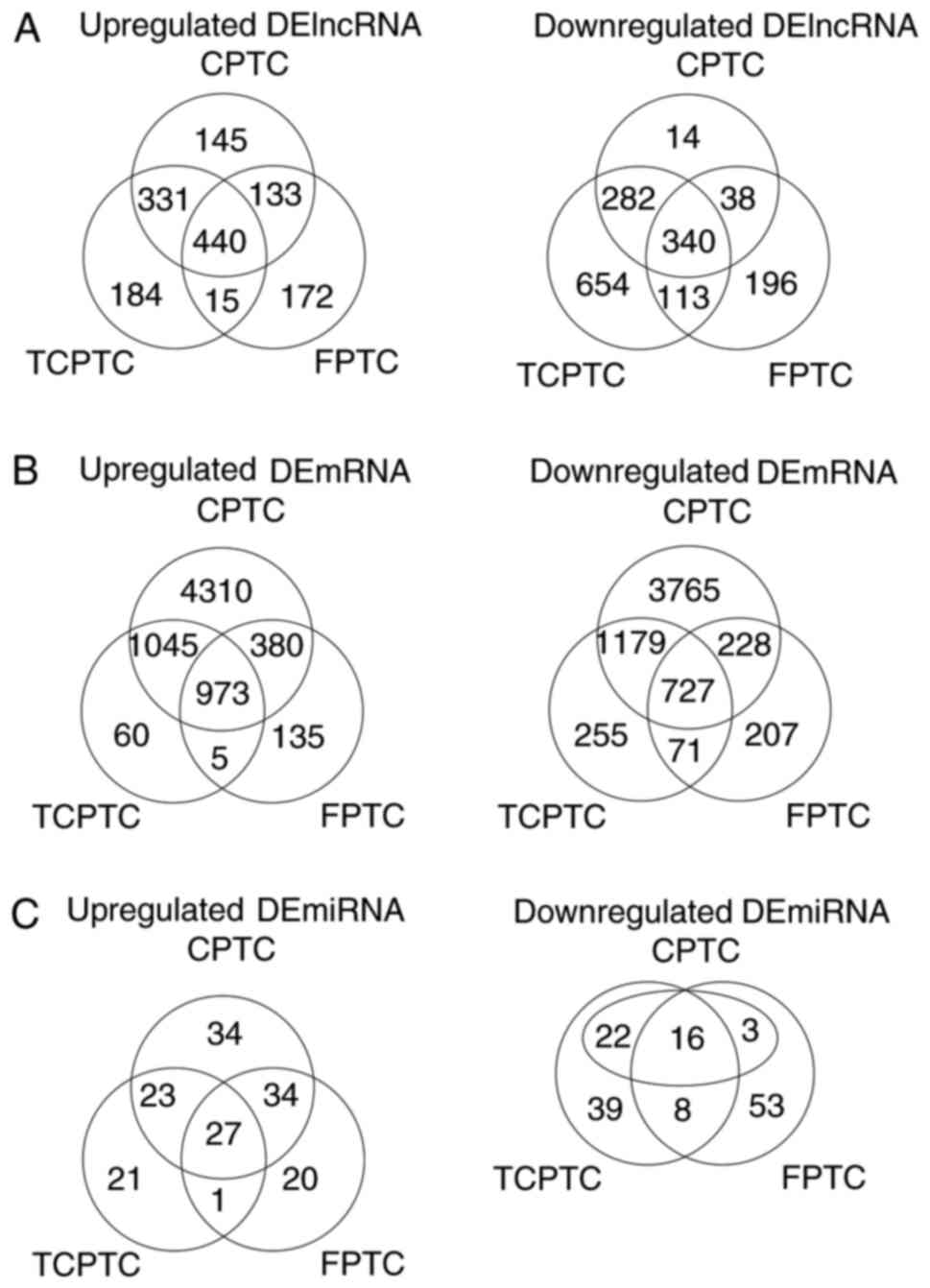
were considered as discriminatively expressed. As a result, a total
of 780 lncRNAs and 1,700 mRNAs were detected to be collectively
dysregulated in carcinoma tissues of 3 PTC variants compared with
normal tissues. Among them, 440 and 340 lncRNAs were commonly
elevated and downregulated, meanwhile, 973 and 727 mRNAs were
collectively upregulated and downregulated, respectively. Filtering
analysis with the same criteria (|log2FC|>1 and FDR
<0.05) also identified 43 miRNAs differentially expressed
between 3 PTC variants and normal thyroid tissues, among them 27
upregulated and 16 downregulated (Fig.
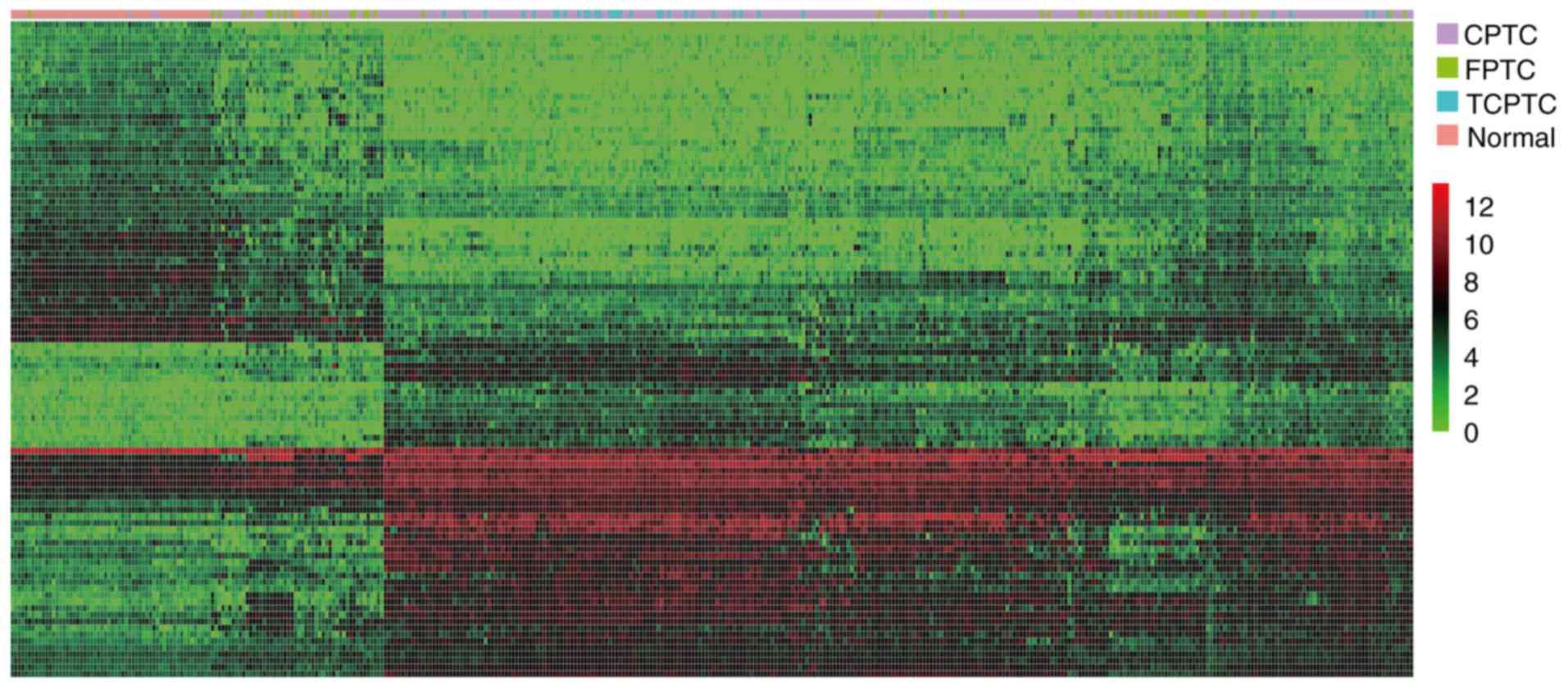
1) (data not shown). To visualize the most significant lncRNAs,
we selected the top 50 upregulated and top 50 downregulated lncRNAs
to construct expression heatmaps (Fig.
2) (data not shown). The data suggested that the expression
profiles of DElncRNAs can distinguish PTC tissues from normal
tissues.
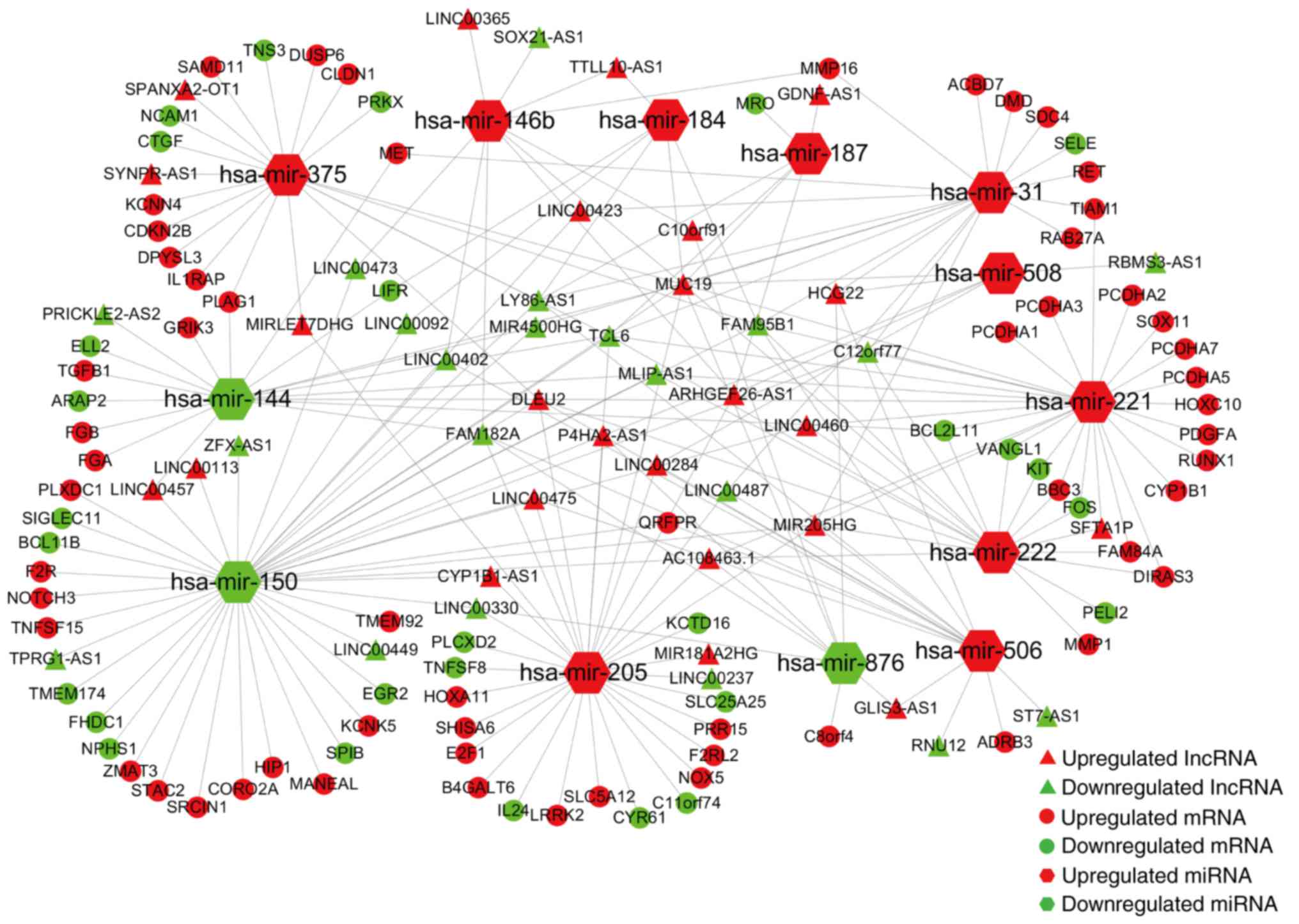
ceRNA network in PTC
In the next step, we predicted the potential
interactions among the above collectively dysregulated genes based
on ceRNA hypothesis by computational analysis to further understand
the function of DElncRNAs. Thirteen specific DEmiRNAs were
identified to interact with 45 DElncRNAs through miRNA response
elements by miRcode online tools (Table II). To improve the predictive
accuracy, TargetScan, miRTarBase and miRanda databases were
combined to predict the candidate mRNA targets of the 11 DEmiRNAs.
After excluding the mRNAs that were not involved in the 1,700
DEmRNAs, 86 mRNAs were enrolled to create the ceRNA network
(Table III). Finally, the ceRNA
network that involved 45 lncRNAs, 13 miRNAs and 86 mRNAs was
visualized using Cytoscape software based on the interactions among
lncRNAs, miRNAs and mRNAs in Tables
II and III (Fig. 3).
 | Table II.Putative miRNAs that may target
lncRNAs by miRNA response elements. |
Table II.
Putative miRNAs that may target
lncRNAs by miRNA response elements.
| lncRNA | miRNAs | lncRNA | miRNAs |
|---|
| AC108463.1 | miR-221, miR-222,
miR-876, miR-150, miR-205 | LINC00475 | miR-506, miR-150,
miR-205 |
| ARHGEF26-AS1 | miR-146b, miR-187,
miR-508, miR-205 | LINC00487 | miR-31, miR-506,
miR-205 |
| C10orf91 | miR-146b,
miR-876 | LY86-AS1 | miR-375, miR-31,
miR-187, miR-506, miR-150, miR-184 |
| C12orf77 | miR-221, miR-222,
miR-31, miR-150 | MIR181A2HG | miR-205 |
| CYP1B1-AS1 | miR-150,
miR-205 | MIR205HG | miR-221, miR-222,
miR-31, miR-506, miR-150, miR-205 |
| DLEU2 | miR-221, miR-222,
miR-144, miR-375, miR-506, miR-150, miR-205 | MIR4500HG | miR-144, miR-31,
miR-150 |
| FAM182A | miR-146b, miR-144,
miR-876, miR-506, miR-150, miR-205 | MIRLET7DHG | miR-375,
miR-205 |
| FAM95B1 | miR-221, miR-222,
miR-375, miR-31, miR-506, miR-150, miR-184 | MLIP-AS1 | miR-221, miR-222,
miR-144, miR-150 |
| GDNF-AS1 | miR-187 | MUC19 | miR-146b, miR-221,
miR-222, miR-144, miR-375, miR-31, miR-187, miR-508, miR-876,
miR-150, miR-205, miR-184 |
| GLIS3-AS1 | miR-876,
miR-506 | P4HA2-AS1 | miR-508, miR-876,
miR-150, miR-205 |
| HCG22 | miR-31, miR-508,
miR-876, miR-506 | PRICKLE2-AS2 | miR-144 |
| LINC00092 | miR-150,
miR-184 | RBMS3-AS1 | miR-508 |
| LINC00113 | miR-150 | RNU12 | miR-506 |
| LINC00237 | miR-205 | SFTA1P | miR-221,
miR-222 |
| LINC00284 | miR-508, miR-506,
miR-205 | SOX21-AS1 | miR-146b |
| LINC00330 | miR-876, miR-150,
miR-205 | SPANXA2-OT1 | miR-375 |
| LINC00365 | miR-146b | ST7-AS1 | miR-506 |
| LINC00402 | miR-146b, miR-506,
miR-150 | SYNPR-AS1 | miR-375 |
| LINC00423 | miR-31,
miR-150 | TCL6 | miR-221, miR-222,
miR-144, miR-375, miR-31, miR-187, miR-150, miR-205 |
| LINC00449 | miR-150 | TPRG1-AS1 | miR-150 |
| LINC00457 | miR-144,
miR-150 | TTLL10-AS1 | miR-146b,
miR-184 |
| LINC00460 | miR-221, miR-222,
miR-150 | ZFX-AS1 | miR-150 |
| LINC00473 | miR-146b,
miR-150 |
|
|
 | Table III.miRNAs targeting mRNAs in papillary
thyroid carcinoma. |
Table III.
miRNAs targeting mRNAs in papillary
thyroid carcinoma.
| miRNA | mRNAs targeted by
miRNA |
|---|
| miR-144 | FGA, ARAP2, PLAG1,
FGB, MET, TGFB1, LIFR, BCL2L11, ELL2, GRIK3 |
| miR-146b | MMP16 |
| miR-150 | BCL11B, STAC2,
CORO2A, TMEM174, SPIB, F2R, PLXDC1, KCNK5, HIP1, NOTCH3, FHDC1,
SRCIN1, TNFSF15, TMEM92, EGR2, NPHS1, MANEAL, QRFPR, ZMAT3,
SIGLEC11 |
| miR-184 | LIFR |
| miR-187 | MRO |
| miR-205 | NOX5, TNFSF8,
KCTD16, CYR61, SLC25A25, PLCXD2, SLC5A12, C11orf74, F2RL2, LRRK2,
E2F1, HOXA11, IL24, B4GALT6, PRR15, SHISA6 |
| miR-221 | PCDHA2, SOX11,
PCDHA1, FOS, PCDHA3, PCDHA7, FAM84A, RUNX1, PDGFA, KIT, PCDHA5,
TIAM1, DIRAS3, BBC3, BCL2L11, VANGL1, HOXC10, CYP1B1 |
| miR-222 | BBC3, DIRAS3, FOS,
VANGL1, KIT, PELI2, FAM84A, MMP1, BCL2L11 |
| miR-31 | DMD, RET, ACBD7,
SELE, MMP16, RAB27A, TIAM1, MET, SDC4 |
| miR-375 | KCNN4, CTGF, DUSP6,
TNS3, IL1RAP, PLAG1, DPYSL3, NCAM1, PRKX, SAMD11, CLDN1,
CDKN2B |
| miR-506 | ADRB3, QRFPR |
| miR-876 | C8orf4 |
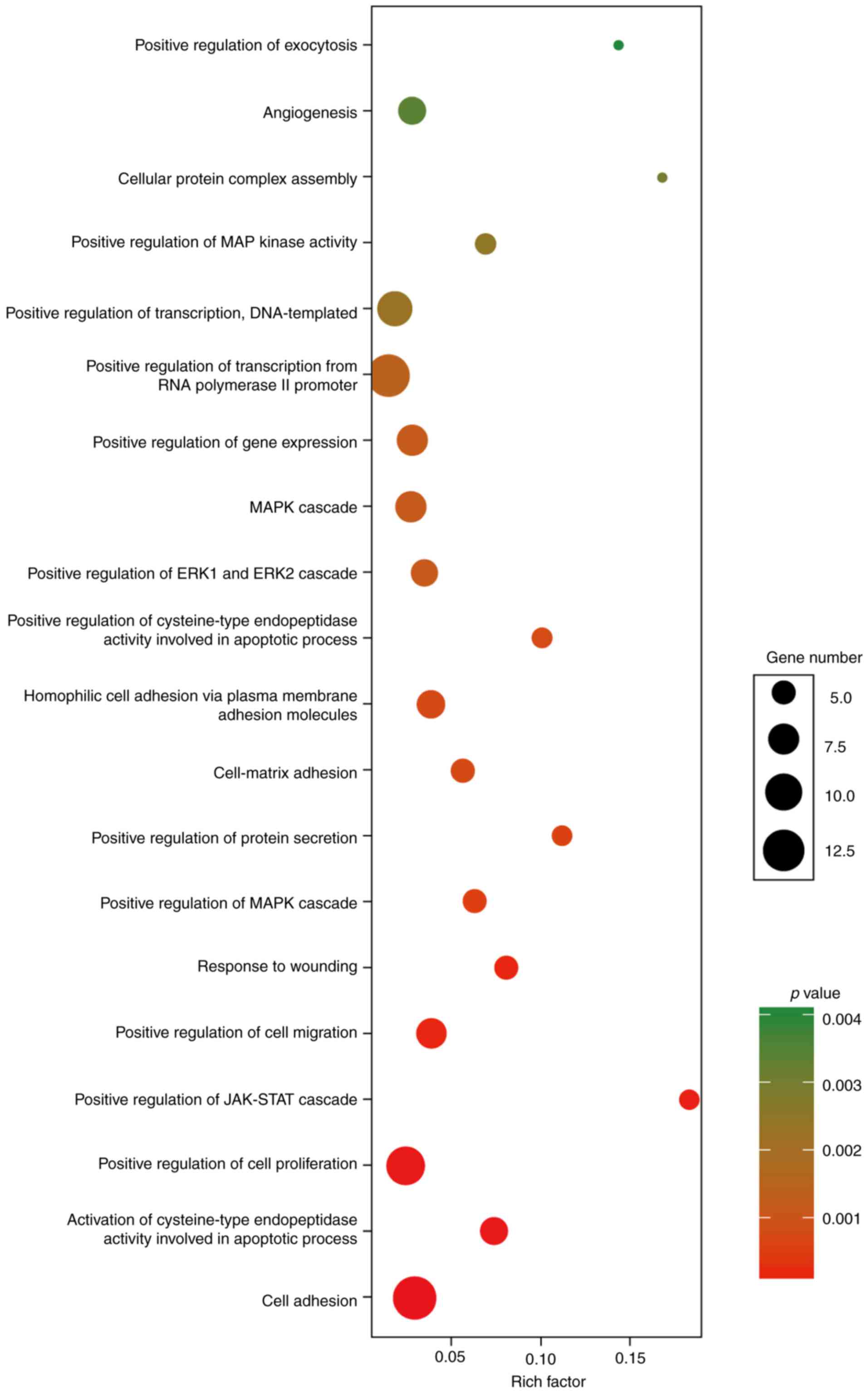
Functional analysis of DEmRNAs in the
ceRNA network
The function analysis revealed that the 86 DEmRNAs
in the above ceRNA network were enriched in 60 GO biological
process categories and 7 KEGG categories (P<0.05). The
significant GO biological processes of dysregulated genes were 3
cell adhesion terms (GO:0007155, GO:0007160 and GO:007156)
(Fig. 4). Table IV contains the significantly
enriched pathways of these DEmRNAs by KEGG analysis. There were two
cancer-related pathways, including pathways in cancer and miRNAs in
cancer. Notably, transcriptional factor E2F1 had an elevated
expression in PTC tissues and was involved as the key component in
both cancer-related pathways.
 | Table IV.KEGG pathways enriched by DEmRNA
involved in the ceRNA network. |
Table IV.
KEGG pathways enriched by DEmRNA
involved in the ceRNA network.
| Pathway ID | Description | P-value | Numbers of
DEmRNAs |
|---|
| hsa05200 | Pathways in
cancer | 8.99E-05 | E2F1, FOS, RET,
CDKN2B, PDGFA, MET, KIT, RUNX1, TGFB1, MMP1, F2R |
| hsa04060 | Cytokine-cytokine
receptor interaction | 4.12E-04 | PDGFA, IL1RAP, MET,
TNFSF15, LIFR, KIT, TGFB1, TNFSF8 |
| hsa04144 | Endocytosis | 0.0043302 | ADRB3, RET, MET,
KIT, ARAP2, TGFB1, F2R |
| hsa05206 | MicroRNAs in
cancer | 0.0070069 | NOTCH3, E2F1,
CYP1B1, PDGFA, MET, MMP16, BCL2L11 |
| hsa05166 | HTLV–I
infection | 0.018641 | E2F1, FOS, EGR2,
CDKN2B, PDGFA, TGFB1 |
| hsa05144 | Malaria | 0.0354242 | MET, SELE,
TGFB1 |
| hsa04015 | Rap1 signaling
pathway | 0.0377107 | TIAM1, PDGFA, MET,
KIT, F2R |
lncRNAs in relation to clinical
outcome
To further understand whether dysregulated lncRNAs
were correlated with patient outcome, the 45 DElncRNAs involved in
the network were analyzed according to clinical characteristics
including sex, race, TNM staging system and pathologic stage. The
expression levels of 9 lncRNAs were aberrantly expressed in
clinical feature comparisons of cohort T (|log2FC|>1
and FDR<0.05) (Table V). Among
them, we identified 3 lncRNAs (FAM182A, LINC00402 and LINC00473)
differentially expressed in race subgroup. Four upregulated lncRNAs
(MIR205HG, C12orf7, TCL6 and LINC00460) and 4 downregulated lncRNAs
(GDNF-AS1, LINC00402, LINC00473 and LINC00237) were markedly
related to tumor progression. LINC00473 may not only inhibit tumor
growth (T3+T4 vs. T1+T2), but also decrease in individuals with
poor pathologic stage, suggesting its potential negative role in
tumor development of PTC. Overexpression lncRNA LINC00460 was
identified to be correlated with the poor pathologic stage.
 | Table V.The correlation between lncRNA in the
ceRNA network and patient characteristics. |
Table V.
The correlation between lncRNA in the
ceRNA network and patient characteristics.
| Comparisons | Downregulated | Upregulated |
|---|
| Race |
| FAM182A, LINC00402
LINC00473 |
| (Asian
vs. White) |
|
|
| Tumor size | GDNF-AS1,
LINC00402, LINC00473 |
|
| (T3+T4
vs. T1+T2) |
|
|
| Lymph node | LINC00237 | MIR205HG, C12orf77,
TCL6 |
| (N1 vs.
N0) |
|
|
| Pathologic
stage | LINC00473 | LINC00460 |
| (Stage
III+IV vs. stage I+II) |
|
|
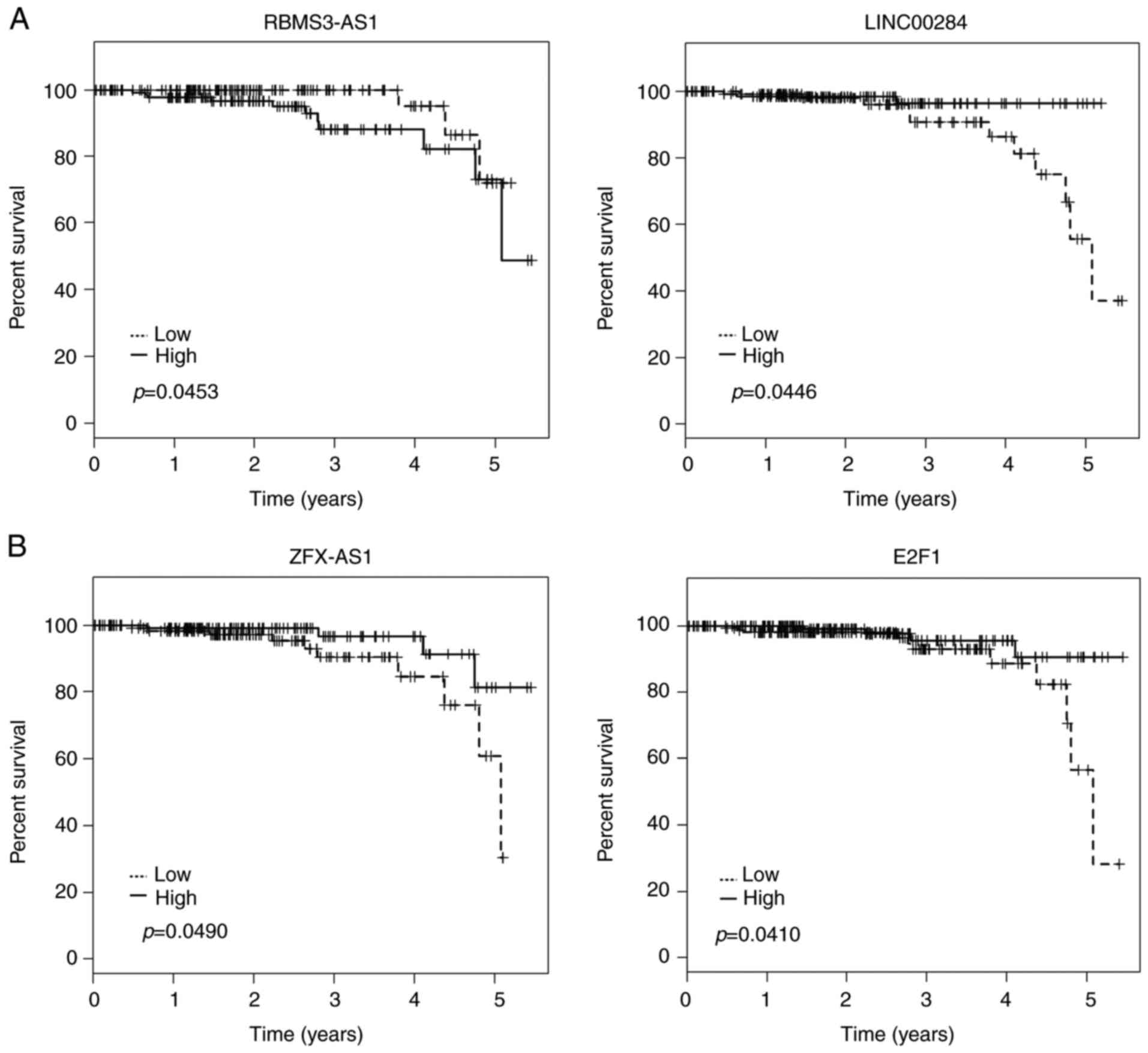
Kaplan-Meier analysis was applied to investigate
overall survival for DElncRNAs in cohort T. Among the 45 DElncRNAs
in the ceRNA network, 3 lncRNAs (LINC00284, RBMS3-AS1 and ZFX-AS1)
were identified to be associated with overall survival by
univariate Cox regression analysis (log-rank, P<0.05).
Kaplan-Meier plots indicated that individuals with relatively
higher RBMS3-AS1 expression tended to have shorter overall survival
time. On the contrary, LINC00284 and ZFX-AS1 could prolong patient
survival time (Fig. 5A).
Kaplan-Meier survival analysis was also assessed to estimate
overall survival for the potentially associated DEmRNAs of these
lncRNAs. Notably, high expression of E2F1 was also predicted to be
positively associated with patient survival time (Fig. 5B).
Discussion
PTC is the most frequent endocrine neoplasia
characterized by a variety of aberrant molecular events driving
tumori-genesis (26). Currently,
much effort has been made to elucidate the roles of ncRNAs involved
in tumorigenesis, disease progression and metastasis of PTC
(17,27,28).
According to the ceRNA hypothesis, increasing evidence has
demonstrated that lncRNAs regulated target genes through microRNAs
(miRNAs) competition (29). To
comprehensively identify the landscape regarding how
lncRNA-associated ceRNA network affects PTC, the present study
analyzed the large-scale sequencing data of PTC patient cohort in
TCGA database. We identified the aberrantly expressed lncRNAs,
mRNAs and miRNAs, and further successfully constructed the
dysregulated lncRNA-associated ceRNA network in PTC by biological
prediction. We also identified several lncRNAs and their related
mRNAs to be potential prognostic indicators for PTC patients.
In previous studies, several groups have identified
numerous abnormally expressed lncRNAs in PTC compared with
non-tumor tissues. Two small cohort studies based on microarray
sequencing of 3 and 5 pairs of PTC samples, respectively, have
identified thousands of significantly dysregulated lncRNAs and
validated several DElncRNAs by real-time PCR (18,30).
Xie et al reported a ceRNA network in PTC containing 29
lncRNAs, 9 miRNAs and 67 mRNAs by microarray analysis of 5-paired
clinical samples (31). However,
lncRNA-associated ceRNA network based on whole-genome gene
expression profiling with large study population in PTC has not
been described.
In the present study, 780 lncRNAs were detected as
commonly dysregulated lncRNAs in 3 PTC variants (CPTC, FPTC and
TCPTC) compared with normal tissues, including 440 upregulated and
340 downregulated. Among them, 45 aberrantly expressed lncRNAs were
identified in the ceRNA network, and 8 out of them were related to
tumor progression. The low expression of an intergenic lncRNA
LINC00473 in PTC tumor tissues (~10-folds) may have potential
capacity to promote tumor growth and pathologic stage. It has been
identified to have prognostic value for lung cancer in a cyclic
AMP-dependent manner (32).
However, whether LINC00473 can utilize similar mechanism to lung
cancer in PTC needs further research. LINC00460, a human gene
transcribed from chromosome 13, is upregulated in various cancers,
including thyroid cancer (33). The
upregulation of LINC00460 was also observed in the present study
and was positively correlated with the pathologic stage of PTC. It
was reported that LINC00460 participated in a series of biological
processes (e.g., metabolic process, chromatin modification, cell
cycle and cell death) and showed prognostic prediction power for
head and neck squamous cell carcinoma (33,34).
These findings implied that LINC00460 may functionally be involved
in the tumorigenesis of PTC. Moreover, RBMS3-AS1 and ZFX-AS1 also
had potential values for PTC patients prognosis. Since these
lncRNAs are reported for the first time as prognostic predictors,
much effort deserves to be made to elucidate their biological
function in neoplasms. Although the lncRNAs listed in Table V had no statistical significance in
Kaplan Meier analysis (data not shown), both the lnRNAs that were
associated with the progression of PTC and with patients' overall
survival may have potential roles in PTC and are worth studying in
the future.
However, having identified several specific lncRNAs
for PTC, the present study drew a comprehensive ceRNA network,
which revealed the complexity of the regulatory relationships among
lncRNAs, miRNAs and mRNAs. E2F1, the best-studied E2F member, was
significantly upregulated in PTC tumor tissues, which was
consistent with a previous study (35). E2F1 was involved in both
cancer-related pathways: pathways in cancer and miRNAs in cancer.
Although previously E2F1 was found to promote thyroid
carcinogenesis (35), our results
demonstrated that high expression of E2F1 could prolong survival
time of PTC patients, which strongly suggested its
tumor-suppressive activity in thyroid cancer. Thus, we supposed
that E2F1 may exhibit both oncogenic and tumor suppressive
properties in thyroid cancer resulting from its capacity to induce
tumor cell proliferation and apoptosis (36–38).
The ceRNA network predicted that E2F1 was a target gene of miR-205.
It was consistent with the previous study that miR-205 induced
cancer senescence by regulating E2F1 and ultimately suppressed
melanoma development (39). Our
bioinformatic analysis predicted that miR-205 may interact with
LINC00284, the increased expression of which was also positively
correlated with the prognosis of PTC patients. Although the
function of LINC00284 is poorly annotated, it is conceivable that
LINC00284 may act as a ceRNA to interact with miR-205 and E2F1 in
PTC.
There are several limitations in the present study.
First, ceRNA hypothesis indicates that lncRNAs can serve as ceRNAs
to regulate expression of the target genes by merging miRNAs.
Therefore, the complex ceRNA network of PTC was built based on this
hypothesis using biological prediction. Second, the ceRNA network
with hundreds of connections suggested that one lncRNA connected
with a number of miRNAs and relevant mRNAs. In this regard, our
further study may focus on conducting experiments to validate
several crucial interactions, particularly to verify the key
interactions we discussed above and determine the roles of these
lncRNAs as ceRNA in PTC. Third, the 3 lncRNAs that were aberrantly
expressed in race need extensive validation studies, which must be
based on a large-scale multiracial cohort.
In conclusion, we identified various dysregulated
lncRNAs, miRNAs and mRNAs between tumor and non-tumor samples by
genome-wide analysis of a large PTC patient cohort from TCGA. The
lncRNA-related ceRNA network will be useful to guide further
investigation concerning the involved lncRNAs of their definite
mechanisms and functions in the tumorigenesis of PTC.
Acknowledgements
We thank The Cancer Genome Atlas (TCGA) Research
Network for providing data and publication permission (http://cancergenome.nih.gov/). The present study was
supported by grants from the Chinese National Natural Science
Foundation Grant (nos. 81671541, 81273202 and 31400773), the
Clinical Medicine Science and Technology Project of Jiangsu
Province of China (BL2013024), the Postgraduate Research and
Practice Innovation Program of Jiangsu Province (KYCX17_1820) and
the China Scholarship Council. The present study was also supported
by the Program of Innovative Research Team of Jiangsu Province, the
Project of the Priority Academic Program Development of Jiangsu
Higher Education Institutions and the Project of the Key Academic
Program Development of Jiangsu University (1291270019).
Glossary
Abbreviations
Abbreviations:
|
TC
|
thyroid cancer
|
|
PTC
|
papillary thyroid cancer
|
|
FTC
|
follicular thyroid cancer
|
|
ATC
|
anaplastic thyroid cancer
|
|
CPTC
|
classical PTC
|
|
FPTC
|
follicular PTC
|
|
TCPTC
|
tall-cell PTC
|
|
ncRNA
|
non-coding RNA
|
|
lncRNA
|
long non-coding RNA
|
|
miRNA
|
microRNA
|
|
ceRNA
|
competitive endogenous RNA
|
|
TCGA
|
The Cancer Genome Atlas
|
|
MREs
|
miRNA response elements
|
|
DAVID
|
The Database for Annotation
Visualization and Integrated Discovery
|
|
GO
|
Gene Ontology
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
|
AJCC
|
American Joint Committee on Cancer
|
|
FC
|
fold change
|
|
FDR
|
false discovery rate
|
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pellegriti G, Frasca F, Regalbuto C,
Squatrito S and Vigneri R: Worldwide increasing incidence of
thyroid cancer: Update on epidemiology and risk factors. J Cancer
Epidemiol. 2013:9652122013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schneider DF and Chen H: New developments
in the diagnosis and treatment of thyroid cancer. CA Cancer J Clin.
63:374–394. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rosenbaum MA and McHenry CR: Contemporary
management of papillary carcinoma of the thyroid gland. Expert Rev
Anticancer Ther. 9:317–329. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brosnan CA and Voinnet O: The long and the
short of noncoding RNAs. Curr Opin Cell Biol. 21:416–425. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fatica A and Bozzoni I: Long non-coding
RNAs: New players in cell differentiation and development. Nat Rev
Genet. 15:7–21. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gibb EA, Brown CJ and Lam WL: The
functional role of long non-coding RNA in human carcinomas. Mol
Cancer. 10:382011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Salmena L, Poliseno L, Tay Y, Kats L and
Pandolfi PP: A ceRNA hypothesis: The Rosetta stone of a hidden RNA
language? Cell. 146:353–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xia T, Liao Q, Jiang X, Shao Y, Xiao B, Xi
Y and Guo J: Long noncoding RNA associated-competing endogenous
RNAs in gastric cancer. Sci Rep. 4:60882014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang J, Fan D, Jian Z, Chen GG and Lai P:
Cancer specific long noncoding RNAs show differential expression
patterns and competing endogenous RNA potential in hepatocellular
carcinoma. PLoS One. 10:e01410422015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang K, Li Q, Kang X, Wang Y and Wang S:
Identification and functional characterization of lncRNAs acting as
ceRNA involved in the malignant progression of glioblastoma
multiforme. Oncol Rep. 36:2911–2925. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sui J, Li YH, Zhang YQ, Li CY, Shen X, Yao
WZ, Peng H, Hong WW, Yin LH, Pu YP, et al: Integrated analysis of
long non-coding RNA-associated ceRNA network reveals potential
lncRNA biomarkers in human lung adenocarcinoma. Int J Oncol.
49:2023–2036. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu L, Yang J, Zhu X, Li D, Lv Z and Zhang
X: Long noncoding RNA H19 competitively binds miR-17-5p to regulate
YES1 expression in thyroid cancer. FEBS J. 283:2326–2339. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Arancio W, Carina V, Pizzolanti G,
Tomasello L, Pitrone M, Baiamonte C, Amato MC and Giordano C:
Anaplastic thyroid carcinoma: A ceRNA analysis pointed to a
crosstalk between SOX2, TP53, and microRNA biogenesis. Int J
Endocrinol. 2015:4393702015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang CT, Oyang YJ, Huang HC and Juan HF:
MicroRNA-mediated networks underlie immune response regulation in
papillary thyroid carcinoma. Sci Rep. 4:64952015. View Article : Google Scholar
|
|
18
|
Lan X, Zhang H, Wang Z, Dong W, Sun W,
Shao L, Zhang T and Zhang D: Genome-wide analysis of long noncoding
RNA expression profile in papillary thyroid carcinoma. Gene.
569:109–117. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Robinson MD, McCarthy DJ and Smyth GK:
edgeR: A Bioconductor package for differential expression analysis
of digital gene expression data. Bioinformatics. 26:139–140. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jeggari A, Marks DS and Larsson E:
miRcode: A map of putative microRNA target sites in the long
non-coding transcriptome. Bioinformatics. 28:2062–2063. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Riffo-Campos ÁL, Riquelme I and
Brebi-Mieville P: Tools for sequence-based miRNA target prediction:
What to choose? Int J Mol Sci. 17(pii): E19872016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hsu SD, Lin FM, Wu WY, Liang C, Huang WC,
Chan WL, Tsai WT, Chen GZ, Lee CJ, Chiu CM, et al: miRTarBase: A
database curates experimentally validated microRNA-target
interactions. Nucleic Acids Res. 39:D163–D169. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
LiVolsi VA: Papillary thyroid carcinoma:
An update. Mod Pathol. 24 Suppl 2:S1–S9. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tunca F, Sormaz IC, Iscan Y, Senyurek YG
and Terzioglu T: Comparison of histopathological features and
prognosis of classical and follicular variant papillary thyroid
carcinoma. J Endocrinol Invest. 38:1327–1334. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xing M: Molecular pathogenesis and
mechanisms of thyroid cancer. Nat Rev Cancer. 13:184–199. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aragon Han P, Weng CH, Khawaja HT,
Nagarajan N, Schneider EB, Umbricht CB, Witwer KW and Zeiger MA:
MicroRNA expression and association with clinicopathologic features
in papillary thyroid cancer: A systematic review. Thyroid.
25:1322–1329. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Q, Li H, Zhang L, Zhang C, Yan W and
Wang C: Identification of novel long non-coding RNA biomarkers for
prognosis prediction of papillary thyroid cancer. Oncotarget.
8:46136–46144. 2017.PubMed/NCBI
|
|
29
|
Ergun S and Oztuzcu S: Oncocers:
ceRNA-mediated cross-talk by sponging miRNAs in oncogenic pathways.
Tumor Biol. 36:3129–3136. 2015. View Article : Google Scholar
|
|
30
|
Yang M, Tian J, Guo X, Yang Y, Guan R, Qiu
M, Li Y, Sun X, Zhen Y, Zhang Y, et al: Long noncoding RNA are
aberrantly expressed in human papillary thyroid carcinoma. Oncol
Lett. 12:544–552. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xie J, Guo B, Ding Z, Kang J, Deng X, Wu B
and Fan Y: Microarray analysis of lncRNAs and mRNAs co-expression
network and lncRNA function as ceRNA in papillary thyroid
carcinoma. J Biomater Tiss Eng. 5:872–880. 2015. View Article : Google Scholar
|
|
32
|
Chen Z, Li J, Lin S, Cao C, Gimbrone NT,
Yang R, Fu DA, Carper MB, Haura EB, Schabath MB, et al:
cAMP/CREB-regulated LINC00473 marks LKB1-inactivated lung cancer
and mediates tumor growth. J Clin Invest. 126:2267–2279. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang Y, Tao Y and Liao Q: Long noncoding
RNA: A crosslink in biological regulatory network. Brief Bioinform.
Apr 24–2017.(Epub ahead of print).
|
|
34
|
Cao W, Liu JN, Liu Z, Wang X, Han ZG, Ji
T, Chen WT and Zou X: A three-lncRNA signature derived from the
Atlas of ncRNA in cancer (TANRIC) database predicts the survival of
patients with head and neck squamous cell carcinoma. Oral Oncol.
65:94–101. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Onda M, Nagai H, Yoshida A, Miyamoto S,
Asaka S, Akaishi J, Takatsu K, Nagahama M, Ito K, Shimizu K and Emi
M: Up-regulation of transcriptional factor E2F1 in papillary and
anaplastic thyroid cancers. J Hum Genet. 49:312–318. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hallstrom TC, Mori S and Nevins JR: An
E2F1-dependent gene expression program that determines the balance
between proliferation and cell death. Cancer Cell. 13:11–22. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tian W, Cui F and Esteban MA: E2F1 in
renal cancer: Mr Hyde disguised as Dr Jekyll? J Pathol.
231:143–146. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhan L, Zhang Y, Wang W, Song E, Fan Y and
Wei B: E2F1: A promising regulator in ovarian carcinoma. Tumour
Biol. 37:2823–2831. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dar AA, Majid S, de Semir D, Nosrati M,
Bezrookove V and Kashani-Sabet M: miRNA-205 suppresses melanoma
cell proliferation and induces senescence via regulation of E2F1
protein. J Biol Chem. 286:16606–16614. 2011. View Article : Google Scholar : PubMed/NCBI
|