Introduction
Multiple myeloma (MM) is an incurable hematologic
neoplasm of plasma cells. Despite the emergence of novel drugs
including proteasome inhibitors and immunomodulating drugs (IMiDs)
that have improved the overall patient outcome (1), the prognosis for MM patients is still
poor, and the pathogenesis of this disease is only partially
understood. Angiogenesis plays a significant role in the
pathogenesis of MM (2). Recent data
have shown that there is a close relationship between MM cells and
adjacent cells in the microenvironment (3) and that angiogenesis in MM is tightly
controlled by angiogenic cytokines and inflammatory factors
(4,5). However, how the release of these
cytokines is regulated and how myeloma cells act on microvascular
endothelial cells and bone marrow stromal cells to promote
angiogenesis remain unclear.
Microvesicles (MVs), a type of extracellular
vesicles (EVs), are also called shedding vesicles or microparticles
(MPs) and have emerged as important players in cell-to-cell
communication (6–8). MVs can load and transmit bioactive
molecules to target cells (9–11) and
induce different signaling cascades (10,12,13). A
previous study found that MVs derived from MM cells (MM MVs) can be
internalized by endothelial cells and promote angiogenesis
(14). However, the mechanism by
which MM MVs promote endothelial cell angiogenesis remains
unclear.
The NF-κB signaling pathway is constitutively active
in MM and regulates the expression of numerous genes, such as
vascular endothelial growth factor (VEGF) and interleukin 6 (IL-6),
that are involved in the pathogenesis of MM including angiogenesis
(15–17). Bortezomib and lenalidomide are
important drugs for treating myeloma, and both of them block NF-κB
activation (18–20). Some studies have examined the MVs
shed by cells after drug treatments. One study demonstrated that
tumor-derived microparticles (TMPs) from mammary carcinoma cells
exposed to paclitaxel chemotherapy contributed to angiogenesis and
tumor regrowth (21). However,
another study showed that the neutralization of VEGF-A with
anti-angiogenic drugs in cultured breast carcinoma cells blocked
TMP-induced angiogenesis (22).
There has been little research on the effect of drugs on MM MVs. A
recent study (23) showed that the
pro-angiogenic activity of MVs derived from bortezomib-treated
myeloma cells was decreased, but the molecular mechanisms behind
this inhibition of angiogenesis have not yet been elucidated.
Moreover, there have been no reports on the effect of IMiDs,
including thalidomide and lenalidomide, on the pro-angiogenic
activity of MM MVs.
In the present study, we determined whether and how
bortezomib and lenalidomide affect the characteristics of MM MVs
derived from RPMI-8226 cells, including their number and expression
of pro-angiogenic factors and their effects on the migration,
invasion and tube formation of endothelial cells. In this context,
we evaluated the expression levels of pro-angiogenic factors and
the activity of NF-κB in endothelial cells.
Materials and methods
Cell lines and culture
The RPMI-8226 human multiple myeloma cell line and
human umbilical vein endothelial cells (HUVECs) were purchased from
the American Type Culture Collection (ATCC, Manassas, VA, USA). The
cells were cultured in medium containing 10% fetal bovine serum
(FBS, Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
1% penicillin-streptomycin (Solarbio, Beijing, China) at 37°C with
5% CO2.
MV isolation
MVs were isolated by continuous differential
centrifugation as previously described (24) from the different conditioned media
of myeloma cells. Briefly, MM cells growing in log phase were
seeded at 5×105 cells/ml and were randomly divided into
5 groups depending on treatment: control group (PBS), 10 or 100 nM
bortezomib (Selleck Chemicals, Houston, TX, USA), 1 or 10 µM
lenalidomide (MedChem Express, Monmouth Junction, NJ, USA). MVs
derived from corresponding RPMI-8226 cells from the 5 groups above
were named: N-MV (PBS), B10-MV (10 nM bortezomib), B100-MV (100 nM
bortezomib), L1-MV (1 µM lenalidomide), and L10-MV (10 µM
lenalidomide). After 24 h of culture, the conditioned media were
centrifuged at 750 × g for 5 min and then at 1500 × g for 20 min to
remove cells and debris. After further centrifugation at 16000 × g
for 45 min at 4°C, the MVs were pelleted. After washing twice with
PBS, the pelleted MVs were resuspended in PBS, stored at 4°C and
used for experiments within a few days.
Flow cytometric analysis of MVs
The number of MVs was determined by flow cytometry
(CytoFLEX, Beckman Coulter, Inc., Brea, CA, USA) following the
manufacturer's protocols. Standard microbeads (1.1 µm,
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) were used for size
calibration, and the number of MVs were calculated using 3-µm
standard microbeads (Sigma-Aldrich; Merck KGaA) as previously
described (24). Briefly, 1.1-µm
standard microbeads were used to set the gate, 3-µm standard
microbeads of an equal concentration were added to the sample as an
internal standard, and the counting endpoint was set at 100,000
events for the 3-µm standard bead population.
Quantitative real-time polymerase
chain reaction (qPCR) and enzyme-linked immunosorbent assay
(ELISA)
To evaluate the expression levels of pro-angiogenic
factors, using qPCR, we analyzed the mRNA expression levels of
VEGF, IL-6 and basic fibroblast growth factor (bFGF) in different
groups of MM MVs and HUVECs, and by ELISA, we analyzed the protein
expression levels of these factors in the conditioned medium of
HUVECs. Additionally, we measured the mRNA expression of NF-κB in
HUVECs. MM MVs from the 5 different groups were isolated and
subjected to RNA preparation using TRIzol (Invitrogen; Thermo
Fisher Scientific, Inc.) and to cDNA synthesis with a cDNA
Synthesis kit (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The total RNA of HUVECs (5×104
cells/ml) co-cultured for 24 h with MM MVs (10 µg/ml) from the 5
different groups above or without MVs was isolated, and cDNA was
synthesized. Each qPCR reaction was prepared in triplicate. PCR was
performed using SuperReal PreMix Plus (SYBR Green) (Tiangen,
Beijing, China) with a real-time PCR system (Rotor-Gene Q, Qiagen,
Hilden, Germany). The following primer sequences (Invitrogen;
Thermo Fisher Scientific, Inc.) were used: VEGF,
5′-AATGCTTTCTCCGCTCTGAA-3′(F) and 5′-GCTTCCTACAGCACAGCAGA-3′ (R)
(96 bp); IL-6, 5′-ATGAGGAGACTTGCCTGGTGAAAAT-3′ (F) and
5′-TCTGGCTTGTTCCTCACTACT-3′ (R) (104 bp); bFGF,
5′-TTCATAGCAAGGTACCGGTTG-3′ (F) and 5′-AGAAGAGCGACCCACACG-3′ (R)
(97 bp); NF-κB, 5′-GGGGACTACGACCTGAATG-3′ (F) and
5′-GGGCACGATTGTCAAAGAT-3′ (R) (118 bp); and β-actin,
5′-GAGCTACGAGCTGCCTGAC-3′ (F) and 5′-GGTAGTTTCGTGGATGCCACAG-3′ (R)
(121 bp). The qPCR conditions were 2 min at 50°C, 2 min at 95°C and
then 40 cycles of 15 sec at 95°C and 30 sec at 60°C. A comparative
∆Ct method was used to determine gene expression and β-actin served
as the normalization gene.
The expression levels of human VEGF, IL-6 and bFGF
in the supernatants of HUVECs were measured by ELISA kits
(NeoBioscience, Shenzhen, China) according to the manufacturer's
instructions. Experiments were performed in triplicate.
Angiogenesis assays
Wound healing assay
Cell migration was determined using a scratch
adhesion test. Briefly, the outside undersurface of a 6-well plate
was scored with a marker pen; HUVECs (5×105 cells/well)
were seeded onto a 6-well plate and cultured overnight to
confluence. Then, at time zero, the cell monolayer was wounded
artificially across the marked line using a 200-µl pipette tip. The
scratched area was captured using an inverted microscope (Axio
Observer D1, Zeiss, Germany) in low-power fields (×40). Then, the
cells were treated with medium (DMEM without FBS) and the 5 groups
of MVs (10 µg/ml) for 24 h. Negative control cells were treated
with PBS. The wounded area was captured again at 24 h and measured
by ImageJ software (National Institutes of Health, Bethesda, MD,
USA). The percentage of recovery was assessed. The experiment was
repeated in triplicate, and each sample was tested in
quadruplicate.
Cell invasion assay
Endothelial cell invasiveness was investigated using
Transwell Boyden chambers (6.5 mm, Costar, USA). The polycarbonate
membrane (8-µm pore size) was coated with a layer of Matrigel
matrix (Corning Inc., Corning, NY, USA). HUVECs (2×105
cells per well) were added to the upper chamber and starved; 750 µl
of complete medium with the indicated group of MVs was added at the
indicated concentration (50 µg/ml) to the lower compartment of the
chamber as a chemoattractant. PBS was used as the negative control.
After incubation at 37°C for 6 h, the cells on the bottom surface
of the membrane were fixed with methanol, stained with Diff-Quik
(Nanjing Jiancheng Bioengineering Institute, Nanjing, China) and
counted in five representative high-power fields (×400).
Tube formation assay
Matrigel (50 µl, Corning Inc.) was added to each
well of a 96-well plate and allowed to polymerize at 37°C for 30
min. Then, HUVECs (2×104 cells per well) were cultured
in DMEM with 6% FBS in the presence of the 5 groups of MVs (10
µg/ml). PBS was used as the negative control. Each treatment was
repeated in three wells. After incubation for 6 and 24 h, images of
the tubular structure that formed in each well were captured with
an inverted microscope (Axio Observer D1, Zeiss, Germany) with an
attached digital camera (Axiocam 506 color, Zeiss, Germany). The
closed tube formation was quantified in five low-power fields
(×40).
Immunofluorescent (IF) measurement of
NF-κB
HUVECs were seeded on climbing slices in 24-well
plates and co-cultured with the 5 groups of MM MVs or PBS for 24 h.
To measure the effect of NF-κB activation, an NF-κB Activation
Nuclear Translocation assay kit (Beyotime Biotech, Haimen, China)
was used according to the manufacturer's protocol and a previous
study (25). Nuclei (blue) and
NF-κB (red) were viewed with a fluorescence-equipped microscope
(BX51, Olympus, Tokyo, Japan). Co-localization of NF-κB with the
nucleus appeared purple.
Western blot analysis
After co-culture with one of the 5 different MVs at
10 µg/ml or with PBS for 24 h, nuclear protein or total protein
were extracted from HUVECs using a Nuclear Protein Extraction kit
(BestBio, Shanghai, China) or a Total Protein Extraction kit
(BestBio), respectively, and then quantified by the BCA method.
Equal amounts (50 µg) of protein extracts were loaded and separated
by SDS-PAGE and then transferred electrophoretically to a
polyvinylidene difluoride (PVDF) membrane. Nonspecific sites were
blocked by incubation with 5% nonfat dry milk in TBST at room
temperature for 1 h. The membranes were incubated with the
indicated primary antibodies [P65 (1:50000, 7R200963-9, Abcam,
Cambridge, MA, USA, rabbit monoclonal), p-p65 (1:1000, 3033S, Cell
Signaling Technology, Inc., Danvers, MA, USA; rabbit monoclonal),
H3 (1:2000, KM9005T, Tianjin Sungene Biotech, Tianjin, China; mouse
monoclonal), and β-actin (1:1000, sc-130657, Santa Cruz
Biotechnology, Santa Cruz, CA, USA; rabbit polyclonal)] in blocking
buffer overnight at 4°C. Anti-rabbit or mouse IgG (1:10000, 21032,
Rockland, Limerick, PA, USA) was used as a secondary antibody and
incubated with the membrane for 2 h at room temperature. After
washing, reactive bands were identified by scanning infrared laser
microscopy (LI-COR Biosciences, Lincoln, NE, USA).
Statistical analysis
All data are expressed as the means ± SEM of at
least three experiments. Differences among groups were analyzed
with one-way ANOVA, and differences were considered statistically
significant at P<0.05.
Results
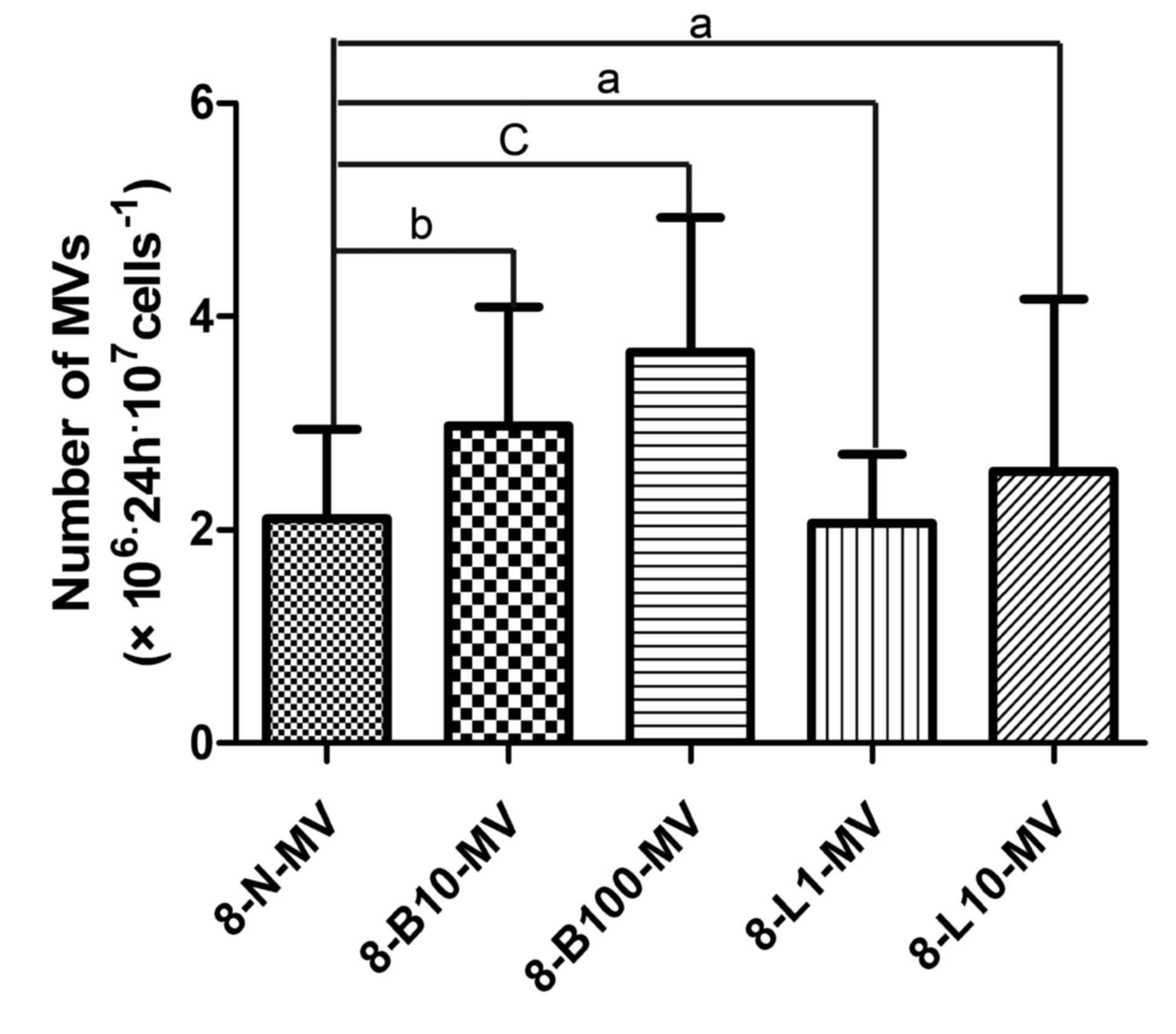
The number of MM MVs was altered
following bortezomib treatment
MM cells had a high rate of MV shedding;
significantly more MVs were observed in the bortezomib groups
(B10-MV and B100-MV) than in the control group, but the number of
MVs was not significantly altered by lenalidomide treatment
(Fig. 1).
Drug treatment significantly decreases
levels of VEGF, IL-6 and bFGF in MM MVs
MM cells in the log phase of growth were seeded at
5×105 cells/ml and were randomly divided into 5 groups
according to treatment: PBS (control), 10 nM bortezomib, 100 nM
bortezomib, 1 µM lenalidomide, or 10 µM lenalidomide. After 24 h,
MVs were isolated from the various conditioned media, and RNA was
extracted from MVs. The PCR results showed that both bortezomib and
lenalidomide treatment significantly decreased the gene expression
of VEGF, IL-6 and bFGF in MVs compared with those treated with PBS,
but no difference was observed between the bortezomib and
lenalidomide groups (Fig. 2A).
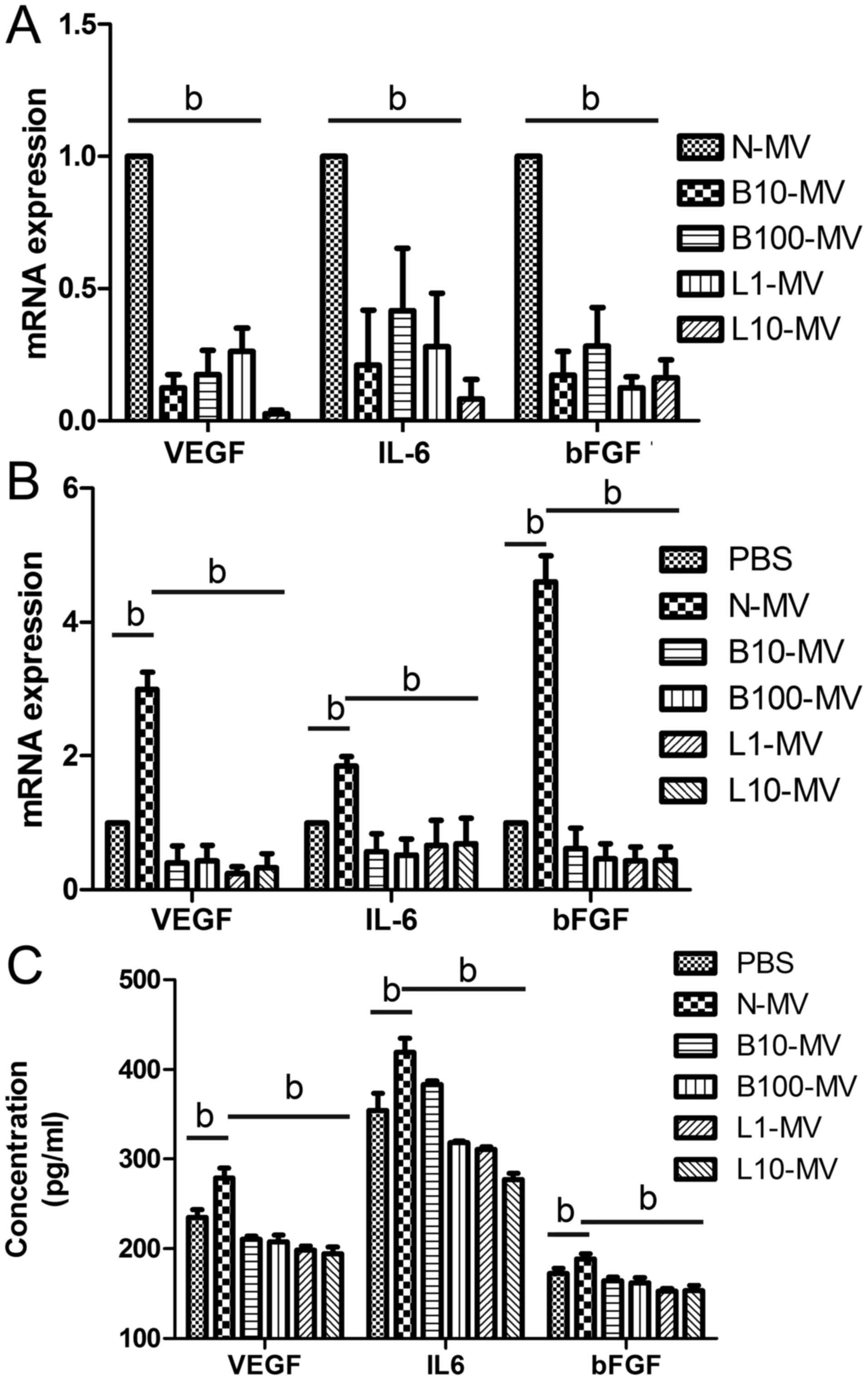 | Figure 2.Drug treatment leads to decreased
pro-angiogenic factor expression. (A) The mRNA expression levels of
VEGF, IL-6 and bFGF were significantly decreased in the
drug-treated microvesicles (MVs) compared with those in the normal
MVs. After incubation with drug-treated MM MVs for 24 h, reduced
expression levels of VEGF, IL-6 and bFGF were found in the cells
(B) and supernatant (C) of HUVECs. bP<0.05. VEGF,
vascular endothelial growth factor; IL-6, interleukin 6; bFGF,
basic fibroblast growth factor; HUVECs, human umbilical vein
endothelial cells; MVs, microvesicles; MM, multiple myeloma; MM
MVs, MVs derived from MM cells. Treatment groups: PBS (cells
treated only with PBS), N-MV (PBS), B10-MV (10 nM bortezomib),
B100-MV (100 nM bortezomib), L1-MV (1 µM lenalidomide), and L10-MV
(10 µM lenalidomide). |
Internalization of MM MVs alters the
level of pro-angiogenic factors in HUVECs
HUVECs were randomly divided into 6 groups and
co-cultured with the different MM MVs (as above) or with PBS
(control group) for 24 h. qPCR and ELISA assays were used to
examine the expression levels of VEGF, IL-6 and bFGF in HUVECs and
in the conditioned medium, respectively. Both qPCR (Fig. 2B) and ELISA (Fig. 2C) results showed that these
cytokines were significantly higher in the normal MV-treated HUVECs
than in the PBS group. Moreover, VEGF, IL-6 and bFGF expression
levels in all drug-treated-MV groups were significantly decreased
compared with those in the normal-MV group (Fig. 2B and C).
Normal MVs (N-MVs) promote the
migration, invasion and tube formation of HUVECs, while
drug-treated MVs (DMVs) inhibit angiogenesis
The angiogenic properties of MM MVs were evaluated
as their ability to induce HUVEC migration, invasion and
tubulogenesis. Cell migration was tested using a wound healing
assay; after treatment with N-MVs for 24 h, HUVEC migration towards
the scratched area was significantly improved. As shown in Fig. 3A, N-MVs (10 µg/ml) induced 55.71%
(P<0.05) of wound recovery compared with 44.95% in the PBS
group. Compared with N-MVs, the DMVs decreased cell motility,
although the B100-MV and L1-MV groups were not statistically
significantly different (Fig. 3A).
Next, the invasive ability of HUVECs was demonstrated by the
Transwell invasion system. In this experiment, different MM MVs
were used as chemoattractants. N-MVs promoted cell invasion through
Matrigel, but cell invasion was significantly inhibited by the 4
groups of DMVs compared with that of the N-MV group (Fig. 3B). Finally, the angiogenic activity
of MVs was investigated by in vitro tube formation assay.
Tubulogenesis was qualified by counting the number of closed tubes
observed at 6 and 24 h. At 6 h, no significant effects were
observed in the N-MVs group compared with the medium control, but
all DMV groups significantly suppressed the tube formation activity
of HUVECs compared with that of the N-MV group (Fig. 3C). At 24 h, the results showed that
N-MVs significantly promoted the tube formation of HUVECs, but tube
formation activity was significantly suppressed by the presence of
DMVs (Fig. 3C).
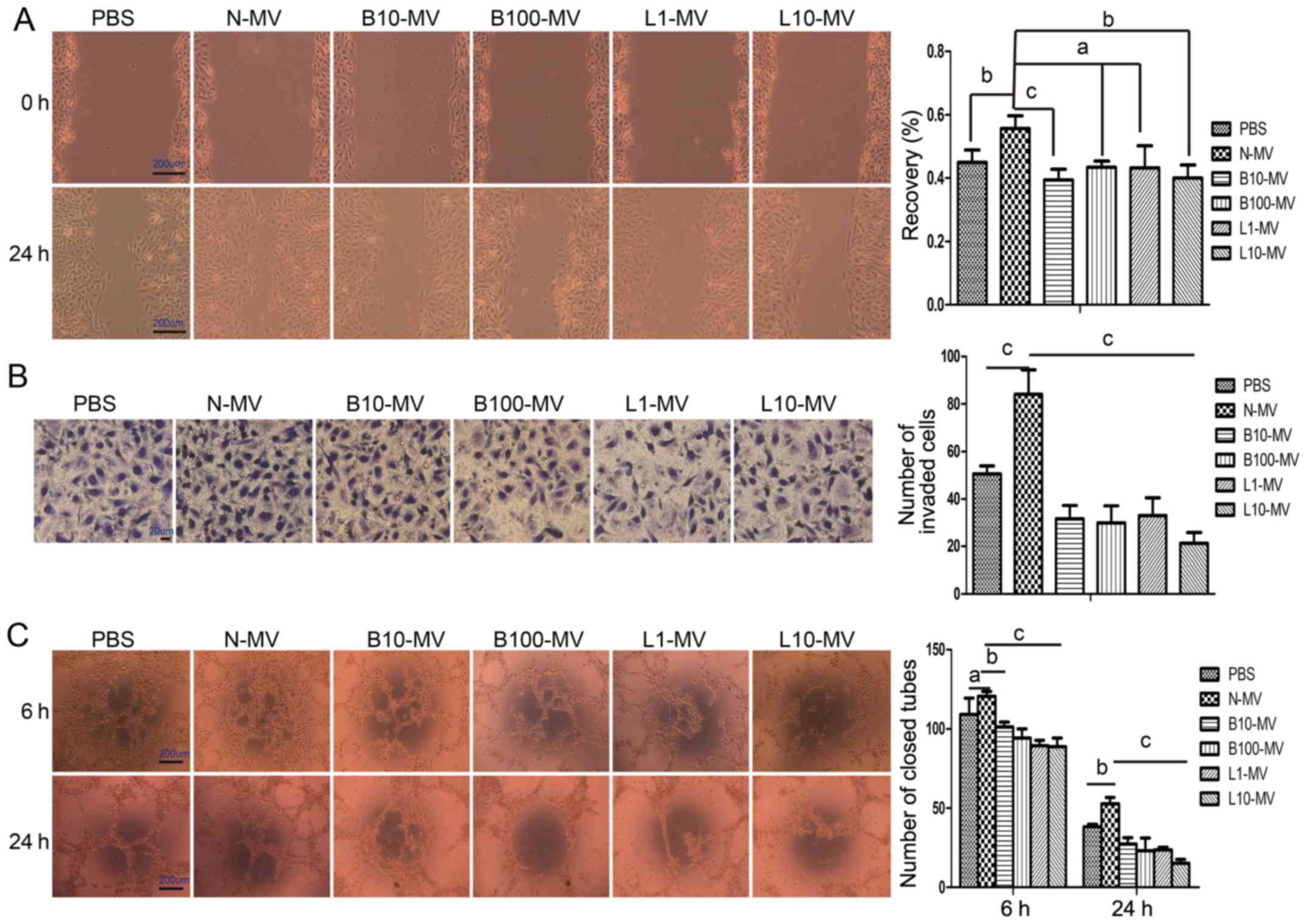 | Figure 3.MM MVs (N-MVs) have angiogenic effects
on HUVECs, while drug-treated MVs (DMVs) inhibit angiogenesis. (A)
N-MVs increased HUVEC migration, while DMVs reduced it. The denuded
area was captured (×40) at 0 and 24 h (left panel), and the
percentage of wound recovery was calculated (right panel). (B)
N-MVs promoted HUVEC invasion, while DMVs reduced it.
Photomicrographs (×400) depict the invading HUVECs at the end of
the experiment (left panel). The number of invading cells was
counted and compared (right panel) among groups. (C) N-MVs enhanced
HUVEC tubulogenesis, while DMVs inhibited it. Typical morphology of
the tube-like structure formed by HUVECs (×40) is shown (left
panel). A quantitative measurement of the closed tubes was
performed, and the results are shown (right panel). (A-C) Data are
presented as the means ± SEM from triplicate experiments.
aP>0.05, bP<0.05,
cP<0.01. MVs, microvesicles; MM, multiple myeloma; MM
MVs, MVs derived from MM cells; HUVECs, human umbilical vein
endothelial cells. Treatment groups: PBS (HUVECs treated only with
PBS), N-MV (PBS), B10-MV (10 nM bortezomib), B100-MV (100 nM
bortezomib), L1-MV (1 µM lenalidomide), and L10-MV (10 µM
lenalidomide). |
Normal MVs (N-MVs) increase NF-κB
activation in HUVECs, while drug-treated MVs (DMVs) reduce NF-κB
activation
The expression of the NF-κB p65 subunit was
determined using qPCR. The expression of NF-κB p65 was increased in
the N-MV group compared with the control group and was
significantly decreased in all DMVs groups compared with the N-MV
group (Fig. 4A). Then, the
expression levels of p65 in the total protein and p-p65 in the
nuclear protein of HUVECs were analyzed by western blot analysis,
and the ratio of p-p65 to p65 was calculated. As shown in Fig. 4B, the protein expression level and
the mean optical density of p-p65 were higher in the N-MV group
than in the control group. However, all DMV treatments caused a
reduction in the protein expression levels and the mean densities
of p-p65 in nuclear proteins compared with those in the
N-MV-treated group. However, p65 exhibited no significant
differences among the MV groups. As shown in Fig. 4C, the same tendency was found in the
immunofluorescence microscopy assay; p65 translocation was greatly
increased in the N-MV group and reduced in the DMV groups. Our data
suggested that N-MVs significantly increased NF-κB activation in
HUVECs, whereas it was decreased by DMVs. These data supported the
regulation of NF-κB activity in target HUVECs by different MVs.
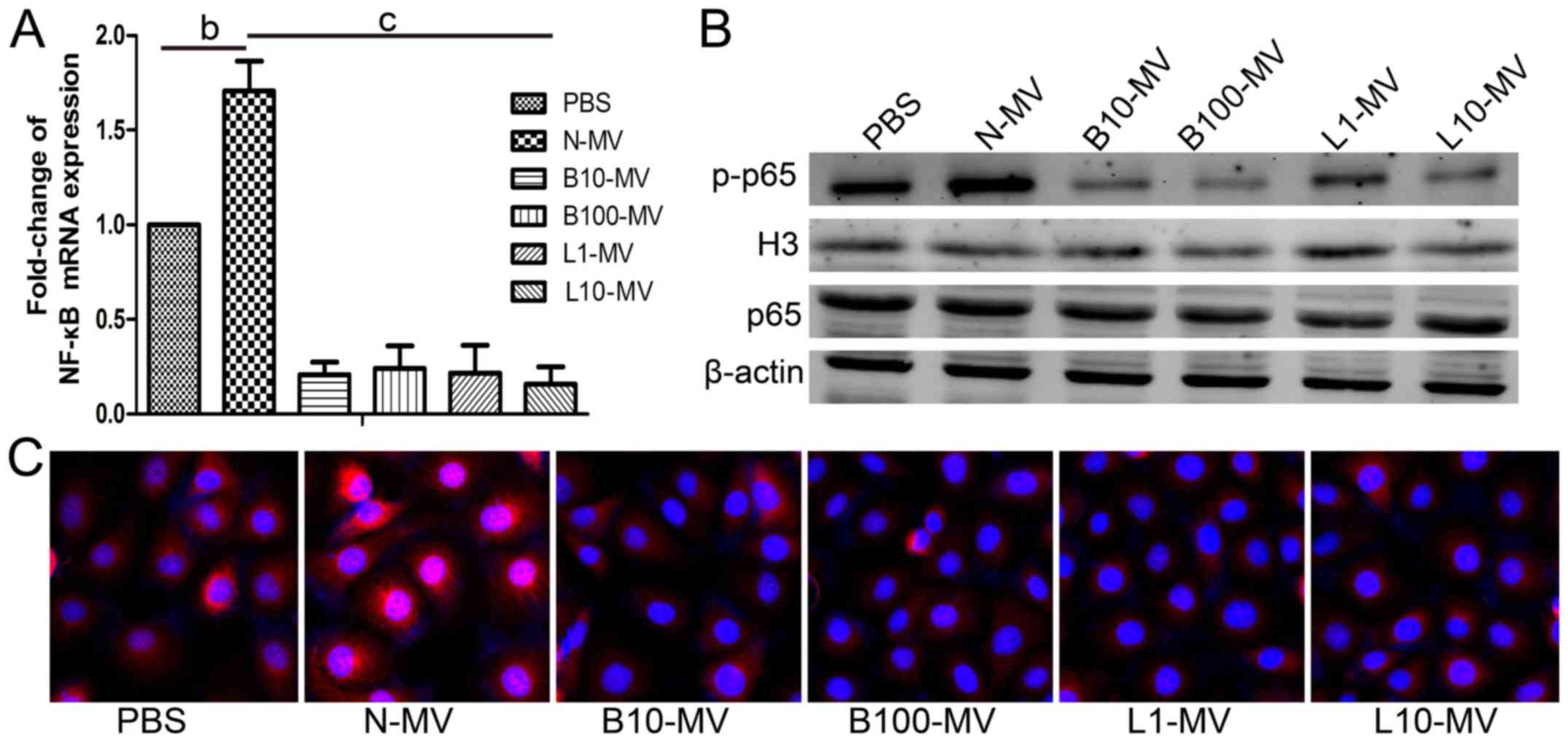 | Figure 4.Effect of MM MVs on the activity of
the NF-κB signaling pathway in HUVECs. N-MV significantly increased
NF-κB activation, whereas DMVs inhibited it. (A) HUVECs
(5×104 cells/ml) were co-cultured with different MM MVs
(10 µg/ml) for 24 h and then analyzed by qPCR. (B) Representative
western blot membranes showing the expression levels of NF-κB p-p65
and H3 in nuclear protein and of NF-κB p65 and β-actin in total
protein. β-actin and H3 were used as internal controls. (C)
Representative immunofluorescence microphotographs showing the
translocation of NF-κB p65 in HUVECs (×100). Red (representative of
the area that contains p65) and blue (representative of the nucleus
part that is DAPI conjugated) area images were overlaid, creating a
purple fluorescence in areas of colocalization. In the control
group, the NF-κB p65 subunit was predominantly localized in the
cytoplasm. Cells stimulated with N-MVs showed a significant
translocation of p65 to the cell nucleus. In cells co-cultured with
DMVs, NF-κB p65 was significantly retained in the cytoplasm. MVs,
microvesicles; MM, multiple myeloma; MM MVs, MVs derived from MM
cells; HUVECs, human umbilical vein endothelial cells. Treatment
groups: PBS (HUVECs treated only with PBS), N-MV (PBS), B10-MV (10
nM bortezomib), B100-MV (100 nM bortezomib), L1-MV (1 µM
lenalidomide), and L10-MV (10 µM lenalidomide) |
Discussion
Multiple myeloma (MM) is a B-cell neoplasm.
Selective supportive conditions of the bone marrow (BM)
microenvironment, especially BM angiogenesis (26), are involved in the development of
MM. Microvesicles (MVs), also called microparticles, range in size
from 100 to 1000 nm in diameter, are formed by the outward blebbing
of the plasma membrane and are known as important players in the
cell-to-cell communication that occurs in normal physiology and in
pathological conditions such as the angiogenic process (10–14). A
recent study has shown that MM-derived MVs (MM MVs) are
internalized by endothelial cells and promote angiogenesis
(14) in vitro and in
vivo. Our results in the present study also indicated that MVs
from normal cultured myeloma cells can induce angiogenesis in
vitro.
The NF-κB signaling pathway is activated in myeloma
under environmental stress, which plays a critical role in disease
oncogenesis and progression (27).
Angiogenesis is critical for the progression of multiple myeloma.
NF-κB regulates the expression of pro-angiogenic genes related to
myeloma pathogenesis including VEGF and IL-6 (28). In addition, interactions between
myeloma cells and the bone marrow environment are critical to NF-κB
activation and myeloma pathogenesis. MM-derived MVs are
acknowledged for their role in the cell-cell communication between
MM cells and the bone marrow microenvironment, and several drugs
that are effective in the treatment of multiple myeloma, including
bortezomib, thalidomide, and lenalidomide, have been found to block
NF-κB activation (18–20).
MVs are released from different cell types under
both normal and stressed conditions. Their biological activity
depends on the origin of the cells, the content they carry and the
environmental conditions of their release, which may have
beneficial or harmful effects. The microenvironment can regulate
their release and the amount and quality of their cargo. Herein, we
studied various differences in MV populations, content and
angiogenic impact under different conditions of normal culture and
drug treatment (bortezomib or lenalidomide). In this study, the
number of MM MVs was substantially increased in a dose-dependent
manner when MM cells were treated with bortezomib compared to the
number of MVs in untreated control cells. In contrast, exposing
cells to lenalidomide did not significantly change the number of
MVs. We speculate that these phenomena may be due to differences
between cytotoxic drugs, which induce tumor cell apoptosis and
anti-angiogenic agents, which inhibit endothelial cells instead of
tumor cells and do not cause apoptosis. This is consistent with
previous reports (21,22). The number of tumor-derived
microparticles (TMPs) was significantly increased in breast
carcinoma cells exposed to paclitaxel chemotherapy compared to the
number in untreated tumor cells (21), but treatment with anti-VEGF-A
neutralizing antibodies, which is a cytostatic drug, did not lead
to a significant change in the number of TMPs (22).
We were surprised to find that MVs from cells
exposed to bortezomib and lenalidomide both exhibited reduced
angiogenic potential compared to MVs from control cells, as
evidenced by the reduced migration, invasion and tubulogenesis of
HUVECs exposed to DMVs. EVs have increased levels of angiogenic
cytokines, such as VEGF (29,30)
and IL-6 (31), which play a
crucial role in MM pathogenesis and tumor progression. In our
study, the MVs derived from normal cultured MM cells contained high
levels of VEGF, IL-6 and bFGF. However, we demonstrated that the
expression levels of these pro-angiogenic factors were decreased in
MVs from bortezomib- and lenalidomide-treated cells, thus we
speculate that these drugs alter the angiogenic activity of MVs by
reducing their content of angiogenic factors.
We also demonstrated that after co-culture with MVs
from bortezomib- or lenalidomide-treated MM cells, endothelial
cells had reduced expression levels of VEGF, IL-6 and VEGF. A
previous study demonstrated that MM MVs exposed to bortezomib had
lower expression levels of angiogenic factors, which limited the
proliferation and migration of these endothelial cells (23). Similarly, another study showed that
the expression level of VEGF-A was decreased in TMPs from breast
carcinoma cells exposed to anti-VEGF-A antibody and thereby reduced
the angiogenic potential (22).
However, the impact of anti-angiogenic therapy with lenalidomide
has not been elucidated; moreover, the effects of bortezomib- or
lenalidomide-exposed MVs on the NF-κB activity of endothelial cells
have not been reported. Here, we showed that the expression levels
of angiogenic factors were reduced in MVs from myeloma cells
exposed to either bortezomib or lenalidomide. Furthermore, both
bortezomib-MV- and lenalidomide-MV-treated endothelial cells had
reduced expression levels of VEGF, IL-6 and bFGF compared with
those of the N-MV-treated group. Consequently, these MVs exhibited
a reduced angiogenic ability, as evaluated by migration, invasion
and tubulogenesis assays. Furthermore, NF-κB was activated in
endothelial cells co-cultured with MVs from untreated cells, while
NF-κB activity was reduced in endothelial cells incubated with MVs
from bortezomib- and lenalidomide-exposed cells.
EVs can modulate angiogenesis by stimulating or
inhibiting it, highly depending on the content of EV and the
expression of surface molecules, which are highly regulated by the
stimulation of EV production (7).
Many mechanisms are involved in the modulation of angiogenesis by
EVs. For example, transfer of proteins such as VEGF (11,32),
bFGF (32) and regulation of
signaling pathways, such as PI3K (10), ERK1/2 (12,32),
Wnt4/β-catenin (33), and NF-κB
(13,34). We speculate that drug-treated MVs
can inhibit angiogenesis by the following mechanisms. i)
Microvesicles contain VEGF, IL-6 and bFGF mRNA, and they can
release these contents after internalization by endothelial cells.
Drug-exposed MVs had decreased mRNA expression levels of VEGF, IL-6
and bFGF, leading to a reduced expression of VEGF, IL-6 and bFGF
mRNA in endothelial cells and a decreased secretion of these
pro-angiogenic factors. ii) Microvesicles act as exogenous
substances that can be internalized by endothelial cells and
activate NF-κB, resulting in increased VEGF, IL-6 and bFGF mRNA
expression and protein secretion in endothelial cells.
Microvesicles exposed to drugs can inhibit NF-κB after fusion with
endothelial cells and thereby lead to decreased expression levels
of angiogenic factors. However, It remains unclear how the
drug-treated MVs inhibit NF-κB activation and to what extent the
inhibition of angiogenesis by drug-treated MVs can be attributed to
NF-κB inhibition. Overall, our data suggest that there are
additional mechanisms worthy of further investigation.
Acknowledgements
Not applicable.
Funding
This study was funded by the National Natural
Science Foundation of China (no. 81500163).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
LS, JML and HMG conceived and designed the study.
HMG, LY and ZYN performed the experiments. HMG wrote the paper. LS,
XJL and JML reviewed and edited the manuscript. All authors read
and approved the manuscript and agree to be accountable for all
aspects of the research in ensuring that the accuracy or integrity
of any part of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kumar SK, Rajkumar SV, Dispenzieri A, Lacy
MQ, Hayman SR, Buadi FK, Zeldenrust SR, Dingli D, Russell SJ, Lust
JA, et al: Improved survival in multiple myeloma and the impact of
novel therapies. Blood. 111:2516–2520. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gkotzamanidou M, Christoulas D, Souliotis
VL, Papatheodorou A, Dimopoulos MA and Terpos E: Angiogenic
cytokines profile in smoldering multiple myeloma: No difference
compared to MGUS but altered compared to symptomatic myeloma. Med
Sci Monit. 19:1188–1194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Accardi F, Toscani D, Bolzoni M, Dalla
Palma B, Aversa F and Giuliani N: Mechanism of action of bortezomib
and the new proteasome inhibitors on myeloma cells and the bone
microenvironment: Impact on myeloma-induced alterations of bone
remodeling. BioMed Res Int. 2015:1724582015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matthes T, Manfroi B, Zeller A,
Dunand-Sauthier I, Bogen B and Huard B: Autocrine amplification of
immature myeloid cells by IL-6 in multiple myeloma-infiltrated bone
marrow. Leukemia. 29:1882–1890. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dankbar B, Padró T, Leo R, Feldmann B,
Kropff M, Mesters RM, Serve H, Berdel WE and Kienast J: Vascular
endothelial growth factor and interleukin-6 in paracrine
tumor-stromal cell interactions in multiple myeloma. Blood.
95:2630–2636. 2000.PubMed/NCBI
|
|
6
|
van der Pol E, Böing AN, Harrison P, Sturk
A and Nieuwland R: Classification, functions, and clinical
relevance of extracellular vesicles. Pharmacol Rev. 64:676–705.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Todorova D, Simoncini S, Lacroix R,
Sabatier F and Dignat-George F: Extracellular Vesicles in
Angiogenesis. Circ Res. 120:1658–1673. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
EL Andaloussi S, Mäger I, Breakefield XO
and Wood MJ: Extracellular vesicles: Biology and emerging
therapeutic opportunities. Nat Rev Drug Discov. 12:347–357. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Canella A, Harshman SW, Radomska HS,
Freitas MA and Pichiorri F: The potential diagnostic power of
extracellular vesicle analysis for multiple myeloma. Expert Rev Mol
Diagn. 16:277–284. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Deregibus MC, Cantaluppi V, Calogero R, Lo
Iacono M, Tetta C, Biancone L, Bruno S, Bussolati B and Camussi G:
Endothelial progenitor cell derived microvesicles activate an
angiogenic program in endothelial cells by a horizontal transfer of
mRNA. Blood. 110:2440–2448. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zou X, Gu D, Xing X, Cheng Z, Gong D,
Zhang G and Zhu Y: Human mesenchymal stromal cell-derived
extracellular vesicles alleviate renal ischemic reperfusion injury
and enhance angiogenesis in rats. Am J Transl Res. 8:4289–4299.
2016.PubMed/NCBI
|
|
12
|
Lombardo G, Dentelli P, Togliatto G, Rosso
A, Gili M, Gallo S, Deregibus MC, Camussi G and Brizzi MF:
Activated Stat5 trafficking via endothelial cell-derived
extracellular vesicles controls IL-3 pro-angiogenic paracrine
action. Sci Rep. 6:256892016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Anderson JD, Johansson HJ, Graham CS,
Vesterlund M, Pham MT, Bramlett CS, Montgomery EN, Mellema MS,
Bardini RL, Contreras Z, et al: Comprehensive proteomic analysis of
mesenchymal stem cell exosomes reveals modulation of angiogenesis
via nuclear factor-kappab signaling. Stem Cells. 34:601–613. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Y, Zhu XJ, Zeng C, Wu PH, Wang HX,
Chen ZC and Li QB: Microvesicles secreted from human multiple
myeloma cells promote angiogenesis. Acta Pharmacol Sin. 35:230–238.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li ZW, Chen H, Campbell RA, Bonavida B and
Berenson JR: NF-kappaB in the pathogenesis and treatment of
multiple myeloma. Curr Opin Hematol. 15:391–399. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chilov D, Kukk E, Taira S, Jeltsch M,
Kaukonen J, Palotie A, Joukov V and Alitalo K: Genomic organization
of human and mouse genes for vascular endothelial growth factor C.
J Biol Chem. 272:25176–25183. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cramer M, Nagy I, Murphy BJ, Gassmann M,
Hottiger MO, Georgiev O and Schaffner W: NF-kappaB contributes to
transcription of placenta growth factor and interacts with metal
responsive transcription factor-1 in hypoxic human cells. Biol
Chem. 386:865–872. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nencioni A, Grünebach F, Patrone F,
Ballestrero A and Brossart P: Proteasome inhibitors: Antitumor
effects and beyond. Leukemia. 21:30–36. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
De Luisi A, Ferrucci A, Coluccia AM, Ria
R, Moschetta M, de Luca E, Pieroni L, Maffia M, Urbani A, Di Pietro
G, et al: Lenalidomide restrains motility and overangiogenic
potential of bone marrow endothelial cells in patients with active
multiple myeloma. Clin Cancer Res. 17:1935–1946. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Singhal S and Mehta J: Lenalidomide in
myeloma. Curr Treat Options Oncol. 8:154–163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fremder E, Munster M, Aharon A, Miller V,
Gingis-Velitski S, Voloshin T, Alishekevitz D, Bril R, Scherer SJ,
Loven D, et al: Tumor-derived microparticles induce bone
marrow-derived cell mobilization and tumor homing: A process
regulated by osteopontin. Int J Cancer. 135:270–281. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Munster M, Fremder E, Miller V, Ben-Tsedek
N, Davidi S, Scherer SJ and Shaked Y: Anti-VEGF-A affects the
angiogenic properties of tumor-derived microparticles. PLoS One.
9:e959832014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zarfati M, Katz T, Avivi I, Brenner B and
Aharon A: PO-45 - The role of microvesicles in multiple myeloma
progression. Thromb Res. 140 Suppl 1:S1932016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun L, Wang HX, Zhu XJ, Wu PH, Chen WQ,
Zou P, Li QB and Chen ZC: Serum deprivation elevates the levels of
microvesicles with different size distributions and selectively
enriched proteins in human myeloma cells in vitro. Acta Pharmacol
Sin. 35:381–393. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ma GF, Chen S, Yin L, Gao XD and Yao WB:
Exendin-4 ameliorates oxidized-LDL-induced inhibition of macrophage
migration in vitro via the NF-κB pathway. Acta Pharmacol Sin.
35:195–202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ribatti D and Vacca A: The role of
microenvironment in tumor angiogenesis. Genes Nutr. 3:29–34. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Klein B: Positioning NK-kappaB in multiple
myeloma. Blood. 115:3422–3424. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Podar K and Anderson KC: Inhibition of
VEGF signaling pathways in multiple myeloma and other malignancies.
Cell Cycle. 6:538–542. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Thompson CA, Purushothaman A, Ramani VC,
Vlodavsky I and Sanderson RD: Heparanase regulates secretion,
composition, and function of tumor cell-derived exosomes. J Biol
Chem. 288:10093–10099. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mineo M, Garfield SH, Taverna S, Flugy A,
De Leo G, Alessandro R and Kohn EC: Exosomes released by K562
chronic myeloid leukemia cells promote angiogenesis in a
Src-dependent fashion. Angiogenesis. 15:33–45. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Roccaro AM, Sacco A, Maiso P, Azab AK, Tai
YT, Reagan M, Azab F, Flores LM, Campigotto F, Weller E, et al: BM
mesenchymal stromal cell-derived exosomes facilitate multiple
myeloma progression. J Clin Invest. 123:1542–1555. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brill A, Dashevsky O, Rivo J, Gozal Y and
Varon D: Platelet-derived microparticles induce angiogenesis and
stimulate post-ischemic revascularization. Cardiovasc Res.
67:30–38. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang B, Wu X, Zhang X, Sun Y, Yan Y, Shi
H, Zhu Y, Wu L, Pan Z, Zhu W, et al: Human umbilical cord
mesenchymal stem cell exosomes enhance angiogenesis through the
Wnt4/β-catenin pathway. Stem Cells Transl Med. 4:513–522. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shabbir A, Cox A, Rodriguez-Menocal L,
Salgado M and Van Badiavas E: Mesenchymal stem cell exosomes induce
proliferation and migration of normal and chronic wound
fibroblasts, and enhance angiogenesis in vitro. Stem Cells Dev.
24:1635–1647. 2015. View Article : Google Scholar : PubMed/NCBI
|