Introduction
Lung cancer is the leading cause of cancer-related
deaths, particularly in underdeveloped countries since it produces
the highest percentage of deaths than any other cancer (1). The tendency of metastasis is very high
in the late stage of lung cancer, and the clinical treatment of
lung cancer is mainly radiotherapy and chemotherapy. However, the
results are often unsatisfactory due to the short survival time of
patients and the tendency of recurrence which is high. In recent
years, there has been more and more research conducted on molecular
targeted therapy of lung cancer, and it is increasingly important
to explore the molecular mechanisms of lung cancer development
(2,3).
The specific mechanism of tumor genesis and
development is complex and changeable. The deletion or mutation of
a tumor-suppressor gene and the expression of a large number of
oncogenes have important impacts on tumorigenesis. In 2002,
Japanese scholars cloned a candidate tumor-suppressor gene, the
Kank1 gene in renal cell carcinoma using heterozygous deletion
analysis (4). The Kank family is
mainly composed of 4 genes, Kank1-Kank4, of which Kankl plays a
certain role in the inhibition of tumorigenesis and development
(5). The present study revealed
that Kankl exhibited a low expression in various tumor tissues
including kidney (6), liver
(7,8) and lung cancer (9,10).
Kankl is mainly distributed in normal tissues, including renal
tubular epithelial cells and the glandular cells of other digestive
organs. Its main function is the polymerization with actin of the
cytoplasm adjusting the cytoskeleton, and driving cell motility
(11). In addition, it can form a
complex with β-catenin shuttling it in the nucleus to regulate
β-catenin subcellular distribution. Studies have revealed that
downregulation of the Kankl gene participates in the genesis and
development of bone tumors by activating the JAK2/Stat
tumor-promoting signaling pathway (12).
In the present study, we found that the expression
of the Kankl gene was low in lung cancer tissues and lung cancer
cells. Following upregulation of the Kankl gene, tumor cell
proliferation was significantly inhibited, and apoptosis occurred.
Concurrently, the expression of Bax decreased and the expression of
Bcl-2 increased, while the expression of the caspase family was
also altered, mainly with the activation of caspase-3 and −9. In
addition, upregulation of the Kankl gene also reduced the invasion
and metastasis of tumor cells. In an in vivo experiment, we
found that the implantation of upregulated Kank1 lung cancer cells
into nude mice resulted in lower tumorigenicity and the significant
decrease of tumor volume in nude mice. In summary, we concluded
that upregulation of the Kankl gene inhibited the progression of
lung cancer both in vitro and in vivo, and its
mechanism was closely related to cell apoptosis and tumor invasion
and metastasis.
Materials and methods
Cell lines and culture
Human lung cancer cell lines NCI-H292, A549 and
PGCL3 cells, and normal human lung cell lines MRC-5 and WI-38 were
purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA). The aforementioned 5 cell lines were all
cultured in RPMI-1640 medium containing 10% fetal bovine serum
(FBS) in an incubator at 37°C, with 5% CO2 and saturated
humidity. RPMI-1640 medium and FBS were purchased from Gibco;
Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
Antibodies and reagents
Kank1 (dilution 1:2,000; cat. no. AM8596b) was
purchased from Abgent, Inc. (San Diego, CA, USA). Bax (dilution
1:1,000; cat. no. 2772T), Bcl-2 (dilution 1:1,000; cat. no. 2872T),
caspase-3 (dilution 1:1,000; cat. no. 9662S), caspase-9 (dilution
1:1,000; cat. no. 9502T), MMP-7 (dilution 1:1,000; cat. no. 71031S)
and MMP-9 (dilution 1:1,000; cat. no. 3852S) antibodies were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
siRNA and plasmids were synthesized by Shanghai GenePharma Co.,
Ltd. (Shanghai, China). An RT-PCR kit was obtained from Takara Bio,
Inc. (Otsu, Japan). PI was purchased from Sigma-Aldrich (St. Louis,
MO, USA). An Annexin V-FITC apoptosis assay kit was purchased from
BD Biosciences (San Jose, CA, USA).
Cell transfection
Human lung cancer cells in the logarithmic growth
phase were collected and cultured in 6-well plates at a density of
6×105 cells/ml. Negative oligonucleotides were used as a
control. The medium was replaced with serum-free and
antibiotic-based medium, according to the procedure on the
transfection of reagents. Following the transfection of siRNA, the
culture plates were placed in a CO2 incubator for 4–6 h,
the medium was then replaced with the serum-containing medium and
cells continued to culture. The transfected cells were screened
after transfection with plasmids. After 3 weeks of selection,
monoclonal antibodies (KANK1 antibody; dilution 1:2,000; cat. no.
AM8596b) were purchased from (Abgent, Inc.) and chosen to expand
the culture to establish an overexpressed Kankl human lung cancer
cell line. Subsequently, the cells underwent RT-PCR detection.
RNA extraction and RT-PCR
detection
Human lung cancer cells were divided into
untransfected, blank plasmid pCMV-vec and the experimental group
pCMV-Kank1. Total RNA was collected and extracted from each group
of cells to assess purity and integrity before reverse
transcription. Following calculation of the RNA concentration, the
RT-PCR reaction was performed using an RT-PCR kit (Takara Bio), and
the procedure was performed according to the manufacturer's
instructions. Kank1 and β-actin gene primers were designed and
synthesized by Invitrogen; Thermo Fisher Scientific, Inc. The
primers were as follows: Kank1 gene upstream primer,
5′-CTTGACACAGTATTTTCACGCTTTTG-3′ and downstream primer,
5′-AAGTAAATGTGACACGGTAAAAAGG-3′; β-actin gene upstream primer,
5′-CTGGGACGACATGGAGAAAA-3′ and downstream primer,
5′-AAGGAAGGCTGGAAGAGTGC-3′. The PCR reaction system was 25 µl and
the reaction conditions were as follows: 94°C for 2 min,
denaturation at 94°C for 30 sec, annealing at 60°C for 30 sec, and
extension at 72°C for 30 sec, for a total of 31 cycles. The
resulting PCR product was electrophoresed on a 1.5% agarose gel and
scanned using a Gel Doc™ XR+ Gel Documentation System (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Cell viability detection
Trypsin was used for the digestion and collection of
the untransfected group, the blank plasmid pCMV-vec group and the
experimental pCMV-Kank1 cell group, and then the cells were
inoculated in 96-well plates, with each set consisting of 5 complex
wells. The transfected group was replaced with complete culture
solution after 24 h, and after 48 h, 20 µl of MTT solution (0.5
mg/ml)/well was added and incubated at 37°C for 4 h. The
supernatant was removed and 150 µl of dimethyl sulphoxide (DMSO)
was added to each well and shaken for 10 min until the purple
crystals were completely dissolved. A microplate reader was used to
detect the optical density of each well at 490 nm, and the average
value of each group was calculated. The cell growth inhibition rate
(%) = (1 - average A value of the treated group/average A value of
the blank group) × 100%. The experiment was repeated 3 times.
Caspase activity assay
Caspase-3 and −9 activities were determined using
the Caspase activity assay kit (Beyotime Institute of
Biotechnology, Shanghai, China) according to the manufacturer's
instructions after transfected human lung cancer cells,
untransfected human lung cancer cells and blank plasmid transfected
cells were collected. The assessment was performed with a
fluorescence spectrophotometer at an excitation wavelength of 400
nm and an onset wavelength of 505 nm.
Flow cytometric analysis detects
apoptosis and the cell cycle
The transfected human lung cancer cells and
untransfected human lung cancer cells as well as the blank plasmid
transfected cells were digested with 0.25% trypsin-EDTA and
collected, then suspended in phosphate-buffered saline (PBS), and
single cell suspensions were prepared. Flow cytometry (BD
Biosciences) was used to detect apoptosis following the Annexin
V-FITC apoptosis assay kit manufacturer's instructions. In
addition, the cells were fixed with 70% ethanol overnight at 4°C.
The cells were then washed with PBS 2 times, add 1 ml of propidium
iodide (PI) comprehensive dye solution (containing RNase 10 µg,
Triton X-100 5 µl), was added to stain the cells in the dark at 4°C
for 30 min in order to assess the cell cycle. The stained cells
were analyzed using a FACSCalibur flow cytometer (Becton-Dickinson;
BD Biosciences).
Assessment of cell invasion and
migration abilities
Each group of cells was cultured in BD 6-well
Transwell culture plates (BD Biosciences). The cells were scratched
using a standard 200 µl pipette tip, and were removed by washing
with serum-free medium. Serial images were obtained at different
time-points using a phase contrast microscope. The cell invasion
assay was performed using Matrigel-coated Transwell chambers. The
8-µm filter in the upper chamber was covered with Matrigel diluted
at 1:6, and each well was seeded with ~2×106 cells.
After 36 h of culturing, the cells that reached the bottom of the
culture well through the membrane were fixed with formalin and
stained with crystal violet. Cells were randomly counted in 10
fields of view (magnification, ×200)/well (Olympus Corp., Tokyo,
Japan).
Western blot analysis
Human lung cancer cells and their stably transfected
cells were harvested and washed twice with PBS and 1 ml of protein
lysis buffer (Sigma-Aldrich) containing 10 µl of protease inhibitor
was added for cell lysis. The protein concentration was determined
using bicinchoninic acid assay (BCA) method. The same amount of
protein was added to each well for loading. The gel was separated
on a 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis
(SDS-PAGE) gel. The protein was then transferred to a
polyvinylidene fluoride (PVDF) membrane using a semi-dry method and
blocked with 5% non-fat dry milk overnight at 4°C. Subsequently, it
was diluted with Tris-buffered saline containing Tween-20 (TBST)
solution and a primary antibody was added at 37°C for 1 h. The
primary antibody dilution ratio (phospho-PI3K antibody, dilution
1:500; cat. no. 4228S; phospho-Akt antibody, dilution 1:1,000; cat.
no. AP3434a; and MMP-7 antibody, dilution 1:800; 71031S; all were
purchased from Cell Signaling Technology, Inc.). The membrane was
washed 3 times with TBST. The secondary antibody anti-rabbit IgG,
HRP-linked antibody (cat. no. 7074P2; Cell Signaling Technology)
was added for incubation at 37°C for 1 h, and the membrane was then
washed with TBST solution 4 times by shaking. An ECL luminescence
reagent was added, X-ray exposure imaging was performed, the bands
were scanned, and the grayscale analysis was performed. β-actin was
used as a reference standard.
Nude mice inoculation
The animal experiment program was approved by the
Medical Ethics Committee of Jiangsu College of Nursing. Twenty nude
male mice, 6–8 weeks old and weighing ~20 g, were purchased from
the Animal Experiment Center of Nanjing University. Housing
conditions were controlled for temperature (18–22°C) and relative
humidity (50–60%) on a light/dark cycle 10 to 14 h. Access to food
and water was ad libitum. Nude mice were randomly divided
into 2 groups and each group consisted of 10 mice. The constructed
PGCL3 cell line and the blank plasmid pCMV-vec cell line with
stably expressed Kank1 gene transfection were implanted into the
subcutaneous right forelimbs of the 2 groups of nude mice,
respectively, to observe the effect of Kank1 on the tumorigenicity
of the transplanted tumors of the nude mice. The growth of the
tumor was observed and assessed at 7, 14, 21 and 28 days. After 4
weeks, the nude mice were sacrificed by cervical spine dislocation,
and the subcutaneously transplanted tumor tissue was excised under
aseptic conditions for index analysis.
Statistical analysis
All experimental data were analyzed using SPSS 18.0
statistical software (SPSS, Inc., Chicago, IL, USA). A t-test and
analysis of variance were used by ANOVA followed by Fishers' least
significant difference. P<0.05 was considered to indicate a
statistically significant result.
Results
The Kank1 gene is downregulated in
human lung cancer cells
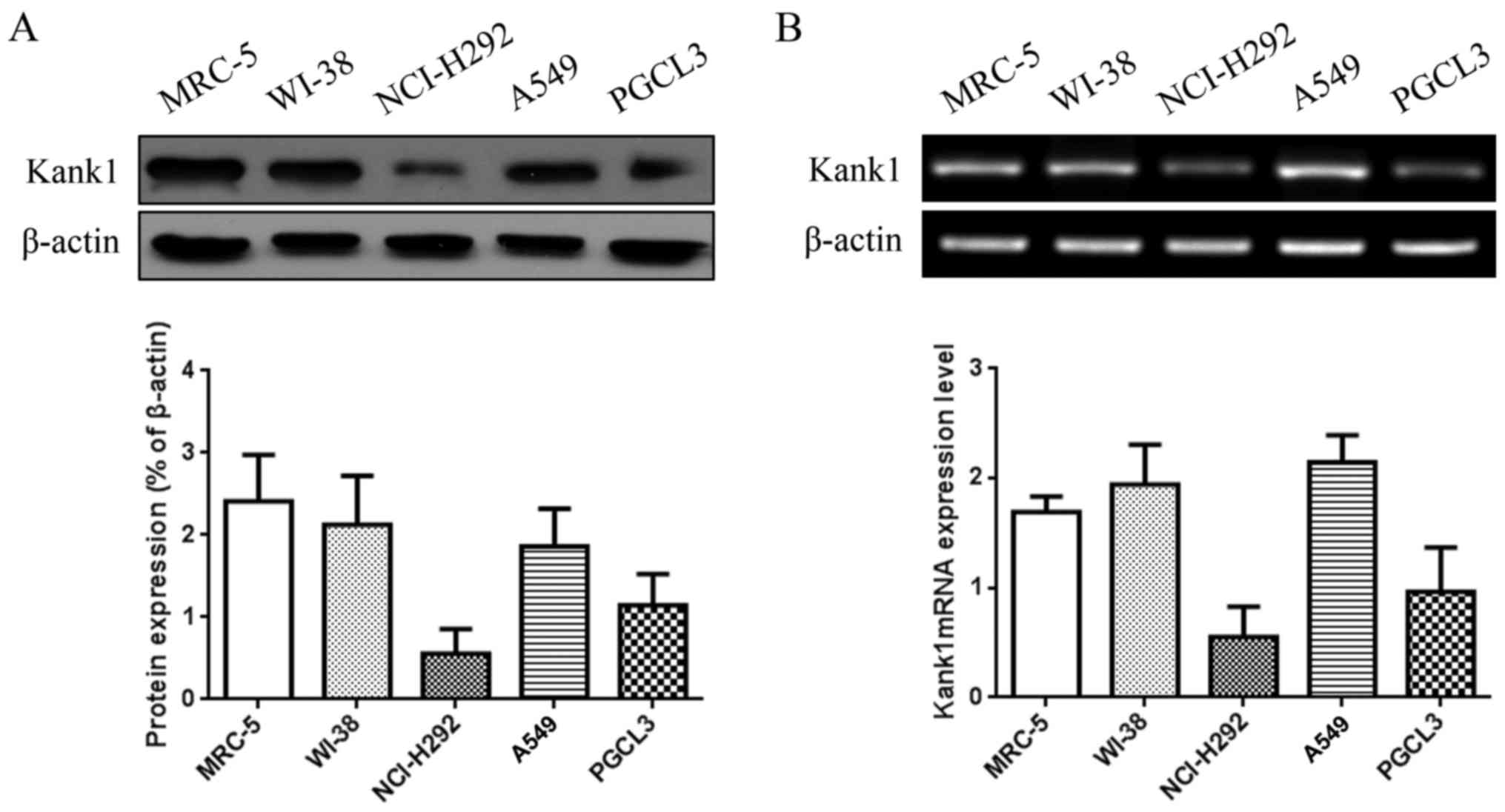
We detected the expression of Kank1 protein and mRNA
in human lung cancer cell lines NCI-H292, A549 and PGCL3 and human
normal lung cell lines MRC-5 and WI-38 by western blotting and
RT-PCR. The results revealed that Kank1 protein and mRNA expression
levels were significantly decreased in NCI-H292 and PGCL3 cells
compared to the normal human lung cell lines (Fig. 1).
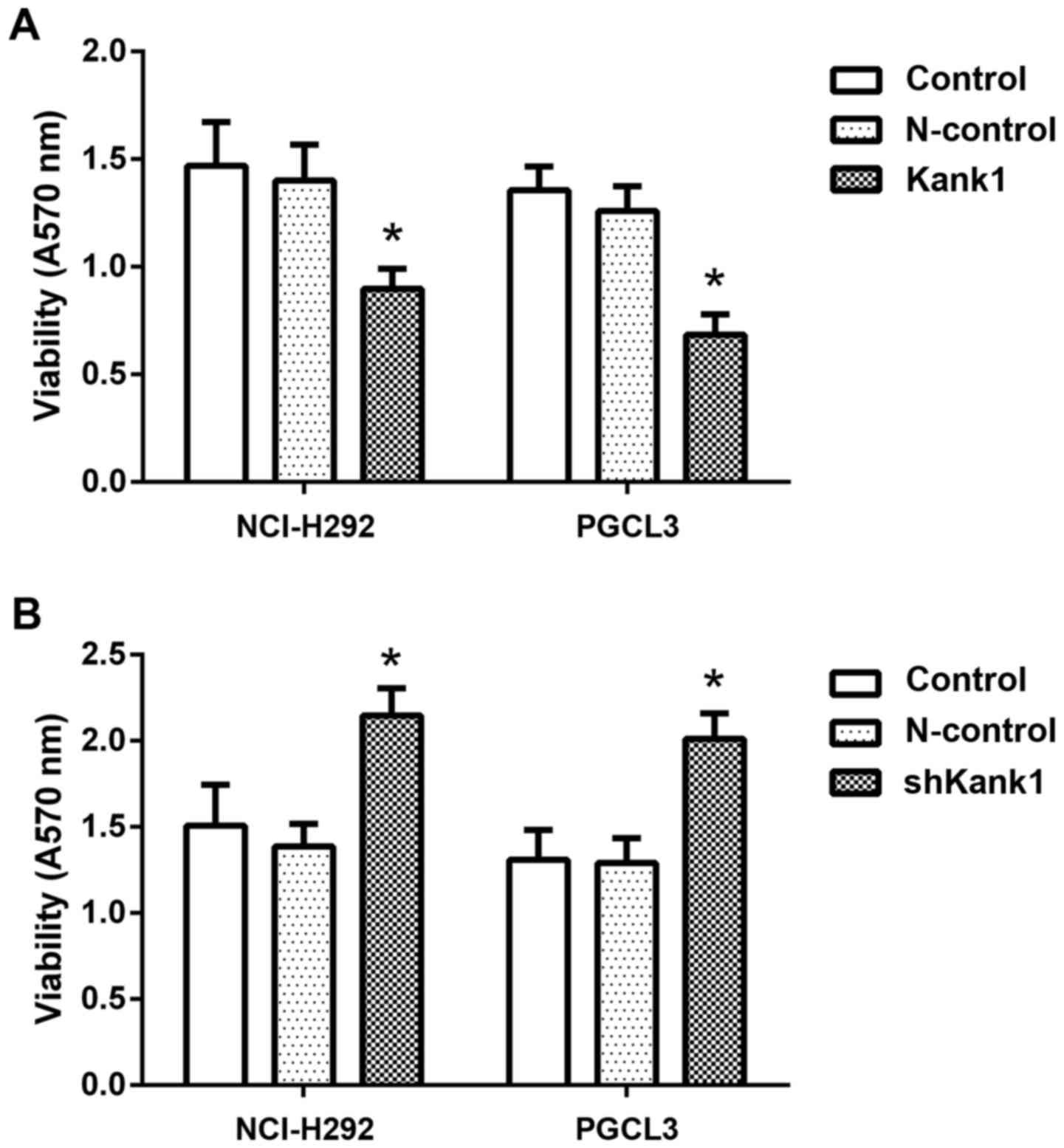
Upregulation of the Kank1 gene
inhibits human lung cancer cell growth
To further confirm the effect of the Kank1 gene on
the growth of human lung cancer cells, we successfully constructed
and transfected Kank1 plasmid to upregulate the expression level of
the Kank1 gene in human lung cancer cells. Concurrently, we
downregulated the expression of the Kank1 gene in human lung cancer
cells by shRNA and used MTT for analysis. We found that the
proliferation of NCI-H292 and PGCL3 cells was significantly
decreased after upregulation of Kank1 gene expression compared with
the control group and negative control oligonucleotide-transfection
group. In contrast, the proliferation of NCI-H292 and PGCL3 cells
was increased upon downregulation of Kank1 gene expression.
Therefore, it was demonstrated that the Kank1 gene had the ability
to regulate the proliferation of human lung cancer cells (Fig. 2).
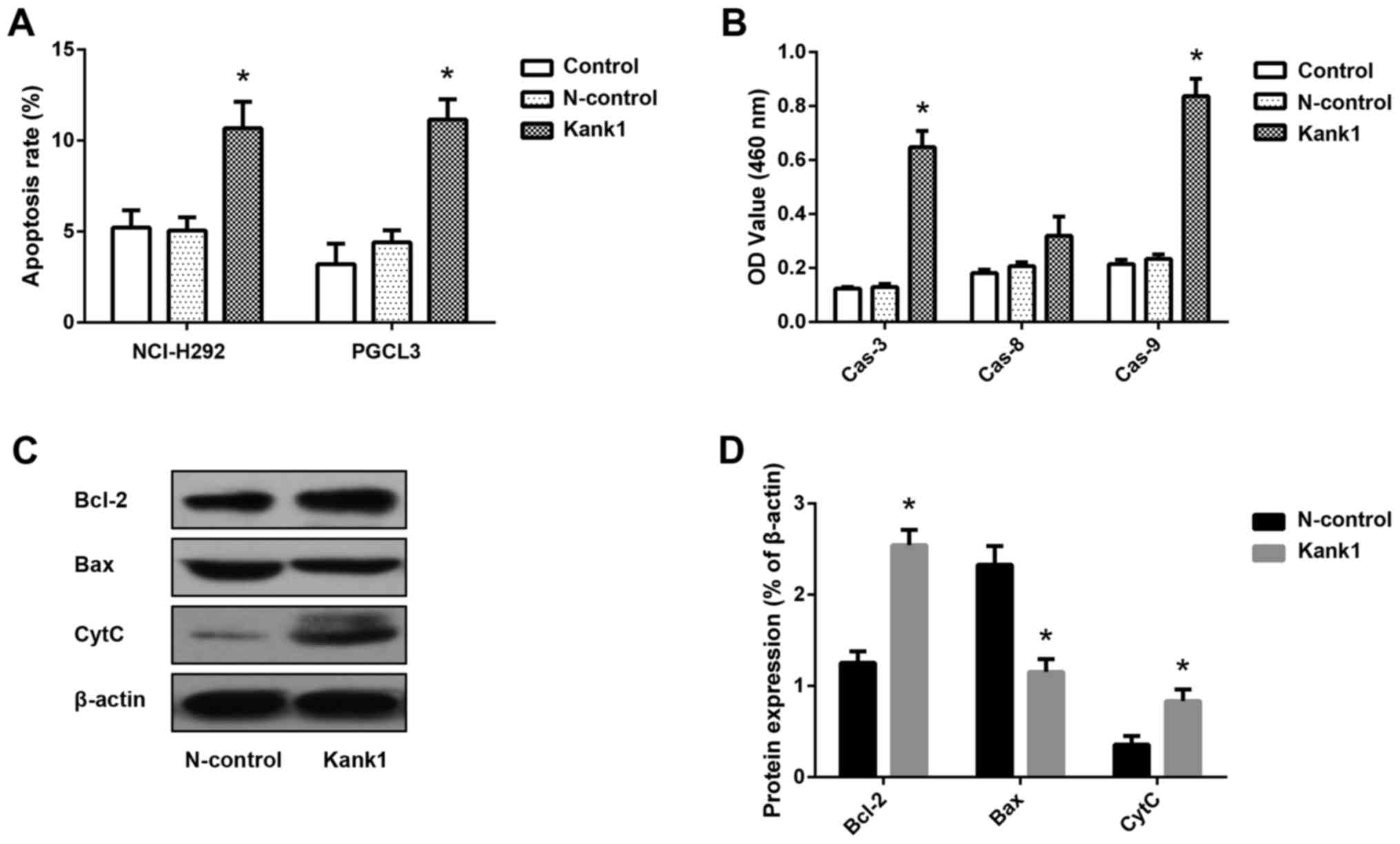
Upregulation of the Kank1 gene induces
apoptosis in human lung cancer cells
To investigate whether the Kank1 gene is associated
with apoptosis in human lung cancer cells, we upregulated the
expression of the Kank1 gene and detected the apoptosis rate by
Annexin V-FITC/PI double-labeling method. We found that the
apoptosis rate of NCI-H292 and PGCL3 cells was significantly
increased after upregulation of Kank1 gene expression compared with
the N-control group (Fig. 3A).
Concurrently, the activity of caspase-3 and −9 increased after
upregulation of Kank1 gene expression. However, the activity of
caspase-8 was not significantly altered (Fig. 3B). In addition, Western blotting was
used to detect the expression of Bcl-2, Bax and CytC in the
cytoplasm, and we found that the levels of Bcl-2 and CytC in the
cytoplasm were significantly increased, while the expression level
of the Bax protein was decreased (Fig.
3C and D). These results indicated that upregulation of the
Kank1 gene led to the mitochondrial apoptosis of human lung cancer
cells.
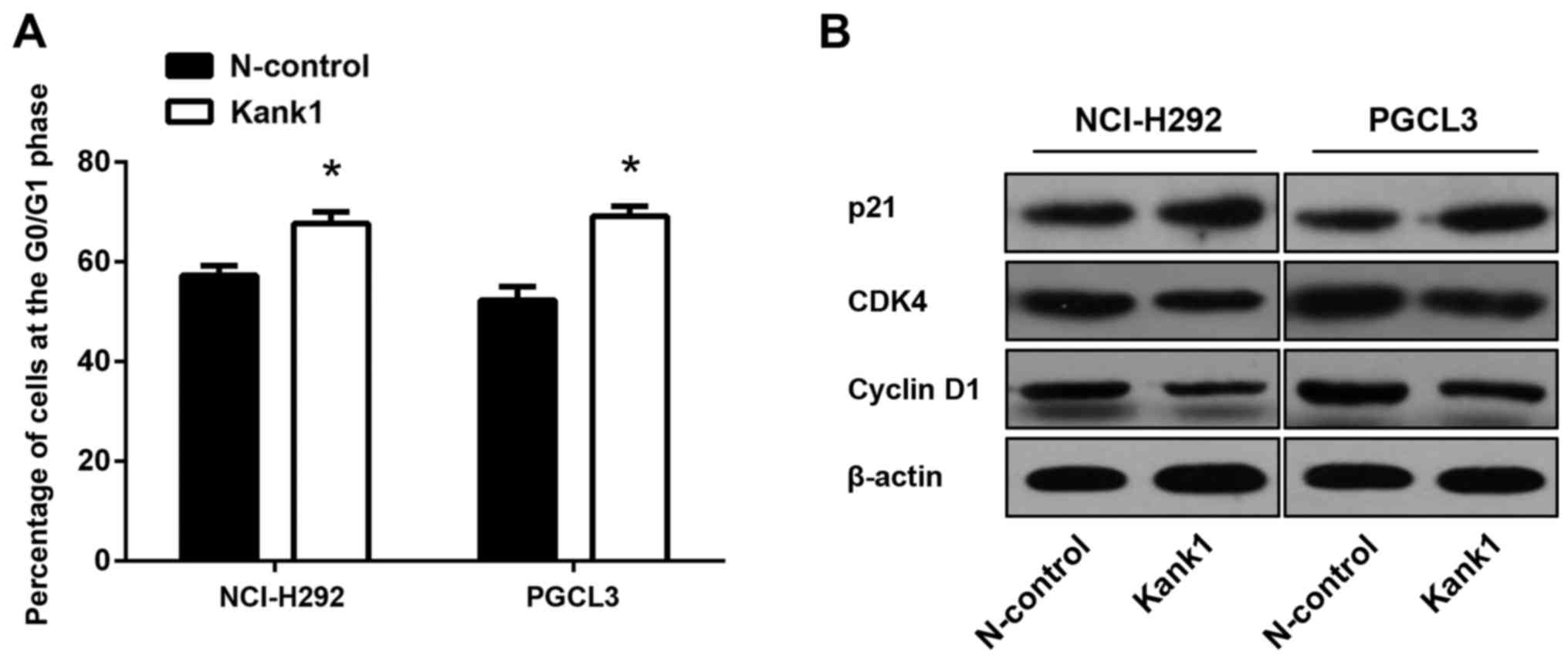
Upregulation of the Kank1 gene induces
cell cycle arrest in human lung cancer cells
To further investigate the molecular mechanism by
which the upregulated Kank1 gene inhibits the proliferation of
human lung cancer cells, we used flow cytometry to analyze the
changes of the cell cycle in lung cancer. It was revealed that the
ratio of the G0/G1 phase of NCI-H292 and PGCL3 cells after
upregulation of the Kank1 gene was significantly higher than that
of the negative control oligonucleotide group (Fig. 4A). Concurrently, the expression
levels of CDK4 and cyclin D1 were significantly decreased, and p21
was significantly increased (Fig.
4B). This demonstrated that upregulation of the Kank1 gene
inhibited human lung cancer cell proliferation by arresting the
cell cycle at the G0/G1 phase.
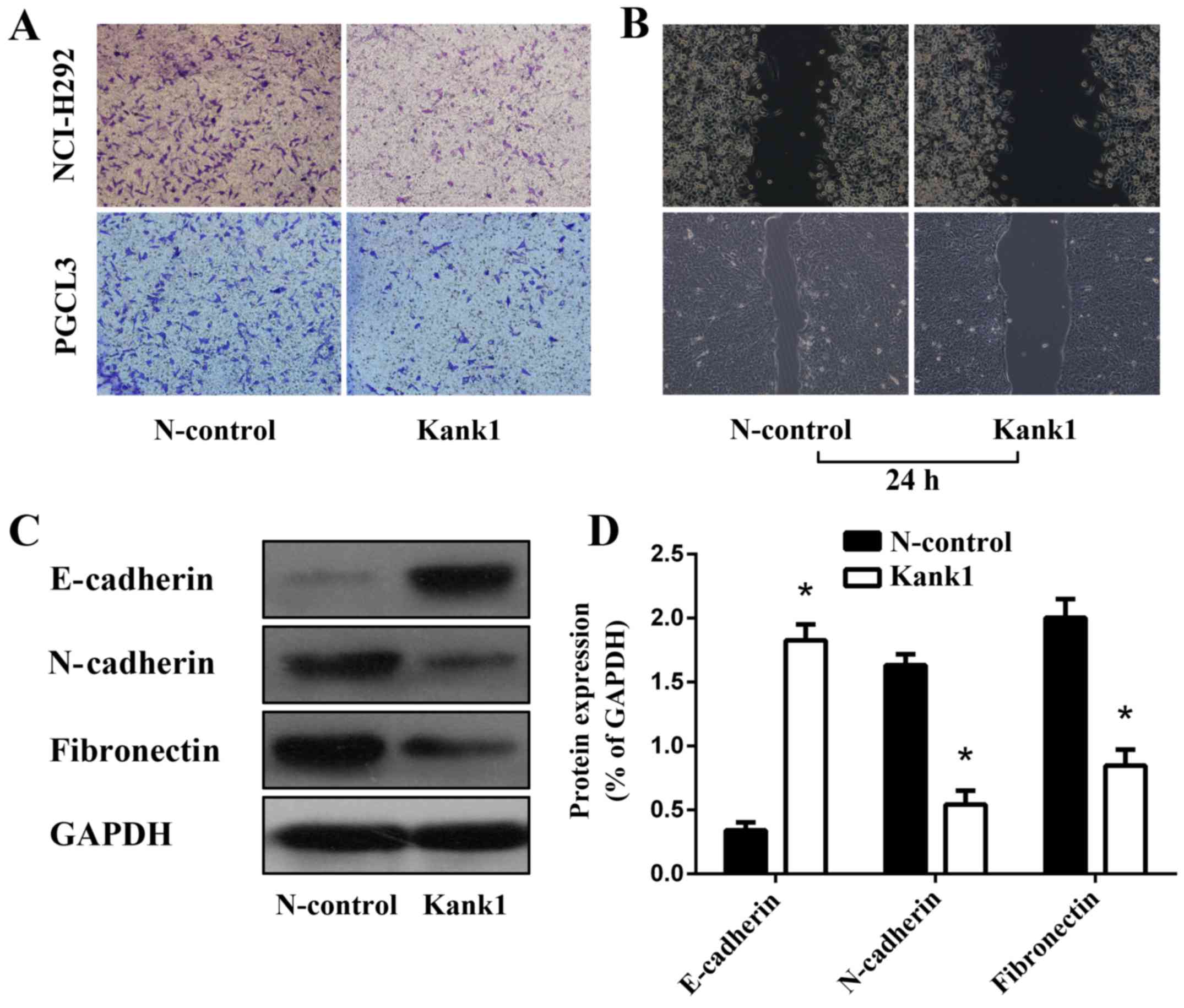
Upregulation of the Kank1 gene
inhibits invasion and metastasis of human lung cancer cells
We found significant decreases in both the invasion
and metastasis of human lung cancer cells with the upregulation of
the Kank1 gene as compared with the negative control
oligonucleotide group (Fig. 5A and
B). The expression of EMT-related proteins E-cadherin,
N-cadherin and fibronectin in human lung cancer cells upon
upregulation of the Kank1 gene was revealed to be significantly
altered as determined by western blotting (Fig. 5C and D). Our results revealed that
upregulation of the Kank1 gene inhibited the invasion and
metastasis of human lung cancer cells.
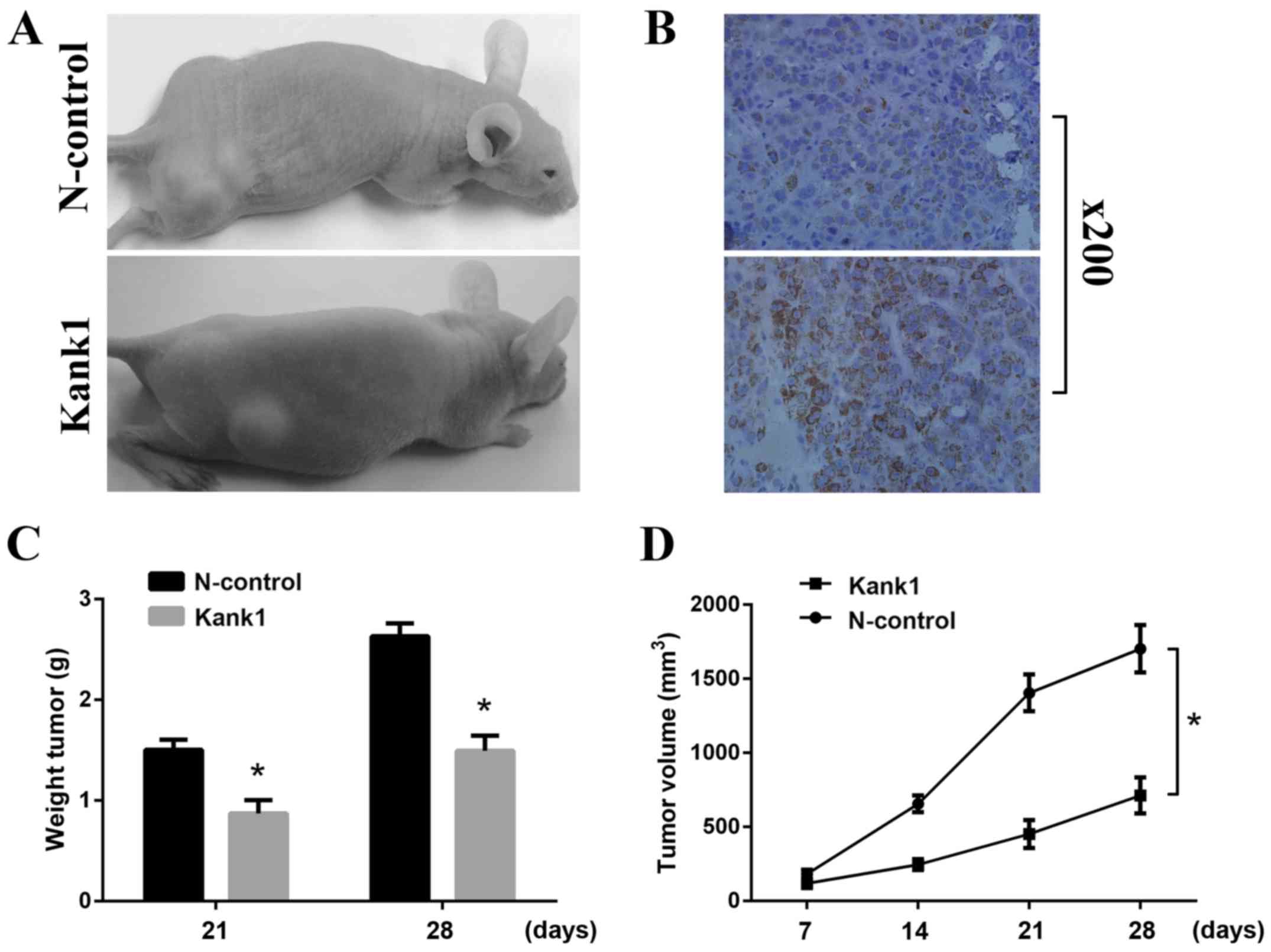
Upregulation of the Kank1 gene
inhibits the growth of xenografts in nude mice
To further determine whether Kankl was associated
with tumor growth, we implanted lung cancer cells with upregulated
Kankl-expression into nude mice. After 4 weeks, we found that
upregulation of the Kank1 gene significantly reduced the rate of
tumor formation in nude mice and significantly reduced the volume
of the tumor xenografts (Fig. 6A and
B). We found that upon surgical resection of the tumor, the
tumor weight in the Kank1-upregulated group was significantly
smaller than that in the control group (Fig. 6C). At each time-point, the volume of
the tumors in the nude mice with the upregulated Kank1 gene was
significantly smaller than that in the control group (Fig. 6D). The in vivo experiments
revealed that upregulation of the Kank1 gene significantly
inhibited the progression of lung cancer in vivo.
Discussion
It is well known that the complicated and changeable
pathogenesis of malignant tumors is closely related to the deletion
or mutation of tumor-suppressor genes and the large number of
oncogenes as well as some signaling pathways. This topic explores
the occurrence and development of lung cancer from the point of
view of tumor suppressor gene deletion or mutation. The Kankl gene,
identified as a candidate tumor suppressor gene in renal cell
carcinoma in 2002 by Japanese scientists using loss of
heterozygosity analysis and other methods, belongs to the Kank
family and is one of its important members (13). Kankl is mainly distributed in the
cytoplasm, and related to cell motility. It affects the occurrence
and development of tumors in a variety of diseases to some extent,
as it often forms a compound with β-catenin and shuttles in the
nucleoplasm, regulating the distribution of β-catenin in the
nucleus and enhancing β-catenin transcription in the occurrence and
development of malignant tumors and invasion and metastasis
(14).
As a candidate tumor-suppressor gene, the Kank1 gene
is expressed at a low level in many malignant tumors, such as
kidney (15) and liver cancer
(8). However, no research on lung
cancer has been reported yet. The incidence of lung cancer and
mortality is ranked first among malignant tumors, however the
pathogenesis is still not clear. In the present study, we found
that the Kank1 gene and protein expression in human lung cancer
cells was significantly lower than in normal cells. Therefore, we
concluded that the Kank1 gene was closely related to the
development and progression of lung cancer, and the Kank1 gene may
be a potential therapeutic target for lung cancer. To further
explain the association between the Kank1 gene and the development
and progression of lung cancer, we upregulated the expression of
the Kank1 gene in human lung cancer cells and observed the
biological changes of human lung cancer cells with overexpressed
Kank1 through a series of basic experiments. We determined that
upregulation of Kank1 gene expression significantly inhibited human
lung cancer cell proliferation with cells arrested in the G0/G1
phase, and cell apoptosis was promoted. Therefore, we surmised that
the low expression of Kank1 in lung cancer plays a certain role in
promoting the proliferation of tumor cells.
The pathways of apoptosis are complex, and the
signaling pathways that regulate apoptosis are diverse. Studies
have shown that the death receptor pathway of the mitochondrial
pathway is a classic pathway of apoptosis in many tumor cells
(16). Bcl-2 and Bax play a key
role in the mitochondrial apoptotic pathway (17). In many tumors, including lung
cancer, the expression of Bcl-2 is increased in tumors and Bax is
decreased (18). To date, the
understanding of how the Kank1 gene regulates the apoptosis
mechanism of human lung cancer cells is still very limited.
Therefore, following upregulation of the Kank1 gene in human lung
cancer cells, we found through western blotting and RT-PCR that Bax
and Bcl-2 translocated, Bax expression decreased, and Bax
translocated from the cytoplasm to the mitochondrial membrane to
alter the permeability of the mitochondrial membrane, promote the
release of cytochrome c from the mitochondria into the
cytoplasm, and then start the cascade of apoptosis to eventually
lead to cell apoptosis. Therefore, we hypothesized that the Kank1
gene promoted apoptosis in human lung cancer cells by regulating
the anti-pro-apoptotic proteins (Bcl-2 and Bax) in the Bcl-2 family
and also confirmed that the Kank1 gene and mitochondrial membrane
permeability finally resulted in tumor cell apoptosis. It is
well-known that caspase family activation and cascade amplification
are the necessary conditions for apoptosis in cells, both from the
external and internal pathways (19). Following upregulation of the Kank1
gene in human lung cancer cells, we found that procaspase-9 and
procaspase-3 expression levels were significantly decreased. In
summary, we found that the Kank1 gene, by regulating the
mitochondrial potential change, activated the mitochondrial
membrane to release caspase to activate caspase and then promote
human lung cancer cell apoptosis.
Tumor invasion and metastasis are important causes
of cancer death. Therefore, elucidating the molecular mechanism of
tumor metastasis has important clinical value for the treatment of
lung cancer. In this study, we found that upregulation of the Kank1
gene significantly inhibited the invasion and metastasis of human
lung cancer cells. In the human lung cancer cells with upregulated
Kank1, the expression levels of EMT-related proteins E-cadherin,
N-cadherin and fibronectin were significantly altered, the
tumorigenicity in nude mice decreased and the volume of xenograft
tumors decreased significantly. The aforementioned results revealed
that the Kank1 gene affected the invasion and metastasis of human
lung cancer cells.
In conclusion, we determined in the present study
that Kank1 gene expression was decreased in lung cancer. After
upregulation of the Kank1 gene, the cells were arrested in the
G0/G1 phase and the proliferation of lung cancer cells was markedly
inhibited. Concurrently, the mitochondrial pathway regulated
Bcl-2/Bax and induced the apoptosis in human lung cancer cells. The
Kank1 gene in the future may become a potential therapeutic target
for the clinical treatment of lung cancer and provide a theoretical
basis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
YG and MZ conceived and designed the study. YG
performed the experiments. MZ wrote the paper. YG and MZ reviewed
and edited the manuscript. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The animal experiment program was approved by the
Medical Ethics Committee of Jiangsu College of Nursing.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hensing T, Chawla A, Batra R and Salgia R:
A personalized treatment for lung cancer: Molecular pathways,
targeted therapies, and genomic characterization. Adv Exp Med Biol.
799:85–117. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Czarnecka-Kujawa K and Yasufuku K:
Molecular alterations in non-small-cell lung cancer: Perspective
for targeted therapy and specimen management for the
bronchoscopist. Respirology. 19:1117–1125. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sarkar S, Roy BC, Hatano N, Aoyagi T,
Gohji K and Kiyama R: A novel ankyrin repeat-containing gene (Kank)
located at 9p24 is a growth suppressor of renal cell carcinoma. J
Biol Chem. 277:36585–36591. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kakinuma N, Zhu Y, Wang Y, Roy BC and
Kiyama R: Kank proteins: Structure, functions and diseases. Cell
Mol Life Sci. 66:2651–2659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Catic A, Kurtovic-Kozaric A, Johnson SH,
Vasmatzis G, Pins MR and Kogan J: A novel cytogenetic and molecular
characterization of renal metanephric adenoma: Identification of
partner genes involved in translocation t(9;15)(p24;q24). Cancer
Genet 214–215. 1–15. 2017.
|
|
7
|
Huang SF, Hsu HC and Fletcher JA:
Investigation of chromosomal aberrations in hepatocellular
carcinoma by fluorescence in situ hybridization. Cancer Genet
Cytogenet. 111:21–27. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shao J, Li Y, Li H, Wu Q, Hou J and Liew
C: Deletion of chromosomes 9p and 17 associated with abnormal
expression of p53, p16/MTS1 and p15/MTS2 gene protein in
hepatocellular carcinomas. Chin Med J. 113:817–822. 2000.PubMed/NCBI
|
|
9
|
Sato M, Takahashi K, Nagayama K, Arai Y,
Ito N, Okada M, Minna JD, Yokota J and Kohno T: Identification of
chromosome arm 9p as themost frequent target of homozygous
deletions in lung cancer. Genes Chromosomes Cancer. 44:405–414.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lo KC, Stein LC, Panzarella JA, Cowell JK
and Hawthorn L: Identification of genes involved in squamous cell
carcinoma of the lung using synchronized data from DNA copynumber
and transcript expression profiling analysis. Lung Cancer.
59:315–331. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Roy BC, Kakinuma N and Kiyama R: Kank
attenuates actin remodeling by preventing interaction between
IRSp53 and Rac1. J Cell Biol. 184:253–267. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kralovics R, Teo SS, Buser AS, Brutsche M,
Tiedt R, Tichelli A, Passamonti F, Pietra D, Cazzola M and Skoda
RC: Altered gene expression in myeloproliferative disorders
correlates with activation of signaling by the V617F mutation of
Jak2. Blood. 106:3374–3376. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Clohisey SM, Dzhindzhev NS and Ohkura H:
Kank is an EB1 interacting protein that localises to muscle-tendon
attachment sites in Drosophila. PLoS One. 9:e1061122014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Y, Kakinuma N, Zhu Y and Kiyama R:
Nucleo-cytoplasmic shuttling of human Kank protein accompanies
intracellular translocation of beta-catenin. J Cell Sci.
119:4002–4010. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Roy BC, Aoyagi T, Sarkar S, Nomura K,
Kanda H, Iwaya K, Tachibana M and Kiyama R: Pathological
characterization of Kank in renal cell carcinoma. Exp Mol Pathol.
78:41–48. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ghavami S, Hashemi M, Ande SR, Yeganeh B,
Xiao W, Eshraghi M, Bus CJ, Kadkhoda K, Wiechec E, Halayko AJ, et
al: Apoptosis and cancer: Mutations within caspase genes. J Med
Genet. 46:497–510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ghasemian M, Mahdavi M, Zare P and Ali
Hosseinpour Feizi M: Spiroquinazolinone-induced cytotoxicity and
apoptosis in K562 human leukemia cells: Alteration in expression
levels of Bcl-2 and Bax. J Toxicol Sci. 40:115–126. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sela B: Survivin: Anti-apoptosis protein
and a prognostic marker for tumor progression and recurrence.
Harefuah. 141:103–107. 1232002.(In Hebrew). PubMed/NCBI
|
|
19
|
Jia J, Furlan A, Gonzalez-Hilarion S,
Leroy C, Gruenert DC, Tulasne D and Lejeune F: Caspases shutdown
nonsense-mediated mRNA decay during apoptosis. Cell Death Differ.
22:1754–1763. 2015. View Article : Google Scholar : PubMed/NCBI
|