Introduction
Esophageal cancer is one of the most aggressive and
fatal gastrointestinal tract malignancies worldwide. Esophageal
squamous cell carcinoma (ESCC) is the predominant histological type
of esophageal cancer in China (1).
Although clinical diagnostic and multidisciplinary therapeutic
progress has been made, the overall prognosis of ESCC patients is
still unfavorable due to rapid progression and metastasis. To date,
the underlying mechanisms involved in the initiation and
progression of ESCC are not fully understood. Therefore, a thorough
understanding of the molecular mechanisms underlying the
carcinogenesis and progression of ESCC is vital for discovering
novel targets and innovative treatment strategies.
Angiogenesis, the process leading to the formation
of new blood vessels from preexisting ones, is one of the major
hallmarks of cancer, and is involved in the progression and growth
of cancer (2,3). Understanding the molecular mechanisms
responsible for tumor angiogenesis can benefit cancer diagnosis and
treatment. Thus, suppression of tumor angiogenesis offers a
promising strategy for targeted therapy of cancer.
IQ-domain GTPase activating protein 1 (IQGAP1) is a
member of a family of scaffolding proteins, which regulate distinct
cellular processes including cell adhesion, proliferation,
migration and other cellular functions through interacting with
diverse proteins (4–6). Thus, IQGAP1 is as a critical
integrator of cellular signaling pathways. Several studies have
shown that IQGAP1 expression is increased in various cancer
tissues, including colorectal carcinoma (7,8),
breast (9), ovarian (10), lung (11), pancreatic (12) and thyroid cancer (13). Furthermore, high expression of
IQGAP1 promotes invasion and metastasis, and exhibits a significant
correlation with poor patient prognosis (7–13). We
reported that IQGAP1 is highly overexpressed in ESCC and the
knockdown of IQGAP1 by small interfering RNA (siRNA) can decrease
cell proliferation and metastasis ability in vitro and in
vivo (14), indicating that
IQGAP1 is a potential target for cancer treatment. However, the
role of IQGAP1 in the angiogenesis of ESCC is not yet known.
In the present study, we investigated the role of
IQGAP1 in regulating the angiogenesis of ESCC and explored its
underlying molecular mechanisms. We report that IQGAP1
overexpression promotes angiogenesis of ESCC by the AKT and
ERK-mediated vascular endothelial growth factor (VEGF)-vascular
endothelial growth factor receptor 2 (VEGFR2) signaling pathway.
These findings suggest an essential role of IQGAP1 in the
angiogenesis of ESCC and provide novel insight into IQGAP1 as an
attractive therapeutic target for cancer anti-angiogenesis
treatment.
Materials and methods
Cell culture and stable
transfections
Human ESCC cell lines EC9706 and KYSE150 were
purchased from the Tumor Cell Bank of the Chinese Academy of
Medical Sciences (Beijing, China). Human umbilical vascular
endothelial cells (HUVECs) were purchased from the CHI Scientific
Inc. (Jiangsu, China). The cells were maintained in Gibco
Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(FBS; HyClone Laboratories; GE Healthcare Life Sciences, Logan, UT,
USA), penicillin and streptomycin at 5% CO2 and 37°C in
a humidified incubator. The GFP-IQGAP1 overexpression and control
plasmids were purchased from GeneCopoeia, Inc. (Rockville, MD, USA)
and transfected into EC9706 cells. Non-specific control and IQGAP1
shRNA (short hairpin RNA) plasmids were purchased from Shanghai
Genechem Co., Ltd., (Shanghai, China) and transfected into KYSE150
cells. Transfection of the plasmids was carried out using
Invitrogen Lipofectamine 2000 transfection reagent (Thermo Fisher
Scientific, Inc.) following the manufacturer's protocol. Stable
cell lines expressing IQGAP1 or IQGAP1 siRNA and control cells were
selected for 2 weeks with 0.4 mg/ml G418 sulfate after
transfection. The supernatant from cells of IQGAP1-overexpressing
or knockdown and control was collected as conditioned medium for
tube formation of HUVECs and chicken embryo chorioallantoic
membrane (CAM) assays (described below). For AKT inhibitor
(LY294002) or ERK inhibitor (PD98059) (Calbiochem, Merck
Biosciences, Merck KGaA, Darmstadt, Germany) treatment,
IQGAP1-overexpressing cells were seeded and after 24 h, cells were
incubated with 20 µM LY294002 or PD98059 in the absence of serum
for 48 h. The supernatant was collected as conditioned medium for
tube formation of HUVECs and chicken embryo CAM assays (described
below).
Western blot analysis
The proteins in the cell lysates were quantified
using the Bradford method. Proteins (70 µg) were processed by
electrophoretic separation on 10% SDS-PAGE and transferred to a
nitrocellulose membrane, which was then blocked with PBS/Tween-20
containing 5% non-fat milk. The membranes were incubated overnight
at 4°C with corresponding primary antibodies. To normalize protein
loading, mouse anti-β-actin antibody (1:5,000; cat. no. A5441;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was used. After
incubation with HRP-conjugated secondary antibody for 2 h at room
temperature, target proteins on the membrane were visualized using
an enhanced chemiluminescence (ECL) detection system (Beijing
ComWin Biotech Co., Ltd, Beijing, China). The band intensity was
analyzed using Bio-Rad's Image Lab software (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). Mouse anti-IQGAP1 antibody was purchased
from BD Biosciences (1:5,000; cat. no. 610612; Franklin Lakes, NJ,
USA). Rabbit anti-GFP (1:1,000; cat. no. D110008) and rabbit
anti-VEGFR2 (1:500; cat. no. D151118) antibodies were purchased
from Sangon Biotech Company (Shanghai, China). Rabbit anti-VEGF
(1:500; cat. no. A12303) and rabbit anti-p-VEGFR2 (1:500; cat. no.
AP0382) antibodies were purchased from ABclonal Biotechnology Co.,
Ltd. (Wuhan, China). Rabbit anti-p-AKT (1:1,000; cat. no. 4060),
total rabbit anti-AKT (1:1,000; cat. no. 9272), rabbit
anti-p-ERK1/2 (1:1,000; cat. no. 9101) and total rabbit anti-ERK1/2
(1:1,000; cat. no. 4695) were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA).
Tube formation assay
A total of 50 µl of chilled Matrigel (BD
Biosciences) was added to a 96-well plate and incubated at 37°C for
30 min. HUVECs (1×104) in 100 µl of conditioned medium
were seeded onto each well and incubated at 37°C in 5%
CO2 for 6 h. Images were captured by phase-contrast
microscopy and the tubular structures were quantified by manual
counting in three random fields per well to obtain the sum.
Chicken embryo CAM assay
Seven-day-old chicken embryos were windowed to
expose the CAM and the conditioned medium was placed onto the CAM.
The windows were sealed with cellophane tape and embryos were
transferred back into the incubator. After 3 days, chicken embryos
were fixed with stationary solution (methanol:acetone 1:1) for 15
min, CAMs were cut and harvested, and then photographed. The
ability of angiogenesis in chicken embryo CAM was quantitated by
measuring the number of vessels.
Statistical analysis
Statistical analysis was conducted using the SPSS
17.0 software package (SPSS, Inc., Chicago, IL, USA). All
experiments were performed in triplicate. Data are presented as the
mean ± SD. The differences between groups were assessed by one-way
ANOVA and followed up using Dunnett's multiple comparison post hoc
test. P<0.05 was considered to indicate a statistically
significant result.
Results
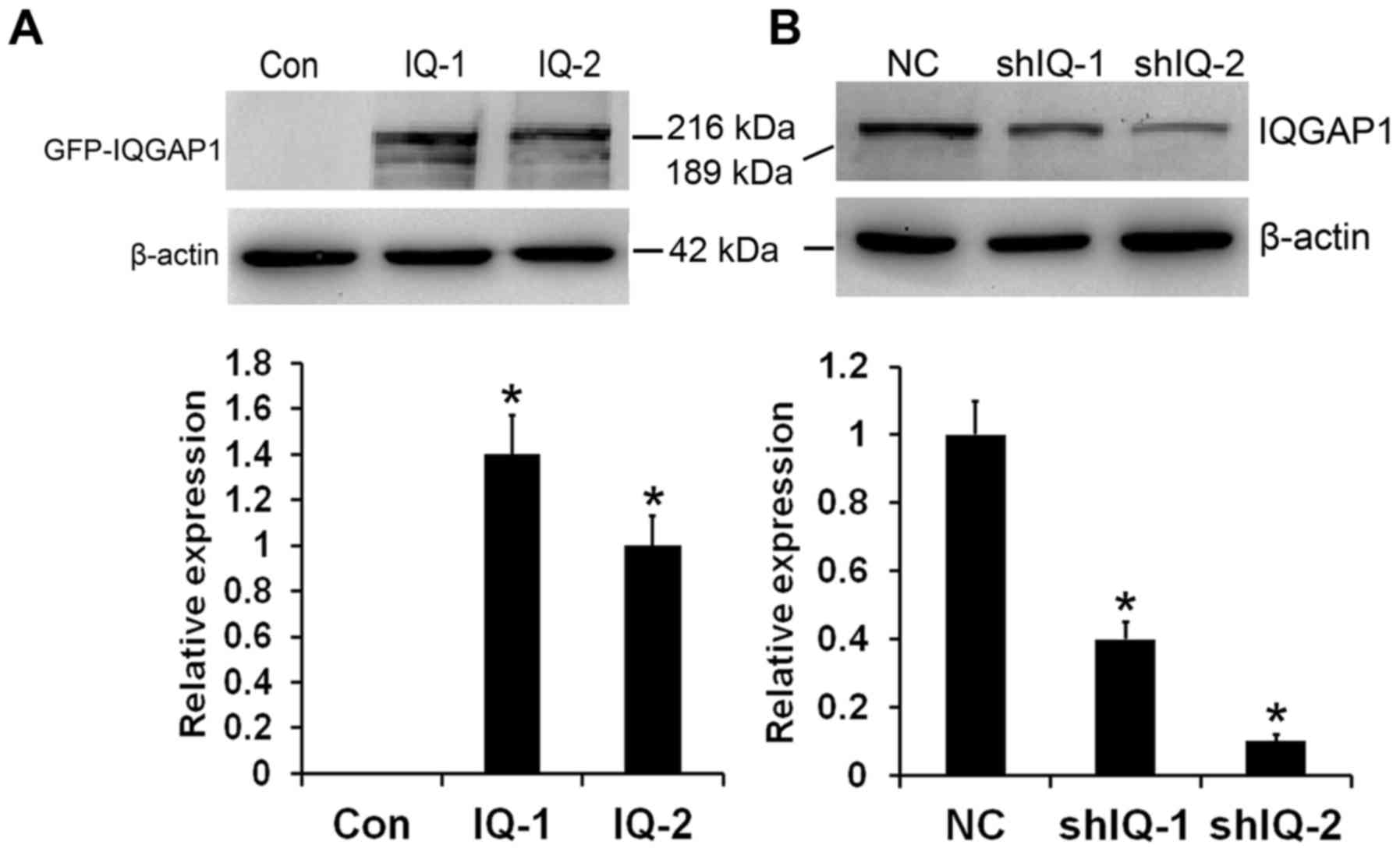
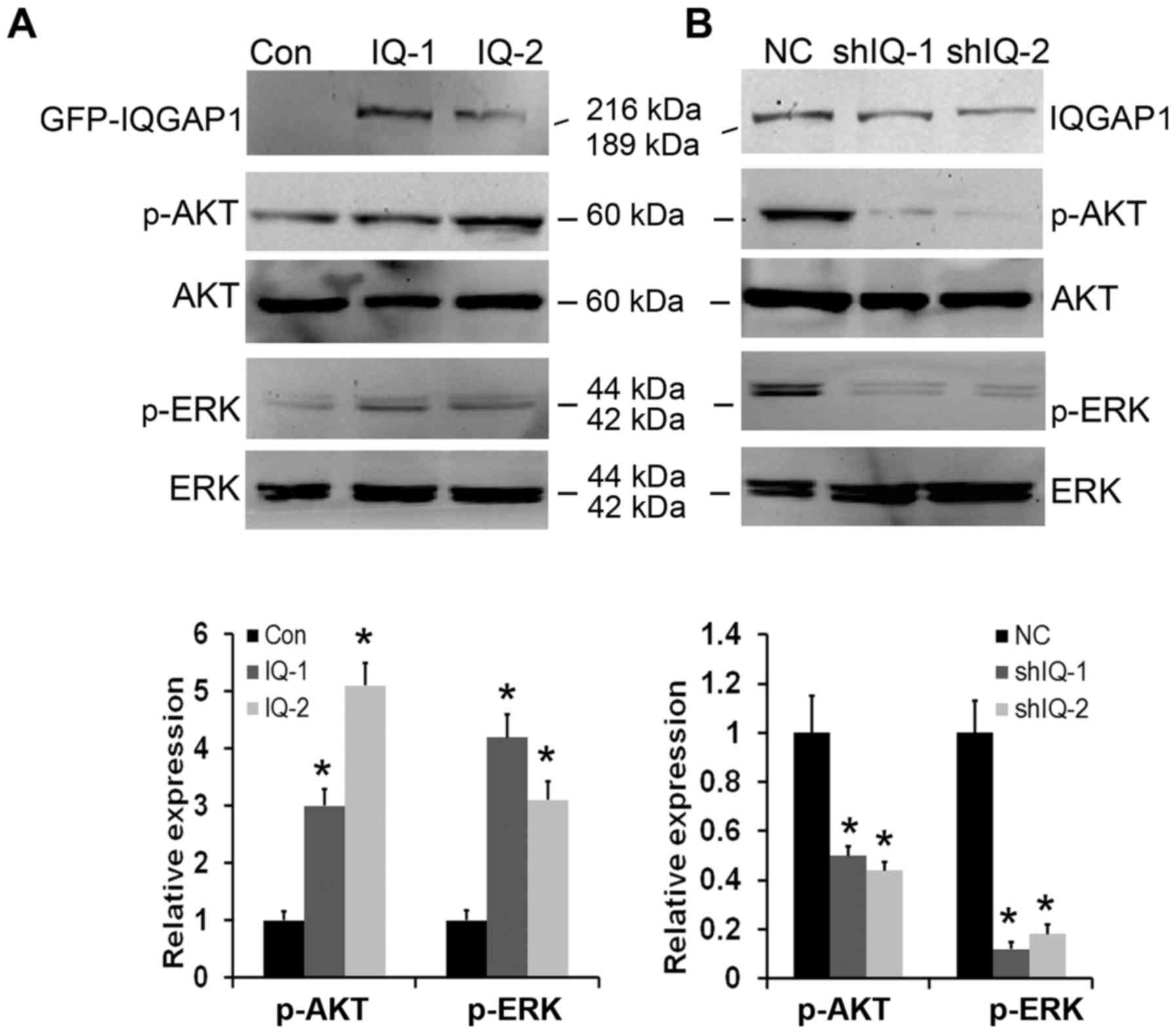
Generation of stable
IQGAP1-overexpressing and silenced clones in the ESCC cell
lines
In order to obtain insight into the effect of IQGAP1
on tumor angiogenesis, we overexpressed IQGAP1 in human ESCC EC9706
cells. Western blot analysis results showed that stable clones
transfected with the expression vector carrying cDNA for human
full-length IQGAP1 (named as IQ-1 and IQ-2) expressed fusion
protein of GFP-IQGAP1, which was not found in the control vector
(named as Con) (Fig. 1A). To
further evaluate the roles of IQGAP1 in tumor angiogenesis and the
potential of IQGAP1 downregulation for ESCC therapy, IQGAP1 stable
knockdown was performed in the human ESCC KYSE150 cell line. As
shown in Fig. 1B, two stable clones
(named as shIQ-1 and shIQ-2) exhibited efficiently reduced
expression levels of IQGAP1 protein compared with the control cells
(named as NC).
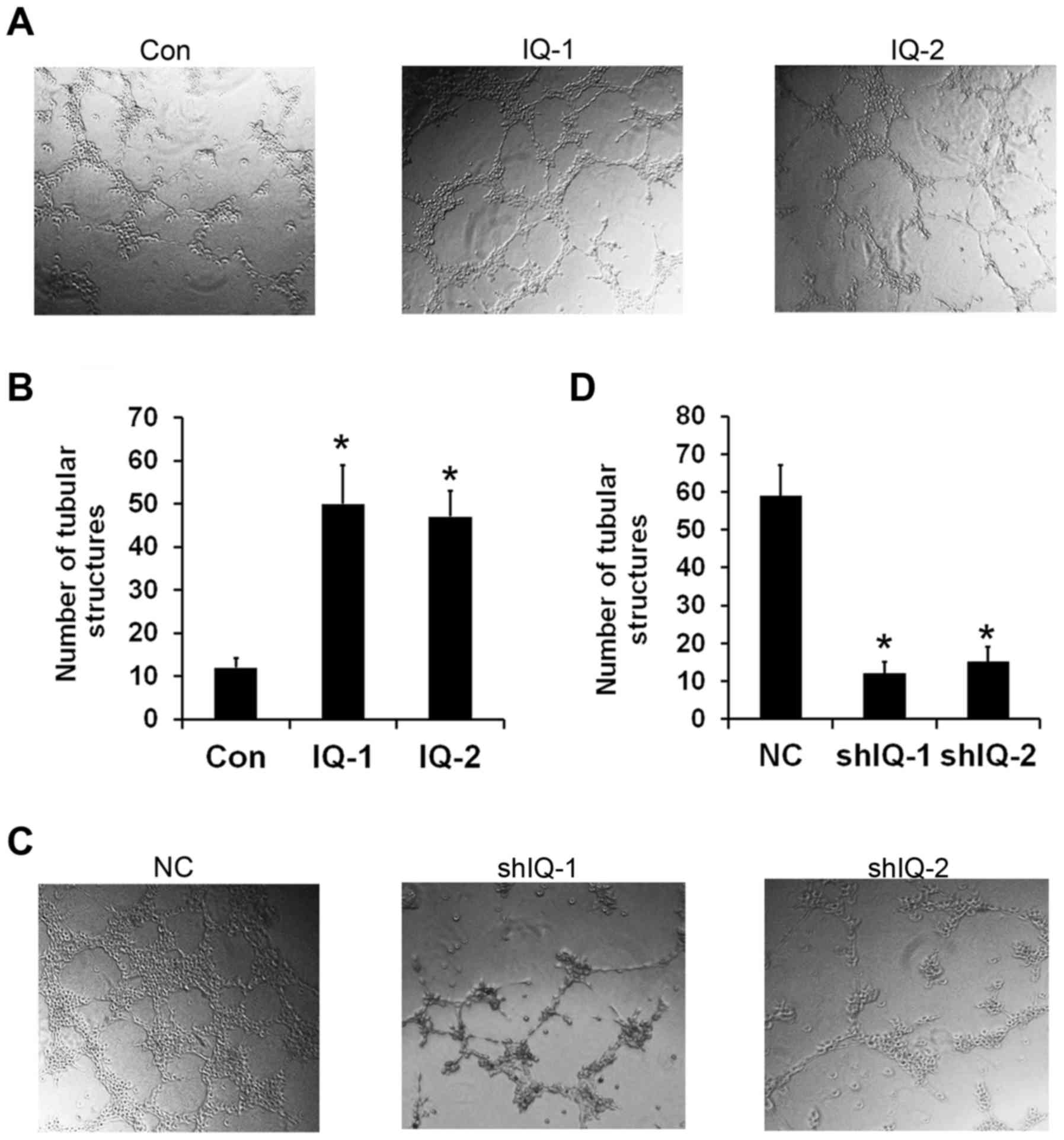
IQGAP1 overexpression promotes tube
formation of HUVECs
The tube formation assay can mimic certain stages of
angiogenesis, which is a well-established in vitro
angiogenesis test (15,16). To assess the functional role of
IQGAP1 in angiogenesis in vitro, we investigated whether
IQGAP1 is involved in capillary tube formation. As shown in
Fig. 2A, the HUVECs spontaneously
formed capillary-like tube structures after 6 h of incubation on
Matrigel. The conditioned medium from IQGAP1 overexpressing cells
increased the number of capillary-like structures. Quantification
of the number of tubular structure showed that IQGAP1
overexpression resulted in a 5-to 6-fold increasing in tube
formation by HUVECs (Fig. 2B). In
contrast, IQGAP1 knockdown resulted in less elongated, broken and
foreshortened tubes compared to the control shRNA-transfected cells
(Fig. 2C). An approximate 6-to
7-fold decrease in tube formation was observed in the IQGAP1
shRNA-transfected cells (Fig. 2D).
As a consequence, this finding confirmed that IQGAP1 functions as a
promoter of tumor angiogenesis in vitro.
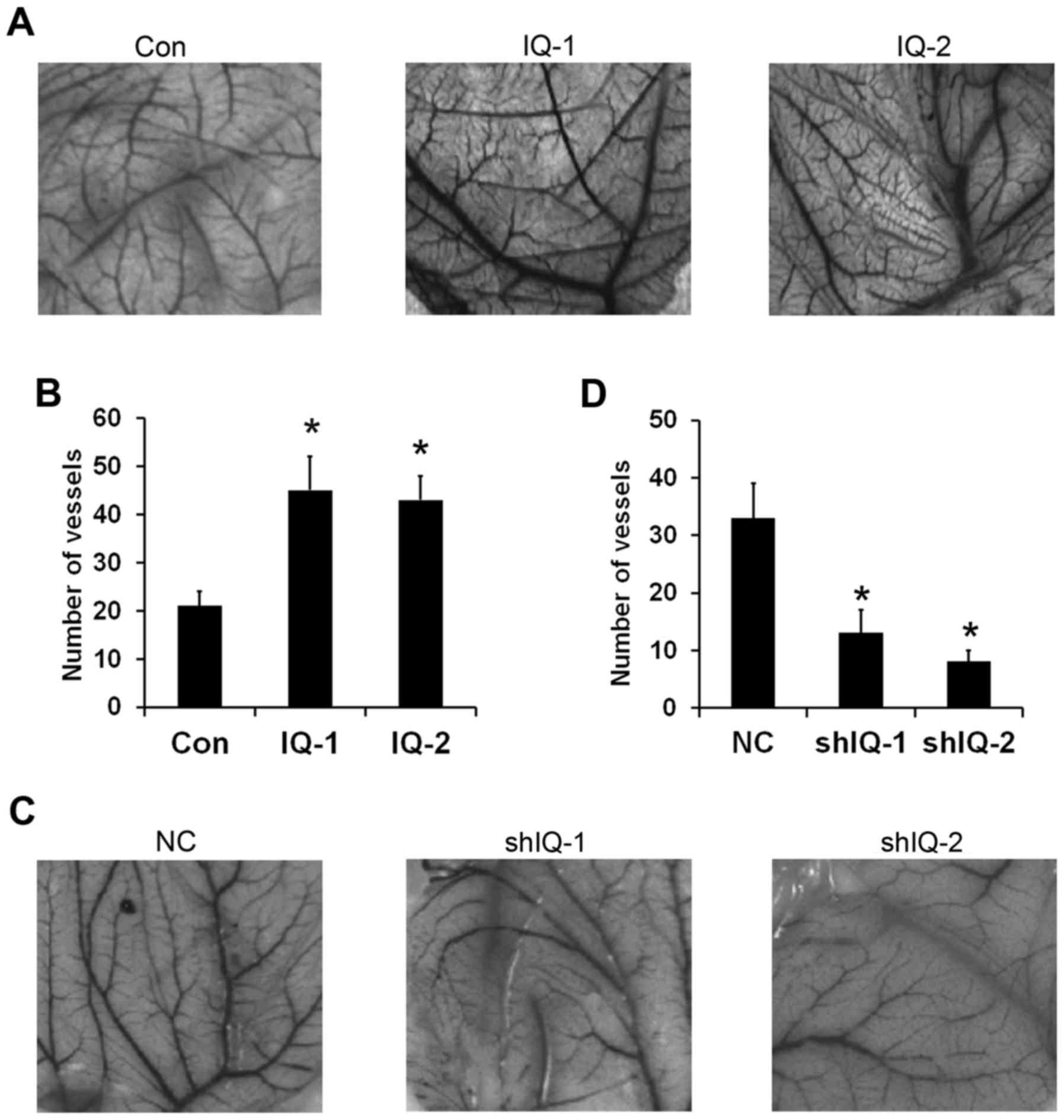
IQGAP1 overexpression stimulates
angiogenesis in the chicken embryo CAM assay
Chicken embryo CAM assay is a well-known model of
angiogenesis that can be widely used to investigate new vessel
formation and inhibition in vivo (17). To further evaluate the potential
effect of IQGAP1 on angiogenesis, chicken embryo CAM assay was
employed. The results showed that IQGAP1 overexpression induced a
stronger proangiogenic response in a chicken embryo CAM assay than
the control (Fig. 3A). The number
of branches of microvessels in the conditioned medium from the
IQGAP1-overexpressing cells increased to 2.5-to 3-fold of the
control (Fig. 3B). Conversely,
IQGAP1 knockdown inhibited angiogenesis (Fig. 3C). Quantitative analysis revealed
that conditioned medium from the IQGAP1-knockdown cells caused a
3.5-to 4-fold reduction in the number of blood vessels (Fig. 3D). These results further confirm
that IQGAP1 overexpression induces tumor angiogenesis in
vivo.
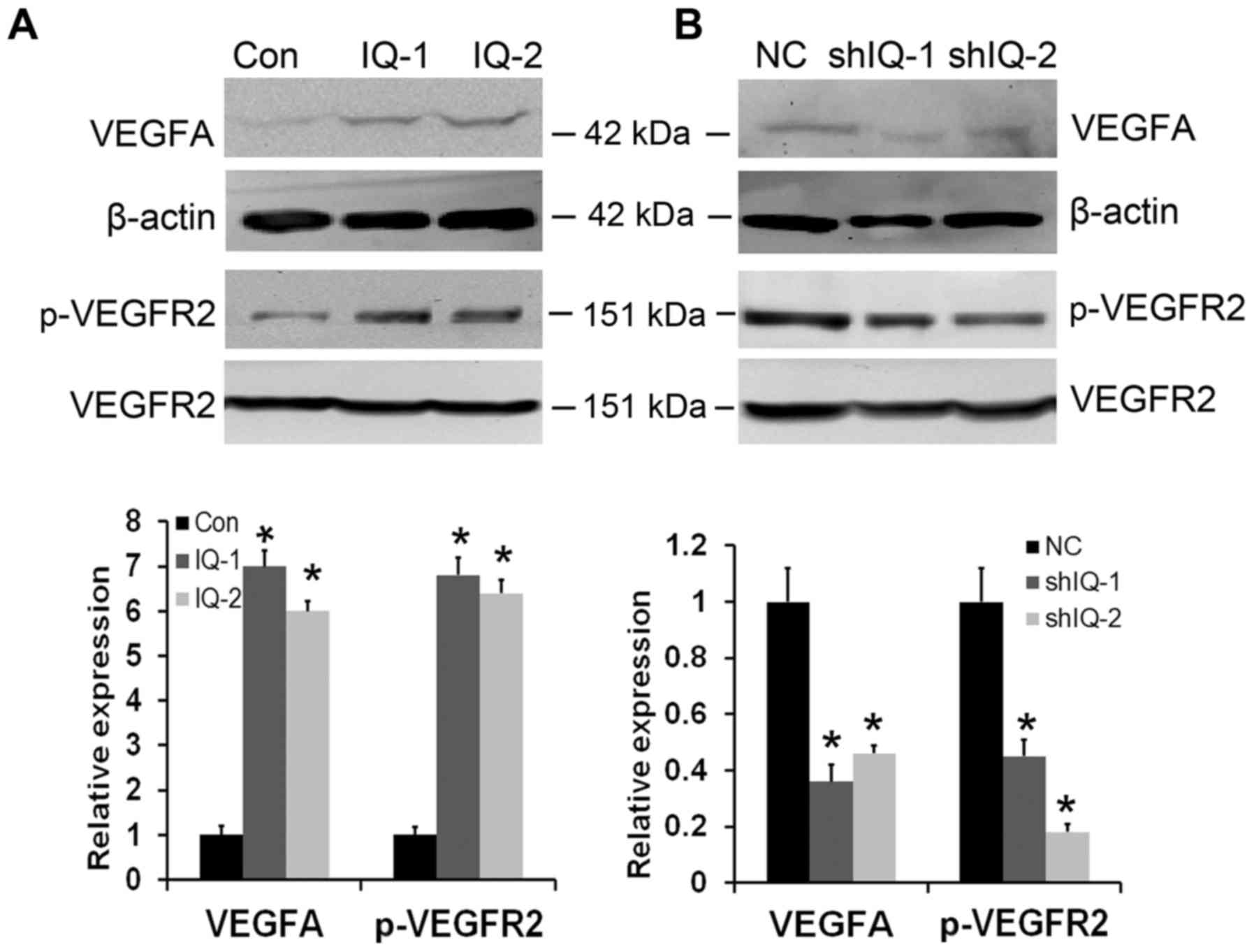
IQGAP1 overexpression enhances
expression of VEGF and activation of VEGFR2
Considering that angiogenic factor VEGF is a prime
regulator of angiogenesis (18), we
first examined the expression of VEGF in IQGAP1-overexpressing and
-silenced ESCC cells. As shown in Fig.
4A and B, IQGAP1 overexpression upregulated the expression
levels of VEGF, whereas VEGF expression was obviously decreased in
the IQGAP1-silenced. VEGFR2 is the most biologically important
receptor for VEGF (19). Thus, we
subsequently examined VEGFR2 expression and activation in the
IQGAP1-overexpressing and -silenced ESCC cells. IQGAP1
overexpression significantly enhanced phosphorylation of VEGFR2,
without obviously affecting overall VEGFR2 expression levels.
Conversely, IQGAP1 knockdown inhibited the phosphorylation of
VEGFR2, while the total levels of VEGFR2 had little change
(Fig. 4A and B). These results
clearly demonstrate that IQGAP1 overexpression can promote tumor
angiogenesis by upregulating VEGF-VEGFR2 signaling.
IQGAP1 overexpression promotes tumor
angiogenesis through AKT and ERK activation
To identify the potential molecular mechanisms of
IQGAP1 in tumor angiogenesis, we analyzed the expression levels of
various signaling proteins by western blot assay. The results
showed that IQGAP1 overexpression markedly increased the levels of
p-AKT and p-ERK, whereas the levels of total AKT and ERK were not
altered (Fig. 5A). Silencing of
IQGAP1 expression led to a significant decrease in the expression
of p-AKT and p-ERK proteins, and had no effect on total AKT and ERK
protein expression (Fig. 5B). To
determine whether the IQGAP1-mediated increase in VEGF and p-VEGFR2
expression as well as tumor angiogenesis is mediated by regulating
AKT or ERK signaling, we analyzed the effect of specific AKT and
ERK inhibitors (LY294002 or PD98059) on IQGAP1-overexpressing
cells. As shown in Fig. 6A and B,
the AKT and ERK inhibitor abolished the role of IQGAP1
overexpression on VEGF and p-VEGFR2 upregulation. Furthermore, we
found that LY294002 or PD98059 could abrogate the effects of
IQGAP1-mediated tumor angiogenesis by in vitro tube
formation of HUVECs (Fig. 6C and D)
and in vivo chicken embryo CAM assay (Fig. 6E and F). Taken together, these
observations demonstrate that IQGAP1 overexpression promotes tumor
angiogenesis by targeting the AKT and ERK-mediated VEGF-VEGFR2
signaling pathway.
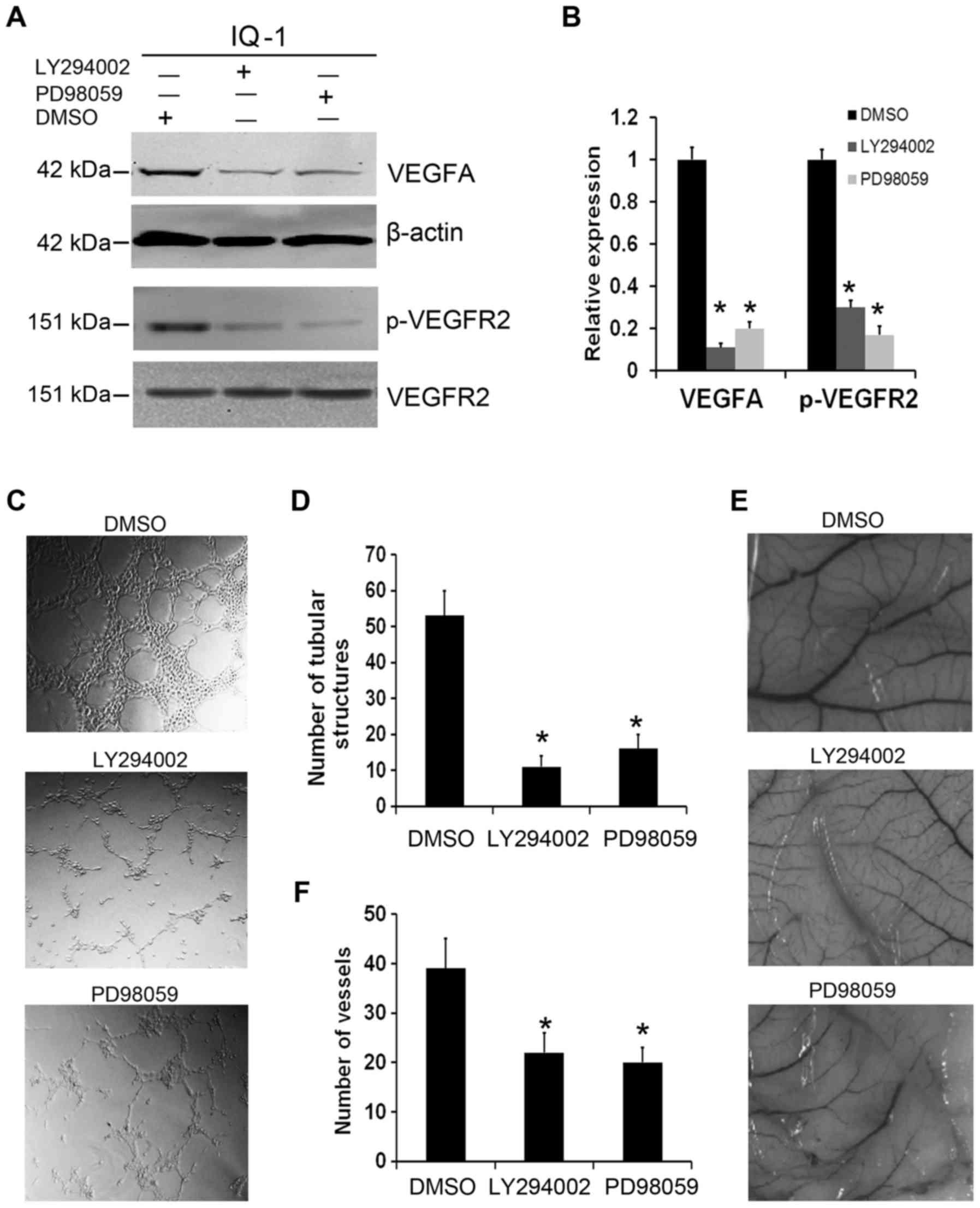 | Figure 6.IQGAP1 regulates expression of VEGF
and phosphorylated (p)-VEGFR2 as well as angiogenesis through AKT
and ERK signaling. (A) AKT inhibitor (LY294002) or ERK inhibitor
(PD98059) (20 µM) was used to treat IQGAP1-overexpressing cells
(IQ-1) for 48 h, and abrogated the effects of IQGAP1
overexpression-mediated upregulation of VEGFA and p-VEGFR2. (B) The
histogram represents quantitative densitometry of proteins. Data
are presented as mean ± SD, n=3, *P<0.01, compared with the
control cells (DMSO). (C) The promoting effect of IQGAP1
overexpression on HUVEC tube formation was attenuated by the AKT or
ERK inhibitor. (D) The number of tubular structures was counted in
each group. Data are presented as mean ± SD, n=3, *P<0.01,
compared with the control cells (DMSO). (E) The pro-angiogenic
function of IQGAP1 overexpression in chicken embryo CAM was
abrogated when IQGAP1 overexpression cells were treated with AKT or
ERK inhibitor. (F) The number of vessels in each group was counted.
Data are presented as mean ± SD, n=3, *P<0.01, compared with the
control cells (DMSO). IQGAP1, IQ-domain GTPase activating protein
1; VEGF, vascular endothelial growth factor; VEGFR2, vascular
endothelial growth factor receptor 2. |
Discussion
Esophageal squamous cell carcinoma (ESCC) is one of
the leading causes of cancer-related death due to the high
incidence of advanced disease, metastasis, and resistance to
radiotherapy and chemotherapy (1).
Thus, it is urgent to identify novel targets and new strategies to
treat this disease. Angiogenesis plays a significant role in the
continuous growth of tumors, invasion and metastasis as capillary
formation in tumors can provide nutrients and oxygen to supply the
growing tumor and also act as conduits for the metastasis of tumors
(2,3,19).
Consequently, more and more attention has been focused on tumor
angiogenesis; and thus, anti-angiogenic therapy has become one of
the most promising and efficient strategy for inhibiting tumor
growth and progression.
The development of ESCC involves the accumulation of
the abnormal expression of oncogenes involved in the initiation and
progression of ESCC. IQGAP1 is a member of the IQGAP family of
multidomain proteins (6,20). Cumulative evidence suggests that
IQGAP1 is an oncogene and is overexpressed in several types of
human cancers (4,21). Consistent with these findings, we
reported that IQGAP1 is upregulated in ESCC tissues and is
correlated with the invasive depth of ESCC tumors (14). However, it has not yet been
elucidated whether IQGAP1 is involved in tumor angiogenesis in ESCC
during which IQGAP1 is upregulated. In the present study, we found
that IQGAP1 overexpression significantly increased the angiogenesis
confirmed by HUVEC tube formation assay in vitro and chicken
embryo CAM assay in vivo, whereas the angiogenesis ability
was markedly suppressed when IQGAP1 expression was silenced. These
results indicate that IQGAP1 is an attractive molecule for
targeting tumor angiogenesis against cancer progression.
Angiogenesis is a complex multistep process which is
regulated by several endogenous angiogenic activators and
inhibitors (2,3,22). Of
the numerous endogenous pro-angiogenic factors, VEGF is well known
as a key regulator of the process of tumor angiogenesis by
stimulating endothelial cell proliferation, migration and invasion.
VEGF exerts its biological effects by binding to specific tyrosine
kinase receptors on the cell surface, called VEGF receptors
(VEGFRs), and VEGFR2 is the major mediator of VEGF-induced
angiogenesis. The binding of VEGF to VEGFR2 leads to the intrinsic
tyrosine kinase activation of the receptors followed by
dimerization and autophosphorylation of VEGFR2, and then triggers a
downstream signaling cascade (19,23,24).
Therefore, we hypothesized that IQGAP1 regulates ESCC angiogenesis
by regulating the VEGF-VEGFR2 pathway. In the present study, we
observed that overexpression of IQGAP1 strongly increased VEGF
expression and phosphorylation of VEGFR2, while knockdown of IQGAP1
obviously decreased VEGF and p-VEGFR2 expression. These findings
showed that IQGAP1 could regulate tumor angiogenesis by controlling
the activation of VEGF-VEGFR2 signaling. It has been reported that
IQGAP1 can directly bind to VEGFR2 and also is necessary for VEGF
to stimulate angiogenesis in MCF-7 and HUVECs (9,25–27),
which is consistent with our findings. Considering that the
VEGF-VEGFR signaling pathway is a significant factor underlying
angiogenesis, numerous therapeutic strategies have been developed
to target angiogenesis by blocking this pathway. Accordingly, this
study further indicates that targeting IQGAP1 represents a
promising therapeutic strategy for tumor angiogenesis.
AKT and ERK are serine/threonine kinases that are
critical for many diverse processes, including cell proliferation,
apoptosis, migration, angiogenesis and metastasis (28–31).
IQGAP1, as a scaffold protein, contains multiple domains which
mediate binding to a number of proteins. It has been reported that
IQGAP1 can combine with AKT and ERK and regulate their activity
(6,21,32–35).
To explore the potential pro-angiogenic mechanisms of IQGAP1 in
ESCC, we detected the expression of the AKT and ERK signaling
pathway. The data showed that IQGAP1 overexpression could increase
phosphorylation of AKT and ERK. Moreover, IQGAP1 knockdown could
inhibit AKT and ERK activation. Furthermore, we observed that AKT
and ERK inhibitors significantly decreased VEGF expression and
VEGFR2 phosphorylation in IQGAP1-overexpressing cells. Moreover,
the pro-angiogenic effect of IQGAP1 overexpression on angiogenesis
in tube formation of HUVECs and a chick embryo CAM angiogenesis
model was abrogated when IQGAP1-overexpressing cells were treated
with the AKT and ERK inhibitor. These findings suggest that IQGAP1
promotes tumor angiogenesis mainly via AKT or ERK/VEGF-VEGFR2
signaling pathway.
In summary, we demonstrated for the first time that
IQGAP1 overexpression could promote angiogenesis in ESCC by
targeting the AKT or ERK/VEGF-VEGFR2 signaling pathway. Moreover,
silencing of the expression of IQGAP1 inhibited tumor angiogenesis.
Our studies not only demonstrated that IQGAP1 regulated the tumor
angiogenesis of ESCC, but also revealed a therapeutic opportunity
in targeting IQGAP1 for cancer treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (no. 81372676) and the Natural
Science Foundation of Shanxi Province (no. 201601D011130).
Availability of data and material
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CHL and XJS carried out the experiments and
interpreted the data. SSN, CYY, YPH and JTK participated in the
collection of the data. XXW and XZL designed the research,
supervised the study, interpreted data and wrote the manuscript.
All authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carmeliet P: Angiogenesis in life, disease
and medicine. Nature. 438:932–936. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Folkman J: Angiogenesis. Annu Rev Med.
57:1–18. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Johnson M, Sharma M and Henderson BR:
IQGAP1 regulation and roles in cancer. Cell Signal. 21:1471–1478.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Noritake J, Watanabe T, Sato K, Wang S and
Kaibuchi K: IQGAP1: A key regulator of adhesion and migration. J
Cell Sci. 118:2085–2092. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
White CD, Erdemir HH and Sacks DB: IQGAP1
and its binding proteins control diverse biological functions. Cell
Signal. 24:826–834. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nabeshima K, Shimao Y, Inoue T and Koono
M: Immunohistochemical analysis of IQGAP1 expression in human
colorectal carcinomas: Its overexpression in carcinomas and
association with invasion fronts. Cancer Lett. 176:101–109. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hayashi H, Nabeshima K, Aoki M, Hamasaki
M, Enatsu S, Yamauchi Y, Yamashita Y and Iwasaki H: Overexpression
of IQGAP1 in advanced colorectal cancer correlates with poor
prognosis-critical role in tumor invasion. Int J Cancer.
126:2563–2574. 2010.PubMed/NCBI
|
|
9
|
Jadeski L, Mataraza JM, Jeong HW, Li Z and
Sacks DB: IQGAP1 stimulates proliferation and enhances
tumorigenesis of human breast epithelial cells. J Biol Chem.
283:1008–1017. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dong P, Nabeshima K, Nishimura N, Kawakami
T, Hachisuga T, Kawarabayashi T and Iwasaki H: Overexpression and
diffuse expression pattern of IQGAP1 at invasion fronts are
independent prognostic parameters in ovarian carcinomas. Cancer
Lett. 243:120–127. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao H, Xie C, Lin X, Zhao Y, Han Y, Fan
C, Zhang X, Du J, Han Y, Han Q, et al: Coexpression of IQ-domain
GTPase-activating protein 1 (IQGAP1) and Dishevelled (Dvl) is
correlated with poor prognosis in non-small cell lung cancer. PLoS
One. 9:e1137132014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang XX, Li XZ, Zhai LQ, Liu ZR, Chen XJ
and Pei Y: Overexpression of IQGAP1 in human pancreatic cancer.
Hepatobiliary Pancreat Dis Int. 12:540–545. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Z, Liu D, Bojdani E, El-Naggar AK,
Vasko V and Xing M: IQGAP1 plays an important role in the
invasiveness of thyroid cancer. Clin Cancer Res. 16:6009–6018.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang XX, Wang K, Li XZ, Zhai LQ, Qu CX,
Zhao Y, Liu ZR, Wang HZ, An QJ, Jing LW, et al: Targeted knockdown
of IQGAP1 inhibits the progression of esophageal squamous cell
carcinoma in vitro and in vivo. PLoS One. 9:e965012014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Arnaoutova I, George J, Kleinman HK and
Benton G: The endothelial cell tube formation assay on basement
membrane turns 20: State of the science and the art. Angiogenesis.
12:267–274. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ponce ML: Tube formation: An in vitro
matrigel angiogenesis assay. Methods Mol Biol. 467:183–188. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ribatti D, Vacca A, Roncali L and Dammacco
F: The chick embryo chorioallantoic membrane as a model for in vivo
research on angiogenesis. Int J Dev Biol. 40:1189–1197.
1996.PubMed/NCBI
|
|
18
|
Verheul HM and Pinedo HM: The role of
vascular endothelial growth factor (VEGF) in tumor angiogenesis and
early clinical development of VEGF-receptor kinase inhibitors. Clin
Breast Cancer. 1 Suppl 1:S80–S84. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zetter BR: Angiogenesis and tumor
metastasis. Annu Rev Med. 49:407–424. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Osman M: An emerging role for IQGAP1 in
regulating protein traffic. ScientificWorldJournal. 10:944–953.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
White CD, Brown MD and Sacks DB: IQGAPs in
cancer: A family of scaffold proteins underlying tumorigenesis.
FEBS Lett. 583:1817–1824. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Detmar M: Tumor angiogenesis. J Investig
Dermatol Symp Proc. 5:20–23. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shibuya M: Vascular endothelial growth
factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: A
crucial target for anti- and pro-angiogenic therapies. Genes
Cancer. 2:1097–1105. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
McMahon G: VEGF receptor signaling in
tumor angiogenesis. Oncologist. 5 Suppl 1:S3–S10. 2000. View Article : Google Scholar
|
|
25
|
Yamaoka-Tojo M, Tojo T, Kim HW, Hilenski
L, Patrushev NA, Zhang L, Fukai T and Ushio-Fukai M: IQGAP1
mediates VE-cadherin-based cell-cell contacts and VEGF signaling at
adherence junctions linked to angiogenesis. Arterioscler Thromb
Vasc Biol. 26:1991–1997. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamaoka-Tojo M, Ushio-Fukai M, Hilenski L,
Dikalov SI, Chen YE, Tojo T, Fukai T, Fujimoto M, Patrushev NA,
Wang N, et al: IQGAP1, a novel vascular endothelial growth factor
receptor binding protein, is involved in reactive oxygen
species-dependent endothelial migration and proliferation. Circ
Res. 95:276–283. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Meyer RD, Sacks DB and Rahimi N:
IQGAP1-dependent signaling pathway regulates endothelial cell
proliferation and angiogenesis. PLoS One. 3:e38482008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fresno Vara JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jiang BH and Liu LZ: AKT signaling in
regulating angiogenesis. Curr Cancer Drug Targets. 8:19–26. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dhillon AS, Hagan S, Rath O and Kolch W:
MAP kinase signalling pathways in cancer. Oncogene. 26:3279–3290.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ding C, Li L, Yang T, Fan X and Wu G:
Combined application of anti-VEGF and anti-EGFR attenuates the
growth and angiogenesis of colorectal cancer mainly through
suppressing AKT and ERK signaling in mice model. BMC Cancer.
16:7912016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sbroggiò M, Carnevale D, Bertero A,
Cifelli G, De Blasio E, Mascio G, Hirsch E, Bahou WF, Turco E,
Silengo L, et al: IQGAP1 regulates ERK1/2 and AKT signalling in the
heart and sustains functional remodelling upon pressure overload.
Cardiovasc Res. 91:456–464. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen F, Zhu HH, Zhou LF, Wu SS, Wang J and
Chen Z: IQGAP1 is overexpressed in hepatocellular carcinoma and
promotes cell proliferation by Akt activation. Exp Mol Med.
42:477–483. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ma Y, Jin Z, Huang J, Zhou S, Ye H, Jiang
S and Yu K: IQGAP1 plays an important role in the cell
proliferation of multiple myeloma via the MAP kinase (ERK) pathway.
Oncol Rep. 30:3032–3038. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cheung KL, Lee JH, Shu L, Kim JH, Sacks DB
and Kong AN: The Ras GTPase-activating-like protein IQGAP1 mediates
Nrf2 protein activation via the mitogen-activated protein
kinase/extracellular signal-regulated kinase (ERK) kinase (MEK)-ERK
pathway. J Biol Chem. 288:22378–22386. 2013. View Article : Google Scholar : PubMed/NCBI
|