Introduction
Ovarian cancer is the leading cause of death from
gynecological cancers. Approximately 50–85% of patients experience
recurrence within 5 years and the median survival time after
recurrence is approximately 2 years (1–3). One
of the reasons for this poor prognosis is disseminated disease
(4,5). Serous carcinoma of surface
epithelial-stromal tumors, which is the major histological type,
often appears in the ascites and results in peritoneal
dissemination to the pelvis and abdomen (6–8).
Patients eventually die from cachexia or bowel obstruction due to
the large intraperitoneal tumor burden (9). For peritoneal dissemination,
carboplatin and paclitaxel are effective as an initial treatment in
advanced ovarian cancer patients (1,3).
However, most patients later present chemoresistance.
During daily workups for cytopathological diagnosis,
we have noticed that flat mesothelial cells are often accompanied
by ovarian cancer cell clusters in the ascites. Mesothelial cells
are the major constituent of the peritoneum covering the
superficial area. This cell type may be involved in the formation
of peritoneal metastasis by producing hyaluronic acid and various
kinds of extracellular matrix (ECM) and adhesion molecules
(10). Thus, ovarian cancer cells
are thought to adhere to the peritoneum via β1 integrins,
hyaluronic acid, and CD44. In addition, the ECM exists in the
connective tissues of the subserosa under the mesothelial cell
layer (11–15).
To explore the genesis of peritoneal dissemination,
many studies have been performed to clarify the mechanism by which
cancer cells attach to the peritoneum, however few studies have
investigated floating ovarian cancer cell clusters in ascites.
Ovarian cancer cells in ascites usually form a papillary
spheroid-like cell cluster and ball pattern (disco or mirror ball
pattern), and the cells proliferate in suspension. Burleson et
al reported that ovarian tumor spheroids contain both
mesothelial and inflammatory cells (12), suggesting that the interaction
between ovarian cancer cells and mesothelial cells in the spheroid
may be important for tumor progression. However, the details of
this mechanism remain largely unknown.
These cells in spheroids appear to acquire some
specific ability to proliferate and survive devoid of tumor
neovascularization in ascitic fluid. As spheroid formation is a
putative feature of cancer stem cells (CSCs), we hypothesized that
mesothelial cells may play a role in the development of tumor
spheroids and give rise to cancer stem-like properties in ovarian
cancer cells. CSCs have been identified in many types of solid
tumors and are relatively quiescent, can self-renew, grow as
spheroids and make up the tumor bulk by generating differentiated
daughter cells through asymmetric division (16,17).
To address this hypothesis, we investigated the role of mesothelial
cells exfoliated from the rat peritoneum in the formation of
ovarian cancer spheroids using a three-dimensional (3D) culture
system and examined whether it is linked to cancer stem-like
properties.
Materials and methods
Cell culture
Human ovarian cancer cell lines CAOV3, A2780, and
SKOV3 and human Met-5A mesothelium cell line were obtained from the
American Type Culture Collection (ATCC; Manassas, VA, USA). The
A2780 and SKOV3 cells were grown in RPMI-1640 (Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; Thermo Fisher Scientific, Inc.), 2 mM
L-glutamine and 50 U/ml penicillin G/streptomycin at 37°C in a
humidified 5% CO2 atmosphere. CAOV3 cells were grown in
Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher Scientific,
Inc.) supplemented with 10% FBS, 2 mM L-glutamine and 50 U/ml
penicillin G/streptomycin at 37°C in a humidified 10%
CO2 atmosphere. Met-5A cells were cultured in DMEM/F12
medium (Nacalai Tesque, Inc., Kyoto, Japan) supplemented with 10%
FBS, 5 ng/ml EGF, hydrocortisone (0.4 µg/ml), hydrocortisone (0.1
µg/ml), insulin (2.5 µg/ml), 50 U/ml penicillin/streptomycin at
37°C in a 5% CO2 atmosphere. A2780, CAOV3 and SKOV3
cells were transfected with pEGFP vector to distinguish between
ovarian cancer cells and mesothelial cells in culture. Green
fluorescent protein (GFP)-positive cells were selected by G418 to
establish stable cell lines.
Isolation and propagation of rat
mesothelial cells
Rat mesothelial cells were isolated from the omentum
and mesenterium of 8-week-old female Sprague-Dawley rats (Charles
River Laboratories Japan, Inc., Yokohama, Japan) according to a
method previously described (18,19).
Briefly, the peritoneum was washed with phosphate-buffered saline
(PBS) and incubated with 0.05% trypsin/EDTA (Nacalai Tesque Inc.)
at 37°C for 50 min. The cells peeled from the peritoneum were
recovered and cultured on a dish coated with 0.1% type 1 collagen
at 37°C in a 5% CO2 atmosphere. The cells were passaged
every 3–4 days and used for assays. Mesothelial cell traits were
confirmed by immunohistochemical staining for calretinin and
α-smooth muscle actin (α-SMA).
Spheroid culture
Spheroids were generated on plates coated with 40%
poly 2-hydroxyethyl methacrylate (Poly-HEMA; Sigma-Aldrich, St.
Louis, MO, USA). Ovarian cancer cell lines CAOV3, A2780 and SKOV3
(GFP-labeled, 2×105) and rat mesothelial cells or Met-5A
human mesothelium cells (2×105) were mixed and cultured
on Poly-HEMA-coated plates for different durations. Parallel
monocultures of ovarian cancer cells or rat mesothelial cells were
prepared as a control.
Immunohistochemical staining
The harvested spheroids were fixed in 10% neutral
buffered formalin for 24 to 48 h. Spheroids were suspended in PBS
and centrifuged to form a pellet in the bottom of a microtube. The
supernatant was removed and 0.1% agarose (Nacalai Tesque, Inc.) was
overlaid in a microtube. Paraffin penetration of solidified agarose
containing spheroids was performed in Tissue-Tek VIP (Sakura
Finetek Japan Co., Ltd., Tokyo, Japan).
Sections (2 to 3-µm thick) were stained with the
following antibodies at 4°C overnight: Anti-calretinin (rabbit
polyclonal, 1:1,000; cat. no. PA5-16681; Thermo Fisher Scientific,
Inc.), anti-α-SMA (1A4, mouse monoclonal, 1:100; cat. no. ab7817;
Abcam, Cambridge, UK), anti-Ki-67 (MIB-1, mouse monoclonal,
1:1,000; cat. no. M724029; Agilent Technologies, Santa Clara, CA,
USA), anti-ALDH1/2 (H-8, mouse monoclonal, 1:5,000; cat. no.
sc-166362; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
anti-CD44 (EPR1013Y, rabbit monoclonal, 1:400; cat. no. ab51037;
Abcam) and anti-CD133 (W6B3C1, mouse monoclonal, 1:400; cat. no.
130-092-395; Miltenyi Biotec, Bergisch Gladbach, Germany). After
sections were deparaffinized, antigen retrieval was performed using
a high pressure chamber (Agilent) at 121°C for 2 min to unmask the
epitopes in 0.1 mol/l citric acid buffer (pH 7.4). An Elite ABC kit
(Vector Laboratories, Inc., Burlingame, CA, USA) was used for the
immunostaining and the antibody complex was visualized by
3,3′-diaminobenzidine tetrahydrochloride (DAB).
RNA extraction and qRT-PCR
Total RNA was collected from cultured cells using
Sepasol-RNA I Super G (Nacalai Tesque) and complementary DNA
synthesized from 1.0 µg of total RNA using oligo (dT) primer and a
Reverse Transcription System (Promega, Madison, WI, USA) according
to the manufacturer's instructions. Quantitative PCR was performed
with specifically designed oligonucleotide primers and LightCycler
FastStart DNA Master SYBR Green I using a LightCycler 2.0
instrument (Roche Diagnostics, Mannheim, Germany). The expression
of the target gene was normalized relative to GAPDH mRNA
expression. The primers used were: GAPDH forward,
5′-CAACTACATGGTTTACATGTTC-3′ and reverse, 5′-GCCAGTGGACTCCACGAC-3′;
CD44s forward, 5′-ATAATAAAGGAGCAGCACTTCAGGA-3′ and reverse,
5′-ATAATTGTGTCTTGGTCTCTGGTAGC-3′; Dclk-1 forward,
5′-AGTCTTCCGATTCCGAGTTGAG-3′ and reverse,
5′-CAGCAACCAGGAATGTATTGGA-3′; Bmi-1 forward,
5′-TGTAAAACGTGTATTGTTCGTTAC-3′ and reverse,
5′-CAATATCTTGGAGAGTTTTATCTGACC-3′.
Xenograft experiments
Ten, 5-week old female SCID-Beige
(CB17.Cg-PrkdcscidLystbg-J/CrlCrlj) mice (16.2 g average body
weight) were obtained from Charles River Laboratories. Food, and
water were autoclaved and changed regularly. All the mice were bred
and maintained in SPF condition at 23°C in a daily cycle of 12 h
light and 12 h darkness at Osaka University Graduate School of
Medicine, Division of Health Sciences, in a controlled state. An
equal number of CAOV3 cells and rat mesothelium cells
(1×106 cells) were cultured on Poly-HEMA-coated dishes
for 24 h. The cells were collected and suspended in 50% Matrigel
(Corning, Corning, NY, USA) in a total volume of 140 µl and then
subcutaneously injected into the lower backs of mice. Tumor growth
was monitored using calipers and tumor volume (v) was calculated as
follows: v = (a × b2)/2, where a is the maximum tumor
axis and b the length of the minor axis. All experiments using mice
were approved by the Institutional Animal Care and Use Committee
Osaka University Graduate School of Medicine and the Committee for
the Ethics of Animal Experiments of Osaka University (approval no.
28-03-001).
Statistical analysis
Data are presented as the mean ± SEM. The
statistical significance of differences between two groups was
calculated by the Student's t-test. When more than two groups were
compared, one-way ANOVA was used followed by Bonferroni's multiple
comparison test to determine the statistical significance of the
differences. Statistical analyses were performed using the JMP 12
software (SAS Institute, Cary, NC, USA) and GraphPad Prism version
6.00 for Mac (GraphPad Software, San Diego, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Calretinin immunostaining
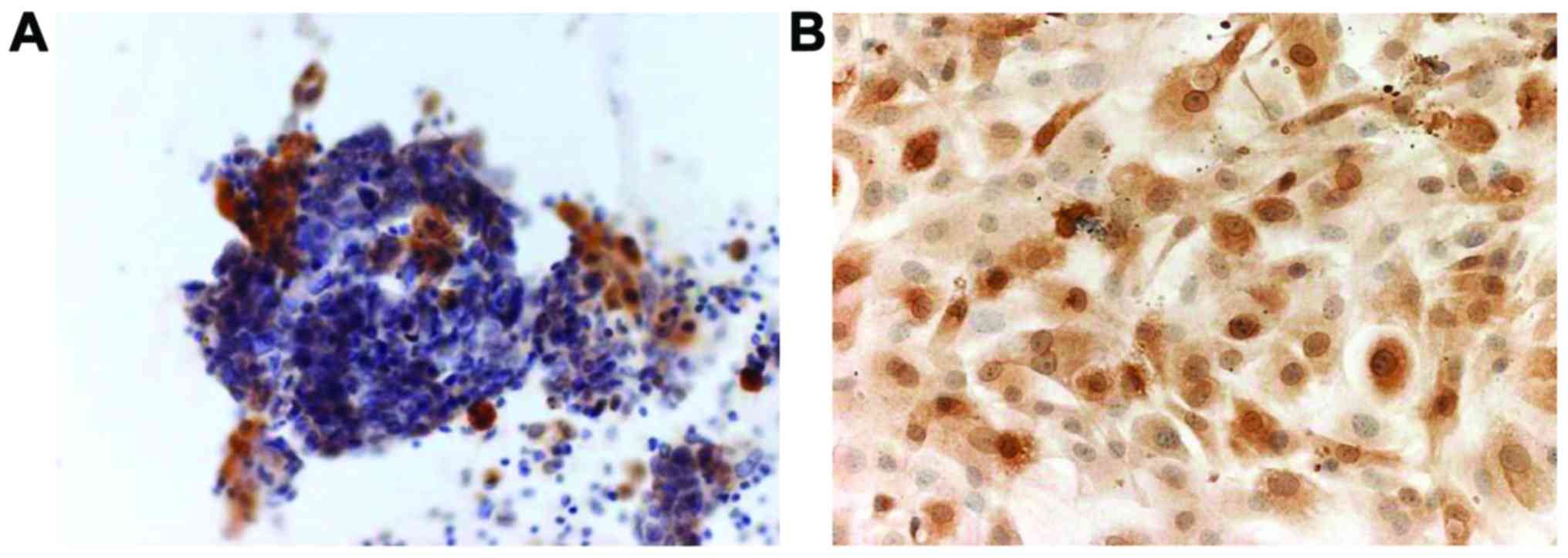
A clinical sample of the spheroids was obtained from
ascitic fluid by abdominal puncture in a 77-year old female
patient. Pathological diagnosis indicated that the spheroid was
composed of ovarian cancer cells and the patient was diagnosed with
carcinomatous peritonitis. Immunohistochemistry revealed that
calretinin, a marker of mesothelial cells (20), was partially expressed in the
spheroids (Fig. 1A). Subsequently,
we assessed the purity of mesothelial cells isolated from the rat
abdominal cavity by immunostaining for calretinin. The cells were
positive in the nucleus and cytoplasm to varying extents (Fig. 1B).
Mesothelial cells promoted in vitro
spheroid formation in CAOV3 and A2780 ovarian cancer cells
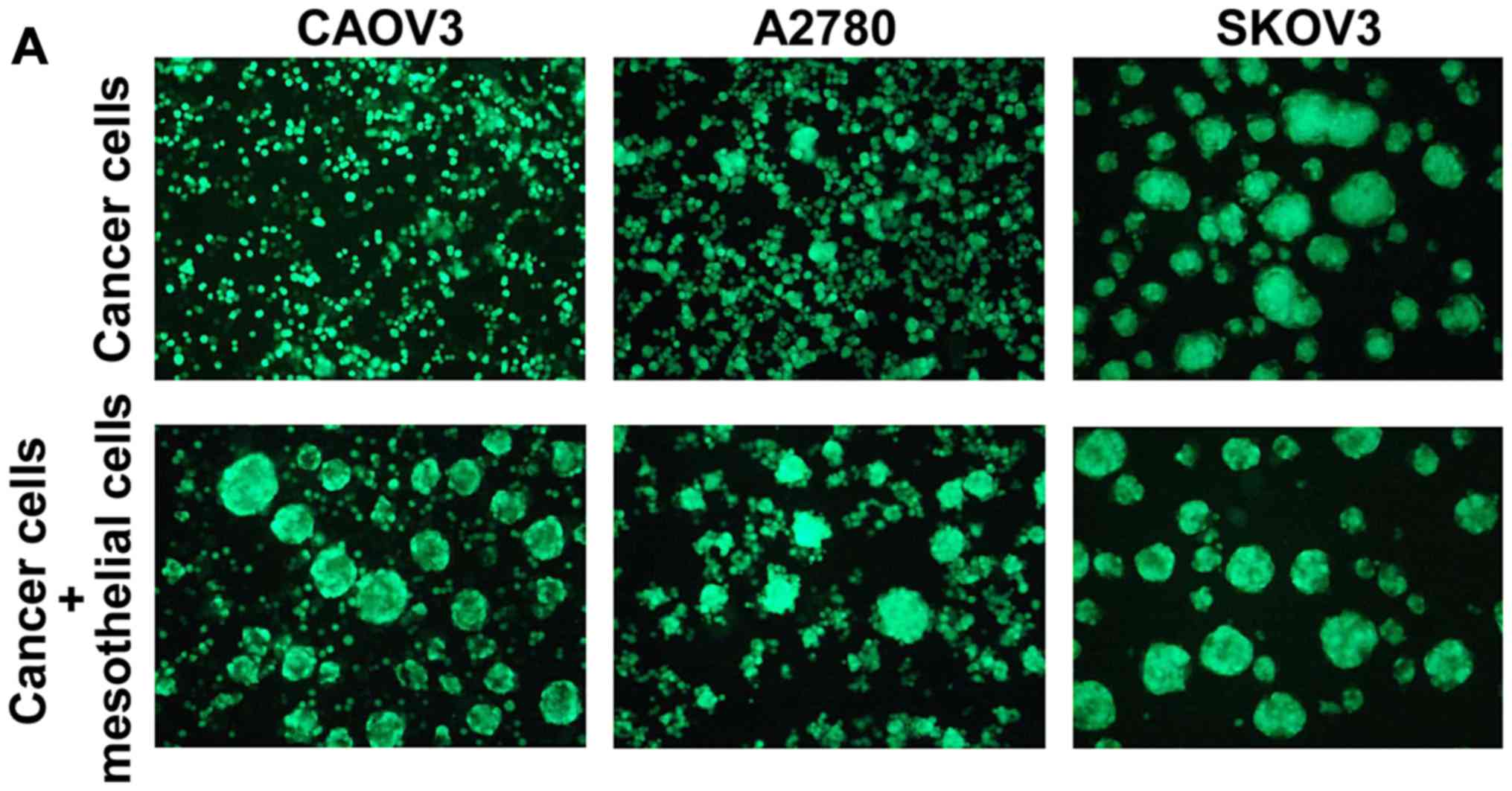
In the 3D cultures on poly-HEMA-coated plates,
GFP-labeled CAOV3, A2780 and SKOV3 cells were grown in the absence
or presence of rat mesothelial cells. The spheroids grew much
larger in the presence of mesothelial cells plus CAOV3 or A2780
cells compared to cancer cells alone. Conversely, the SKOV3 cells
formed large spheroids even in the absence of mesothelial cells
(Fig. 2A). The time course studies
at 24, 48, 72 and 120 h are displayed in Fig. 2B-a-c. H&E staining of the 3D
cultures of rat mesothelial cells is also displayed in Fig. 2B-d.
Assessment of the proliferative
activity using Ki-67 antibody
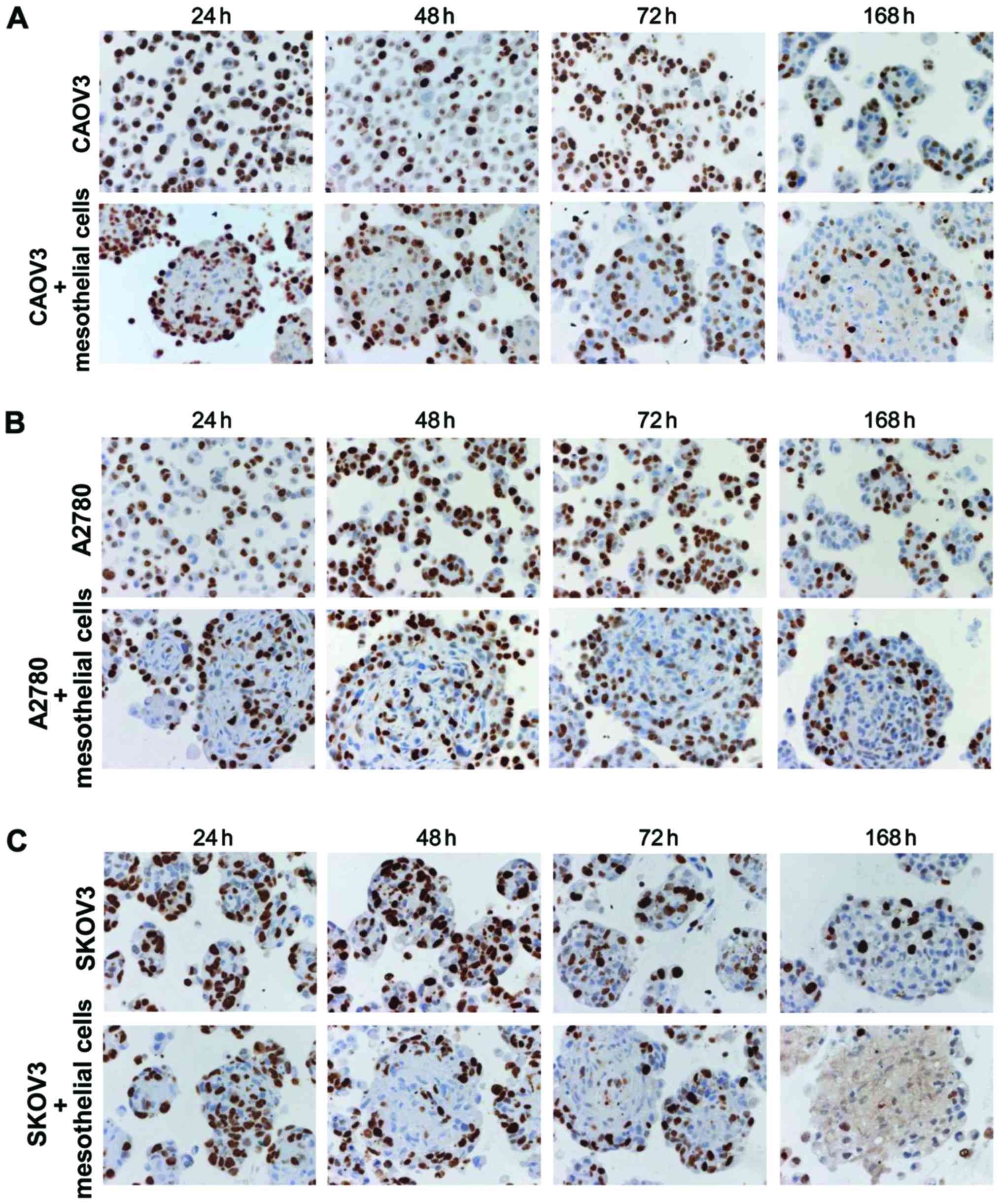
To examine the proliferative activity of cells, cell
blocks were made at the indicated time-points. Ki-67-positive cells
were evaluated in monoculture (cancer cells alone) and co-culture
(cancer cells plus mesothelial cells) conditions. In monocultures
of CAOV3 and A2780 cells, Ki-67 positivity was randomly observed,
whereas the expression of Ki-67 was high in the peripheral zone and
low in the central area of spheroids in co-culture (Fig. 3A and B). In SKOV3 cells,
proliferative cells were randomly distributed in monoculture
spheroids (Fig. 3C). Conversely,
the co-culture spheroids had Ki-67-positive cells at the peripheral
zone at 48 and 72 h (Fig. 3C).
Immunostaining of CSC markers
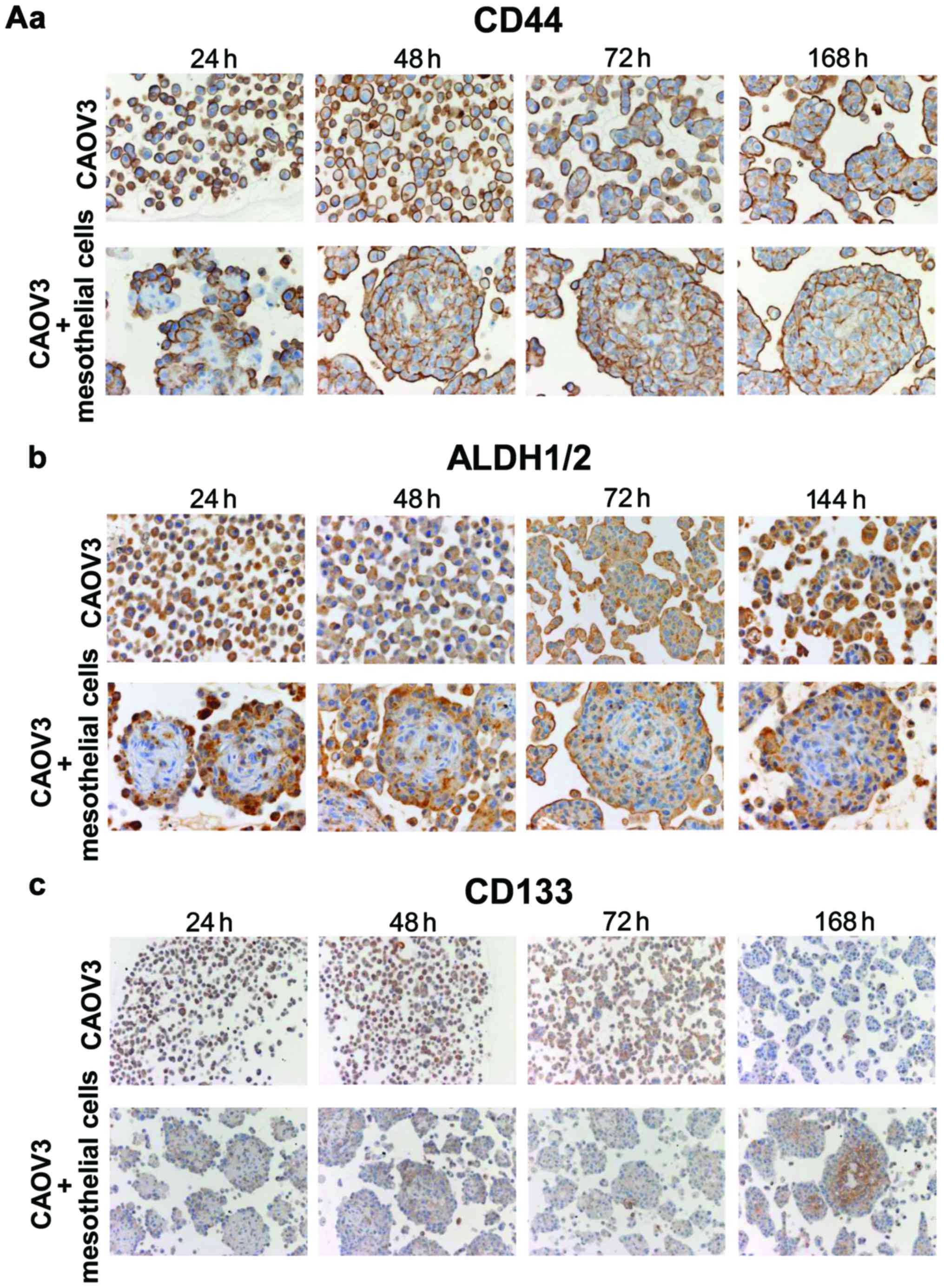
The majority of CAOV3 cells in monoculture exhibited
intense expression of CD44 and ALDH1/2 and positive staining was
observed at the periphery of spheroids at 24 h in co-culture with
mesothelial cells (Fig. 4A). At
subsequent time-points, the expression of CD44 and ALDH1/2
continued and extended to the whole spheroid body (Fig. 4A). The CAOV3 cells exhibited weak
CD133 expression in both monoculture and co-culture at 24–72 h
(Fig. 4A). However, intense
expression of CD133 was observed in the cell membrane in the inner
portion of spheroids at 96 h (Fig.
4B-a) and 168 h (Fig. 4A-c).
Ki-67 staining revealed that the proliferative activity of the
cancer cells was high at the periphery and low in the inner portion
of spheroids at 96 h (Fig. 4B-a).
Staining mesothelial cells with α-SMA antibody revealed that they
were located in the central area at 48 h and then shrunk (144 h) as
the cancer cells expanded (Fig.
4B-b). Calretinin staining of mesothelial cells also revealed
concordant results at 48 and 168 h (Fig. 4B-c). Similar CSC marker expression
was observed in A2780 cells (Fig.
4C).
Effect of mesothelial cells on cancer
stem cell marker mRNA expression
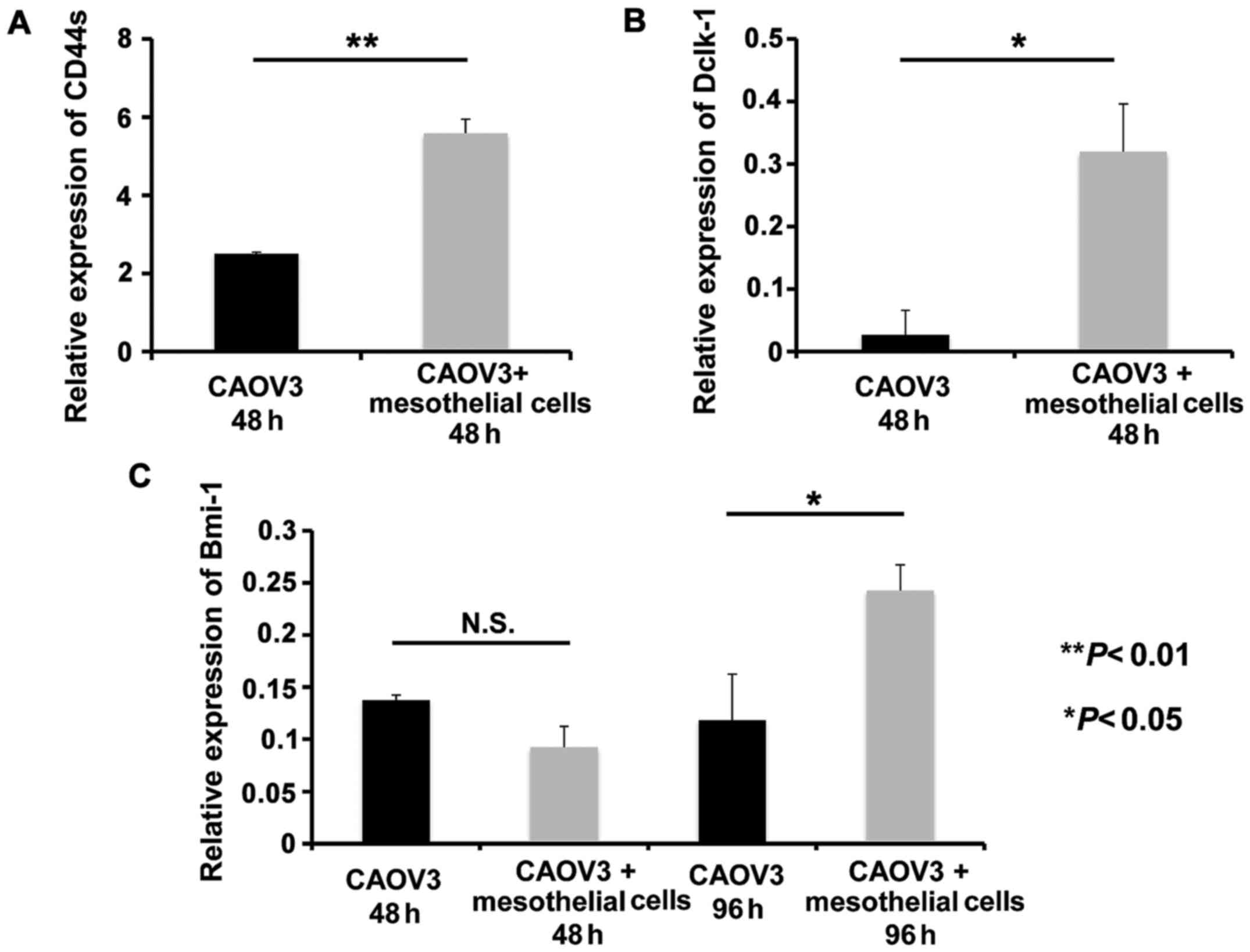
Subsequently, we examined the mRNA levels of the
stem cell markers in CAOV3 cells using human-specific primers for
CD44s, Dclk-1 and Bmi-1, which do not detect mRNA derived from rat
mesothelial cells. We observed that CD44s and Dclk-1 transcripts
significantly increased in co-culture with mesothelial cells at 48
h compared to CAOV3 cells alone (P<0.01 and P<0.05,
respectively; Fig. 5A and B).
Conversely, Bmi-1 mRNA did not increase at 48 h, but significantly
increased at 96 h in co-culture with mesothelial cells (P<0.05,
Fig. 5C).
Mesothelial cells promote in vivo
ovarian cancer growth in xenografts
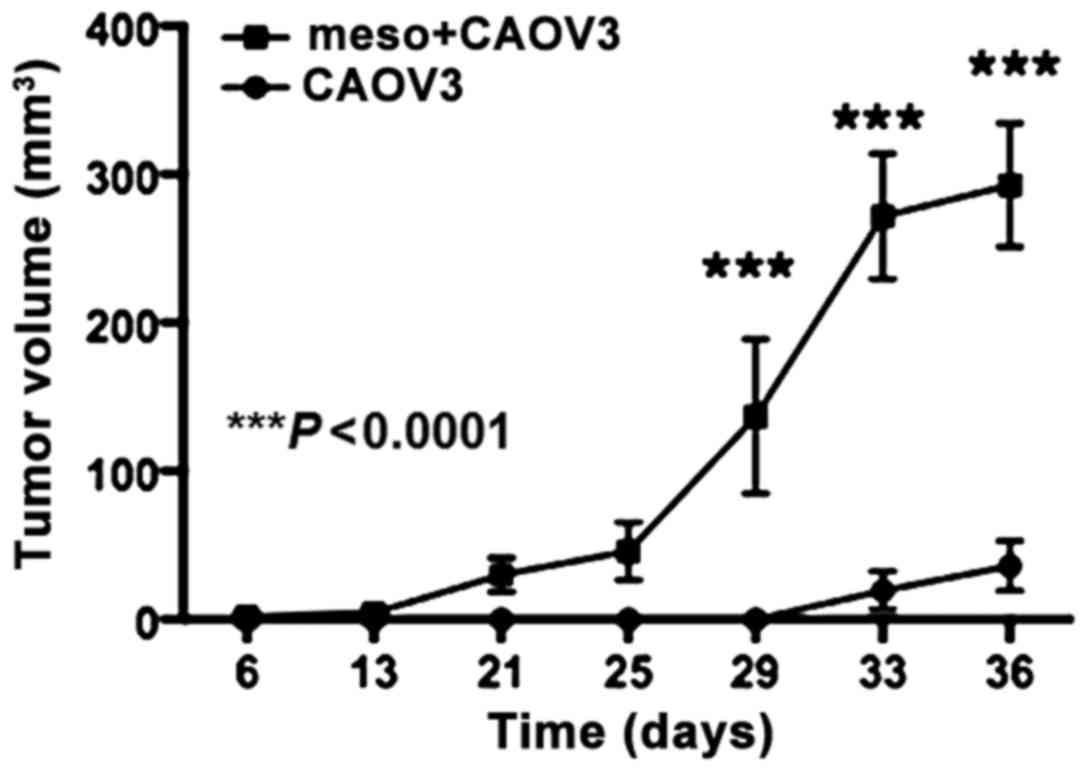
Subcutaneous injection of the mixture of CAOV3 and
mesothelial cells resulted in tumors on day 25 in 7 of 7 mice
(100%). Subcutaneous tumors derived from CAOV3 cells alone were
detected in 4 of 7 mice (57%) on day 36. Injection of mesothelial
cells did not produce tumors in the three injected mice. The tumor
volume was significantly larger in the CAOV3 and mesothelial cell
group than the CAOV3 alone group (P<0.0001, Fig. 6). The tumors had a solid
histological pattern and no significant morphological difference
was observed between the two CAOV3 groups (data not shown).
Spheroid formation by ovarian cancer
cells in the presence of human mesothelial cells
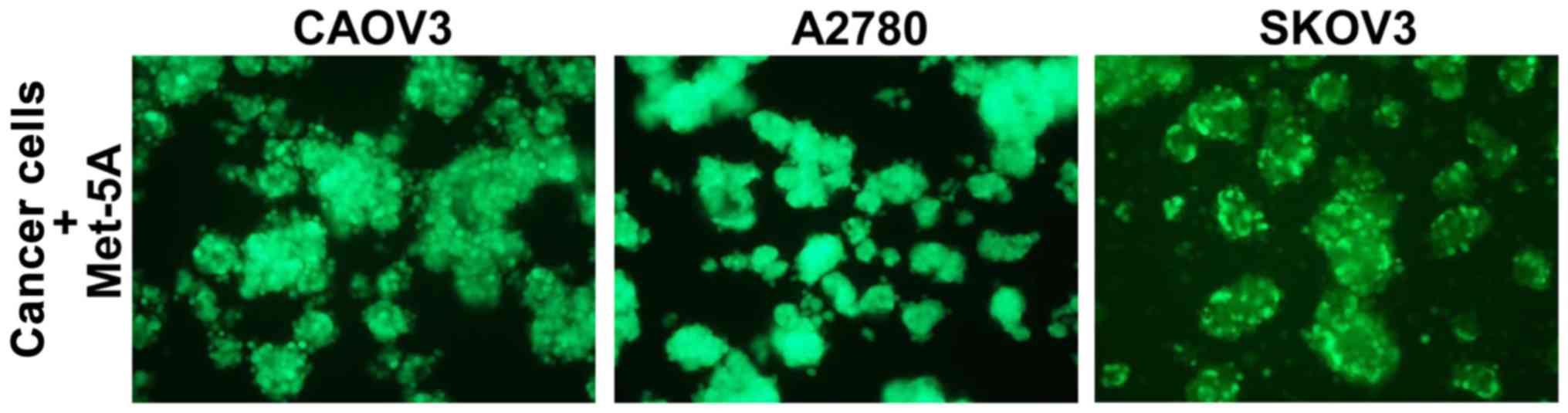
Finally, we used human Met-5A mesothelial cells
instead of rat mesothelial cells. Three ovarian cancer cell lines,
CAOV3, A2780 and SKOV3, were stably labeled with GFP and cultured
with human Met-5A mesothelial cells for 48 h in suspension. All
three ovarian cancer cell lines formed spheroids in the presence of
mesothelial cells (Fig. 7).
Discussion
During daily workups for cytopathological diagnosis,
the first author of the present study, noticed morphological
features in H&E staining indicating that the mesothelial cells
were often mixed in the cancerous cluster balls called ‘spheroids’
in the ascites of ovarian cancer patients. The presence of
mesothelial cells was ascertained in clinical spheroids based on
calretinin or α-SMA staining. The present study was performed to
address her long-time question regarding the role of mesothelial
cells in ovarian cancer spheroids.
In GFP-tagged 3D-culture studies, we observed that
co-culturing ovarian cancer cells and rat mesothelial cells
facilitated spheroid formation. In CAOV3 and A2780 cells,
significantly larger spheroids were produced by the mixture of
mesothelial cells. In contrast, SKOV3 cells formed spheroids
without mesothelial cells, as previously reported (21). The SKOV3 spheroids were reported to
express a high level of cancer stem markers, such as CD133 and
CD117. Nevertheless, it is of note that the initial spheroid
formation at 24–48 h appeared to be facilitated by mesothelial
cells (Fig. 2B-c). Recent
molecular-based analysis has uncovered that the high grade serous
ovarian carcinoma is of fimbriae tubae origin (22). Furthermore, the genetic expression
profile of SKOV3 cells was different from that of other serous
carcinoma types (23–25). This may explain, at least in part,
the enhanced spheroid formation ability of SKOV3 cells.
Long-term 3D-culture is one of the characteristics
of the present study. Repeated experiments indicated that the
spheroids generated in co-cultures appeared to be healthier after
120 h of culture compared to those produced in monocultures
(Fig. 2B). Based on the findings,
we hypothesized that such spheroids may be endowed with cancer
stemness.
Experiments on the proliferative activity assessed
by Ki-67 staining revealed a specific proliferation pattern of the
co-cultured spheroids. Cell block studies revealed that
Ki-67-positive cells were located at the peripheral region of
spheroids in each cell type, especially after 72 h in culture. This
contrasted with the random distribution of Ki-67-stained cells in
the spheroids consisting of SKOV3 cells alone (Fig. 3C). When we examined the localization
of mesothelial cells, we observed that they stayed in the central
portion of spheroids. These findings indicated that tumor cells at
the periphery should have high proliferative activity in spheroids
produced from co-culture.
Spheroid or sphere formation is one of the hallmarks
of CSCs. Bone morphogenetic protein (BMP) 2 promoted the
phosphorylation of SMAD5 and induced epithelial-mesenchymal
transition (EMT) (26). Taking into
consideration that the CAOV3 and A2780 cells exhibited prominent
spheroid formation with the aid of mesothelial cells, we examined
the expression of the cell surface markers of CSCs. Since the
majority of CAOV3 and A2780 cells initially expressed CD44 and
ALDH1/2, time-course studies indicated that tumor cells expanded
from the periphery to the central area. For example, CD44-positive
cells were partially located at the periphery at 24 h, continued to
grow, and occupied almost the entire spheroid at 168 h. This was
consistent with the observation that mesothelial cells residing in
the central area gradually disappeared.
CD133 (also known as prominin-1) is a putative cell
surface marker for ovarian CSCs and other types of solid tumors
(27,28). One of the major findings of the
present study was that CD133-positive cancer cells emerged in the
late phase (72–168 h) in the inner portion of the spheroids of
CAOV3 and A2780 cells. Since this antibody reacted with the human
CD133 membranous protein (29,30),
and based on the morphological assessment of epithelial-like cells
in the high-power image, the CD133-expressing cells were tumor
cells, probably arresting at the G0-G1 phase of the cell cycle. The
expression of CD133 was consistently reported to be associated with
the CSC population at the G0/G1 phase in osteosarcoma and gastric
cancer (31,32). Bmi-1 and Lrig-1 are known quiescent
stem markers, which contrast active CSC markers, such as Lgr5 and
Dclk-1 (33–36). Notably, RNA expression of CSC
markers CD44s and Dclk-1 was increased at 48 h in co-culture, and
Bmi-1 mRNA increased relatively late (i.e. 96 h), when the CAOV3
tumor cells were positive for CD133 expression. In animal
experiments, we observed that co-cultured cells exhibited enhanced
tumorigenicity (7/7 vs. 4/7) and a significant increase in tumor
volume compared to CAOV3 monoculture. High tumorigenicity is the
best hallmark of CSCs, indicating that co-cultured cells acquire
cancer stem-like properties.
Our findings indicated a scenario for the genesis
and survival of spheroids in carcinomatous peritonitis from ovarian
cancer. With the mesothelial cells in ascites as the core, ovarian
cancer cells attached to it, possibly via the adhesion mechanism of
α2β1, α5β1, α3β1, αvβ1 or α6β1 integrins, hyaluronic acid or CD44
(14,37–39).
Tumor cells gradually grew and expanded from the periphery to the
central area of the spheroid body. After quiescent CSCs were
generated in the inner region, the spheroid acquired eternal
survival, through the continuous self-renewal of CSCs and
asymmetric division, producing differentiated daughter cells. At
the later phase, the initially required mesothelial cells were no
longer necessary. Furthermore, one of the reasons for disease
recurrence after chemotherapy was assumed to be due to the presence
of CSCs in spheroids.
In the present study, we used rat mesothelial cells,
which may raise a concern about xenogenic (interspecies) reactions
in the process of spheroid formation, and this is a possibility
that cannot be denied. Prior to the study, we confirmed that human
Met-5A mesothelial cells can make spheroids in cooperation with
ovarian cancer cells (Fig. 7).
However, we considered that immortalized Met-5A cells transfected
with pRSV-T plasmid containing the SV40 early region and Rous
sarcoma virus long terminal repeat may not be suitable for
analyzing the physiological interaction between tumor cells and
non-tumor mesothelial cells. To emphasize non-tumor features, we
eventually decided to prepare rat mesothelial cells from the
omentum and mesenterium, when they would be necessary. We observed
that such non-tumor cells were going away from spheroids after a
long time in culture, whereas tumor cells gradually expanded and
survived long-term with the acquisition of cancer stem-like
properties. With immortal Met-5A cells, this dynamic physiological
reaction may have proceeded differently.
In addition, it is important to investigate whether
mesothelial cells are located at the central portion of spheroids
in clinical samples. During daily workups for cytopathological
diagnosis, it is demanding to examine the parts inside of
spheroids, since cell block is required for this purpose. In
addition, since spheroids are not common among the various
cytological specimens, a confirmation study may be considered as a
future research direction.
In conclusion, the present study provided a novel
in vitro 3D spheroid model of ovarian cancer cells with rat
mesothelial cells. Our results revealed that mesothelial cells
enhanced stem cell-related gene expression and facilitated spheroid
formation in 3D-culture and tumor formation in a xenograft model,
which is considered a hallmark of CSCs. Further studies of
carcinomatous peritonitis using clinically collected mesothelial
cells are essential in order to fully comprehend the pathogenesis
of this disease from one stage to the next.
Acknowledgements
Not applicable.
Funding
The present study was supported by a Grant-in-Aid
for Scientific Research (grant no. 17K08775) from the Japan Society
for the Promotion of Science (to SM). The funders had no role in
the study design, data collection and analysis, decision to
publish, or preparation of the manuscript.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
AS, SM and HY designed the study. AS, SM, YY, KM,
YQ, JW, HH and XW performed the experiments. AS, SM, YY and HY
analyzed the data and compiled the figures. AS, SM and HY wrote the
manuscript. YH, NK, SN and NM reviewed and edited the manuscript
and also participated in the conception of the study. All authors
read and approved the contents of the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
All experiments using mice were approved by the
Institutional Animal Care and Use Committee of Osaka University
Graduate School of Medicine and the Committee for the Ethics of
Animal Experiments of Osaka University (approval nos. 28-03-001;
Osaka, Japan).
Consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
References
|
1
|
Ozols RF: Systemic therapy for ovarian
cancer: Current status and new treatments. Semin Oncol. 33 2 Suppl
6:S3–S11. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liao J, Qian F, Tchabo N,
Mhawech-Fauceglia P, Beck A, Qian Z, Wang X, Huss WJ, Lele SB,
Morrison CD, et al: Ovarian cancer spheroid cells with stem
cell-like properties contribute to tumor generation, metastasis and
chemotherapy resistance through hypoxia-resistant metabolism. PLoS
One. 9:e849412014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Foster R, Buckanovich RJ and Rueda BR:
Ovarian cancer stem cells: Working towards the root of stemness.
Cancer Lett. 338:147–157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shield K, Ackland ML, Ahmed N and Rice GE:
Multicellular spheroids in ovarian cancer metastases: Biology and
pathology. Gynecol Oncol. 113:143–148. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Burleson KM, Boente MP, Pambuccian SE and
Skubitz AP: Disaggregation and invasion of ovarian carcinoma
ascites spheroids. J Transl Med. 4:62006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shen-Gunther J and Mannel RS: Ascites as a
predictor of ovarian malignancy. Gynecol Oncol. 87:77–83. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ayantunde AA and Parsons SL: Predictors of
poor prognosis in patients with malignant ascites: A prospective
study. Clin Med Diagnostics. 2:1–6. 2012. View Article : Google Scholar
|
|
8
|
Mackey JR and Venner PM: Malignant
ascites: Demographics, therapeutic efficacy and predictors of
survival. Can J Oncol. 6:474–480. 1996.PubMed/NCBI
|
|
9
|
Mills GB, May C, Hill M, Campbell S, Shaw
P and Marks A: Ascitic fluid from human ovarian cancer patients
contains growth factors necessary for intraperitoneal growth of
human ovarian adenocarcinoma cells. J Clin Invest. 86:851–855.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mutsaers SE: Mesothelial cells: Their
structure, function and role in serosal repair. Respirology.
7:171–191. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lengyel E: Ovarian cancer development and
metastasis. Am J Pathol. 177:1053–1064. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Burleson KM, Casey RC, Skubitz KM,
Pambuccian SE, Oegema TR Jr and Skubitz AP: Ovarian carcinoma
ascites spheroids adhere to extracellular matrix components and
mesothelial cell monolayers. Gynecol Oncol. 93:170–181. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Strobel T and Cannistra SA:
Beta1-integrins partly mediate binding of ovarian cancer cells to
peritoneal mesothelium in vitro. Gynecol Oncol. 73:362–367. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mikuła-Pietrasik J, Sosińska P and Książek
K: Resveratrol inhibits ovarian cancer cell adhesion to peritoneal
mesothelium in vitro by modulating the production of α5β1 integrins
and hyaluronic acid. Gynecol Oncol. 134:624–630. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cannistra SA, Kansas GS, Niloff J,
DeFranzo B, Kim Y and Ottensmeier C: Binding of ovarian cancer
cells to peritoneal mesothelium in vitro is partly mediated by
CD44H. Cancer Res. 53:3830–3838. 1993.PubMed/NCBI
|
|
16
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: Accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Skubitz AP, Taras EP, Boylan KL, Waldron
NN, Oh S, Panoskaltsis-Mortari A and Vallera DA: Targeting CD133 in
an in vivo ovarian cancer model reduces ovarian cancer progression.
Gynecol Oncol. 130:579–587. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Akedo H, Shinkai K, Mukai M, Mori Y,
Tateishi R, Tanaka K, Yamamoto R and Morishita T: Interaction of
rat ascites hepatoma cells with cultured mesothelial cell layers: A
model for tumor invasion. Cancer Res. 46:2416–2422. 1986.PubMed/NCBI
|
|
19
|
Stylianou E, Jenner LA, Davies M, Coles GA
and Williams JD: Isolation, culture and characterization of human
peritoneal mesothelial cells. Kidney Int. 37:1563–1570. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Doglioni C, Dei Tos AP, Laurino L,
Iuzzolino P, Chiarelli C, Celio MR and Viale G: Calretinin: A novel
immunocytochemical marker for mesothelioma. Am J Surg Pathol.
20:1037–1046. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma L, Lai D, Liu T, Cheng W and Guo L:
Cancer stem-like cells can be isolated with drug selection in human
ovarian cancer cell line SKOV3. Acta Biochim Biophys Sin.
42:593–602. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kindelberger DW, Lee Y, Miron A, Hirsch
MS, Feltmate C, Medeiros F, Callahan MJ, Garner EO, Gordon RW,
Birch C, et al: Intraepithelial carcinoma of the fimbria and pelvic
serous carcinoma: Evidence for a causal relationship. Am J Surg
Pathol. 31:161–169. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Domcke S, Sinha R, Levine DA, Sander C and
Schultz N: Evaluating cell lines as tumour models by comparison of
genomic profiles. Nat Commun. 4:21262013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Elias KM, Emori MM, Papp E, MacDuffie E,
Konecny GE, Velculescu VE and Drapkin R: Beyond genomics: Critical
evaluation of cell line utility for ovarian cancer research.
Gynecol Oncol. 139:97–103. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Anglesio MS, Wiegand KC, Melnyk N, Chow C,
Salamanca C, Prentice LM, Senz J, Yang W, Spillman MA, Cochrane DR,
et al: Type-specific cell line models for type-specific ovarian
cancer research. PLoS One. 8:e721622013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
McLean K, Gong Y, Choi Y, Deng N, Yang K,
Bai S, Cabrera L, Keller E, McCauley L, Cho KR and Buckanovich RJ:
Human ovarian carcinoma-associated mesenchymal stem cells regulate
cancer stem cells and tumorigenesis via altered BMP production. J
Clin Invest. 121:3206–3219. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Grosse-Gehling P, Fargeas CA, Dittfeld C,
Garbe Y, Alison MR, Corbeil D and Kunz-Schughart LA: CD133 as a
biomarker for putative cancer stem cells in solid tumours:
Limitations, problems and challenges. J Pathol. 229:355–378. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ajani JA, Song S, Hochster HS and
Steinberg IB: Cancer stem cells: The promise and the potential.
Semin Oncol. 42 Suppl 1:S3–S17. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Motegi H, Kamoshima Y, Terasaka S,
Kobayashi H and Houkin K: Type 1 collagen as a potential niche
component for CD133-positive glioblastoma cells. Neuropathology.
34:378–385. 2014.PubMed/NCBI
|
|
30
|
Okudela K, Woo T, Mitsui H, Tajiri M,
Masuda M and Ohashi K: Expression of the potential cancer stem cell
markers, CD133, CD44, ALDH1, and β-catenin, in primary lung
adenocarcinoma - their prognostic significance. Pathol Int.
62:792–801. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yano S, Tazawa H, Hashimoto Y, Shirakawa
Y, Kuroda S, Nishizaki M, Kishimoto H, Uno F, Nagasaka T, Urata Y,
et al: A genetically engineered oncolytic adenovirus decoys and
lethally traps quiescent cancer stem-like cells in S/G2/M phases.
Clin Cancer Res. 19:6495–6505. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li J, Zhong XY, Li ZY, Cai JF, Zou L, Li
JM, Yang T and Liu W: CD133 expression in osteosarcoma and
derivation of CD133+ cells. Mol Med Rep. 7:577–584.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sangiorgi E and Capecchi MR: Bmi1 is
expressed in vivo in intestinal stem cells. Nat Genet. 40:915–920.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Powell AE, Wang Y, Li Y, Poulin EJ, Means
AL, Washington MK, Higginbotham JN, Juchheim A, Prasad N, Levy SE,
et al: The pan-ErbB negative regulator Lrig1 is an intestinal stem
cell marker that functions as a tumor suppressor. Cell.
149:146–158. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Barker N, van Es JH, Kuipers J, Kujala P,
van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H,
Peters PJ and Clevers H: Identification of stem cells in small
intestine and colon by marker gene Lgr5. Nature. 449:1003–1007.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nakanishi Y, Seno H, Fukuoka A, Ueo T,
Yamaga Y, Maruno T, Nakanishi N, Kanda K, Komekado H, Kawada M, et
al: Dclk1 distinguishes between tumor and normal stem cells in the
intestine. Nat Genet. 45:98–103. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ahmed N, Riley C, Rice G and Quinn M: Role
of integrin receptors for fibronectin, collagen and laminin in the
regulation of ovarian carcinoma functions in response to a matrix
microenvironment. Clin Exp Metastasis. 22:391–402. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shield K, Riley C, Quinn MA, Rice GE,
Ackland ML and Ahmed N: Alpha2beta1 integrin affects metastatic
potential of ovarian carcinoma spheroids by supporting
disaggregation and proteolysis. J Carcinog. 6:112007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sawada K, Mitra AK, Radjabi AR, Bhaskar V,
Kistner EO, Tretiakova M, Jagadeeswaran S, Montag A, Becker A,
Kenny HA, et al: Loss of E-cadherin promotes ovarian cancer
metastasis via alpha 5-integrin, which is a therapeutic target.
Cancer Res. 68:2329–2339. 2008. View Article : Google Scholar : PubMed/NCBI
|