Introduction
In recent years, increasing data have indicated the
importance of microRNAs (miRNAs) in the progression of cancers
including oral squamous cell carcinoma (OSCC) (1–4).
miRNAs are small non-coding RNAs of ~22 nucleotides in length,
existing as single stranded RNAs that act by binding to partially
complementary sequences in the 3′untranslated regions (UTRs) of
target gene mRNAs to regulate protein expression. miR-21, which is
considered to be a proto-oncogene, is frequently reported as
overexpressed in various cancer types and has been implicated in
tumorigenesis (5,6). In addition, miR-21 has been reported
to serve an important role in several signaling pathways, including
Wnt/β-catenin and phosphatase and tensin homolog deletion on
chromosome 10 (PTEN)/phosphatidylinositol 3-kinase (PI3K)/Akt
(7,8). Furthermore, miR-21 may negatively
regulate multiple target genes, including programmed cell death
protein 4 (PDCD-4), tissue inhibitor of
metalloproteinases-3, integrin subunit β 4 and PTEN
(9–12).
PTEN is a tumor suppressor gene that has frequently
been reported to be involved in the regulation of cell
proliferation, invasion, migration and apoptosis in many types of
cancer, and the expression of PTEN is downregulated in a wide range
of malignancies, including breast cancer, glioblastoma, colorectal
carcinoma, pancreatic cancer and OSCC (13–17).
Previous studies have demonstrated that the PI3K/Akt signaling
pathway is involved in multiple biological processes and that PTEN
functions as a tumor suppressor by negatively regulating the
PI3K/Akt signaling pathway, which reduces cell growth and increases
cell apoptosis (18,19). miRNAs, including miR-136, miR-181a
and miR-221/222, have been reported to regulate the expression of
PTEN (20–22). However, the role of miR-21 with
regard to the expression of PTEN in OSCC is not well
established.
Since the therapeutic potential of miR-21 inhibitor,
to the best of our knowledge, has not been investigated in OSCC, in
the present study the biological effects and molecular mechanism of
miR-21 involved in the apoptosis of OSCC cells were evaluated. The
results obtained support that miR-21 inhibitor, via its influence
on PTEN expression, may be a novel agent for the treatment of
OSCC.
Materials and methods
Tissue acquisition
Surgically resected OSCC specimens were obtained
from 35 patients (aged 45 to 70, including 19 males and 16 females)
with OSCC between June 2015 and June 2017 at Jining No. 1 People's
Hospital (Jining, China) with written informed consent. According
to the Union for International Cancer Control (UICC) standards in
2002, all the cases were classified as phases I–IV. In addition,
individual oral tissue samples from 10 normal subjects (aged 35–65,
including 6 males and 4 females) were collected as controls with
written informed consent. The experimental procedures were approved
by the Research Ethics Committee of Jining No. 1 People's
Hospital.
Hematoxylin and eosin (H&E)
staining
H&E staining is a popular staining method in
histology and it is one of the most widely used staining in medical
diagnosis. The prepared 4-µm paraffin sections were incubated for 2
h in a 60°C incubator. The sections were deparaffinized by
turpentine oil (TO) and sequentially soaked in 100, 95, 85 and 70%
alcohol solutions for 2 min, and then hydrated using distilled
water for 5 min. The sections were stained with hematoxylin for
5–15 min. Then, the excess dyeing solution on the slide was washed
with water. Next, the sections were stained with eosin for 1–5 min.
Subsequently, the sections were dehydrated using 70, 85, 95 and
100% alcohol, respectively, and were transparent through TO.
Finally, the excess TO transparent agent around the section was
wiped off, and a suitable amount of neutral resin was added, and
the slide was covered.
Immunohistochemical staining
Immunohistochemistry was used to detect PTEN
expression in the OSCC specimens. The samples were fixed, embedded
and cut into 4-µm thick sections. The sections were deparaffinized
using xylene and rehydrated by increased grades of ethanol. A total
of 0.5 µg primary rabbit polyclonal antibodies against PTEN (1:200;
cat. no. 9188S; Cell Signaling Technology, Inc., Danvers, MA, USA)
were added following antigen retrieval and incubated at 4°C
overnight. Subsequently, immunohistochemical staining was performed
using VECTASTAIN® ABC immunohistochemistry kit (Cowin
Biosciences, Co., Ltd., Beijing, China) according to the
manufacturer's instructions.
In situ hybridization (ISH)
ISH analysis was performed on deparaffinized 4-µm
OSCC tissue sections using an ISH tissue implementation kit. OSCC
sections were incubated at 60°C for 1 h and deparaffinized.
Subsequently, the sections were rehydrated in decreasing
concentrations of ethanol and washed in deionized water. The slides
were incubated in 0.2 mol/l HCl for 5 min at room temperature and
washed 3 times in phosphate-buffered saline (PBS) for 5 min.
Proteins were digested for 15 min at 37°C following the addition of
pepsin solution. Dehydration of sections was performed through
incubation with 70, 95 and 100% ethanol for 1 min at each
concentration. miR-21 probes labeled with digoxin were added to the
hybrids. Sections were denatured at 90°C for 4 min, followed by
incubation in ISH slide denaturation and hybridization solution at
37°C overnight. Post-incubation, coverslips and glue were removed
and the slides were washed. Finally, the slides were observed under
an inverted microscope.
Cell culture and transfection
The OSCC cell lines SCC15 and SCC25 were obtained
from the American Type Culture Collection (ATTC; Manassas, VA, USA)
and cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (FBS), 100 µg/ml penicillin, and 100 µg/ml
streptomycin at 37°C in a humidified 5% CO2 environment.
miR-21 inhibitor and empty vector were chemically synthesized by
Suzhou GenePharma Co., Ltd. (Suzhou, China) and transfected into
SCC15 and SCC25 cells using Lipofectamine™ RNAiMAX
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
recommended protocol. Cells in which Lipofectamine™ RNAiMAX was
only added were used as a control group. Both SCC15 and SCC25 Cells
in each of the 3 groups (control, scramble and miR-21 inhibitor)
were collected to be used for subsequent analysis at different
time-points following transfection.
Dual luciferase reporter assay
293T cells were obtained from ATTC and cultured in
24-well plates and co-transfected with 0.5 µg pMIR vectors
containing PTEN 3′UTR or mutant (mut)PTEN 3′UTR and 20 µM miR-21
mimics or 20 µM miR-21 inhibitor using Lipofectamine®
RNAiMAX. Cells were lysed with Passive Lysis Buffer and collected
at 48 h post-transfection. Luciferase activity was detected with a
dual luciferase reporter assay kit according to the manufacturer's
protocol. Renilla luciferase activity was used for
normalization.
Real-time reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) of
miRNAs and mRNAs
Total miRNAs and mRNAs were extracted from SCC15 and
SCC25 cells using an miRNeasy Mini kit (Qiagen GmbH, Hilden,
Germany) and Total RNA Purification kit (Qiagen GmbH) according to
the manufacturer's instructions. Real-time RT-qPCR analysis was
performed to validate miRNA and mRNA expression using a One-Step
RT-PCR Kit. U6 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH;
Invitrogen; Thermo Fisher Scientific, Inc.) were used as endogenous
controls. Each test was repeated in triplicate.
Western blot analysis
After transfection for 48 h, SCC15 and SCC25 cells
were washed 3 times with cold PBS and lysed in a buffer composed of
radio-immunoprecipitation assay buffer (Cowin Biosciences Co.,
Ltd.) and phenylmethanesulfonyl fluoride (Cowin Biosciences Co.,
Ltd.) (100:1). Total proteins were heated at 95°C for 10 min
following measurement of protein concentration with an Enhanced BCA
Protein Assay kit (Cowin Biosciences Co., Ltd.). Equal quantities
(25 µg) of heated proteins from each sample were separated by 12%
SDS-PAGE and transferred to polyvinylidene difluoride membranes.
The blots were blocked in Tris-buffered saline with Tween-20 (TBST)
containing 5% non-fat milk at room temperature for 1 h, then
incubated with specific primary antibodies against against PTEN
(1:1,000; cat. no. 9188S), phosphorylated (p)Akt (1:2,000; cat. no.
4060T), Akt (1:1,000; cat. no. 4691T) and GAPDH (1:1,000; cat. no.
5174S) (Cell Signaling Technology, Inc.) overnight at 4°C.
Subsequently, the blots were rinsed 3 times with TBST and incubated
with the secondary antibody for 1 h at room temperature. Protein
bands were imaged using the AlphaView SA Western blot detection
system (Carl Zeiss AG, Oberkochen, Germany) and quantified
following normalization to the density of GAPDH using ImageJ
software (National Institutes of Health, Bethesda, MD, USA). Each
test was repeated in triplicate.
Cell proliferation assay
Cell proliferation was assessed using Cell Counting
Kit-8 (CCK-8; Cowin Biosciences Co., Ltd.) according to the
manufacturer's instructions. Following treatment with miR-21
inhibitor, SCC15 and SCC25 cells were seeded into 96-well plates at
a density of 4,000 cells/well and subsequently allowed to attach
overnight. A 10 µl quantity of CCK-8 was added to each well at 0,
24, 48 and 72 h after transfection, and the cells were incubated
for 1 h. Optical density (OD) was determined by spectrophotometric
analysis at a wavelength of 450 nm. Each experiment was repeated in
triplicate.
Transwell cell migration assay
After transfection, SCC15 and SCC25 cells were
transferred to 8-µm pore inserts and placed in companion wells
containing DMEM and 10% FBS (Gibco; Thermo Fisher Scientific,
Inc.). The inserts were removed after a 12-h incubation, and the
non-migrated cells on the upper surface were harvested. Cells on
the lower surface were fixed with 5% glutaraldehyde (Beyotime
Institute of Biotechnology, Shanghai, China) and stained with
Giemsa (Beyotime Institute of Biotechnology) and counted under a
fluorescent microscope. Each test was repeated in triplicate.
Cell apoptosis assay
SCC15 and SCC25 cells were washed with PBS and
resuspended in buffer at a concentration of 106
cells/ml. Cells were mixed with 5 µl fluorescein
isothiocyanate-conjugated Annexin V reagent and 5 µl propidium
iodide (PI) (Invitrogen; Thermo Fisher Scientific, Inc.). At 15 min
after incubation in the dark at room temperature, the samples were
analyzed by flow cytometry (Beckman Coulter GmbH, Krefeld,
Germany). Each test was repeated in triplicate.
Statistical analysis
All experiments were repeated at least 3 times and
the data were presented as the mean ± standard deviation (SD). The
results were analyzed by Student's t-test for the comparison of two
and ANOVA (followed by Tukey's post hoc test) for the comparison of
multiple samples using SPSS 19.0 software (IBM Corp., Armonk, NY,
USA). P<0.05 was considered to indicate a statistically
significant result.
Results
Expression of miR-21 and PTEN in OSCC
tissues
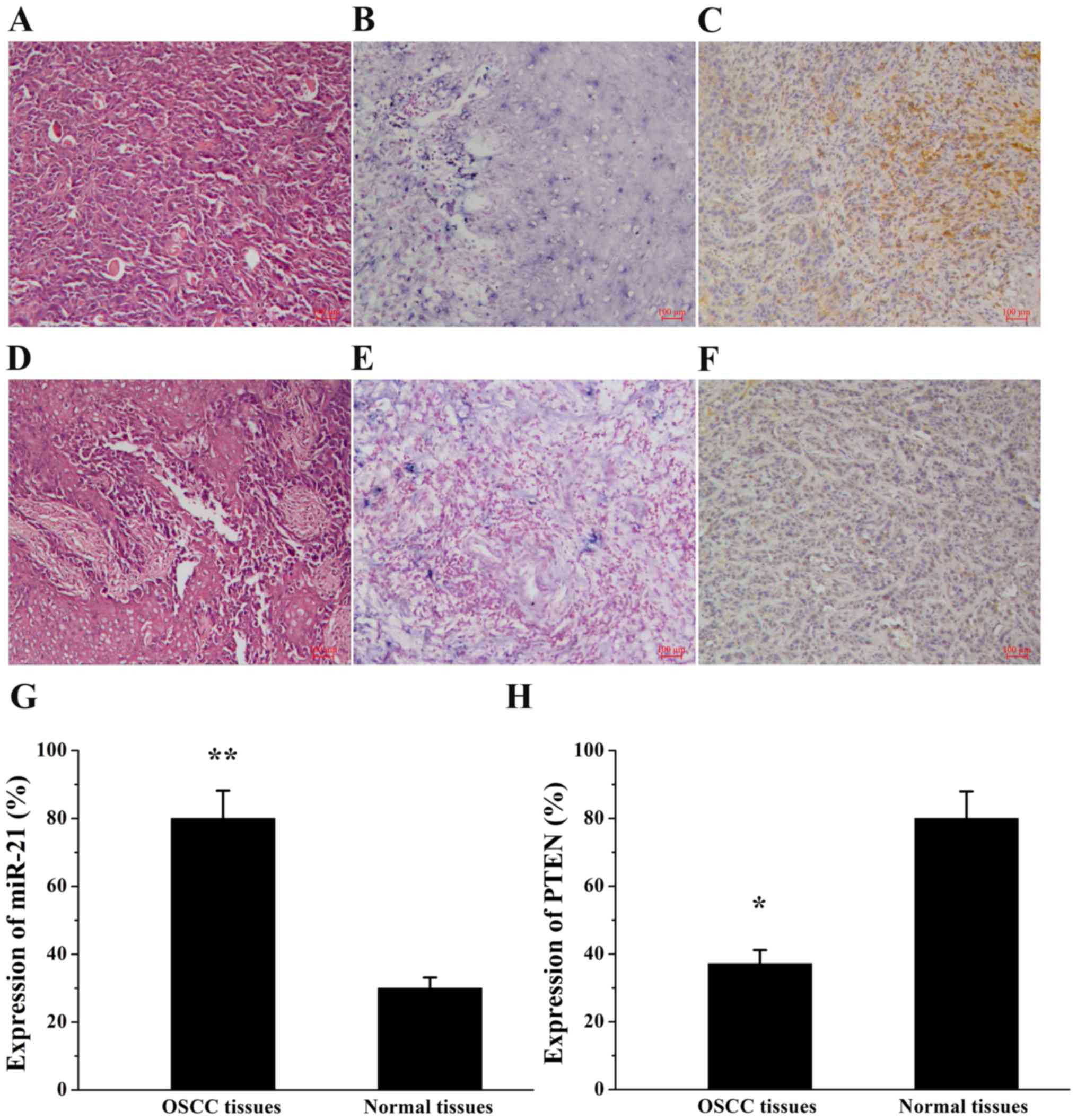
The expression of miR-21 and PTEN was examined by
ISH and immunohistochemical staining, respectively, in human OSCC
tissues and normal tissues (Fig.
1). As depicted in Table I,
miR-21 expression was observed in 80.0% (28/35) of OSCC tissues and
in 30.0% (3/10) of normal tissues (P=0.003). By contrast, PTEN
expression exhibited an opposite trend in OSCC tissues (37.1%,
13/35) and normal tissues (80.0%, 8/10; P=0.017) compared with the
expression of miR-21. Furthermore, the association between miR-21
and PTEN expression and the clinicopathological profiles of
patients was evaluated (Table II).
The data indicated that the expression of miR-21 and PTEN was
associated with tumor stage. miR-21 expression was lower in early
stages (I+II) than in advanced stages (III+IV; P=0.028) and the
expression of PTEN was significantly higher in early stages (I+II)
than in advanced stages (III+IV; P=0.010). miR-21 and PTEN
expression levels were not significantly associated with patient
sex or age, tumor differentiation or lymph node metastases
(P>0.05).
 | Table I.miR-21 and PTEN expression in OSCC and
normal tissues. |
Table I.
miR-21 and PTEN expression in OSCC and
normal tissues.
|
|
| miR-21
expression | PTEN expression |
|---|
|
|
|
|
|
|---|
| Group | No. of tumor
specimens, n | n | χ2 | P-value | n | χ2 | P-value |
|---|
| OSCC tissues | 35 | 28 | 9.073 | 0.003 | 13 | 5.740 | 0.017 |
| Normal tissues | 10 | 3 |
|
| 8 |
|
|
 | Table II.Associations of miR-21 and PTEN
expression with clinicopathological profiles. |
Table II.
Associations of miR-21 and PTEN
expression with clinicopathological profiles.
|
|
| miR-21
expression | PTEN expression |
|---|
|
|
|
|
|
|---|
| Clinicopathological
profile | No. of tumor
specimens, n | n | χ2 | P-value | n | χ2 | P-value |
|---|
| Sex |
|
Male | 19 | 16 | 0.461 | 0.497 | 8 | 0.438 | 0.508 |
|
Female | 16 | 12 |
|
| 5 |
|
|
| Age, years |
|
>60 | 20 | 17 | 0.729 | 0.393 | 7 | 0.092 | 0.762 |
|
≤60 | 15 | 11 |
|
| 6 |
|
|
| Stage |
|
I+II | 17 | 11 | 4.833 | 0.028 | 10 | 6.655 | 0.010 |
|
III+IV | 18 | 17 |
|
| 3 |
|
|
|
Differentiation |
|
Well | 9 | 7 | 0.043 | 0.979 | 3 | 0.092 | 0.955 |
|
Moderate | 16 | 13 |
|
| 6 |
|
|
|
Poor | 10 | 8 |
|
| 4 |
|
|
| Lymph node
metastasis |
|
Negative | 23 | 18 | 0.127 | 0.722 | 9 | 0.114 | 0.736 |
|
Positive | 12 | 10 |
|
| 4 |
|
|
| Total | 35 | 28 |
|
| 13 |
|
|
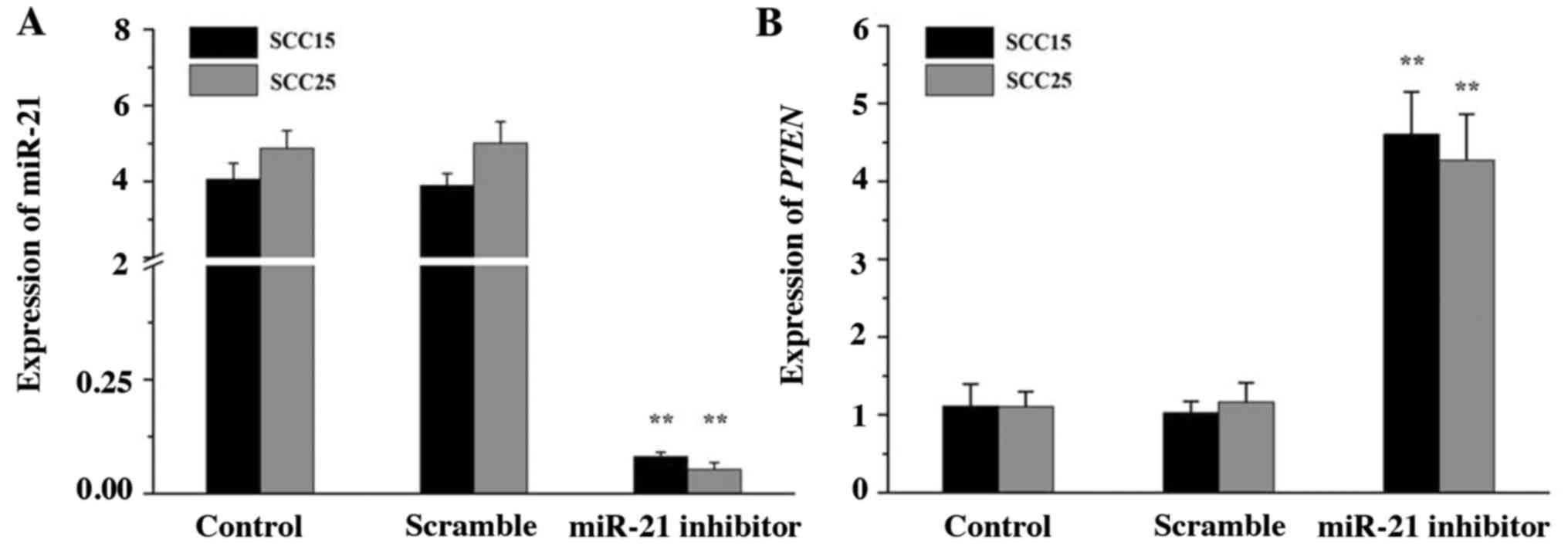
miR-21 and PTEN expression in SCC15
and SCC25 cells
To knockdown endogenous miR-21, miR-21 inhibitor was
synthesized and transfected into SCC15 and SCC25 cells. The
expression of miR-21 and PTEN was then analyzed by RT-qPCR. The
data indicated that miR-21 inhibitor efficiently silenced the
expression of miR-21 compared with that in the control groups
(Fig. 2A; P<0.01). By contrast,
the gene expression of PTEN was notably upregulated in the miR-21
inhibitor group (Fig. 2B;
P<0.01). No significant differences between the scramble and
control groups were noted (P>0.05). The results indicated that
miR-21 may be negatively associated with PTEN expression in SCC15
and SCC25 cells.
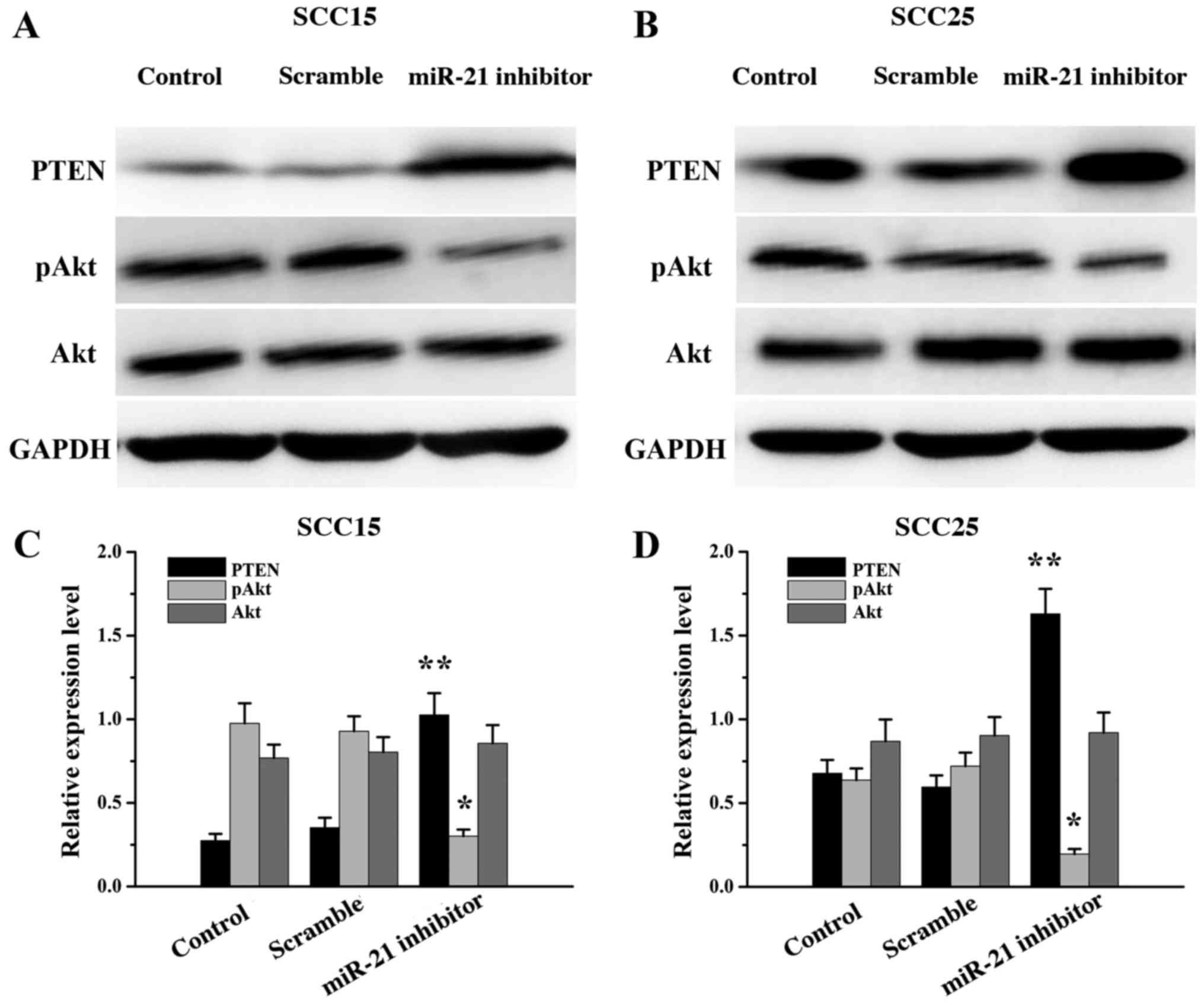
miR-21 inhibitor transfection altered
the expression of PTEN, Akt and pAkt
To investigate the molecular mechanism of miR-21 in
the apoptosis of SCC15 and SCC25 cells, western blot analysis was
performed following miR-21 silencing to assess the expression of
PTEN, Akt and pAkt. The expression of PTEN was significantly
increased (P<0.01) and the expression of pAkt decreased
(P<0.05) in the miR-21 inhibitor groups compared with that in
the control groups. There were no significant differences in Akt
expression between the miR-21 inhibitor and control groups
(P>0.05; Fig. 3). The results
indicated that the level of PTEN expression in SCC15 and SCC25
cells was negatively regulated by miR-21.
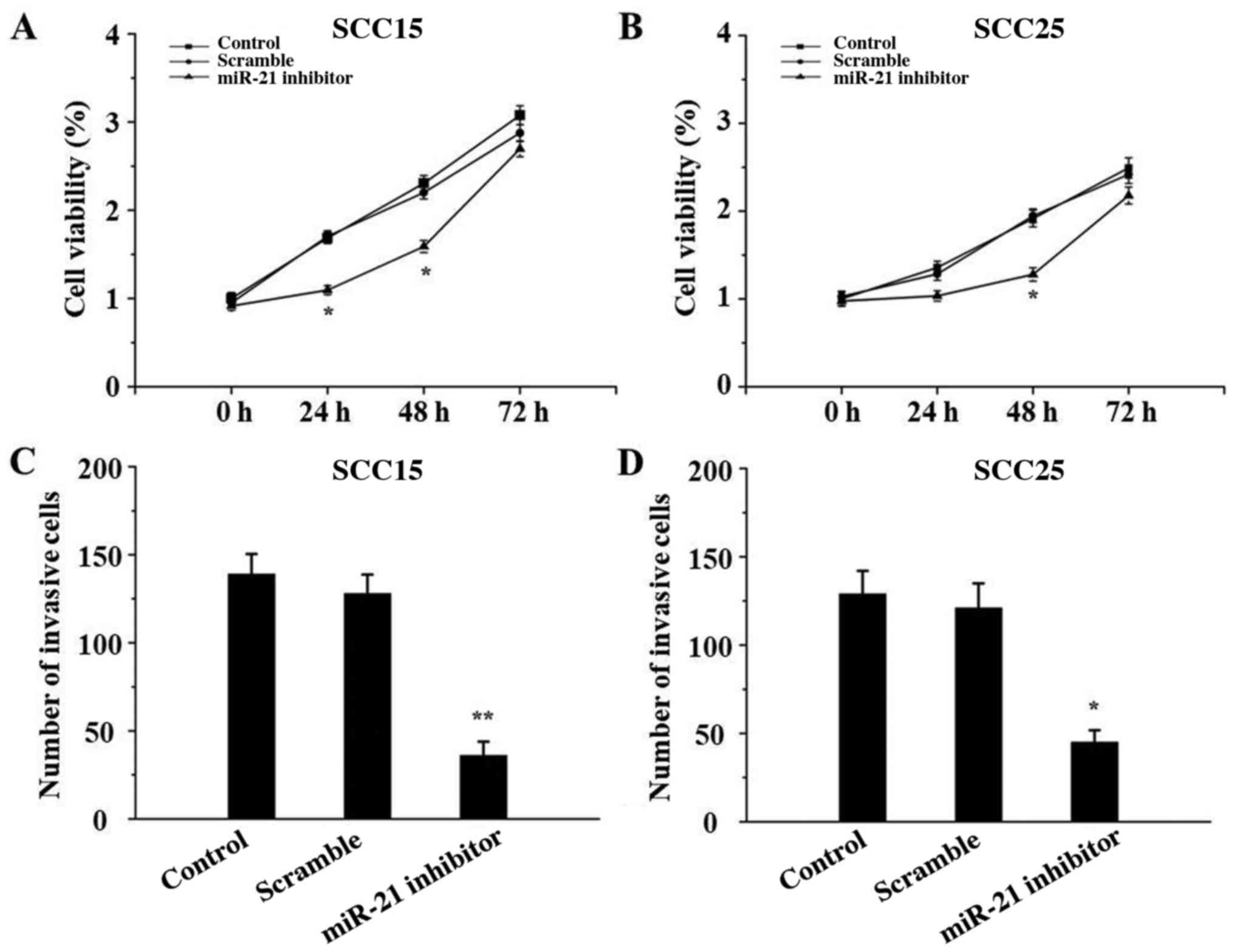
miR-21 silencing inhibits SCC15 and
SCC25 cell proliferation and invasion
The effect of miR-21 inhibitor on the proliferation
and invasion of SCC15 and SCC25 cells was determined by CCK-8 and
Transwell assays, respectively. As depicted in Fig. 4, both the proliferative and invasive
abilities of SCC15 and SCC25 cells in the miR-21 inhibitor groups
were significantly suppressed compared with those in the control
groups (P<0.05). There were no significant differences between
the control and scramble groups (P>0.05).
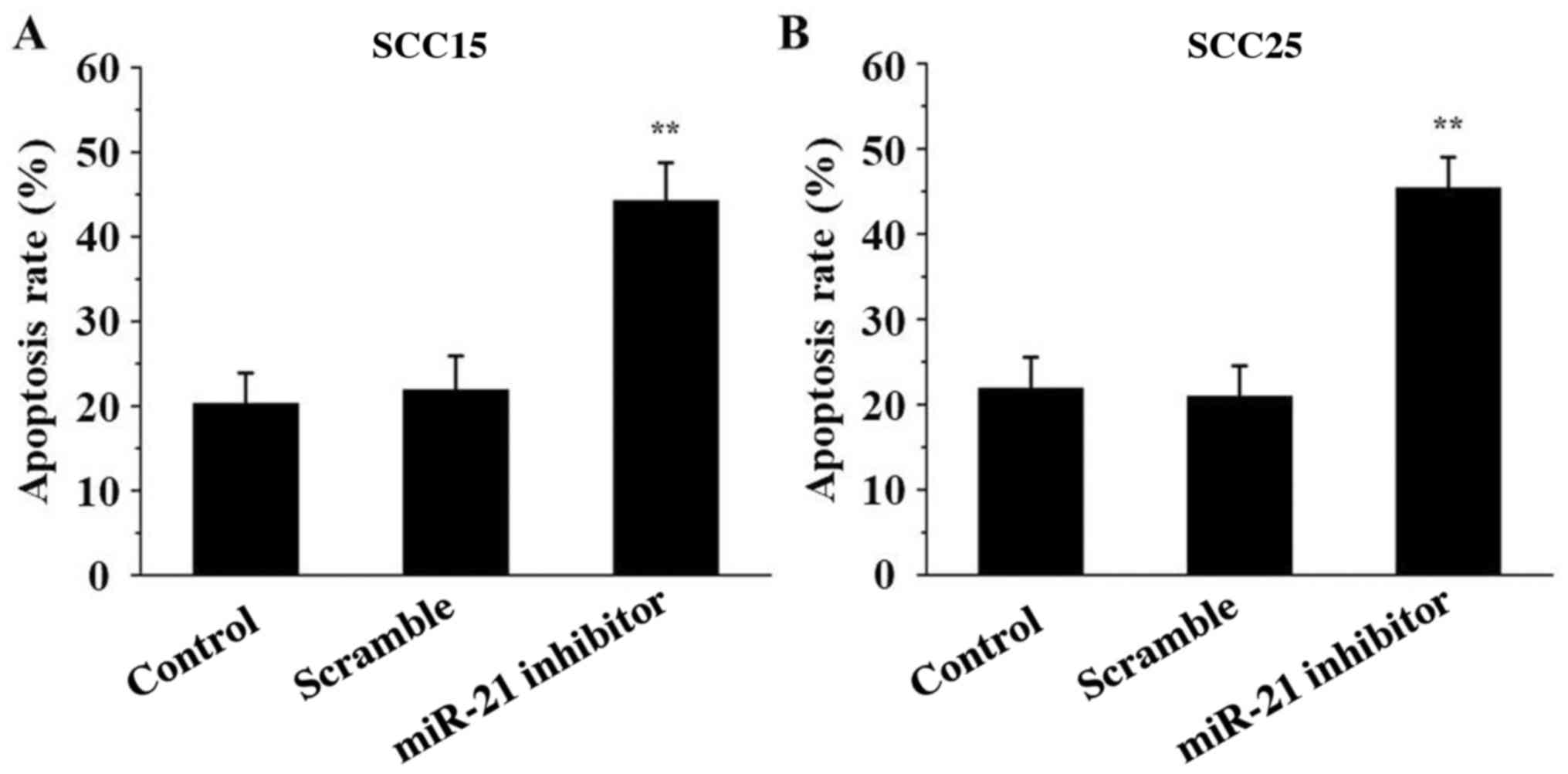
miR-21 inhibitor induces SCC15 and
SCC25 cell apoptosis
To detect the effect of miR-21 inhibitor on SCC15
and SCC25 cell apoptosis, Annexin V/PI analysis was performed. The
data indicated that the percentage of apoptotic cells was markedly
induced in the miR-21 inhibitor groups compared with that in the
control groups (P<0.01; Fig. 5),
indicating that miR-21 inhibitor transfection induced cell
apoptosis.
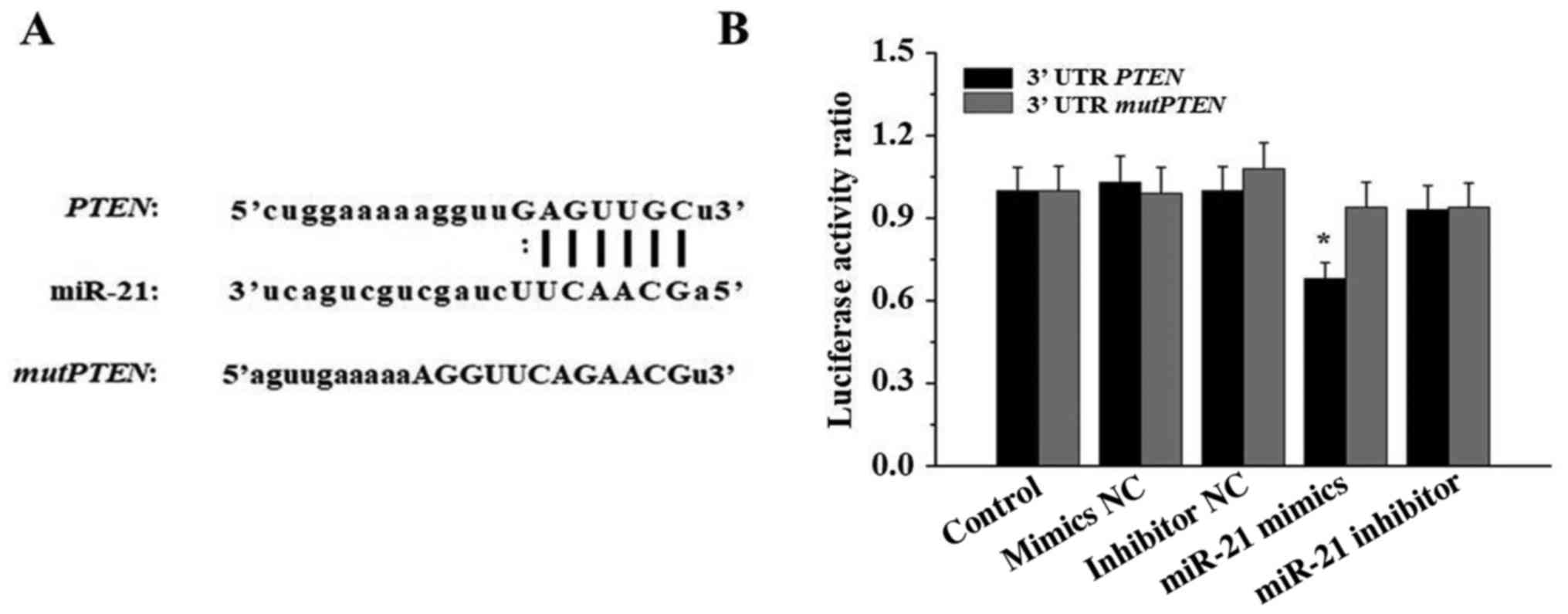
miR-21 acts directly on PTEN mRNA
3′UTR
To validate whether PTEN is a direct target of
miR-21, PTEN 3′UTR and mutPTEN 3′UTR luciferase
constructs were transfected into 293T cells with negative control
(NC) mimics, miR-21 mimics, inhibitor NC or miR-21 inhibitor.
Luciferase activity was assessed using a dual-luciferase reporter
assay system. As illustrated in Fig.
6, compared with the cells in the other groups, the luciferase
activity of 293T cells transfected with miR-21 mimics and
PTEN 3′UTR was significantly reduced (P<0.05).
Discussion
OSCC is the most common cancer of the head and neck
and presents a poor prognosis, with a 5-year survival rate of
<60% (23,24). As one of the current treatment
strategies for OSCC, gene therapy serves an important role
alongside other treatment modalities including surgery,
chemotherapy and radiotherapy. However, poor target activity is a
pivotal reason that restricts the clinical applications of gene
therapy in treating cancers. Therefore, there is a critical need to
identify sensitive gene targets and to understand the molecular
mechanism involved in the aggressive growth characteristics of
OSCC.
miRNAs are considered to be important regulators of
cell proliferation, differentiation, the cell cycle and cell death.
They are also considered to be novel molecular targets in the
diagnosis and treatment of human carcinomas. As a member of the
oncomiRs, miR-21 has been confirmed to be overexpressed in various
types of tumors and to be capable of negatively regulating multiple
target genes. Li et al (25)
demonstrated that miR-21 was overexpressed in tongue SCC (TSCC)
tissues compared with that in the adjacent normal tissues, and that
it may regulate TSCC development by inhibiting TSCC cell apoptosis
in part via tropomyosin α-1 chain silencing. Koenig et al
(26) compared the expression of
miR-21 targets in 377 patients with liver cancer and revealed that
the levels of 402 miR-21 targets were altered in hepatocellular
carcinoma. Their analysis identified novel miR-21 targets (CAMSAP1,
DDX1, MARCKSL1 and RMND5A) that appeared likely to serve a causal
role in hepatocarcinogenesis. Yan et al (27) reported that miR-21 may promote
salivary adenoid cystic carcinoma (SACC) progression through
PDCD-4, PTEN and B-cell lymphoma 2, and suggested that miR-21 may
be a novel target for SACC therapy.
The present study on OSCC determined that the
expression of miR-21 had a negative association with the expression
of PTEN protein. In OSCC cells, real-time RT-qPCR results revealed
that the expression of miR-21 was significantly reduced in SCC15
and SCC25 cells transfected with miR-21 inhibitor compared with
that in the control groups. By contrast, upregulation of PTEN gene
expression was observed following the treatment with miR-21
inhibitor. The data indicated that miR-21 may negatively regulate
PTEN gene expression in both OSCC tissues and cells. Furthermore,
bioinformatics and luciferase assays indicated that miR-21
modulates PTEN expression by directly targeting a binding site
within the mRNA 3′UTR. Collectively these findings revealed that
PTEN is directly regulated by miR-21.
As the first tumor suppressor gene identified with
phosphatase activity, PTEN has been confirmed as a target gene in
colorectal, gastric, cervical and non-small cell lung cancer. Wu
et al (28) reported that
miR-21 could modulate malignant phenotypes including proliferation,
invasion, cell cycle progression and anti-apoptosis in colorectal
cancer cells by downregulating PTEN protein expression. A previous
study indicated that the PI3K/Akt signaling pathway was activated
in multiple types of cancers, and notably that the mechanisms
activating PI3K/Akt signaling included loss of function of PTEN
(29). Wang et al (30) observed that miR-155 suppressed PTEN
expression, enhanced PI3K/Akt/mTOR signaling and inhibit human
osteosarcoma MG-63 cell apoptosis and autophagy was induced by
adrenomedullin. In the present study, upregulation of PTEN and
downregulation of pAkt proteins was observed following treatment
with miR-21 inhibitor. Additionally, the results revealed that when
the expression of miR-21 was suppressed, the proliferative and
invasive abilities of SCC15 and SCC25 cells were inhibited, while
cell apoptosis was promoted. These data indicated that cell
proliferation, invasion and apoptosis may be associated with the
expression of PTEN and the PI3K/Akt signaling pathway in OSCC cell
lines.
In conclusion, the present study revealed that
miR-21 inhibitor transfection significantly inhibited the growth of
SCC15 and SCC25 cells by reducing cell proliferation and promoting
cell apoptosis. It was also revealed by luciferase assay that PTEN
was a direct target of miR-21. Furthermore, the results indicated
that the modulation of miR-21 activity may regulate the expression
of PTEN, and that the PI3K/Akt signaling pathway may be involved
through this targeting of PTEN. Overall, these present results
indicate that the miR-21/PTEN axis may be a potential novel
therapeutic target in OSCC.
Acknowledgements
The authors thank Mr. Shenghui Yang for his
technical support.
Funding
The present study was supported by the Shandong
Medical and Health Technology Development Project (grant no.
2017WS343).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
YZ, JX and HM conceived and designed the study. JX,
FJ, YL and GC performed the experiments. YL, GC and HM analyzed the
data. YZ and FJ wrote the manuscript. All authors read and approved
the manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The experimental procedures were approved by the
Research Ethics Committee of Jining No. 1 People's Hospital and
written informed consent was obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shi Z, Li Y, Qian X, Hu Y, Liu J, Zhang S
and Zhang J: MiR-340 inhibits triple-negative breast cancer
progression by reversing EZH2 mediated miRNAs dysregulated
expressions. J Cancer. 8:3037–3048. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hawa Z, Haque I, Ghosh A, Banerjee S,
Harris L and Banerjee SK: The miRacle in pancreatic cancer by
miRNAs: Tiny angels or devils in disease progression. Int J Mol
Sci. 17:E8092016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang M, Matyunina LV, Walker LD, Chen W,
Xiao H, Benigno BB, Wu R and McDonald JF: Evidence for the
importance of post-transcriptional regulatory changes in ovarian
cancer progression and the contribution of miRNAs. Sci Rep.
7:81712017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pereira CM, Sehnem D, da Fonseca EO,
Barboza HFG, de Carvalho ACP, DaSilva AFM, Moura-Neto V and
DosSantos MF: miRNAs: Important targets for oral cancer pain
research. Biomed Res Int. 2017:40435162017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li L, Wang X, Li W, Yang L, Liu R, Zeng R,
Wu Y and Shou T: miR-21 modulates prostaglandin signaling and
promotes gastric tumorigenesis by targeting 15-PGDH. Biochem
Biophys Res Commun. 495:928–934. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang Q, Xu E, Dai J, Wu J, Zhang S, Peng B
and Jiang Y: miR-21 regulates
N-methyl-N-nitro-N'-nitrosoguanidine-induced
gastric tumorigenesis by targeting FASLG and BTG2.
Toxicol Lett. 228:147–156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou B, Wang J, Zheng G and Qiu Z:
Methylated urolithin A, the modified ellagitannin-derived
metabolite, suppresses cell viability of DU145 human prostate
cancer cells via targeting miR-21. Food Chem Toxicol. 97:375–384.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang P, Guan Q, Zhou D, Yu Z, Song Y and
Qiu W: miR-21 inhibitors modulate biological functions of gastric
cancer cells via PTEN/PI3K/mTOR pathway. DNA Cell Biol. 37:38–45.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Frankel LB, Christoffersen NR, Jacobsen A,
Lindow M, Krogh A and Lund AH: Programmed cell death 4 (PDCD4) is
an important functional target of the microRNA miR-21 in breast
cancer cells. J Biol Chem. 283:1026–1033. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou S, Zhang S, Wang Y, Yi S, Zhao L,
Tang X, Yu B, Gu X and Ding F: MiR-21 and miR-222 inhibit apoptosis
of adult dorsal root ganglion neurons by repressing TIMP3 following
sciatic nerve injury. Neurosci Lett. 586:43–49. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ferraro A, Kontos CK, Boni T, Bantounas I,
Siakouli D, Kosmidou V, Vlassi M, Spyridakis Y, Tsipras I, Zografos
G, et al: Epigenetic regulation of miR-21 in colorectal cancer:
ITGB4 as a novel miR-21 target and a three-gene network
(miR-21-ITGBeta4-PDCD4) as predictor of metastatic tumor potential.
Epigenetics. 9:129–141. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chanyshev MD, Ushakov DS and Gulyaeva LF:
Expression of miR-21 and its Acat1, Armcx1, and
PTEN target genes in liver of female rats treated with DDT
and benzo[a]pyrene. Mol Biol. 51:664–670. 2017.(In Russian).
View Article : Google Scholar
|
|
13
|
Guo Y, Chang H, Li J, Xu XY, Shen L, Yu ZB
and Liu WC: Thymosin alpha 1 suppresses proliferation and induces
apoptosis in breast cancer cells through PTEN-mediated inhibition
of PI3K/Akt/mTOR signaling pathway. Apoptosis. 20:1109–1121. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li XT, Wang HZ, Wu ZW, Yang TQ, Zhao ZH,
Chen GL, Xie XS, Li B, Wei YX, Huang YL, et al: miR-494-3p
regulates cellular proliferation, invasion, migration, and
apoptosis by PTEN/AKT signaling in human glioblastoma cells. Cell
Mol Neurobiol. 35:679–687. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Y, Sun J, Cai Y, Jiang Y, Wang X, Huang
X, Yin Y and Li H: MiR-200a acts as an oncogene in colorectal
carcinoma by targeting PTEN. Exp Mol Pathol. 101:308–313. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao ZQ, Wang JF, Chen DH, Ma XS, Wu Y,
Tang Z and Dang XW: Long non-coding RNA GAS5 suppresses pancreatic
cancer metastasis through modulating miR-32-5p/PTEN axis. Cell
Biosci. 7:662017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sushma PS, Jamil K, Kumar PU,
Satyanarayana U, Ramakrishna M and Triveni B: PTEN and p16 genes as
epigenetic biomarkers in oral squamous cell carcinoma (OSCC): A
study on south Indian population. Tumour Biol. 37:7625–7632. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nan Y, Guo L, Song Y, Wang L, Yu K, Huang
Q and Zhong Y: Combinatorial therapy with adenoviral-mediated PTEN
and a PI3K inhibitor suppresses malignant glioma cell growth in
vitro and in vivo by regulating the PI3K/AKT signaling pathway. J
Cancer Res Clin Oncol. 143:1477–1487. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu X, Li Z, Li T, Long F, Lv Y, Liu L,
Liu X and Zhan Q: Osthole inhibits the PI3K/AKT signaling pathway
via activation of PTEN and induces cell cycle arrest and apoptosis
in esophageal squamous cell carcinoma. Biomed Pharmacother.
102:502–509. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen X, Huang Z and Chen R: Microrna-136
promotes proliferation and invasion ingastric cancer cells through
Pten/Akt/P-Akt signaling pathway. Oncol Lett. 15:4683–4689.
2018.PubMed/NCBI
|
|
21
|
Geletina NS, Kobelev VS, Babayants EV,
Feng L, Pustylnyak VO and Gulyaeva LF: PTEN negative correlates
with miR-181a in tumour tissues of non-obese endometrial cancer
patients. Gene. 655:20–24. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li B, Lu Y, Yu L, Han X, Wang H, Mao J,
Shen J, Wang B, Tang J, Li C and Song B: miR-221/222 promote cancer
stem-like cell properties and tumor growth of breast cancer via
targeting PTEN and sustained Akt/NF-κB/COX-2 activation. Chem Biol
Interact. 277:33–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Taghavi N and Yazdi I: Prognostic factors
of survival rate in oral squamous cell carcinoma: Clinical,
histologic, genetic and molecular concepts. Arch Iran Med.
18:314–319. 2015.PubMed/NCBI
|
|
24
|
Tamaki S, Kawakami M, Ishitani A,
Kawashima W, Kasuda S, Yamanaka Y, Shimomura H, Imai Y, Nakagawa Y,
Hatake K, et al: Soluble MICB serum levels correlate with disease
stage and survival rate in patients with oral squamous cell
carcinoma. Anticancer Res. 30:4097–4101. 2010.PubMed/NCBI
|
|
25
|
Li J, Huang H, Sun L, Yang M, Pan C, Chen
W, Wu D, Lin Z, Zeng C, Yao Y, et al: MiR-21 indicates poor
prognosis in tongue squamous cell carcinomas as an apoptosis
inhibitor. Clin Cancer Res. 15:3998–4008. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Koenig AB, Barajas JM, Guerrero MJ and
Ghoshal K: A comprehensive analysis of argonaute-clip data
identifies novel, conserved and species-specific targets of mir-21
in human liver and hepatocellular carcinoma. Int J Mol Sci.
19:E8512018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yan F, Wang C, Li T, Cai W and Sun J: Role
of miR-21 in the growth and metastasis of human salivary adenoid
cystic carcinoma. Mol Med Rep. 17:4237–4244. 2018.PubMed/NCBI
|
|
28
|
Wu Y, Song Y, Xiong Y, Wang X, Xu K, Han
B, Bai Y, Li L, Zhang Y and Zhou L: MicroRNA-21 (Mir-21) promotes
cell growth and invasion by repressing tumor suppressor PTEN in
colorectal cancer. Cell Physiol Biochem. 43:945–958. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jung S, Li C, Jeong D, Lee S, Ohk J, Park
M, Han S, Duan J, Kim C, Yang Y, et al: Oncogenic function of
p34SEI-1 via NEDD41 mediated PTEN
ubiquitination/degradation and activation of the PI3K/AKT pathway.
Int J Oncol. 43:1587–1595. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang L, Tang B, Han H, Mao D, Chen J, Zeng
Y and Xiong M: miR-155 affects osteosarcoma MG-63 cell autophagy
induced by adriamycin through regulating PTEN-PI3K/AKT/mTOR
signaling pathway. Cancer Biother Radiopharm. 33:32–38. 2018.
View Article : Google Scholar : PubMed/NCBI
|