Introduction
Juvenile myelomonocytic leukemia (JMML) is an
infrequent but aggressive hematological system tumor of infancy and
childhood with poor prognosis. Signs and symptoms include fever,
thrombocytopenia, hemorrhage, lymphadenopathy, high fetal
hemoglobin levels and progressive hematologic anemia,
hepatosplenomegaly, and clonal proliferation of myelomonocytic
cells (1–3). The most effective treatment currently
available is allogeneic hematopoietic stem cell transplantation
(HSCT). However, the overall survival at 5 years after transplant
is only 52–63% due to treatment-related toxicity and frequent
disease relapse (3–7). In addition, some patients lack a
suitable human leukocyte antigen-matched donor, thus novel
chemotherapeutic regimens are needed to further improve
outcome.
Malignant tumors are a major cause of mortality in
children, and traditional cancer treatments such as chemotherapy,
radiotherapy and surgery carry severe side effects or do not
markedly improve prognosis. As an aggressive myeloproliferative
neoplasm, response to conventional chemotherapy is weak, thus most
JMML patients require early allotransplantation (6,7).
Alternatively, gene module analysis of JMML may facilitate the
screening of anticancer drugs targeting specific anomalies in gene
expression. In the present study, we performed gene module analysis
to identify gene expression signatures of JMML and to illuminate
the functions of these altered genes and protein networks in
disease pathogenesis.
While there are voluminous studies on anticancer
drug pharmacokinetics and efficacy against solid tumors, there have
been few studies focusing on JMML. Drugs that target
JMML-associated genes can be an effective anticancer approach,
either alone or in combination with traditional modalities. Modular
analysis of bioinformatic data is emerging as a valuable strategy
for systematic and comprehensive analysis of regulatory signaling
pathways relevant to disease etiology and drug treatment effects
(8,9). In the present study, we used modular
analysis bioinformatics methods to search for differentially
expressed genes and their key functional group(s) and networks in
JMML.
Genes differentially expressed in leukemic cells
compared to normal cells were classified into upregulated and
downregulated groups. These differentially expressed genes were
then compared to differentially expressed genes in cells treated
with small molecules from the CMap database by converting them into
probe setters based on the HG-U133A platform. Genes were then
assigned enrichment values between-1 and 1 according to whether
specific small molecules stimulated the expression state in
leukemia cells (closer to 1) or normal cells (closer to −1). Based
on this analysis, we identified drugs that regulate key nodes of
JMML-associated regulatory networks. The large number of genes and
drugs identified provide many possible targets and effective
chemotherapies for JMML.
Materials and methods
Screening for differentially expressed
genes in JMML
We downloaded JMML microarray data from the GEO
platform (https://www.ncbi.nlm.nih.gov/geo/) [data code GSE71449
(human)] (10).
The criteria for data selection were gene chip
expression profiling data accrued over the last three years (since
2014) with clear disease and normal control groups in the data set.
The disease queried was ‘Juvenile myelomonocytic leukemia’. The
largest number of GSE71449 samples were obtained by such a
standard. While our analysis is indeed limited to all
disease-related data sets in the database, further analysis of
other data sets, including miRNA chips, is already under way as no
single data set represents all genes associated with a disease. The
mechanisms of any disease are only revealed by direct experimental
study, but these gene chip results may identify genes with greater
probability of involvement. The single-chip DEG analysis can only
reveal that in the current data set, these differentially expressed
genes (DEGs) are most likely to be related to disease.
Array Express was retrieved, and the results
retrieved with the same search criteria coincided with those in
GEO. There was no larger sample size under the same screening
criteria. GEO data sets are included in the EBI database, thus
there is no mention of it.
The database provides tissue sample results from 44
JMML patients and seven healthy donors obtained using the
Agilent-041648 CMGG Human V1.1 60k [Probe Name Version] microarray
platform. We used the R software bagaffy (11) (Version1.50.0, http://www.bioconductor.org/packages/release/bioc/html/affy.html)
to read the downloaded microarray matrix data and the robust
multi-array average method (12,13) to
perform standardized data preprocessing (including Background
correction, Normalization, and Expression calculation). We
performed annotation for probes in the platform annotation file and
deleted probes that did not match the gene symbols used. When the
same gene was reflected by different probes, we adopted the average
as the final expression value.
For the two data groups (JMML and controls), we
screened for differentially expressed genes using the R bag Limma
microarray analysis package (14)
and adopted the BH-corrected T test to identify genes significantly
over- or under expressed. For every differentially expressed gene,
we assumed a threshold P<0.05 and log|FC| >0.58.
Differentially expressed genes were selected based on a threshold
|logFC| >0.58, corresponding to a fold change >1.5 or ≤1.5
(15). We then used the common
enrichment analysis tool DAVID (16) (version 6.8) and subjected genes with
enrichment ≥2 and significance threshold of hypergeometric test
P<0.05 to Gene Ontology (GO) (17) function and Kyoto Encyclopedia of
Genes and Genomes (KEGG) (18)
pathway analyses.
For observing the function of differentially
expressed genes intuitively, we used the ClueGO (19) plug-in (version 2.2.6, http://apps.cytoscape.org/apps/ClueGO)
of Cytoscape software (20) and
constructed separate GO BP and KEGG pathway cross-linked enrichment
graphs for upregulated and downregulated genes using P<0.05 as
the significantly enriched threshold.
Building and analyzing modules of
differentially expressed genes
We used the STRING (21) database bank to predict possible
protein-protein interaction (PPI) networks for all differentially
expressed proteins and genes with parameter PPI score 0.4 (medium
confidence). Networks with average interaction strengths at protein
nodes were constructed using Cytoscape.
Interaction networks may contain nodes in which the
interactions are closed among the constituent genes. These nodes
thus represent distinct biological processes. There are many
methods to define nodes by PPI cluster analysis but MCODE (22) was chosen because it is the most
common. We applied MCODE to calculate the scores of every node,
with higher scores indicating a greater degree of separation from
other nodes and stronger association with specific processes. We
choose nodes with score ≥5 and node number ≥5 for subsequent GO and
KEGG pathway analyses.
Screening for drugs that regulate
functional modules
The CMap database (23,24)
stores the genome-wide expression profiles of human cells treated
with various active small molecules. In total, CMap contains data
from 6,100 small molecule interference experiments (with normal
control groups) using 1,309 small molecules, for a total of 7,056
different expression profiles.
We analyzed gene expression differences between
normal cells and leukemia cells and then compared the responses of
differentially expressed genes to identify those with similar or
opposite effects (upregulation vs. downregulation) on normal cells.
We divided the genes differentially expressed between normal and
leukemia cells into upregulated and downregulated subgroups. Then
we transformed the HG-U133A platform probe set results and compared
them to the set of differentially expressed genes under small
molecule treatment from the CMap database to obtain enrichment
values. In this case, enrichment values range from-1 and 1, with
those closer to 1 influenced to a greater extent by small molecules
in normal cells and those with values closer to −1 influenced to a
greater extent by small molecules in leukemia cells.
For key modular gene screening, we constructed
connectivity maps and used the gene expression differences in human
cells treated with small molecules to identify drugs affecting the
expression levels of genes associated with the disease.
Identification of possible miRNAs
regulating target genes
We identified potential miRNA targeting genes within
PPIs using Webgestalt (http://www.webgestalt.org/) (25) tools and overrepresentation
enrichment analysis methods with a threshold P<0.05 by
hypergeometric tests and BH correction for enriched gene number and
count ≥5.
Identification of possible
transcription factors regulating target genes
Based on the transcription factor-regulated network
data in the ITFP database and TRANSFAC bank, we searched for
transcription factors regulating differentially expressed genes and
further screened the differentially expressed target genes
regulated by these transcription factors for network integration.
We performed network integration for the obtained TF-Target,
miRNA-Target, and PPI network and constructed integration networks
using Cytoscape.
Statistical analysis
t-tests were used to screen for differentially
expressed genes and to evaluate the enrichment obtained by GO_BP-
and KEGG-based methods. A P<0.01 was considered statistically
significant. With respect to each herbal component, Fisher's exact
test was used to evaluate the genes in modules. Based on the above
data, we set P<0.01 for each component.
Results
Screening for differentially expressed
genes in JMML
We downloaded the JMML expression data from the GEO
database, and identified 700 upregulated and 737 downregulated
genes at threshold levels of P<0.05 and log|FC| >0.58. From
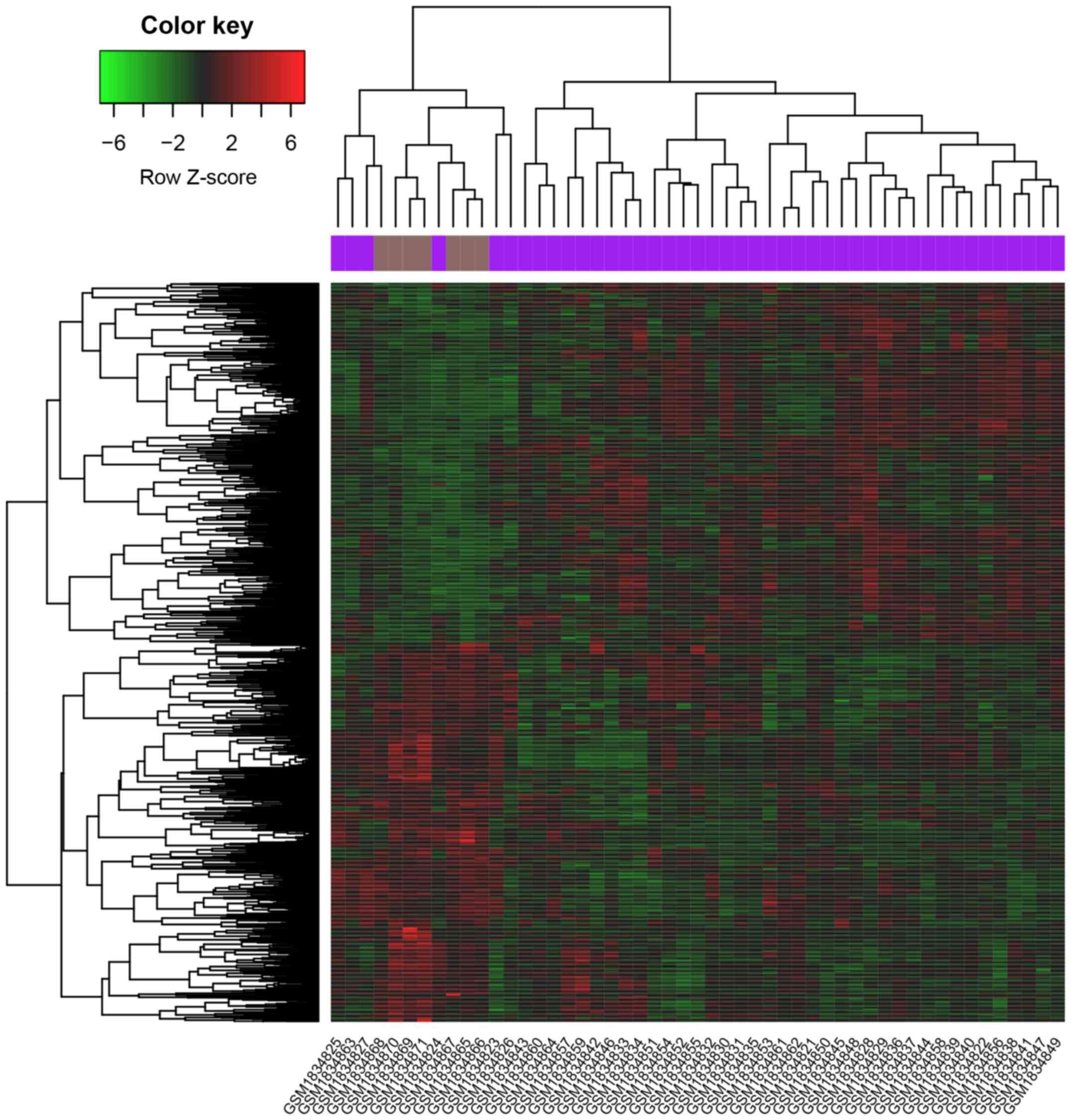
these genes, we constructed heat maps (Fig. 1). We then performed GO and KEGG
pathway enrichment analyses for upregulated and downregulated mRNA
muster, and according to the screening threshold value obtained GO
terms and KEGG pathways including these differentially expressed
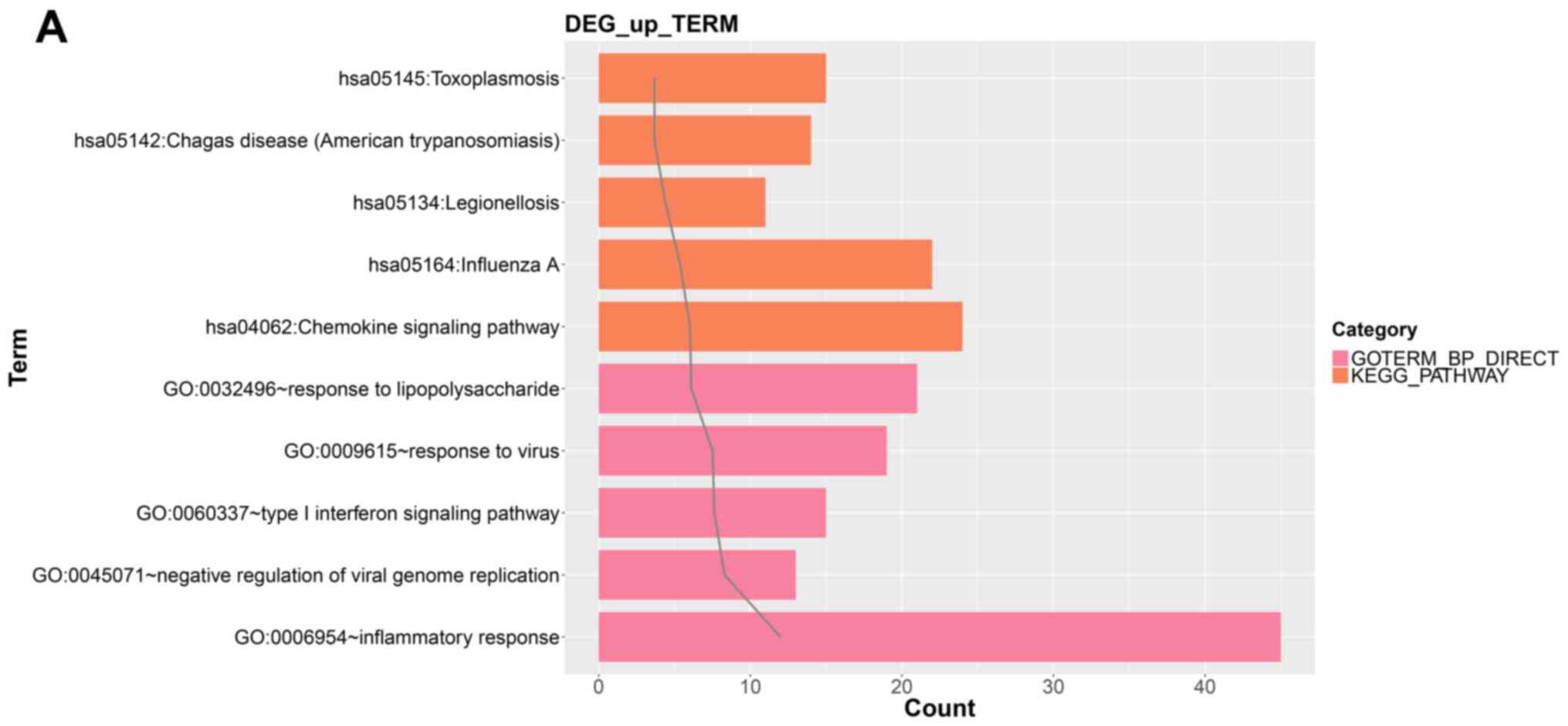
genes. Fig. 2A shows the top five
GO Biological Process (BP) terms and KEGG pathways including
upregulated mRNAs and Fig. 2B shows
these same results for downregulated mRNAs.
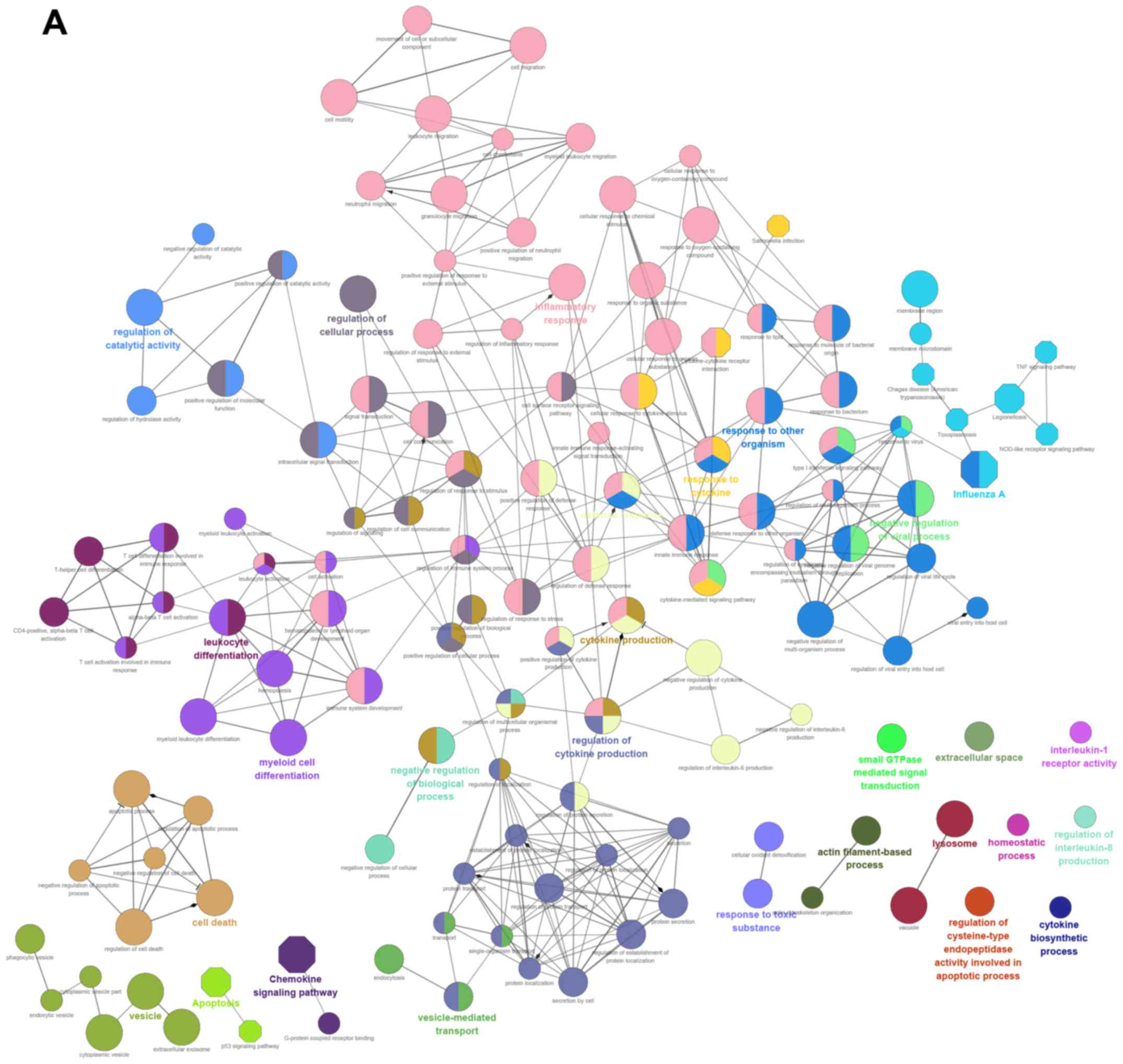
We used clueGO to perform GO and KEGG pathway
analysis for all upregulated and downregulated differentially
expressed genes (Fig. 3A and B).
ClueGO provides kappa coefficients that can be used to divide the
pathways into functional groups. In the figure, different colors
indicate pathway enrichment results and correlations between two
pathways are reflected by a connecting line. The sizes of nodes
reflect the P-value. Fusion of enrichment results was performed
when different terms were enriched for the same gene. In the
figure, GO terms are indicated by different colors. Thus, one color
indicates a functional group. The size of the term nodes depends on
the threshold P-value, increasing as P gets smaller.
Key genes and PPI networks constructed
from differentially expressed genes
Combined with the PPI database, we constructed the
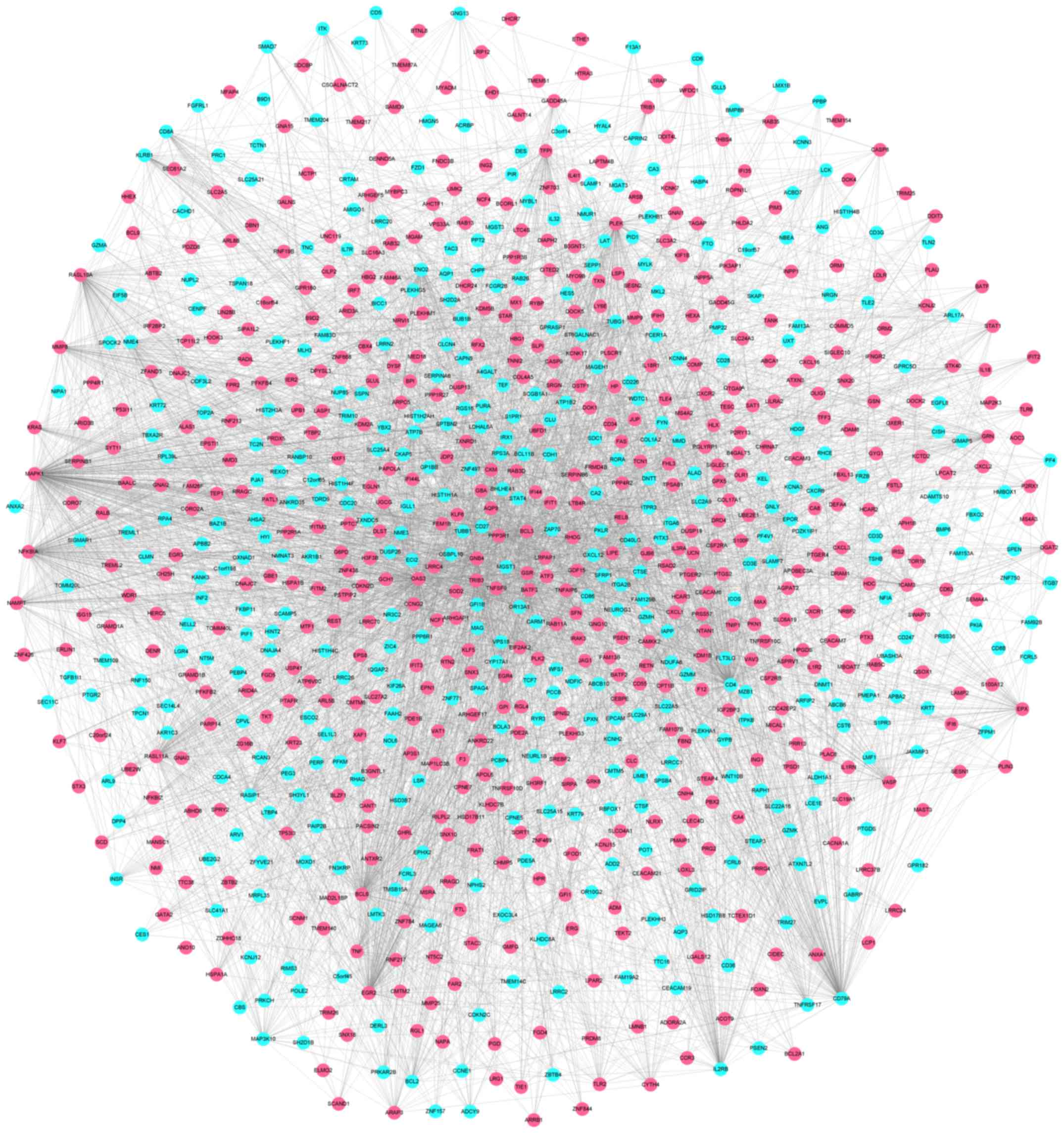
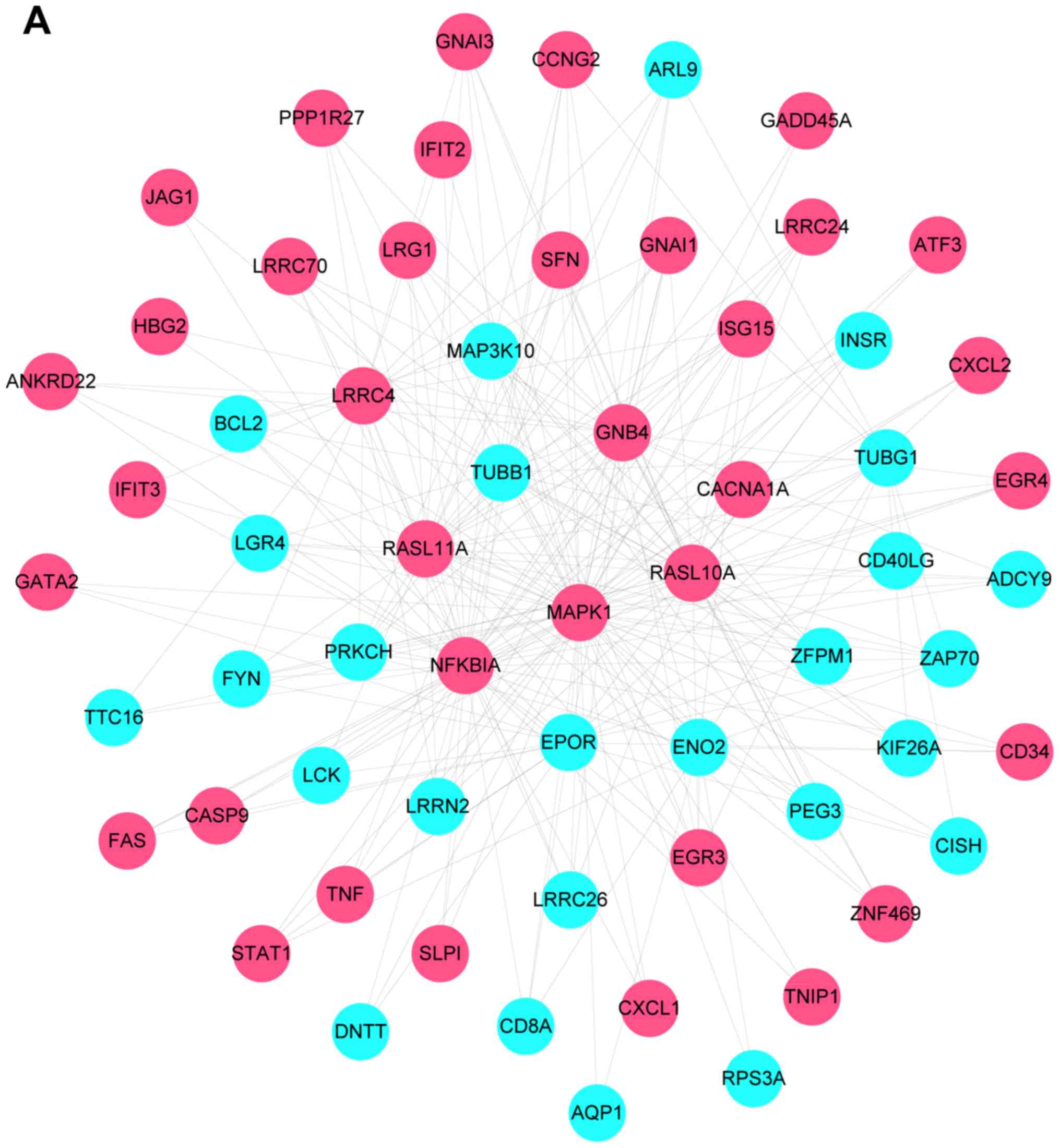
network figures for differentially expressed genes (Fig. 4). The PPI network of differentially
expressed genes contained 908 nodes and 5,053 related pairs. The
relationships among the nodes are closed. Table I lists the top 10 highest degree
nodes obtained from the PPI network and Fig. 4 shows the PPI network constructed
from the differentially expressed genes, with pink nodes indicating
upregulated and blue nodes the downregulated genes.
 | Table I.The top 10 highest degree nodes
obtained from the PPI network. |
Table I.
The top 10 highest degree nodes
obtained from the PPI network.
| Gene | Degree | Betweenness | Closeness |
|---|
| MAPK1 | 283.0 | 142874.9 | 0.54246414 |
| CD4 | 218.0 | 93239.1 | 0.49266702 |
| OAS3 | 212.0 | 110066.46 | 0.48373333 |
| CD79A | 173.0 | 59164.633 | 0.47862798 |
| NFKBIA | 157.0 | 37323.453 | 0.48424986 |
| BCL6 | 154.0 | 42434.684 | 0.4748691 |
| EGR2 | 138.0 | 33878.51 | 0.4512438 |
| PLEK | 134.0 | 53153.11 | 0.45509282 |
| TRIB3 | 115.0 | 24556.303 | 0.44591936 |
| RASL11A | 112.0 | 16269.245 | 0.44923228 |
Modular analysis
We obtained a total of 10 modules from the PPI
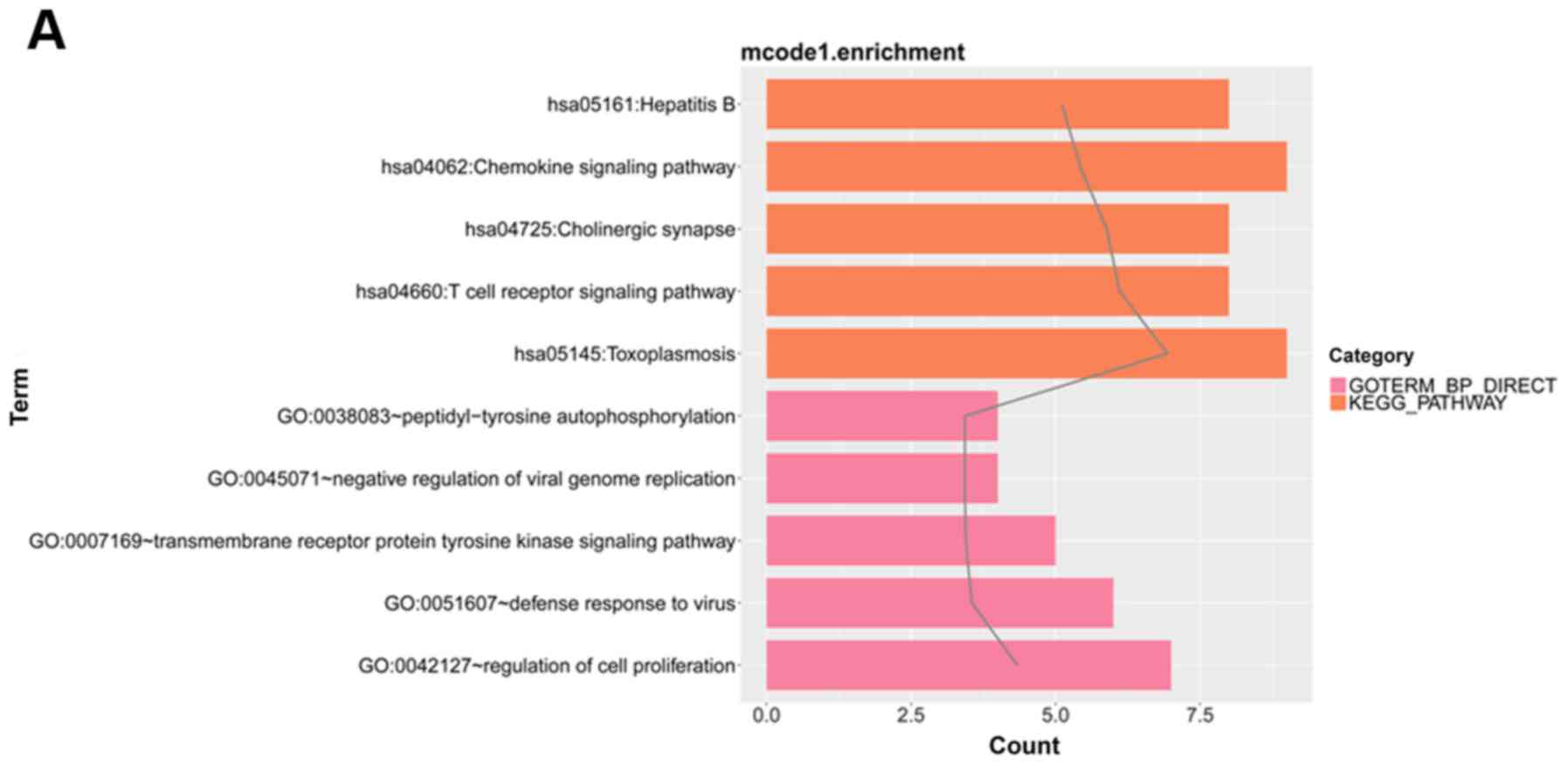
network, of which four had scores >3 (Fig. 5). We also performed functional
enrichment analysis for the genes of modules. Fig. 6 shows the top five GO BP terms and
KEGG pathways in module 1, the four KEGG pathways and top five GO
BP terms in module 2, the four GO BP terms in module 3 (no KEGG
pathways were enriched), and the top five GO BP terms and KEGG
pathways in module 4.
For module 1, pathway analysis indicated that
differentially expressed genes were most strongly (lowest P-value)
related to Regulation of cell proliferation. For module 2,
differentially expressed genes were most strongly related to
Pathways in cancer. In module 3, the strongest relationship was
with Transcription, DNA-templated, and in module 4 the strongest
relationship was with Immune response.
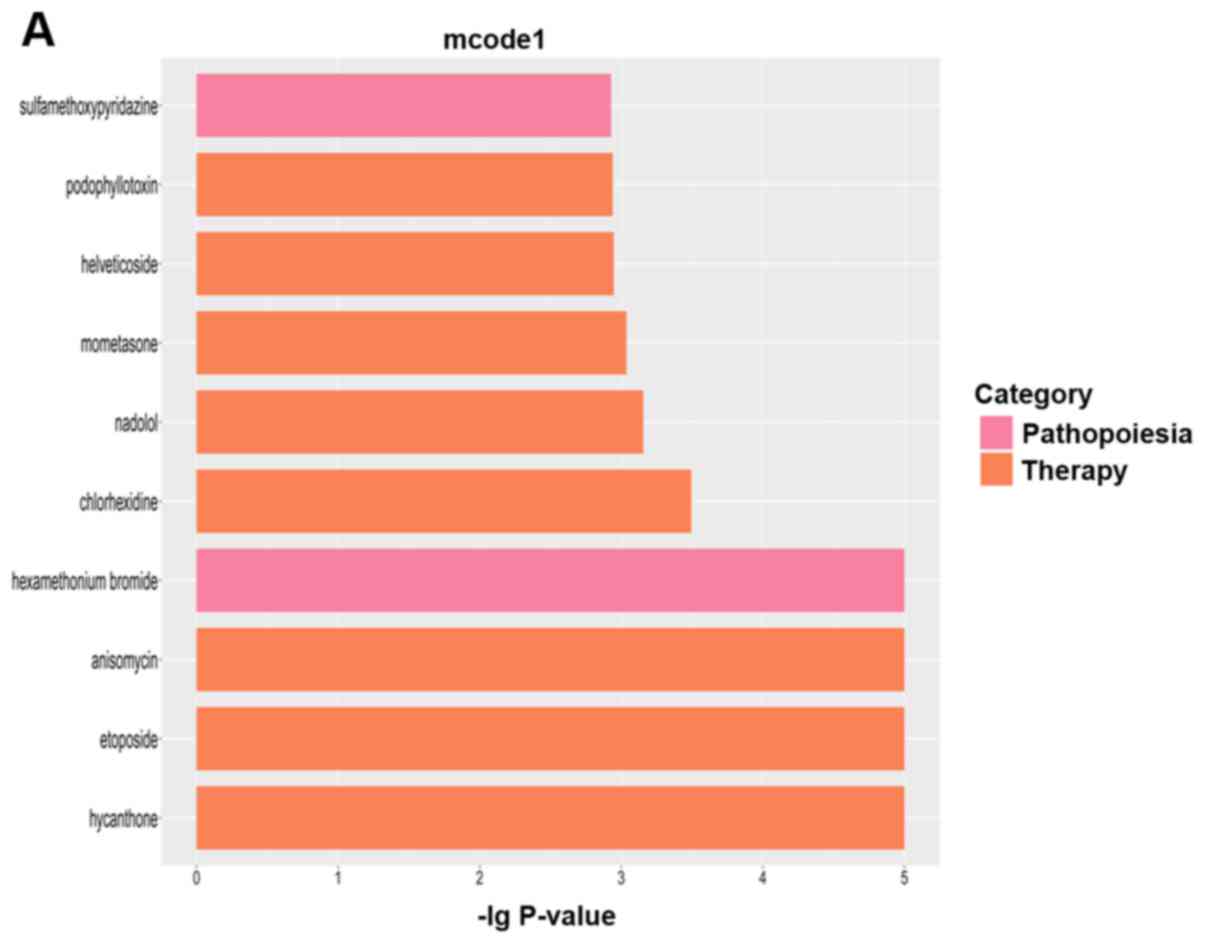
Small drug molecules related to
diseases
We performed small molecule drug analysis for the 4
modules using the CMap database and threshold P<0.05. The
regulatory effects on each module are shown in Fig. 7.
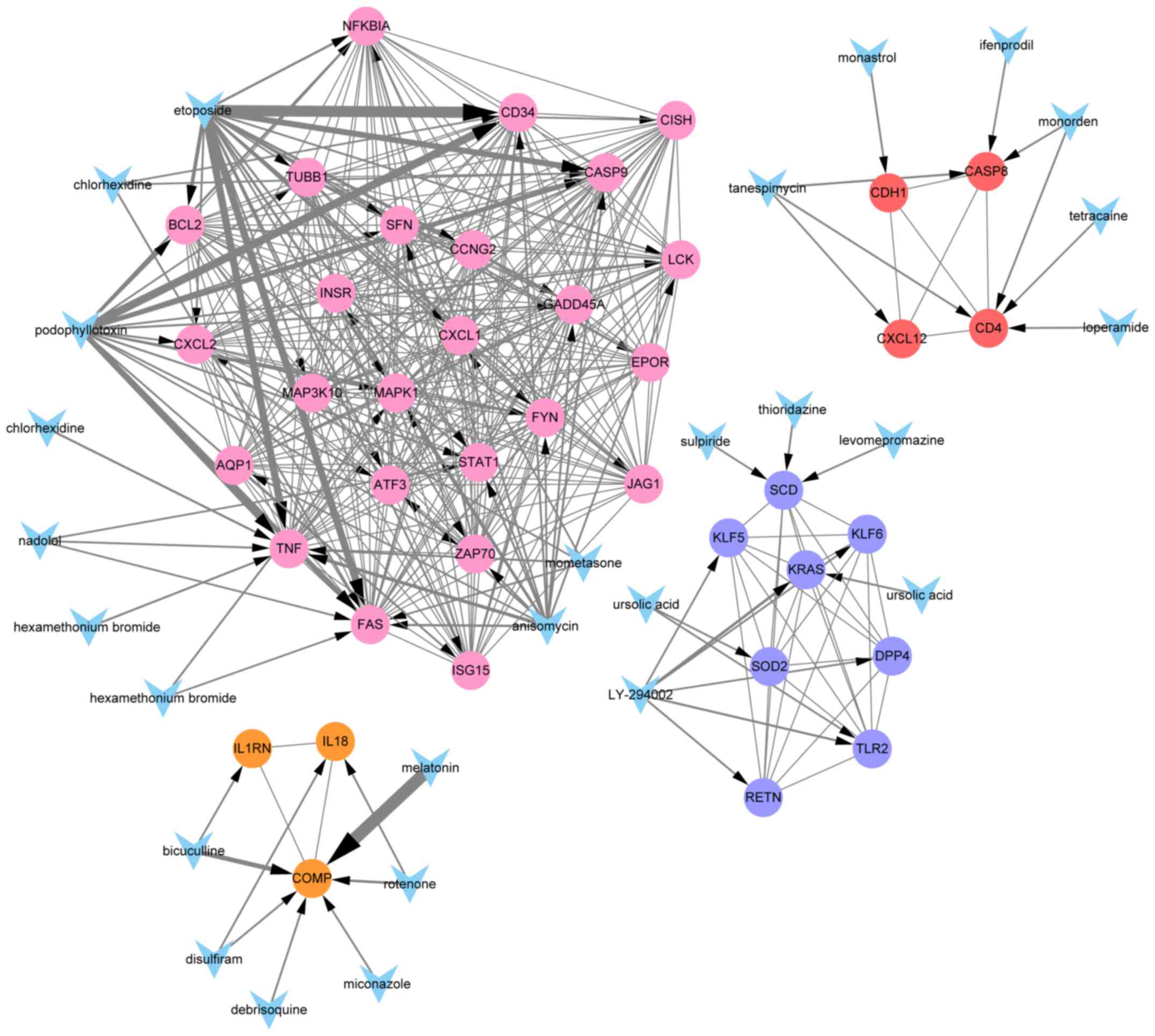
Statistical relationships between
modular genes and small drugs
We used local perl script and performed Pubmed
searches for the analyzed modular genes and the corresponding small
molecules, and constructed the network figures shown in Fig. 8. The P-values were considered in
descending order, and the drugs with the 10 smallest P-values were
used for subsequent analyses. A total of 40 drugs were identified
as possible regulators of the 4 modules. To obtain novel anticancer
drugs, we conducted a literature review of the cancer-related
research on the 24 drugs with greatest effects on gene expression.
In cancer treatment, the number of publications reflects the extent
of research on a given drug's potential efficacy. Multiple drugs
have been researched extensively and already certified to be
effective anticancer drugs, such as etoposide and
phosphonothreonine (the number of relevant publications was
>100). Others, however, have not been fully studied for their
anticancer efficacies, such as disulfiram, ursolic acid,
miconazole, thioridazine, loperamide, monastrol (the number of
relevant publications was ≤10), and others, such as nadolol,
tetracaine and levomepromazine have never been studied with respect
to cancer. Publication numbers for drug are shown in Fig. 9.
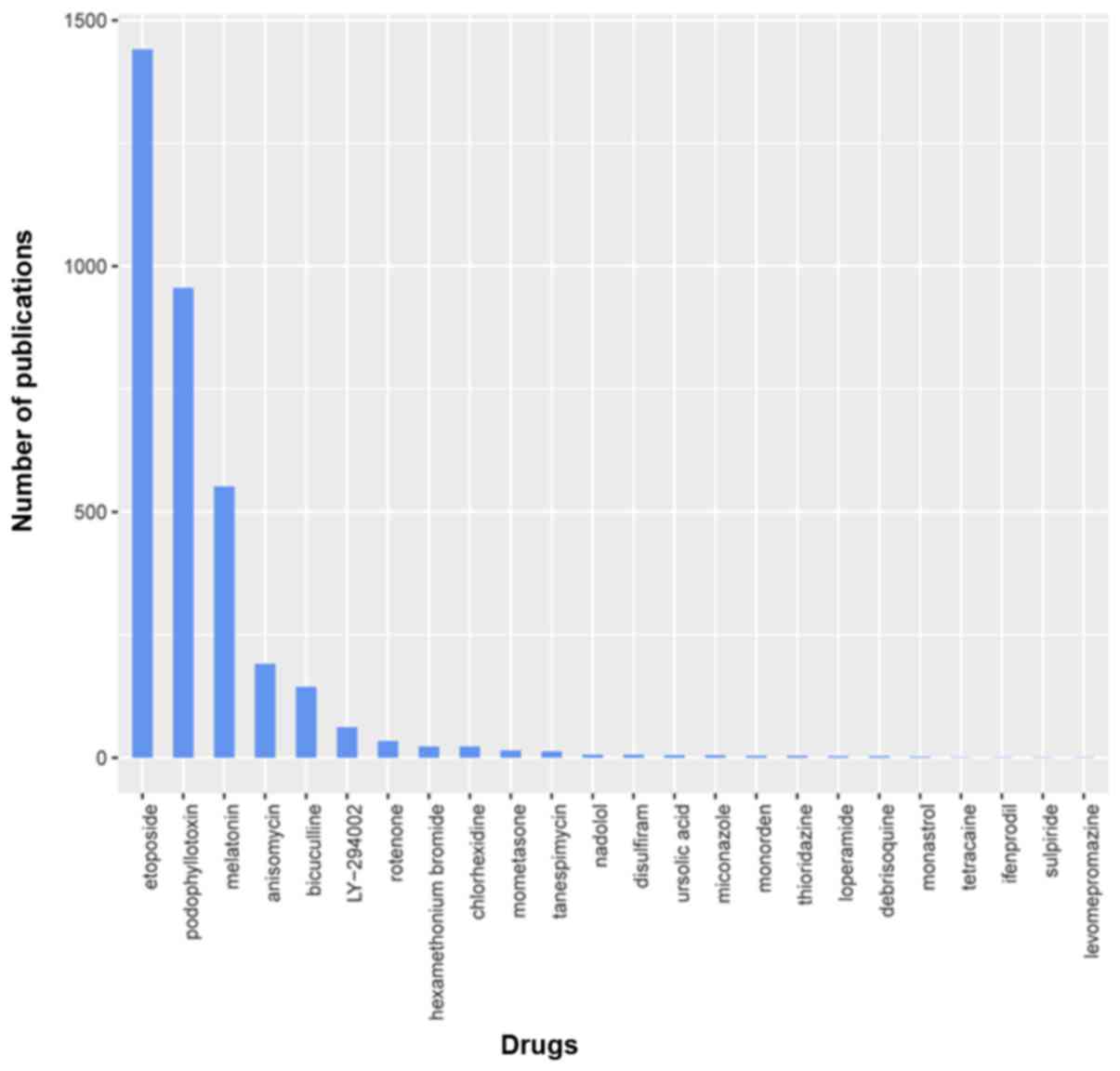 | Figure 9.To evaluate potential efficacy, we
conducted a literature appraisal of 24 small molecules. The number
of publications is indicative of the extent of study on cancer.
Those with fewer publications may be novel anticancer drugs. Of
those identified, many are confirmed to be anticancer drugs, for
example etoposide and phosphonothreonine (>100 relevant
publications), while others have not been studied extensively for
anticancer efficacy, for example disulfiram, ursolic acid,
miconazole, thioridazine, loperamide and monastrol (≤10 relevant
publications). A few, such as nadolol, tetracaine, and
levomepromazine, have never been examined for anticancer
properties. |
Identification of candidate miRNAs
regulating differentially expressed genes
We identified candidate miRNAs that may regulate the
differentially expressed genes using a screening threshold of
P<0.05. For upregulated genes, we identified 10 candidate miRNAs
(Table IIA) and for the
downregulated genes four candidate miRNAs (Table IIB).
 | Table II.Identification of candidate miRNAs
regulating differentially expressed genes (DEGs). |
Table II.
Identification of candidate miRNAs
regulating differentially expressed genes (DEGs).
| A, miRNAs for
upregulated DEGs |
|---|
|
|---|
| miRNA | P-value |
|---|
| AGTCAGC,
MIR-345 | 0.002846737 |
| CAGGTCC,
MIR-492 | 0.003876902 |
| ACCAATC,
MIR-509 | 0.011602863 |
| CCAGGTT,
MIR-490 | 0.017555181 |
| TGCACTG, MIR-148A,
MIR-152, MIR-148B | 0.023681008 |
| TGAGATT,
MIR-216 | 0.030489785 |
| TCCAGAT,
MIR-516-5P | 0.033806763 |
| AGTCTTA,
MIR-499 | 0.034998955 |
| GTGTCAA,
MIR-514 | 0.037442107 |
| AAAGGGA, MIR-204,
MIR-211 | 0.040666691 |
|
| B, miRNAs for
downregulated DEGs |
|
| miRNA | P-value |
|
| GGCACTT,
MIR-519E | 0.002921036 |
| TCCGTCC,
MIR-184 | 0.037734308 |
| GTAAGAT,
MIR-200A | 0.040359704 |
| AGCATTA,
MIR-155 | 0.04249545 |
Identification of candidate
transcription factors regulating differentially expressed
genes
For the upregulated differentially expressed genes,
we identified 22 differentially expressed transcription factors
(TFs). For the downregulated differentially expressed genes, we
identified 19 differentially expressed TFs. The results are
summarized in Table III.
 | Table III.Identification of candidate
transcription factors (TFs) regulating differentially expressed
genes (DEGs). |
Table III.
Identification of candidate
transcription factors (TFs) regulating differentially expressed
genes (DEGs).
| TFs for upregulated
DEGs | TFs for
downregulated DEGs |
|---|
| ABTB2, AHCTF1,
BATF, CORO7, ERG, FGD5, FOXN2, IFIH1, IFIT1, IFIT2, IFIT3, ING1,
INPP5A, LASP1, PARP14, PLEK, PTBP2, SAMD9, STAT1, TANK, TTC7B,
WDR1 | CCNE1, CDC20,
CKAP5, FAM129B, KRT7, LGR4, MKL2, NFIA, REXO1, SMARCA4, SPEN, TLE2,
TRIM10, TRIM27, TTC16, ZBTB4, ZNF497, ZNF750, ZNF771 |
Discussion
Currently the only curative treatment option for
JMML is hematopoietic stem cell transplantation (HSCT). However,
disease recurrence remains a major cause of treatment failure
(7). Clinical symptoms are caused
by hematopoietic insufficiency and excessive proliferation of
leukemic monocytes and granulocytes, leading to hepatosplenomegaly,
lymphadenopathy, skin rash and respiratory failure (3,7,26). A
serious obstacle to the research of JMML is the lack of suitable
experimental models, impeding the development and pre-clinical
evaluation of novel therapeutic approaches. Primary JMML leukemia
cells cannot be maintained in culture as they differentiate and
become apoptotic (27), while an
immortalized cell line derived from JMML cells has not yet been
successfully established (28). The
generation of induced pluripotent stem cell lines originating from
JMML cells was reported, but conceptually such systems are limited
by their artificial nature and the risk of further transformation
during reprogramming (29).
Therefore, it is important to identify tumor-promoting processes,
such as dysregulation of specific genes and networks, and develop
targeted therapies.
In the present study, we combined the genome-wide
gene chip information already available from JMML tissues and used
modular analysis bioinformatics procedures to identify molecular
targets and small molecule compounds that affect their expression.
For this purpose, modular analysis is a valuable and well
established bioinformatic method that is useful for analysis of
both large-scale protein networks and single proteins and genes.
Moreover, this method has been used to study the development and
treatment of multiple diseases. After the enrichment analysis, we
found that genes related to the hematopoietic cell lineage and to
regulation of immune responses were significantly downregulated,
while chemokine signaling pathway and inflammatory response genes
were significantly upregulated compared to healthy tissue.
Hematopoietic progenitors produce a myriad of
diverse lineages, including progenitors with lymphoid, myeloid, and
erythroid potential, prior to hematopoietic stem cells. Previous
pathway analyses revealed that genes related to hematopoietic cell
lineages were significantly downregulated in JMML (30–34),
and we further found that genes related to regulation of immune
responses were also significantly downregulated. Anticancer
treatment leads to dense immune infiltrate, including many
CD8+ and CD4+ cells, concomitant with tumor
regression, at both treated and untreated lesions, consistent with
generation of a tumor-specific systemic immune response (35). Several studies have indicated the
synergistic potential of various immunomodulatory agents (36–39).
Therapies that enhance or impede immune responses are essential but
optimal timing and the administration route are important
detemising results, such as increased survival and immune
responses, leading to complete tumor regression in some cases
(40–42).
Rminants of efficacy. Preclinical studies combining
these agents have shown proThe PI3K/Akt and MAPK signaling pathways
have been shown to mediate chemokine-induced migration of multiple
cell types. Modular analysis suggested that the Chemokine signaling
pathway (module 1) was strongly related to JMML morbidity. In
addition to regulation of cell proliferation, differentiation,
invasion, and inflammation, chemokines are widely involved in the
regulation of cancer. Analysis of module 1 revealed that regulation
of cell proliferation was significantly increased and strongly
related to JMML occurrence. Further, analysis of all 4 functional
modules revealed complex interactions among a number of genes
involved in Chemokine signaling pathway and Regulation of cell
proliferation. Chemokines are small proteins expressed in response
to injury or infection and during normal immune surveillance.
Chemokines are involved in leukocyte trafficking and regulate tumor
metastasis, proliferation, differentiation and angiogenesis
(43–46).
Module 2 contained genes primarily involved in
Pathways in cancer, Transcriptional dysregulation in cancer, and
Signal transduction, suggesting that module 2 contains clusters of
key tumor-promoting genes. Accumulation of driver somatic
alterations in genes modifies critical cellular processes leading
to cancer (47,48). In recent years, the catalog of
driver genes known to take part in the development of malignancies
has expanded due to whole-exome and whole-genome analyses of large
tumor sets by large international consortia (49,50).
In addition to upregulation of oncogenes, downregulation of
anticancer proteins or dysregulation of subcellular localization
may also contribute to JMML.
Cancer cells scatter from tumors in hoards and build
new tumors in distant tissues and organs (51,52).
In over 90% of fatal cancers, metastasis is the cause of death.
Tumors contain a heterogeneous population of cells in an organized
hierarchy akin to normal tissue. Tumor progression, invasiveness,
and self-renewal are attributes of smaller subfractions of cancer
cells. Tumor initiation by disseminated cancer cells relies on
their ability to self-renew and initiate metastatic tumors.
Genes in module 3 were primarily involved in
Response to hypoxia, Negative regulation of transcription from RNA
polymerase II promoter, Regulation of transcription, DNA-templated,
and Transcription, DNA-templated. We found that among these
pathways, transcription, DNA-templated was most strongly related to
JMML occurrence. The identification and detection of specific
nucleic acids (either DNA or RNA) is an enabling technology for
forensic analysis (53),
recognition of genetic mutations (54) and pathogen identification (55). For many methods, quantification of
nucleic acids is important for identifying a given DNA sequence.
For example, in many forms of cancer (56), oncogene copy number increases and
deletion of tumor-suppressor genes can be found. In addition to
measuring changes in gene copy number, quantitative detection of
circulating DNAs can be a useful diagnostic tool for identifying
cancer (57).
The genes in module 4 are closely associated with
extracellular matrix organization and immune responses. Much effort
has been devoted to determining how cellular components of the
tumor promote cancer development and initiate formation of a niche
conducive to growth (58).
Alternatively, the importance of non-cellular components of the
niche during cancer progression is well documented, particularly
extracellular matrix (ECM) organization (59–62).
In addition to a stable structure with supportive functions in
maintaining tissue morphology, the ECM is a surprisingly dynamic
and versatile part of the cell milieu influencing fundamental
aspects of cell biology (63). For
major developmental processes, the ECM directly or indirectly
regulates almost all cellular behaviors (64–67).
Indeed, in diseases such as cancer, abnormal ECM dynamics may be a
strong determinant of clinical outcome (68). How disruption of ECM dynamics
contributes to tumor development is a challenging issue in cancer
biology.
The local immune response has also emerged as an
important element in the multistep process of cancer development
(69). Observations that some
tumors arise from chronic inflammation sites have led to
speculation of a strong connection between tumor onset and
inflammatory pathways. In addition, some tumors are infiltrated by
both the innate and adaptive arms of the immune system, thus many
different immune cells are present within the tumor
microenvironment (70). Elements of
both the innate and adaptive immune systems have been reported to
act both as pro- or anti-tumorigenic factors depending on the
relative balance. The intercellular communication between
infiltrating immune cells and cancer cells modulates this immune
response so as to positively influence tumor development (71).
We found the module 2 was most strongly related to
JMML occurrence. There are likely synergistic interactions among
module 2 genes.
Many investigations have attempted to screen for
well-tolerated and affordable anti-neoplastic medications, and a
myriad of drugs with cytotoxicity against human cancer cell lines
have been described (72,73). In our study, we also screened for
drugs with curative potential against JMML through modulation of
associated gene pathways. In 1999, Mayer et al (74) first identified monastrol, a
cell-permeable small molecule with antimitotic activity but without
neuronal cytotoxicity. Through inhibition of kinesin Eg5, monastrol
can induce the mono-astral conformation of microtubules (75,76).
Several subsequent studies have clarified the anti-mitotic
mechanisms of monastrol (77–79),
but few studies have investigated its anticancer activity (80–82).
In both canine and human cancer patients, the
peripherally acting µ-opiate receptor agonist loperamide
hydrochloride is recommended as a treatment for
chemotherapy-related diarrhea (83,84).
Loperamide was shown to dose-dependently induce apoptosis and
suppress the proliferation of human liver, lung, bone, and breast
cancer cell lines (85). In human
cancer cell lines, the mechanism underlying apoptosis induction has
not been fully elucidated, although the caspase 3 pathway has been
implicated (85) and loperamide
will exert anticancer properties at clinically relevant doses. In
the clinic, loperamide would be an attractive drug for JMML because
of its minimal side effects and low price. Our bioinformatic
analysis revealed a potential role for loperamide in JMML
treatment. Further studies are needed to establish mechanisms of
antitumor activity.
Originally, thioridazine (TDZ) was used as a therapy
for psychotic disease (86,87). In addition, it has been used to
treat drug-resistant microorganisms (88,89).
Recently TDZ was reported to have potent effects on various types
of cancer cells, including anti-angiogenesis and apoptosis
promotion of breast and ovarian cancers cells (90,91).
In addition, TDZ induced cytotoxicity of cervical (92), prostate (93), gastric (94), and pancreatic cancer cells (95). TDZ also has selectivity in leukemia
as a dopamine receptor inhibitor (96,97).
A few publications have suggested that miconazole
has anticancer effects (98).
Miconazole is a common treatment for superficial fungal infection
and a prominent systemic antifungal agent. In different human
neoplastic cell lines, Wu et al demonstrated that miconazole
could induce cell cycle arrest. This growth arrest was
dose-dependent and related to the p53 signaling pathway (99). Anticancer effects of miconazole were
reported on 4T1 (breast cancer) and 5637 (bladder cancer) cell
lines.
Ursolic acid (UA) was found to have a biphasic
response against three breast cancer cell lines (100). Another study (101) reported that disulfiram suppressed
tumor growth by killing cancer cells and was even effective in
combination with DHA. Indeed, UA could act as a substitute for
clioquinol.
Compared to treatments targeting individual genes,
treatment with agents affecting larger gene groups may have better
efficacy as anticancer therapy. The aim of the present research was
to identify possible molecular targets for cancer treatment and
potential anticancer drugs. We searched for drugs that regulate the
essential functional modules of JMML using the DrugBank Small
Molecule database, and found several drugs already shown to have
anticancer effects, including nadolol, disulfram, ursolic acid,
micronazole, thioridazine, loperamide, monastrol, tetracaine, and
levomepromazine. In contrast, other drugs identified have never
been examined for anticancer efficacy, such as tetracaine,
levomepromazine and nadolol. Nonetheless, effects on differentially
expressed genes in JMML suggest therapeutic potential.
The nine drugs identified are potential therapies
for JMML. Many widely used and studied drugs have never been
examined for effects against cancer, such as tetracaine,
levomepromazine and nadolol. According to our enrichment analyses,
these agents hold potential for JMML treatment. We searched the
Pubmed database and found several RCTs on these drugs, but no
published clinical RCTs supporting anticancer effects on JMML. In
the future, we plan to perform RCTs on these nine drugs examining
possible therapeutic efficacy against JMML.
When designing therapeutic regimens for JMML,
possible adverse reactions must be considered. It is thus
noteworthy that the drugs identified here are in clinical use with
well described safety profiles. When tumor cell proliferation
reaches the highest activity, drugs interfering with the cell cycle
or targeting proliferation pathways are significantly more
potent.
MicroRNAs are endogenous 20–25 nucleotide non-coding
RNAs found in eukaryotes that regulate gene expression at the level
of translation. Mature miRNAs are produced by sequential cleavage
of longer primary transcripts. Then the RNA-induced silencing
complex RISC induced by RNA is assembled to recognize target mRNAs
by base complementary pairing, and the target mRNA is degraded or
suppressed according to the degree of complementarity. Recent
studies have shown that the silencing complex degrades the target
mRNA or inhibits the translation of the target mRNA. Through gene
silencing, miRNAs regulate diverse processes including growth,
virus defense, hematopoiesis, organ formation, cell propagation,
apoptosis, and fat metabolism, among others. Transcription factors
(TFs) are proteins with special structures that regulate gene
expression at the level of the genome. The transcription initiation
of eukaryotes is complex and requires the assistance of multiple
protein cofactors. Transcription factors form a transcription
initiation complex with RNA polymerase II. Both miRNAs and TFs
regulate genes, thus differentially expressed genes (DEGs) are
predicted by miRNAs and TFs, which further extends the number of
potential targets. Like differentially expressed genes, the
prediction of miRNAs and TFs involved in differential expression
also provides clues to disease mechanisms.
In conclusion, our study used multiple bioinformatic
methods to identify 4 gene modules associated with JMML. In
addition, we identified 40 drugs based on the CMap database that
can alter expression and function of the 4 modules. We performed
Fisher's exact test to precisely screen for drugs with the
strongest effects on module regulation. Through our study, we
identified nine drugs that have considerable potential as new
anticancer drugs. Moreover, the study provides a new research
template for future research on JMML-targeted anticancer treatments
through detailed analyses of core functions. In the future, we will
conduct follow-up studies to verify the anticancer effects of the
selected drugs.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Research
Project of Heilongjiang Health and Family Planning Commission
(project no. 2016-038).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
WZ and YY conceived and designed the study. WZ, LW
and YY performed the experiments. WZ and LW wrote the manuscript.
WZ, LW and YY reviewed and edited the manuscript and were also
involved in the conception of the study. All authors read and
approved the manuscript and agree to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chan RJ, Cooper T, Kratz CP, Weiss B and
Loh ML: Juvenile myelomonocytic leukemia: A report from the 2nd
International JMML Symposium. Leuk Res. 33:355–362. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Niemeyer CM and Kratz CP: Paediatric
myelodysplastic syndromes and juvenile myelomonocytic leukaemia:
Molecular classification and treatment options. Br J Haematol.
140:610–624. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Niemeyer CM, Arico M, Basso G, Biondi A,
Rajnoldi Cantu A, Creutzig U, Haas O, Harbott J, Hasle H, Kerndrup
G, et al: Chronic myelomonocytic leukemia in childhood: A
retrospective analysis of 110 cases. European working group on
myelodysplastic syndromes in childhood (EWOG-MDS). Blood.
89:3534–3543. 1997.PubMed/NCBI
|
|
4
|
Bergstraesser E, Hasle H, Rogge T, Fischer
A, Zimmermann M, Noellke P and Niemeyer CM: Non-hematopoietic stem
cell transplantation treatment of juvenile myelomonocytic leukemia:
A retrospective analysis and definition of response criteria.
Pediatr Blood Cancer. 49:629–633. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dvorak CC and Loh ML: Juvenile
myelomonocytic leukemia: Molecular pathogenesis informs current
approaches to therapy and hematopoietic cell transplantation. Front
Pediatr. 2:252014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Locatelli F, Nollke P, Zecca M, Korthof E,
Lanino E, Peters C, Pession A, Kabisch H, Uderzo C, Bonfim CS, et
al: Hematopoietic stem cell transplantation (HSCT) in children with
juvenile myelomonocytic leukemia (JMML): Results of the
EWOGMDS/EBMT trial. Blood. 105:410–419. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Locatelli F and Niemeyer CM: How I treat
juvenile myelomonocytic leukemia. Blood. 125:1083–1090. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang PI and Marcotte EM: It's the machine
that matters: Predicting gene function and phenotype from protein
networks. J Proteomics. 73:2277–2289. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hedges SB: The origin and evolution of
model organisms. Nat Rev Genet. 3:838–849. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Helsmoortel HH: Gene expression profiling
of 44 JMML patients and 7 healthy donors (discovery cohort). Homo
sapiens. Dec 23–2015.
|
|
11
|
Gautier L, Cope L, Bolstad BM and Irizarry
RA: Affy-analysis of Affymetrix GeneChip data at the probe level.
Bioinformatics. 20:307–315. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bolstad BM, Irizarry RA, Astrand M and
Speed TP: A comparison of normalization methods for high density
oligonucleotide array data based on variance and bias.
Bioinformatics. 19:185–193. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Irizarry RA, Hobbs B, Collin F,
Beazer-Barclay YD, Antonellis KJ, Scherf U and Speed TP:
Exploration, normalization, and summaries of high density
oligonucleotide array probe level data. Biostatistics. 4:249–264.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Smyth GK: Limma: Linear models for
microarray data, in Bioinformatics and computational biology
solutions using R and Bioconductor. Springer. 397–420. 2005.
|
|
15
|
Liu H, Xu R, Liu X, Sun R and Wang Q:
Bioinformatics analysis of gene expression in
peripheralbloodmononuclearcells from children with type 1 diabetes
in 3 periods. Exp Clin Endocrinol Diabetes. 122:477–483. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
da Huang W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2008. View Article : Google Scholar
|
|
17
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: Gene Ontology: Tool for the unification of biology. Nat
Genet. 25:25–29. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kanehisa M and Goto S: KEGG: Kyoto
encyclopedia of genes and genomes. Nucleic Acids Res. 28:27–30.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bindea G, Mlecnik B, Hackl H, Charoentong
P, Tosolini M, Kirilovsky A, Fridman WH, Pagès F, Trajanoski Z and
Galon J: ClueGO: A Cytoscape plug-in to decipher functionally
grouped gene ontology and pathway annotation networks.
Bioinformatics. 25:1091–1093. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Szklarczyk D, Franceschini A, Wyder S,
Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos
A, Tsafou KP, et al: STRING v10: Protein-protein interaction
networks, integrated over the tree of life. Nucleic Acids Res.
43:D447–D452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bandettini WP, Kellman P, Mancini C,
Booker OJ, Vasu S, Leung SW, Wilson JR, Shanbhag SM, Chen MY and
Arai AE: MultiContrast Delayed Enhancement (MCODE) improves
detection of subendocardial myocardial infarction by late
gadolinium enhancement cardiovascular magnetic resonance: A
clinical validation study. J Cardiovasc Magn Reson. 14:832012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lamb J, Crawford ED, Peck D, Modell JW,
Blat IC, Wrobel MJ, Lerner J, Brunet JP, Subramanian A, Ross KN, et
al: The Connectivity Map: Using gene-expression signatures to
connect small molecules, genes, and disease. Science.
313:1929–1935. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lamb J: The connectivity map: A new tool
for biomedical research. Nat Rev Cancer. 7:54–60. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang B, Kirov S and Snoddy J: WebGestalt:
An integrated system for exploring gene sets in various biological
contexts. Nucleic Acids Res. 33:W741–W748. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chang TY, Dvorak CC and Loh ML: Bedside to
bench in juvenile myelomonocytic leukemia: Insights into
leukemogenesis from a rare pediatric leukemia. Blood.
124:2487–2497. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sakashita K, Kato I, Daifu T, Saida S,
Hiramatsu H, Nishinaka Y, Ebihara Y, Ma F, Matsuda K, Saito S, et
al: In vitro expansion of CD34+CD38− cells
under stimulation with hematopoietic growth factors on AGM-S3 cells
in juvenile myelomonocytic leukemia. Leukemia. 29:606–614. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gualtieri RJ, Castleberry RP, Gibbons J,
Miller DM, Berkow RL, Parmley RT and Banks J: Cell culture studies
and oncogene expression in juvenile chronic myelogenous leukemia.
Exp Hematol. 16:613–619. 1988.PubMed/NCBI
|
|
29
|
Gandre-Babbe S, Paluru P, Aribeana C, Chou
ST, Bresolin S, Lu L, Sullivan SK, Tasian SK, Weng J, Favre H, et
al: Patient-derived induced pluripotent stem cells recapitulate
hematopoietic abnormalities of juvenile myelomonocytic leukemia.
Blood. 121:4925–4929. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hadland BK, Huppert SS, Kanungo J, Xue Y,
Jiang R, Gridley T, Conlon RA, Cheng AM, Kopan R and Longmore GD: A
requirement for Notch1 distinguishes 2 phases of definitive
hematopoiesis during development. Blood. 104:3097–3105. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kobayashi M, Shelley WC, Seo W, Vemula S,
Lin Y, Liu Y, Kapur R, Taniuchi I and Yoshimoto M: Functional B-1
progenitor cells are present in the hematopoietic stem
cell-deficient embryo and depend on Cbfβ for their development.
Proc Natl Acad Sci USA. 111:12151–12156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin Y, Yoder MC and Yoshimoto M: Lymphoid
progenitor emergence in the murine embryo and yolk sac precedes
stem cell detection. Stem Cells Dev. 23:1168–1177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yoshimoto M, Montecino-Rodriguez E,
Ferkowicz MJ, Porayette P, Shelley WC, Conway SJ, Dorshkind K and
Yoder MC: Embryonic day 9 yolk sac and intra-embryonic hemogenic
endothelium independently generate a B-1 and marginal zone
progenitor lacking B-2 potential. Proc Natl Acad Sci USA.
108:1468–1473. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yoshimoto M, Porayette P, Glosson NL,
Conway SJ, Carlesso N, Cardoso AA, Kaplan MH and Yoder MC:
Autonomous murine T-cell progenitor production in the
extra-embryonic yolk sac before HSC emergence. Blood.
119:5706–5714. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mastrangelo MJ, Maguire HC Jr, Eisenlohr
LC, Laughlin CE, Monken CE, McCue PA, Kovatich AJ and Lattime EC:
Intratumoral recombinant gm-csf-encoding virus as gene therapy in
patients with cutaneous melanoma. Cancer Gene Ther. 6:409–422.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
McNeel DG, Chen YH, Gulley JL, Dwyer AJ,
Madan RA, Carducci MA and DiPaola RS: Randomized phase II trial of
docetaxel with or without PSA-TRICOM vaccine in patients with
castrate-resistant metastatic prostate cancer: A trial of the
ECOG-ACRIN cancer research group (E1809). Hum Vaccin Immunother.
11:2469–2474. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Oudard S, Rixe O, Beuselinck B, Linassier
C, Banu E, Machiels JP, Baudard M, Ringeisen F, Velu T,
Lefrere-Belda MA, et al: A phase II study of the cancer vaccine
TG4010 alone and in combination with cytokines in patients with
metastatic renal clear-cell carcinoma: Clinical and immunological
findings. Cancer Immunol Immunother. 60:261–271. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ottolino-Perry K, Acuna SA, Angarita FA,
Sellers C, Zerhouni S, Tang N and McCart JA: Oncolytic vaccinia
virus synergizes with irinotecan in colorectal cancer. Mol. Oncol.
9:1539–1552. 2015.
|
|
39
|
Quoix E, Lena H, Losonczy G, Forget F,
Chouaid C, Papai Z, Gervais R, Ottensmeier C, Szczesna A,
Kazarnowicz A, et al: TG4010 immunotherapy and first-line
chemotherapy for advanced non-small-cell lung cancer (time):
Results from the phase 2b part of a randomised, double-blind,
placebo-controlled, phase 2B/3 trial. Lancet Oncol. 17:212–223.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Foy SP, Sennino B, dela Cruz T, Cote JJ,
Gordon EJ, Kemp F, Xavier V, Franzusoff A, Rountree RB and Mandl
SJ: Poxvirus-based active immunotherapy with PD-1 and LAG-3 dual
immune checkpoint inhibition overcomes compensatory immune
regulation, yielding complete tumor regression in mice. PLoS One.
11:e01500842016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Foy SP, Mandl SJ, dela Cruz T, Cote JJ,
Gordon EJ, Trent E, Delcayre A, Breitmeyer J, Franzusoff A and
Rountree RB: Poxvirus-based active immunotherapy synergizes with
CTLA-4 blockade to increase survival in a murine tumor model by
improving the magnitude and quality of cytotoxic T cells. Cancer
Immunol Immunother. 65:537–549. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cappuccini F, Stribbling S, Pollock E,
Hill AV and Redchenko I: Immunogenicity and efficacy of the novel
cancer vaccine based on simian adenovirus and MVA vectors alone and
in combination with PD-1 mAb in a mouse model of prostate cancer.
Cancer Immunol Immunother. 65:701–713. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dimberg A: Chemokines in angiogenesis.
Curr Top Microbiol Immunol. 341:59–80. 2010.PubMed/NCBI
|
|
44
|
Speyer CL and Ward PA: Role of endothelial
chemokines and their receptors during inflammation. J Invest Surg.
24:18–27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ben-Baruch A: The multifaceted roles of
chemokines in malignancy. Cancer Metastasis Rev. 25:357–371. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Luther SA and Cyster JG: Chemokines as
regulators of T cell differentiation. Nat Immunol. 2:102–107. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Vogelstein B, Papadopoulos N, Velculescu
VE, Zhou S, Diaz LA Jr and Kinzler KW: Cancer genome landscapes.
Science. 339:1546–1558. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cancer Genome Atlas Research Network, .
Weinstein JN, Collisson EA, Mills GB, Shaw KR, Ozenberger BA,
Ellrott K, Shmulevich I, Sander C and Stuart JM: The cancer genome
atlas pan-cancer analysis project. Nat Genet. 45:1113–1120. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
International Cancer Genome Consortium, .
Hudson TJ, Anderson W, Artez A, Barker AD, Bell C, Bernabé RR, Bhan
MK, Calvo F, Eerola I, Gerhard DS, et al: International network of
cancer genome projects. Nature. 464:993–998. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Braun S, Vogl FD, Naume B, Janni W,
Osborne MP, Coombes RC, Schlimok G, Diel IJ, Gerber B, Gebauer G,
et al: A pooled analysis of bone marrow micrometastasis in breast
cancer. N Engl J Med. 353:793–802. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Janni W, Vogl FD, Wiedswang G, Synnestvedt
M, Fehm T, Juckstock J, Borgen E, Rack B, Braun S, Sommer H, et al:
Persistence of disseminated tumor cells in the bone marrow of
breast cancer patients predicts increased risk for relapse-a
European pooled analysis. Clin Cancer Res. 17:2967–2976. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Budowle B, Allard MW, Wilson MR and
Chakraborty R: Forensics and mitochondrial DNA: Applications,
debates, and foundations. Ann Rev Genom Hum Genet. 4:119–141. 2003.
View Article : Google Scholar
|
|
54
|
International HapMap Consortium, . Frazer
KA, Ballinger DG, Cox DR, Hinds DA, Stuve LL, Gibbs RA, Belmont JW,
Boudreau A, Hardenbol P, Leal SM, et al: A second generation human
haplotype map of over 3.1 million SNPs. Nature. 449:851–861. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Cheng X, Chen G and Rodriguez WR:
Micro-and nanotechnology for viral detection. Anal Bioanal Chem.
393:487–501. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Pinkel D, Segraves R, Sudar D, Clark S,
Poole I, Kowbel D, Collins C, Kuo WL, Chen C, Zhai Y, et al: High
resolution analysis of DNA copy number variation using comparative
genomic hybridization to microarrays. Nat Genet. 20:207–211. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sozzi G, Conte D, Mariani L, Lo Vullo S,
Roz L, Lombardo C, Pierotti MA and Tavecchio L: Analysis of
circulating tumor DNA in plasma at diagnosis and during follow-up
of lung cancer patients. Cancer Res. 61:4675–4678. 2001.PubMed/NCBI
|
|
58
|
Bhowmick NA, Neilson EG and Moses HL:
2004. Stromal fibroblasts in cancer initiation and progression.
Nature. 432:332–337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sternlicht MD, Lochter A, Sympson CJ, Huey
B, Rougier JP, Gray JW, Pinkel D, Bissell MJ and Werb Z: The
stromal proteinase MMP3/stromelysin-1 promotes mammary
carcinogenesis. Cell. 98:137–146. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Paszek MJ, Zahir N, Johnson KR, Lakins JN,
Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M,
Boettiger D, et al: Tensional homeostasis and the malignant
phenotype. Cancer Cell. 8:241–254. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Erler JT, Bennewith KL, Nicolau M,
Dornhöfer N, Kong C, Le QT, Chi JT, Jeffrey SS and Giaccia AJ:
Lysyl oxidase is essential for hypoxia-induced metastasis. Nature.
440:1222–1226. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Levental KR, Yu H, Kass L, Lakins JN,
Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, et
al: Matrix crosslinking forces tumor progression by enhancing
integrin signaling. Cell 139. 1–906. 2009.
|
|
63
|
Hynes RO: The extracellular matrix: Not
just pretty fibrils. Science. 326:1216–1219. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wiseman BS, Sternlicht MD, Lund LR,
Alexander CM, Mott J, Bissell MJ, Soloway P, Itohara S and Werb Z:
Site-specific inductive and inhibitory activities of MMP-2 and
MMP-3 orchestrate mammary gland branching morphogenesis. J Cell
Biol. 162:1123–1133. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Stickens D, Behonick DJ, Ortega N, Heyer
B, Hartenstein B, Yu Y, Fosang AJ, Schorpp-Kistner M, Angel P and
Werb Z: Altered endochondral bone development in matrix
metalloproteinase 13-deficient mice. Development. 131:5883–5895.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Rebustini IT, Myers C, Lassiter KS, Surmak
A, Szabova L, Holmbeck K, Pedchenko V, Hudson BG and Hoffman MP:
MT2-MMP-dependent release of collagen IV NC1 domains regulates
submandibular gland branching morphogenesis. Dev Cell. 17:482–493.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Lu P, Takai K, Weaver VM and Werb Z:
Extracellular matrix degradation and remodeling in development and
disease. Cold Spring Harb Perspect Biol. 3:a0050582011. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Cox TR and Erler JT: Remodeling and
homeostasis of the extracellular matrix: Implications for fibrotic
diseases and cancer. Dis Model Mech. 4:165–178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kitamura T, Qian BZ and Pollard JW: Immune
cell promotion of metastasis. Nat Rev Immunol. 15:73–86. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
DeNardo DG and Coussens LM: Inflammation
and breast cancer. Balancing immune response: Crosstalk between
adaptive and innate immune cells during breast cancer progression.
Breast Cancer Res. 9:2122007. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hatzoglou A, Ouafik L, Bakogeorgou E,
Thermos K and Castanas E: Morphine cross-reacts with somatostatin
receptor SSTR2 in the T47D human breast cancer cell line and
decreases cell growth. Cancer Res. 55:5632–5636. 1995.PubMed/NCBI
|
|
73
|
Rasmussen M, Zhu W, Tønnesen J, Cadet P,
Tønnesen E and Stefano GB: Effects of morphine on tumor growth.
Neuro Endocrinol Lett. 23:193–198. 2002.PubMed/NCBI
|
|
74
|
Mayer TU, Kapoor TM, Haggarty SJ, King RW,
Schreiber SL and Mitchison TJ: Small molecule inhibitor of mitotic
spindle bipolarity identified in a phenotype-based screen. Science.
286:971–974. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Crews CM and Mohan R: Small-molecule
inhibitors of the cell cycle. Curr Opin Chem Biol. 4:47–53. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Liu X, Gong H and Huang K: Oncogenic role
of kinesin proteins and targeting kinesin therapy. Cancer Sci.
104:651–656. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Kapoor TM, Mayer TU, Coughlin ML and
Mitchison TJ: Probing spindle assembly mechanisms with monastrol, a
small molecule inhibitor of the mitotic kinesin, Eg5. J Cell Biol.
150:975–988. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
DeBonis S, Simorre JP, Crevel I, Lebeau L,
Skoufias DA, Blangy A, Ebel C, Gans P, Cross R, Hackney DD, et al:
Interaction of the mitotic inhibitor monastrol with human kinesin
Eg5. Biochemistry. 42:338–349. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Cochran JC and Gilbert SP: ATPase
mechanism of Eg5 in the absence of microtubules: Insight into
microtubule activation and allosteric inhibition by monastrol.
Biochemistry. 44:16633–16648. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Haque SA, Hasaka TP, Brooks AD, Lobanov PV
and Baas PW: Monastrol, a prototype anti-cancer drug that inhibits
a mitotic kinesin, induces rapid bursts of axonal outgrowth from
cultured postmitotic neurons. Cell Motil Cytoskeleton. 58:10–16.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Maliga Z, Kapoor TM and Mitchison TJ:
Evidence that monastrol is an allosteric inhibitor of the mitotic
kinesin Eg5. Chem Biol. 9:989–996. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Müller C, Gross D, Sarli V, Gartner M,
Giannis A, Bernhardt G and Buschauer A: Inhibitors of kinesin Eg5:
Antiproliferative activity of monastrol analogues against human
glioblastoma cells. Cancer Chemother Pharmacol. 59:157–164. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Cascinu S, Bichisao E, Amadori D,
Silingardi V, Giordani P, Sansoni E, Luppi G, Catalano V,
Agostinelli R and Catalano G: High-dose loperamide in the treatment
of 5-fluorouracil-induced diarrhea in colorectal cancer patients.
Support Care Cancer. 8:65–67. 2000.PubMed/NCBI
|
|
84
|
Vail DM: Supporting the veterinary cancer
patient on chemotherapy: Neutropenia and gastrointestinal toxicity.
Top Companion Anim Med. 24:122–129. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Gong XW, Xu YH, Chen XL and Wang YX:
Loperamide, an antidiarrhea drug, has antitumor activity by
inducing cell apoptosis. Pharmacol Res. 65:372–378. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Ohman R and Axelsson R: Relationship
between prolactin response and antipsychotic effect of thioridazine
in psychiatric-patients. Eur J Clin Pharmacol. 14:111–116. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Realmuto GM, Erickson WD, Yellin AM,
Hopwood JH and Greenberg LM: Clinical comparison of thiothixene and
thioridazine in schizophrenic adolescents. Am J Psychiatry.
141:440–442. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
van Soolingen D, Hernandez-Pando R, Orozco
H, Aguilar D, Magis-Escurra C, Amaral L, van Ingen J and Boeree MJ:
The antipsychotic thioridazine shows promising therapeutic activity
in a mouse model of multidrug-resistant tuberculosis. PLoS One.
5:e126402010. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Thorsing M, Klitgaard JK, Atilano ML, Skov
MN, Kolmos HJ, Filipe SR and Kallipolitis BH: Thioridazine induces
major changes in global gene expression and cell wall composition
in methicillin-resistant Staphylococcus aureus USA300. PLoS One.
8:e645182013. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Byun HJ, Lee JH, Kim BR, Kang S, Dong SM,
Park MS, Lee SH, Park SH and Rho SB: Anti-angiogenic effects of
thioridazine involving the FAK-mTOR pathway. Microvasc Res.
84:227–234. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Park MS, Dong SM, Kim BR, Seo SH, Kang S,
Lee EJ, Lee SH and Rho SB: Thioridazine inhibits angiogenesis and
tumor growth by targeting the VEGFR-2/PI3K/mTOR pathway in ovarian
cancer xenografts. Oncotarget. 5:4929–4934. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Kang S, Dong SM, Kim BR, Park MS, Trink B,
Byun HJ and Rho SB: Thioridazine induces apoptosis by targeting the
PI3K/Akt/mTOR pathway in cervical and endometrial cancer cells.
Apoptosis. 17:989–997. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Csonka Á, Spengler G, Martins A, Ocsovszki
I, Christensen JB, Hendricks O, Kristiansen JE, Amaral L and Molnar
J: Effect of thioridazine stereoisomers on the drug accumulation of
mouse lymphoma and human prostate cancer cell lines in vitro. In
Vivo. 27:815–820. 2013.PubMed/NCBI
|
|
94
|
Mu J, Xu H, Yang Y, Huang W, Xiao J, Li M,
Tan Z, Ding Q, Zhang L, Lu J, et al: Thioridazine, an antipsychotic
drug, elicits potent antitumor effects in gastric cancer. Oncol
Rep. 31:2107–2114. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Nagel D, Spranger S, Vincendeau M, Grau M,
Raffegerst S, Kloo B, Hlahla D, Neuenschwander M, von Kries Peter
J, Hadian K, et al: Pharmacologic inhibition of MALT1 protease by
phenothiazines as a therapeutic approach for the treatment of
aggressive ABC-DLBCL. Cancer Cell. 22:825–837. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Sachlos E, Risueño RM, Laronde S,
Shapovalova Z, Lee JH, Russell J, Malig M, McNicol JD, Fiebig-Comyn
A, Graham M, et al: Identification of drugs including a dopamine
receptor antagonist that selectively target cancer stem cells.
Cell. 149:1284–1297. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Ke XY, Lin Ng VW, Gao SJ, Tong YW, Hedrick
JL and Yang YY: Co-delivery of thioridazine and doxorubicin using
polymeric micelles for targeting both cancer cells and cancer stem
cells. Biomaterials. 35:1096–1108. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Nudelman A, Ruse M, Aviram A, Rabizadeh E,
Shaklai M, Zimrah Y and Rephaeli A: Novel anticancer prodrugs of
butyric acid. 2. J Med Chem. 35:687–694. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Wu CH, Jeng JH, Wang YJ, Tseng CJ, Liang
YC, Chen CH, Lee HM, Lin JK, Lin CH, Lin SY, et al: Antitumor
effects of miconazole on human colon carcinoma xenografts in nude
mice through induction of apoptosis and G0/G1 cell cycle arrest.
Toxicol Appl Pharmacol. 180:22–35. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Lewinska A, Adamczyk-Grochala J,
Kwasniewicz E, Deregowska A and Wnuk M: Ursolic acid-mediated
changes in glycolytic pathway promote cytotoxic autophagy and
apoptosis in phenotypically different breast cancer cells.
Apoptosis. 22:800–815. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Jiao Y, Hannafon BN, Zhang RR, Fung KM and
Ding WQ: Docosahexaenoic acid and disulfiram act in concert to kill
cancer cells: A mutual enhancement of their anticancer actions.
Oncotarget. 8:17908–17920. 2017. View Article : Google Scholar : PubMed/NCBI
|