Introduction
Cutaneous squamous cell carcinoma (cSCC) is the
second most common non-melanoma skin cancer (NMSC) and accounts for
20–50% of skin cancers (1,2). An overall 263% increase in the
incidence of cSCC between 1976 and 1984, and between 2000 and 2010,
was reported by the Rochester Epidemiology Project, conducted by
the Mayo Clinic (3). Metastatic
cSCC is lethal, with a mortality rate of 70% as demonstrated by
several large studies (4). Among
all of the risk factors, UV radiation is the most detrimental.
Tumors most commonly arise on sun-exposed skin in fair-skinned
individuals who are at the highest risk, especially the head, neck,
and backs of the arms and hands (4). UV radiation acts as a carcinogen both
directly, by inducing cell damage, such as DNA mutations, and
indirectly, by inducing immunosuppression (5). Therefore, the development of cSCC is
closely associated with genomic perturbations, genetic mutations
and the altered expression of key molecules, through a highly
complex pathological process involving many dynamic and interacting
biological processes and molecular pathways.
In recent years, with the rising incidence of cSCC
and increasing expectations of patients, the selection of an
appropriate treatment to maximize the preservation of function and
minimize the recurrence and metastasis rate remains a challenge. In
the clinic, surgical excision is considered the gold standard in
the treatment of cSCC. Non-surgical approaches, including
photodynamic therapy (PDT), radiation therapy, cryotherapy and
chemotherapy, are widely used in late-stage cSCC (6,7).
However, research into drug therapy remains limited, and further
studies are needed to provide a rationale for the development of
novel therapeutic modalities.
While the efficacy of the available drug therapies
is limited and discovering new drug therapies using traditional
methods is likely to take a long time, drug repositioning may speed
up the process of discovering other conditions that existing drugs
could treat more effectively, and may be less expensive (8). This study aimed to investigate new
drug therapies for cSCC by using computational methods, including
text mining, biological process and pathway analysis,
protein-protein interaction (PPI) analysis to mine public
databases, and bioinformatics tools to systematically identify
interaction networks between drugs and gene targets (9,10). By
using data analytical tools, we were able to analyze the
characteristics of candidate genes for the purpose of drug
selection. Based on the drug-gene interaction analysis of the final
genes, candidate drugs were then derived.
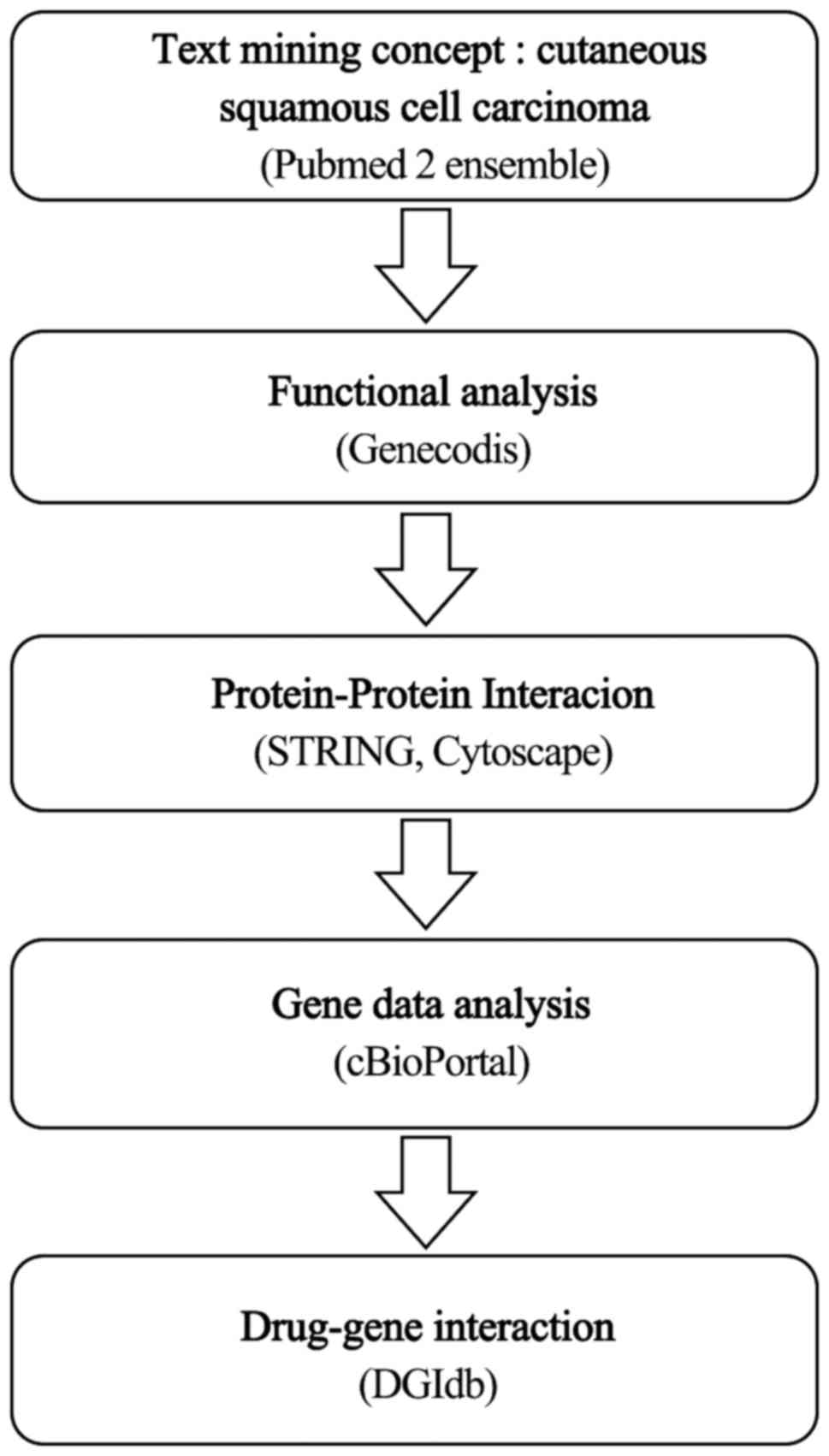
Materials and methods
Text mining
Text mining makes it possible to collect
disease-gene associations automatically from large volumes of
biological literature. We used pubmed2ensembl (http://pubmed2ensembl.ls.manchester.ac.uk/) to perform
text mining. Pubmed2ensembl has been developed as an extension to
the BioMart system that links over 2,000,000 articles in PubMed to
nearly 150,000 genes in Ensembl from 50 species (11). Pubmed2ensembl provides links between
the literature and genes for data exploration, which means that
when queries are performed, Pubmed2ensembl extracts all of the
genes related to the search concepts from the biological literature
available. In this study, we performed the query with the concept
‘cutaneous squamous cell carcinoma’ (cSCC). We chose ‘Homo
sapiens’ as the species dataset, then selected ‘Ensembl Gene
ID’ and ‘Associated Gene Name’ under GENE and deselected ‘MEDLINE:
PubMed ID’ under PUBMED2ENSEMBL FEATURES. After entering ‘cutaneous
squamous cell carcinoma’ in the search box, we selected the ‘search
for PubMed IDs’ and ‘filter on Entrez: PMID’ drop-down menus. Then,
the query returned all the gene hits that were used in the next
step.
Biological process and pathway
analysis
GeneCodis (http://genecodis.cnb.csic.es/) is a web-based tool
that integrates various sources of information to search for
annotations that frequently co-occur in a group of genes and rank
them by statistical significance, which is essential in the
biological interpretation of high-throughput experiments (12). We used GeneCodis for an enrichment
analysis of the genes related to cSCC. We put the genes from the
text mining step into the input set and analyzed this set of genes
using the GO biological process categories. The most significantly
enriched biological processes were selected. Genes with the
selected annotations were used for the next step, which was an
additional GeneCodis analysis performed with annotations of the
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways. Among
these highly enriched pathways above the P-value cutoff, those most
relevant to cSCC pathology were selected. Genes belonging to the
selected pathways were used for further analysis.
Protein interaction network
The STRING database (http://string-db.org) integrates the protein-protein
interactions of selected genes (13). In the first page of the STRING
database, we selected ‘Multiple proteins’ from the left menu bar,
entered the genes selected from the previous step, and ‘Homo
sapiens’ was selected as the organism. With respect to the
confidence score, the stronger the evidence that two proteins
interact with each other is, the higher the confidence scores
observed. However, although a lower confidence score may decrease
the confidence of the network, given the fact that it may broaden
the inclusion criteria, the confidence score was set to medium
(score 0.400) in this study. Then, the protein-protein interaction
network of the target genes was obtained.
Next, we used the Cytoscape software platform to
visualize and analyze the interaction network. Cytoscape is a
software tool for the visual exploration of biomedical networks
composed of proteins, genes and other types of interactions,
supported by diverse annotations and experimental data (14). We imported data in the format ‘.tsv’
from the STRING EXPORT channel. CentiScaPe, an app which calculates
a larger number of network parameters, was then used to analyze the
topological characteristics of each node and to select the key
nodes. We chose ‘Degree’ and ‘Betweenness’ as parameters to select
the key genes. The higher the Degree of the node, the greater the
number of gene products that interact with each other, which means
the greater the role that the node plays in cSCC. The Betweenness
value of the node indicates the tendency of a node to connect to
the core of other nodes. In this study, the criteria for the
selection of key genes was set in such a way that the nodes for
which Degree and Betweenness were both greater than or equal to the
mean were the key nodes. Candidate genes were selected for final
analysis in the next step.
Gene data analysis
The cBioPortal for cancer genomics (http://cbioportal.org) is a web-based tool for
exploring, visualizing and analyzing multidimensional cancer
genomics data. Datasets in the portal are from 10 published cancer
studies and for each tumor sample, data may be available from
multiple genomic analysis platforms (15). We selected ‘Cutaneous squamous cell
carcinoma’ as the filter, and then performed the query with the
final list of genes from the previous step as the input set.
Results were described in each tab, including OncoPrint, Mutations,
Survival and Network, which allowed us to explore genomic
alterations and provided a graphical summary of the mutations
identified in each query gene, survival analysis, patient-centric
queries, and network visualization and analysis.
Drug-gene interactions
We used DGIdb (http://www.dgidb.org) to explore drug-gene
interactions in the final list of genes, which were used as the
potential targets in a search for existing drugs (16). At least one drug was found to
correspond to each gene. These candidate drugs targeting the
genes/pathways relevant to cSCC may represent potential
treatments.
Results
Results of text mining, biological
process and pathway analysis
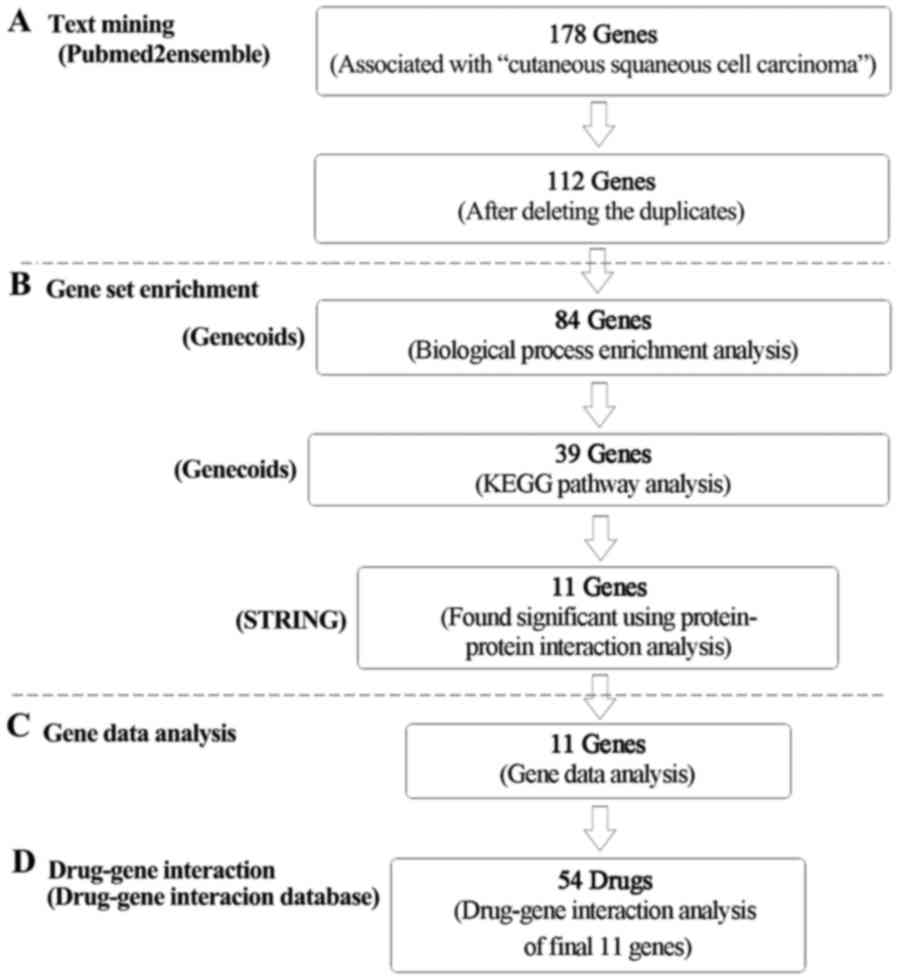
In the process of exploring potential drugs for cSCC
(Fig. 1), 178 genes were found to
be related to cSCC from the text mining searches. After deleting
the duplicates, 121 genes were left (Fig. 2). GeneCodis gene enrichment analysis
was first performed using biological processes analysis to find the
most enriched terms related to cSCC pathology. During this process,
to make sure that only the most enriched annotations were selected,
a P-value cutoff (P=1.00E-06) was set. Among the most significantly
enriched biological processes above the cutoff, those most relevant
to cSCC pathology based on the available literature and research
were selected. Therefore, the analysis of enriched biological
process annotations resulted in 21 sets of annotations containing
84 genes (Table I). The five most
enriched biological process annotations were: i) ‘Negative
regulation of apoptotic process’ (P=3.94548E-14); ii) ‘positive
regulation of cell proliferation’ (P=1.70535E-12); iii)
‘anti-apoptosis’ (P=5.19303E-10); iv) ‘positive regulation of
transcription from RNA polymerase II promoter’ (P=1.04587E-09); and
v) ‘negative regulation of transcription from RNA polymerase II
promoter’ (P=1.06495E-09), containing 17, 17, 12, 17 and 15 genes
from the query set, respectively. Other highly enriched biological
process annotations included ‘response to cytokine stimulus’,
‘positive regulation of ERK1 and ERK2 cascade’, ‘positive
regulation of MAPK cascade’ and ‘apoptotic process’.
 | Table I.Summary of biological process gene
set enrichment analysis. |
Table I.
Summary of biological process gene
set enrichment analysis.
| Process | Genes in query
set | Total genes in
genome | Corrected
hypergeo-metric P-value | Genes |
|---|
| Negative regulation
of apoptotic process | 17 | 272 | 3.94548E-14 | ERBB2, CD44,
THBS1, CDKN1A, RPS6KB1, MDM2, NOTCH1, TP53, BCL2L1, AKT2, AKT1,
BRAF, IGF1R, MYC, EGFR, TIMP1, RELA |
| Positive regulation
of cell proliferation | 17 | 361 | 1.70535E-12 | FGFR2, HRAS,
ERBB2, FGFR4, CCND1, MDM2, NOTCH1, BCL2L1, FOXM1, FGFR3, TNFSF13,
IGF1R, MYC, EGFR, TIMP1, RELA, ITGB1 |
| Anti-apoptosis | 12 | 200 | 5.19303E-10 | IL1A, TERT,
NFKB1, THBS1, BCL2L1, FAS, AKT1, BRAF, TP63, IGF1R, BIRC5,
RELA |
| Positive regulation
of transcription from | 17 | 578 | 1.04587E-09 | FGFR2, IL1A,
NFKB1, EGR1, FOXE1, TP53, VDR, FOXP3, FOXM1, AKT1, |
| RNA polymerase II
promoter |
|
|
| AKT2, TP63, MYC,
MAPK14, KLF4, STAT3, RELA |
| Negative regulation
of transcription from | 15 | 416 | 1.06495E-09 | FGFR2, NFKB1,
EGR1, MDM2, FOXE1, NOTCH1, SKIL, TP53, VDR, |
| RNA poly merase II
promoter |
|
|
| FOXM1, ID1,
TP63, MYC, KLF4, STAT3 |
| Negative regulation
of cell proliferation | 14 | 341 | 1.07819E-09 | FGFR2, IL1A,
HRAS, CDKN1A, CDKN2B, TP53, VDR, FOXP3, CDH13, TIMP2, E2F7, KLF4,
IGFBP3, IGFBP3 |
| Response to
cytokine stimulus | 8 | 62 | 1.88982E-09 | CDKN2B, AANAT,
BCL2L1, FAS, JUNB, TIMP1, STAT3, RELA |
| Inflammatory
response | 12 | 259 | 3.37207E-09 | IL1A, NFKB1,
THBS1, LY75, CD163, PDPN, P2RX7, AXL, AKT1, SELE, PLAA,
RELA |
| Positive regulation
of ERK1 and ERK2 cascade | 8 | 70 | 3.43595E-09 | FGFR2, IL1A,
HRAS, CD44, FGFR4, FGFR3, BRAF, SRC |
| Positive regulation
of transcription, | 15 | 468 | 3.50444E-09 | BRCA2, NFKB1,
EGR1, FOXE1, NOTCH1, TP53, FOXP3, FOXM1, TP63, |
| DNA-dependent |
|
|
| CDH1, CDKN2A,
MYC, KLF4, STAT3, RELA |
| Cell adhesion | 16 | 556 | 3.61519E-09 | L1CAM, MCAM,
COL7A1, CD44, THBS1, COL19A1, VCAM1, SELE, CDH13, CDH13, SRC,
LAMB3, DSG2, CDH3, COL12A1, ITGB1 |
| Epidermis
development | 8 | 81 | 8.50065E-09 | COL7A1, FOXN1,
NOTCH1, FABP5, KRT2, KRT5, LAMB3 |
| Cell cycle
arrest | 9 | 129 | 1.30308E-08 | HRAS, THBS1,
CDKN1A, CDKN2B, SKIL, TP53, MSH2, CDKN2A, MYC |
| Signal
transduction | 21 | 1176 | 1.31225E-08 | HRAS, NFKB1,
S100A6, ERBB2, P2RX5, RPS6KB1, VDR, FAS, PDPN, AKT2, TNFSF13, AXL,
AKT1, IGF1R, SRC, PLAA, EGFR, MTA1, MAPK14, MET, STAT3 |
| Response to growth
factor stimulus | 5 | 15 | 1.81127E-08 | P2RY1, SKIL,
FAS, MYC, P2RY2 |
| Positive regulation
of MAPK cascade | 7 | 61 | 2.4874E-08 | FGFR2, HRAS,
ERBB2, P2RX7, FGFR3, IGF1R, ITGB1 |
| Protein
autophosphorylation | 9 | 148 | 3.32277E-08 | FGFR2, ERBB2,
FGFR4, FGFR3, AKT1, IGF1R, EGFR, MAPK14, MET |
| Apoptotic
process | 15 | 594 | 3.38731E-08 | FGFR2, IL1A,
HRAS, NFKB1, TP53, BCL2L1, FAS, AKT1, CLPTM1L, WWOX, ID1, TP63,
BIRC5, BIRC5 |
| Response to
mechanical stimulus | 6 | 45 | 9.94563E-08 | P2RY1, RPS6KB1,
P2RX7, JUNB, RELA, MMP9 |
| Positive regulation
of apoptotic process | 9 | 178 | 1.22103E-07 | NOTCH1, TP53,
BCL2L1, FAS, P2RX7, TOP2A, MMP9, IGFBP3, ITGB1 |
| Cell
proliferation | 11 | 312 | 1.23121E-07 | IL1A, ERBB2,
TP53, PDPN, AREGB, AKT1, UCHL1, EGFR, MCM7, MET, STAT3 |
In the process of KEGG pathway enrichment analysis,
the P-value cutoff was set at 1.00E-05. Among the most
significantly enriched pathway annotations above the cutoff, those
most relevant to cSCC pathology based on the available literature
and research were selected. The analysis of enriched pathway
annotations resulted in 10 pathways containing a total of 39 unique
genes (Table II). The three most
significantly enriched pathways were: i) ‘Pathways in cancer’
(P=3.24886E-35); ii) ‘MAPK signaling pathway’ (P=1.71374E-15); and
iii) ‘ErbB signaling pathway’ (P=2.49437E-15), containing 29, 15
and 11 genes from the query set, respectively. Other highly
enriched pathways included ‘focal adhesion’, ‘p53 signaling
pathway’, ‘apoptosis’, ‘cell cycle’, ‘endocytosis’, ‘VEGF signaling
pathway’ and ‘TGF-β signaling pathway’.
 | Table II.Summary of KEGG process gene set
enrichment analysis. |
Table II.
Summary of KEGG process gene set
enrichment analysis.
| Process | Genes in query
set | Total genes in
genome | Corrected
hypergeometric P-value | Genes |
|---|
| Pathways in
cancer | 29 | 324 | 3.24886e-35 | MSH2, MMP9,
MDM2, MET, BCL2L1, HRAS, BIRC5, ERBB2, 1GF1R, FGFR3, STAT3, CCND1,
FAS, BRAF, CDKN2B, LAMB3, CDKN1A, AKT1, EGFR, NFKB1, MYC, BRCA2,
ITGB1, RELA, CDKN2B, TP53, CDKN2A, TP53, CDH1, AKT2, FGFR2 |
| MAPK signaling
pathway | 15 | 262 | 1.71374e-15 | HRAS, FGFR3,
FAS, IL1A, FGFR4, BRAF, AKT1, EGFR, NFKB1, MYC, MAPK14, RELA, TP53,
AKT2, FGFR2 |
| ErbB signaling
pathway | 11 | 87 | 2.49437e-15 | HRAS, ERBB2,
RPS6KB1, BRAF, SRC, CDKN1A, AKT1, EGFR, MYC, AKT2, AREGB |
| Focal adhesion | 13 | 197 | 2.00274e-14 | MET, HRAS,
ERBB2, IGF1R, CCND1, THBS1, BRAF, SRC, LAMB3, AKT1, EGFR, ITGB1,
AKT2 |
| p53 signaling
pathway | 8 | 67 | 1.49997e-11 | MDM2, IGFBP3,
CCND1, FAS, THBS1, CDKN1A, CDKN2A, TP53 |
| Apoptosis | 8 | 86 | 9.36792e-11 | BCL2L1, FAS,
IL1A, AKT1, NFKB1, RELA, TP53, AKT2 |
| Cell cycle | 8 | 123 | 1.36384e-09 | MDM2, MCM7,
CCND1, CDKN2B, CDKN1A, MYC, CDKN2A, TP53 |
| Endocytosis | 9 | 193 | 2.05544e-09 | MDM2, MET, HRAS,
IGF1R, FGFR3, FGFR4, SRC, EGFR, FGFR2 |
| VEGF signaling
pathway | 5 | 75 | 1.32444e-06 | HRAS, SRC, AKT1,
MAPK14, AKT2 |
| TGF-β signaling
pathway | 5 | 82 | 2.05828e-06 | THBS1, RPS6KB1,
ID1, CDKN2B, MYC |
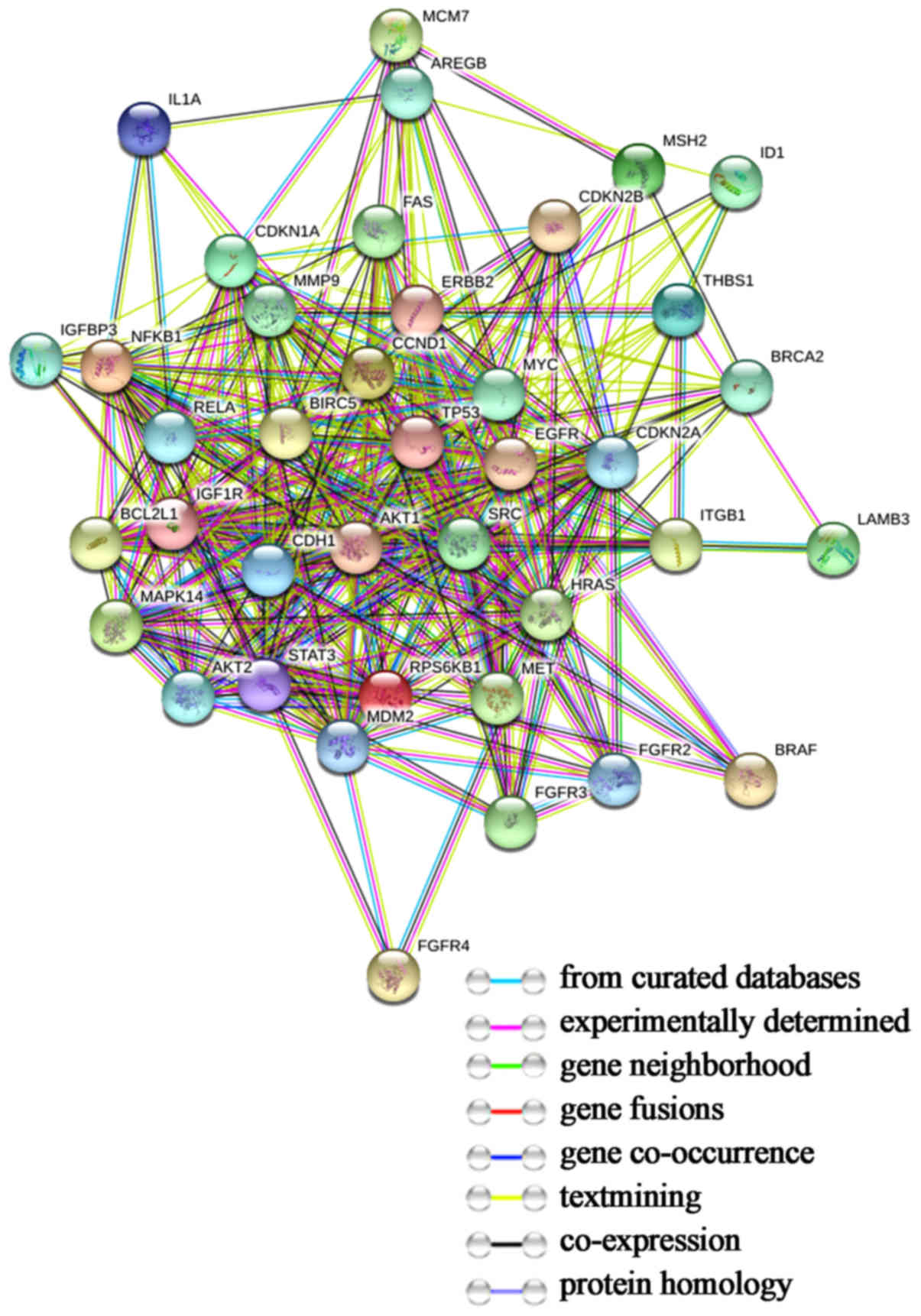
Results of protein-protein
interaction
The protein-protein interaction network of the 39
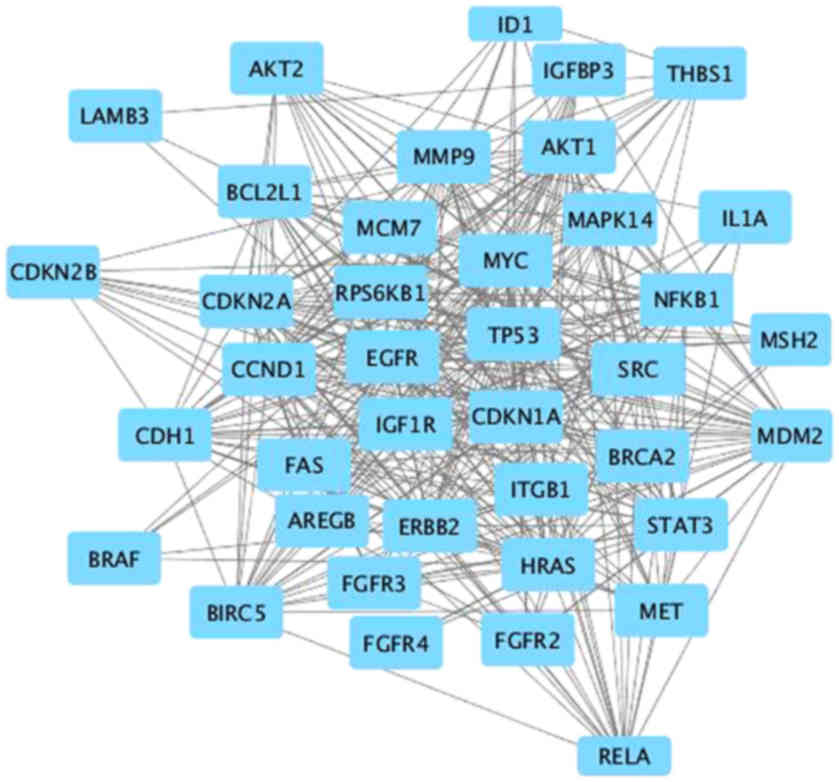
target genes was illustrated using STRING (Fig. 3). We imported data in the format
‘.tsv’ from the STRING EXPORT channel to Cytoscape (Fig. 4). Then, we used CentiScaPe, an app
which calculates a larger number of network parameters, to analyze
the topological characteristics of each node and select the key
nodes. Node Degree represents the total number of edges incident to
the node. In this study, the maximum and minimum Degree values were
34.00 and 3.00 respectively, with an average value of 18.62.
Betweenness centrality for each node refers to the number of
shortest paths that pass through the node. The maximum value was
99.60, with a minimum of 0.00 and an average of 20.10. We set up
the criteria for the selection of key genes so that the nodes for
which Degree and Betweenness were both greater than or equal to the
mean were the key nodes. Eleven genes were selected based on these
criteria, forming a tight interaction network, as determined by
Cytoscape.
Results of gene data analysis
The 11 genes included TP53, MDM2, CCND1, CDKN2A,
HRAS, EGFR, MYC, ERBB2, AKT1, STAT3 and SRC. The results of the
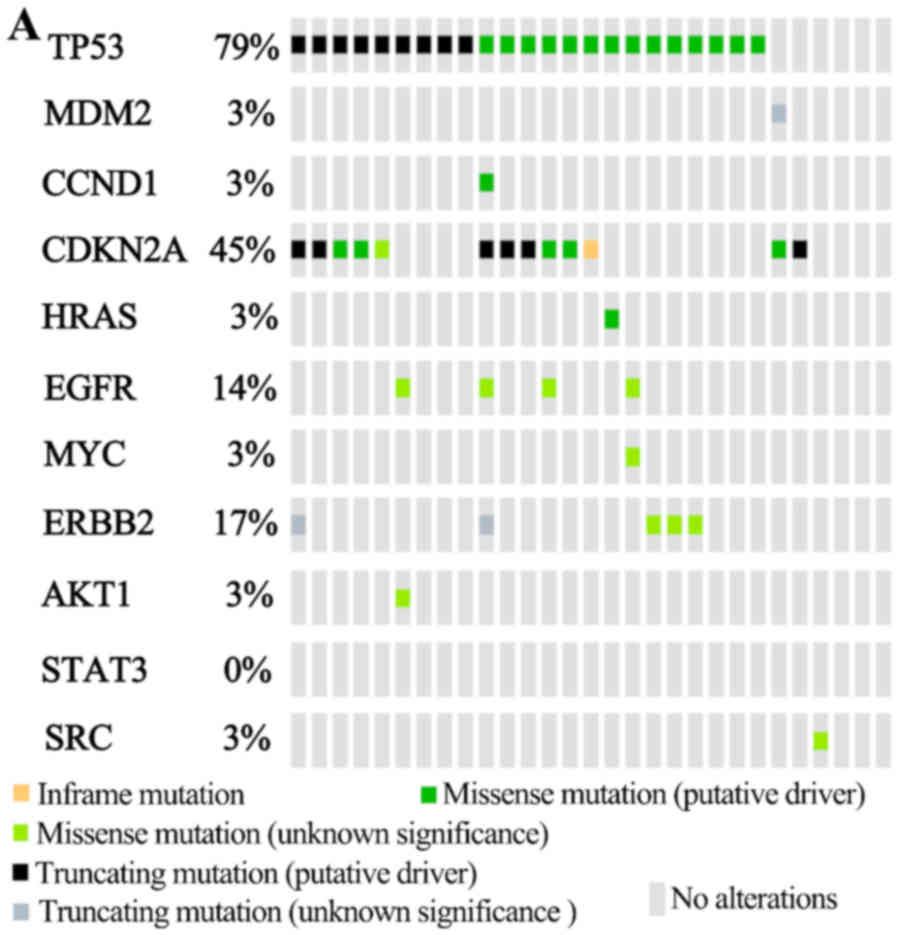
cBioPortal analysis are shown in each tab (Fig. 5). From the OncoPrint, 26 cases (90%)
had an alteration in at least one of the 11 genes, with the
frequency of alteration in each of the 11 selected genes presented
in Fig. 5A. For TP53, most of the
alterations were missense mutations and truncating mutations. The
alterations in MDM2 were truncating mutations with unknown
significance. Events associated with CDKN2A included an in-frame
mutation, and several missense and truncating mutations. The
alterations in CCND1 and HRAS were missense mutations. For EGFR,
MYC, AKT1 and SRC, the alterations were missense mutations with
unknown significance. The alterations in ERBB2 included missense
mutations and truncating mutations with unknown significance. No
alterations occurred in STAT3 in these cases. To further identify
the details of all mutations in each query gene, the Mutations tab
was used. For example, the graphical summary of the mutations
associated with TP53 showed that there were 35 mutations, including
1 duplicate mutation, in patients with multiple samples. In the
Survival tab, results displayed as Kaplan-Meier plots with P-values
are available (Fig. 5B). The
overall survival of cSCC patients with or without alterations in
the query genes are presented with a log rank text P-value of
0.139. The Network tab provides interactive analysis and
visualization of networks consisting of genes, pathways, drugs and
interactions (Fig. 5C). The network
contained 61 nodes, including 11 query genes and the 50 most
frequently altered neighboring genes. By selecting ‘show all
drugs’, a network containing gene-centric drug-target information
was generated. For example, gefitinib and erlotinib, which are
epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors
(TKIs) (EGFR-TKIs) that target the intracellular domain of EGFR,
and cetuximab and panitumumab, which are antibodies that block the
extracellular domain of EGFR and ERBB2, respectively, were shown
with edges connecting them to their targets.
Results of drug-gene interactions
Using the final list of 11 genes as the potential
targets in the drug-gene interaction analysis, a list of 54 drugs
was selected as possible drug treatments for cSCC (Table III). Most of the drugs are used
for cancer treatment, which is in accordance with the fact that the
most enriched pathway was ‘pathways in cancer’. These drugs can be
divided into several major categories, including chemotherapy
agents, tyrosine kinase inhibitors (TKIs), HER2 inhibitors, EGFR
inhibitors, AKT/PI3K inhibitors, mTOR inhibitors, mitogen-activated
protein kinase (MAPK) inhibitors, cyclin-dependent kinase (CDK)
inhibitors, histone deacetylase (HDAC) inhibitors, nonsteroidal
anti-inflammatory drugs (NSAIDs) and others. Fifteen of the 54
drugs were chemotherapy agents. Among these, platins and
5-fluorouracil (5-FU) are used as palliative treatments alone or in
combination with radiotherapy (17). Other drugs which were previously
studied, including capecitabine, carboplatin, doxorubicin,
gemcitabine and methotrexate, are used for either advanced or
metastatic disease, alone or in combination (18). Certain chemotherapy agents work by
disrupting the normal function of microtubules and thereby stopping
cell division, such as docetaxel and vinorelbine (19). Irinotecan serves as a topoisomerase
inhibitor by blocking topoisomerase I, which results in DNA damage
and cell death (20). While
azacitidine, a chemical analog of the nucleoside cytidine, is used
in the treatment of myelodysplastic syndrome (21), most of the drugs had been previously
approved to be used in different types of cancer, including
non-small cell lung cancer (docetaxel), colon and small cell lung
cancer (irinotecan), colorectal cancer (oxaliplatin), and
astrocytoma and glioblastoma multiforme (temozolomide) (19–21).
 | Table III.Candidate drug targeting genes with
cSCC. |
Table III.
Candidate drug targeting genes with
cSCC.
| Number | Drug | Gene | Score | Approved? | Aprroved use in
cSCC? | Reference (Pubmed
ID) |
|---|
| 1 | Azacitidine | MYC | 2 | Yes | No |
9006118 |
| 2 | Capecitabine | ERBB2 | 8 | Yes | No | 17192538 |
| 3 | Carboplatin | TP53 | 7 | Yes | No | 25567130 |
| 4 |
Cyclophosphamide | TP53 | 4 | Yes | No | 17388661 |
| 5 | Docetaxel | ERBB2 | 7 | Yes | Yes | 22149875 |
| 6 | Doxorubicin | TP53 | 10 | Yes | Yes | 25658463 |
| 7 | Fluorouracil | ERBB2 | 4 | Yes | No | 28784859 |
| 8 | Gemcitabine | TP53 | 7 | Yes | No | 27167172 |
| 9 | Irinotecan | EGFR | 3 | Yes | Yes | 23209031 |
| 10 | Oxaliplatin | ERBB2,
TP53 | 4 | Yes | No | 24957073 |
| 11 | Paclitaxel | ERBB2 | 7 | Yes | No | 24886365 |
| 12 | Tegafur | ERBB2 | 2 | Yes | Yes | 24982373 |
| 13 | Temozolomide | EGFR | 3 | Yes | No | 25910950 |
| 14 | Vinorelbine | ERBB2 | 3 | Yes | No | 27992451 |
| 15 | Methotrexate | CCND1 | 2 | Yes | No | 12972956 |
| 16 | Lapatinib | ERBB2 | 91 | Yes | Yes | 26619011 |
| 17 | Afatinib | EGFR | 141 | Yes | Yes | 26619011 |
| 18 | Sorafenib | EGFR | 4 | Yes | No | 23629727 |
| 19 | Sunitinib | EGFR,
MYC | 2 | Yes | No | 27149458 |
| 20 | Nintedanib | SRC | 3 | Yes | No | 18559524 |
| 21 | Masitinib | SRC | 1 | No | No | None found |
| 22 | Ibrutinib | ERBB2 | 4 | Yes | No | 27256378 |
| 23 | Trastuzumab | ERBB2 | 99 | Yes | No | 27900589 |
| 24 | Pertuzumab | ERBB2 | 39 | Yes | No | 17192538 |
| 25 | Dacomitinib | EGFR | 20 | Yes | No | 24857124 |
| 26 | Gefitinib | EGFR | 125 | Yes | No | 24533047 |
| 27 | Cetuximab | EGFR | 110 | Yes | No | 26619011 |
| 28 | Osimertinib | EGFR | 31 | Yes | No | 26522274 |
| 29 | Panitumumab | EGFR | 32 | Yes | No | 17355997 |
| 30 | Erlotinib | EGFR | 167 | Yes | Yes | 26619011 |
| 31 | Dasatinib | TP53, HERAS,
EGFR | 2 | Yes | No | 27222538 |
| 32 | Imatinib | EGFR, MYC,
CDKN2A | 2 | Yes | No | 28514312 |
| 33 | Bosutinib | EGFR | 2 | Yes | No | 28416483 |
| 34 | Ponatinib | EGFR,
ERBB2 | 2 | Yes | No | 22238366 |
| 35 | Ceritinib | SRC | 2 | Yes | No | 25394791 |
| 36 | Crizotinib | EGFR | 6 | Yes | No | 27595477 |
| 37 | Perifosine | AKT1 | 6 | No | No | 25519148 |
| 38 | Omipalisib | AKT1 | 1 | No | No | None found |
| 39 | Apitolisib | AKT1 | 1 | No | No | None found |
| 40 | Ipatasertib | AKT1 | 9 | No | No | 27872130 |
| 41 | Everolimus | ERBB2 | 6 | Yes | No | 21107682 |
| 42 | Sirolimus | EGFR | 8 | Yes | No | 24934779 |
| 43 | Temsirolimus | EGFR, ERBB2,
AKTI | 2 | Yes | No | 24470557 |
| 44 | Trametinib | HERAS,
EGFR | 5 | Yes | No | 27222538 |
| 45 | Doramapimod | SRC | 2 | No | No | 27154914 |
| 46 | Palbociclib | CDKN2A | 18 | Yes | No | 26715889 |
| 47 | Ribociclib | CDKN2A,
CCND1 | 1 | Yes | No | None found |
| 48 | Vorinostat | TP53 | 3 | Yes | No | 26009011 |
| 49 | Indomethacin | MYC | 2 | Yes | No | 10403534 |
| 50 | Sulindac | MYC | 2 | Yes | No | 12414619 |
| 51 | Asprin | TP53 | 2 | Yes | No | 21475861 |
| 52 | Acitretin | STAT3 | 1 | Yes | No | None found |
| 53 | Dimethyl
sulfoxide | HERAS | 2 | Yes | No |
3856429 |
| 54 | Bevacizumab | TP53 | 8 | Yes | No | 27466356 |
Among these candidate drugs, 21 drugs targeted
receptor tyrosine kinases (RTKs) and interacted in signaling
pathways responsible for cell proliferation, survival,
angiogenesis, invasion and metastasis. These drugs included
EGFR-TKIs, HER2 receptor inhibitor, Bruton's TKI (BTKI), Bcr-Abl
TKI, anaplastic lymphoma kinase (ALK) inhibitor, and multi-target
TKIs. Gefitinib and erlotinib are EGFR-TKIs, which block EGFR
signaling selectively through competitive reversible binding at the
intracellular EGFR-TK domain. Cetuximab, a recombinant chimeric
monoclonal antibody that competitively inhibits EGFR, was reported
to be used as a first-line therapy for patients with unresectable
cSCC expressing EGFR in a phase II trial of 36 patients. The
disease control rate was 69% overall, with eight partial responses
and two complete responses (22).
Drugs targeting HER2 included the monoclonal antibodies trastuzumab
and pertuzumab, used for breast cancer that is HER2
receptor-positive (23,24). Ibrutinib, a BTKI, is used to treat
B-cell cancers such as chronic lymphocytic leukemia, mantle cell
lymphoma, and Waldenström's macroglobulinemia (25). TKIs that work by blocking the
Bcr-Abl tyrosine-kinase included dasatinib, imatinib, bosutinib and
ponatinib, which have been used to treat chronic myelogenous
leukemia (CML) and acute lymphocytic leukemia (ALL), Philadelphia
chromosome-positive (Ph+) (26). ALK inhibitors, crizotinib and
ceritinib, were approved for the treatment of some NSCLC cases in
the US and other countries, but have not been formally evaluated in
the setting of cSCC (27). Other
drugs such as masitinib, sorafenib and sunitinib inhibit cellular
signaling by targeting multiple RTKs, including platelet-derived
growth factor (PDGFR), vascular endothelial growth factor receptor
(VEGFR), fibroblast growth factor receptor (FGFR) and RAF family
kinases, which play an important role in both tumor angiogenesis
and tumor cell proliferation (28–30).
PI3K/AKT/mTOR is one of the major signaling pathways
that have been identified to be important in cancer. mTOR is a key
kinase downstream of the PI3K/AKT pathway, which regulates tumor
cell proliferation, growth, angiogenesis and survival (31). Drugs targeting this signaling
pathway include AKT/PI3K inhibitors (perifosine, omipalisib
apitolisib and ipatasertib) and mTOR inhibitors (everolimus,
sirolimus and temsirolimus). Another important pathway, the MAPK
signaling pathway, is closely related to the pathology of cSCC,
which is consistent with the results of the KEGG pathway analysis.
Drugs related to this signaling pathway include the p38-MAPK
inhibitor doramapimod, and the MEK inhibitor trametinib, which was
approved for use in combination with dabrafenib for the treatment
of patients with BRAF V600E/K-mutant metastatic melanoma (32).
Among the list, two of the drugs work as CDK4 and
CDK6 inhibitors, palbociclib and ribociclib, and are used for the
treatment of ER-positive and HER2-negative breast cancer. One drug,
vorinostat, an inhibitor of HDACs, was reported to block
proliferation by inhibiting cell cycle regulatory machinery
(33). Non-steroidal
anti-inflammatory drugs (NSAIDs), such as aspirin, indomethacin and
sulindac, are commonly used in the treatment of acute or chronic
inflammatory conditions. Other drugs included acitretin, a
second-generation retinoid used for psoriasis, bevacizumab, a
recombinant monoclonal antibody that blocks angiogenesis by
inhibiting vascular endothelial growth factor A (VEGF-A), and
dimethyl sulfoxide (DMSO), used as a topical analgesic,
anti-inflammatory and antioxidant (34).
Discussion
Cutaneous squamous cell carcinoma (cSCC) is the
second most common skin cancer, with a high metastasis rate and an
>70% mortality rate when metastasized. It is generally accepted
that the majority of cSCCs are treated with surgical excision.
However, research into drugs as an adjuvant therapy is limited. In
this study, we identified 11 target genes following gene set
enrichment analysis, targetable by 54 drugs for possible cSCC
treatment (Table III). Genetic
changes in this carcinogenic process alter cell function, allowing
for self-sufficiency in growth signals, insensitivity to antigrowth
signals, escape from apoptosis, unlimited replicative potential,
invasion, angiogenesis and metastasis, which is consistent with the
enriched biological processes identified using GeneCodis analysis
(Table I), such as ‘apoptotic
process’, ‘cell proliferation’, ‘cell adhesion’ and ‘signal
transduction’. Potential drugs identified by the drug-gene
interaction search were divided into chemotherapy agents, TKIs,
HER2 inhibitors, EGFR inhibitors, AKT/PI3K inhibitors, mTOR
inhibitors, MAPK inhibitors, CDK inhibitors, HDAC inhibitors,
NSAIDs and others, acting upon signaling pathways including ‘MAPK
signaling pathway’, ‘ErbB signaling pathway’, ‘p53 signaling
pathway’, ‘apoptosis’ and ‘VEGF signaling pathway’ (Table II).
Chemotherapy plays a crucial role alone or in
combination with other treatment modalities in cSCC. Current
chemotherapeutic agents used in advanced or metastatic disease are
as follows: Platin derivates (e.g. cisplatin or carboplatin),
5-fluorouracil (5-FU), bleomycin, methotrexate, adriamycin,
taxanes, gemcitabine, or ifosfomide alone or in combination, 6 of
which appeared in our drug list. Carboplatin and docetaxel have
been used previously in cSCC as a routine clinical treatment with
good overall and progression-free survival (35,36),
while a study has shown that 5-FU, when administered orally in 14
patients with aggressive cSCC, achieved some success, with 64.3%
exhibiting measurable improvement and 14.3% partially responding
(37).
Mutations in tyrosine kinase receptor genes have
been observed in cSCC. The frequent amplification of EGFR and the
closely related ERBB2 can contribute to elevated receptor activity,
which is consistent with our gene search results. EGFR, a member of
the ERBB growth factor receptor family, has an extracellular
ligand-binding domain, a transmembrane region and a transduction
module consisting of a TK motif and several autophosphorylation
sites, and initiates signal transduction via activation of a
receptor-associated TK. The family also includes ERBB2 (HER2),
ERBB3, and ERBB4 (38). They
regulate apoptosis, cell cycle progression, differentiation,
angiogenesis, migration and development. There are at least two
types of EGFR inhibitors. One is EGFR small molecule inhibitors,
including gefitinib and erlotinib, which are illustrated in the
gene-drug interaction results; these block tyrosine kinase binding
at the intracellular domain. The other is antibodies against EGFR,
including cetuximab and panitumumab, which block the extracellular
domain of the receptor and inhibit ligand binding. The downstream
pathways including the P53 signaling pathway, MAPK signaling
pathway, PI3K/AKT pathway and nuclear factor-κB (NF-κB) pathway,
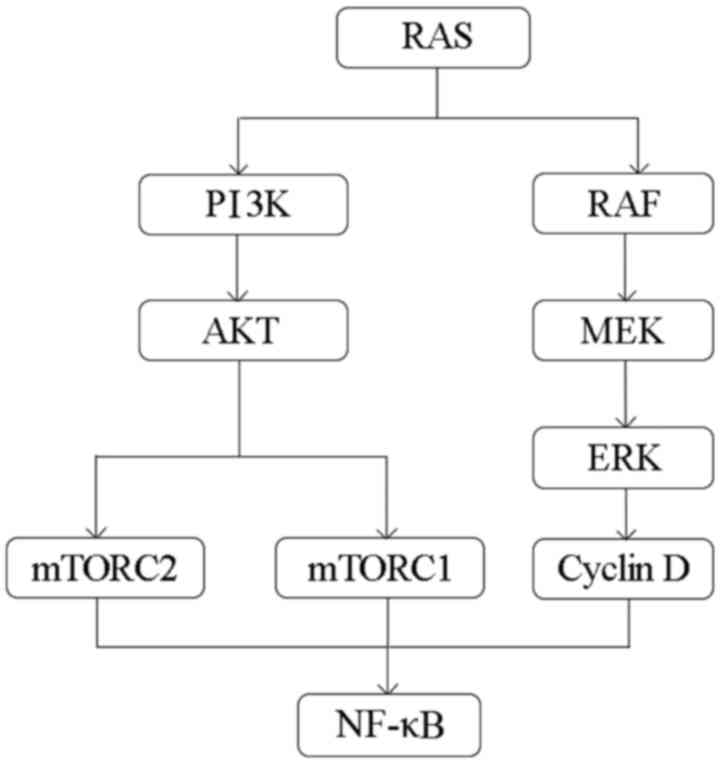
are activated by signaling through EGFR family members (Fig. 6) (39).
Gefitinib is a reversible EGFR-TKI, which regulates
the EGFR/MAPK/Pak1 pathways (40).
A phase II trial to evaluate the efficacy of gefitinib in
aggressive cSCC has shown that gefitinib, as a neoadjuvant therapy
prior to definitive treatment, was well tolerated in patients with
aggressive cSCC and did not interfere with the definitive
treatment, with 18.2% having a complete response and 27.3% having a
partial response (41). A
single-arm phase II study in 40 patients with cSCC not amenable to
curative therapy showed an overall response rate of 16% with a
favorable adverse event profile, which demonstrated modest activity
of gefitinib in incurable cSCC (42). Analogously, erlotinib, another
orally available reversible EGFR inhibitor, demonstrates responses
in advanced cSCC alone or in combination with other therapies
(43). Although many results of
trials using either gefitinib or erlotinib have been promising,
especially considering the acceptance of oral therapy by patients
and drug tolerance, none of the trials can be considered as
definitive due to their lack of controls. Cetuximab, a monoclonal
antibody that competitively inhibits EGFR, administered in
combination with simultaneous high-dose radiotherapy, showed a good
result with respect to significantly improved local tumor control
and survival compared to irradiation alone in a randomized phase
III clinical trial in HNSCC patients (44). The potential of cetuximab to treat
unresectable advanced cSCC alone or combined with radiotherapy was
confirmed in several studies, and further randomized studies are
still needed (45,46). Lapatinib, a dual tyrosine kinase
inhibitor which blocks the HER2/neu and EGFR pathways, was reported
to induce the inhibition of PI3K/AKT/mTOR and ErK1/2 signaling
pathways, leading to autophagy-inducing and EMT-suppressing effects
in A431 cell lines (43). Thus,
lapatinib may represent a promising anticancer drug for cSCC
treatment.
The PI3K/AKT/mTOR pathway is frequently activated in
cSCC, compared to actinic keratosis or normal skin (47). Class I PI3Ks phosphorylate
phosphatidylinositol 4,5-bisphosphate (PIP2) at the 3-OH of the
inositol ring to generate phosphatidylinositol 3,4,5-trisphosphate
(PIP3), which in turn activates AKT and the downstream mTOR complex
to play key roles in carcinogenesis (48). Activation of AKT will generate
downstream effects, including the promotion of cell proliferation,
migration and invasion, increasing cellular glycolytic flux and
inhibiting cellular apoptosis (49). Phosphorylated mTORC1 activates p70
S6 kinase, which enhances mRNA translation and drives cell growth
by activating the ribosomal protein S6 and elongation factor 2,
while mTORC2 directly phosphorylates AKT on serine 473, which
promotes cell survival and proliferation via the activation of
NF-κB and upregulation of cyclin D1 (50,51).
Therefore, the PI3K/AKT/mTOR pathway has become a potential
therapeutic target in cancer treatment (Fig. 6). As a result, inhibitors of
PI3K/AKT/mTOR have been explored, among which a selective p110
inhibitor, idelalisib, in PIK3CA mutation-positive cancer has been
approved for the treatment of chronic lymphocytic leukemia (CLL)
and follicular B-cell lymphoma of non-Hodgkin's lymphoma (48). However, no clinical studies have
reported the role of PI3K/AKT pathway-targeting drugs in cSCC to
date.
Apitolisib, a dual PI3K and mTOR inhibitor found in
our drug list, demonstrated favorable pharmacokinetics and evidence
of biological activity in a phase I study in patient with solid
tumors. However, the therapeutic window was narrow for the putative
increased risk of pulmonary toxicity (48). Everolimus, an mTORC1 inhibitor, is
undergoing a trial as part of a Phase I/II study investigating
induction chemotherapy with weekly everolimus plus carboplatin and
paclitaxel in locally unresectable advanced HNSCC. The treatment is
well tolerated with an overall response rate of 79% (52). Another Phase I and pharmacokinetic
study of everolimus in combination with cetuximab and carboplatin
for recurrent or metastatic HNSCC showed an encouraging response
rate, with 61.5% and 8.15 months progression-free survival, which
suggested possible clinical efficacy in a select group of patients
with cSCC (49).
The MAPK pathway is another major oncogenic pathway
in human cancer (Fig. 6). PI3K and
RAF can be activated by RAS, a monomeric membrane-associated
GTP-binding protein. RAF kinase transduces signals through a MAPK
cascade. Therefore, the PI3K/AKT/mTOR and MAPK signaling pathways
are compensatory pathways that mediate cell survival through
co-regulated proteins, and negatively regulate each other. AKT
directly phosphorylates and inactivates RAF, while MEK suppresses
PI3K signaling by promoting the membrane localization of
phosphatase and tensin homolog (PTEN) (31). The MAPK signaling pathway was the
second most highly enriched pathway annotation in our pathway
analysis process. Two drugs, including trametinib, a MEK inhibitor,
and doramapimod, a p38 MAPK inhibitor, were included in our final
drug list. Trametinib was approved by the US Food and Drug
Administration and European Medicines Agency as a single agent for
the treatment of patients with BRAF V600E-mutated metastatic
melanoma (53). However, according
to previous studies, patients treated with the BRAF V600E
inhibitors vemurafenib and dabrafenib rapidly develop cSCC
(17). Evidence indicates that
these inhibitors may result in a paradoxical activation of the MAPK
pathway, which in turn acts in tandem with mutations in other tumor
suppressors or oncogenes such as TP53 and HRAS (54). Therefore, inhibition of the MAPK
pathway may provide a potential treatment option for cSCC.
The NF-κB signaling pathway is a major pathway
mediating several fatal processes in cancer cells. The PI3K/AKT or
MAPK pathways have been shown to regulate the expression and
activity of the NF-κB transcription factor in cancer (55). NF-κB cooperates with many signaling
molecules and pathways. Its crosstalk with other transcription
factors includes STAT3, P53 and the ETS family member ERG, which
either directly interacts with NF-κB subunits or affects NF-κB
target genes (56,57). NF-κB can also be regulated by other
signaling pathways. Kinases activing or regulating NF-κB include
members of the MAPK family [Jun-N-terminal kinase (JNK) and p38],
protein kinase C (PKC), AKT and PI3K, which regulate the expression
and activity of NF-κB or affect upstream signaling pathways
(58,59). Inhibitors of either the PI3K/AKT or
MAPK signaling pathways may contribute to the inhibition of the
NF-κB signaling cascade, which verifies our research findings to a
certain degree.
One drug in our final drug list, the HDAC inhibitor
vorinostat, has shown potential to inhibit the growth of human
xenograft tumors. A study conducted by Kurundkar et al
showed that vorinostat impaired proliferation and migration, and
induced apoptosis, by downregulating the ERK and AKT/mTOR signaling
pathways; more specifically, by inhibiting mTOR signaling, which
was accompanied by a reduction in cell survival-associated AKT and
ERK signaling pathways (33). A
study on osteoarthritis demonstrated that vorinostat inhibits the
IL-1β-induced expression of matrix metalloproteinase (MMP)-1,
MMP-13 and inducible nitric oxide synthase (iNOS) through
inhibition of the phosphorylation of p38 and ERK1/2, and the
regulation of NF-κB by inhibiting the degradation of I-kBα and
attenuating NF-κB p65 translocation to the nucleus. This suggested
that vorinostat targets multiple proliferation and growth
regulatory pathways by inhibiting HDACs, which provides the
rationale for a novel mechanism-based therapeutic intervention for
cSCC (33).
Three of the drugs found in our drug list are
NSAIDs, which are closely related to cSCC pathology as exposure to
UV radiation damages skin cells, leading to the release of
inflammatory cytokines such as cyclooxygenase (COX)-2 and its
product prostaglandin E2, which promote skin carcinogenesis
(23). Overexpression of COX-2 has
been observed in cSCC (60). NSAIDs
inhibit COX-2 and suppress the production of prostaglandin E2,
which prevents E2 and E4 receptors from upregulating inflammatory
cytokines that recruit immune cells and promote NF-κB activation. A
meta-analysis reported that the use of non-aspirin NSAIDs or any
NSAIDs significantly reduced the risk of developing cSCC by 15 and
18%, respectively, suggesting that NSAIDs have the potential to
prevent the development of cSCC collectively (61).
The limitations of this study are associated with
the databases we used and the criteria we set in each screening
step. These databases may have limited data sources, thus our
analysis may have to be repeated as databases become more
comprehensive. The criteria that we set are likely to be
subjective. Different rational criteria could be set up to further
explore the best outcome.
In conclusion, we presented a method to explore
candidate drugs which target the genes/pathways that are relevant
to cSCC, with the aim of elucidating potential treatments. Such
methods may be used routinely as databases and analytical tools
evolve and improve. As a result, in this method, we identified a
total of 54 potential drugs, including 25 chemotherapy agents, 21
TKIs, 7 inhibitors of AKT/PI3K/mTOR, 2 inhibitors of MAPK, 2 CDK
inhibitors, 1 HDAC inhibitor, 3 NSAIDs and 3 other drugs.
Forty-nine of the 54 have not yet been tested in cSCC, which
provides a basis for new trials and the development of novel
targeted therapies as potential treatments for cSCC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YP, YZ and JL designed the research, performed the
experiments, analyzed the data and wrote the manuscript. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Eisemann N, Waldmann A, Geller AC,
Weinstock MA, Volkmer B, Greinert R, Breitbart EW and Katalinic A:
Non-melanoma skin cancer incidence and impact of skin cancer
screening on incidence. J Invest Dermatol. 134:43–50. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Que SKT, Zwald FO and Schmults CD:
Cutaneous squamous cell carcinoma: Incidence, risk factors,
diagnosis, and staging. J Am Acad Dermatol. 78:237–247. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Muzic JG, Schmitt AR, Wright AC, Alniemi
DT, Zubair AS, Lourido Olazagasti JM, Seda Sosa IM, Weaver AL and
Baum CL: Incidence and trends of basal cell carcinoma and cutaneous
squamous cell carcinoma: A population-based study in olmsted
county, minnesota, 2000 to 2010. Mayo Clin Proc. 92:890–898. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Burton KA, Ashack KA and Khachemoune A:
Cutaneous squamous cell carcinoma: A review of high-risk and
metastatic disease. Am J Clin Dermatol. 17:491–508. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Diepgen T, Fartasch M, Drexler H and
Schmitt J: Occupational skin cancer induced by ultraviolet
radiation and its prevention. Br J Dermatol. 167 Suppl 2:S76–S84.
2012. View Article : Google Scholar
|
|
6
|
Boeckx C, Baay M, Wouters A, Specenier P,
Vermorken JB, Peeters M and Lardon F: Anti-epidermal growth factor
receptor therapy in head and neck squamous cell carcinoma: Focus on
potential molecular mechanisms of drug resistance. Oncologist.
18:850–864. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dotto GP and Rustgi AK: Squamous cell
cancers: A unified perspective on biology and genetics. Cancer
Cell. 29:622–637. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moosavinasab S, Patterson J, Strouse R,
Rastegar-Mojarad M, Regan K, Payne PR, Huang Y and Lin SM: ‘RE:fine
drugs’: An interactive dashboard to access drug repurposing
opportunities. Database (Oxford). 2016:pii: baw0832016. View Article : Google Scholar
|
|
9
|
Andronis C, Sharma A, Virvilis V,
Deftereos S and Persidis A: Literature mining, ontologies and
information visualization for drug repurposing. Brief Bioinform.
12:357–368. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu H, Beck TN, Golemis EA and
Serebriiskii IG: Integrating in silico resources to map a signaling
network. Methods Mol Biol. 1101:197–245. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Baran J, Gerner M, Haeussler M, Nenadic G
and Bergman CM: pubmed2ensembl: A resource for mining the
biological literature on genes. PLoS One. 6:e247162011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Carmona-Saez P, Chagoyen M, Tirado F,
Carazo JM and Pascual-Montano A: GENECODIS: A web-based tool for
finding significant concurrent annotations in gene lists. Genome
Biol. 8:R32007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Szklarczyk D, Franceschini A, Wyder S,
Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos
A, Tsafou KP, et al: STRING v10: Protein-protein interaction
networks, integrated over the tree of life. Nucleic Acids Res.
43:D447–D452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Su G, Morris JH, Demchak B and Bader GD:
Biological network exploration with Cytoscape 3. Curr Protoc
Bioinformatics. 47:8.13.1–24. 2014. View Article : Google Scholar
|
|
15
|
Gao J, Aksoy BA, Dogrusoz U, Dresdner G,
Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al:
Integrative analysis of complex cancer genomics and clinical
profiles using the cBioPortal. Sci Signal. 6:pl12013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wagner AH, Coffman AC, Ainscough BJ, Spies
NC, Skidmore ZL, Campbell KM, Krysiak K, Pan D, McMichael JF,
Eldred JM, et al: DGIdb 2.0: Mining clinically relevant drug-gene
interactions. Nucleic Acids Res. 44:D1036–D1044. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ribas A and Flaherty KT: BRAF targeted
therapy changes the treatment paradigm in melanoma. Nat Rev Clin
Oncol. 8:426–433. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stratigos A, Garbe C, Lebbe C, Malvehy J,
del Marmol V, Pehamberger H, Peris K, Becker JC, Zalaudek I, Saiag
P, et al: Diagnosis and treatment of invasive squamous cell
carcinoma of the skin: European consensus-based interdisciplinary
guideline. Eur J Cancer. 51:1989–2007. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wright TJ, McKee C, Birch-Machin MA, Ellis
R, Armstrong JL and Lovat PE: Increasing the therapeutic efficacy
of docetaxel for cutaneous squamous cell carcinoma through the
combined inhibition of phosphatidylinositol 3-kinase/AKT signalling
and autophagy. Clin Exp Dermatol. 38:421–423. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wood JP, Smith AJ, Bowman KJ, Thomas AL
and Jones GD: Comet assay measures of DNA damage as biomarkers of
irinotecan response in colorectal cancer in vitro and in vivo.
Cancer Med. 4:1309–1321. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cogle CR, Scott BL, Boyd T and
Garcia-Manero G: Oral azacitidine (CC-486) for the treatment of
myelodysplastic syndromes and acute myeloid leukemia. Oncologist.
20:1404–1412. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maubec E, Petrow P, Scheer-Senyarich I,
Duvillard P, Lacroix L, Gelly J, Certain A, Duval X, Crickx B,
Buffard V, et al: Phase II study of cetuximab as first-line
single-drug therapy in patients with unresectable squamous cell
carcinoma of the skin. J Clin Oncol. 29:3419–3426. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Balduzzi S, Mantarro S, Guarneri V,
Tagliabue L, Pistotti V, Moja L and D'Amico R:
Trastuzumab-containing regimens for metastatic breast cancer.
Cochrane Database Syst Rev: CD006242. 2014. View Article : Google Scholar
|
|
24
|
Harbeck N, Beckmann MW, Rody A,
Schneeweiss A, Müller V, Fehm T, Marschner N, Gluz O, Schrader I,
Heinrich G, et al: HER2 dimerization inhibitor pertuzumab-mode of
action and clinical data in breast cancer. Breast Care (Basel).
8:49–55. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim ES and Dhillon S: Ibrutinib: A review
of its use in patients with mantle cell lymphoma or chronic
lymphocytic leukaemia. Drugs. 75:769–776. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang K and Fu LW: Mechanisms of resistance
to BCR-ABL TKIs and the therapeutic strategies: A review. Crit Rev
Oncol Hematol. 93:277–292. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Roberts PJ: Clinical use of crizotinib for
the treatment of non-small cell lung cancer. Biologics. 7:91–101.
2013.PubMed/NCBI
|
|
28
|
Hahn KA, Ogilvie G, Rusk T, Devauchelle P,
Leblanc A, Legendre A, Powers B, Leventhal PS, Kinet JP, Palmerini
F, et al: Masitinib is safe and effective for the treatment of
canine mast cell tumors. J Vet Intern Med. 22:1301–1309. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wilhelm SM, Adnane L, Newell P, Villanueva
A, Llovet JM and Lynch M: Preclinical overview of sorafenib, a
multikinase inhibitor that targets both Raf and VEGF and PDGF
receptor tyrosine kinase signaling. Mol Cancer Ther. 7:3129–3140.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gan HK, Seruga B and Knox JJ: Sunitinib in
solid tumors. Expert Opin Investig Drugs. 18:821–834. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ersahin T, Tuncbag N and Cetin-Atalay R:
The PI3K/AKT/mTOR interactive pathway. Mol Biosyst. 11:1946–1954.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Flaherty KT, Infante JR, Daud A, Gonzalez
R, Kefford RF, Sosman J, Hamid O, Schuchter L, Cebon J, Ibrahim N,
et al: Combined BRAF and MEK inhibition in melanoma with BRAF V600
mutations. N Engl J Med. 367:1694–1703. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kurundkar D, Srivastava RK, Chaudhary SC,
Ballestas ME, Kopelovich L, Elmets CA and Athar M: Vorinostat, an
HDAC inhibitor attenuates epidermoid squamous cell carcinoma growth
by dampening mTOR signaling pathway in a human xenograft murine
model. Toxicol Appl Pharmacol. 266:233–244. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shih T and Lindley C: Bevacizumab: An
angiogenesis inhibitor for the treatment of solid malignancies.
Clin Ther. 28:1779–1802. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jarkowski A III, Hare R, Loud P, Skitzki
JJ, Kane JM III, May KS, Zeitouni NC, Nestico J, Vona KL, Groman A
and Khushalani NI: Systemic therapy in advanced cutaneous squamous
cell carcinoma (CSCC): The roswell park experience and a review of
the literature. Am J Clin Oncol. 39:545–548. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sola Biete A, Querol Marruecos J, Manuel
Calvo FA, Fransoy Verger E, Casino Rovirosa A, de Castro Grau JJ,
de Las Heras González M, Aguerri Ramos A, Eito Palacios A, Candal
Veiras C and López Solano MV: Phase II trial: Concurrent
radio-chemotherapy with weekly docetaxel for advanced squamous cell
carcinoma of head and neck. Clin Transl Oncol. 9:244–250. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cartei G, Cartei F, Interlandi G,
Meneghini G, Jop A, Zingone G, Tabaro G and Mazzoleni F: Oral
5-fluorouracil in squamous cell carcinoma of the skin in the aged.
Am J Clin Oncol. 23:181–184. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Roskoski R Jr: ErbB/HER protein-tyrosine
kinases: Structures and small molecule inhibitors. Pharmacol Res.
87:42–59. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ratushny V, Gober MD, Hick R, Ridky TW and
Seykora JT: From keratinocyte to cancer: The pathogenesis and
modeling of cutaneous squamous cell carcinoma. J Clin Invest.
122:464–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Barnes CJ, Bagheri-Yarmand R, Mandal M,
Yang Z, Clayman GL, Hong WK and Kumar R: Suppression of epidermal
growth factor receptor, mitogen-activated protein kinase, and Pak1
pathways and invasiveness of human cutaneous squamous cancer cells
by the tyrosine kinase inhibitor ZD1839 (Iressa). Mol Cancer Ther.
2:345–351. 2003.PubMed/NCBI
|
|
41
|
Lewis CM, Glisson BS, Feng L, Wan F, Tang
X, Wistuba II, El-Naggar AK, Rosenthal DI, Chambers MS, Lustig RA
and Weber RS: A phase II study of gefitinib for aggressive
cutaneous squamous cell carcinoma of the head and neck. Clin Cancer
Res. 18:1435–1446. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
William WN Jr, Feng L, Ferrarotto R,
Ginsberg L, Kies M, Lippman S, Glisson B and Kim ES: Gefitinib for
patients with incurable cutaneous squamous cell carcinoma: A
single-arm phase II clinical trial. J Am Acad Dermatol.
77:1110–1113.e2. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yao M, Shang YY, Zhou ZW, Yang YX, Wu YS,
Guan LF, Wang XY, Zhou SF and Wei X: The research on lapatinib in
autophagy, cell cycle arrest and epithelial to mesenchymal
transition via Wnt/ErK/PI3K-AKT signaling pathway in human
cutaneous squamous cell carcinoma. J Cancer. 8:220–226. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bonner JA, Harari PM, Giralt J, Azarnia N,
Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, et al:
Radiotherapy plus cetuximab for squamous-cell carcinoma of the head
and neck. N Engl J Med. 354:567–578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Preneau S, Rio E, Brocard A, Peuvrel L,
Nguyen JM, Quéreux G and Dreno B: Efficacy of cetuximab in the
treatment of squamous cell carcinoma. J Dermatolog Treat.
25:424–427. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Samstein RM, Ho AL, Lee NY and Barker CA:
Locally advanced and unresectable cutaneous squamous cell
carcinoma: Outcomes of concurrent cetuximab and radiotherapy. J
Skin Cancer. 2014:2845822014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Einspahr JG, Calvert V, Alberts DS,
Curiel-Lewandrowski C, Warneke J, Krouse R, Stratton SP, Liotta L,
Longo C, Pellacani G, et al: Functional protein pathway activation
mapping of the progression of normal skin to squamous cell
carcinoma. Cancer Prev Res (Phila). 5:403–413. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhao W, Qiu Y and Kong D: Class I
phosphatidylinositol 3-kinase inhibitors for cancer therapy. Acta
Pharm Sin B. 7:27–37. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Saba NF, Hurwitz SJ, Magliocca K, Kim S,
Owonikoko TK, Harvey D, Ramalingam SS, Chen Z, Rogerio J, Mendel J,
et al: Phase 1 and pharmacokinetic study of everolimus in
combination with cetuximab and carboplatin for recurrent/metastatic
squamous cell carcinoma of the head and neck. Cancer.
120:3940–3951. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Rogers SJ, Box C, Harrington KJ, Nutting
C, Rhys-Evans P and Eccles SA: The phosphoinositide 3-kinase
signalling pathway as a therapeutic target in squamous cell
carcinoma of the head and neck. Expert Opin Ther Targets.
9:769–790. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Song G, Ouyang G and Bao S: The activation
of Akt/PKB signaling pathway and cell survival. J Cell Mol Med.
9:59–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Raymond E, Tourneau CL, Gatineau M, Delord
JP, Fayette J, Dreyer C, Tijeras-Raballand A, Albert S, Granier M,
Chibaudel B, et al: CAPRA: Safety, efficacy, and translational
biomarkers of weekly everolimus, carboplatin, and paclitaxel as
induction therapy for locally advanced head and neck squamous cell
carcinoma (HNSCC). An Am J Roentgenol. 177:1041–1044. 2013.
|
|
53
|
Lugowska I, Kosełapaterczyk H, Kozak K and
Rutkowski P: Trametinib: A MEK inhibitor for management of
metastatic melanoma. Onco Targets Ther. 8:2251–2259.
2015.PubMed/NCBI
|
|
54
|
Cammareri P, Rose AM, Vincent DF, Wang J,
Nagano A, Libertini S, Ridgway RA, Athineos D, Coates PJ, McHugh A,
et al: Inactivation of TGFβ receptors in stem cells drives
cutaneous squamous cell carcinoma. Nat Commun. 7:124932016.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sun SC: The non-canonical NF-κB pathway in
immunity and inflammation. Nat Rev Immunol. 17:545–558. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Grivennikov SI and Karin M: Dangerous
liaisons: STAT3 and NF-kappaB collaboration and crosstalk in
cancer. Cytokine Growth Factor Rev. 21:11–19. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Schneider G, Henrich A, Greiner G, Wolf V,
Lovas A, Wieczorek M, Wagner T, Reichardt S, von Werder A, Schmid
RM, et al: Cross talk between stimulated NF-kappaB and the tumor
suppressor p53. Oncogene. 29:2795–2806. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Schulzeosthoff K, Ferrari D, Riehemann K
and Wesselborg S: Regulation of NF-kappa B activation by MAP kinase
cascades. Immunobiology. 198:35–49. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Dan HC, Cooper MJ, Cogswell PC, Duncan JA,
Ting JP and Baldwin AS: Akt-dependent regulation of NF-{kappa}B is
controlled by mTOR and Raptor in association with IKK. Genes Dev.
22:1490–1500. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kuzbicki L, Lange D, Stanek-Widera A and
Chwirot BW: Different expression of cyclooxygenase-2 (COX-2) in
selected nonmelanocytic human cutaneous lesions. Folia Histochem
Cytobiol. 49:381–388. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Muranushi C, Olsen CM, Pandeya N and Green
AC: Aspirin and nonsteroidal anti-inflammatory drugs can prevent
cutaneous squamous cell carcinoma: A systematic review and
meta-analysis. J Invest Dermatol. 135:975–983. 2015. View Article : Google Scholar : PubMed/NCBI
|