Introduction
Glucocorticoids have been used for the treatment of
various disorders including skin-related diseases and cancers
(1,2) and in recent years, several trials have
been performed to assess their use as adjuvant therapy in various
applications (2–4). They have inhibitory effects in
choroidal neovascularization and were revealed to be generally
effective in providing anti-inflammatory effects and short-term
relief in mice (5). Kenalog
(triamcinolone acetonide), a synthetic glucocorticoid is commonly
used to treat various diseases such as ocular and choroidal
melanoma (6,7). However, like many other
glucocorticoids, treatment with Kenalog aggravates side effects and
rarely achieves sustainable results for patients and in cases
requiring chronic therapy (8,9). This
may be due to the ability of some tissues such as skin cells
including melanoma to produce glucocorticoids from cholesterol due
to intramolecular rearrangements caused by UV light that may impair
the effectiveness of the drug (10,11).
In recent years, several approaches have been
devised to increase the bioactivity of glucocorticoids to overcome
the problems related to low efficacy and increased adverse effects
(12). One of the approaches is
incrementally modified drugs (IMD) (13). IMD involves the addition of
functional groups and/or synthesis of a conventional drug
chemically. Using this innovative technology, several modified
glucocorticoid drugs, for example nitro-steroids 21-NO-prednisolone
and many other NO-releasing nonsteroidal anti-inflammatory drugs
(NSAIDs), have been demonstrated to have enhanced efficacy
(14). However, efforts made thus
far in IMD do not cater enough to the use of physical means such as
ionizing radiation (IR). With this in mind, the use of ionizing
radiation was developed to improve the potency and efficacy of
glucocorticoids in cancer treatment. Various studies have reported
the use of ionizing radiation to improve the efficacy of
glucocorticoids (15). In a
previous study, we revealed that rotenone derivatives modified by
ionizing radiation had potential anti-carcinogenic activity in
hepatic cancer cells (16). The aim
of the present study, was to further improve our knowledge on
γ-irradiated glucocorticoids as potential anticancer drugs for the
treatment of various types of cancer. In the present study,
Kenalog-IR was reported as a potential candidate for the treatment
of skin cancer.
To elucidate the contribution of IR in IMD
approaches, the in vitro cytotoxic effects of Kenalog-IR
were assessed in melanoma cancer cells. It was found that
Kenalog-IR generated more effective anticancer activity when
compared to Kenalog and was confirmed as a suitable candidate for
cancer treatment. The present results revealed that the induction
of the intrinsic apoptosis pathway was associated with the
production of ROS and exhibited anticancer activity potential and
the results were in line with a previous study which revealed
γ-radiation-modified dexamethasone as a potential target in human
lung cancer treatment (17).
Although, the structure of Kenalog-IR remains to be determined, its
potential anticancer activity cannot be ruled out for the treatment
of melanoma skin cancer.
Materials and methods
Preparation of Kenalog suspension and
irradiation
The Kenalog drug was obtained from Sigma-Aldrich;
Merck KGaA (Darmstadt, Germany) and 1 g of the stock was dissolved
in a liter of methanol. The suspension was irradiated at 50 kGy at
a dose rate of 10 kGy/h generated by a 60Co irradiator
(MDS Nordion, Ottawa, ON, Canada) at the Advanced Radiation
Technology Institute, Korea Atomic Energy Research Institute.
Irradiated samples were evaporated in vacuo, and crude
compounds were analyzed by liquid chromatography coupled with mass
spectrometry (LC-MS) and liquid chromatography (HPLC). To determine
the chromatograms of the Kenalog-IR, irradiated samples were
evaporated in vacuo and analyzed by high performance HPLC or
LC-MS (Agilent Technologies, Inc., Palo Alto, CA, USA) in a
YMC-Pack ODS-A-302 column (4.6 mm i.d. × 150 mm; YMC Co., Ltd.,
Kyoto, Japan). The mobile phase comprised of linear gradient
starting with 0.1% (v/v) HCOOH at 40°C and increased to 50% (v/v)
MeCN in 0.1% (v/v) CHOOH/H2O over 20 min, and then
increased to 100% MeCN for a further 20 min (detection, UV 254 nm;
flow rate, 1.0 ml/min). This irradiated solution was named
Kenalog-IR and a final concentration of 1 mg/ml was used for
analysis.
Cell culture
The cell lines used for the present study, SK-Mel-5
was purchased from the American Type Culture Collection (ATCC;
Rockville, MD, USA) and CCD-986sk skin fibroblast was obtained from
the Korean Cell Line Bank (KCLB; Seoul, Korea). SK-Mel-5 cells were
prepared in Dulbecco's modified Eagle's medium (DMEM) and CCD-986sk
in Roswell Park Memorial Institute (RPMI)-1640 medium with 100 U/ml
penicillin and 100 µg/ml streptomycin and 10% heat-inactivated
fetal bovine serum (FBS) (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Cells were maintained at 37°C, in an incubator
until 80–90% confluence was reached, and an equal number of cells
were incubated without or with various concentrations of Kenalog
and Kenalog-IR (0, 5, 10, 25, 50 and 100 µg/ml) for each set of
experimental conditions. Cells were washed with 1X
phosphate-buffered saline pH 7.4 (PBS) and harvested with 0.5%
trypsin-0.2% EDTA (Gibco; Thermo Fisher Scientific, Inc.) and were
either used directly for analysis or stored at −80°C for further
analysis.
Cell viability and proliferation
Cell viability was measured by determining
mitochondrial function using MTT assay kit
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(Roche Diagnostics, Indianapolis, IN, USA)] and cell toxicity was
assessed by trypan blue staining. The SK-Mel-5 cells were seeded at
a density of 0.3×104 cells/well in a 96-well flat bottom
plate. After 24 h, the cells were treated with various
concentrations of Kenalog or Kenalog-IR for 24 and 48 h. After
treatment, 20 µl of 5 mg/ml MTT solution was added to each well and
incubated for 2.5 h at 37°C, in a 5% CO2 atmosphere. The
supernatant was removed and solubilized with dimethyl sulfoxide
(DMSO) for 10 min to dissolve the formazan produced. The absorbance
was measured at 570 nm using a microplate reader (Tecan Group Ltd.,
Männedorf, Switzerland). Cell viability was expressed as the
percentage of difference from the control at the corresponding
concentration points. For the cytotoxic (cell death) assay, the
cells were treated with various concentrations of compounds as
aforementioned at a density of 0.3×106 in a 65-mm
culture dish. The staining was carried out by trypan blue
dye-exclusion using a counting chamber and dead and live cells were
counted by an Olympus IX71fluorescence microscope (Olympus Corp.,
Tokyo, Japan). To assess the non-specific cytotoxic effect of
Kenalog-IR, normal skin fibroblast cells CCD-986sk were used.
Briefly, 0.3×106 cells/well were seeded into a 96-well
plate for 96 h and subsequently treated with Kenalog or Kenalog-IR
for 24 and 48 h. Then, cell viability was assessed by MTT assay as
aforementioned.
Apoptotic assay determination
For quantitative analysis of apoptotic and necrotic
dead cells, Muse Annexin V and Dead Cell Assay kit (MCH100105; EMD
Millipore, Billerica, MA, USA) was used. SK-Mel-5 cells
(3×105) were seeded in a 65-mm culture dish for 24 h and
then treated with 100 µg/ml of Kenalog or Kenalog-IR for 24 h at
37°C, in a 5% CO2 atmosphere. Cells were harvested and
washed with PBS pH 7.4 as aforementioned. Furthermore, the cells
were stained with Annexin V and Dead Cell reagent for 20 min and
flow cytometric assessment was performed by Muse™ Cell Analyzer
(EMD Millipore). The live and apoptotic cells were distinguished
from necrotic cells as follows: Live cells (Annexin
V-FITC−/PI−) called double negative, early
apoptotic cells (Annexin V-FITC+/PI−), late
apoptotic cells (Annexin V-FITC+/PI+) called
double positive and necrotic cells (Annexin
V-FITC−/PI+). The apoptotic cells were
expressed as the percentage of live cells, early/late apoptotic
cells, and dead cells determined by Muse analysis software (Muse
1.1.2; EMD Millipore).
Determination of DNA
fragmentation
The chromosomal DNA fragments were identified using
agarose gel electrophoresis. Briefly, 3×105 SK-Mel-5
cells were seeded and treated with 100 µg/ml Kenalog or Kenalog-IR
and 2 µM doxorubicin for 18 h. The 0.1% DMSO contained media and
the fresh media-treated cells were used as vehicle and control,
respectively. After treatment, the cells were washed two times with
PBS and cell lysates were harvested by DNA lysis buffer-1 (10 mM
EDTA, 0.25% Triton X-100 and 2.5 mM Tris-HCl at pH 8) and incubated
at room temperature (RT) for 15 min. Cells were centrifuged at
13,000 × g for 20 min at 4°C and an equal volume of supernatant and
isopropanol were mixed and incubated at −80°C for 1 h. After cold
incubation, the samples were centrifuged at 13,000 × g for 20 min
at 4°C and the pellets were washed three times with cold 75%
ethanol by centrifugation at 13,000 × g for 20 min at 4°C. Pellets
were then left to dry at RT and suspended with 100 µl of DNA lysis
buffer-2 (10 mM EDTA, and 2.5 mM Tris-HCl at pH 8). The samples
were further incubated with 0.1 mg/ml RNase A for 30 min at RT and
mixed with 0.25 mg/ml proteinase K for 1 h at RT. Then, the samples
were mixed with 6X loading dye to a final concentration of 1X, and
loaded in 1.2% agarose gel containing 1X gel red stain.
Electrophoresis was run for 30 min at 100 V/cm.
Cell cycle assessment
For cell cycle assessment, Muse cell cycle reagent
(EMD Millipore) was used. The analysis of differential DNA content
in each phase of the cell cycle (G0/G1, S,
and G2/M) was determined in melanoma cells. Briefly,
3×105 SK-Mel-5 cells were seeded and treatment was
carried out as described in the apoptosis assay aforementioned.
Nocodazole (400 nM) was used to induce G2/M phase cell arrest.
Cells were then stained and incubated for 30 min with Muse cell
cycle reagent at 4°C and flow cytometric assessment was performed
by Muse™ Cell Analyzer (EMD Millipore). The DNA content was
expressed as the percentage of cells in the respective cell cycle
phase.
Assessment of reactive oxygen species
(ROS)
Intracellular ROS produced by stressed cells were
assessed by Muse oxidative stress reagent assay (EMD Millipore).
SK-Mel-5 cells (1×105) were seeded in a 65-mm culture
dish for 24 h and then treated with 100 µg/ml of Kenalog and
Kenalog-IR or 2 µM doxorubicin as the positive control. Cells were
harvested and the cell suspension was incubated with assay reagent
for 30 min and the oxidized red dihydroethidium (DHE) fluorescence
intensity was assessed by flow cytometer using Muse™ Cell Analyzer
(EMD Millipore). The ROS-positive and ROS-negative cells were
expressed as a percentage of the ROS gated profile. For the
intracellular source of ROS determination, MitoSOX assay
(Invitrogen; Thermo Fisher Scientific, Inc.) was used. Cells were
pre-labeled with MitoSOX reagent before treatment. Then cells were
treated as indicated above for 18 h and harvested with trypsin. The
cells were harvested, washed and further incubated with a buffer
containing 5 µM MitoSOX for 10 min at 37°C in the dark. After the
incubation time, the cells were washed twice and suspended for
measurements with a flow cytometer.
Western blot analysis
Treated and untreated SK-Mel-5 cells were harvested,
lysed with radioimmunoprecipitation assay buffer (RIPA; Rockland
Immunochemicals, Inc., Limerick, PA, USA) and cytosolic and
mitochondria fractions were separated by Cytochrome c
Release Apoptosis Assay kit (cat. no. ab65311; Abcam, Cambridge,
UK) following the manufacturer's instructions. Cell debris was
removed by centrifugation and the protein concentration was
determined by bicinchoninic acid protein assay kit (Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Cell lysates containing an equal amount of protein (40 µg) were
prepared and separated by 10 or 15% SDS-PAGE and 10 µg of cytosolic
fractions and 15 µg of both mitochondria and debris fractions were
loaded onto 12% SDS-PAGE and transferred to polyvinylidene
difluoride membranes (PVDF; Merck Millipore). The membranes were
then blocked with 5% non-fat milk in Tris-buffered saline
containing Tween-20 (TBST) for 1 h at RT. The membranes were
subsequently probed overnight at 4°C with primary antibodies at
dilution of 1:1,000 for anti-Bcl-2 (cat. no. 2870), anti-Bax (cat.
no. 5023), anti-Bad (cat. no. 9292), anti-caspase-9 and cleaved
caspase-9 (cat. no. 9502), anti-caspase-7 (cat. no. 9492) and
cleaved caspase-7 (cat. no. 9491), anti-caspase-3 (cat. no. 9662)
and cleaved caspase-3 (cat. no. 9664), anti-PARP (cat. no. 9532)
and cleaved PARP (cat. no. 9541), anti-AIF, anti-AKT (cat. no.
4691) and p-AKT (cat. no. 4060), anti-mTOR (cat. no. 2983) and
p-mTOR (cat. no. 5536), anti-β-actin (cat. no. 4970) and anti-GAPDH
(cat. no. 2118) (all primary antibodies; Cell Signaling Technology,
Danvers, MA, USA) and sodium dismutase-1 (cat. no. SC-11407) (Santa
Cruz Biotechnology, Santa Cruz, CA, USA). The blots were then
incubated with secondary antibody horseradish peroxidase
(HRP)-conjugated anti-mouse (cat. no. 7076 at 1:2,500) or
anti-rabbit IgG (cat. no. 7074 at 1:3,000) both from Cell Signaling
Technology (Danvers, MA, USA) for 1 h at RT. The membranes were
incubated with the chemiluminescence (ECL) reagent (Thermo Fisher
Scientific, Inc.) for protein band detection.
Statistical analysis
All data were evaluated by one-way ANOVA, followed
by Tukey's post hoc test and results were considered statistically
significant when the P-value was <0.05. Experiments were
performed at least of three times independently.
Results
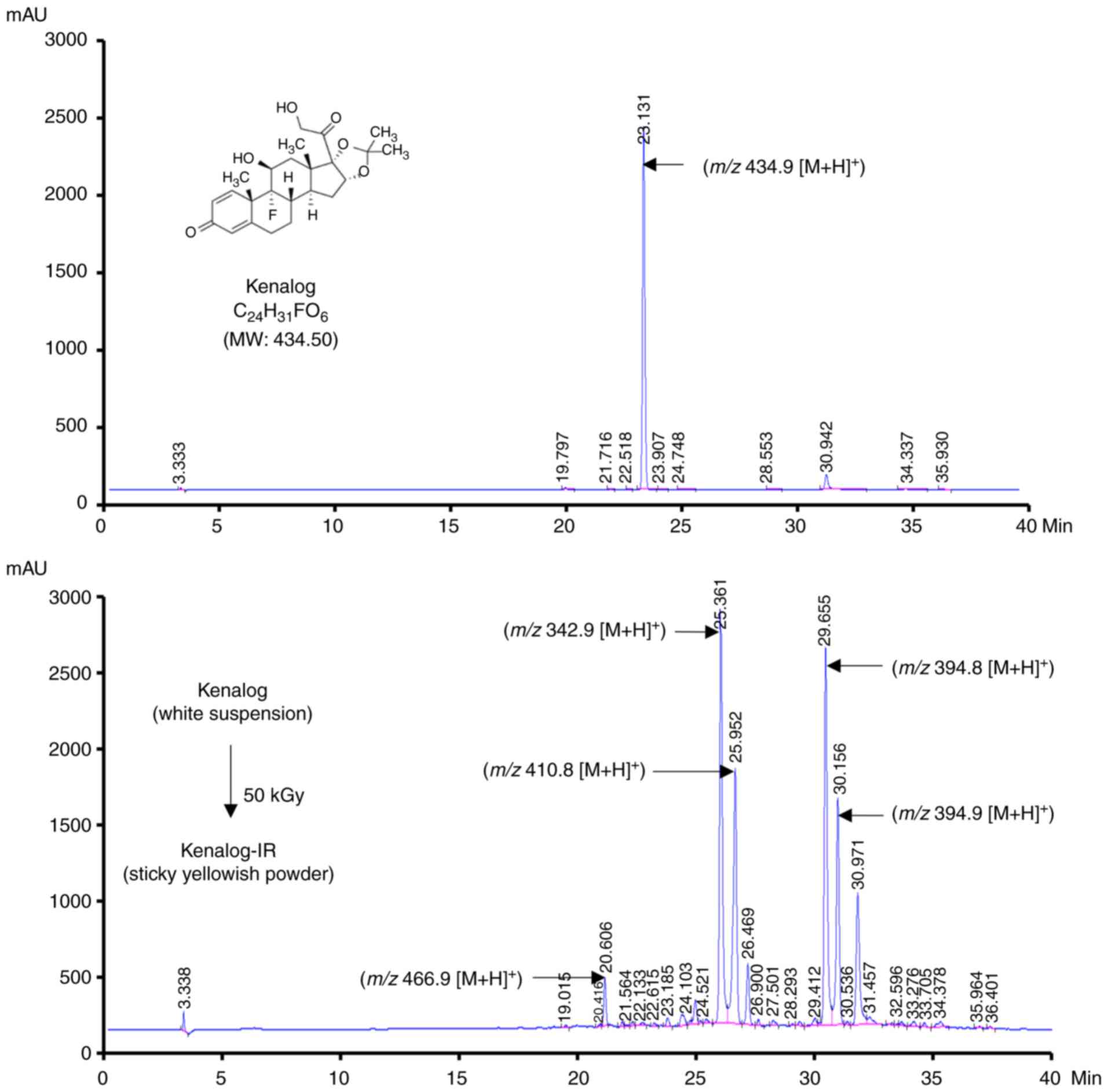
Effects of ionizing radiation on
Kenalog
To investigate the radiolytic effects of ionizing
radiation on Kenalog, 50 kGy of γ radiation was used to modify the
chemical structures of the drug. Sample solutions of Kenalog and
Kenalog-IR were analyzed by LC-MS and as revealed in Fig. 1, one major peak was observed in the
chromatogram of Kenalog (Fig. 1,
upper panel) and four peaks in the Kenalog-IR chromatogram at a
various retention times (Fig. 1,
lower panel). These newly generated peaks indicated that ionizing
radiation caused alterations to Kenalog and that the changes may
contribute to the cytotoxic effects of Kenalog-IR.
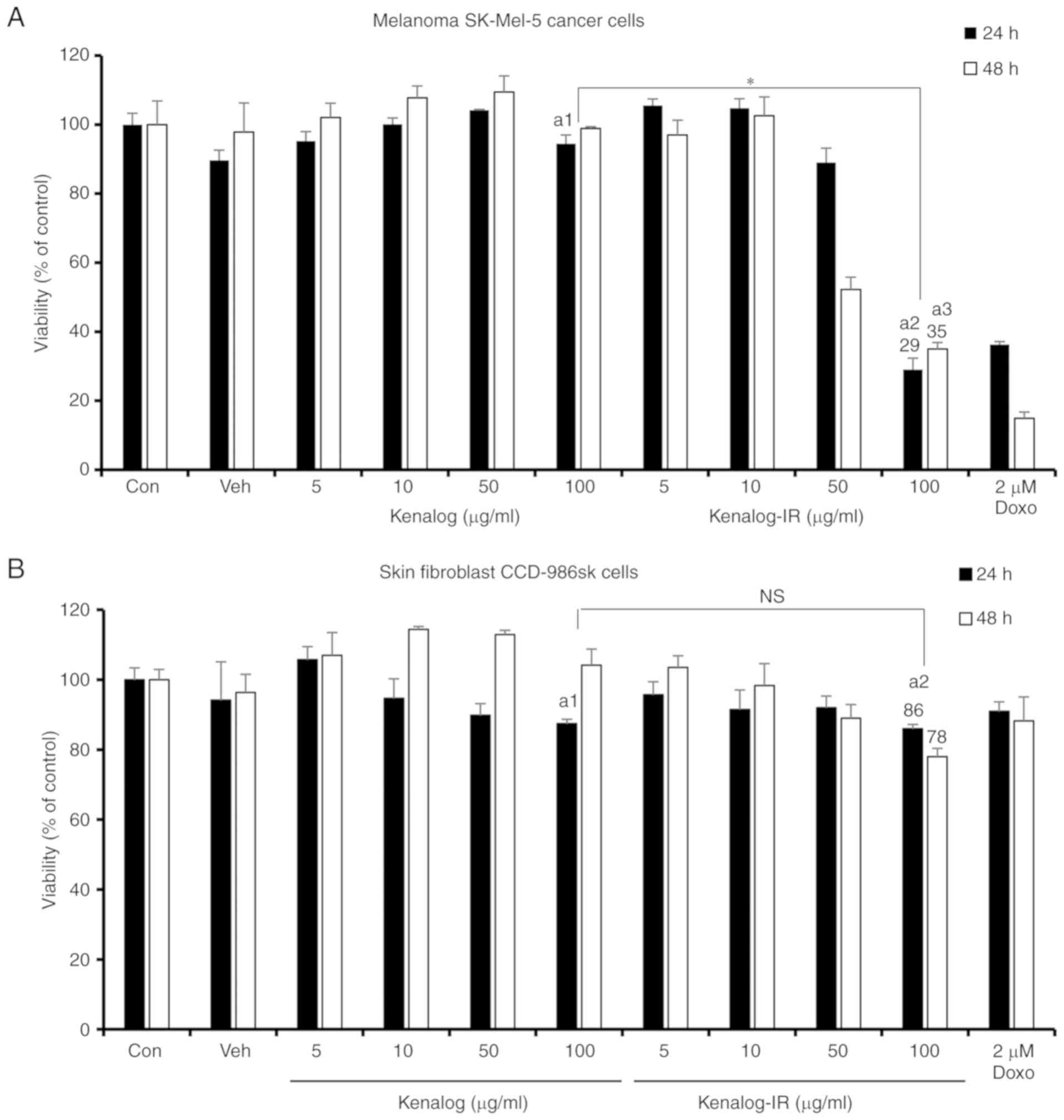
Kenalog-IR inhibits proliferation and
induces cytotoxic effects in SK-Mel 5 cells
In order to investigate the anticancer activity of
Kenalog-IR, MTT and trypan blue assays were used to evaluate the
cytotoxic effects in the SK-Mel-5 cell line. It has been previously
reported that the clinically available form of Kenalog has less
cytotoxic effects compared to other corticosteroids (9). Thus, we first compared the cytotoxic
effect of Kenalog and Kenalog-IR. As anticipated, Kenalog-treated
cells exhibited a lesser effect on the viability of SK-Mel-5 cells
in a concentration and time-dependent manner compared to
Kenalog-IR. At 24 and 48 h, 100 µg/ml Kenalog-IR significantly
reduced cell viability to 29% (P=1×10−11) and 35%
(P=2×10−10), respectively (Fig. 2A) compared to the control and 100
µg/ml of Kenalog. In addition to the enhanced specific desired
cytotoxic effect of the drug, attaining a platform that will not
induce toxic effects in normal cells is very important in cancer
treatment. The non-specific cytotoxic effects of Kenalog-IR in the
skin fibroblast cell line CCD-986sk was observed. At 24 h,
Kenalog-IR significantly reduced cell viability to 86% (P=0.004)
but no significant differences (P=0.87) were observed between
Kenalog and Kenalog-IR-treated groups (Fig. 2B). This indicated that treatment of
SK-Mel-5 cells with Kenalog-IR induced less toxicity to normal
cells. Furthermore, trypan blue assay also revealed that Kenalog-IR
significantly induced 79% (P=6×10−7) of cell death as
compared to 8% (P=0.01) observed in Kenalog-treated cells (Fig. 2C). For the total number of cells, a
similar trend was observed where Kenalog-IR reduced the number of
cells to approximately half (Fig.
2D) when compared to the control and Kenalog-treated cells. In
addition, the cytotoxic effects related to apoptosis were also
observed by morphological changes in Kenalog-IR-treated cells. The
morphological assessment revealed that, 100 µg/ml of Kenalog-IR
caused plasma membrane protrusion and changes in pigmentation as
observed with white patches (Fig.
2E). Collectively the results revealed that Kenalog-IR
exhibited a potential effect in anticancer treatment compared to
the original drug, Kenalog.
Kenalog-IR induces apoptosis and
aggravates DNA integrity
As observed in Fig.
2, Kenalog-IR treated cells exhibited morphological changes and
cell death when compared to Kenalog-treated cells. To understand
the nature of Kenalog-IR-induced cell death, apoptosis and the cell
cycle were assessed in SK-Mel-5 cells for 24 h. As indicated by
flow cytometry, Kenalog-IR treated cells resulted in 62%
(P=2.5×10−12) of total apoptosis and 37%
(P=1.6×10−12) of live cells (Fig. 3A and B). In comparison to the live
cells in the control group, the reduction of a number of live cells
in Kenalog-IR treated cells indicated that at 24 h many cells had
progressed into apoptosis. Since apoptosis is known to be triggered
through the intrinsic (caspase-mediated) pathway or extrinsic
(death receptor-mediated) pathway, it was of interest to determine
which pathway was involved in Kenalog-IR-induced cell death. To
investigate this phenomenon, we blocked the intrinsic apoptosis
pathway with caspase pan-inhibitor Z-VAD FMK and treated the cells
as aforementioned. The results revealed that blocking caspase
activation decreased total apoptosis to 28%
(P=1.09×10−5) in Kenalog-IR-treated cells (Fig. 3C and D) which indicated the
involvement of intrinsic apoptosis in cell death. Furthermore, DNA
fragmentation analysis revealed that Kenalog-IR induced cell death
as confirmed by DNA fragments observed in a gel electrophoresis
result (Fig. 3E, lane 5). To assess
that no extrinsic apoptosis was involved, we investigated
caspase-8, a key molecule in the extrinsic pathway. The result
revealed no activation of caspase-8 in Kenalog-IR treated cells
(Fig. 3F). Furthermore, it was of
interest to reveal whether Kenalog-IR-induced apoptosis was related
to cell cycle arrest. As revealed (Fig.
3G and H), Kenalog-IR induced 36.3% (P=0.003) cell arrest in
the G2/M phase even when combined with nocodazole a known inducer
of G2/M arrest, the same effect was observed (38.6%,
P=2.5×10−4). In sum, these data indicated that
Kenalog-IR induced intrinsic apoptosis with a compromised cell
cycle.
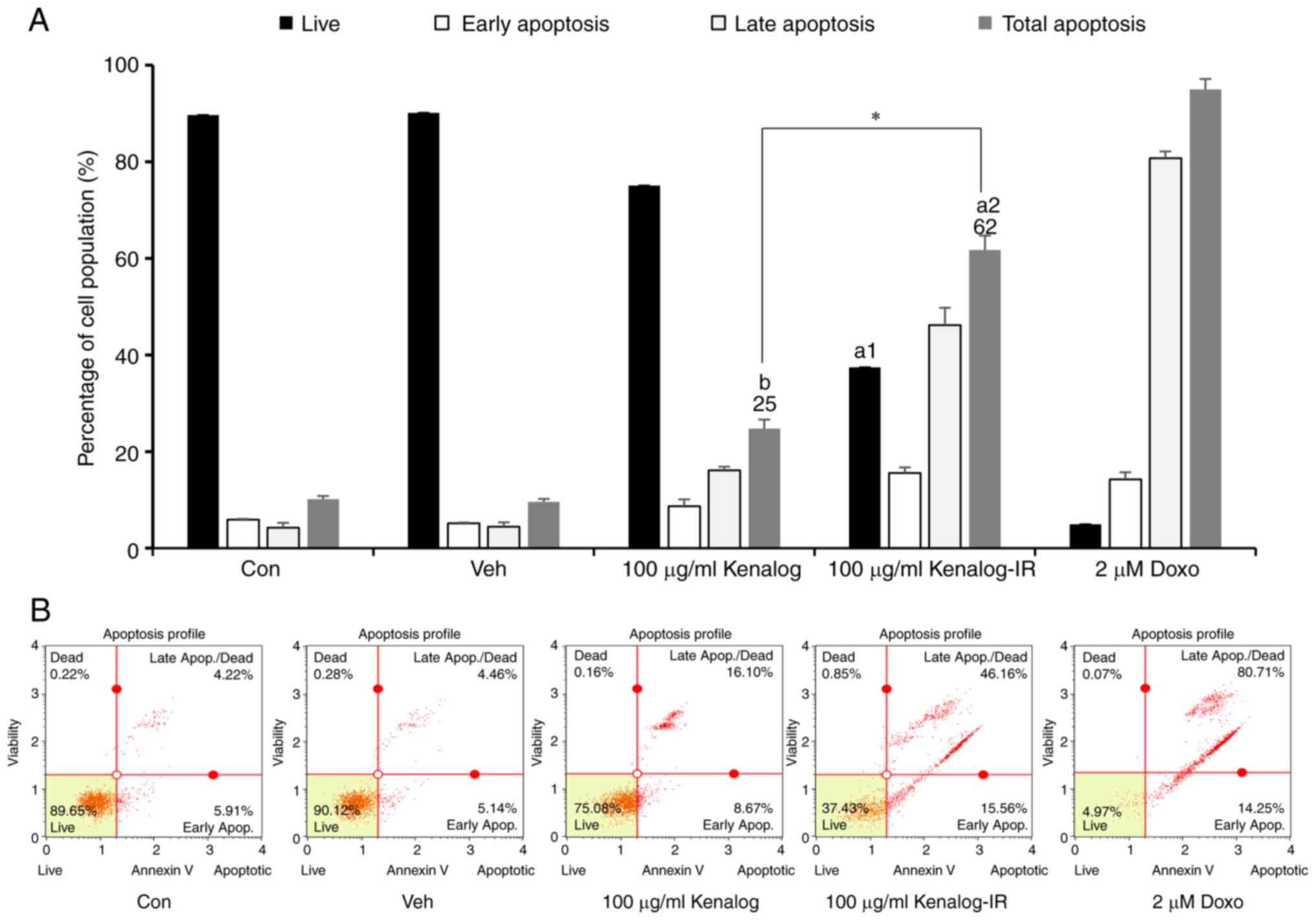 | Figure 3.Kenalog-IR induces apoptosis with
aggravating DNA integrity. Apoptosis and cell cycle analysis of
Kenalog- and Kenalog-IR-treated cells was determined by Muse
Annexin V-FITC/PI and a Muse cell cycle flow cytometer. (A)
Percentage of live, early, late and total apoptosis. The population
of positively stained propidium iodine cells was used to calculate
the percentage of viable cells (a1=2.5×10−12,
a2=1.6×10−12, b=0.9,
*=2.5×10−11). (B) Quadrants indicating Annexin V-FITC/PI
stained SK-Mel-5 cells exhibiting live, early apoptosis, late
apoptosis, and dead cells. (C and D) Cells were pretreated with 40
µM of Z-VAD-FMK for 1 h and further treated with treatments +
Z-VAD-FMK for 24 h. (C) Cells were harvested for percentage
apoptosis analysis (a=1.09×10−5,
b=0.84 and n.s=0.73) and (D) Annexin
V-FITC/PI quadrants. (E) DNA fragmentation assay. Cells were
treated with treatments as indicated for 24 h and DNA was harvested
for 1.2% gel electrophoresis. In addition, a DNA ladder (1 Kb) was
used as the marker at 100 V/cm for 30 min. (F) Verification of
extrinsic apoptosis. Cells were treated as indicated with Z-VAD-FMK
for western blot assay. The anti-caspase-8 antibody was used to
detect the extrinsic apoptosis pathway and GAPDH was used as a
control. (G and H) Cell cycle analysis after 24 h of treatment.
Nocodazole (400 nM) was used to induce G2/M cell cycle arrest. (G)
The cell cycle summary (a1=0.01, a2=0.003,
a3=2.5×10−6, *=1.8×10−5,
**=1.3×10−7), and the phases indicated (H) the DNA
content index profiles in each group and cell cycle phases G1/0, S
and G2/M, expressed as the percentage of positively-stained cells
(G). Data are presented as the mean ± SEM of three independent
experiments (*P<0.05 between treatment groups, ‘NS’ indicates
not significant between treatment groups, small letters ‘a’ and ‘b’
indicate significant and not significant differences between
treatments and the control respectively). IR, ionizing
radiation. |
Kenalog-IR induces apoptosis through
the intrinsic mitochondrial pathway
To further examine the apoptosis pathway involved in
Kenalog-IR-induced cell death, the expression of several programmed
cell death markers was examined by immunoblotting. Apoptosis is
induced either by the intrinsic pathway based on caspase activation
or the extrinsic pathways involving death receptors (18). The expression of pro- and anti-Bcl-2
family proteins, caspase-3 and PARP was gradually determined at 100
µg/ml (Fig. 4A). Further analysis
of the Bcl-2 family 24 h after Kenalog-IR treatment, revealed
increased levels of pro-apoptotic members Bax (P=0.0042) and Bad
(P=4×10−8) and decreased expression levels of
anti-apoptotic member Bcl-2 (P=0.041) (Fig. 4B). Executioner caspases, markers of
the intrinsic pathway were also found to be activated. Kenalog-IR
activated the expression of cleaved caspase-7
(P=2.8×10−10) and cleaved caspase-3
(P=2.4×10−11) while it decreased the expression of both
the total and the cleaved form of caspase-9 (Fig. 4C) at 24 h of treatment. The
activation of caspase-7 and −3 indicated the involvement of the
intrinsic pathway. Furthermore, the mediator of the intrinsic
pathway involved in the release of the PARP enzyme was
investigated. PARP is involved in the release of mitochondrial
proteins such as cytochrome c or apoptosis-inducing factor
(AIF) and regulates its translocation to the cytoplasm (19). Kenalog-IR decreased AIF protein
levels in the mitochondria fraction (Fig. 4E, mitochondria fraction lane 3) and
induced the expression of cleaved PARP at 24 h of treatment
(Fig. 4D). These results
demonstrated that Kenalog-IR induced apoptosis through activation
of the intrinsic mitochondrial pathway in SK-Mel-5 cells.
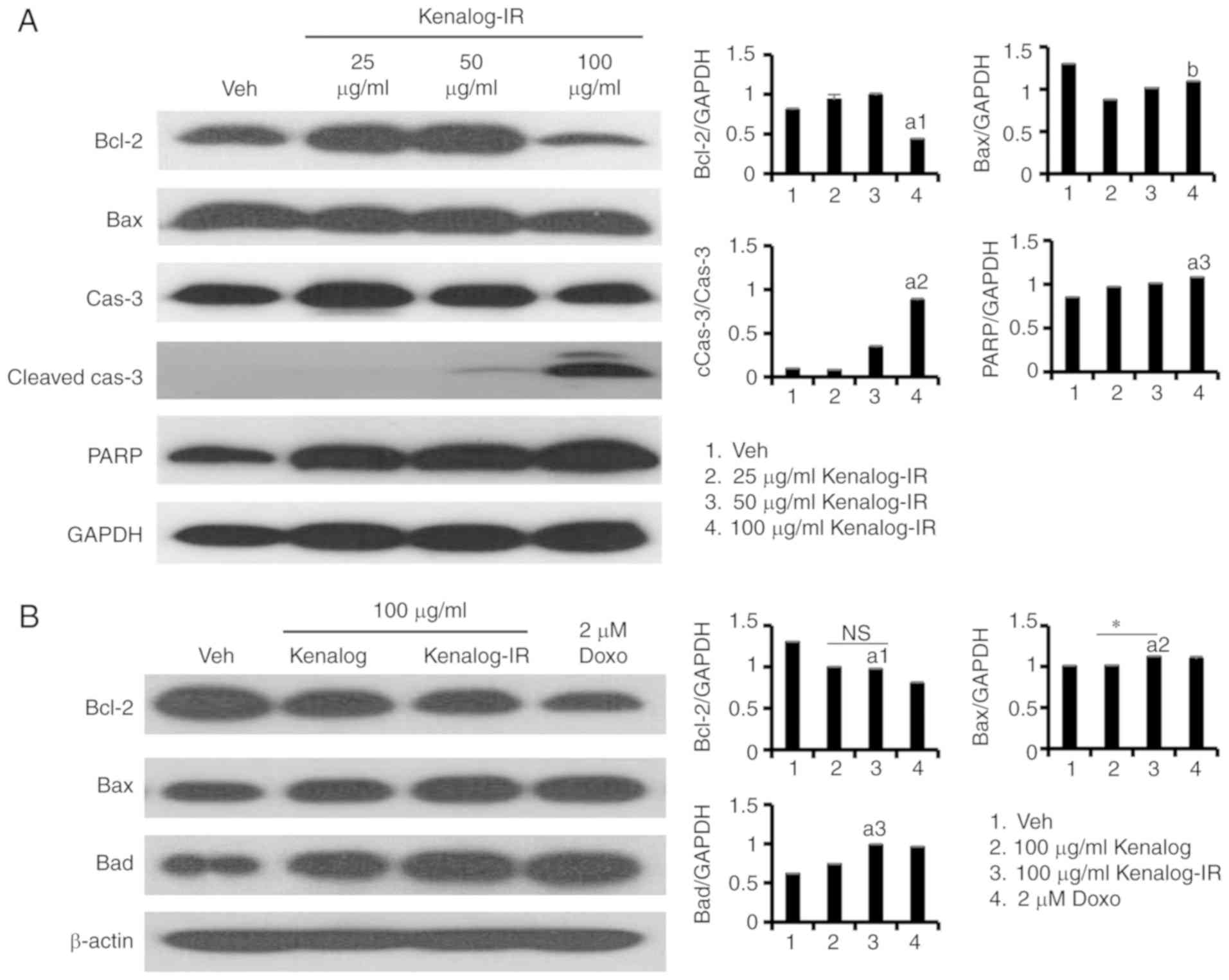 | Figure 4.Kenalog-IR induces apoptosis through
the intrinsic mitochondrial pathway. Analysis of apoptotic protein
markers. Whole cell-lysates were extracted by RIPA and an equal
amount of protein was analyzed by western blotting. (A)
Dose-dependent expression of Bcl-2 family proteins, caspase-3 and
PARP gradually determined at 100 µg/ml of Kenalog-IR treatment
(a1=1.2×10−7,
a2=3.4×10−12, a3=0.0065 and
b=0.072). (B) Analysis of pro- and anti-apoptotic Bcl-2
family proteins 24 h after Kenalog-IR treatment
(a1=0.041, a2=0.0042,
a3=4×10−8, n.s=0.18 and
*=0.00017). (C) The Kenalog-IR activated expression of intrinsic
apoptosis pathways proteins (a1=1.1×10−13,
a2=2.8×10−10 and
a3=2.4×10−11) and (D) Kenalog-IR induced the
expression of cleaved PARP 24 h of treatment (a=0.00006
and *=1×10−7). (E) SK-Mel-5 cells were cultured in
absence (line 1) or presence of Kenalog (line 2), Kenalog-IR (line
3) and Doxo as a positive control (line 4). Cytosolic and
mitochondria fractions were separated by 12% SDS-PAGE. The
histograms shown in the right panel of parts A-D indicate ImageJ
extracted intensities as normalized against the control protein
(GAPDH) or the total form of respective proteins. Data are
presented as the mean ± SEM of three independent experiments
[*P<0.05 between treatment groups, ‘NS’ indicates not
significant between treatment groups, a small letter ‘a’ indicates
significant differences between treatments and the control or
calibrator (GAPDH) or the total form of the protein of interest].
IR, ionizing radiation; Doxo, doxorubicin. |
Kenalog-IR increases production of ROS
which in turn increases mitochondria-mediated apoptosis
Reactive oxygen species (ROS) is the hallmark of
apoptosis and a significant indicator of cells undergoing oxidative
stress. Normally, the induction of the intrinsic pathway is linked
with the leakage of mitochondrial proteins which pass through the
distracted electron transport chain (ETC) and stimulate further
production of ROS and executor proteins. Based on our
aforementioned results, caspase activation in particular caspase-3
and −7 were regarded as key molecules revealing impaired
mitochondrial function (20,21).
Given these facts, investigation of whether apoptotic induction was
linked with ROS production was performed. Using intracellular
detection of superoxide radicals by dihydroethidium (DHE), it was
revealed that Kenalog-IR significantly induced 84%
(P=3.7×10−7) of ROS positive cells (Fig. 5A, lane 4) compared to 31% (P=0.990)
of Kenalog-treated cells (Fig. 5A,
lane 3). Furthermore, blocking of ROS by N-acetyl-Cysteine (NAC)
revealed a reduction of ROS positive cells to 27% (P=0.06)
(Fig. 5A, lane 5). These results
indicated that Kenalog-IR-induced apoptosis was mediated by ROS
production. It is well known that accumulation of superoxide and
other oxidative stress molecules are associated with increased
levels of ROS which cause increased expression of the enzyme sodium
dismutase (SOD) and subsequently a downstream pathway related to
ROS induction (Fig. 5C). To
ascertain this, the source of ROS induced by Kenalog-IR was first
revealed. The MitoSOX assay results revealed that the majority of
ROS were generated from the mitochondria (Fig. 5D). To establish whether the
increased levels of apoptosis were related to ROS, western blot
analysis was performed for the ROS-related pathway. The results
revealed that the expression level SOD1 was increased in
Kenalog-IR-treated cells (Fig. 5E,
lane 3) demonstrating that ROS was increased and scavenged by this
enzyme. Furthermore, decreased expression of both phosphorylated
AKT (P=2.86×10−9) and phosphorylated mTOR
(P=2×10−5) molecules (Fig.
5E) was observed indicating the involvement of the AKT/mTOR
pathway in the induction of apoptosis. The experiments in Fig. 5, revealed that ROS production was
the source of Kenalog-IR-induced apoptosis in SK-Mel-5 cells.
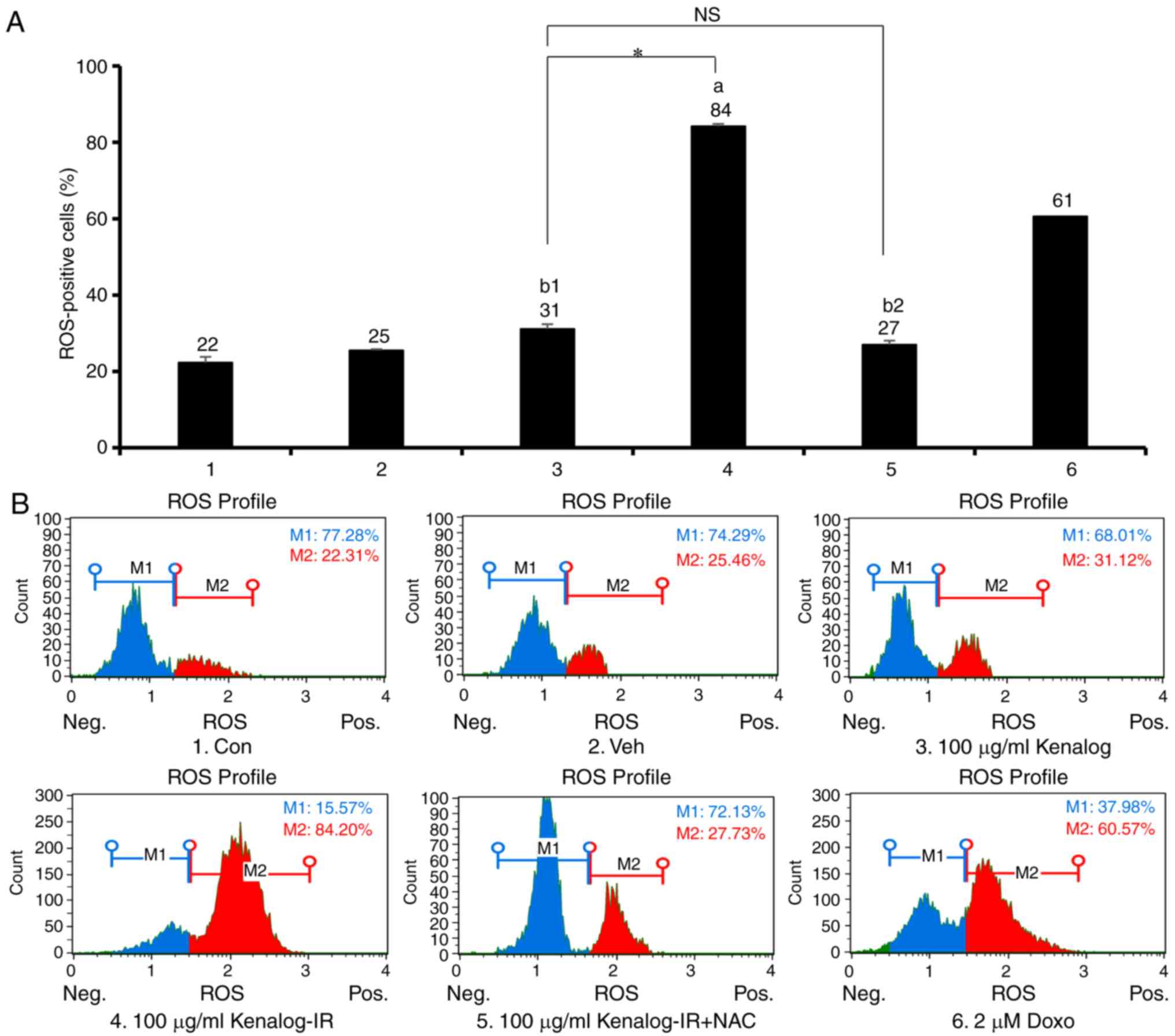 | Figure 5.Kenalog-IR increases production of
ROS which in turn increases mitochondria-mediated apoptosis.
Intracellular ROS levels of SK-Mel-5 cells. Cells treated with
Kenalog and Kenalog-IR for 24 h were assessed by flow cytometer.
(A) The ROS-negative (M1) and ROS-positive (M2) cells were
expressed as a percentage in M1 and M2 gates respectively
(a=3.7×10−7, b1=0.99,
b2=0.065, *=2.9×10−9,
n.s=0.175). (B) The population of positive ROS-stained
cells was used to calculate the percentage of ROS-stained cells as
shown in the histogram. (C) Hypothetical illustration of
Kenalog-IR-induced ROS. (D) Mitochondrial ROS determination by flow
cytometer. Cells were pre-labeled with 5 µM MitoSOX reagent for 1 h
and exposed to treatment for 18 h. Cells were harvested, washed and
further incubated with MitoSOX reagent for 10 min at 37°C in the
dark. Quadrants indicate ROS-positive cells. (E) Analysis of the
ROS-mediated pathway. Cells were treated with Kenalog or Kenalog-IR
and cell lysates were subjected to 10 or 15% SDS-PAGE. SOD1 was
used to denote increased levels of ROS while GAPDH was used as a
control. The histograms shown in the right panel indicate ImageJ
extracted intensities as normalized against the total protein
(a1=2.86×10−9,
a2=2×10−5, *=1.04×10−8, **=0.002).
Data are presented as the mean ± SEM of three independent
experiments (*P<0.05 between treatment groups, ‘NS’ indicates
not significant between treatment groups, small letters ‘a’ and ‘b’
indicate significant and not significant differences between
treatments and the control respectively). IR, ionizing radiation;
ROS, reactive oxygen species. |
Discussion
Kenalog is a synthetic glucocorticoid used to treat
various cancers and skin diseases. Treatment with Kenalog rarely
achieves sustainable results for patients and has adverse effects.
To improve its efficacy, ionizing radiation was used to modify the
structure of Kenalog to develop a potential anticancer drug. As
analyzed by LC-MS, Kenalog-IR formed four peaks (Fig. 1, lower panel) which indicated a
promising role of IR in the field of IMD. While the single
structures of Kenalog-IR remain to be isolated, its enhanced
anticancer activity cannot be ruled out especially in the treatment
of melanoma skin cancer. Previously, research by our group revealed
the role of IR in enhancing the activity of anticancer drugs in the
treatment of hepatic and lung cancer cells (16,17).
The findings of these two studies proposed the innovative use of IR
to modify the active structure of various glucocorticoids such as
Kenalog to increase their potency. While some studies have revealed
the use of Kenalog in the management of ocular inflammatory
(7) and choroidal melanoma
(6), its effectiveness is
constantly challenged like many other glucocorticoids (6,22,23).
Furthermore, the biogenesis of melanocytes that produce several
intermediate proteins which are highly immunogenic and the ability
of skin to produce glucocorticoids from cholesterol limit
therapeutic use of several glucocorticoids in skin cancer
applications (24). However, in the
present study, the enhanced role of Kenalog by IR was reported to
improve anticancer activities in melanoma cancer cells.
Generation of apoptosis and apoptosis-related
markers in drug treatment trials is a superlative approach in
cancer treatment. Apoptosis is an important phenomenon in the
mechanism of homeostasis, it balances several processes including
cell division and cell demise (25,26).
Activation of the apoptosis pathway can be initiated from different
entry points, either at the plasma membrane upon death receptor
activation (receptor/extrinsic pathway) or at the mitochondria
(mitochondrial/intrinsic pathway) (18). While it is generally accepted that
the downstream key molecule for the extrinsic pathway is caspase-8,
the intrinsic pathway has several key molecules that initiate a
cytochrome c/Apaf-1/caspase-9-containing apoptosome complex
to form a unique network of intrinsic apoptosis (27). The present study, proposed a new
modified Kenalog compound, Kenalog-IR which induced characteristic
intrinsic apoptosis cell death as observed by suspended apoptotic
bodies and plasma membrane protrusion (Fig. 2D) with induction of 62% of total
apoptosis (Fig. 3). The results
were further confirmed by observed DNA fragmentation (Fig. 3E) in the Kenalog-IR-treated group
and the activation of cleaved caspases indicated the involvement of
the intrinsic pathway (Figs. 3C,
4E and 5A). The control and regulation of cancer
cell death through the intrinsic apoptotic pathway involved
induction of protein markers including the release of cytochrome
c as a result of an imbalance between Bcl-2 family proteins
(21,28). The imbalance between these proteins
caused the release of caspase proteins from cleaved caspase-9
downstream to cleaved-caspase-3 which was associated with impaired
mitochondria, the critical site of ROS production. Manifestation of
impaired mitochondria was revealed by an excessive imbalance of
Bcl-2 family proteins as observed in Fig. 4A and B, and increased production of
mROS observed in Fig. 5D following
Kenalog-IR treatment. Furthermore, the use of antioxidant NAC
(Fig. 5A and B) indicated that ROS
was the main cause of Kenalog-IR-induced apoptosis associated with
intrinsic cell death in melanoma cancer cells. Elevated levels of
ROS further triggered the release of intrinsic mitochondrial
proteins to the cytosol with subsequent activation of cleaved forms
of caspase-7 and −3 and deactivation of cleaved caspase-9 (Fig. 4C) possibly causing the programmed
death of melanoma cancer cells (29).
However, initiation and execution of apoptosis are
mediated by cysteine-dependent aspartate such as caspase-3 in
association with the activation of cleaved poly(ADP-ribose)
polymerase PARP (30–32). The PARP enzyme is important in the
release of mitochondrial proteins and a crucial molecule in the
intrinsic apoptosis pathway (30).
Attenuation of AIF in the mitochondria fraction (Fig. 4E) and increased levels of cleaved
PARP observed in Fig. 4D indicated
the regulatory role of PARP in the translocation of mitochondrial
proteins involved in programmed cell death. To relate the mechanism
of intrinsic apoptosis with the ROS-mediated cell death induced by
Kenalog-IR, increased G2/M phase cell arrest was observed even when
the cells were treated with nocodazole a known inducer of G2/M cell
arrest. This observation indicated that Kenalog-IR affected the
cell cycle associated with ROS through the AKT/mTOR signaling
pathway (Fig. 5D). The
PI3K/AKT/mTOR pathway plays an important role in cell
proliferation, migration, cellular metabolism, protein synthesis
and apoptosis (33). Phosphorylated
AKT (p-AKT) activates many downstream targets including mTOR and
GSK-3β, which are important in controlling the cell cycle and
apoptosis (34). Since, Kenalog-IR
attenuated phosphorylation of AKT and mTOR in SK-Mel-5 cells, this
may indicate that cell death involved with cell cycle progression
and proliferation may also induce cell death.
Collectively, the findings of the present study
elucidated the mechanism of cell death induced by Kenalog-IR during
melanoma cancer treatment, however the use of such a therapeutic
approach especially in skin cancers still remains challenging.
Several corticosteroids and glucocorticoids have been reported to
have low solubility, low bioavailability and longer duration of
action (12). The increased
cytotoxic effect of Kenalog-IR may suggest improved bioavailability
and shortened duration of action in melanoma cells when compared to
Kenalog (Fig. 2). Since many
glucocorticoids are immunosuppressive, we suggested that the
actions induced by Kenalog-IR may be attributed to commonly known
transcriptional effects of glucocorticoid receptor (GR) agonists
which alter the transcription of many genes involved in cell death.
This may lead to increased binding affinity of GR(s), known to be
G2/M cell-cycle dependent (Fig.
3G), which partly enhanced cellular death by Kenalog-IR
(35,36). This may also indicate that intrinsic
apoptosis is not the only mechanism of cell death induced by
Kenalog-IR, instead other glucocorticoid-induced signaling pathways
may lead to cell death. Furthermore, due to the biogenesis of
melanocytes (24), the cytotoxic
effects induced to the surrounding environment by both melanoma
cells and the drug remain a challenge which requires a
resolution.
In conclusion, the present study, elucidated the
importance of an IR-modified drug in cancer treatment. Kenalog-IR
inhibited melanoma cancer cell proliferation and caused cell death
by the intrinsic apoptosis pathway. Moreover, Kenalog-IR-induced
cell death was associated with increased production of ROS
modulated by the release of caspases and activation of cleaved
PARP. In general, the results of the present study, justify the
hypothesis that incrementally modified drugs by IR may potentially
be used as anticancer candidates in various cancer treatment
strategies. However, further studies will have to be conducted in
the future to determine the structures of Kenalog generated by IR
and the stability of these compounds before being subjected to
clinical trials.
Acknowledgements
Not applicable.
Funding
This research was funded by the Ministry of Science
and ICT through the Basic Science Research Program by the Korean
government.
Availability of data and materials
The analyzed datasets generated during the study are
available from the corresponding author upon reasonable
request.
Authors' contributions
RAK, EHL and HWB conceived, designed and wrote the
study. RAK, FJR, HJC and CHP performed the experiments and analyzed
data. BYC and HWB were involved in the conception of the study,
supervised the experimental work and revised the manuscript. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patients consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
IR
|
ionizing radiation
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
ROS
|
reactive oxygen species
|
|
IMD
|
incrementally modified drugs
|
References
|
1
|
Haller J, Mikics É and Makara GB: The
effects of non-genomic glucocorticoid mechanisms on bodily
functions and the central neural system. A critical evaluation of
findings. Front Neuroendocrinol. 29:273–291. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wooldridge JE, Anderson CM and Perry MC:
Corticosteroids in advanced cancer. Oncology. 15:225–234.
2001.PubMed/NCBI
|
|
3
|
Kim DW, Kim YI, Ud Din F, Cho KH, Kim JO
and Choi HG: Development of a novel triamcinolone acetonide-loaded
spray solution for the treatment of stomatitis. Pharmazie.
69:512–517. 2014.PubMed/NCBI
|
|
4
|
Paspulati A, Punjabi OS, Theodoropoulou S
and Singh RP: Triamcinolone acetonide as an adjuvant to membrane
peeling surgery: A pilot study. Ophthalmic Surg, Lasers Imaging
Retina. 44:41–45. 2013. View Article : Google Scholar
|
|
5
|
Takata S, Masuda T, Nakamura S, Kuchimaru
T, Tsuruma K, Shimazawa M, Nagasawa H, Kizaka-Kondoh S and Hara H:
The effect of triamcinolone acetonide on laser-induced choroidal
neovascularization in mice using a hypoxia visualization
bio-imaging probe. Sci Rep. 5:98982015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shields CL, Demirci H, Dai V, Marr BP,
Mashayekhi A, Materin MA, Manquez ME and Shields JA: Intravitreal
triamcinolone acetonide for radiation maculopathy after plaque
radiotherapy for choroidal melanoma. Retina. 25:868–874. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yeung CK, Chan KP, Chan CK, Pang CP and
Lam DS: Cytotoxicity of triamcinolone on cultured human retinal
pigment epithelial cells: Comparison with dexamethasone and
hydrocortisone. Jap J Ophthalmol. 48:236–242. 2004. View Article : Google Scholar
|
|
8
|
Perretti M, Paul-Clark M, Mancini L and
Flower R: Generation of innovative anti-inflammatory and
anti-arthritic glucocorticoid derivatives that release NO: The
nitro-steroids. Dig Liver Dis. 35:S41–S48. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wyles CC, Houdek MT, Wyles SP, Wagner ER,
Behfar A and Sierra RJ: Differential cytotoxicity of
corticosteroids on human mesenchymal stem cells. Clin Orthop Relat
Res. 473:1155–1164. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Slominski A, Gomez-Sanchez CE, Foecking MF
and Wortsman J: Metabolism of progesterone to DOC, corticosterone
and 18OHDOC in cultured human melanoma cells. FEBS Lett.
455:364–366. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Slominski A, Zjawiony J, Wortsman J, Semak
I, Stewart J, Pisarchik A, Sweatman T, Marcos J, Dunbar C and C
Tuckey R: A novel pathway for sequential transformation of
7-dehydrocholesterol and expression of the P450scc system in
mammalian skin. Eur J Biochem. 271:4178–4188. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Henderson NK and Sambrook PN: Relationship
between osteoporosis and arthritis and effect of corticosteroids
and other drugs on bone. Curr Opin Rheumatol. 8:365–369. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ha D, Choi Y, Kim DU, Chung KH and Lee EK:
A comparative analysis of the impact of a positive list system on
new chemical entity drugs and incrementally modified drugs in South
Korea. Clin Ther. 33:926–932. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Paul-Clark M, Del Soldato P, Fiorucci S,
Flower RJ and Perretti M: 21-NO-prednisolone is a novel nitric
oxide-releasing derivative of prednisolone with enhanced
anti-inflammatory properties. Br J Pharmacol. 131:1345–1354. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo Z, Guo A, Guo Q, Rui M, Zhao Y, Zhang
H and Zhu S: Decomposition of dexamethasone by gamma irradiation:
kinetics, degradation mechanisms and impact on algae growth.
Chemical Engineering J. 307:722–728. 2017. View Article : Google Scholar
|
|
16
|
Badaboina S, Bai HW, Na YH, Park CH, Kim
TH, Lee TH and Chung BY: Novel radiolytic rotenone derivative,
rotenoisin B with potent anti-carcinogenic activity in hepatic
cancer cells. Int J Mol Sci. 16:16806–16815. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee EH, Park CH, Choi HJ, Kawala RA, Bai
HW and Chung BY: Dexamethasone modified by gamma-irradiation as a
novel anticancer drug in human non-small cell lung cancer. PLoS
One. 13:e01943412018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Seong YA, Shin PG and Kim GD: Anacardic
acid induces mitochondrial-mediated apoptosis in the A549 human
lung adenocarcinoma cells. Int J Oncol. 42:1045–1051. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cai J, Yang J and Jones DP: Mitochondrial
control of apoptosis: The role of cytochrome c. Biochim
Biophys Acta 1366. 139–149. 1998.
|
|
21
|
Wu CC and Bratton SB: Regulation of the
intrinsic apoptosis pathway by reactive oxygen species. Antioxid
Redox Signal. 19:546–558. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
el Filali M, Homminga I, Maat W, van der
Velden PA and Jager MJ: Triamcinolone acetonide and anecortave
acetate do not stimulate uveal melanoma cell growth. Mol Vis.
14:1752–1759. 2008.PubMed/NCBI
|
|
23
|
Gao H, Qiao X, Gao R, Mieler WF, McPherson
AR and Holz ER: Intravitreal triamcinolone does not alter basal
vascular endothelial growth factor mRNA expression in rat retina.
Vision Res. 44:349–356. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Slominski AT and Carlson JA: Melanoma
resistance: A bright future for academicians and a challenge for
patient advocates. Mayo Clin Proc. 429–433. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheng YL, Lee SC, Lin SZ, Chang WL, Chen
YL, Tsai NM, Liu YC, Tzao C, Yu DS and Harn HJ: Anti-proliferative
activity of Bupleurum scrozonerifolium in A549 human lung
cancer cells in vitro and in vivo. Cancer Lett. 222:183–193. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ye YT, Zhong W, Sun P, Wang D, Wang C, Hu
LM and Qian JQ: Apoptosis induced by the methanol extract of
Salvia miltiorrhiza Bunge in non-small cell lung cancer
through PTEN-mediated inhibition of PI3K/Akt pathway. J
Ethnopharmacol. 200:107–116. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fulda S and Debatin KM: Extrinsic versus
intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene.
25:47982006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma Y, Zhu B, Yong L, Song C and Liu X, Yu
H, Wang P, Liu Z and Liu X: Regulation of intrinsic and extrinsic
apoptotic pathways in osteosarcoma cells following oleandrin
treatment. Int J Mol Sci. 17:E19502016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kroemer G, Galluzzi L and Brenner C:
Mitochondrial membrane permeabilization in cell death. Phys Rev.
87:99–163. 2007.
|
|
30
|
Cregan SP, Dawson VL and Slack RS: Role of
AIF in caspase-dependent and caspase-independent cell death.
Oncogene. 23:2785–2796. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hwang JT, Kwak DW, Lin SK, Kim HM, Kim YM
and Park OJ: Resveratrol induces apoptosis in chemoresistant cancer
cells via modulation of AMPK signaling pathway. Ann NY Acad Sci
1095. 441–448. 2007. View Article : Google Scholar
|
|
32
|
Hwang JT, Ha J, Park IJ, Lee SK, Baik HW,
Kim YM and Park OJ: Apoptotic effect of EGCG in HT-29 colon cancer
cells via AMPK signal pathway. Cancer Lett. 247:115–121. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Morgensztern D and McLeod HL:
PI3K/Akt/mTOR pathway as a target for cancer therapy. Anticancer
Drugs. 16:797–803. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dobbin ZC and Landen CN: The importance of
the PI3K/AKT/MTOR pathway in the progression of ovarian cancer. Int
J Mol Sci. 14:8213–8227. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ashwell JD, Lu FW and Vacchio MS:
Glucocorticoids in T cell development and function. Ann Rev
Immunol. 18:309–345. 2000. View Article : Google Scholar
|
|
36
|
Coutinho AE and Chapman KE: The
anti-inflammatory and immunosuppressive effects of glucocorticoids,
recent developments and mechanistic insights. Mol Cell Endocrinol.
335:2–13. 2011. View Article : Google Scholar : PubMed/NCBI
|