Introduction
Gastric cancer (GC) is one of the most common solid
tumors and the third leading cause of cancer-related deaths
worldwide (1,2). Despite improvements in treatment
approaches, prognosis of patients with advanced GC remains poor
even after curative resection.
Among various tumor subtypes, α-fetoprotein
(AFP)-producing GC (AFPGC) is recognized as a most aggressive tumor
with subsequent poor prognosis compared with common GC subtypes,
with a high propensity for liver metastasis (3–7). AFPGC
has been reported to have high proliferative, proangiogenic and
reduced apoptotic activity by immunohistochemical examination
(7). However, the molecular
mechanism remains to be elucidated.
Among various molecules, microRNAs (miRNAs) have
attracted attention as having oncogenic or tumor-suppressive
functions and also tumor progression-related factors. miRNAs are
endogenous, small, non-coding, single-stranded RNAs of 20–25
nucleotides that regulate the expression of target genes at the
post-transcriptional level by binding to complementary sequences
(8). A single miRNA can influence
the expression of hundreds of genes. Therefore, we hypothesized
that some specific miRNAs may play crucial roles in AFPGC.
Previously, we demonstrated that miR-122-5p was
significantly higher in AFPGC tissues and that the plasma
expression level may be a useful biomarker in patients with AFPGC
(9). In the present study, we
examined the biological function of miR-122-5p and the
molecular mechanism underlying tumor progression in AFPGC.
Materials and methods
Culture of GC cell lines and primary
tumor samples
The AFPGC cell line FU97 and the other GC cell lines
MKN7, MKN74 and NUGC-3 were purchased from the Japanese Collection
of Research Bioresources Cell Bank (Osaka, Japan). FU97 was
cultured in Dulbecco's modified Eagle's medium (DMEM; Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (FBS; Invitrogen; Thermo Fisher Scientific,
Inc.), penicillin-streptomycin (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) and 10 mg/l insulin (Sigma-Aldrich; Merck
KGaA). The other GC cell lines were cultured in Roswell Park
Memorial Institute (RPMI)-1640 medium (Invitrogen; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS and
penicillin-streptomycin (Sigma-Aldrich; Merck KGaA). All cells were
cultured in 5% carbon dioxide at 37°C in a humidified chamber.
All primary AFPGC and non-AFPGC samples at various
stages as controls from the same cohort were obtained from 25
patients (18 males and 7 females) who underwent gastrectomy for GC
at the University of Yamanashi Hospital between January 2012 and
December 2018 (age, 68.04±10.97 years). Tumor specimens and
resected lymph nodes obtained at surgery were immediately fixed in
10% neutral-buffered formalin and embedded in paraffin after
fixation. AFPGC was defined on the basis of a plasma AFP level
>10 ng/ml or positive AFP immunoreactivity in tissue
samples.
The present study was approved by the Ethics
Committee of the Yamanashi University and was performed in
accordance with the ethical standards of the Declaration of
Helsinki and its amendments. Informed written consent was obtained
from all patients.
Transfection of FU97 with miRNA
inhibitors
The miRNA inhibitors for miR-122-5p (cat. no.
MH11012) (UGGAGUGUGACAAUGGUGUUUG) and negative control anti-miRNA
(mirVana miRNA Inhibitor Negative Control #1)
(UUACGUCGUCGCGUCGUUAU) were purchased from Thermo Fisher
Scientific, Inc. miRNA inhibitors were transfected into cells at
the indicated concentration using Lipofectamine RNAiMAX (Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. The transfection concentration of miR-122-5p
inhibitor and negative control used in the present study was 30 nM.
Total RNA and protein were subsequently collected at 48 h following
transfection.
Quantitative reverse
transcription-polymerase chain reaction (qRT-PCR)
Total RNA was extracted using the miRNeasy Mini kit
(Qiagen GmbH, Hilden, Germany) following the manufacturer's
protocols. Subsequently, a NanoDrop 2000 spectrophotometer (Thermo
Fisher Scientific, Inc.) was used to measure the total RNA
concentration. The reverse transcription reaction was conducted
with a High-Capacity cDNA Reverse Transcription kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.) and TaqMan MicroRNA
Reverse Transcription kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.). Total RNA and miRNA levels were quantified by
qRT-PCR according to standard procedures. qRT-PCR conditions were
as follows: Pre-heating for 10 min at 95°C; repeating 40 cycles at
95°C for 15 sec and 60 sec at 60°C. Total RNA levels were
normalized to the endogenous control gene
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and miRNA
levels were normalized to RNU6B. The following primers were
used for the TaqMan assay (Thermo Fisher Scientific, Inc.):
AFP (cat. no. Hs01040598_m1), forkhead box O3 (FOXO3;
cat. no. Hs00818121_m1), human hsa-miR-122-5p (cat. no.
002245) (UGGAGUGUGACAAUGGUGUUUG), GAPDH (. no. Hs02786624_g1)
(forward, 5′-AATGGACAACTGGTCGTGGAC-3′ and reverse,
5′-CCCTCCAGGGGATCTCTGTTTG-3′) and RNU6B (cat. no. 001093).
qRT-PCR was performed in triplicate. ΔCq values for all RNAs
relative to the control gene GAPDH and RNU6B were
determined. The relative mRNA expression was assessed using
2−ΔΔCq method (10).
Targeted prediction of miR-122-5p was analyzed by TargetScan
(www.targetscan.org).
Western blotting
Anti-AFP (dilution 1:200; cat. no. ab54745),
anti-bcl-2 (dilution 1:500; cat. no. ab692), anti-caspase-3
(dilution 1:5,000; cat. no. 32351) and anti-β-actin (dilution
1:1,000; cat. no. ab8227) were purchased from Abcam (Cambridge, MA,
USA). The cells were harvested in RIPA buffer (Thermo Fisher
Scientific, Inc.) supplemented with protease inhibitors (Cell
Signaling Technology, Inc., Danvers, MA, USA) and the protein
concentrations were determined using the bicinchoninic acid (BCA)
protein assay kit (Thermo Fisher Scientific, Inc.). Cell lysate
proteins (40 µg/lane) were separated by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then
transferred onto polyvinylidene fluoride (PVDF) membranes (Merck
USA, Minneapolis, MN, USA). The PVDF membranes were then incubated
in Odyssey blocking buffer (PBS) (LI-COR Biosciences, Lincoln, NE,
USA) for 1 h at room temperature. The PVDF membranes were then
incubated with primary antibodies overnight at 4°C. Then, the
membranes were washed four times for 5 min in PBS and incubated
with goat anti-rabbit and anti-mouse IgG conjugated to horseradish
peroxidase (dilution 1:1,000; cat. nos. ab6721 and ab6789; Abcam)
buffer for 1 h at room temperature. The membranes then were probed
with the indicated antibodies, and proteins were detected by an ECL
Prime Western Blotting Detection reagent (Thermo Fisher Scientific,
Inc.).
Proliferation assay and cell cycle
analysis
To assess cell growth, the number of viable cells at
various time-points after transfection was assessed by the
colorimetric water-soluble tetrazolium salt assay (Cell Counting
Kit-8; Dojindo Molecular Technologies, Inc., Rockville, MD, USA).
Cell viability was determined by reading the optical density at 450
nm. The cell cycle was evaluated 24 h after transfection by
fluorescence-activated cell sorting (FACS) (BD Biosciences, Fanklin
Lakes, NJ, USA). For the FACS analysis, harvested cells were fixed
in 70% cold ethanol and treated with RNase (Sigma-Aldrich; Merck
KGaA) and propidium iodide (PI; Sigma-Aldrich; Merck KGaA).
Apoptotic cell analysis
The apoptotic cells were evaluated 24 h after
transfection by FACS. Transfected cells were harvested and stained
with fluorescein isothiocyanate FITC-conjugated Annexin V
and phosphatidylinositol (PI) using an Annexin V kit (Beckman
Coulter, Inc., Brea, CA, USA).
Statistical analysis
Statistical significance was determined using
GraphPad Prism version 5 (GraphPad Software, Inc., San Diego, CA,
USA). Quantitative values were expressed as the means ± standard
deviation (SD) unless noted otherwise. Statistical significance was
evaluated using Student's t-test and one-way analysis of variance
(ANOVA) for each time-point, followed by Tukey's post hoc test. The
correlation between miR-122-5p and FOXO3 in GC tissue
samples was analyzed by Pearson's correlation analysis. P<0.05
was considered to indicate a statistically significant result.
Results
Association between AFP and miR-122-5p
levels in GC cell lines
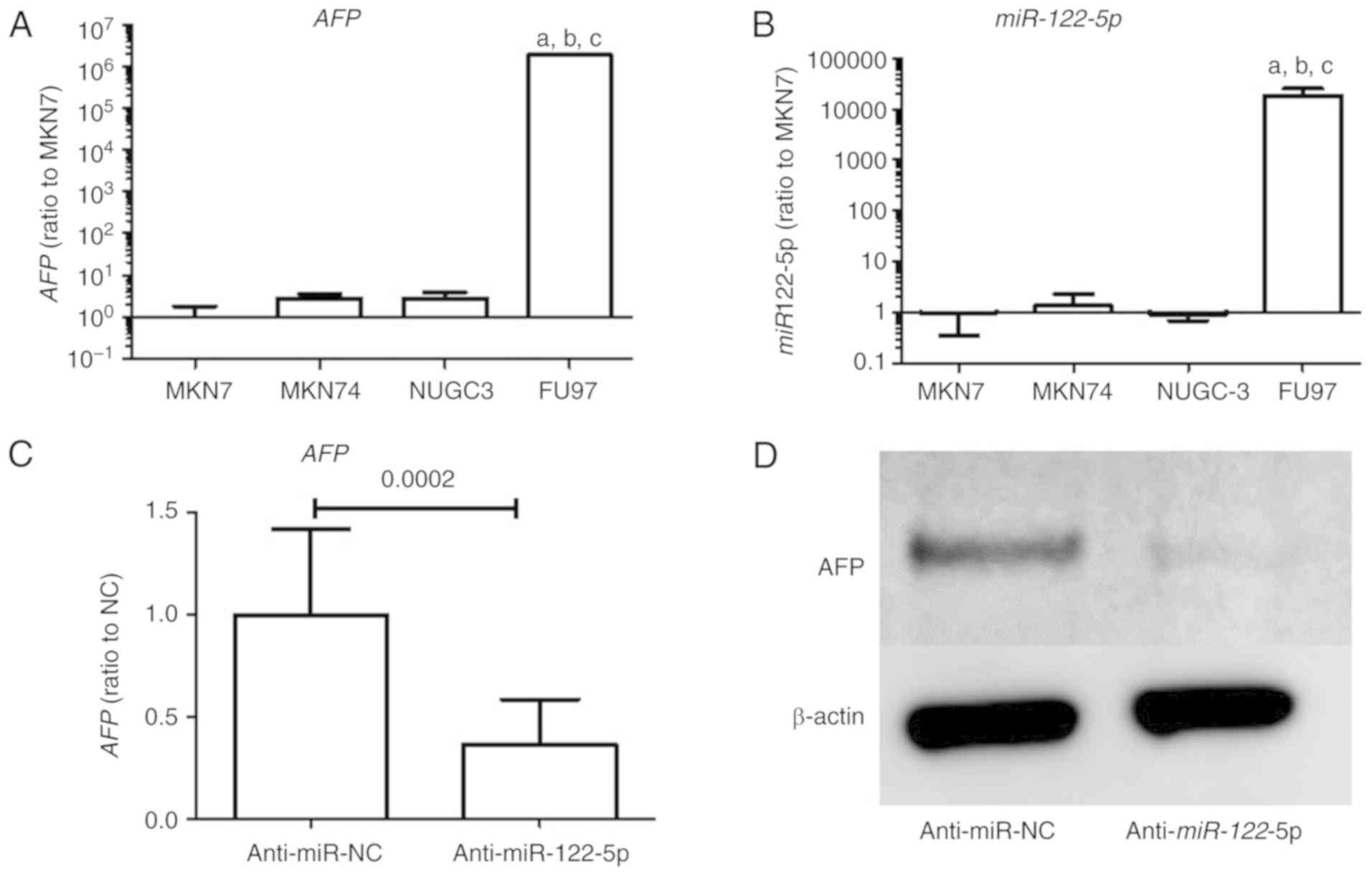
The expression levels of AFP and miR-122-5p
in GC cell lines were significantly higher in FU97 than in the
other non-AFPGC cell lines as determined by qRT-PCR (Fig. 1A and B). Moreover, suppression of
miR-122-5p reduced AFP levels in the FU97 cell line
as determined by qRT-PCR (Fig. 1C).
Protein expression of AFP was also decreased by miR-122-5p
suppression in the FU97 cell line as determined by western blotting
(Fig. 1D).
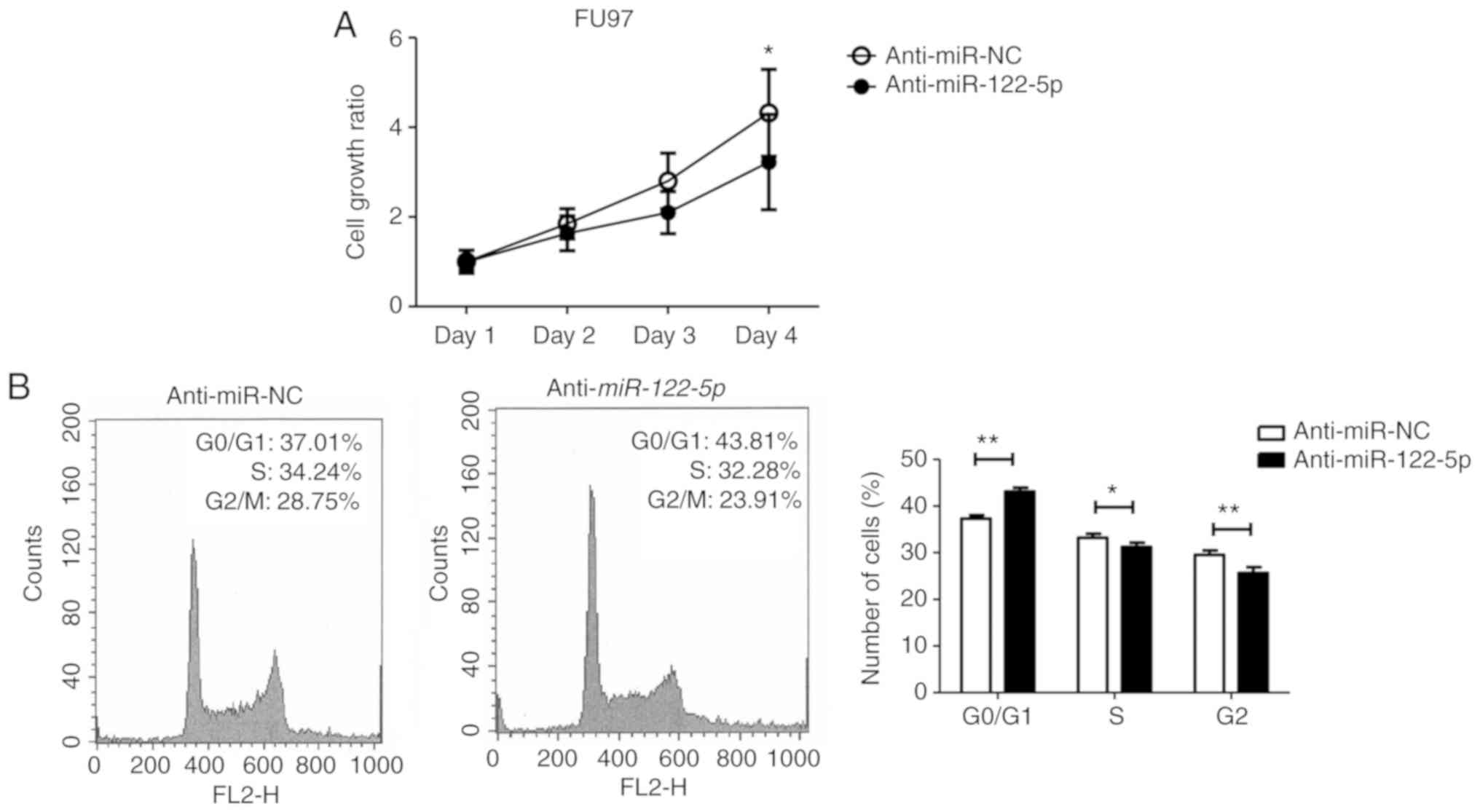
Investigation of the function of
miR-122-5p in the AFPGC cell line
To investigate the function of miR-122-5p in
AFPGC, we first performed a cell proliferation assay using
miR-122-5p inhibitor. Proliferation of FU97 was
significantly inhibited with miR-122-5p inhibitor
transfection compared with negative control inhibitor transfection
(Fig. 2A). FACS analysis revealed
that inhibition of miR-122-5p induced G0/G1 cell cycle
arrest (Fig. 2B).
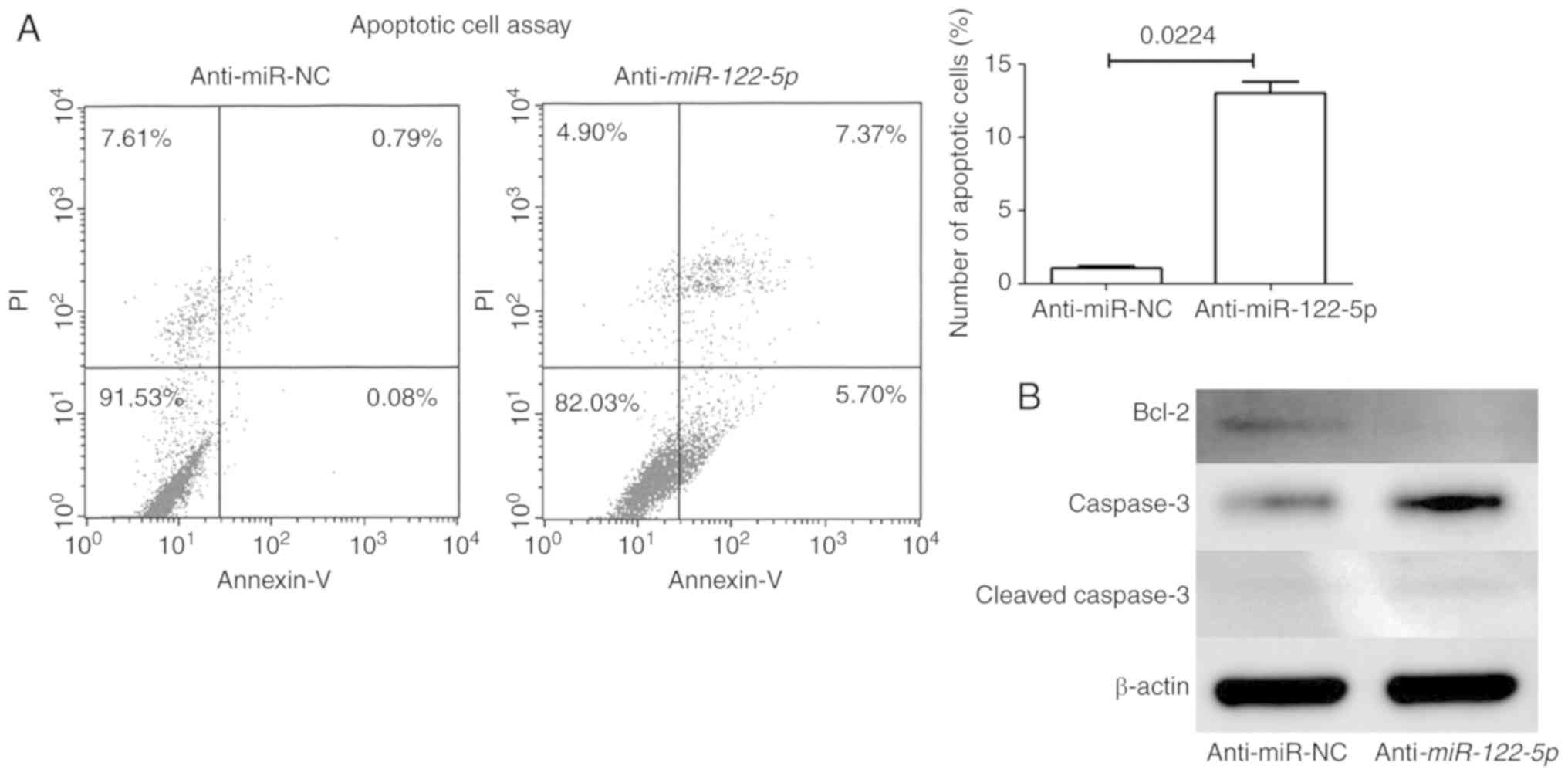
Next, apoptotic cell analysis revealed that
inhibition of miR-122-5p increased early (Annexin
V-positive/PI-negative) and late (Annexin V-positive/PI-positive)
apoptosis (Fig. 3A). Western
blotting revealed that the expression of anti-apoptotic protein
(Bcl-2) was decreased and that of pro-apoptotic protein (caspase-3
and cleaved caspase-3) was increased with miR-122-5p
inhibition in FU97 cells (Fig.
3B).
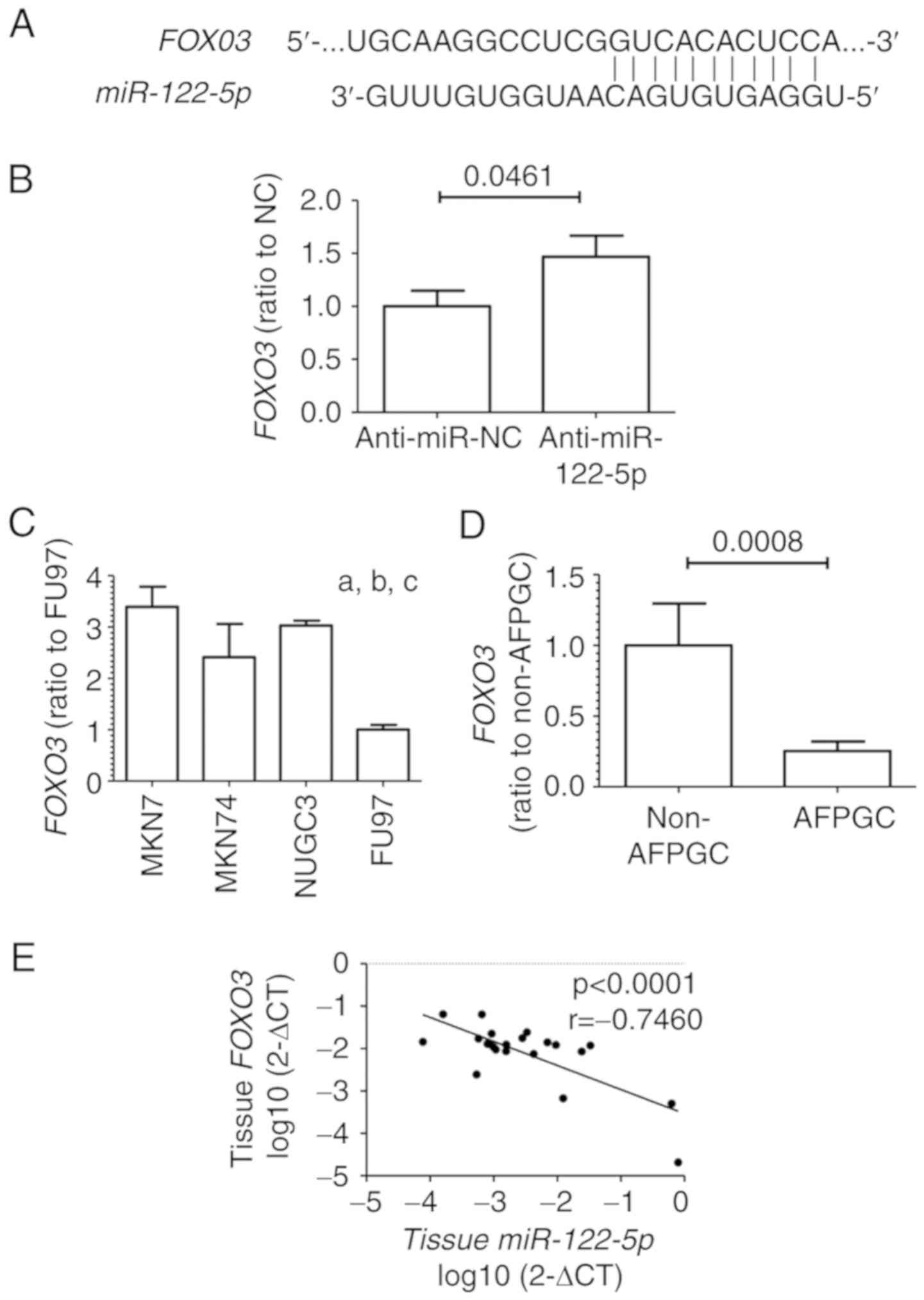
Association between FOXO3 and
miR-122-5p levels in GC cell lines and GC tissue samples
To further investigate the molecular mechanism of
miR-122-5p suppressing tumor progression in AFPGC, the miRNA
target analysis tool (TargetScan; www.targetscan.org) was used to predict potential
targets of miR-122-5p. Among numerous potential targets, we
selected FOXO3, which is involved in cell cycle arrest and
apoptosis, for further analyses in the present study (Fig. 4A). Inhibition of miR-122-5p
increased FOXO3 levels in the FU97 cell line as determined
by qRT-PCR (Fig. 4B). Moreover,
among the GC cell lines, the expression levels of FOXO3 were
significantly lower in the FU97 cells than in the other non-AFPGC
cell lines as revealed by qRT-PCR (Fig.
4C).
We also investigated the FOXO3 expression in
25 GC tissue samples including 5 AFPGC patients. FOXO3
levels were significantly lower in AFPGC than in non-AFPGC tissue
samples (Fig. 4D). Moreover,
miR-122-5p expression levels were inversely correlated with
FOXO3 levels (r=−0.7460, P<0.0001; Fig. 4E).
Discussion
Although α-fetoprotein-producing gastric cancer
(AFPGC) has been recognized as an aggressive gastric cancer
(11,12), to the best of our knowledge no
comprehensive molecular analysis has been reported for this
uncommon subtype. We previously identified that miR-122-5p
was significantly highly expressed in AFPGC tissues and could be a
useful plasma biomarker in patients (9). Moreover, we revealed that
miR-122-5p exhibited a strong correlation with malignant
potential in clinical settings (9).
In the present study, we confirmed that the expression level of
miR-122-5p was significantly increased in the AFPGC cell
line than in common gastric cancer (GC) cell lines, and also
inhibition of miR-122-5p significantly reduced AFP levels in
the AFPGC cell line. Conversely, several studies have clearly
demonstrated that the AFP molecule itself directly contributes to
the promotion of tumor progression (13–15).
With regard to these findings, we hypothesized that
miR-122-5p may directly regulate tumor progression in
AFPGC.
Current functional analyses by the gene transfection
approach clearly demonstrated that inhibition of miR-122-5p
significantly reduced proliferation in AFPGC cells through
induction of apoptosis. Several previous studies have revealed that
resistance to apoptosis could play a crucial role in the aggressive
characteristics of AFPGC (14,15).
Apoptotic resistance may be a major cause of the low
chemosensitivity in AFPGC.
Next, we investigated the possible targets of
miR-122-5p and identified FOXO3 as a likely candidate. FOXO3
is a critical protein involved in cell cycle arrest and apoptosis
(16,17). Therefore, FOXO3 is recognized as a
tumor suppressor, and decreased FOXO3 expression is associated with
progression of various tumors, including GC (18–20).
Guo et al (21) revealed
that miR-122-5p downregulated FOXO3 expression by
binding to the 3′UTR of its mRNA. A luciferase reporter assay was
carried out by them, and it revealed direct interaction between
miR-122-5p with FOXO3 (21). We also revealed that
miR-122-5p overexpression inhibited FOXO3 by qRT-PCR and
western blotting in GC cell line NUGC-3 (Fig. S1). We confirmed that suppression of
miR-122-5p increased FOXO3 levels, and miR-122-5p
expression levels were inversely correlated with FOXO3
levels in GC tissue samples. Moreover, expression of FOXO3
was significantly lower in AFPGC than in non-AFPGC cell lines and
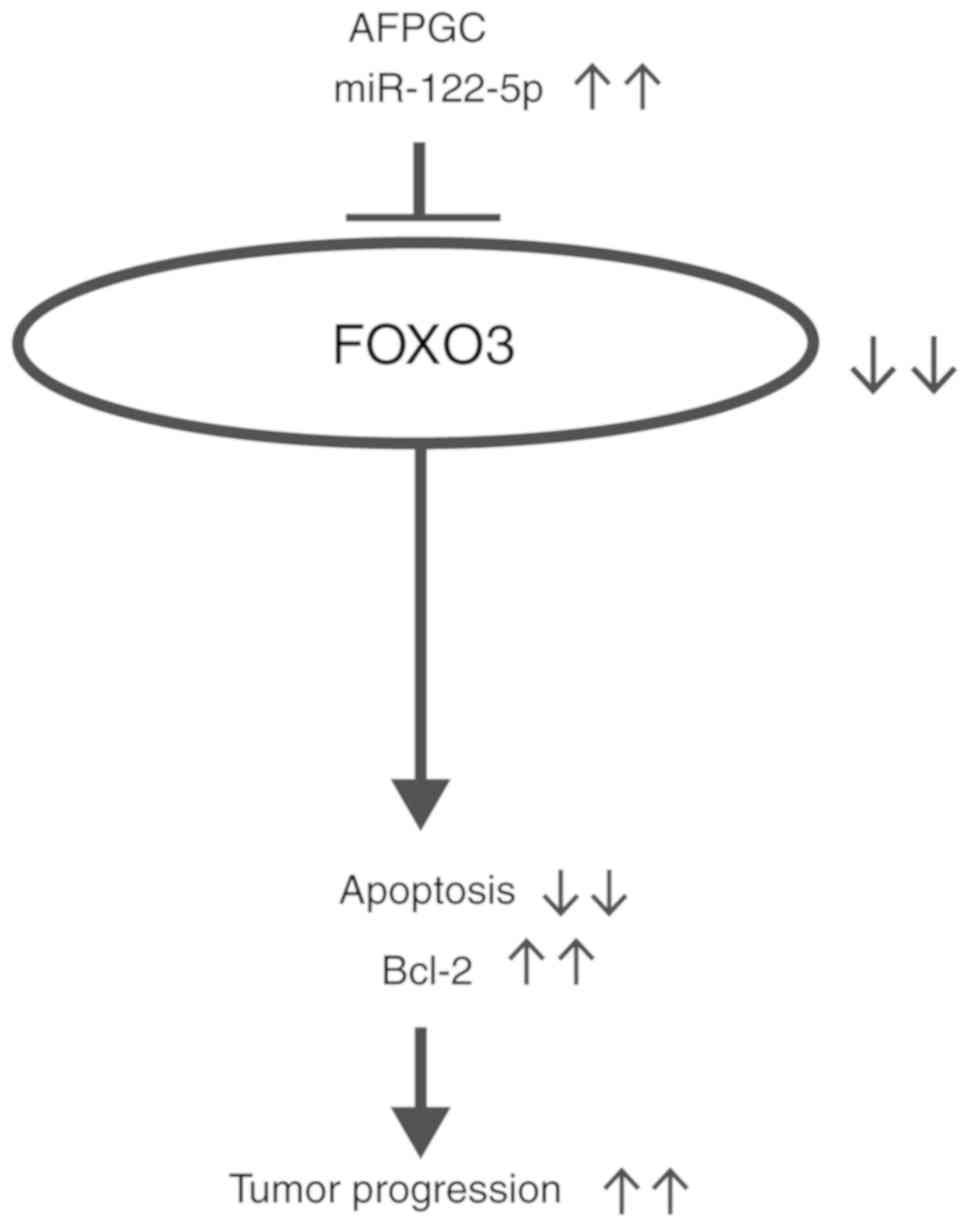
clinical tumor tissues. Collectively, FOXO3 is an important target
of miR-122-5p in AFPGC, which inhibits apoptosis and
subsequently facilitates tumor progression (Fig. 5).
In several previous studies, miR-122-5p was
downregulated and functioned as a tumor suppressor in various
cancers, such as hepatocellular (22), non-small cell lung (23), gallbladder (24), bladder (25) and breast cancer (26) as well as GC (27–29).
However, we clearly revealed that miR-122-5p facilitated
tumor progression in AFPGC cells and speculated that the function
of miR-122-5p and mechanisms underlying tumor progression
may be distinct between AFPGC and non-AFPGC.
Why AFPGC causes liver-specific metastasis, the most
distinct clinical characteristic of the disease, remains unknown.
In the present study, the migration and invasion abilities of FU97
were not significantly altered by inhibition of miR-122-5p
(data not shown). Moreover, miR-122-5p was not correlated
with liver metastasis of non-AFPGC in clinical settings (data not
shown). He et al (30)
revealed that upregulated Bcl-2 (anti-apoptotic protein) was
correlated with liver metastasis of AFPGC in clinical settings.
Similarly, the protein level of Bcl-2 was decreased by inhibition
of miR-122-5p in the present study. We surmised that the
anti-apoptotic effect may be important for the progression of liver
metastasis in AFPGC. However, further studies are warranted to
demonstrate the biological function of miR-122-5p for liver
metastasis in AFPGC.
In conclusion, miR-122-5p inhibited apoptosis
and facilitated tumor progression by targeting FOXO3 in AFPGC,
which indicated the possibility of miR-122-5p as a potential
therapeutic target in AFPGC.
Supplementary Material
Supporting Data
Acknowledgements
The authors are grateful to Ms. Motoko Inui and Ms.
Makiko Mishina (University of Yamanashi, Chuo, Japan) for their
expert technical assistance.
Funding
No funding was received.
Availability of data and materials
Data were collected at the University of Yamanashi
and are not publicly available.
Authors' contributions
SM performed the majority of the experiments and
wrote the manuscript; SF and RS performed the research; KS, HAk and
YK provided the tissue samples and the clinical data, and reviewed
and edited the manuscript; HS, NH, HAm, HKa, MS, SI and HKo made
substantial contributions to the data analysis and interpretation.
DI designed the study and helped to draft the manuscript. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Yamanashi University (approval no. 1825) and was
performed in accordance with the ethical standards of the
Declaration of Helsinki and its amendments. Written informed
consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: Defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cancer Genome Atlas Research Network, .
Comprehensive molecular characterization of gastric adenocarcinoma.
Nature. 513:202–209. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bourreille J, Metayer P, Sauger F, Matray
F and Fondimare A: Existence of alpha feto protein during
gastric-origin secondary cancer of the liver. Presse Med.
78:1277–1278. 1970.(In French). PubMed/NCBI
|
|
4
|
Chang YC, Nagasue N, Abe S, Kohno H,
Yamanoi A, Uchida M and Nakamura T: The characters of AFP-producing
early gastric cancer. Nihon Geka Gakkai Zasshi. 91:1574–1580.
1990.(In Japanese). PubMed/NCBI
|
|
5
|
Motoyama T, Aizawa K, Watanabe H, Fukase M
and Saito K: alpha-Fetoprotein producing gastric carcinomas: A
comparative study of three different subtypes. Acta Pathol Jpn.
43:654–661. 1993.PubMed/NCBI
|
|
6
|
Chang YC, Nagasue N, Abe S, Taniura H,
Kumar DD and Nakamura T: Comparison between the clinicopathologic
features of AFP-positive and AFP-negative gastric cancers. Am J
Gastroenterol. 87:321–325. 1992.PubMed/NCBI
|
|
7
|
Koide N, Nishio A, Igarashi J, Kajikawa S,
Adachi W and Amano J: Alpha-fetoprotein-producing gastric cancer:
Histochemical analysis of cell proliferation, apoptosis, and
angiogenesis. Am J Gastroenterol. 94:1658–1663. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ha M and Kim VN: Regulation of microRNA
biogenesis. Nat Rev Mol Cell Biol. 15:509–524. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maruyama S, Furuya S, Shiraishi K, Shimizu
H, Akaike H, Hosomura N, Kawaguchi Y, Amemiya H, Kawaida H, Sudo M,
et al: miR-122-5p as a novel biomarker for
alpha-fetoprotein-producing gastric cancer. World J Gastrointest
Oncol. 10:344–350. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kono K, Amemiya H, Sekikawa T, Iizuka H,
Takahashi A, Fujii H and Matsumoto Y: Clinicopathologic features of
gastric cancers producing alpha-fetoprotein. Dig Surg. 19:359–365;
discussion 365. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu X, Cheng Y, Sheng W, Lu H, Xu Y, Long
Z, Zhu H and Wang Y: Clinicopathologic features and prognostic
factors in alpha-fetoprotein-producing gastric cancers: Analysis of
104 cases. J Surg Oncol. 102:249–255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li M, Li H, Li C, Wang S, Jiang W, Liu Z,
Zhou S, Liu X, McNutt MA and Li G: Alpha-fetoprotein: A new member
of intracellular signal molecules in regulation of the PI3K/AKT
signaling in human hepatoma cell lines. Int J Cancer. 128:524–532.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jia Y, Liu D, Xiao D, Ma X, Han S, Zheng
Y, Sun S, Zhang M, Gao H, Cui X, et al: Expression of AFP and STAT3
is involved in arsenic trioxide-induced apoptosis and inhibition of
proliferation in AFP-producing gastric cancer cells. PLoS One.
8:e547742013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu S, Ma Y, Sun T, Ren R, Zhang X and Ma
W: Expression of α-fetoprotein in gastric cancer AGS cells
contributes to invasion and metastasis by influencing anoikis
sensitivity. Oncol Rep. 35:2984–2990. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zheng F, Tang Q, Wu J, Zhao S, Liang Z, Li
L, Wu W and Hann S: p38α MAPK-mediated induction and interaction of
FOXO3a and p53 contribute to the inhibited-growth and
induced-apoptosis of human lung adenocarcinoma cells by berberine.
J Exp Clin Cancer Res. 33:362014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu HB, Gao XX, Zhang Q, Liu J, Cui Y, Zhu
Y and Liu YF: Expression and prognostic implications of FOXO3a and
Ki67 in lung adenocarcinomas. Asian Pac J Cancer Prev.
16:1443–1448. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang XB, Zhao JJ, Huang CY, Wang QJ, Pan
K, Wang DD, Pan QZ, Jiang SS, Lv L, Gao X, et al: Decreased
expression of the FOXO3a gene is associated with poor prognosis in
primary gastric adenocarcinoma patients. PLoS One. 8:e781582013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park SH, Jang KY, Kim MJ, Yoon S, Jo Y,
Kwon SM, Kim KM, Kwon KS, Kim CY and Woo HG: Tumor suppressive
effect of PARP1 and FOXO3A in gastric cancers and its clinical
implications. Oncotarget. 6:44819–44831. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang LJ, Tang Q, Wu J, Chen Y, Zheng F,
Dai Z and Hann SS: Inter-regulation of IGFBP1 and FOXO3a unveils
novel mechanism in ursolic acid-inhibited growth of hepatocellular
carcinoma cells. J Exp Clin Cancer Res. 35:592016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guo D, Ma J, Li T and Yan L: Up-regulation
of miR-122 protects against neuronal cell death in ischemic stroke
through the heat shock protein 70-dependent NF-κB pathway by
targeting FOXO3. Exp Cell Res. 369:34–42. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang N, Wang Q, Shen D, Sun X, Cao X and
Wu D: Downregulation of microRNA-122 promotes proliferation,
migration, and invasion of human hepatocellular carcinoma cells by
activating epithelial-mesenchymal transition. Onco Targets Ther.
9:2035–2047. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qin H, Sha J, Jiang C, Gao X, Qu L, Yan H,
Xu T, Jiang Q and Gao H: miR-122 inhibits metastasis and
epithelial-mesenchymal transition of non-small-cell lung cancer
cells. Onco Targets Ther. 8:3175–3184. 2015.PubMed/NCBI
|
|
24
|
Lu W, Zhang Y, Zhou L, Wang X, Mu J, Jiang
L, Hu Y, Dong P and Liu Y: miR-122 inhibits cancer cell malignancy
by targeting PKM2 in gallbladder carcinoma. Tumour Biol. Nov
6–2015.(Epub ahead of print).
|
|
25
|
Wang Y, Xing QF, Liu XQ, Guo ZJ, Li CY and
Sun G: MiR-122 targets VEGFC in bladder cancer to inhibit tumor
growth and angiogenesis. Am J Transl Res. 8:3056–3066.
2016.PubMed/NCBI
|
|
26
|
Ergün S, Ulasli M, Igci YZ, Igci M,
Kirkbes S, Borazan E, Balik A, Yumrutaş Ö, Camci C, Cakmak EA, et
al: The association of the expression of miR-122-5p and its target
ADAM10 with human breast cancer. Mol Biol Rep. 42:497–505. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen Q, Ge X, Zhang Y, Xia H, Yuan D, Tang
Q, Chen L, Pang X, Leng W and Bi F: Plasma miR-122 and miR-192 as
potential novel biomarkers for the early detection of distant
metastasis of gastric cancer. Oncol Rep. 31:1863–1870. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rao M, Zhu Y, Zhou Y, Cong X and Feng L:
MicroRNA-122 inhibits proliferation and invasion in gastric cancer
by targeting CREB1. Am J Cancer Res. 7:323–333. 2017.PubMed/NCBI
|
|
29
|
Xu X, Gao F, Wang J, Tao L, Ye J, Ding L,
Ji W and Chen X: MiR-122-5p inhibits cell migration and invasion in
gastric cancer by down-regulating DUSP4. Cancer Biol Ther.
19:427–435. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
He L, Ye F, Qu L, Wang D, Cui M, Wei C,
Xing Y, Lee P, Suo J and Zhang DY: Protein profiling of
alpha-fetoprotein producing gastric adenocarcinoma. Oncotarget.
7:28448–28459. 2016.PubMed/NCBI
|