Introduction
Persistent Hepatitis C virus (HCV) infection may
result in liver cancer. In 2011, 130–170 million people were
infected with HCV worldwide. Liver cancer develops in 1–4% patients
with HCV-induced cirrhosis annually (1). Understanding the mechanisms that
underlie the development of HCV into liver cancer is important for
HCV-associated liver cancer treatment. HCV nonstructural 5A (NS5A)
encodes a 447 amino acid phosphoprotein (2). This protein serves as a
transcriptional activator of cell growth. It interacts with other
proteins and has a crucial role in hepatocarcinogenesis (3). According to a previous study, NS5A
inhibits cell apoptosis in vivo and in vitro
(4). Peng et al (5) reported that NS5A decreases caspase-3
cleavage (5). Lan et al
(2) demonstrated that HCV NS5A
suppresses p53-mediated transcriptional transactivation and
apoptosis during HCV infection, contributing to
hepatocarcinogenesis. In addition, NS5A significantly increases the
expression of inducible nitric oxide synthase, cyclin D1 and
nuclear factor-κB, but decreases p53 protein expression in HepG2
cells (6). By regulating the
expression of several genes in host liver cells, NS5A also induces
cellular proliferation, and influences the curative effect of
interferon (7).
Autophagy provides energy to tumor cells for
survival and metabolic reprogramming, in order to accommodate rapid
cell growth and proliferation (8).
Increasing amounts of evidence indicate that autophagy is induced
by a number of stressors in tumor cells, such as starvation, growth
factor deprivation, hypoxia, damage stimulation and therapeutic
drugs, and is an important survival mechanism in response to
cellular stress (9). Beclin l, the
mammalian counterpart of the yeast Atg6 gene, is an essential
protein in autophagy (10). Using
cDNA microarray screens and northern blot analysis, a previous
study showed that Beclin 1 is upregulated in liver cancer tissues
(11). In addition, Beclin 1 gene
deletion results in tumor cell apoptosis, specifically in hypoxic
regions (12). Liu et al
(13) reported that Beclin 1 gene
deletion by either RNA interference or the autophagy inhibitor
3-methyladenine (MA) significantly enhances melatonin-induced
apoptosis in mouse hepatoma H22 cells. Guo et al (14) found that autophagy inhibition
significantly increases liver cancer cell apoptosis during nutrient
starvation or hypoxia in vitro. NS5A upregulates Beclin 1
mRNA and protein expression in a HCV NS5A-transactivated protein 9
(NS5ATP9)-dependent manner (15).
In addition, NS5A could induce autophagy in a Beclin 1-dependent
manner. Thus, the present study hypothesized that NS5A inhibits
apoptosis by inducing Beclin 1-dependent autophagy.
Materials and methods
Construction of plasmids
pcDNA3.1/myc-His(−)-NS5A (pNS5A) was constructed as
previously described (15). Beclin
1 was amplified using the primer set
5′-GATATCATGGAAGGGTCTAAGACGTC-3′ and
5′-GGATCCTCATTTGTTATAAAATTGTGAGG-3 ′. Total RNA from L02 cells was
prepared using a total RNA kit (R6834; Omega, Norcross, GA, USA)
according to the manufacturer's instructions. mRNA was reverse
transcribed into cDNA using a PrimeScript RT reagent kit (Takara
Biotechnology Co., Ltd., Dalian, China). The RT temperature
protocol was 37°C for 15 min and 85°C for 5 sec. The amplified
product was digested with EcoRV and BamHI (Takara
Biotechnology Co., Ltd.) and inserted into the vector
pcDNA3.1/myc-His(−).
siRNA oligonucleotides
Beclin 1 small interfering RNA [(siRNA) cat. no.
sc-29797] and negative control siRNA (cat. no. sc-37007) were
purchased from Santa Cruz Biotechnology Co., Ltd. (Dallas, TX,
USA).
Cell culture and transfection
Hepatoblastoma HepG2 cells were obtained from the
Chinese Academy of Science Cell Bank (Shanghai, China) and cultured
in Dulbecco's modified Eagle's medium (DMEM; Gibco, Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin G and 100 µg/ml streptomycin in a humidified incubator
at 37°C in a 5% CO2 atmosphere. Cells were cultured to
60–80% confluence and transiently transfected with 50 nM Beclin 1
siRNA, 50 nM siRNA negative control for 48–72 h, 2 µg pNS5A or 2 µg
plasmid control for 48–72 h using Polyplus transfection reagent
(Polyplus-transfection SA, Illkirch, France) according to the
manufacturer's protocols.
Starvation was induced by amino acid deprivation in
Earle's Balanced Salt Solution (EBSS; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) at different time points (24–48 h).
3-methyladenine 10 mM (3-MA; Sigma-Aldrich, M9281) and chloroquine
10 µM (CQ, InvivoGen, Shatin, Hong Kong, tlrl-chq) were added to
the medium for 24 h to block autophagy.
Western blotting
Protein was extracted from HepG2 cells using lysis
buffer containing a protease inhibitor cocktail (Cell Signaling
Technology, Inc., Danvers, MA, USA). Equal amounts of protein
(40–60 µg/lane) were separated on 12% Bis-Tris Gel/MOPS (cat. no.
NP0034; Invitrogen; Thermo Fisher Scientific, Inc.) and transferred
to polyvinylidene difluoride membranes by electroblotting.
Following blocking with 5% non-fat dry milk, the membranes were
incubated with primary antibodies against LC3B (cat. no. 3868; Cell
Signaling Technology, Inc.), GAPDH (cat. no. 5174; Cell Signaling
Technology, Inc.), Beclin 1 (cat. no. 3495; Cell Signaling
Technology, Inc.), p53 (cat. no sc-126; Santa Cruz Biotechnology
Co., Ltd.), apoptosis regulator BAX (Bax; cat. no. sc-493; Santa
Cruz Biotechnology Co., Ltd.), cleaved caspase-3 (cat. no. 9579s;
Cell Signaling Technology, Inc.) and anti-His (cat. no. sc-803;
Santa Cruz Biotechnology Co., Ltd.). Protein bands were detected by
an enhanced chemiluminescence system (cat. no. 32209; Thermo Fisher
Scientific, Inc.). Western blotting data were quantified using
Bio1D software (S:11.640150; Vilber Lourmat, Marne-la-Vallée,
France).
Flow cytometry
Following transfection and starvation at different
times, cells were harvested and washed two times with cold
BioLegend cell staining buffer (cat. no. 420201; BioLegend, Inc.,
San Diego, CA, USA), and then resuspended in the Annexin V binding
buffer (cat. no. 422201; BioLegend, Inc.) at a concentration of 106
cells/ml. Subsequently, 2 µl fluorescein isothiocyanate-Annexin V
(cat. no. 640906; BioLegend, Inc.) and 2 µl 7-aminoactinomycin D
(7-AAD; cat. no. 420401; BioLegend, Inc.) were added and incubated
for 15 min at room temperature (25°C) in the dark. FACSCalibur
(cat. no. 342975; BD Biosciences, Franklin Lakes, NJ, USA) was used
to flow cytometric analysis.
Cell proliferation assay
Prior to transfection, HepG2 cells were implanted
into 96-well plates at a density of 5,000 cells/well. Cell Counting
Kit-8 (CCK-8) solution (10 µl; cat. no. EQ645; Dojindo Molecular
Technologies, Inc., Kumamoto, Japan) was added to each well, which
contained 200 µl culture medium, 48 h post-transfection. After 40
min of incubation at 37°C, optical density values were read at 450
nm.
Caspase-3/-7 activity
Cells were seeded in 96-well plates at a density of
10,000 cells/well (100 µl DMEM with 10% FBS) 24 h before
transfection. Caspase-Glo 3/7 reagent (100 µl; cat. no. G8092;
Promega Corporation, Madison, WI, USA) was added to each well 48 h
post-transfection. A plate shaker was used to lyse cells at 15 × g
and room temperature for 1 h. The luminescence of each sample was
measured using a plate-reading luminometer (TURNER 9000-001;
Promega Corporation), according to the manufacturer's
instructions.
Statistical analysis
All experiments were repeated at least three times.
Two groups were compared using the paired Student's t-test.
Multiple groups were compared by two-way analysis of variance
followed by Tukey's post hoc test. All data were expressed as the
mean ± standard error of the mean. P<0.05 was considered to
indicate a statistically significant difference.
Results
NS5A inhibits apoptosis under
starvation
pNS5A or control vector was transiently transfected
into HepG2 cells, and Annexin V/7-AAD staining was used to
determine the number of cells undergoing apoptotic or necrotic
processes under different stress conditions by flow cytometry, in
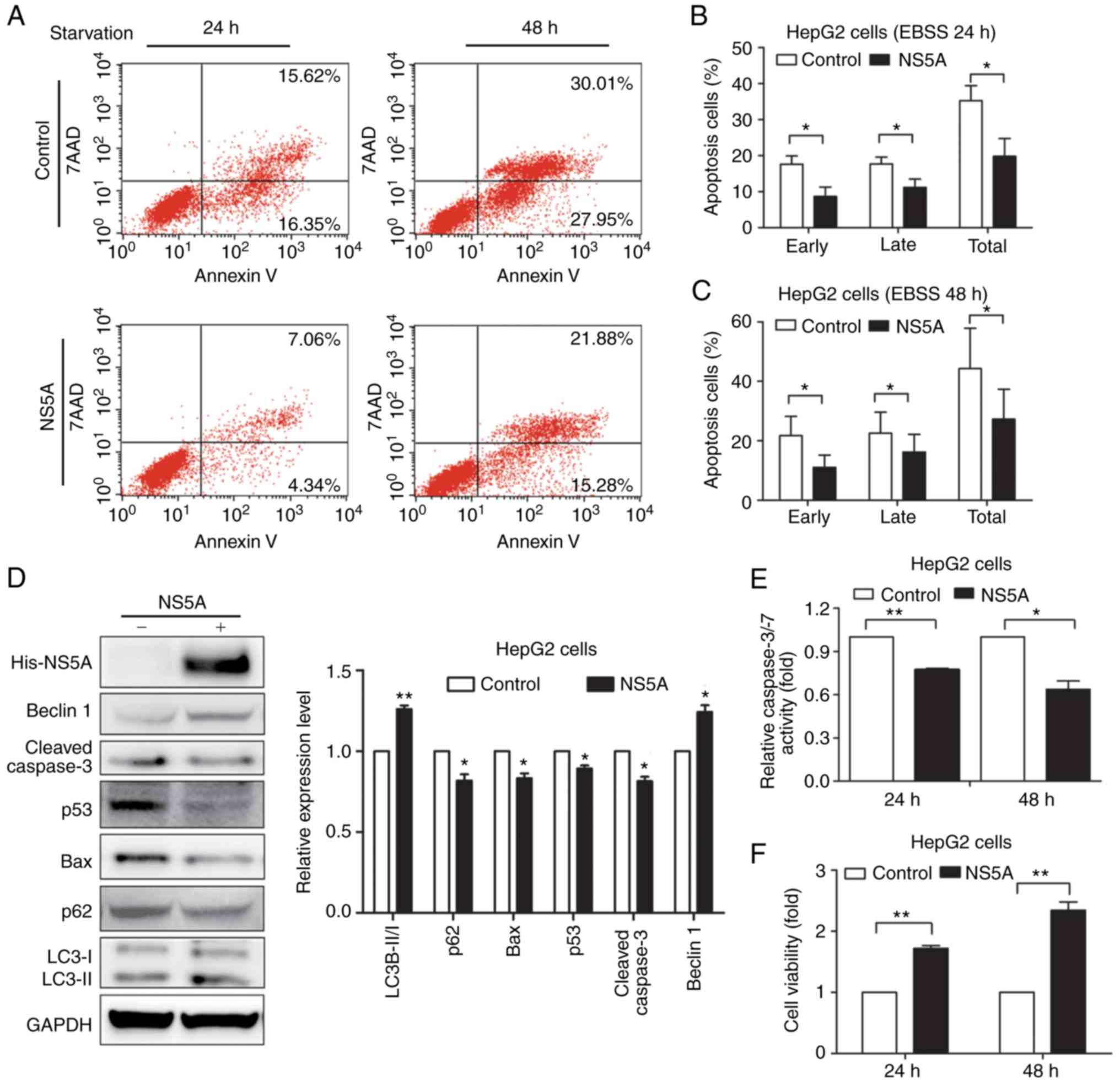
order to examine whether NS5A functioned in starvation-induced
apoptosis (Fig. 1A). The early,
late and total apoptotic cells were quantified and data were
displayed as the percentage of apoptotic cells. It was found that
NS5A transfection significantly repressed HepG2 cell apoptosis
under starvation, compared with the control group under starvation
for 24 and 48 h (Fig. 1B and C).
However, how NS5A repressed the apoptosis induced by starvation was
unclear. Therefore, the protein expression of p53, Bax and cleaved
caspase-3 was detected by western blotting (Fig. 1D). Compared with the control group,
transfection with the pNS5A plasmid for 48 h significantly
decreased the total p53 and Bax expression levels (Fig. 1D). Furthermore, the conversion of
LC3-I to LC3-II was detected, as well as decreased p62 expression
compared with the control group, indicating that NS5A may have
induced autophagy (Fig. 1D).
Caspase-3/-7 activity was attenuated in the pNS5A-transfected
group, compared with the control (Fig.
1E), which was consistent with the flow cytometry results under
starvation for 24 h. Furthermore, HepG2 cell viability increased in
the pNS5A-transfected group, compared with the control group, as
detected by CCK-8 assays (Fig.
1F).
Inhibition of autophagy increased the
apoptosis of hepatoblastoma cells during starvation
A previous study reported that NS5A protein
expression upregulates the mRNA and protein expression of Beclin 1,
and also increases Beclin 1 promoter activity by upregulating the
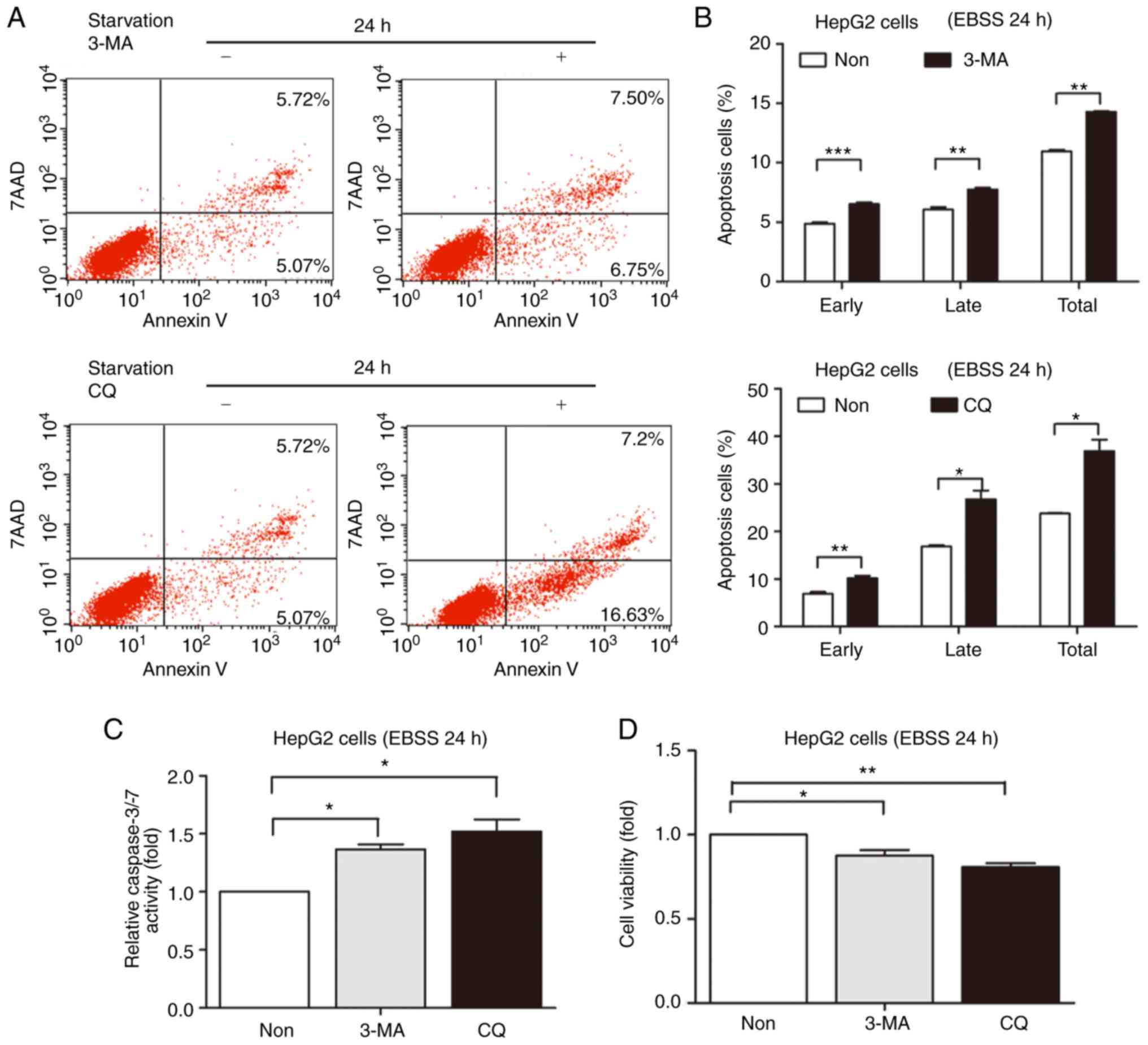
NS5ATP9 expression in HepG2 cells (15). The autophagy inhibitors 3-MA and CQ
were used to inhibit autophagy to examine whether NS5A-induced
autophagy had a role in cell survival or death. HepG2 cell death
was detected following incubation with EBSS for 24 h. The present
study also detected cell apoptosis under starvation for 48 h. HepG2
cell resistance to 3-MA or CQ was diminished by the longer
starvation period (48 h). Therefore, 24 h was selected. Prior
studies have shown that 3-MA or CQ inhibit autophagic activity
(16). The present study
demonstrated that HepG2 cell apoptosis increased in the 3-MA- or
CQ-treated groups (Fig. 2A). The
quantified results showed that the apoptotic cells increased
compared with the control under starvation for 24 h (Fig. 2B). In addition, caspase-3/-7
activity was enhanced in 3-MA- or CQ-treated groups, which was
consistent with the flow cytometry results under starvation for 24
h (Fig. 2C). In addition, cell
viability increased in the 3-MA- or CQ-treated groups (Fig. 2D).
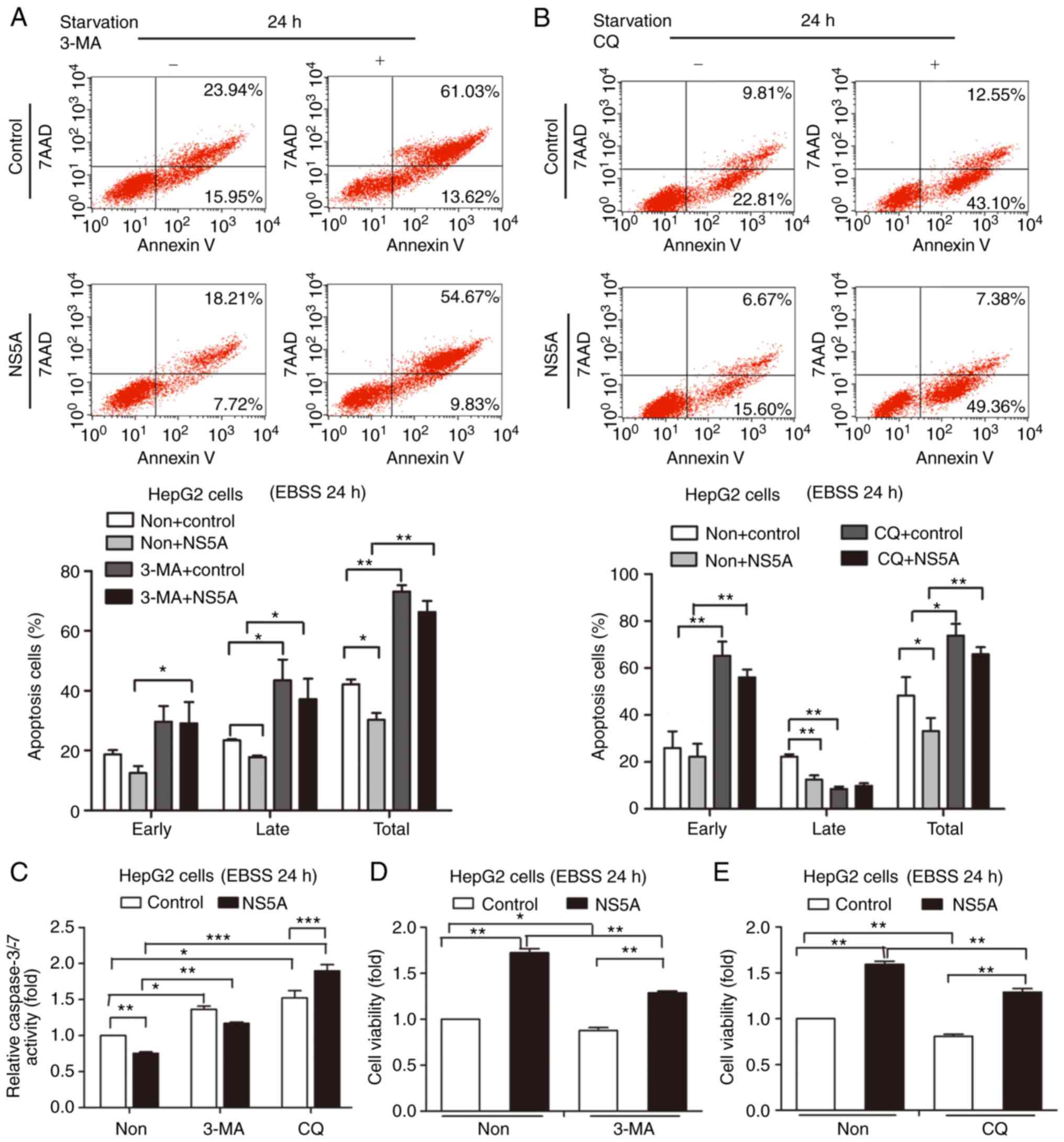
NS5A-mediated apoptosis inhibition is weakened by
autophagy inhibitors 3-MA or CQ. 3-MA and CQ were used to inhibit
autophagy to further analyze whether this process was involved in
NS5A-mediated apoptosis. Compared with the control group, apoptosis
was increased in NS5A-transfected cells treated with 3-MA or CQ
(Fig. 3A and B). The quantified
results revealed that the number of apoptotic cells treated with
3-MA or CQ increased compared with the control under starvation for
24 h (Fig. 3A and B). This
indicated that autophagy was involved and protected cells from
apoptosis. Although NS5A reduced apoptosis, this protective effect
was eliminated by autophagy inhibition. Furthermore, caspase-3/-7
activity increased in the 3-MA- or CQ-treated groups transfected
with NS5A plasmid compared with the control groups (Fig. 3C). This result was consistent with
flow cytometry results. In addition, cell viability was decreased
in the 3-MA- or CQ-treated groups compared with the control groups
(Fig. 3D and E). These results
showed that autophagic inhibition may prevent the protective
effects of NS5A under starvation.
Apoptosis inhibition mediated by NS5A
is attenuated by Beclin 1 siRNA
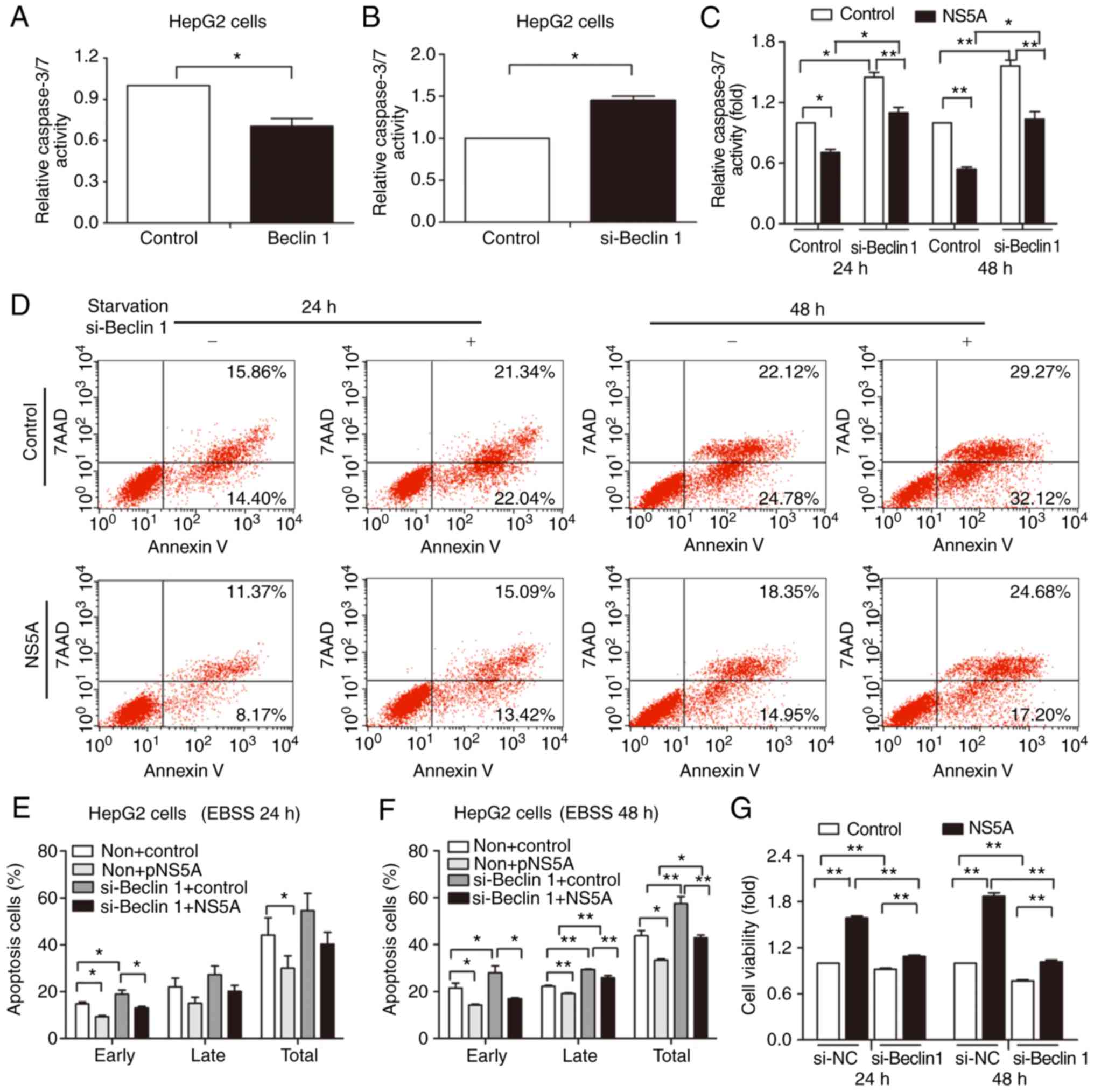
Beclin 1 has a central role in regulating autophagy
and apoptosis for cellular protection (17). The present study used siRNA and
Beclin 1 plasmid to silence and overexpress Beclin 1, respectively,
to further confirm the role of Beclin 1 in autophagy and
NS5A-mediated apoptosis. Relative caspase-3/-7 activity decreased
in the Beclin 1-overexpressed group (Fig. 4A), and significantly increased in
the siRNA group (Fig. 4B), compared
with the controls.
HepG2 cells were transfected with either control or
Beclin 1 siRNA for 24 h, followed by transfection with pNS5A or
control for 48 h and subsequent starvation for 24 or 48 h.
Apoptosis was determined by caspase-3/-7 activity (Fig. 4C) and flow cytometry (Fig. 4D). The percentage of early, late,
and total apoptotic cells was quantified. Compared with the pNS5A
transfected group without Beclin 1 siRNA transfection, apoptosis
was increased in the pNS5A and Beclin 1 siRNA-transfected group
under starvation for 48 h (Fig. 4E and
F). The results revealed that Beclin 1 gene silencing
attenuated the NS5A-mediated reduction in apoptosis, demonstrating
that Beclin 1 served a role in apoptosis inhibition. Furthermore,
the viability of NS5A-transfected cells decreased when Beclin 1 was
silenced (Fig. 4G).
Beclin 1 mediates the inhibition of
apoptosis by NS5A in HepG2 cells
Beclin 1 is expressed in liver tumor tissues and
cancer cell lines, and cannot be detected in healthy liver tissues
(6). In addition, HCV infection
transcriptionally upregulates the expression of Beclin 1 (18). The increased expression of Beclin 1
may therefore have an important role in starvation-induced cell
apoptosis, or Beclin 1-mediated autophagy may be involved in liver
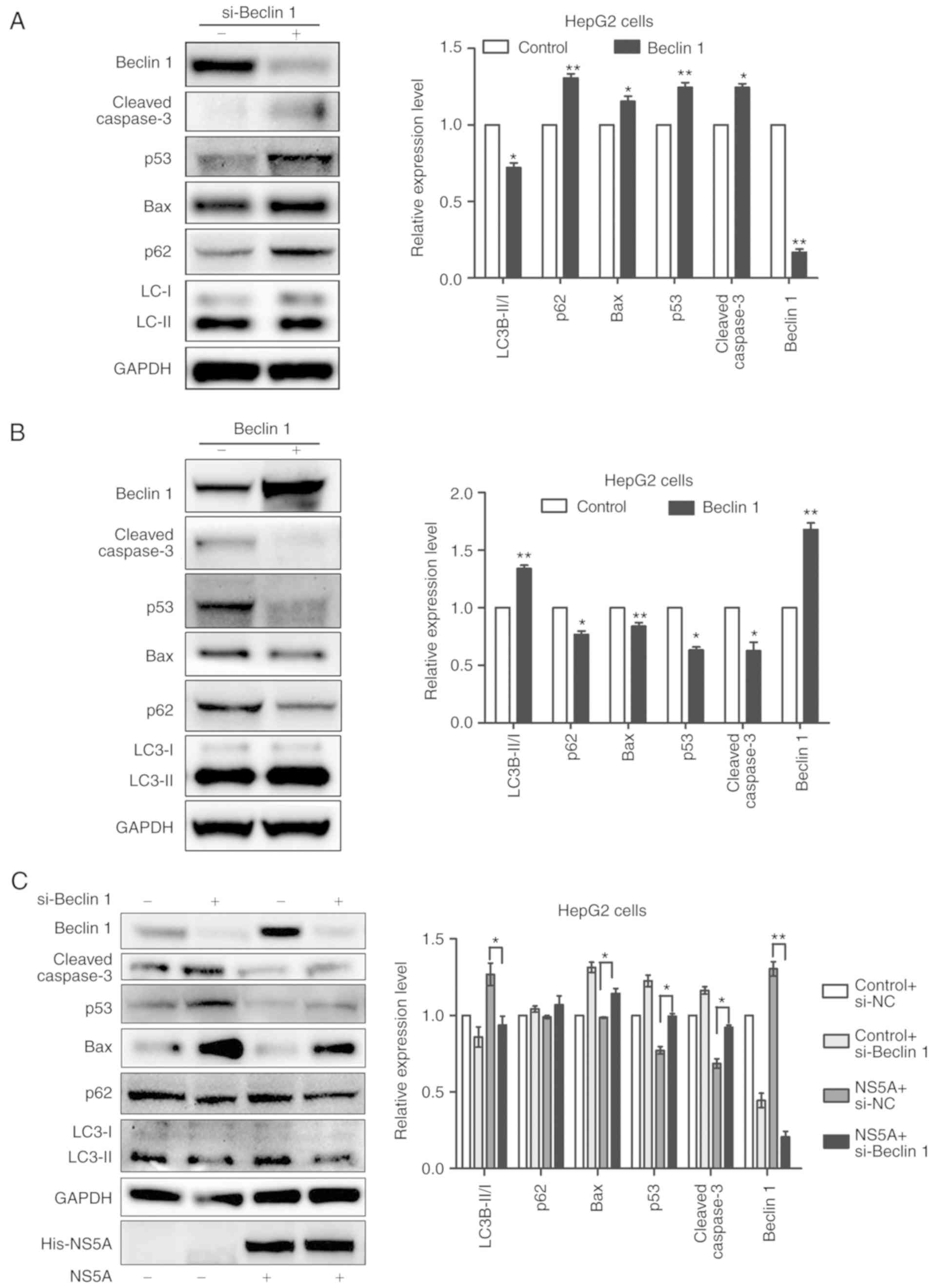
cancer induced by HCV infection. siRNA was used to knockdown Beclin
1 by transfection into HepG2 cells. The western blot results showed
that Beclin 1 siRNA effectively inhibited its expression in HepG2
cells, compared with the siRNA negative control (Fig. 5A and B). In addition, the ratio of
LC3-I/LC3-II was significantly decreased as a result of the Beclin
1 siRNA transfection (Fig. 5A),
whereas it was increased by Beclin 1 plasmid transfection (Fig. 5B). The protein expression of p53,
Bax and cleaved caspase-3 in HepG2 cells under starvation for 24 h
was also detected by western blotting. Compared with the control
group, transfection with Beclin 1 siRNA significantly increased
total p53, Bax, and cleaved caspase-3 expression (Fig. 5A), whereas Beclin 1 plasmid
transfection had the opposite effect (Fig. 5B). Next, NS5A plasmid was
transfected into the HepG2 cells with Beclin 1 gene silencing to
identify the importance of Beclin 1 in NS5A-inhibited
apoptosis.
Compared with the pNS5A transfected group without
si-Beclin 1, p53, Bax and cleaved caspase-3 protein expression
increased in the pNS5A-transfected Beclin 1 siRNA group under
starvation for 48 h, compared with the pNS5A-transfected negative
control siRNA group (Fig. 5C).
These results suggested that the inhibitory effect of Beclin 1
siRNA on autophagy recovered the sensitivity of hepatoblastoma
cells to starvation. Therefore, autophagy induced by NS5A may have
facilitated the tolerance of hepatoblastoma cells to starvation in
a Beclin 1-dependent manner.
Discussion
Apoptosis is a critical process in liver cancer. In
chronic HCV, downregulation of apoptosis and enhanced cell
proliferation not only causes HCV infection persistency in the
majority of patients, but also promotes liver cancer (19). It has been reported that several
components of HCV influence hepatocarcinogenesis, including NS5A,
the envelope protein E2 and the core protein (20,21).
NS5A may serve an essential role in HCV-associated liver cancer
development by inhibiting cell apoptosis (22). The present study demonstrated that
NS5A overexpression inhibited the apoptosis induced by starvation,
and enhanced cell viability. HCV NS5A abrogates p53-mediated
apoptosis in Hep3B cells by suppressing the transcriptional
transactivation activity of p53 in a dose-dependent manner
(2). The results of the present
study revealed that p53, Bax, and cleaved caspase-3 protein
expression was decreased in NS5A-expressing HepG2 cells. Our
previous studies showed that NS5A induces autophagy in a Beclin
1-dependent manner (15) and
autophagy mediates the proliferation of HepG2 cells (16). Autophagy, which provides nutrients
by degrading existing cellular components, is considered an
adaptive response to various cellular pressures, including hypoxia
and starvation (23,24). It has been speculated that the
effects of autophagy on cell survival and apoptosis may vary
depending on the cell type, micro-environment, and the extent of
autophagy induction (25).
Autophagy in tumor therapy is associated with chemotherapy,
radiation and immune tolerance (25,26).
However, autophagy is also an indispensable physiological response
required to maintain the viability of cells during starvation
(27). In addition, increased
autophagy following treatment with antitumor drugs reduces their
effects (28). Furthermore,
inhibition of autophagy in starved HeLa cells promotes apoptosis
and caspase-3 activation (29,30).
The present study demonstrated that the inhibition of autophagy by
3-MA or CQ significantly increased the apoptosis of starved cells.
Further, Beclin 1 expression was also silenced to inhibit
autophagy, which also increased HepG2 cell apoptosis. Therefore, it
was speculated that Beclin 1-dependent autophagy may have mediated
apoptosis inhibition by NS5A.
Beclin 1 is a major regulator of autophagy (31). In a previous study, the results of a
cDNA microarray screen and northern blot analysis demonstrated that
the Beclin 1 gene expression is upregulated in liver cancer tissues
(11). It has also been shown that
Beclin 1 serves a major role in liver cancer. The overexpression of
Beclin 1 increases cell survival by inhibiting apoptosis (32,33),
and its silencing results in the increased sensitivity of cells to
stress (34). Furthermore, miR-216a
enhances the radiosensitivity of pancreatic cancer cells by
suppressing Beclin 1-mediated autophagy (35). The combined delivery of Beclin 1
siRNA and FTY720 (a novel immunosuppressive agent with effective
anticancer properties) more efficiently inhibits liver cancer cell
progression by suppressing protective autophagy and increasing
apoptosis (36), which is
consistent with the results of the present study. The present study
found that Beclin 1 inhibited p53, Bax, and cleaved caspase-3
expression in starved HepG2 cells. Furthermore, it was confirmed
that Beclin 1 expression by NS5A was involved in the negative
regulation of starvation-induced liver cancer apoptosis, which was
accompanied by reduced p53 and apoptosis regulator Bax expression,
as well as decreased caspase-3/-7 activation. Combining siRNA and
anticancer drugs into the same vector has unique advantages
(36). Therefore, it was speculated
that the combined treatment of Beclin 1 siRNA with anticancer drugs
may effectively promote the drug's efficacy and significantly
improve HCC treatment.
In conclusion, HCV NS5A inhibited starvation-induced
apoptosis by downregulating p53-associated signaling pathways, and
this process was mediated by upregulating Beclin 1-dependent
autophagy in human hepatoblastoma cells. This suggested that Beclin
1 was vital in starvation-induced apoptosis and mediated
NS5A-inhibited apoptosis. However, this was not the only mechanism
by which NS5A inhibited the apoptosis of liver cancer cells. Even
following autophagy inhibition or Beclin 1 silencing, the
NS5A-induced effects of HepG2 cell apoptosis inhibition and
proliferation increase was blocked. However, the inhibition was
significant and thus may provide treatment clues for HCV-associated
liver cancer. Recently, the combination of siRNA and anticancer
drugs into the same vector has been shown to downregulate the
expression of cancer-associated genes and promote the effect of
cancer drugs on tumor sites to effectively inhibit tumor
progression (37,38). Therefore, the results of the present
study may aid in developing a novel therapeutic strategy to prevent
cancer cells from adapting to stress. Inhibition of autophagy or
Beclin 1 targeted siRNA may be promising strategy for use in
HCV-associated liver cancer therapy. Therefore, in view of Beclin 1
and its role in autophagy, it is likely that Beclin 1 targeting in
the treatment of HCV-associated liver cancer may have better
therapeutic effect. However, NS5A-mediated hepatocarcinogenesis is
only one part of HCV-mediated tumorigenesis. Therefore, the exact
mechanism of HCV-associated liver tumor remains to be elucidated.
Further animal experiments research is needed to verify the
experimental results of the present study.
Acknowledgements
Not applicable.
Funding
This study was funded by Beijing Key Laboratory of
Emerging Infectious Diseases Project (grant no. DTKF201704), the
National Natural Science Foundation of China (grant no. 81470863),
and the Beijing Municipal Administration of Hospitals' Ascent Plan
(grant no. DFL20151701).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MQ and JC designed the experiments. MQ wrote and
revised the manuscript. SF and SL performed the flow cytometric
analysis. LZ and YZ cultured the HepG2 cells and conducted the
transfection experiment. SF and SL performed the caspase-3/-7
activity assay and cell proliferation detection. MQ performed
plasmid construction and western blotting. JC supervised all
experiments and analyses. All authors read and approved the
manuscript, and agreed to be accountable for all aspects of the
work.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hajarizadeh B, Grebely J and Dore GJ:
Epidemiology and natural history of HCV infection. Nat Rev
Gastroenterol Hepatol. 10:553–562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lan KH, Sheu ML, Hwang SJ, Yen SH, Chen
SY, Wu JC, Wang YJ, Kato N, Omata M, Chang FY, et al: HCV NS5A
interacts with p53 and inhibits p53-mediated apoptosis. Oncogene.
21:4801–4811. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shi L, Zhang SL, Li K, Hong Y, Wang Q, Li
Y, Guo J, Fan WH, Zhang L and Cheng J: NS5ATP9, a gene up-regulated
by HCV NS5A protein. Cancer Lett. 259:192–197. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tamura R, Kanda T, Imazeki F, Wu S,
Nakamoto S, Tanaka T, Arai M, Fujiwara K, Saito K, Roger T, et al:
Hepatitis C Virus nonstructural 5A protein inhibits
lipopolysaccharide-mediated apoptosis of hepatocytes by decreasing
expression of Toll-like receptor 4. J Infect Dis. 204:793–801.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Peng L, Liang D, Tong W, Li J and Yuan Z:
Hepatitis C virus NS5A activates the mammalian target of rapamycin
(mTOR) pathway, contributing to cell survival by disrupting the
interaction between FK506-binding protein 38 (FKBP38) and mTOR. J
Biol Chem. 285:20870–20881. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tang H, Da L, Mao Y, Li Y, Li D, Xu Z, Li
F, Wang Y, Tiollais P, Li T, et al: Hepatitis B virus X protein
sensitizes cells to starvation-induced autophagy via up-regulation
of beclin 1 expression. Hepatology. 49:60–71. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schmitz U and Tan SL: NS5A-from obscurity
to new target for HCV therapy. Recent Pat Antiinfect Drug Discov.
3:77–92. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin CI, Whang EE, Abramson MA, Jiang X,
Price BD, Donner DB, Moore FD Jr and Ruan DT: Autophagy: A new
target for advanced papillary thyroid cancer therapy. Surgery.
146:1208–1214. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Song X, Zhang X, Wang X, Zhu F, Guo C,
Wang Q, Shi Y, Wang J, Chen Y and Zhang L: Tumor suppressor gene
PDCD4 negatively regulates autophagy by inhibiting the
expression of autophagy-related gene ATG5. Autophagy.
9:743–755. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kihara A, Kabeya Y, Ohsumi Y and Yoshimori
T: Beclin-phosphatidylinositol 3-kinase complex functions at the
trans-Golgi network. EMBO Rep. 2:330–335. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Song H, Xia SL, Liao C, Li YL, Wang YF, Li
TP and Zhao MJ: Genes encoding Pir51, Beclin 1, RbAp48 and aldolase
b are up or down-regulated in human primary hepatocellular
carcinoma. World J Gastroenterol. 10:509–513. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Degenhardt K, Mathew R, Beaudoin B, Bray
K, Anderson D, Chen G, Mukherjee C, Shi Y, Gélinas C, Fan Y, et al:
Autophagy promotes tumor cell survival and restricts necrosis,
inflammation, and tumorigenesis. Cancer Cell. 10:51–64. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu C, Jia Z, Zhang X, Hou J, Wang L, Hao
S, Ruan X, Yu Z and Zheng Y: Involvement of melatonin in
autophagy-mediated mouse hepatoma H22 cell survival. Int
Immunopharmacol. 12:394–401. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo XL, Li D, Sun K, Wang J, Liu Y, Song
JR, Zhao QD, Zhang SS, Deng WJ, Zhao X, et al: Inhibition of
autophagy enhances anticancer effects of bevacizumab in
hepatocarcinoma. J Mol Med (Berl). 91:473–483. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Quan M, Liu S, Li G, Wang Q, Zhang J,
Zhang M, Li M, Gao P, Feng S and Cheng J: A functional role for
NS5ATP9 in the induction of HCV NS5A-mediated autophagy. J Viral
Hepat. 21:405–415. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Quan M, Liu S, Wang Q, Li G, Zhang Y, Feng
S, Liang J and Cheng J: NS5ATP9 promotes Beclin 1-dependent
starvation-induced autophagy of hepatoblastoma cells. J Cell
Biochem. 116:1574–1582. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cao Y and Klionsky DJ: Physiological
functions of Atg6/Beclin 1: A unique autophagy-related protein.
Cell Res. 17:839–849. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shrivastava S, Bhanja CJ, Steele R, Ray R
and Ray RB: Hepatitis C virus upregulates Beclin1 for induction of
autophagy and activates mTOR signaling. J Virol. 86:8705–8712.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jahan S, Ashfaq UA, Khaliq S, Samreen B
and Afzal N: Dual behavior of HCV Core gene in regulation of
apoptosis is important in progression of HCC. Infect Genet Evol.
12:236–239. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jahan S, Khaliq S, Ijaz B, Ahmad W and
Hassan S: Role of HCV Core gene of genotype 1a and 3a and host gene
Cox-2 in HCV-induced pathogenesis. Virol J. 8:1552011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Núñez O, Fernández-Martínez A, Majano PL,
Apolinario A, Gómez-Gonzalo M, Benedicto I, López-Cabrera M, Boscá
L, Clemente G, García-Monzón C, et al: Increased intrahepatic
cyclooxygenase 2, matrix metalloproteinase 2, and matrix
metalloproteinase 9 expression is associated with progressive liver
disease in chronic Hepatitis C virus infection: Role of viral core
and NS5A proteins. Gut. 53:1665–1672. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang YF, He B, Li NP, Ma J, Gong GZ and
Zhang M: The oncogenic role of NS5A of Hepatitis C virus is
mediated by up-regulation of survivin gene expression in the
hepatocellular cell through p53 and NF-κB pathways. Cell Biol Int.
35:1225–1232. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhong J, Kong X, Zhang H, Yu C, Xu Y, Kang
J, Yu H, Yi H, Yang X and Sun L: Inhibition of CLIC4 enhances
autophagy and triggers mitochondrial and ER stress-induced
apoptosis in human glioma U251 cells under starvation. PLoS One.
7:e393782012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li J, Yang B, Zhou Q, Wu Y, Shang D, Guo
Y, Song Z, Zheng Q and Xiong J: Autophagy promotes hepatocellular
carcinoma cell invasion through activation of
epithelial-mesenchymal transition. Carcinogenesis. 34:1343–1351.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang GD, Tan YZ, Wang HJ and Zhou P:
Autophagy promotes degradation of polyethyleneimine-alginate
nanoparticles in endothelial progenitor cells. Int J Nanomedicine.
12:6661–6675. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kanamori H, Takemura G, Maruyama R, Goto
K, Tsujimoto A, Ogino A, Li L, Kawamura I, Takeyama T, Kawaguchi T,
et al: Functional significance and morphological characterization
of starvation-induced autophagy in the adult heart. Am J Pathol.
174:1705–1714. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ou L, Lin S, Song B, Liu J, Lai R and Shao
L: The mechanisms of graphene-based materials-induced programmed
cell death: A review of apoptosis, autophagy, and programmed
necrosis. Int J Nanomedicine. 12:6633–6646. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Boya P, González-Polo RA, Casares N,
Perfettini JL, Dessen P, Larochette N, Métivier D, Meley D,
Souquere S, Yoshimori T, et al: Inhibition of macroautophagy
triggers apoptosis. Mol Cell Biol. 25:1025–1040. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ryter SW and Choi AM: Autophagy in the
lung. Proc Am Thorac Soc. 7:13–21. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kuma A, Hatano M, Matsui M, Yamamoto A,
Nakaya H, Yoshimori T, Ohsumi Y, Tokuhisa T and Mizushima N: The
role of autophagy during the early neonatal starvation period.
Nature. 432:1032–1036. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liang XH, Kleeman LK, Jiang HH, Gordon G,
Goldman JE, Berry G, Herman B and Levine B: Protection against
fatal Sindbis virus encephalitis by beclin, a novel
Bcl-2-interacting protein. J Virol. 72:8586–8596. 1998.PubMed/NCBI
|
|
33
|
Shimizu S, Kanaseki T, Mizushima N, Mizuta
T, Arakawa-Kobayashi S, Thompson CB and Tsujimoto Y: Role of Bcl-2
family proteins in a non-apoptotic programmed cell death dependent
on autophagy genes. Nat Cell Biol. 6:1221–1228. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mizushima N, Yamamoto A, Matsui M,
Yoshimori T and Ohsumi Y: In vivo analysis of autophagy in response
to nutrient starvation using transgenic mice expressing a
fluorescent autophagosome marker. Mol Biol Cell. 15:1101–1111.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang X, Shi H, Lin S, Ba M and Cui S:
MicroRNA-216a enhances the radiosensitivity of pancreatic cancer
cells by inhibiting beclin-1-mediated autophagy. Oncol Rep.
34:1557–1564. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu JY, Wang ZX, Zhang G, Lu X, Qiang GH,
Hu W, Ji AL, Wu JH and Jiang CP: Targeted co-delivery of Beclin 1
siRNA and FTY720 to hepatocellular carcinoma by calcium phosphate
nanoparticles for enhanced anticancer efficacy. Int J Nanomedicine.
13:1265–1280. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Biswas S, Deshpande PP, Navarro G,
Dodwadkar NS and Torchilin VP: Lipid modified triblock PAMAM-based
nanocarriers for siRNA drug co-delivery. Biomaterials.
34:1289–1301. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cheng D, Cao N, Chen J, Yu X and Shuai X:
Multifunctional nanocarrier mediated co-delivery of doxorubicin and
siRNA for synergistic enhancement of glioma apoptosis in rat.
Biomaterials. 33:1170–1179. 2012. View Article : Google Scholar : PubMed/NCBI
|