Introduction
Lung cancer is the primary contributor towards
cancer mortality and morbidity in the developed world, including in
countries such as China. The latest global cancer statistics report
an estimated 2,093,876 new cases and 1,761,007 deaths due to lung
cancer worldwide in 2018 (1). These
statistics are reflected in China, where there were 733,300 new
lung cancer cases and 610,200 deaths due to lung cancer in 2015
(2). Lung cancer presents as either
non-small cell (NSCLC) or small cell lung cancer, with the latter
further classified into lung adenocarcinoma (LUAD) and lung
squamous cell carcinoma (LUSC). In recent years, an increased
number of cases of LUAD has been observed, which has surpassed the
incidence of LUSC. LUAD is mostly associated with genetic factors,
environmental and other external factors, including smoking.
Genetic factors are able to function as more objective biomarkers
for the diagnosis, treatment and prognosis of lung cancer.
The kinesin (KIF) family member genes are mainly
found in eukaryotic cells, primarily in microtubules. In
vitro experiments have demonstrated that the transport of
proteins is unidirectional, moving along the negative pole of
microtubule towards the positive pole. Therefore, the KIF family
genes control mass protein transfer both intracellularly and
extracellularly, including functions, such as transporting
organelles and material vesicles, and participating in cell mitosis
(3–5).
The use of whole-genome sequencing data combined
with bioinformatics analysis is an effective method with which to
explore prospective molecular mechanisms. The Cancer Genome Atlas
(TCGA, http://portal.gdc.cancer.gov) is an
open-source project using large-scale genomic sequencing to map the
genomes of 33 types of human cancer (6,7), including
complete RNA sequencing (RNA-seq) data for LUAD. Numerous studies
have reported that KIF family member genes are dysregulated in
multiple types of cancer, and can be used as diagnostic and
prognostic biomarkers for cancers (5,8–10). In our previous study, we analyzed
genome-wide breast cancer RNA-seq dataset from the TCGA database
and found that multiple genes belonging to the KIF family could be
used as biomarkers for the diagnosis and prognosis of breast cancer
(11). Therefore, we concluded that
some of the KIF family genes may also be used as diagnostic and
prognostic biomarkers of LUAD. In addition, previous studies have
also reported that some of the KIF family genes may be used as
prognostic indicators of LUAD (12–14).
However, the comprehensive systemic analysis of KIF family genes in
LUAD has not yet been reported, at least to the best of our
knowledge, and thus the potential underlying molecular mechanism
still require further investigation. In order to fill this gap in
knowledge, the present study aimed to elucidate the potential
molecular mechanisms of KIF family member genes, and to determine
their prognostic value in LUAD.
Materials and methods
Data source and pre-processing
Clinical data, as well as the complete RNA
sequencing (RNA-seq) library of TCGA LUAD cohort were derived from
the TCGA database (https://portal.gdc.cancer.gov/projects/TCGA-LUAD)
(6,7,15). Raw
RNA-seq was normalized using the R platform of the DESeq package
(http://www.bioconductor.org/packages/release/bioc/html/DESeq.html),
allowing the identification of the differentially expressed genes
(DEGs) of KIF family members between LUAD tumor and adjacent
non-cancerous tissues (16). This
study does not contain any experiments using human participants or
animals performed by any of the authors. Since all datasets
included in tge current study were downloaded from the TCGA
database and data acquisition and application are consistent with
the publication guidelines of TCGA, additional approval by an
ethics committee is thus not necessary.
Prognostic KIF gene screening
The inclusion criteria and exclusion criteria of the
patients with LUAD for survival analysis were as follows: Inclusion
criteria: i) LUAD tumor tissues RNA sequencing data set were
available; ii) overall survival (OS) time was available and not
zero. Exclusion criteria: i) Patient tumor tissues were not
subjected RNA sequencing; ii) the OS time was zero or unavailable.
Survival analysis was performed using the normalized mRNA gene
expression dataset of KIF-related genes and clinical outcome
parameters. The subjects were grouped as having either a low or
high-expression based on the median expression value of each gene.
The prognostic values of KIF family member genes were evaluated via
multivariate Cox proportional hazards regression analysis using the
R platform of the survival package (https://cran.r-project.org/web/packages/survival/index.html).
The group that had a low KIF gene expression was used as the
reference group, with all data adjusted for tumor stage. A P-value
<0.05 was considered to indicate a statistically significant
difference, with the respective gene designated as a prognostic KIF
genes.
Construction of a prognostic gene
signature based on KIF gene expression
A prognostic gene signature was constructed based on
the linear combination of gene expression levels multiplied by a
regression coefficient (β), which was derived from multivariate Cox
proportional hazards regression analysis. The prognostic KIF family
member genes were inserted into the multivariate Cox regression
model using overall survival as the dependent variable. The risk
score formula of the prognosis signature was as follows (17–22): Risk
score = expression of KIF1 × β1
KIF1 + expression of KIF2 × β2
KIF2 + … expression of KIFn × βn
KIFn. Patients were classified as having low or high
risks based on the median value of risk scores. A time-dependent
receiver operating characteristic (ROC) curve was drawn by the R
platform of the survivalROC package (https://cran.r-project.org/web/packages/survivalROC/index.html)
in order to evaluate the predictive accuracy of KIF genes
expression based prognostic signature for the prognosis of LUAD
(23).
Comprehensive survival analysis of
mRNA expression-based prognostic signature
The association between LUAD clinical features and
the contrasted prognostic signature was investigated using
stratified and joint effects survival analysis. A nomogram was
generated to evaluate the individualized prognosis risk score based
on clinical characteristics and KIF gene expression-based
prognostic signature.
Gene set enrichment analysis
(GSEA)
To further assess the biological pathways that
underlie prognostic KIF genes in LUAD OS, GSEA (http://software.broadinstitute.org/gsea/index.jsp) was
performed (24,25). GSEA uncovered the potential mechanisms
of prognostic-KIF genes using the Molecular Signatures Database
(MSigDB, http://software.broadinstitute.org/gsea/msigdb/index.jsp)
c2(c2.all.v6.2.symbols.gmt) and c5(c5.all.v6.2.symbols.gmt)
(26). The results of GSEA that had a
false discovery rate (FDR) <0.25, |Normalized Enrichment Score
(NES)| >1 and a nominal P-value <0.05 were considered to
indicate a statistically significant difference.
Statistical analysis
SPSS version 20.0 software (IBM Corp.) and R3.3.1
(https://www.r-project.org). were used to
compute all statistical analyses. The diagnostic receiver operating
characteristic (ROC) curves of KIF genes between tumor and adjacent
non-cancerous tissues were analyzed and plotted by SPSS version
20.0. The independent samples t-test was used to compare the mRNA
expression levels of tumor and adjacent normal tissues. The
co-expression correlation between KIF family member genes was
assessed by Pearson's correlation coefficient. Survival analyses
were assessed using the Kaplan-Meier method and Cox proportional
hazard regression model. Clinical parameters with a log-rank test
P-value <0.05 in LUAD OS were subjected to further multivariate
Cox proportional hazards regression model for adjustment. A value
of P<0.05 was considered to indicate a statistically significant
difference.
Results
Study cohort
A total of 515 patients that contributed 535 tumor
tissues and 59 adjacent non-cancerous tissues were extracted from
the TCGA database LUAD project. In total, 500 patients with LUAD
had complete clinical outcome parameters and RNA-seq data, and
these were included into further survival analysis. Univariate
survival analysis of the clinical parameters in LUAD OS suggested
that tumor stage was significantly associated with LUAD OS
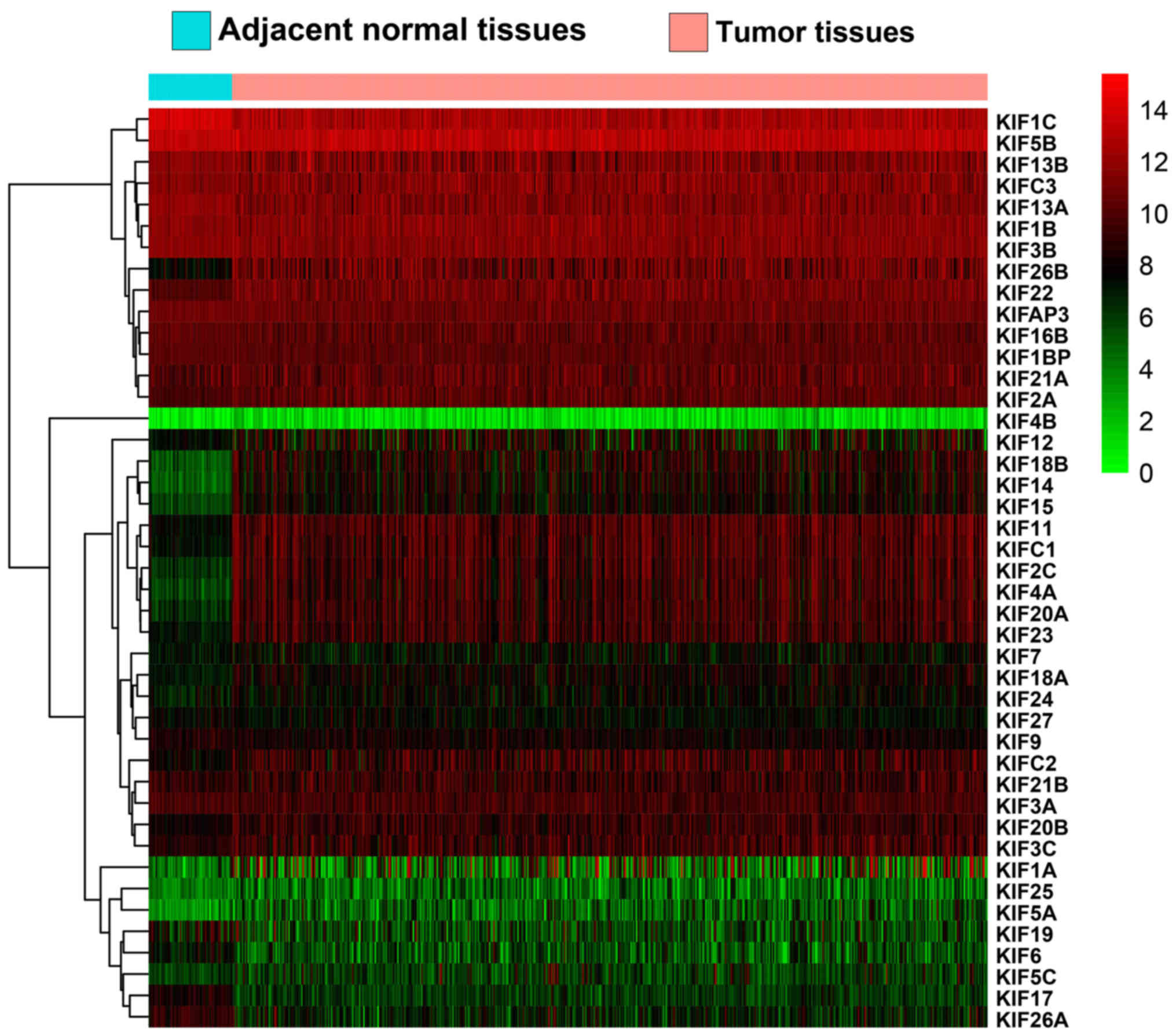
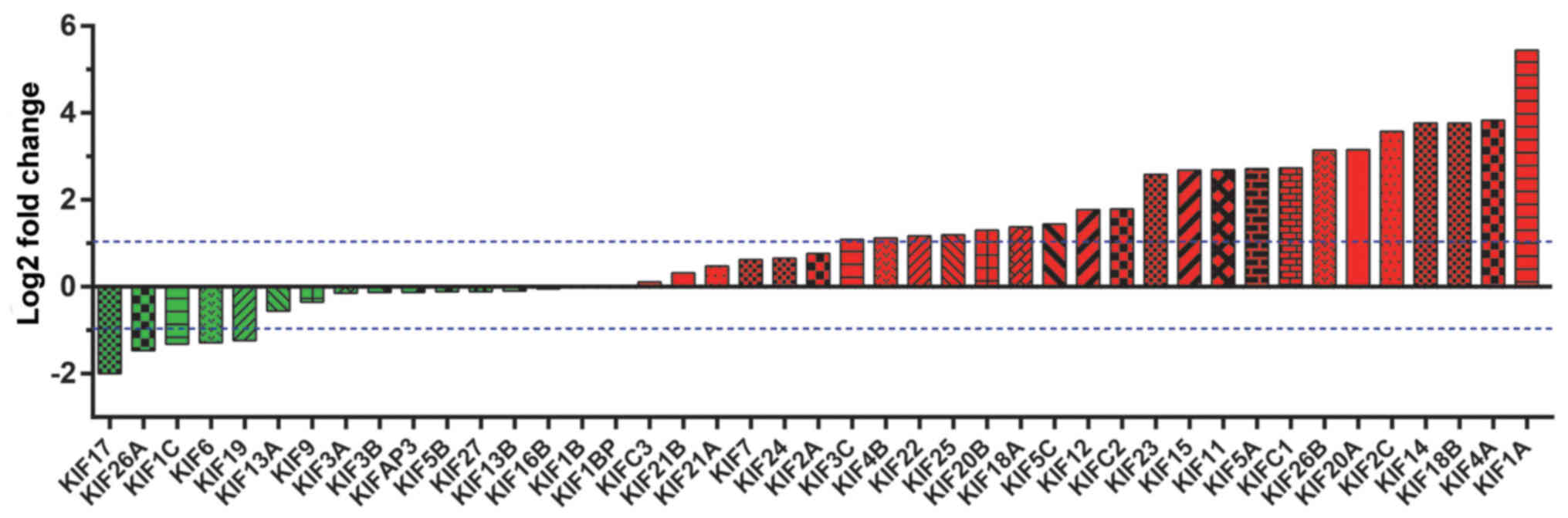
(Table I). Expression heatmaps and
differential expression fold changes are shown in Figs. 1 and 2,
respectively. In total, 25 KIF genes were found to be significantly
dysregulated between the LUAD tumor and adjacent non-cancerous
tissues, of these, 23 KIF genes were identified as DEGs based on
the following criteria: |log2 Fold Change(FC)| ≥1, P-value <0.05
and FDR <0.05. In total, 5 DEGs were found to be downregulated
in the LUAD tumor tissues, whereas the others were upregulated
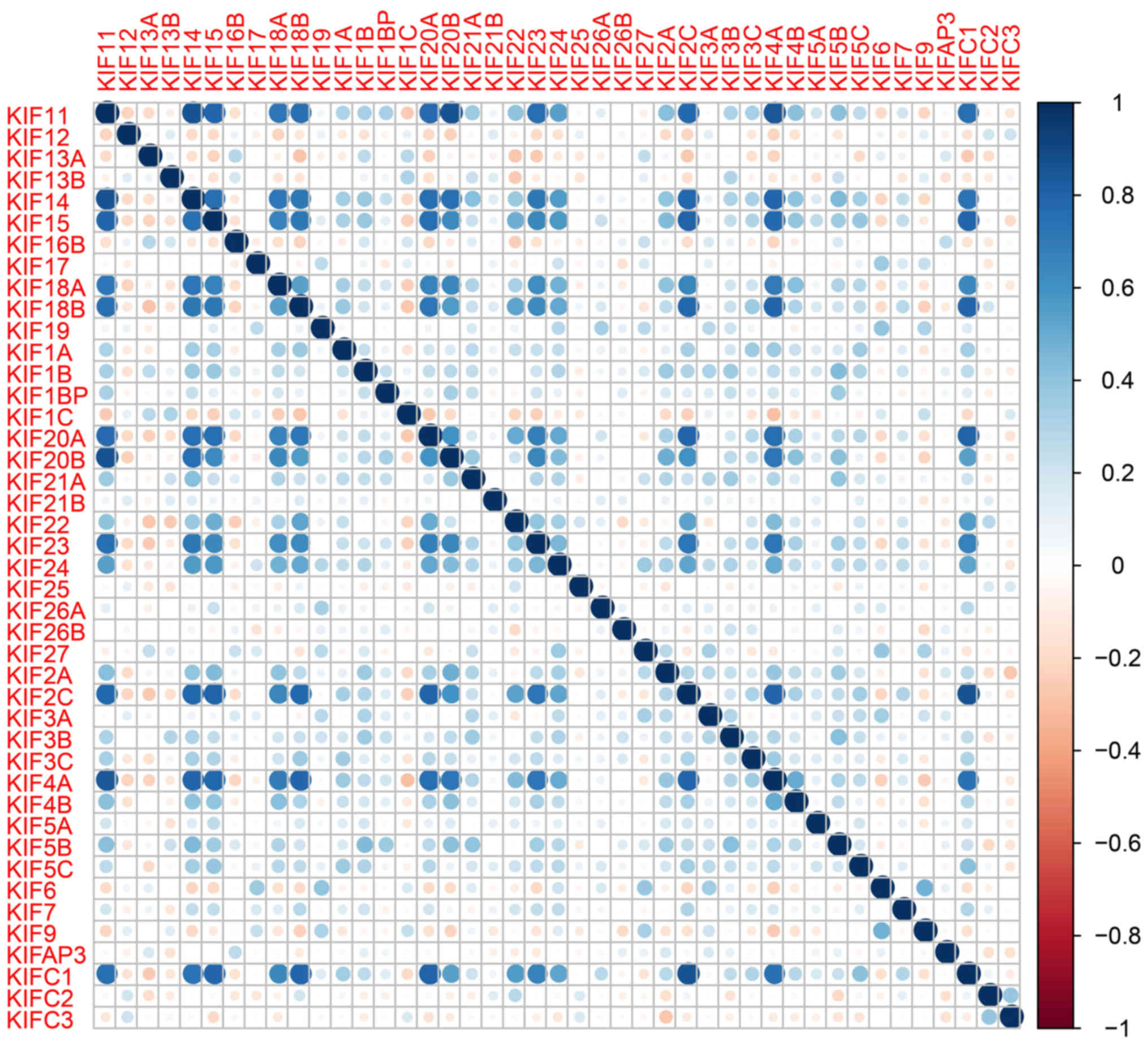
(Table II). Further analysis of the
co-expressed KIF genes in the tumor tissues revealed that a
majority of KIF genes existed in complex co-expression associations
(Fig. 3 and Table SI).
 | Table I.Clinical parameters of patients with
LUAD from TCGA. |
Table I.
Clinical parameters of patients with
LUAD from TCGA.
| Variables | Patients
(n=500) | MST (days) | Crude HR (95%
CI) | Log-rank
P-value |
|---|
| Age
(years)a |
|
|
| 0.386 |
|
≤65 | 215 | 1,499 | 1 |
|
|
>65 | 264 | 1,454 | 1.143
(0.845–1.546) |
|
| Sex |
|
|
| 0.754 |
|
Female | 270 | 1,454 | 1 |
|
|
Male | 230 | 1,528 | 1.048
(0.783–1.403) |
|
| Tumor
stageb |
|
|
| <0.0001 |
| Stage
I | 268 | 2,620 | 1 |
|
| Stage
II | 119 | 1,209 | 2.473
(1.719–3.559) |
|
| Stage
III | 80 |
879 | 3.495
(2.383–5.126) |
|
| Stage
IV | 25 |
826 | 3.819
(2.201–6.629) |
|
| Tumor
stageb |
|
|
| <0.0001 |
| Stage
I+II | 387 | 1,632 | 1 |
|
| Stage
III+IV | 105 |
826 | 2.585
(1.894–3.528) |
|
 | Table II.Differential expression analysis and
survival analysis of KIF family genes in patients with LUAD. |
Table II.
Differential expression analysis and
survival analysis of KIF family genes in patients with LUAD.
|
| Differential
expression analysis | Survival
analysis |
|---|
|
|
|
|
|---|
| Genes | log2 Fold
change | P-value | FDR | HRa | Low 95% CI | High 95% CI |
P-valueb |
|---|
| KIF14 | 3.761199 | <0.0001 | <0.0001 | 1.686098 | 1.246964 | 2.279878 | 0.000689 |
| KIF18A | 1.373027 | 0.002178 | 0.00919 | 1.610726 | 1.190265 | 2.179715 | 0.002012 |
| KIF23 | 2.582725 | <0.0001 | <0.0001 | 1.51088 | 1.117299 | 2.043104 | 0.007355 |
| KIF20A | 3.14896 | <0.0001 | <0.0001 | 1.478097 | 1.092943 | 1.998979 | 0.011181 |
| KIF11 | 2.689239 | <0.0001 | <0.0001 | 1.437782 | 1.064161 | 1.94258 | 0.01803 |
| KIF20B | 1.301735 | <0.0001 | 0.000271 | 1.43011 | 1.059873 | 1.929678 | 0.019265 |
| KIF18B | 3.766396 | <0.0001 | <0.0001 | 1.421903 | 1.052975 | 1.920092 | 0.021631 |
| KIF4A | 3.830272 | <0.0001 | <0.0001 | 1.405441 | 1.04261 | 1.894539 | 0.025494 |
| KIF1B | 0.008518 | 0.925271 | 0.972874 | 0.749876 | 0.558315 | 1.007161 | 0.055805 |
| KIF27 | −0.11005 | 0.603581 | 0.758646 | 0.763263 | 0.567524 | 1.02651 | 0.073954 |
| KIFC1 | 2.734491 | <0.0001 | <0.0001 | 1.307037 | 0.968747 | 1.763459 | 0.079741 |
| KIF4B | 1.122955 | 0.585909 | 0.74532 | 1.282052 | 0.954614 | 1.721803 | 0.098684 |
| KIFC2 | 1.792038 | <0.0001 | <0.0001 | 0.79411 | 0.590222 | 1.068429 | 0.127819 |
| KIF2C | 3.573061 | <0.0001 | <0.0001 | 1.25987 | 0.93572 | 1.696312 | 0.127964 |
| KIFAP3 | −0.1271 | 0.49553 | 0.668045 | 0.820523 | 0.610544 | 1.102719 | 0.189646 |
| KIF16B | −0.04692 | 0.831639 | 0.914865 | 0.822771 | 0.611296 | 1.107406 | 0.198118 |
| KIF1BP | 0.032733 | 0.883941 | 0.946259 | 1.196995 | 0.890981 | 1.608112 | 0.232603 |
| KIF13A | −0.5605 | 0.00104 | 0.00482 | 0.838206 | 0.624352 | 1.125311 | 0.240241 |
| KIF5A | 2.712286 | 0.007712 | 0.026645 | 0.850435 | 0.633679 | 1.141335 | 0.280467 |
| KIF17 | −1.99907 | <0.0001 | 0.000566 | 0.854866 | 0.637723 | 1.145947 | 0.29427 |
| KIF21B | 0.316249 | 0.296082 | 0.472991 | 0.863796 | 0.641166 | 1.163729 | 0.335624 |
| KIF3C | 1.08788 | 0.000112 | 0.000682 | 1.155325 | 0.860101 | 1.551884 | 0.337567 |
| KIF21A | 0.471146 | 0.078692 | 0.175791 | 0.869257 | 0.647914 | 1.166216 | 0.35006 |
| KIF5B | −0.11057 | 0.509291 | 0.680306 | 1.149144 | 0.857356 | 1.540238 | 0.352275 |
| KIF26A | −1.46358 | 0.000133 | 0.000793 | 0.877007 | 0.652637 | 1.178514 | 0.38403 |
| KIF12 | 1.77177 | <0.0001 | <0.0001 | 0.878517 | 0.6541 | 1.179931 | 0.389461 |
| KIF24 | 0.658401 | 0.275437 | 0.450236 | 1.132135 | 0.843729 | 1.519126 | 0.408083 |
| KIF15 | 2.685009 | <0.0001 | <0.0001 | 1.133063 | 0.842076 | 1.524602 | 0.40941 |
| KIF25 | 1.190456 | 0.336607 | 0.51667 | 0.918596 | 0.685346 | 1.23123 | 0.569947 |
| KIF7 | 0.621628 | 0.265307 | 0.439337 | 1.083857 | 0.807299 | 1.455156 | 0.592125 |
| KIF6 | −1.28474 | 0.013328 | 0.042003 | 0.924712 | 0.688201 | 1.242503 | 0.603526 |
| KIF13B | −0.09452 | 0.4315 | 0.610314 | 1.07943 | 0.804877 | 1.447637 | 0.609761 |
| KIF19 | −1.23658 | 0.000403 | 0.002133 | 0.931921 | 0.694829 | 1.249914 | 0.637848 |
| KIF5C | 1.444426 | 0.067916 | 0.156645 | 0.937923 | 0.697936 | 1.260429 | 0.670823 |
| KIF26B | 3.141348 | <0.0001 | <0.0001 | 0.938876 | 0.698727 | 1.261564 | 0.675623 |
| KIF1A | 5.445563 | <0.0001 | <0.0001 | 1.064054 | 0.791266 | 1.430885 | 0.681207 |
| KIF2A | 0.760907 | 0.001016 | 0.004726 | 1.062085 | 0.791335 | 1.42547 | 0.688285 |
| KIF22 | 1.166602 | <0.0001 | <0.0001 | 1.057282 | 0.787208 | 1.420012 | 0.711293 |
| KIFC3 | 0.108768 | 0.504868 | 0.675814 | 1.056382 | 0.787667 | 1.41677 | 0.714182 |
| KIF3B | −0.13168 | 0.409013 | 0.588436 | 0.954955 | 0.712533 | 1.279855 | 0.757712 |
| KIF3A | −0.14052 | 0.500277 | 0.671849 | 0.955389 | 0.71235 | 1.281346 | 0.760587 |
| KIF1C | −1.31875 | <0.0001 | <0.0001 | 0.990563 | 0.737947 | 1.329656 | 0.949668 |
| KIF9 | −0.34716 | 0.253979 | 0.425275 | 0.992495 | 0.739871 | 1.331377 | 0.959913 |
Prognostic KIF gene screening
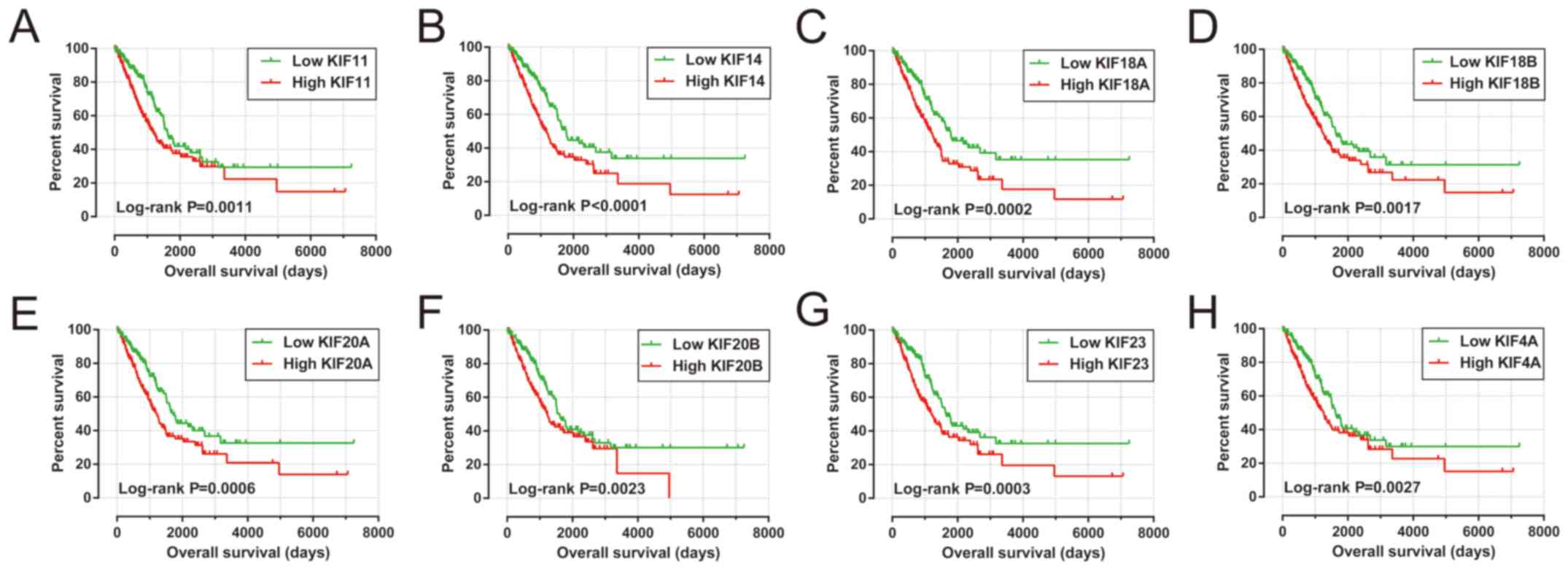
Survival analysis of KIF genes in the present study
cohort based on multivariate Cox proportional hazards regression
model demonstrated a total of 8 KIF genes that were significantly
associated with LUAD OS (Table II
and Fig. 4). The upregulation of
these 8 prognostic KIF genes was associated with significantly
higher mortality risks in the patients with LUAD. In addition, we
also observed that these 8 prognostic KIF genes were notably
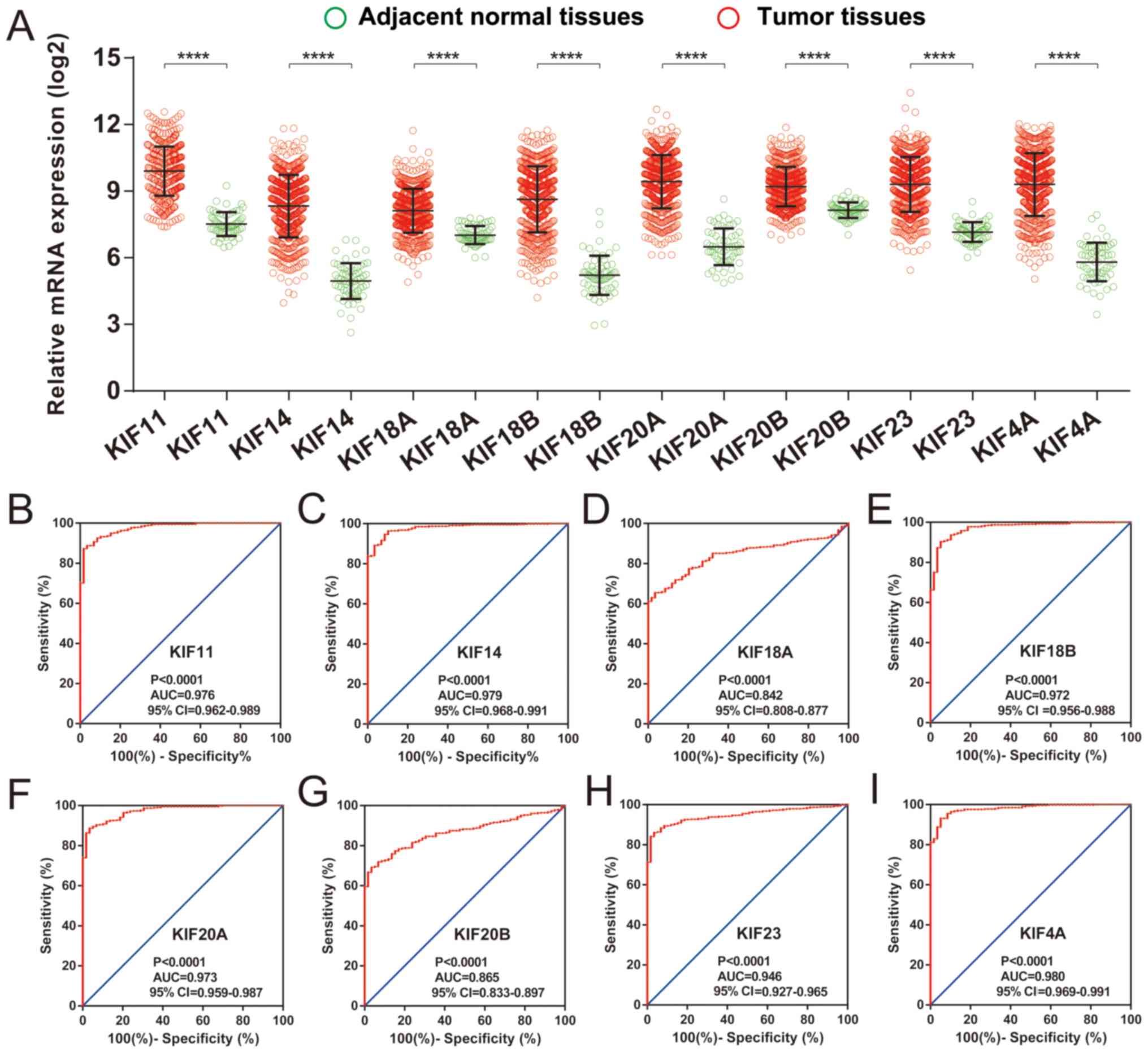
upregulated in the LUAD tumor tissues (Fig. 5A), and ROC curve analysis also
substantiated that these 8 prognostic KIF genes may serve as
potential diagnostic biomarkers for LUAD (Fig. 5B-I).
Construction of a prognostic gene
signature
The 8 KIF genes that were significantly associated
with LUAD OS on single gene survival analysis were subjected to
screening for potential prognostic gene signature combination using
the ‘step’ function. The most significant KIF candidate gene
combinations of these 8 KIF genes were further screened for
prognostic signature construction. Finally, KIF14, KIF18B
and KIF20A were used for the prognostic signature
construction based on the following formula: Expression of
KIF14 × (0.2437) + expression of KIF18B × (−0.1541) +
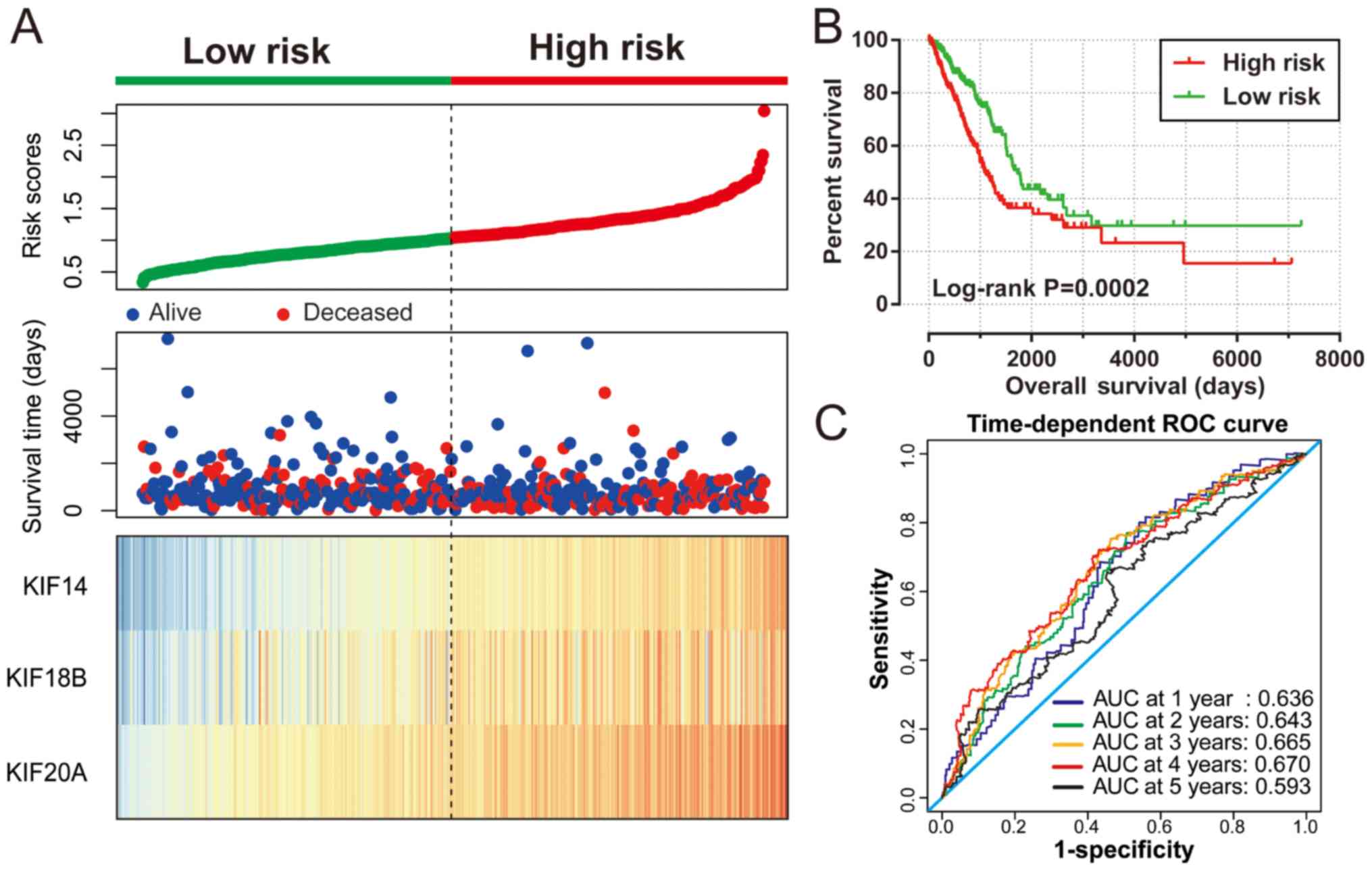
expression of KIF20A × (0.1926). Survival analysis revealed
that patients with high risk scores were more likely to have an
increased risk of death (log-rank P=0.0002, adjusted P=0.003;
adjusted HR, 1.576; 95% CI, 1.166–2.129; Fig. 6A and B) and a poorer clinical outcome
(median survival time, high risk vs. low risk: 1,081 vs. 1,725
days). The predictive accuracy of this prognostic signature was
determined using time-dependent ROC curve analysis, with the
results suggesting that the constructed signature was able to
accurately predict the 1-, 2-, 3-, 4- and 5-year patient survival,
based on the respective area under curves 0.636, 0.643,0.665, 0.670
and 0.593 (Fig. 6C), respectively. We
also noted that the expression levels of the KIF14, KIF18B
and KIF20A genes exhibited a strongly and positive
correlation with each other (Pearson's correlation coefficient
r=0.713 for KIF14 and KIF18B; r=0.760 for
KIF14 and KIF20A; r=0.722 for KIF20A and
KIF18B; Table SI).
Stratified and joint effects
analysis
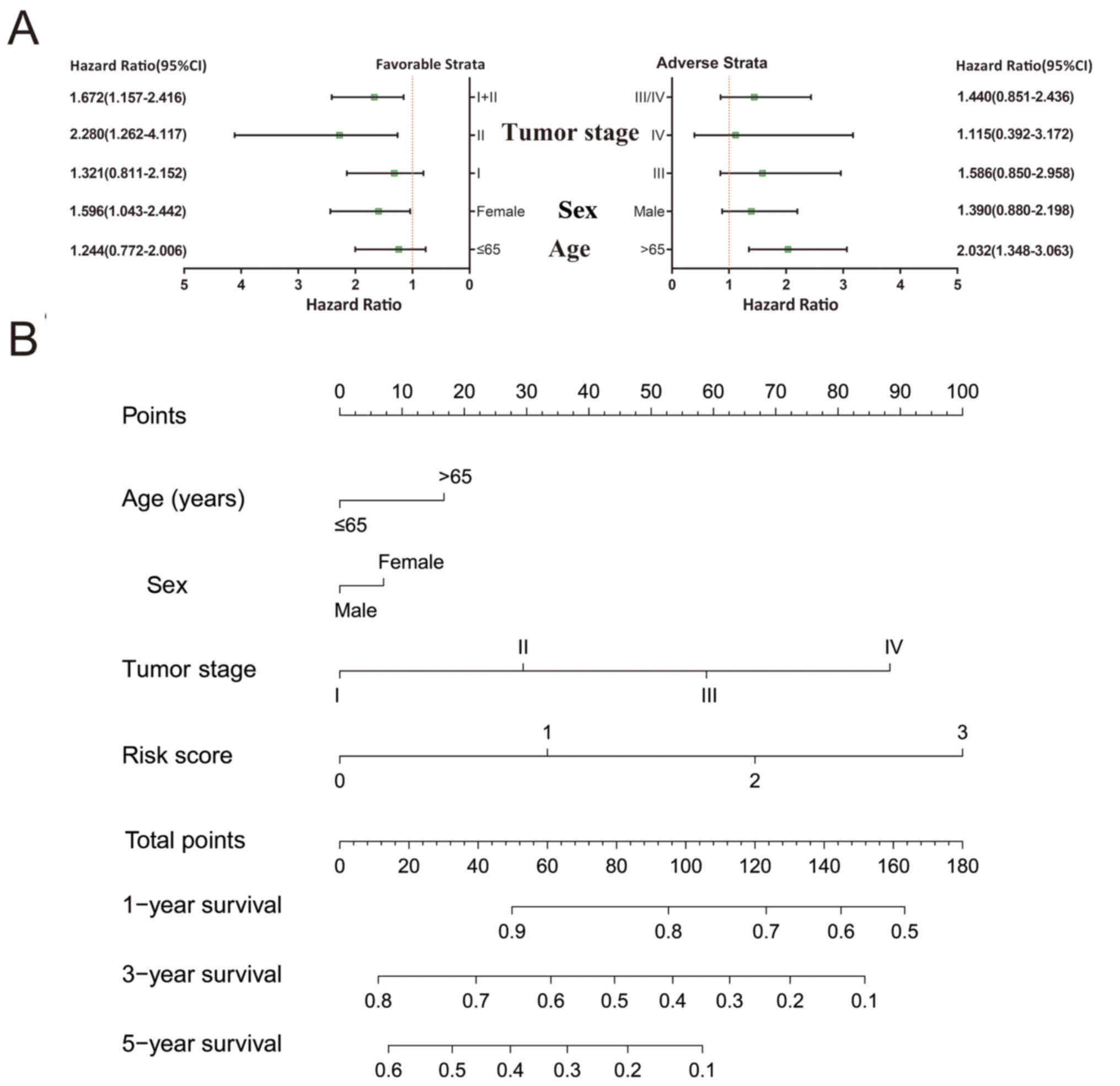
A comprehensive analysis of the nomogram and
stratified and joint effects survival analysis was used to further
investigate the association between clinical parameters and the
prognostic gene signature. Patients that had stage I and stage I+II
disease, were of the female sex and were >65 years of age were
more likely to succumb to the disease if they also had higher risk
scores (Fig. 7A). A nomogram
constructed of the risk scores and clinical LUAD parameters
demonstrated that the KIF gene expression-based prognostic
signature was more accurate compared to other parameters (Fig. 7B).
Joint effects survival analysis between the KIF gene
expression-based clinical parameters and prognostic gene signatures
indicated that the constructed signature was able to accurately
predict the OS of patients with LUAD, particularly when combined
with clinical parameters (Fig. 8 and
Table III).
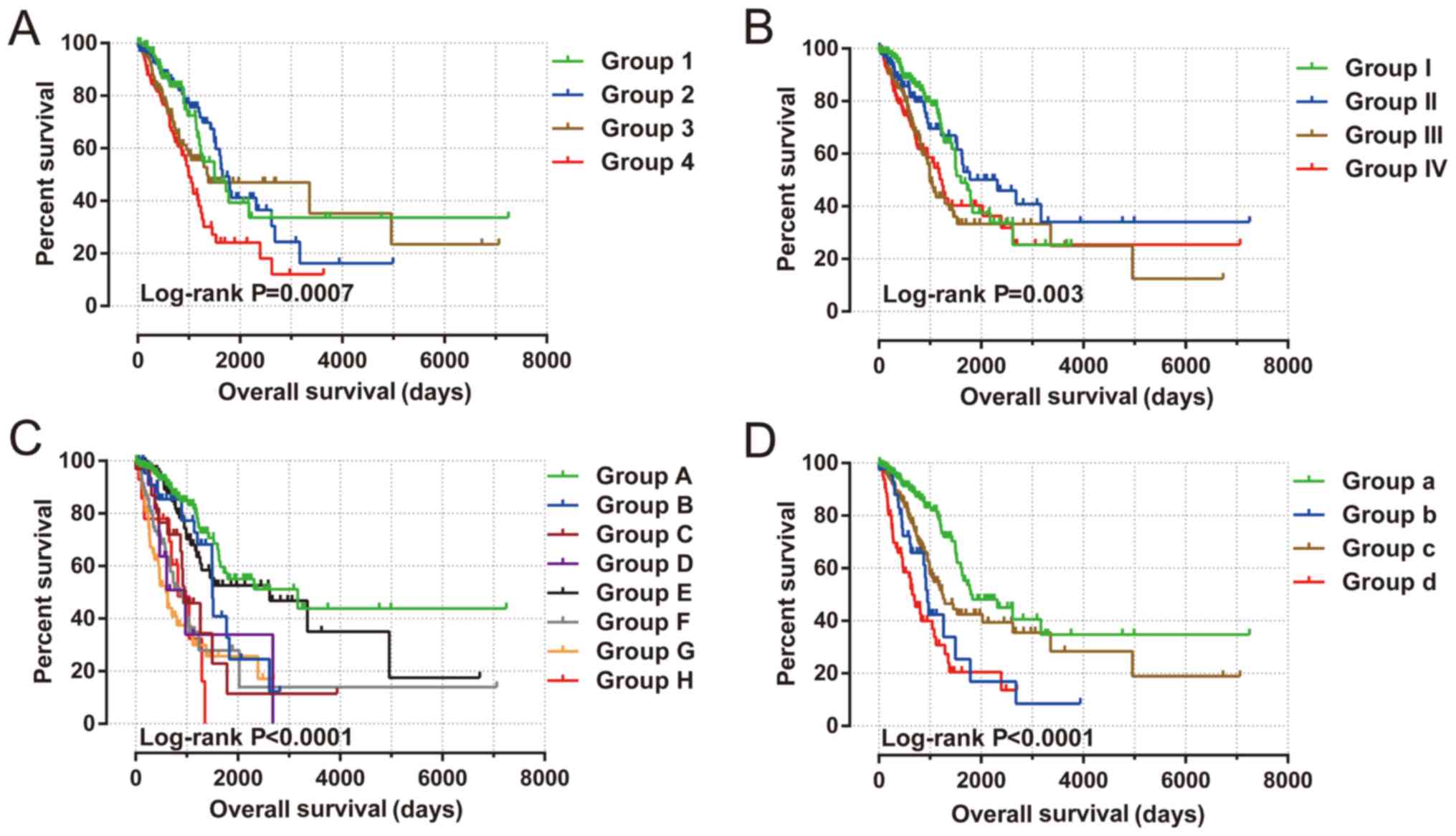 | Figure 8.Joint effects analysis of risk score
and clinical features in patients with LUAD. Joint effects analysis
of risk score stratified by the following clinical features in
patients with LUAD: (A) Age: Group 1 is low risk and age ≤65
combination, group 2 is low risk and age >65 combination, group
3 is high risk and age ≤65 combination, and group 4 is high risk
and age >65 combination. (B) Sex: Group I is low risk and female
combination, group II is low risk and male combination, group III
is high risk and female combination, and group IV is high risk and
male combination. (C) Tumor stage: Group A is low risk and stage I
combination, group B is low risk and stage II combination, group C
is low risk and stage III combination, group D is low risk and
stage IV combination, group E is high risk and stage I combination,
group F is high risk and stage II combination, group G is high risk
and stage III combination, and group H is high risk and stage IV
combination. (D) Tumor stage stratified by early stage and advanced
stage: Group a is low risk and stage I+II combination, group b is
low risk and stage III+IV combination, group c is high risk and
stage I+II combination, and group d is high risk and stage III+IV
combination. LUAD, lung adenocarcinoma. |
 | Table III.Joint effects survival analysis of
clinical parameters and the risk score in LUAD patients from
TCGA. |
Table III.
Joint effects survival analysis of
clinical parameters and the risk score in LUAD patients from
TCGA.
| Group | Risk score | Variables | Events/total
(n=500) | MST (days) | Crude HR (95%
CI) | Crude P | Adjusted HR (95%
CI) | Adjusted
P-valuea |
|---|
|
|
| Age (years) |
|
|
|
|
|
|
| 1 | Low risk | ≤65 | 29/95 | 1,501 | 1 |
| 1 |
|
| 2 | Low risk | >65 | 43/144 | 1653 | 0.958
(0.598–1.536) |
0.86 | 0.984
(0.607–1.595) |
0.948 |
| 3 | High risk | ≤65 | 45/120 | 1,357 | 1.362
(0.853–2.173) |
0.195 | 1.223
(0.760–1.967) |
0.406 |
| 4 | High risk | >65 | 56/120 | 999 | 2.021
(1.288–3.171) |
0.002 | 1.937
(1.226–3.060) |
0.005 |
|
|
| Sex |
|
|
|
|
|
|
| I | Low risk | Female | 43/151 | 1,600 | 1 |
| 1 |
|
| II | Low risk | Male | 32/99 | 2,318 | 1.011
(0.638–1.601) |
0.963 | 0.935
(0.582–1.503) |
0.781 |
| III | High risk | Female | 53/119 | 999 | 1.763
(1.177–2.640) |
0.006 | 1.644
(1.088–2.4830 |
0.018 |
| IV | High risk | Male | 54/131 | 1,235 | 1.747
(1.169–2.609) |
0.006 | 1.434
(0.952–2.159) |
0.084 |
|
|
| Tumor stage |
|
|
|
|
|
|
| A | Low risk | Stage I | 34/154 | 3,169 | 1 |
| 1 |
|
| B | Low risk | Stage II | 17/48 | 1,501 | 1.751
(0.977–3.140) |
0.06 | 1.751
(0.977–3.140) |
0.06 |
| C | Low risk | Stage III | 15/32 | 952 | 3.025
(1.643–5.571) |
0.0004 | 3.025
(1.643–5.571) |
0.0004 |
| D | Low risk | Stage IV |
7/11 | 976 | 3.957
(1.749–8.952) |
0.001 | 3.957
(1.749–8.952) |
0.001 |
| E | High risk | Stage I | 31/114 | 2,620 | 1.317
(0.809–2.144) |
0.268 | 1.317
(0.809–2.144) |
0.268 |
| F | High risk | Stage II | 37/71 | 864 | 3.883
(2.421–6.227) | <0.0001 | 3.883
(2.421–6.227) | <0.0001 |
| G | High risk | Stage III | 30/48 | 593 | 4.697
(2.868–7.694) | <0.0001 | 4.697
(2.868–7.694) | <0.0001 |
| H | High risk | Stage IV | 9/14 | 826 | 4.712
(2.244–9.892) | <0.0001 | 4.712
(2.244–9.892) | <0.0001 |
|
|
| Tumor stage |
|
|
|
|
|
|
| a | Low risk | Stage I+II | 51/202 | 1,798 | 1 |
| 1 |
|
| b | Low risk | Stage III+IV | 22/43 | 952 | 2.767
(1.674–4.574) | <0.0001 | 3.948
(2.024–7.699) | <0.0001 |
| c | High risk | Stage I+II | 68/185 | 1,258 | 1.750
(1.216–2.520) | 0.003 | 1.644
(1.139–2.373) |
0.008 |
| d | High risk | Stage III+IV | 39/62 | 656 | 3.974
(2.612–6.047) | <0.0001 | 5.700
(3.061–10.613) | <0.0001 |
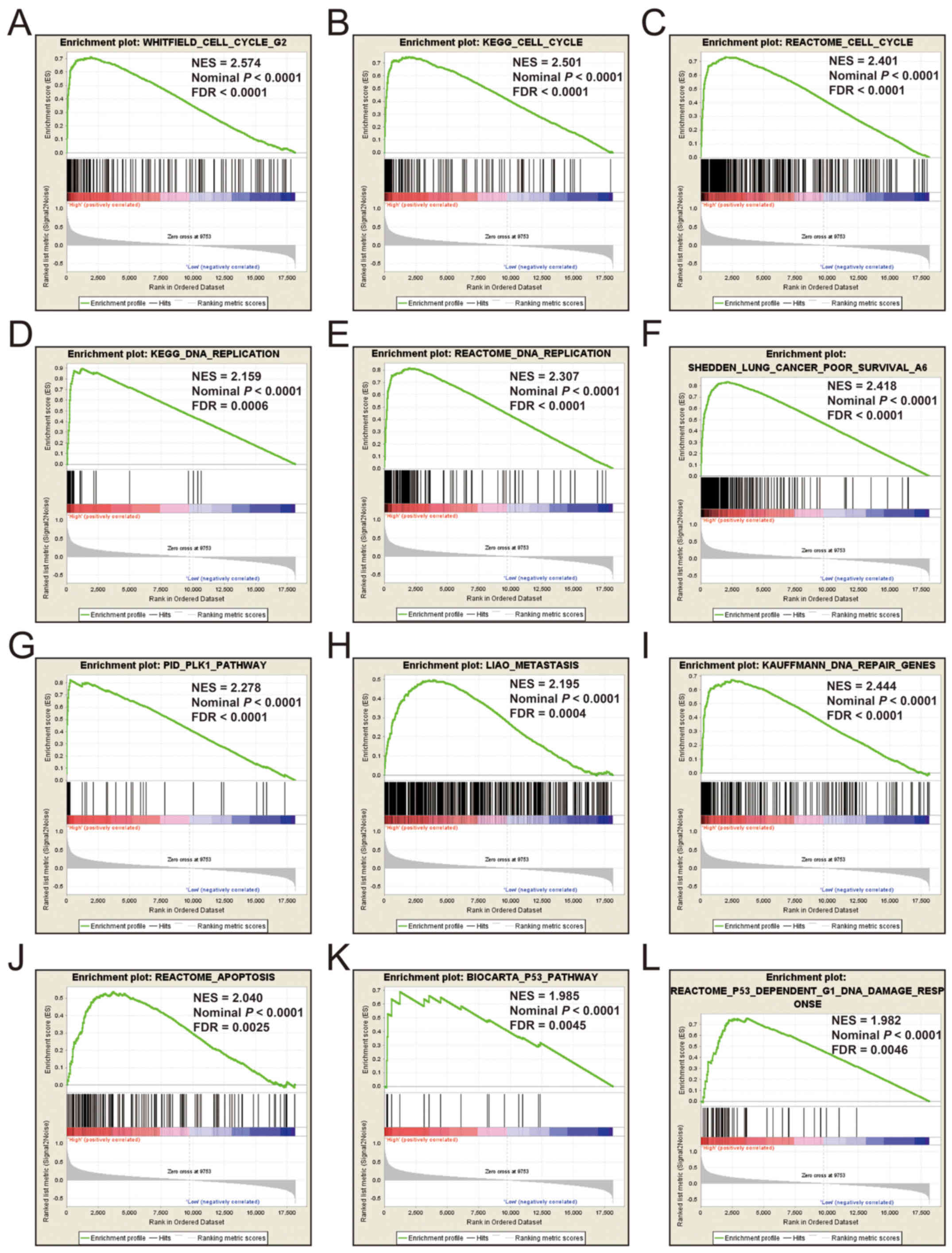
GSEA
Additional exploration of the biological pathways of
the selected KIF genes in relation to LUAD was carried out using a
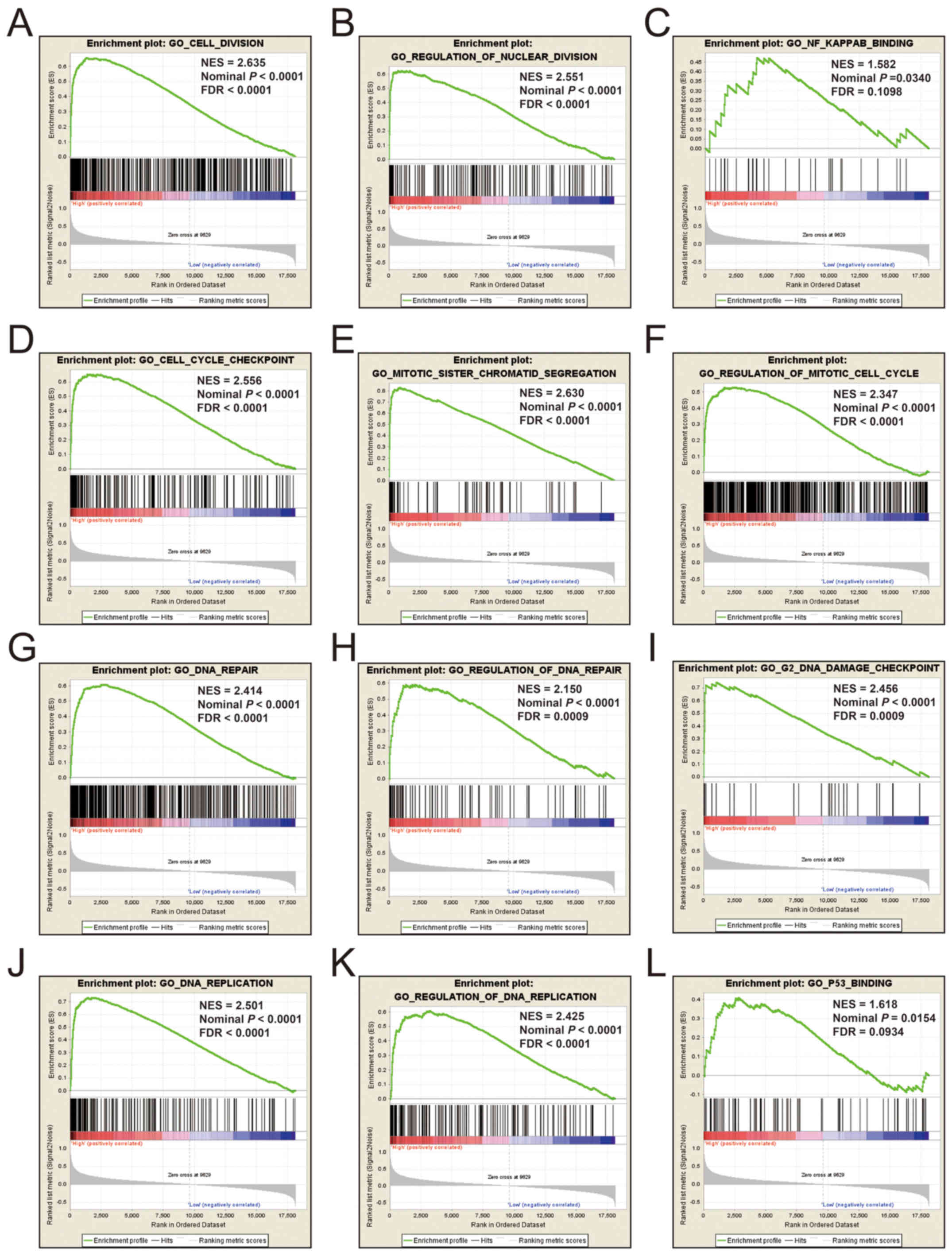
single gene GSEA. An enrichment of c5 suggested that a high
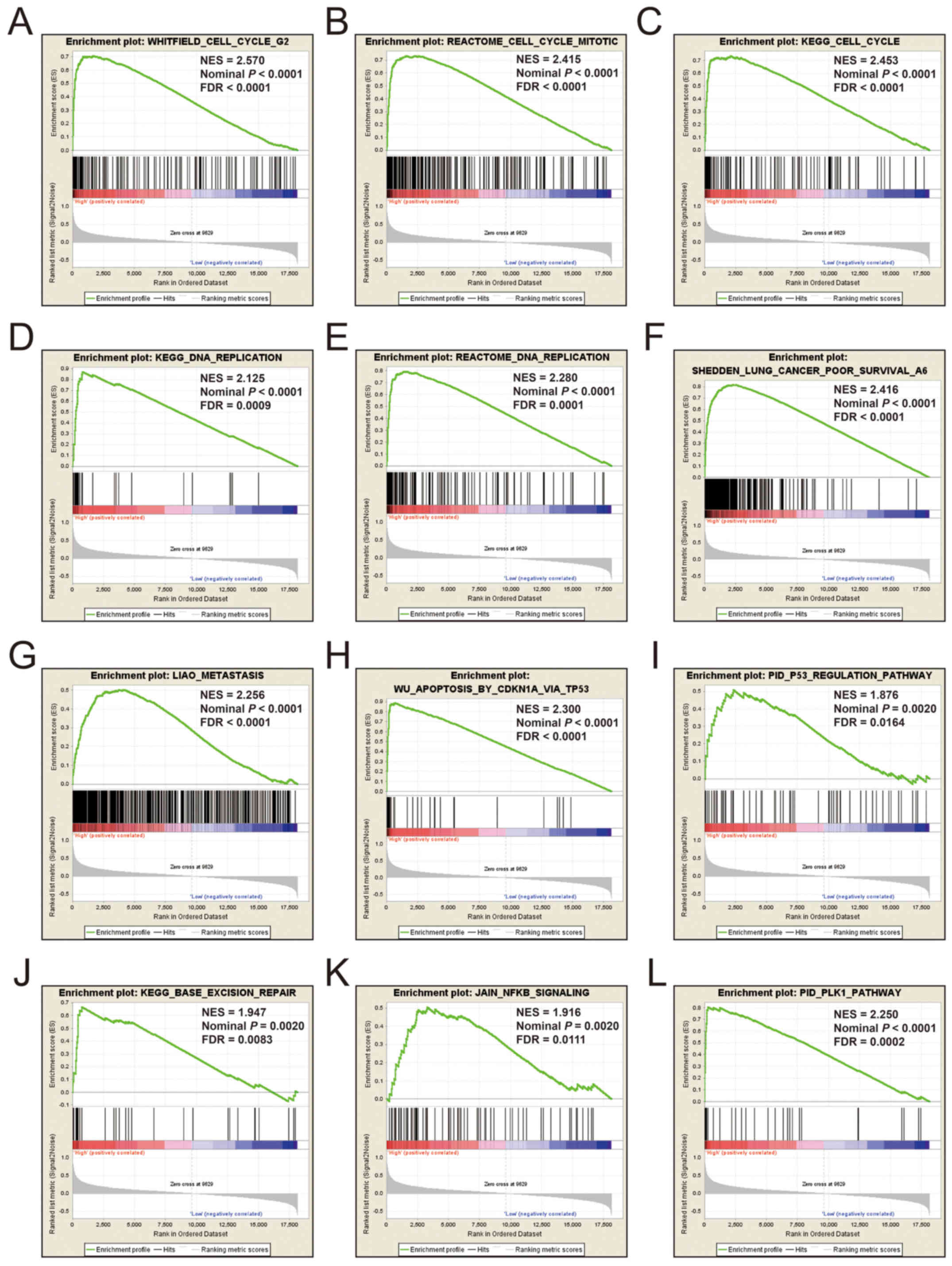
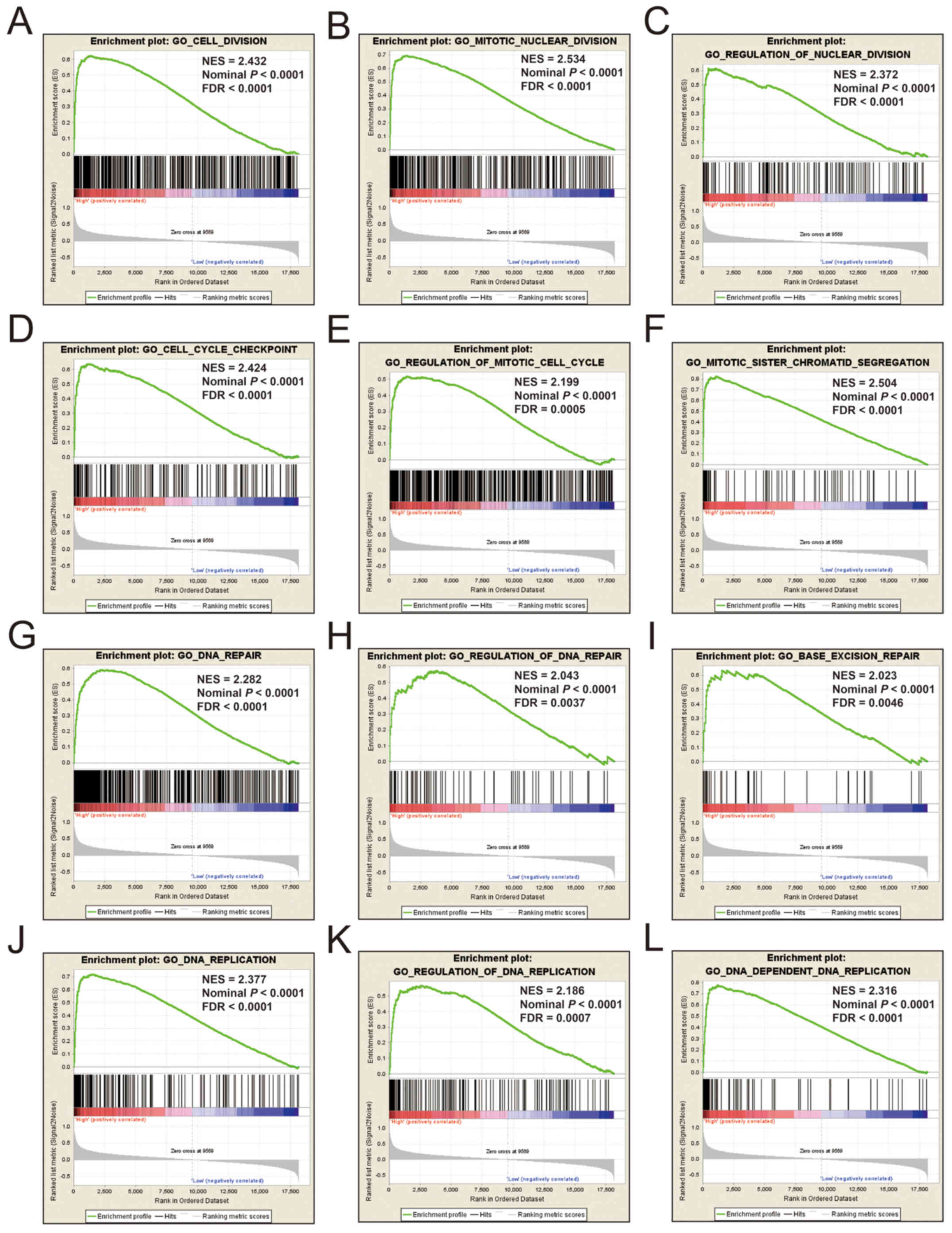
expression of KIF14 was involved in DNA repair, DNA
replication, cell cycle, tumor protein p53 (TP53) binding and
mitotic sister chromatid separation biological processes (Fig. 9 and Table
SII). Whereas, an enrichment of c2 indicated that a high
expression of KIF14 influenced the cell cycle, DNA
replication, lung cancer poor survival, metastasis, base excision
repair, the PLK1 pathway, nuclear factor-κB (NF-κB) and the TP53
pathway (Fig. 10 and Table SIII). c5 enrichment suggested that a
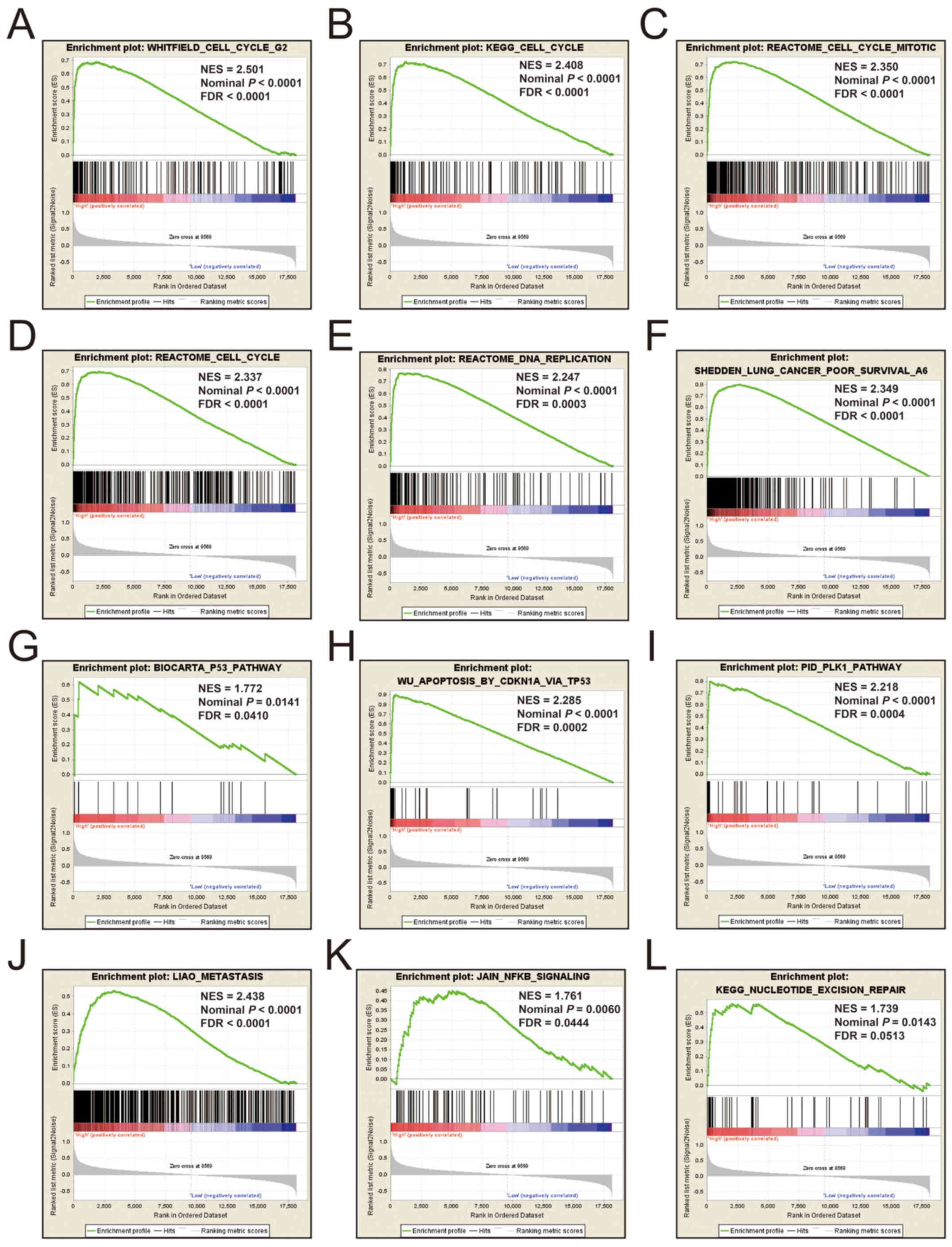
high expression of KIF18B was also involved in cell
division, the cell cycle, DNA replication and DNA repair (Fig. 11 and Table
SIV), whereas c2 enrichment suggested that a high expression of
KIF18b was involved in the cell cycle, DNA replication, lung
cancer poor survival, metastasis, base excision repair, the PLK1
pathway, NF-κB and TP53 pathway (Fig.
12 and Table SV). Similar
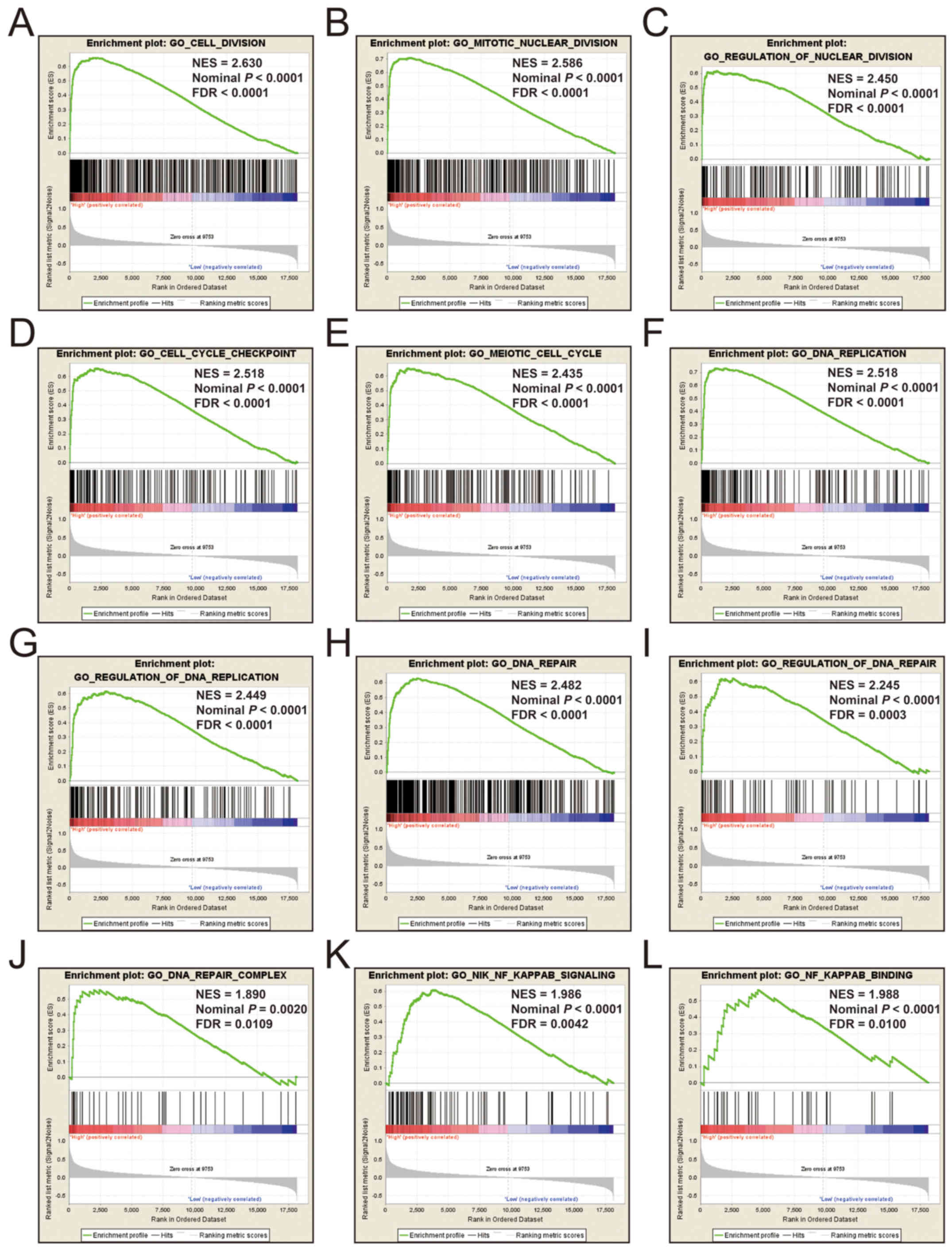
results were also found for KIF20A, where c5 enrichment
suggested that a high expression of KIF20A was involved in
cell division, cell cycle, DNA replication, DNA repair and the
NF-κB pathway (Fig. 13 and Table SVI), whereas c2 enrichment suggested
that high expression of KIF20A was involved in cell cycle,
DNA replication, lung cancer poor survival, apoptosis, metastasis,
DNA repair, the PLK1 and TP53 pathway (Fig. 14 and Table
SVII). It is evident that the potential mechanisms of KIF14,
KIF18B and KIF20A are likely mediated through their
influence on cell cycle regulation, DNA replication and DNA repair.
Furthermore, all the c2 enrichment analyses of KIF14, KIF18B
and KIF20A were enriched in the gene set of
SHEDDEN_LUNG_CANCER_POOR_SURVIVAL_A6, which indicated that the
upregulated expression levels of the genes of this gene set in
patients with lung cancer were predictors of a poor survival
outcome.
Discussion
KIF family member genes encoded proteins are
required for numerous processes, including intracellular transport,
chromosome segregation, mitotic spindle formation and cytokinesis,
and multiple family member genes have been reported to be
dysregulated in various types of cancer (5,8,12,27–29). The
prognostic and diagnostic capabilities of KIF family member genes
have been demonstrated in various types of cancer. In a previous
study, immumohistochemical staining suggested that KIF3A
expression was significantly higher in breast cancer (BC) tumor
tissues than healthy adjacent tissues (8). Previous studies have demonstrated that
an increased KIF2A expression is a predictor of an
unfavorable clinical outcome in patients with LUAD and diffuse
large B cell lymphoma (12,30). An increased KIF4A expression
has also been shown to be strongly associated with a poorer
prognosis of patients with BC (9),
prostate cancer (PCa) (10) and
hepatocellular carcinoma (HCC) (31,32).
Similar prognostic values of KIF11 in oral cancer (33) and BC (34) have also been reported. Other members
of the KIF gene family have also exhibited similar prognostic
values in other types of cancer, such as KIF26B in ovarian
cancer (OC) (35) and KIF20B
in HCC (36). In the present study,
we observed that 26 KIF family member genes were differentially
expressed in LUAD and healthy adjacent tissues and identified as
DEGs, with 5 DEGs were downregulated and 18 DEGs were upregulated.
In total, 8 of these DEGs were identified as diagnostic and
prognostic genes for LUAD, which was consistent with the findings
of the above-mentioned studies. Three of these genes were used to
construct a potential prognostic signature for LUAD.
For 3 KIF genes of the prognostic signature that
were identified in the present study, previous studies have
observed that KIF14 is notably upregulated in tumor tissues
of OC (37,38), pancreatic carcinoma (39), cervical cancer (40), BC (41),
PCa (28), glioma (42) and gastric cancer (GC) (43). The prognostic analysis of previous
studies has demonstrated that an elevated KIF14 expression
confers unfavorable clinical outcomes in patients with OC (37,38),
pancreatic carcinoma (39), cervical
cancer (40), BC (41), PCa (28), glioma (42), medulloblastoma (44), lung cancer (45), HCC (46)
and GC (43). The function of
KIF14 may serve as an oncogene in cancers, and inhibiting
KIF14 has been shown to inhibit the growth of PCa and LUAD
cell lines (13,28), to suppress the proliferation of
medulloblastoma and NSCLC (44,45), to
decrease cancer cell migration and induce apoptosis in HCC
(46), as well as to inhibit tumor
metastasis in GC (43), PCa (28) and LUAD (13). In the present study, our results of
KIF14 in LUAD were also consistent with those of these
previous studies. Our results suggest that KIF14 may be
adopted as diagnostic and prognostic indicator for LUAD. Similar
with the KIF14, we also identified that KIF18B was
upregulated in LUAD tumor tissues, suggesting its utility as a
diagnostic and prognostic biomarker in patients with LUAD. Wu et
al demonstrated that KIF18B expression was increased in
cervical cancer tumor tissues with an advanced tumor grade and
stage. This gene may also function as a cervical cancer oncogene,
as the downregulation of KIF18B has been shown to inhibit
cervical cancer migration, invasion and cell in vitro
(47). Itzel et al identified
KIF18B as a novel oncogene that drives carcinogenesis in HCC
(48). Our results were very
consistent with the results of these previous studies. To the best
of our knowledge, this study was the first to suggest that
KIF18B may serve as potential diagnostic and prognostic
indicator for LUAD.
Another of our candidate prognostic signature gene,
KIF20A, has also been reported to be strongly expressed in
nasopharyngeal carcinoma (NPC) (49),
NSCLC (14,50,51), HCC
(52), cervical cancer (53), glioma (54), OC (55)
and clear cell renal cell carcinoma (ccRCC) (56). Furthermore, a high expression of
KIF20A in these types of cancer has also been shown to be
associated with an increased risk of an unfavorable prognosis
(14,49–53,55,56).
In addition, previous studies have also demonstrated that a high
KIF20A expression is associated with a poor clinical outcome
in patients with melanoma (57) and
ovarian clear cell carcinoma cells (58). Previous studies have also observed
that KIF20A is significantly related to tumor progression,
and advanced stage tumor tissues exhibit an inceased KIF20A
expression level (55,56). Functional experiment assessment in
cancers infers that KIF20A may play a carcinogenic role in
cancer, and cancer cell proliferation can be regulated by the
overexpression or inhibition of KIF20A (14,52,54,55,58).
In the present study, we also identified the
prospective molecular mechanisms using GSEA. KIF family genes play
critical roles in chromosome segregation, mitotic spindle formation
and cytokinesis. GSEA analysis further verified that KIF14,
KIF18B and KIF20A were significant participators in cell
cycle regulation, thereby influencing the clinical outcome of
patients with LUAD. Previous studies have demonstrated that
KIF14 functions to regulate cell apoptosis and
proliferation, cytokinesis and cell division (46,59,60). Xu
et al demonstrated that inhibiting KIF14 in HCC cell
lines can influence the cell cycle and cytokinesis biological
process (29). The overexpression of
KIF14 in colorectal cancer (CRC) has been shown to promote
cell proliferation and accelerate cell cycle progression (61). A similar oncogenic function of
KIF20A in the cell cycle and proliferation has also been
reported in pan-cancers (14,55,58,62). Itzel
et al observed that the overexpression of KIF18B
increased the proliferation of HCC cells (48). Based on literature reviewing and
prospective molecular mechanism analysis from the current study, it
can be concluded that the one of the molecular mechanisms of KIF
family genes is the involvement in the prognosis of LUAD mainly by
affecting cell cycle-related biological processes and pathways.
Among one of the limitations of this study is that
clinical information derived from TCGA was not comprehensive,
barring a complete assessment of risk profiles. The results of the
current study were also based on a single cohort and lack
additional validation cohorts, with verification in larger sample
sizes across differing cohorts needed to further verify the
findings. Furthermore, the results of this study were derived from
RNA sequencing data from the TCGA LUAD cohort and were not
validated in additional cohorts by RT-PCR and immunohistochemistry
in both the mRNA and protein level. Nevertheless, the resultant 3
KIF gene-signature developed in this study was proven to be a more
accurate prognosticator in contrast to other clinical data. These
results lay the foundation for further studies into the mechanistic
functions of KIF genes as regards the prognosis of patients with
LUAD, allowing for further development targeted LUAD therapy.
In conclusion, in this study, using an integrated
assessment of KIF family member genes RNA-seq dataset and clinical
data of LUAD derived from the TCGA database, we systematically
evaluated the differential expression and prognostic values of KIF
family member genes, and found that 23 KIF genes were DEGs between
LUAD tumor and adjacent normal tissues. In total, 8 of these were
found to be potential prognostic and diagnostic biomarkers in
patients with LUAD. In addition, we also developed a novel 3 KIF
gene-expression-based signature, including KIF14, KIF18B and
KIF20A, which may aid in the prognosis of patients with
LUAD.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank the contributors of
The Cancer Genome Atlas (https://cancergenome.nih.gov/) for sharing the LUAD
dataset on open access. In addition, we would also like to
acknowledge the helpful comments on this manuscript received from
our reviewers.
Funding
This study was supported in part by the self-raised
Scientific Research Fund of the Ministry of Health of Guangxi
Province (Z2016318 and Z2016364), The Basic Ability Improvement
Project for Middle-aged and Young Teachers in Colleges and
Universities in Guangxi (2018KY0110).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable request.
All raw data of LUAD, which were included in the current study, can
be downloaded from TCGA (https://portal.gdc.cancer.gov/).
Authors' contributions
LZ and PW designed this study; LZ, GZ, XW, XL, RH,
CH, PH, JZ and PW conducted and further performed the study,
processed and analyzed the data, as well as interpret the results.
LZ wrote this manuscript, and PW guided the writing. All authors
have read and approved the final manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The current study does not contain any studies with
human participants or animals performed by any of the authors.
Since all LUAD datasets included in this manuscript were obtained
from The Cancer Genome Atlas, therefore, additional approval by an
Ethics Committee was not necessary. In addition, the procedures of
this manuscript were in accordance with the Helsinki declaration of
1964 and its later amendments.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hirokawa N, Noda Y, Tanaka Y and Niwa S:
Kinesin superfamily motor proteins and intracellular transport. Nat
Rev Mol Cell Biol. 10:682–696. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lu W and Gelfand VI: Moonlighting motors:
kinesin, dynein, and cell polarity. Trends Cell Biol. 27:505–514.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rath O and Kozielski F: Kinesins and
cancer. Nat Rev Cancer. 12:527–539. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weinstein JN, Collisson EA, Mills GB, Shaw
KR, Ozenberger BA, Ellrott K, Shmulevich I, Sander C and Stuart JM;
Cancer Genome Atlas Research Network, : The Cancer Genome Atlas
Pan-Cancer analysis project. Nat Genet. 45:1113–1120. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tomczak K, Czerwińska P and Wiznerowicz M:
The Cancer Genome Atlas (TCGA): An immeasurable source of
knowledge. Contemp Oncol (Pozn) 19A. A68–A77. 2015.
|
|
8
|
Xia P, Chu S, Liu G, Chen G, Yi T, Feng S
and Zhou H: High expression of KIF3A is a potential new parameter
for the diagnosis and prognosis of breast cancer. Biomed Rep.
8:343–349. 2018.PubMed/NCBI
|
|
9
|
Xue D, Cheng P, Han M, Liu X, Xue L, Ye C,
Wang K and Huang J: An integrated bioinformatical analysis to
evaluate the role of KIF4A as a prognostic biomarker for breast
cancer. OncoTargets Ther. 11:4755–4768. 2018. View Article : Google Scholar
|
|
10
|
Gao H, Chen X, Cai Q, Shang Z and Niu Y:
Increased KIF4A expression is a potential prognostic factor in
prostate cancer. Oncol Lett. 15:7941–7947. 2018.PubMed/NCBI
|
|
11
|
Song X, Zhang T, Wang X, Liao X, Han C,
Yang C, Su K, Cao W, Gong Y, Chen Z, et al: Distinct diagnostic and
prognostic values of kinesin family member genes expression in
patients with breast cancer. Med Sci Monit. 24:9442–9464. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xie T, Li X, Ye F, Lu C, Huang H, Wang F,
Cao X and Zhong C: High KIF2A expression promotes proliferation,
migration and predicts poor prognosis in lung adenocarcinoma.
Biochem Biophys Res Commun. 497:65–72. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hung PF, Hong TM, Hsu YC, Chen HY, Chang
YL, Wu CT, Chang GC, Jou YS, Pan SH and Yang PC: The motor protein
KIF14 inhibits tumor growth and cancer metastasis in lung
adenocarcinoma. PLoS One. 8:e616642013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao X, Zhou LL, Li X, Ni J, Chen P, Ma R,
Wu J and Feng J: Overexpression of KIF20A confers malignant
phenotype of lung adenocarcinoma by promoting cell proliferation
and inhibiting apoptosis. Cancer Med. 7:4678–4689. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cancer Genome Atlas Research Network, .
Comprehensive molecular profiling of lung adenocarcinoma. Nature.
511:543–550. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Anders S and Huber W: Differential
expression analysis for sequence count data. Genome Biol.
11:R1062010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liao X, Zhu G, Huang R, Yang C, Wang X,
Huang K, Yu T, Han C, Su H and Peng T: Identification of potential
prognostic microRNA biomarkers for predicting survival in patients
with hepatocellular carcinoma. Cancer Manag Res. 10:787–803. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liao X, Liu X, Yang C, Wang X, Yu T, Han
C, Huang K, Zhu G, Su H, Qin W, et al: Distinct diagnostic and
prognostic values of minichromosome maintenance gene expression in
patients with hepatocellular carcinoma. J Cancer. 9:2357–2373.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liao X, Wang X, Huang K, Yang C, Yu T, Han
C, Zhu G, Su H, Huang R and Peng T: Genome-scale analysis to
identify prognostic microRNA biomarkers in patients with early
stage pancreatic ductal adenocarcinoma after
pancreaticoduodenectomy. Cancer Manag Res. 10:2537–2551. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liao X, Yang C, Huang R, Han C, Yu T,
Huang K, Liu X, Yu L, Zhu G, Su H, et al: Identification of
potential prognostic long non-coding RNA biomarkers for predicting
survival in patients with hepatocellular carcinoma. Cell Physiol
Biochem. 48:1854–1869. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang R, Liao X and Li Q: Identification
and validation of potential prognostic gene biomarkers for
predicting survival in patients with acute myeloid leukemia.
OncoTargets Ther. 10:5243–5254. 2017. View Article : Google Scholar
|
|
22
|
Liao X, Huang K, Huang R, Liu X, Han C, Yu
L, Yu T, Yang C, Wang X and Peng T: Genome-scale analysis to
identify prognostic markers in patients with early-stage pancreatic
ductal adenocarcinoma after pancreaticoduodenectomy. OncoTargets
Ther. 10:4493–4506. 2017. View Article : Google Scholar
|
|
23
|
Heagerty PJ and Zheng Y: Survival model
predictive accuracy and ROC curves. Biometrics. 61:92–105. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Subramanian A, Tamayo P, Mootha VK,
Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub
TR, Lander ES, et al: Gene set enrichment analysis: A
knowledge-based approach for interpreting genome-wide expression
profiles. Proc Natl Acad Sci USA. 102:15545–15550. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mootha VK, Lindgren CM, Eriksson KF,
Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E,
Ridderstråle M, Laurila E, et al: PGC-1alpha-responsive genes
involved in oxidative phosphorylation are coordinately
downregulated in human diabetes. Nat Genet. 34:267–273. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liberzon A, Birger C, Thorvaldsdóttir H,
Ghandi M, Mesirov JP and Tamayo P: The Molecular Signatures
Database (MSigDB) hallmark gene set collection. Cell Syst.
1:417–425. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sheng N, Xu YZ, Xi QH, Jiang HY, Wang CY,
Zhang Y and Ye Q: Overexpression of KIF2A is suppressed by miR-206
and associated with poor prognosis in ovarian cancer. Cell Physiol
Biochem. 50:810–822. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Y, Yuan Y, Liang P, Zhang Z, Guo X,
Xia L, Zhao Y, Shu XS, Sun S, Ying Y, et al: Overexpression of a
novel candidate oncogene KIF14 correlates with tumor progression
and poor prognosis in prostate cancer. Oncotarget. 8:45459–45469.
2017.PubMed/NCBI
|
|
29
|
Xu H, Choe C, Shin SH, Park SW, Kim HS,
Jung SH, Yim SH, Kim TM and Chung YJ: Silencing of KIF14 interferes
with cell cycle progression and cytokinesis by blocking the
p27(Kip1) ubiquitination pathway in hepatocellular carcinoma. Exp
Mol Med. 46:e972014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang Y, You X, Liu H, Xu M, Dang Q, Yang
L, Huang J and Shi W: High KIF2A expression predicts unfavorable
prognosis in diffuse large B cell lymphoma. Ann Hematol.
96:1485–1491. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang Y, Wang H, Lian Y, Wu X, Zhou L,
Wang J, Deng M and Huang Y: Upregulation of kinesin family member
4A enhanced cell proliferation via activation of Akt signaling and
predicted a poor prognosis in hepatocellular carcinoma. Cell Death
Dis. 9:1412018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hou G, Dong C, Dong Z, Liu G, Xu H, Chen
L, Liu L, Wang H and Zhou W: Upregulate KIF4A enhances
proliferation, invasion of hepatocellular carcinoma and indicates
poor prognosis across human cancer types. Sci Rep. 7:41482017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Daigo K, Takano A, Thang PM, Yoshitake Y,
Shinohara M, Tohnai I, Murakami Y, Maegawa J and Daigo Y:
Characterization of KIF11 as a novel prognostic biomarker and
therapeutic target for oral cancer. Int J Oncol. 52:155–165.
2018.PubMed/NCBI
|
|
34
|
Pei YY, Li GC, Ran J and Wei FX: Kinesin
family member 11 contributes to the progression and prognosis of
human breast cancer. Oncol Lett. 14:6618–6626. 2017.PubMed/NCBI
|
|
35
|
Yang X, Zhang L and Xie L: Upregulation of
KIF26B, cell migration and proliferation of human ovarian cancer
cell lines in vitro, and patient outcomes from human bioinformatic
analysis. Med Sci Monit. 24:3863–3872. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu X, Li Y, Zhang X, Liu XY, Peng A, Chen
Y, Meng L, Chen H, Zhang Y, Miao X, et al: Inhibition of kinesin
family member 20B sensitizes hepatocellular carcinoma cell to
microtubule-targeting agents by blocking cytokinesis. Cancer Sci.
109:3450–3460. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Qiu HL, Deng SZ, Li C, Tian ZN, Song XQ,
Yao GD and Geng JS: High expression of KIF14 is associated with
poor prognosis in patients with epithelial ovarian cancer. Eur Rev
Med Pharmacol Sci. 21:239–245. 2017.PubMed/NCBI
|
|
38
|
Thériault BL, Pajovic S, Bernardini MQ,
Shaw PA and Gallie BL: Kinesin family member 14: An independent
prognostic marker and potential therapeutic target for ovarian
cancer. Int J Cancer. 130:1844–1854. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou Z, Cheng Y, Jiang Y, Liu S, Zhang M,
Liu J and Zhao Q: Ten hub genes associated with progression and
prognosis of pancreatic carcinoma identified by co-expression
analysis. Int J Biol Sci. 14:124–136. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang W, Shi Y, Li J, Cui W and Yang B:
Up-regulation of KIF14 is a predictor of poor survival and a novel
prognostic biomarker of chemoresistance to paclitaxel treatment in
cervical cancer. Biosci Rep. 36:e003152016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Corson TW and Gallie BL: KIF14 mRNA
expression is a predictor of grade and outcome in breast cancer.
Int J Cancer. 119:1088–1094. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang Q, Wang L, Li D, Deng J, Zhao Z, He
S, Zhang Y and Tu Y: Kinesin family member 14 is a candidate
prognostic marker for outcome of glioma patients. Cancer Epidemiol.
37:79–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang Z, Li C, Yan C, Li J, Yan M, Liu B,
Zhu Z, Wu Y and Gu Q: KIF14 promotes tumor progression and
metastasis and is an independent predictor of poor prognosis in
human gastric cancer. Biochim Biophys Acta Mol Basis Dis 1865.
181–192. 2019. View Article : Google Scholar
|
|
44
|
Li KK, Qi Y, Xia T, Chan AK, Zhang ZY,
Aibaidula A, Zhang R, Zhou L, Yao Y and Ng HK: The kinesin KIF14 is
overexpressed in medulloblastoma and downregulation of KIF14
suppressed tumor proliferation and induced apoptosis. Lab Invest.
97:946–961. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Corson TW, Zhu CQ, Lau SK, Shepherd FA,
Tsao MS and Gallie BL: KIF14 messenger RNA expression is
independently prognostic for outcome in lung cancer. Clin Cancer
Res. 13:3229–3234. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yang T, Zhang XB and Zheng ZM: Suppression
of KIF14 expression inhibits hepatocellular carcinoma progression
and predicts favorable outcome. Cancer Sci. 104:552–557. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wu Y, Wang A, Zhu B, Huang J, Lu E, Xu H,
Xia W, Dong G, Jiang F and Xu L: KIF18B promotes tumor progression
through activating the Wnt/β-catenin pathway in cervical cancer.
OncoTargets Ther. 11:1707–1720. 2018. View Article : Google Scholar
|
|
48
|
Itzel T, Scholz P, Maass T, Krupp M,
Marquardt JU, Strand S, Becker D, Staib F, Binder H, Roessler S, et
al: Translating bioinformatics in oncology: Guilt-by-profiling
analysis and identification of KIF18B and CDCA3 as novel driver
genes in carcinogenesis. Bioinformatics. 31:216–224. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu SL, Lin HX, Qiu F, Zhang WJ, Niu CH,
Wen W, Sun XQ, Ye LP, Wu XQ, Lin CY, et al: Overexpression of
kinesin family member 20A correlates with disease progression and
poor prognosis in human nasopharyngeal cancer: A retrospective
analysis of 105 patients. PLoS One. 12:e01692802017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yang G, Chen Q, Xiao J, Zhang H, Wang Z
and Lin X: Identification of genes and analysis of prognostic
values in nonsmoking females with non-small cell lung carcinoma by
bioinformatics analyses. Cancer Manag Res. 10:4287–4295. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ni M, Liu X, Wu J, Zhang D, Tian J, Wang
T, Liu S, Meng Z, Wang K, Duan X, et al: Identification of
candidate biomarkers correlated with the pathogenesis and prognosis
of non-small cell lung cancer via integrated bioinformatics
analysis. Front Genet. 9:4692018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Shi C, Huang D, Lu N, Chen D, Zhang M, Yan
Y, Deng L, Lu Q, Lu H and Luo S: Aberrantly activated Gli2-KIF20A
axis is crucial for growth of hepatocellular carcinoma and predicts
poor prognosis. Oncotarget. 7:26206–26219. 2016.PubMed/NCBI
|
|
53
|
Zhang W, He W, Shi Y, Gu H, Li M, Liu Z,
Feng Y, Zheng N, Xie C and Zhang Y: High expression of KIF20A is
associated with poor overall survival and tumor progression in
early-stage cervical squamous cell carcinoma. PLoS One.
11:e01674492016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Saito K, Ohta S, Kawakami Y, Yoshida K and
Toda M: Functional analysis of KIF20A, a potential
immunotherapeutic target for glioma. J Neurooncol. 132:63–74. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li H, Zhang W, Sun X, Chen J, Li Y, Niu C,
Xu B and Zhang Y: Overexpression of kinesin family member 20A is
associated with unfavorable clinical outcome and tumor progression
in epithelial ovarian cancer. Cancer Manag Res. 10:3433–3450. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yuan L, Chen L, Qian K, Qian G, Wu CL,
Wang X and Xiao Y: Co-expression network analysis identified six
hub genes in association with progression and prognosis in human
clear cell renal cell carcinoma (ccRCC). Genom Data. 14:132–140.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yamashita J, Fukushima S, Jinnin M, Honda
N, Makino K, Sakai K, Masuguchi S, Inoue Y and Ihn H: Kinesin
family member 20A is a novel melanoma-associated antigen. Acta Derm
Venereol. 92:593–597. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kawai Y, Shibata K, Sakata J, Suzuki S,
Utsumi F, Niimi K, Sekiya R, Senga T, Kikkawa F and Kajiyama H:
KIF20A expression as a prognostic indicator and its possible
involvement in the proliferation of ovarian clear-cell carcinoma
cells. Oncol Rep. 40:195–205. 2018.PubMed/NCBI
|
|
59
|
Carleton M, Mao M, Biery M, Warrener P,
Kim S, Buser C, Marshall CG, Fernandes C, Annis J and Linsley PS:
RNA interference-mediated silencing of mitotic kinesin KIF14
disrupts cell cycle progression and induces cytokinesis failure.
Mol Cell Biol. 26:3853–3863. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Singel SM, Cornelius C, Zaganjor E, Batten
K, Sarode VR, Buckley DL, Peng Y, John GB, Li HC, Sadeghi N, et al:
KIF14 promotes AKT phosphorylation and contributes to
chemoresistance in triple-negative breast cancer. Neoplasia.
16:247–256, 256 e242. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang ZZ, Yang J, Jiang BH, Di JB, Gao P,
Peng L and Su XQ: KIF14 promotes cell proliferation via activation
of Akt and is directly targeted by miR-200c in colorectal cancer.
Int J Oncol. 53:1939–1952. 2018.PubMed/NCBI
|
|
62
|
Xiu G, Sui X, Wang Y and Zhang Z: FOXM1
regulates radiosensitivity of lung cancer cell partly by
upregulating KIF20A. Eur J Pharmacol. 833:79–85. 2018. View Article : Google Scholar : PubMed/NCBI
|