Introduction
Urinary bladder cancer (BC) is the most common tumor
of the urinary system. In the United States of America alone,
79,030 new cases and 16,870 mortalities were registered in 2017 due
to urinary BC (1). These account for
50% of all tumors of the urinary system (1). Cystoscopy remains as the diagnostic
‘gold standard’ for primary diagnosis and follow-up of bladder
cancer. At the point of diagnosis, the majority of BC cases (75%)
are limited to the organ and are characterized as non-muscle
invasive bladder tumors (NMIBC) which affect only the mucosa or
submucosa (2). The remaining tumors
are muscle-invasive tumors (MIBC) which spread to the deeper layers
of tissue. For NMIBC, alterations in the fibroblast growth factor
(FGF) receptor 3 gene are common (3), while for MIBC, mutations in P53,
Rb1 and phosphatase and tensin homolog have been reported
(4). After initial diagnosis and
treatment, recurrence is likely to occur; thus, patients must
undergo a long period of surveillance (2). Repeated cystoscopies are uncomfortable
for patients, time consuming for physicians and expensive. The main
objectives of urological research are to improve early diagnosis
and the surveillance of patients (5),
and reduce the subsequent cystoscopies by analyzing biomarkers in
body fluids. Furthermore, the identification of novel markers of
therapeutic responses is required to reduce the number of necessary
transurethral resection of bladder tumor (TURB) procedures for
patients with BC. In this respect, the altered expression of
microRNAs (miRNAs/miRs) in the body fluids of patients, including
blood and urine, is of considerable interest. A systematic review
reported this for such patients and those with other urological
cancers (6). The advantage of miRNAs
is their stability in body fluids and the possibility of
non-invasive collection of samples (7).
Six studies have investigated differentially
expressed miRNAs in the plasma of BC patients; the findings
suggested that circulating miRNAs in the plasma could be novel
non-invasive biomarkers for bladder cancer (8–13).
However, the focus of these studies was primarily the diagnostic
validity of miRNAs. The potential monitoring capacity during the
surveillance of these patients has not yet been studied. In
addition, inconsistent results have been reported from such
studies, not only from the heterogeneity of study cohorts but also
due to analytical differences in the sampling processes and
different normalization approaches (14). Considering these critical points, the
objectives of the present study were: i) To examine a broad panel
of plasma miRNAs and identify potential diagnostic and surveillance
monitoring markers and useful reference miRNAs; and ii) to focus on
the pre-analytical process to exclude samples with interfering
analytical factors, such as the hemolysis of plasma samples. Based
on the promising results of previous studies using plasma (8–13), we also
decided to determine miRNAs in plasma samples to avoid
preanalytical/analytical issues with urine samples (stability of
mRNA, hematuria and the selection of urine fractions, including
non-centrifuged urine, urine sediments, supernatant following
centrifugation and exosome preparation). We proposed that
addressing the preanalytical/analytical issues of plasma samples
are better to control than that of urine samples. As hemolysis
serves as an analytical limitation, we aimed to isolate samples
free of this factor. Hematuria is a typical marker for urological
disease and is difficult to avoid as of the possible contamination
of miRNAs from blood cells. A cell-free plasma could be prepared
only with two centrifugation steps. miRNAs in plasma and serum
samples have high stability in harsh conditions such as
temperature, pH or long-time storage in frozen conditions (15). miRNAs are protected by RNA-binding
proteins (Argonaute) and high-density lipoproteins, and are
embedded inside microvesicles (16).
To increase the validity of the present study, a
two-step procedure was performed: A discovery phase, including
patients of an early cancer stage and a subsequent validation phase
using samples from patients of different cancer stages. To the best
of our knowledge, the present study is the first to obtain plasma
samples from the same patient at different stages of disease for
surveillance monitoring.
Materials and methods
Sample collection
Plasma samples were collected from patients with
papillary BC before undergoing TURB or radical cystectomy (RC) at
the Department of Urology, Charité-Universitätsmedizin Berlin
between May 2014 and August 2016. In addition, we collected samples
after primary tumor removal prior to the control TURB or some days
after the radical cystectomy. The pathological classification of
tissues was performed according to the Union of International
Cancer Control 2010 (17). All
patients provided written informed consent; the present study
approved by the Ethics Committee of the Hospital
Charité-Universitätsmedizin Berlin (EA1/134/12). The pathological
classification for all BC patients is summarized in Tables I and II. The study was performed in two
steps.
 | Table I.Clinicopathological characteristics
of the discovery cohort of patients with and without bladder
cancer. |
Table I.
Clinicopathological characteristics
of the discovery cohort of patients with and without bladder
cancer.
| Features | Non-tumor | Bladder cancer |
|---|
| All cases | 10 | 10 |
|
Urolithiasis | 3 |
|
|
Cystitis cystica | 3 |
|
|
BPH | 1 |
|
|
Healthy | 3 |
|
| Age, years | 48–85 | 48–77 |
| Median | 65.0 | 66.5 |
| Sex |
|
|
|
Male | 7 | 6 |
|
Female | 3 | 4 |
| Grading |
|
|
|
Low |
| 10 |
| Tumor stage |
|
|
| NMIBC
(9×pTa, 1×pT1) |
| 10 |
| Distant
metastasis |
| 0 |
| Lymph nodes |
| 0 |
 | Table II.Clinicopathological characteristics
of the validation cohort of patients with bladder cancer. |
Table II.
Clinicopathological characteristics
of the validation cohort of patients with bladder cancer.
| Features | TURB aPatients with tumor aNon-malignant in follow-up | aPatients with tumor aMalignant in follow-up | RC aPatients with tumor aNon-malignant in follow-up |
|---|
| All cases | 13 | 15 | 8 |
| Age (years) | 55–85 | 48–87 | 52–79 |
| Median | 70.0 | 74.0 | 67.0 |
| Sex |
|
Male | 12 | 10 | 7 |
|
Female | 1 | 5 | 1 |
| Time interval
(days) | 30–147 | 28–365 | 7–98 |
| Median | 43 | 86 | 7 |
| Grading |
|
Low | 6 | 10 | 0 |
|
High | 7 | 5 | 8 |
| Tumor stage |
|
NMIBC | 13 | 13 | 0 |
|
MIBC | 0 | 2 | 8 |
| Distant
metastasis | 0 | 0 | 0 |
| Lymph vessels | 0 | 0 | 1 |
Firstly, a small discovery cohort of each 10
patients with (6 males, 4 females) and without (7 males, 3 females)
BC was analyzed (Table I). The group
of patients without BC included 3 healthy persons and 7 patients
with other urologic diseases not related to any type of malignancy
(Table I). All BC patients underwent
TURB or RC. They did not received any pretreatments. In all groups
patients with metastasis or any other type of malignancy were
excluded from BC group. The result of the first analysis was
validated in a second cohort of 36 patients (Table II) patients were distinguished
according to the result of the second surgery. The first group
included 13 patients (12 males, 1 female) where the tumor was
completely removed during the first TURB and the second TURB after
some weeks showed a non-malignant result. The second group
comprised 15 patients (10 males, 5 females) with a tumor after the
second TURB, and a third group included 8 patients (7 males, 1
female) who received RC and had postoperative plasma samples.
Plasma samples were also collected prior to the second
surgeries.
Sample preparation and the control of
interfering hemolysis
Peripheral venous blood was collected in a 6.0 ml
K-EDTA-Vacutainer (BD Biosciences). Two centrifugation steps at
room temperature prepared cell-free plasma immediately after
collection. The first centrifugation step was conducted at 2,500 ×
g for 15 min. The supernatant from this step was centrifuged at
1,500 × g for 10 min. Aliquots of the cell-free plasma were stored
at −80°C. For a reliable miRNA analysis, it is important that the
plasma is free from hemolysis. To exclude this interference, the
absorption of plasma samples was measured at 414 nm using NanoDrop
1000 spectrohotometer (NanoDrop Techologies; Thermo Fisher
Scientific, Inc.). Only samples with an absorption lower than 0.3
were used for further analysis (18).
In addition to measuring the absorption of samples, hemolysis was
assessed by determining the levels of miR-451 and miR-23a via
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR). The first is expressed in red blood cells and the other
is relatively stable in plasma and is not affected by hemolysis
(19–21). Quantification cycle (Cq) differences
between miR-23a and miR-451 higher than 7.0 indicate a high risk of
hemolysis (19). Corresponding
samples were excluded from further analysis.
RNA preparation and RT-qPCR of
miRNAs
Exiqon Services performed all preparation and
analytical steps for process in the present study, including total
RNA extraction from the plasma, quality control and the RT-qPCR of
miRNAs. During the discovery phase, total RNA was extracted from
the plasma samples using the miRCURY™ RNA isolation kit-biofluids
(Exiqon). An aliquot of 200 µl plasma per sample was used. All
following steps were performed according to the instruction manual
v1.5 section B.
During the validation phase, total RNA was extracted
from 200 µl plasma using the miRCURY RNA isolation kit-biofluids, a
high-throughput bead-based protocol v.1 (Exiqon) in an automated
96-well format. In both phases, the purified total RNA was eluted
in a final volume of 50 µl.
In the discovery phase, 7 µl RNA was reverse
transcribed in a 35 µl reaction volume using the miRCURY LNA™
Universal RT microRNA PCR, Polyadenylation, and cDNA synthesis kit
(Exiqon). In the validation phase, the volume was reduced to 2 µl
RNA and 10 µl reaction volume for RT. cDNA was diluted 50X and
assayed in 10 µl PCR reactions according to the protocol for
miRCURY LNA™ Universal RT microRNA PCR. Each miR was assayed once
by qPCR on the microRNA Ready-to-Use PCR, Serum/Plasma Focus panel
v.4.0, including 179 microRNAs and using ExiLENT SYBR®
Green master mix (Roche Diagnostics GmbH). In both phases, the qPCR
reaction was performed at 95°C for 10 min, followed by 45
amplification cycles at 95°C for 10 sec, and 60°C for 60 sec. The
primer sequences are proprietary information of Exiqon Services,
available from QIAGEN GmbH. The miRNA sequences and primer sets
used were listed in Table III.
After selecting for reference miRNAs, the geometric mean of
let-7i-5p and miR-29c-3p was used for relative quantification of
RT-qPCR data in both phases. The amplification was performed using
a LightCycler® 480 Real-Time PCR system (Roche GmbH) in
384-well plates. The amplification curves were analyzed using the
Roche LC software version 1.5.0.39, both for determination of Cq
(by the 2nd derivative method) and for melting curve analysis
(22).
 | Table III.miRs analyzed via reverse
transcription-quantitative polymerase chain reaction. |
Table III.
miRs analyzed via reverse
transcription-quantitative polymerase chain reaction.
| A, miRs |
|---|
|
|---|
| Human miR | miR target sequence
(5′-3′) | Corresponding LNA™
microRNA PCR primer set (cat. no.) | Cat.
no.a |
|---|
| miR-29b-3p |
UAGCACCAUUUGAAAUCAGUGUU | 204679 | YP00204679 |
| miR-590-5p |
GAGCUUAUUCAUAAAAGUGCAG | 204222 | YP00204222 |
| miR-10b-5p |
UACCCUGUAGAACCGAAUUUGUG | 205637 | YP00205637 |
| miR-15b-5p |
UAGCAGCACAUCAUGGUUUACA | 204243 | YP00204243 |
| miR-144-5p |
GGAUAUCAUCAUAUACUGUAAG | 204670 | YP00204670 |
| miR-29c-3p |
UAGCACCAUUUGAAAUCGGUUA | 204729 | YP00204729 |
| let-7i-5p |
UGAGGUAGUAGUUUGUGCUGUU | 204394 | YP00204394 |
|
| B, Internal RNA
spike-in references |
|
Reference | Sequence
(5′-3′) | Corresponding
LNA™ microRNA PCR primer set (cat. no.) | Cat.
no.a |
|
| UniSp2 | –b | 203950 | YP00203950 |
| UniSp4 | – | 203953 | YP00203953 |
| UniSP5 | – | 203955 | YP00203955 |
| cel-miR39-3p | – | 203952 | YP00203952 |
| Interplate
calibrator |
| Not for sale | Not for sale |
Data analysis
Data were presented as median values with the
interquartile range, or with the confidence interval. The miRNA
levels of the Serum/Plasma Focus panel v.4.0 were assessed using
the qBasePlus (Biogazelle) software version 3.2
(20180721216) and the geNorm computer program. This software was
used to identify reference miRNAs and to detect differential miRNA
levels between controls and patients based on the global
normalization and reference miRNA-based approaches described below.
Statistical analyses and area under the curves (AUC) of
receiver-operating characteristics (ROC) analyses were performed
using GraphPad Prism version 5.04 for Windows (GraphPad Software,
Inc.) and MedCalc Statistical Software version 18.5 (MedCalc
Software bvba). Non-parametric tests, a Mann-Whitney U test for
independent and a Wilcoxon test for paired samples, logistic
regression analysis, χ2 test, Fisher's exact test,
Spearman rank correlation coefficients, McNemar test and sample
size calculations were applied. To evaluate the clinical validity
of the miRNAs with respect to avoiding type I and type II errors,
the conventional thresholds of α=0.05 (significance level) and a
power of 0.80 were selected for sample size calculations. An
internal validation of relevant results was performed using the
bias-corrected and accelerated bootstrap method via SPSS 25
software (IBM Corp.) with 2,000 bootstrap replicates. Calculations
concerning the within-subject and between-subject components of
biological variation were conducted in consideration of the
recommendations reported by Fraser (23). P<0.05 (two-tailed) was considered
to indicate a statistically significant difference.
Results
Discovery phase: Differential plasma
miRNA levels between controls and BC patients
The BC cohort consisted of low grade and early stage
patients (Table I). These patients
were deliberately selected to establish a miRNA profile
particularly for patients suffering from an early cancer stage and
facilitate therefore the identification of single sensitive markers
for diagnostic purposes. The control group included healthy
individuals and non-tumor patients with other urological diseases
(Table I) that did not exhibit
differential miRNA levels. Of the total 179 miRNAs on the Exiqon
panel, 122 miRNAs were detected in the 20 samples of the
non-malignant and BC group. Based on the global normalization
approach, the levels of nine miRNAs (Table IV) were different between the two
groups.
 | Table IV.Differences in the expression levels
of miRs in plasma samples of patients with bladder cancer compared
with the control group of discovery cohort. |
Table IV.
Differences in the expression levels
of miRs in plasma samples of patients with bladder cancer compared
with the control group of discovery cohort.
|
| Normalization |
|
|---|
|
|
|
|
|---|
|
| Two reference
miRNAs | Global mean |
|
|---|
|
|
|
|
|
|---|
| miR | P-value | Fold change | P-value | Fold change | Cq (range) |
|---|
| has-miR-19a-3p | 0.01 | 1.25 | 0.08 | 1.24 | 25.1–28.2 |
|
hsa-miR-29b-3p | 0.01 | 1.33 | 0.02 | 1.32 | 29.6–32.8 |
|
hsa-miR-590-5p | 0.01 | 1.34 | 0.03 | 1.33 | 30.5–33.3 |
| has-miR-2110 | 0.02 | −2.26 | 0.02 | −2.28 | 34.6-ND |
| has-miR-144-3p | 0.02 | 1.30 | 0.11 | 1.29 | 24.8–27.1 |
|
hsa-miR-144-5p | 0.02 | −1.85 | 0.02 | −1.86 | 29.5–33.8 |
| has-let-7b-3p | 0.03 | −2.01 | 0.01 | −2.02 | 33.2–37.2 |
| has-miR-19b-3p | 0.04 | 1.24 | 0.13 | 1.24 | 23.8–27.4 |
| has-miR-425-5p | 0.04 | 1.20 | 0.15 | 1.19 | 28.2–31.5 |
|
hsa-miR-10b-5p | 0.04 | −1.46 | 0.04 | −1.47 | 30.9–33.1 |
| has-miR-324-5p | 0.04 | 1.41 | 0.03 | 1,41 | 31.7-ND |
| has-miR-93-5p | 0.05 | 1.33 | 0.08 | 1.32 | 26.3–30.4 |
|
hsa-miR-15b-5p | 0.05 | 1.57 | 0.02 | 1.57 | 29.6–33.1 |
| has-miR-29a-3p | 0.09 | −1.32 | 0.03 | −1.32 | 29.7–32.1 |
For the reference miRNA search, the inclusion
criteria included a fold change of +1.09 to −1.09 between controls
and patients; Cq<31. These criteria fulfilled 17 miRNAs of the
122 detectable miRNAs. According to these criteria, the miRNAs
let-7i-5p and miR-29c-3p were identified as useful normalizers
according to the geNorm approach in the software
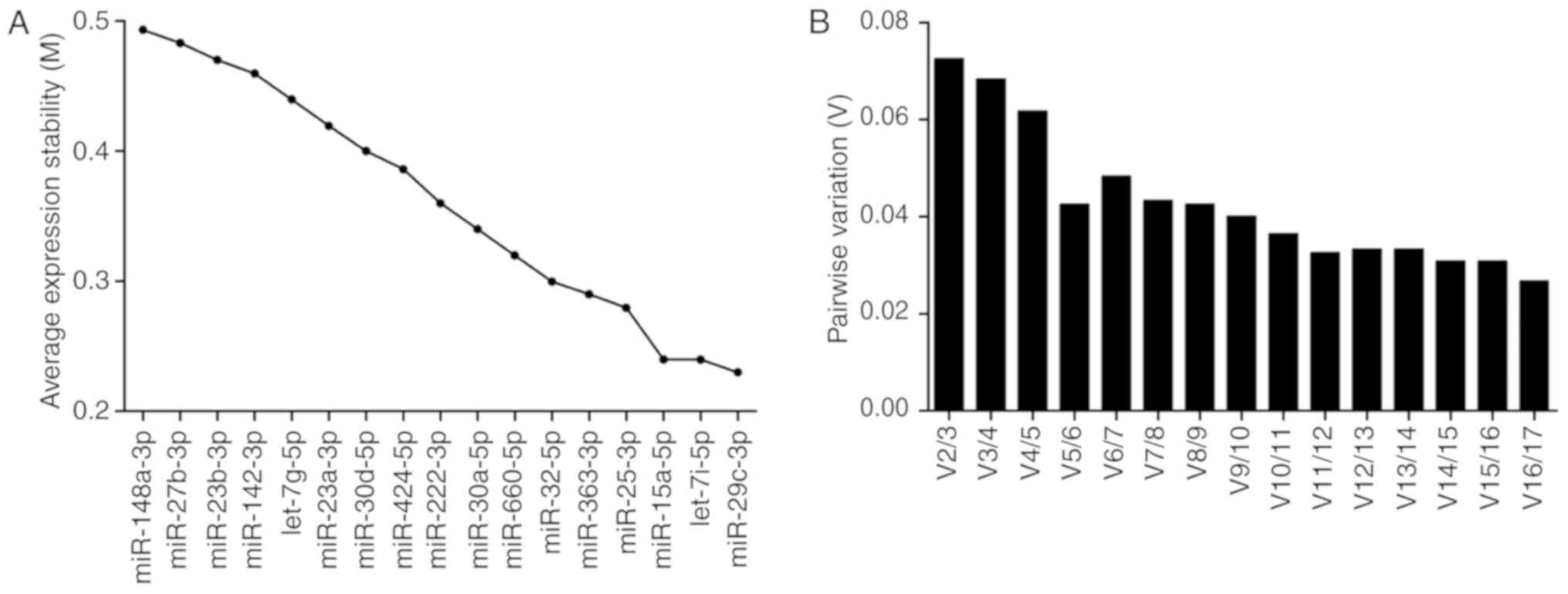
qBasePlus (Fig. 1). The
average expression stability value (M) of all candidate reference
miRNA between 0.235–0.5 denote good stability for all candidate
reference miRNAs that were distinctly lower than the default limit
of 1.5 in the geNorm program. The lowest M-value indicates a gene
with the highest stability (Fig. 1A).
Furthermore, the program calculates a normalization factor (V),
which is a criterion for determining the number of reference
miRNAs. The program recommends V<0.15 for reliable
normalization. The pair of miRNAs (let-7i-5p and miR-29c-3p)
achieved this cut-off value (Fig.
1B). Therefore, the geometric mean of miRNAs let-7i-5p and
miR-29c-3p served as the normalization factor for calculating the
differential expression levels of miRNAs between the control and BC
patients.
Based on this reference miRNA approach, 13
differentially expressed miRNAs were identified between the two
cohorts (Table IV). Eight of these
13 miRNAs were identical with the nine miRNAs detected using the
global normalization approach. To select miRNAs for further
validation, we compared the two normalization approaches and
applied the following criteria: i) A fold change level higher or
lower than 1.2 obtained in both normalization approaches; ii)
Cq-values <35 in controls and patients; and iii) equal
significant P-values with both normalization methods. According to
these criteria, three miRNAs with elevated levels (miR-15b-5p,
miR-590-5p and miR-29b-3p) and two miRNAs with reduced levels
(miR-10b-5p and miR-144-5p) were selected as potential miRNA
candidates for further validation in patient samples.
Validation phase: Plasma miRNA
candidates as diagnostic and surveillance markers
Based on the summarized mean difference of the five
significant candidate miRNAs between controls and BC patients in
the discovery phase, together with the standard deviations in the
two groups, the required number of cases for the validation of the
discovery results were calculated with a power of 0.80 to obtain
data for statistical significance based on α=0.05. As the
disease-monitoring capacity of miRNAs following tumor removal was
included in the validation phase, the ratio of controls to tumor
patients was altered to 1:3. Under these conditions, necessary
sample sizes of 10 controls and 30 patients with BC were
calculated. The validation cohort included 36 BC cases, and 10
controls from the discovery phase control group included three
healthy patients among non-tumor patients with other urological
diseases (Table I); their values did
not significantly differ from the values of healthy patients
(P=0.517 and 0.732 for miR-15b-5p and miR-590-5p, respectively;
data not shown).
The consistent long-term quality control of the
miRNA measurements performed by Exiqon justified the use of the
control group for discovery as controls in the validation phase. In
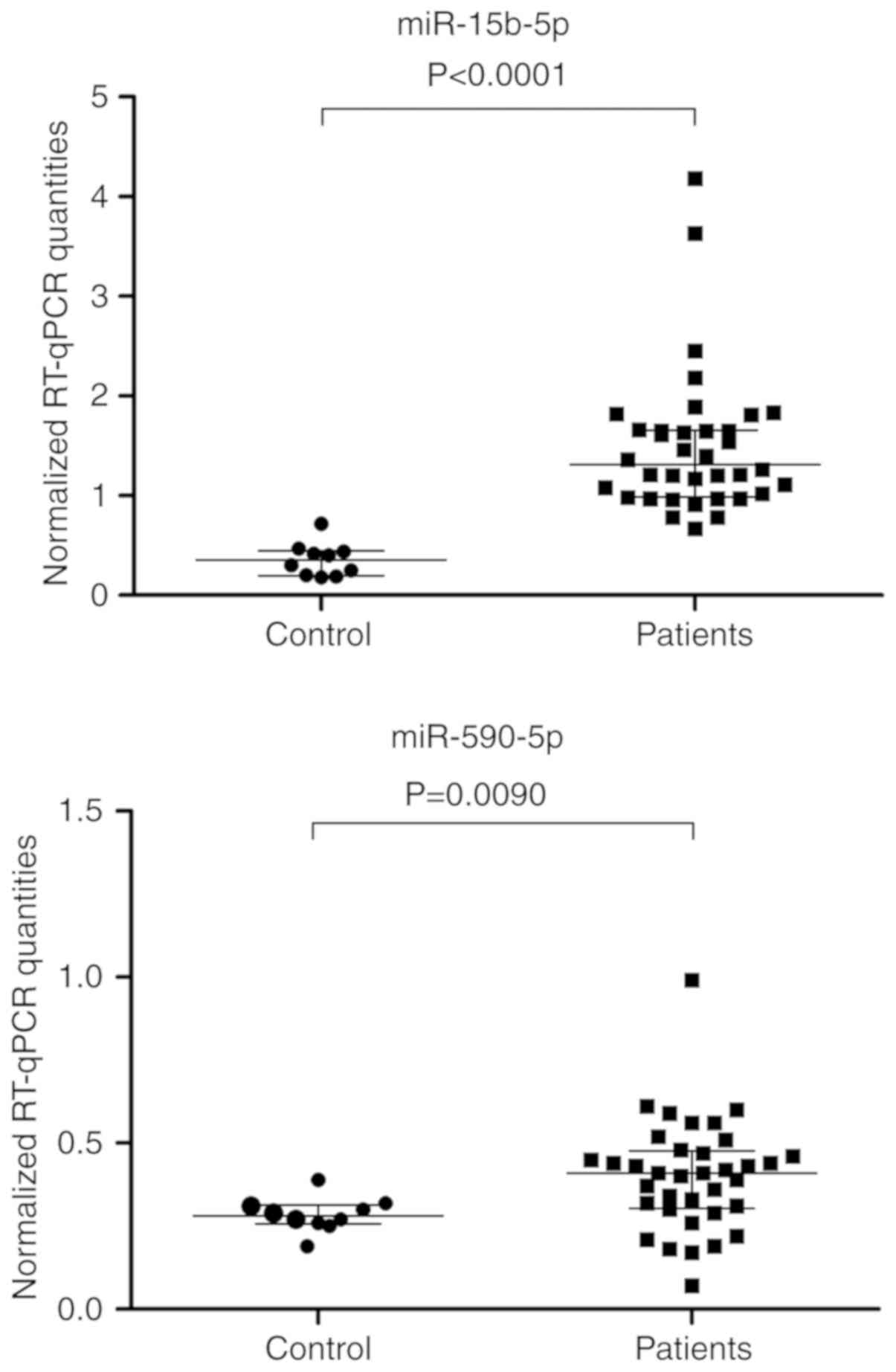
line with the findings from the discovery phase, the plasma levels
of miR-15b-5p and miR-590-5p were significantly elevated in
patients, indicating diagnostic benefit (Fig. 2). As the control group included not
only healthy individuals but also patients with typical urological
diseases without cancer (Table I), a
selection bias could therefore be ruled out. The internal
validation of data by bootstrapping supported the statistical
results (Fig. 2).
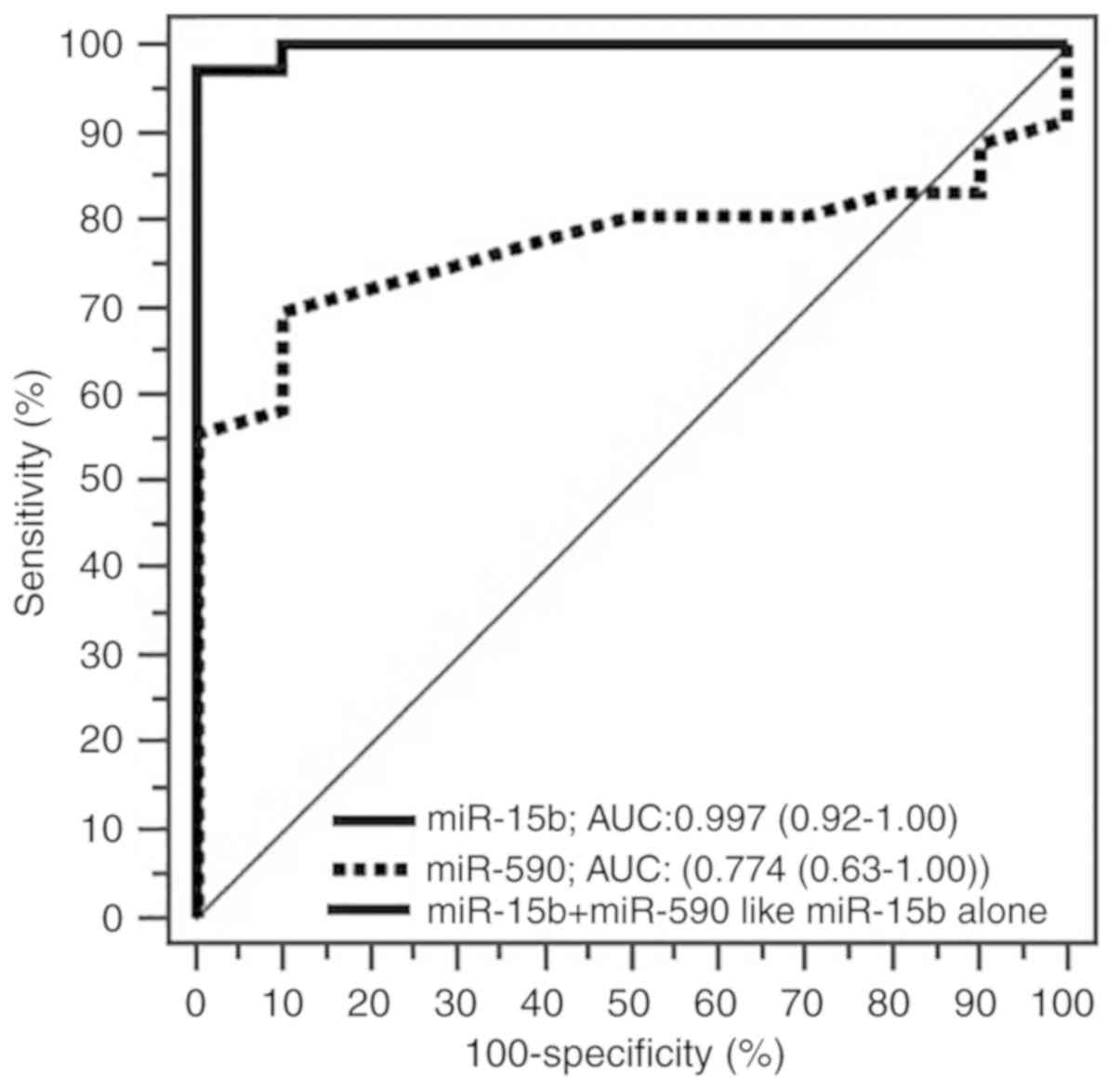
The ROC curve analysis demonstrated an AUC of 0.997
[95% confidence interval (CI), 0.918–1.000] for miR-15b-5p and an
AUC of 0.774 (95% CI, 0.626–0.884) for miR-590-5p as diagnostic
biomarkers. The levels of miR-15b-5p discriminated BC patients from
control subjects with a sensitivity of 97.2% and specificity of
100.0% with a Youden index of 0.9722. For miR-590-5p, the
sensitivity and specificity of miR-590-5p were 69.4 and 90.0%,
respectively, with a Youden index of 0.5944. The combination of the
two miRNAs based on a logistic regression model did not further
improve the discrimination ability achieved by miR-15b-5p alone
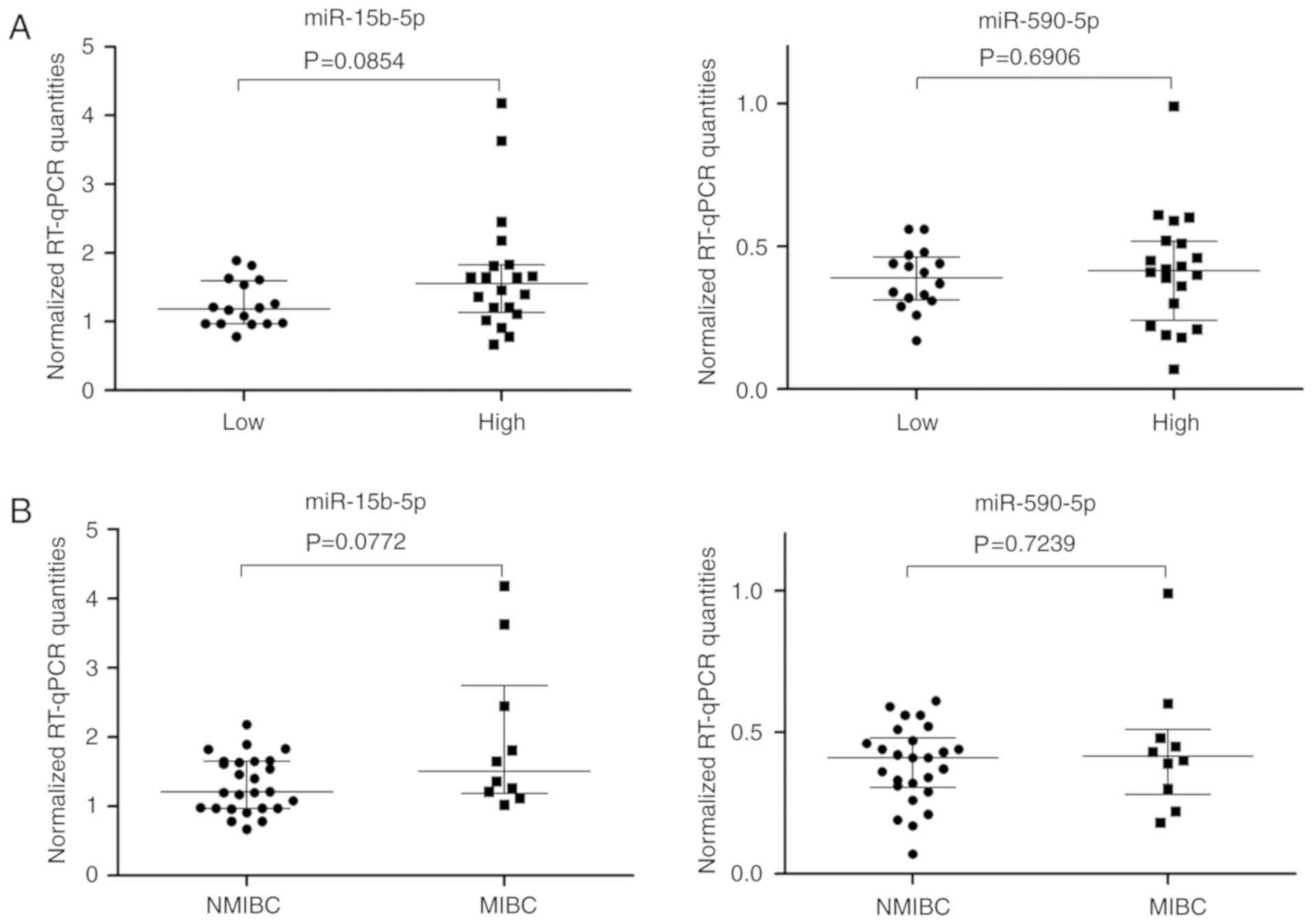
(Fig. 3). Increased miR-15-5p levels
were evident and were also supported by bootstrapping calculations
for high grade tumors and MIBCs; the levels of miR-590-5p were
markedly unchanged (Fig. 4). These
data correspond to Spearman rank correlation coefficients
(rs) obtained between tumor stage or grade and the two
miRNAs as follows: NMIBC/MIBC with miR-15b-5p (rs=0.308,
P=0.0677) and with miR-590-5p (rs=−0.023, P=0.8930) and
low/high grade with miR-15b-5p (rs=0.293, P=0.0823) and
with miR-590 (rs=−0.070, P=0.6851). The results of
correlation analysis for clinicopathological characteristics were
summarized in Table V. The different
levels of miR-29b-3p, miR-10b-5p, and miR-144-5p between tumor
patients and controls found in the discovery phase could not be
confirmed.
 | Table V.Spearman correlation coefficient
between miR and clinicopathological characteristics. |
Table V.
Spearman correlation coefficient
between miR and clinicopathological characteristics.
|
| Validation cohort
n=36 |
|---|
|
|
|
|---|
| Features | miR-15b-5p | miR-590-5p |
|---|
| Sex |
| rs | −0.1740 | −0.0800 |
|
P-value | 0.3108 | 0.6410 |
| Age |
| rs | −0.2360 | 0.1990 |
|
P-value | 0.1655 | 0.2442 |
| Grade
(low/high) |
| rs | 0.2930 | 0.0700 |
|
P-value | 0.0823 | 0.6851 |
| Stage
(NMIBC/MIBC) |
| rs | 0.3080 | 0.0230 |
|
P-value | 0.0677 | 0.8930 |
To assess the validity of the five selected miRNAs
as surveillance biomarkers, the 36 BC patients were classified into
three groups according to the tumor presence in the control TURB
specimens and the collection after RC (Table II). Fig.
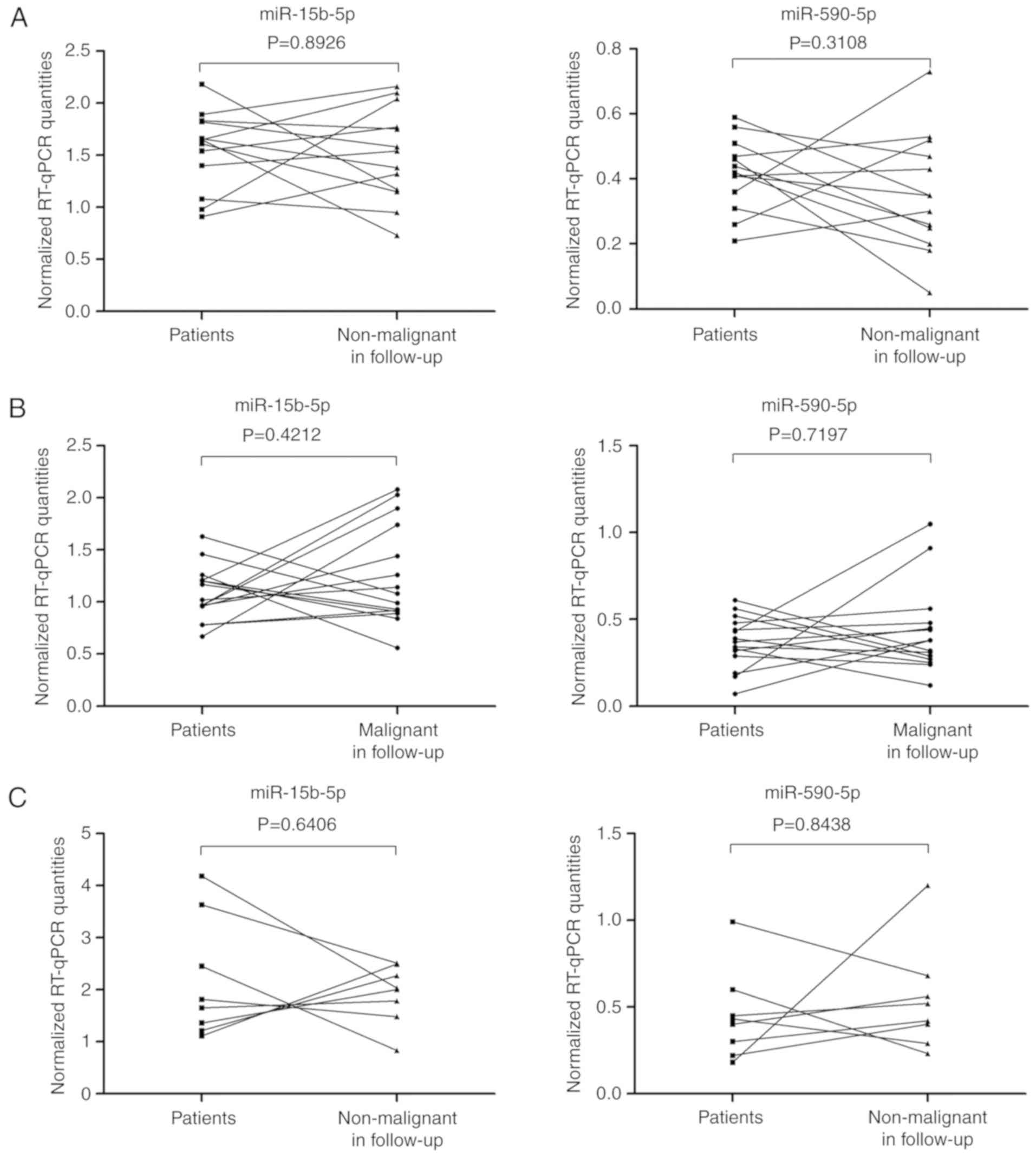
5 presented the expression levels of the two potentially
diagnostic miRNAs in the three groups. In 13 patients, the bladder
tumor was completely removed at the first TURB, while non-malignant
results were reported for the second control TURB (Fig. 5A). In the second group with 15
patients, malignant lesions were detected in the control TURB after
a median follow-up of 86 days (Fig.
5B). Plasma samples were also collected from 8 patients a few
days after the radical cystectomy (Fig.
5C). None of the five selected miRNAs for validation showed any
differences between tumor samples before the first TURB, and
follow-up samples before the control TURB or after RC from the same
patient. Paired Wilcoxon analyses revealed that the levels of
miRNAs in the plasma were not statistically different in patients
with non-malignant or malignant histological results after TURB
compared with the data before the treatment (Fig. 5A and B). After BC removal, the
expected reduction of miR-15b-5p and miR-590-5p miRNA levels
occurred only in 7 and 8 of 13 patients, respectively (Fig. 5A). Similarly, notably decreased or
unchanged miRNA levels were observed when the malignant lesions
were detected in the follow-up control TURB. Considering that the
interval between the first and the second sampling could be too
short, which may account for these unexpected values, a
time-dependent regression calculation of the miRNA levels in the
two TURB cohorts was performed. A time-dependent effect was not
observed (Fig. S1). Furthermore,
both TURB cohorts did not differ in their tumor aggressiveness
(tumor grade and tumor stage: Fisher's exact test with P=0.445 and
1.000, respectively). In addition, in the cohort with the remaining
tumor, tumor grade and tumor stage did not differ in the paired
samples in the first and second TURB specimen (McNemar analysis,
P=1.000). We also conducted multivariate logistic regression
analysis for clinical parameters, tumor stage and grade, and the
plasma levels of both miRNAs between the first and second TURB, but
did not improve the prediction of complete tumor removal. Only the
tumor stage remained as independent variable (data not shown). A
change in the miRNA level was not observed a few days after radical
cystectomy (Fig. 5C). In all cases,
these statistically unchanged miRNA patterns could not be
attributed to obvious clinical manifestations.
To support these statistical data, we investigated
the effects of within-subject or intra-individual biological
variation of miR-15b-5p and miR-590-5p in these two time-dependent
measurements of our study. For that purpose, the database from
Ammerlaan and Betsou (24), which
summarized the within-subject variation of numerous miRNAs in blood
plasma and serum, was used. For example, miR-15b-5p was
characterized by a mean coefficient of variation of ~65%.
Considering an analytical variation of only 10%, the reference
change value (also known as ‘critical values’ between serial data)
of ~180% would result; 95% of the dispersions of miR-15b-5p (and
also of miR-590-5p) of the two values of the patients considerably
overlapped due to this inherent intra-individual biological
variation. Therefore, the results were not significantly different.
Monitoring of the follow-up of disease would be hardly possible
using these miRNAs due their high biological variation, resulting
in a large fluctuation of the miRNA levels.
Discussion
miRNAs have been proposed as optional novel
biomarkers in a variety of diseases (6,25). miRNAs
may be applied as sensitive and specific tools for diagnostic,
prognostic and predictive purposes, particularly in different types
of cancer (26,27). The urgent need for reliable
non-invasive markers for patients with BC has been emphasized as an
important demand in the European Association of Urology guidelines
(2). However, miRNAs in the blood
plasma from BC patients have been examined in only a few studies,
exclusively as diagnostic biomarkers (8–11,13). Thus, we performed this study with aim
to provide insight into miRNAs as diagnostic tools and investigate
the monitoring capacity of miRNAs with strict control of potential
interfering effect of hemolysis in plasma samples. miR-15b-5p and
miR-590-5p were finally confirmed in the validation phase, based on
five potential miRNAs candidates identified in the primary
discovery phase, as significant discriminative markers between
patients with BC and controls. However, none of these miRNAs were
determined to be suitable for monitoring the disease.
To the best of our knowledge, neither plasma
miR-15b-5p or miR-590-5p have been reported in previous studies
with regards patients with BC (8–13).
Previous studies revealed a total of 22 miRNAs (Table SI) as potential significant
diagnostic markers but only three miRNAs (miR-92a-3p, miR-99 and
miR-100) were reported by at least two working groups. Four miRNAs
(miR-25-3p, miR-33b-5p, miR-92a-3p and miR-194) may be influenced
by hemolysis or could detectable in blood cells (20,28). Due
to a lack of consistent results, none of these miRNA panels has
been applied in practice. The present study reported the degree of
hemolysis with two methods (absorption measurement and the
detection of specific miRNAs) and rigorously disclosed hemolyzed
plasma samples.
miR-15-5p and miR-590-5p were determined to be
upregulated in BC in a previous own study (29) and confirmed by others (30–32).
Armstrong et al (33) reported
on the top 25 miRNAs with highest concentrations in BC tissue,
white-blood cells, urine and plasma only in patients with BC but
not in controls. In tissue and urine samples, the authors detected
miR-15b-5p in the aforementioned top 25 miRNA whereas miR-15b-5p in
plasma samples was not among the top 25 miRNAs. However, it should
be considered that Armstrong et al (33) did not compare the data between
controls and patients. Thus, the plasma concentration of miR-15b-5p
between BC patients remained unknown in the report of Armstrong
et al (33), whereas our data
reflect the different levels between healthy individuals and BC
patients. In addition, in the plasma samples of our study for the
discovery phase, 122 miRNAs were detected, while miR-15b-5p was the
53rd miRNA ranked in tumor patients and 64th in controls according
to the measured levels. However, as shown in Table IV, this miRNA had the highest
increase in expression in cancer patients compared with controls.
Thus, this result corresponds with the findings of Armstrong et
al (33) in which miR-15b-5p was
not observed in the top 25 miRNAs with the highest abundance in the
plasma. This phenomenon of non-concordance between tissue and
circulating miRNA levels was particularly evident in the study of
Xie et al (34). These authors
reported diagnostic markers for BC based on a meta-analysis of
studies of tissue data of BC. The meta-analysis included 26
publications; 21 were related to tissue, four to urine and only one
to serum. Therefore, the suggested potentially diagnostic miRNAs
may be used for the differentiation of tissue material. The were
notable discrepancies between the six common differentially
expressed miRNAs in BC tissue of the meta-analysis by Xie et
al (34); none of them were found
in our analysis of plasma samples. This phenomenon termed
distortion between cellular and circulating markers (35) reflects the lack of concordance in
changes of circulating miRNAs with tissue expression levels
described in other studies (36,37). The
possible explanation for this are non-uniform releasing processes
of miRNAs by cells (38). The levels
of miR-15b-5p were increased by 1.2- and 2.4-fold in urothelial
carcinoma than in adjacent normal bladder tissue (29,30).
Furthermore, based on bioinformatics analysis of The Cancer Genome
Atlas, Wang et al (32)
reported a relationship between miR-15b-5p and age-associated genes
in BC. This is interesting as the incidence of BC increases after
the 40th year of life and reaches a maximum in the sixth and
seventh decade (39). The examined
patient collective were aged in their 60s and 70s in the analysis
conducted by Wang et al (32).
Moreover, the long-life expectancy in western countries has been
linked to an increase in BC cases as the risk of disease increases
with age (1). The expression of the
other relevant miRNA miR-590-5pwas determined to be 2.98- and
3.14-fold higher in malignant than in non-malignant bladder tissue
(29,31). Thus, these tissue expression data
support that as of the altered levels of these miRNAs due to the
extent of their release from carcinoma tissue, these miRNAs may
serve as potential circulating biomarkers (38). Under the stringent conditions of the
present study, the diagnostic aspect of the increased levels of
miR-15b-5p and miR-590-5p in the plasma of BC patients could be
confirmed by further validation and may be an indication for the
presence of BC. In addition, our study reported that the tendency
of increased levels of miR-15b-5p depending on the aggressiveness
of the cancer supported its potential as a cancer biomarker.
Furthermore, miR-15b was identified as a diagnostic marker in urine
(40). Jiang et al (41) also reported on the use of miR-15b-5p
with a decreased level in serum of patients, although it is
recommended that carefully prepared plasma samples from selected
specimens should be used for the quantification of circulating
miRNAs (42). Therefore, we proposed
that this discrepancy could be a result of a hemolysis-impaired
normalization approach as Jiang et al (41) used miR-16-5p, in addition to
miR-193-5p, for normalization. These miRNAs was typically found to
be increased in samples that could be characterized as hemolytic by
hemoglobin sensitivity measurements or the ratio miR-451/miR-23a,
whereas the effects of hemolysis are difficult to observe (20,21).
Furthermore, the use of different RNA isolation kits and different
PCR systems could not be excluded as other analytical reasons for
discrepancies in the levels of miR-15-5p in our study and that by
Jiang et al (41). On the
other hand, comparable increases in plasma miR-15b-5p of NMIBC and
MIBC patients, and the use of a similar patient cohort to Jiang
et al (41) support the
conclusion that pre-analytical/analytical reasons are may be more
likely to explain for these different expression levels between the
studies, than biological reasons. Other researchers have detected
miR-15b-5p in plasma samples in relation to other tumor types, such
as colorectal carcinomas (43),
hepatocellular carcinoma (44) and
melanoma (45).
Few studies have reported the potential functions of
miR-15b and miR-590-5p, especially in bladder cancer. Wang et
al (46) detected an increased
level of miR-15b-5p relative to the decreased expression of long
noncoding RNA MAGI2-AS3 and the tumor suppressor CCDC19 in BC
tissues and cell lines. Transfection experiments with cell cultures
indicated miR-15b-5p as a potential an oncogene in BC (46). Similarly, overexpression of miR-15b-5p
in gastric cancer was associated with the expression of the tumor
suppressor progestin and adipoQ receptor family member 3 (47). Furthermore, the process of
epithelial-mesenchymal transition was promoted (47).
To the best of our knowledge regarding miR-590-5p in
plasma, only one report exists for patients undergoing
chemoradiotherapy for the treatment of head and neck cancer
(48). miR-590-5p was described as
part of a six-miRNA panel that reflects the response to
chemoradiotherapy (48). One target
of the miR-590-5p is Yes associated protein (YAP) (49), which induces cell growth and invasion
in BC (50). Overexpression of YAP is
partly responsible for poor prognosis in BC (50,51).
Furthermore, for NMIBC, epigenetic regulation of the FGF gene
family was described (52), which is
involved in a variety of biological processes, including tumor
growth (53). Zhang et al
(53) revealed that FGF18 is
negatively regulated by miR-590-5p, which leads to a promotion of
gastric tumorigenesis.
In terms of disease follow-up, we assumed that the
dysregulated miRNAs in plasma samples from BC patients may return
to the levels recorded for the non-tumor samples after some time.
In the present study, the levels of the five selected miRNAs in the
plasma samples, particularly miR-15b-5p and miR-590-5p, did not
independently return to that for the control after the second TURB.
This suggested these two miRNAs as potential diagnostic biomarkers.
The median time of 50 days corresponds to the usual time interval
between the first and second TURB (2); 7 days after RC, the miRNA levels had not
yet returned to normal. Time-dependent regression line calculations
of the miRNA levels measured before the control TURB and percentage
related to the levels before the first TURB, did not show a
time-dependent effect. The half-lives of different cellular miRNAs
differ between 1 and 120 h (54–56). Based
on these data, it could be assumed that circulating miRNAs released
by cancer cells may rapidly degrade or at least notably decrease in
their levels after cancer removal. In BC patients, a tumor reversal
based on miRNA changes was observed only for urinary cell-free
miRNAs (57–59). A postoperative decrease of circulating
miRNAs was also reported in hepatocellular, gastric, colorectal,
lung and breast cancer within 7–10 days after surgery (16,44,60–62).
However, compared with the preoperative levels of tumor-associated
miRNAs in lung and prostate carcinoma, both unchanged and decreased
miRNA levels, depending on specific miRNAs and the cancer type,
were observed after surgery (61,63–66). This
phenomenon of statistically comparable pre-and postoperative miRNA
levels corresponds with our results.
Additionally, a selection bias is very unlikely for
this contradictory observation in the clinical use as diagnostic
and monitoring markers in our case-control study. However, the
relatively high biological variation in comparison to the
analytical variation of the two miRNAs should be considered as one
essential reason for this phenomenon. The result is an overlap of
the 95% dispersion of the repeated values and the lack of
statistical significance. A similar fluctuating miRNA pattern was
measured in blood samples collected before, and 7–17 days and six
subsequent 3 month intervals after lung cancer resection (64). This effect may impair the clinical
validity of biomarkers particularly for monitoring purposes
(23). At present, only scarce data
(24,67) are available regarding the
within-subject variability of circulating miRNA levels to support
this view. However, we believe that this aspect in the application
of circulating miRNAs as monitoring markers requires further
investigation. This issue requires greater attention in the future,
taking into account the recent recommendations for evaluating
studies on biological variation (68).
Certain limitations of the present study should be
addressed, including the low number of included patients, their
short follow-up times, and the different composition of the
discovery and validation cohorts with regard to tumor stage and
grade. To avoid possible type I and type II errors with the low
number of patients, we performed an additional internal validation
using a bootstrapping approach with confirming results. The limited
number of patients was evident for the lack of statistically
significant associations between the increased levels of miR-15b-5p
depending on the cancer aggressiveness. As more patients could not
be recruited, we believe that the data of the post hoc power
analysis are appropriate to support this influencing effect. To
further investigate this, a post hoc power analysis was conducted
based on these population-related effect sizes of median
differences, the study cohort proportions, and a power of 0.80 to
obtain data for statistically significant results on a level of
α=0.05. For the low/high tumors, NMIBCs and MIBCs, the participants
of the present study would have to increase from 36 to 72 and 94,
respectively. It could be criticized that the time interval between
the first and second TURB was too short to achieve stable
circulating miRNA levels in plasma. This interval corresponds to
the regular follow-up examinations within the risk management of
these patients. In addition, the time-dependent regression line
calculations could not confirm this concern of a time-dependent
effect. Furthermore, there are differences in patient cohorts in
the discovery and validation phase. The discovery phase included
only low grade tumors and NMIBCs while the validation phase
included high grade tumors and MIBCs. However, as explained in the
objective of our study, this design was deliberately selected in
the discovery phase in order to identify potential diagnostic
markers already in early cancer stages.
In conclusion, despite these limitations but based
on the selected stringent pre-analytical and analytical conditions,
and the evident statistical significances of the two circulating
miRNAs, miR-15b-p and miR-590-5p could be verified as
discriminative biomarkers between cancer cases and controls to
provide an initial indication of a possible tumorigenic disease.
Further diagnostic methods must confirm our first hypothesis.
However, for the surveillance of BC patients, neither of the
investigated miRNAs seem to be informative. An expected
postoperative decline of the levels of miR-15b-5p and miR-590-5p as
the miRNAs of interest could not be observed in some patients after
tumor removal. This contradictory behavior of miRNA patterns could
not be attributed to any notable or latent clinical manifestations.
On the other hand, calculations of the ‘critical differences’
(23) of the miRNA levels in the
follow-up reported in our study may explain this phenomenon caused
by a high biological variation of the examined circulating miRNAs.
Similar patterns have been described in the literature and confirm
our observations (61,63–66). Our
findings support the view that diagnostic markers are by no means
always suitable as monitoring markers (69); however, current knowledge of the
effects of biological variation of circulating miRNAs is markedly
limited. Particular attention and further research to this critical
point will be necessary to evaluate the true validity of
circulating miRNAs in clinical practice. Further prospective
investigations of circulating miRNAs are warranted to consider the
aforementioned issues for a suitable study design.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The project was supported by Stiftung Urologische
Forschung.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contribution
AT, LB and KJ were responsible for the
conceptualization, investigation, methodology, validation,
visualization and writing of the manuscript. AT and KJ contributed
to the data curation, formal analysis of the data, and project
administration. LB and KJ were involved in the funding acquisition,
and carried out the writing and editing of the manuscript. AT was
responsible for the data acquisition, including reagents,
materials, patients and laboratory samples; and supervision of the
research was conducted by KJ. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All patients provided their informed consent to this
study, which was approved by the Hospital
Charité-Universitätsmedizin Berlin (EA1/134/12; Berlin,
Germany).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AUC
|
area under the curve
|
|
BC
|
bladder cancer
|
|
CI
|
confidence interval
|
|
Cq
|
quantification cycle
|
|
miRNAs
|
microRNAs
|
|
RC
|
radical cystectomy
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
TURB
|
transurethral resection
|
|
YAP
|
Yes associated protein
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Babjuk M, Böhle A, Burger M, Capoun O,
Cohen D, Comperat EM, Hernandez V, Kaasinen E, Palou J, Roupret M,
et al: EAU Guidelines on Non-Muscle-invasive urothelial carcinoma
of the bladder: Update 2016. Eur Urol. 71:447–461. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pandith AA, Shah ZA and Siddiqi MA:
Oncogenic role of fibroblast growth factor receptor 3 in
tumorigenesis of urinary bladder cancer. Urol Oncol. 31:398–406.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu XR: Biology of urothelial
tumorigenesis: Insights from genetically engineered mice. Cancer
Metastasis Rev. 28:281–290. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim WJ and Bae SC: Molecular biomarkers in
urothelial bladder cancer. Cancer Sci. 99:646–652. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Etheridge A, Lee I, Hood L, Galas D and
Wang K: Extracellular microRNA: A new source of biomarkers. Mutat
Res. 717:85–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zen K and Zhang CY: Circulating microRNAs:
A novel class of biomarkers to diagnose and monitor human cancers.
Med Res Rev. 32:326–348. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Adam L, Wszolek MF, Liu CG, Jing W, Diao
L, Zien A, Zhang JD, Jackson D and Dinney CP: Plasma microRNA
profiles for bladder cancer detection. Urol Oncol. 31:1701–1708.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Feng Y, Liu J, Kang Y, He Y, Liang B, Yang
P and Yu Z: miR-19a acts as an oncogenic microRNA and is
up-regulated in bladder cancer. J Exp Clin Cancer Res. 33:672014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Feng Y, Kang Y, He Y, Liu J, Liang B, Yang
P and Yu Z: microRNA-99a acts as a tumor suppressor and is
down-regulated in bladder cancer. BMC Urol. 14:502014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Du M, Shi D, Yuan L, Li P, Chu H, Qin C,
Yin C, Zhang Z and Wang M: Circulating miR-497 and miR-663b in
plasma are potential novel biomarkers for bladder cancer. Sci Rep.
5:104372015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fang Z, Dai W, Wang X, Chen W, Shen C, Ye
G and Li L: Circulating miR-205: A promising biomarker for the
detection and prognosis evaluation of bladder cancer. Tumour Biol.
37:8075–8082. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Motawi TK, Rizk SM, Ibrahim TM and Ibrahim
IA: Circulating microRNAs, miR-92a, miR-100 and miR-143, as
non-invasive biomarkers for bladder cancer diagnosis. Cell Biochem
Funct. 34:142–148. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tölle A, Blobel CC and Jung K: Circulating
miRNAs in blood and urine as diagnostic and prognostic biomarkers
for bladder cancer: An update in 2017. Biomark Med. 12:667–676.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K,
Guo J, Zhang Y, Chen J, Guo X, et al: Characterization of microRNAs
in serum: A novel class of biomarkers for diagnosis of cancer and
other diseases. Cell Res. 18:997–1006. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang Z, Huang D, Ni S, Peng Z, Sheng W
and Du X: Plasma microRNAs are promising novel biomarkers for early
detection of colorectal cancer. Int J Cancer. 127:118–126. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wittekind C, Asamura H and Sobin LH: TNM
Atlas. Hoboken. (New Jersey, US, Wiley-Backwell Publishing). 2014.
View Article : Google Scholar
|
|
18
|
Shah JS, Soon PS and Marsh DJ: Comparison
of methodologies to detect low levels of hemolysis in serum for
accurate assessment of serum microRNAs. PLoS One. 11:e01532002016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Blondal T, Jensby NS, Baker A, Andreasen
D, Mouritzen P, Wrang Teilum M and Dahlsveen IK: Assessing sample
and miRNA profile quality in serum and plasma or other biofluids.
Methods. 59 (Suppl):S1–S6. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pritchard CC, Kroh E, Wood B, Arroyo JD,
Dougherty KJ, Miyaji MM, Tait JF and Tewari M: Blood cell origin of
circulating MicroRNAs: A cautionary note for cancer biomarker
studies. Cancer Prev Res (Phila). 5:492–497. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kirschner MB, Edelman JJ, Kao SC, Vallely
MP, van Zandwijk N and Reid G: The impact of hemolysis on cell-free
microRNA biomarkers. Front Genet. 4:942013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fraser CG: Biological variation-from
principles to practice. Washington. (DC, U.S., AACC Press).
2001.
|
|
24
|
Ammerlaan W and Betsou F: Intraindividual
temporal miRNA variability in serum, plasma, and white blood cell
subpopulations. Biopreserv Biobank. 14:390–397. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ciesla M, Skrzypek K, Kozakowska M, Loboda
A, Jozkowicz A and Dulak J: MicroRNAs as biomarkers of disease
onset. Anal Bioanal Chem. 401:2051–2061. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang W, Peng B, Wang D, Ma X, Jiang D,
Zhao J and Yu L: Human tumor microRNA signatures derived from
large-scale oligonucleotide microarray datasets. Int J Cancer.
129:1624–1634. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang X, Liang M, Dittmar R and Wang L:
Extracellular microRNAs in urologic malignancies: Chances and
challenges. Int J Mol Sci. 14:14785–14799. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
MacLellan SA, MacAulay C, Lam S and Garnis
C: Pre-profiling factors influencing serum microRNA levels. BMC
Clin Pathol. 14:272014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ratert N, Meyer HA, Jung M, Lioudmer P,
Mollenkopf HJ, Wagner I, Miller K, Kilic E, Erbersdobler A, Weikert
S, et al: miRNA profiling identifies candidate mirnas for bladder
cancer diagnosis and clinical outcome. J Mol Diagn. 15:695–705.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Song T, Xia W, Shao N, Zhang X, Wang C, Wu
Y, Dong J, Cai W and Li H: Differential miRNA expression profiles
in bladder urothelial carcinomas. Asian Pac J Cancer Prev.
11:905–911. 2010.PubMed/NCBI
|
|
31
|
Zhao F, Ge YZ, Zhou LH, Xu LW, Xu Z, Ping
WW, Wang M, Zhou CC, Wu R and Jia RP: Identification of hub miRNA
biomarkers for bladder cancer by weighted gene coexpression network
analysis. Onco Targets Ther. 10:5551–5559. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang C, Chen L, Yang Y, Zhang M and Wong
G: Identification of bladder cancer prognostic biomarkers using an
ageing gene-related competitive endogenous RNA network. Oncotarget.
8:111742–111753. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Armstrong DA, Green BB, Seigne JD, Schned
AR and Marsit CJ: MicroRNA molecular profiling from matched tumor
and bio-fluids in bladder cancer. Mol Cancer. 14:1942015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xie Y, Ma X, Chen L, Li H, Gu L, Gao Y,
Zhang Y, Li X, Fan Y, Chen J, et al: MicroRNAs with prognostic
significance in bladder cancer: A systematic review and
meta-analysis. Sci Rep. 7:56192017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Friedel R, Diederichs F and Lindena J:
Advances in clinical enzymology. Basel, S. Karger AG; Switzerland:
1979, pp. 70–105
|
|
36
|
Hauser S, Wulfken LM, Holdenrieder S,
Moritz R, Ohlmann CH, Jung V, Becker F, Herrmann E,
Walgenbach-Brunagel G, von Ruecker A, et al: Analysis of serum
microRNAs (miR-26a-2*, miR-191, miR-337-3p and miR-378) as
potential biomarkers in renal cell carcinoma. Cancer Epidemiol.
36:391–394. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wulfken LM, Moritz R, Ohlmann C,
Holdenrieder S, Jung V, Becker F, Herrmann E, Walgenbach-Brunagel
G, von Ruecker A, Muller SC, et al: MicroRNAs in renal cell
carcinoma: Diagnostic implications of serum miR-1233 levels. PLoS
One. 6:e257872011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fendler A, Stephan C, Yousef GM,
Kristiansen G and Jung K: The translational potential of microRNAs
as biofluid markers of urological tumours. Nat Rev Urol.
13:734–752. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
White MC, Holman DM, Boehm JE, Peipins LA,
Grossman M and Henley SJ: Age and cancer risk: A potentially
modifiable relationship. Am J Prev Med 46 (3 Suppl 1). S7–S15.
2014. View Article : Google Scholar
|
|
40
|
Miah S, Dudziec E, Drayton RM, Zlotta AR,
Morgan SL, Rosario DJ, Hamdy FC and Catto JW: An evaluation of
urinary microRNA reveals a high sensitivity for bladder cancer. Br
J Cancer. 107:123–128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jiang X, Du L, Wang L, Li J, Liu Y, Zheng
G, Qu A, Zhang X, Pan H, Yang Y and Wang C: Serum microRNA
expression signatures identified from genome-wide microRNA
profiling serve as novel noninvasive biomarkers for diagnosis and
recurrence of bladder cancer. Int J Cancer. 136:854–862. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Binderup HG, Madsen JS, Heegaard NHH,
Houlind K, Andersen RF and Brasen CL: Quantification of microRNA
levels in plasma-Impact of preanalytical and analytical conditions.
PLoS One. 13:e02010692018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kanaan Z, Roberts H, Eichenberger MR,
Billeter A, Ocheretner G, Pan J, Rai SN, Jorden J, Williford A and
Galandiuk S: A plasma microRNA panel for detection of colorectal
adenomas: A step toward more precise screening for colorectal
cancer. Ann Surg. 258:400–408. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen Y, Chen J, Liu Y, Li S and Huang P:
Plasma miR-15b-5p, miR-338-5p, and miR-764 as biomarkers for
hepatocellular carcinoma. Med Sci Monit. 21:1864–1871. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fogli S, Polini B, Carpi S, Pardini B,
Naccarati A, Dubbini N, Lanza M, Breschi MC, Romanini A and Nieri
P: Identification of plasma microRNAs as new potential biomarkers
with high diagnostic power in human cutaneous melanoma. Tumour
Biol. 39:10104283177016462017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang F, Zu Y, Zhu S, Yang Y, Huang W, Xie
H and Li G: Long noncoding RNA MAGI2-AS3 regulates CCDC19
expression by sponging miR-15b-5p and suppresses bladder cancer
progression. Biochem Biophys Res Commun. 507:231–235. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhao C, Li Y, Chen G, Wang F, Shen Z and
Zhou R: Overexpression of miR-15b-5p promotes gastric cancer
metastasis by regulating PAQR3. Oncol Rep. 38:352–358. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Summerer I, Niyazi M, Unger K, Pitea A,
Zangen V, Hess J, Atkinson MJ, Belka C, Moertl S and Zitzelsberger
H: Changes in circulating microRNAs after radiochemotherapy in head
and neck cancer patients. Radiat Oncol. 8:2962013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yu M, Luo Y, Cong Z, Mu Y, Qiu Y and Zhong
M: MicroRNA-590-5p inhibits intestinal inflammation by targeting
YAP. J Crohns Colitis. 12:993–1004. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Dong L, Lin F, Wu W, Liu Y and Huang W:
Verteporfin inhibits YAP-induced bladder cancer cell growth and
invasion via Hippo signaling pathway. Int J Med Sci. 15:645–652.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu JY, Li YH, Lin HX, Liao YJ, Mai SJ,
Liu ZW, Zhang ZL, Jiang LJ, Zhang JX, Kung HF, et al:
Overexpression of YAP 1 contributes to progressive features and
poor prognosis of human urothelial carcinoma of the bladder. BMC
Cancer. 13:3492013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
van Kessel KE, Van Neste L, Lurkin I,
Zwarthoff EC and Van Criekinge W: Evaluation of an epigenetic
profile for the detection of bladder cancer in patients with
hematuria. J Urol. 195:601–607. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang J, Zhou Y, Huang T, Wu F, Pan Y,
Dong Y, Wang Y, Chan AK, Liu L, Kwan JS, et al: FGF18, a prominent
player in FGF signaling, promotes gastric tumorigenesis through
autocrine manner and is negatively regulated by miR-590-5p.
Oncogene. 38:33–46. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Marzi MJ, Ghini F, Cerruti B, de Pretis S,
Bonetti P, Giacomelli C, Gorski MM, Kress T, Pelizzola M, Muller H,
et al: Degradation dynamics of microRNAs revealed by a novel
pulse-chase approach. Genome Res. 26:554–565. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gantier MP, McCoy CE, Rusinova I, Saulep
D, Wang D, Xu D, Irving AT, Behlke MA, Hertzog PJ, Mackay F and
Williams BR: Analysis of microRNA turnover in mammalian cells
following Dicer1 ablation. Nucleic Acids Res. 39:5692–5703. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Guo Y, Liu J, Elfenbein SJ, Ma Y, Zhong M,
Qiu C, Ding Y and Lu J: Characterization of the mammalian miRNA
turnover landscape. Nucleic Acids Res. 43:2326–2341. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Juracek J, Peltanova B, Dolezel J, Fedorko
M, Pacik D, Radova L, Vesela P, Svoboda M, Slaby O and Stanik M:
Genome-wide identification of urinary cell-free microRNAs for
non-invasive detection of bladder cancer. J Cell Mol Med.
22:2033–2038. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhang X, Zhang Y, Liu X, Fang A, Wang J,
Yang Y, Wang L, Du L and Wang C: Direct quantitative detection for
cell-free miR-155 in urine: A potential role in diagnosis and
prognosis for non-muscle invasive bladder cancer. Oncotarget.
7:3255–3266. 2016.PubMed/NCBI
|
|
59
|
Du L, Jiang X, Duan W, Wang R, Wang L,
Zheng G, Yan K, Wang L, Li J, Zhang X, et al: Cell-free microRNA
expression signatures in urine serve as novel noninvasive
biomarkers for diagnosis and recurrence prediction of bladder
cancer. Oncotarget. 8:40832–40842. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tsujiura M, Ichikawa D, Komatsu S,
Shiozaki A, Takeshita H, Kosuga T, Konishi H, Morimura R, Deguchi
K, Fujiwara H, et al: Circulating microRNAs in plasma of patients
with gastric cancers. Br J Cancer. 102:1174–1179. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Le HB, Zhu WY, Chen DD, He JY, Huang YY,
Liu XG and Zhang YK: Evaluation of dynamic change of serum miR-21
and miR-24 in pre- and post-operative lung carcinoma patients. Med
Oncol. 29:3190–3197. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Heneghan HM, Miller N, Lowery AJ, Sweeney
KJ, Newell J and Kerin MJ: Circulating microRNAs as novel minimally
invasive biomarkers for breast cancer. Ann Surg. 251:499–505. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Aushev VN, Zborovskaya IB, Laktionov KK,
Girard N, Cros MP, Herceg Z and Krutovskikh V: Comparisons of
microRNA patterns in plasma before and after tumor removal reveal
new biomarkers of lung squamous cell carcinoma. PLoS One.
8:e786492013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Leidinger P, Keller A, Backes C, Huwer H
and Meese E: MicroRNA expression changes after lung cancer
resection: A follow-up study. RNA Biol. 9:900–910. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Leidinger P, Galata V, Backes C, Stähler
C, Rheinheimer S, Huwer H, Meese E and Keller A: Longitudinal study
on circulating miRNAs in patients after lung cancer resection.
Oncotarget. 6:16674–16685. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Egidi MG, Cochetti G, Serva MR, Guelfi G,
Zampini D, Mechelli L and Mearini E: Circulating microRNAs and
kallikreins before and after radical prostatectomy: Are they really
prostate cancer markers? Biomed Res Int. 2013:2417802013.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Keller A, Rounge T, Backes C, Ludwig N,
Gislefoss R, Leidinger P, Langseth H and Meese E: Sources to
variability in circulating human miRNA signatures. RNA Biol.
14:1791–1798. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Aarsand AK, Roraas T, Fernandez-Calle P,
Ricos C, Diaz-Garzon J, Jonker N, Perich C, Gonzalez-Lao E,
Carobene A, Minchinela J, et al: The biological variation data
critical appraisal checklist: A standard for evaluating studies on
biological variation. Clin Chem. 64:501–514. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Burke HB: Increasing the power of
surrogate endpoint biomarkers: The aggregation of predictive
factors. J Cell Biochem. (Suppl 19):S278–S282. 1994.
|