Introduction
Cervical cancer remains the fourth most common and
the fourth most fatal type of cancer in women. Based on GLOBOCAN
estimates, there will be a total of 569,847 new cases of cervical
cancer and 311,365 deaths due to cervical cancer in 2018 worldwide
(1). The main underlying cause of
cervical cancer is human papillomavirus (HPV) infection. HPV
infections may cause mild dysplasia which usually regresses
spontaneously but some infections caused by high-risk HPV types,
may become persistent and progress to high grade lesions and
eventually invasive carcinoma (2).
Although cervical cancer is not easily detected and
treatable, the long window of time between precancerous lesions and
invasive cancer provides the opportunity for early detection and
prevention. Indeed, regular cytology-based screening has
significantly reduced but not eliminated cervical cancer mortality
(3), while the most recent advances
concerning HPV testing and HPV vaccines have further improved the
disease control, mainly in high-income countries (4). However, a significant number of new
cases are still diagnosed.
The treatment of cervical cancer relies on surgical
interventions that are usually accompanied by chemotherapy and
radiation. Elimination of the cancer and patient survival are the
top priorities; however, since childbirth is often delayed in
developed countries, preservation of fertility is a major factor
when considering cervical cancer treatment approaches. Fertility
sparing techniques have been developed but they can only be applied
after careful evaluation of the cancer and in early stages
(5,6).
Targeted pharmaceutical treatment approaches could be the solution
to this problem, but despite the intensive efforts during the last
decades, no novel drugs have been applied in clinical practice
(7,8).
A need for deeper understanding of the underlying
mechanisms of cervical cancer pathology is critical. Towards this
goal, our group has already investigated the secretome (9) and the membrane proteomes (10) of three cervical cancer cell lines in
comparison to normal cervical keratinocytes with liquid
chromatography coupled with tandem mass spectrometry (LC-MS/MS).
These studies highlighted the inhibition of matrix metalloproteases
(9) and the deregulation of HIPPO,
PI3K/Akt, and EIF2 signaling pathways (10) in cervical cancer as well as many
differentially expressed proteins that are potentially crucial for
cervical malignancy. Along the same lines and in an attempt to
elucidate the molecular mechanisms underlining cervical
carcinogenesis, we analyzed the intracellular proteome of three
cervical cancer cell lines against normal cervical keratinocytes
employing high resolution LC-MS/MS. The differentially expressed
proteins between the cervical cancer cell lines and the control
cell line were integrated into molecular pathways that are possibly
deregulated during the malignant transformation of the cervical
epithelium. Through an extensive review of the existing literature,
we shortlisted the consistent proteomic changes providing a
valuable dataset of proteins for further investigation and
functional analyses. These proteins could be used as molecular
markers or drug targets.
Materials and methods
Cell culture
HeLa (HPV 18+), SiHa (HPV 16+)
and C33A (HPV−) cervical cancer cell lines, were
purchased from the American Type Culture Collection (ATCC)
(Manassas, VA, USA) and cultured in Dulbecco's modified Eagle's
medium (DMEM) supplemented with 10% fetal bovine serum (FBS)
(Gibco/Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and 1% penicillin-streptomycin at 37°C in 5% CO2 as
previously described (11). ΗCK1T
cells were a kind gift from Dr Tohru Kiyono of the National Cancer
Center Research Institute (Chuo-ku, Tokyo, Japan) (12) and were cultured as proposed (13) in Defined Keratinocyte Serum-Free
Medium (SFM) (Gibco; Thermo Fisher Scientific, Inc.) supplemented
with 5 ng/ml epidermal growth factor (EGF) (Gibco; Thermo Fisher
Scientific, Inc.) and 50 µg/ml of bovine pituitary extract (BPE)
(Gibco; Thermo Fisher Scientific, Inc.). When the cells reached a
concentration of 106 cells per ml, they were
trypsinized, harvested, and the pellets were washed in 1X
phosphate-buffered saline (PBS) 3 times before storage at −80°C
until further use.
Sample preparation
Pellets were homogenized in homogenization buffer (7
M urea, 2 M thiourea, 4% CHAPS, 1% DTE) using mild sonication
(water bath sonication). After centrifugation at 16,000 × g for 20
min, the total cell extract was obtained as a supernatant. Protein
concentration was measured with the Bradford assay. The sample
preparation was based on the GeLC-MS protocol described by
Makridakis and Vlahou (14). Briefly,
10 µg of protein extract from each sample were analyzed by 12% SDS
PAGE and stained with Coomassie Colloidal Blue (Fluka; Thermo
Fisher Scientific, Inc.) overnight. The gel bands were excised and
cut in small pieces (1–2 mm side squares). Gel pieces were
destained in 40% acetonitrile (Thermo Fisher Scientific, Inc.), 50
mM NH4HCO3 (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany), reduced in 10 mM dithioerythritol
(Sigma-Aldrich; Merck KGaA), 100 mM NH4HCO3,
and alkylated in 50 mM iodoacetamide (Applichem, Inc., Omaha, NE,
USA), 100 mM NH4HCO3. Samples were dried
using the Savant Speedvac™ concentrator (Thermo Fisher Scientific,
Inc.) and trypsinized overnight at room temperature with 600 ng
trypsin (Roche Diagnostics, Basel, Switzerland) in 10 mM
NH4HCO3. Peptide extraction was performed
with a wash of the trypsinized gel pieces with 50 mM
NH4HCO3, followed by two washes with 50%
acetonitrile (Thermo Fisher Scientific, Inc.), 5% formic acid
(Sigma-Aldrich; Merck KGaA) for 15 min at room temperature, under
shaking conditions. Extracted peptides were dried using the Savant
Speedvac™ concentrator and analyzed by LC-MS/MS.
LC-MS/MS (liquid chromatography
coupled with tandem mass spectrometry) analysis
Analysis of the protein extracts was conducted with
an UltiMate 3000 Nano HPLC Dionex Ultimate® 3000 RSLS
system (Dionex™; Thermo Fisher Scientific, Inc.) as previously
reported (9). Dried peptides were
solubilized in 10 µl mobile phase A (0.1% formic acid) and 5 µl
from each sample was loaded on a 0.1×20 mm, 5-µm C18 nanotrap
column. The loading was performed at a flow rate of 5 µl/min in 98%
mobile phase A [0.1% formic acid (Sigma-Aldrich; Merck KGaA)] and
2% mobile phase B [100% acetonitrile (Thermo Fisher Scientific,
Inc.)], 0.1% formic acid. Then, the sample was analyzed using an
Acclaim PepMap C18 nanocolumn 75 µm × 50 cm (Dionex™; Thermo Fisher
Scientific, Inc), at a flow rate of 0.3 µl/min. The trap and the
nanoflow column were maintained at 35°C. The samples were eluted
with a gradient of solvent B, starting at 1% B for 5 min, rising to
5% B at 10 min, 25% B at 180 min and 65% B at 240 min. The column
was then washed and re-equilibrated prior to injection of the next
sample. The eluent was ionized using a Proxeon Nano Spray Electron
Spray Ionization (ESI) source, operating in positive ion mode into
an Orbitrap Elite FTMS (Thermo Fisher Scientific, Inc). Ionization
voltage was at 2.6 kV and the capillary temperature was at 200°C.
The mass spectrometer was operated using the MS/MS mode scanning
from 380 to 2,000 amu (atomic mass units). The resolution of ions
in MS1 was 60,000, and 15,000 for HCD MS2. The top 20
multiply-charged ions were selected from each scan for MS/MS
analysis using HCD at 35% collision energy. Dynamic exclusion was
set to 30 sec.
MS data analysis
Peptide identification was performed with Proteome
Discoverer 1.4 software package (Thermo Scientific, Inc.), using
the SEQUEST search engine and the UniProt (http://www.uniprot.org/) human reviewed database,
updated on May 30, 2016, including 20,204 entries. The search was
performed using carbamidomethylation of cysteine as static, and
oxidation of methionine as dynamic modifications. Two missed
cleavage sites, a precursor mass tolerance of 10 ppm and fragment
mass tolerance of 0.05 Da were allowed. False discovery rate (FDR)
validation was based on q value: target FDR (strict), 0.01; target
FDR (relaxed), 0.05. SEQUEST results were filtered for
false-positive identifications. Peptide quantification was
performed as previously described (15). Only peptides being present in 75% of
the samples in at least one group (cervical cancer or normal cell
line) were considered confident identifications and were used for
protein quantification. Statistical analysis was based on the
Mann-Whitney test performed in R (version 3.3.1). Differences with
P-value ≤0.05 were considered statistically significant and an
expression change threshold of 2 (cancer/normal ≥2 or cancer/normal
≤0.5) was also applied for differential expression. The protein
expression heatmap was created at http://www.heatmapper.ca/ (16).
Functional analysis
Functional analysis was performed with the ClueGO
plug-in (17) in Cytoscape 3.4.0
(18). Ontologies were retrieved from
REACTOME pathways database (updated on September 4, 2017) and only
statistically significant pathways (Bonferroni corrected P-value
≤0.05, two-sided hypergeometric test) were taken into account. For
the remaining parameters, default settings were used. Results were
simplified based on biological relevance and only the leading term
from each group is presented.
Western blot analysis
Four 30-µg samples of cell extract dissolved in
Laemli's buffer from each cell line were loaded on 10%
SDS-polyacrylamide gel after incubation at 90°C for 10 min (2 gels
in total with 2 replicates from each cell line on each gel). The
gel was run at 40 V for 15 min and then at 120 V in
Tris-glycine-SDS buffer. The transfer was performed in transfer
buffer (3.03 g Tris, 14.4 g glycine, 200 ml methanol for 1 liter
total volume) for 2 h at 290 mA at 4°C. Then, the membrane was
stained with Ponceau-S stain for 5 min, washed with ultrapure water
for 5 min three times, followed by the addition of blocking
solution (5% w/v non-fat dried milk in TBS-Tween 0.1% v/v) and
incubation for 2 h. The membrane was washed with TBS-Tween 0.1% v/v
successively for 15, 5 and 5 min, and the primary antibodies: LIMA1
(sc-136399; dilution, 1:200; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), PON2 (sc-373981; dilution, 1:100; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and α-tubulin (T6199;
dilution, 1:5000; Sigma-Aldrich; Merck KGaA) were added at the
respective dilutions and left at 4°C overnight. The next day, the
three washes were repeated and the secondary goat anti-mouse
antibody IgG-HRP (sc-2005; Santa Cruz Biotechnology, Inc.) was
added at a 1:2,000 dilution and left at room temperature for 2 h.
The three washes were repeated, ECL was added and left for 1 min;
its excess was removed, followed by film exposure and development.
The films were scanned and the quantification of the bands was
performed with Quantity One software 4.4.1 (Bio-Rad Laboratories,
Hercules, CA, USA). For LIMA1, the bands corresponding to 110 and
90 kDa were quantified. For PON2, the bands corresponding to 53 and
43 kDa were quantified. Both antibodies (LIMA1 and PON2) were
blotted on the same membranes (since the difference in molecular
weights allowed this) and quantification of α-tubulin was used as a
loading control for both antibodies. One-way ANOVA and Tukey HSD
tests were performed in SPSS Statistics 22.0 (IBM, Armonk, NY,
USA).
Results
Differential expression analysis
Towards a better understanding of the cervical
cancer pathology and the molecular mechanisms underlying the
malignant transformation of the cervical epithelium, a comparative
proteomic analysis of cervical cell lines was performed, utilizing
the GeLC-MS protocol (14). The cell
lines that were analyzed included the following: HCK1T (human
cervical keratinocytes), a normal cervical epithelium cell line;
HeLa, a cervical cancer cell line positive for HPV18
(HPV18+); SiHa, a cervical cancer cell line positive for
HPV16 (HPV16+); and C33A, a cervical cancer cell line
negative for HPV (HPV−). Four biological replicates were
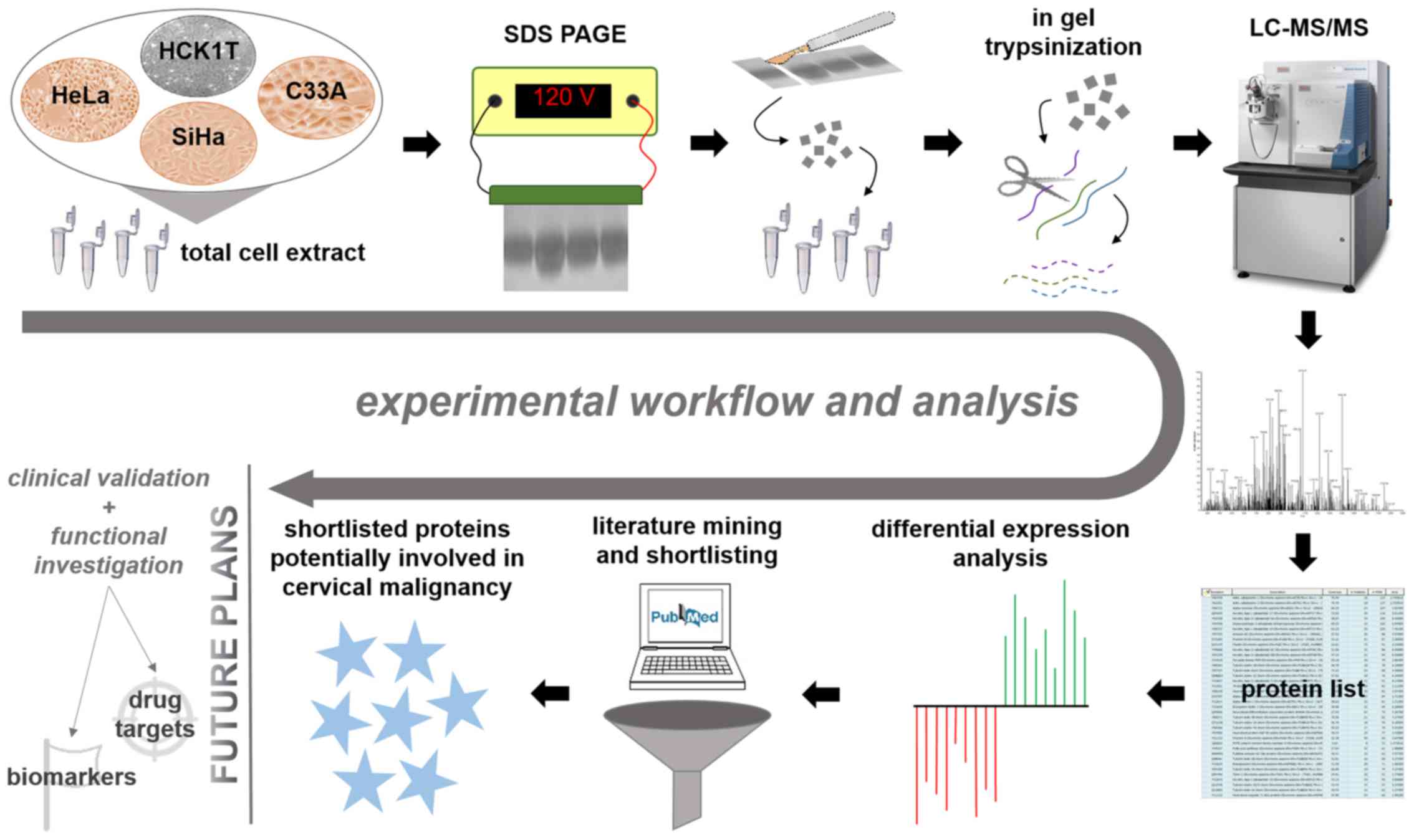
processed from each cell line. A graphical representation of our
workflow is presented in Fig. 1.
The total number of protein identifications was
2,607 for HCK1T; 2,859 for HeLa; 2,902 for SiHa; and 3,405 for
C33A. Each of the three cancer cell lines (HeLa, SiHa, C33A) was
compared to the normal cell line (HCK1T) and only peptides detected
in at least 75% of the samples (3 out of 4 biological replicates)
in at least one group (cervical cancer or normal cell line) were
taken into account for the analysis. The differentially expressed
proteins in cancer vs. normal cells were considered the proteins
that showed a statistically significant change (Mann-Whitney
P-value ≤0.05) and had an expression change of at least 2
(cancer/normal ≥2 or cancer/normal ≤0.5). In addition, proteins
uniquely identified only in one cell line per comparison were also
considered as differentially expressed proteins.
In the comparison between the HeLa and HCK1T cells,
919 proteins were differentially expressed. Out of these, 321 were
upregulated and 165 were downregulated in cancer, while 257
proteins were uniquely identified in HeLa and 176 proteins were
uniquely identified in HCK1T cells (Supplementary Table I, available at Figshare, doi:
10.6084/m9.figshare.7121540; http://figshare.com/articles/Supplementary_data/7121540).
When comparing SiHa to HCK1T cells, 826 proteins were
differentially expressed in total, out of which 310 were
upregulated in cancer, 138 were downregulated in cancer, 224
proteins were detected only in SiHa and 154 proteins were only
detected only in HCK1T (Supplementary Table II, available at
Figshare, doi: 10.6084/m9.figshare.7121540; http://figshare.com/articles/Supplementary_data/7121540).
Finally, in regards to the comparison of C33A to HCK1T cells, the
total number of differentially expressed proteins was 1,370. Out of
these proteins, 497 were upregulated in cancer, 174 were
downregulated in cancer, 472 were identified only in C33A cells and
227 proteins were only identified in HCK1T cells (Supplementary
Table III, available at Figshare, doi: 10.6084/m9.figshare.7121540;
http://figshare.com/articles/Supplementary_data/7121540).
 | Table I.Shortlist of 21 proteins consistently
deregulated in cervical cancer cell lines compared to normal
cervical keratinocytes that are novel findings based on existing
literature. |
Table I.
Shortlist of 21 proteins consistently
deregulated in cervical cancer cell lines compared to normal
cervical keratinocytes that are novel findings based on existing
literature.
|
|
| HeLa vs. HCK1T | SiHa vs. HCK1T | C33A vs. HCK1T |
|---|
|
|
|
|
|
|
|---|
| Symbol | Name | No. of
peptides | Ratio | No. of
peptides | Ratio | No. of
peptides | Ratio |
|---|
| LIMA1 | LIM domain and
actin-binding protein 1 | 6 | 0.20 | 7 | 0.24 | 6 | 0.09 |
| KPNA2 | Importin subunit
α-1 | 9 | 124.09 | 6 | 46.84 | 13 | 351.64 |
| PON2 | Serum
paraoxonase/arylesterase 2 | 4 | 12.93 | 2 | 4.80 | 3 | 11.99 |
| DNAJA1 | DnaJ homolog
subfamily A member 1 | 4 | 7.70 | 7 | 6.70 | 6 | 7.44 |
| NSUN2 | tRNA [cytosine
(34)-C (5)]-methyltransferase | 8 | 6.63 | 11 | 9.60 | 7 | 4.20 |
| FUS | RNA-binding protein
FUS | 4 | 5.22 | 4 | 5.08 | 3 | 6.45 |
| EEF1A2 | Elongation factor
1-α2 | 12 | 5.75 | 6 | 4.03 | 11 | 5.41 |
| RANGAP1 | Ran
GTPase-activating protein 1 | 5 | 3.18 | 7 | 4.65 | 7 | 7.30 |
| PSMB4 | Proteasome subunit
β type-4 | 3 | 5.10 | 2 | 3.88 | 3 | 5.74 |
| AHSA1 | Activator of 90 kDa
heat shock protein ATPase 1 homolog | 2 | 2.84 | 4 | 3.25 | 5 | 8.02 |
| GMPS | GMP synthase
(glutamine-hydrolyzing) | 12 | 5.73 | 6 | 2.40 | 13 | 5.93 |
| PSMB7 | Proteasome subunit
β type-7 | 3 | 4.76 | 2 | 4.36 | 3 | 4.60 |
| CAPRIN1 | Caprin-1 | 4 | 4.52 | 4 | 3.70 | 4 | 4.44 |
| SSRP1 | FACT complex
subunit SSRP1 | 12 | 3.01 | 12 | 3.15 | 14 | 6.34 |
| TOMM34 | Mitochondrial
import receptor subunit TOM34 | 6 | 4.02 | 4 | 2.48 | 7 | 5.56 |
| DHX9 | ATP-dependent RNA
helicase A | 8 | 3.73 | 5 | 2.36 | 10 | 5.50 |
| MAT2A |
S-adenosylmethionine synthase isoform
type-2 | 4 | 2.55 | 5 | 2.89 | 8 | 6.13 |
| PABPC1 |
Polyadenylate-binding protein 1 | 8 | 3.40 | 9 | 3.31 | 8 | 4.19 |
| KHSRP | Far upstream
element-binding protein 2 | 6 | 2.29 | 9 | 3.63 | 9 | 4.93 |
| G3BP1 | Ras
GTPase-activating protein-binding protein 1 | 5 | 2.65 | 5 | 2.57 | 9 | 5.39 |
| ACAT1 | Acetyl-CoA
acetyltransferase, mitochondrial | 6 | 2.26 | 8 | 3.32 | 8 | 3.68 |
Functional analysis
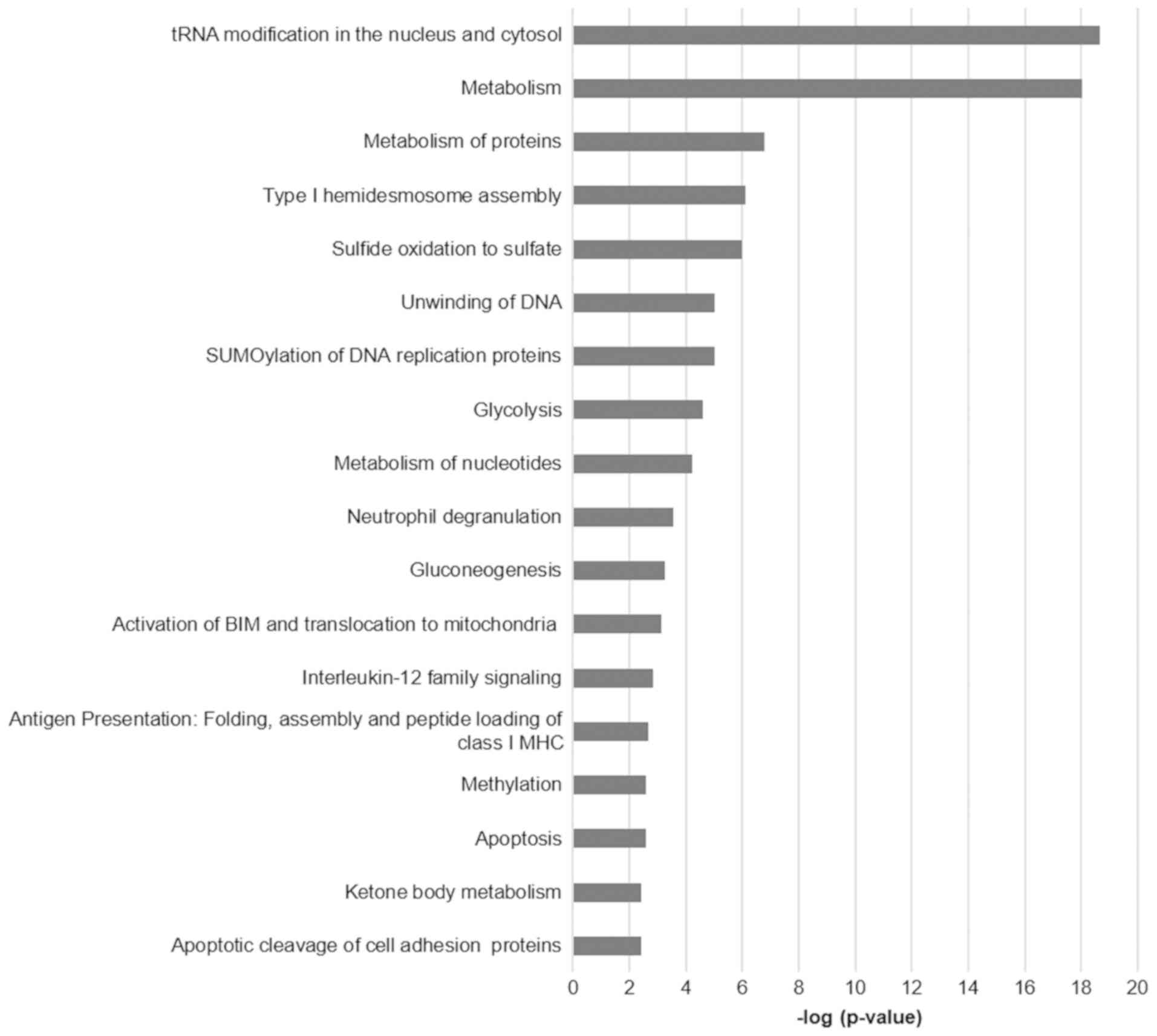
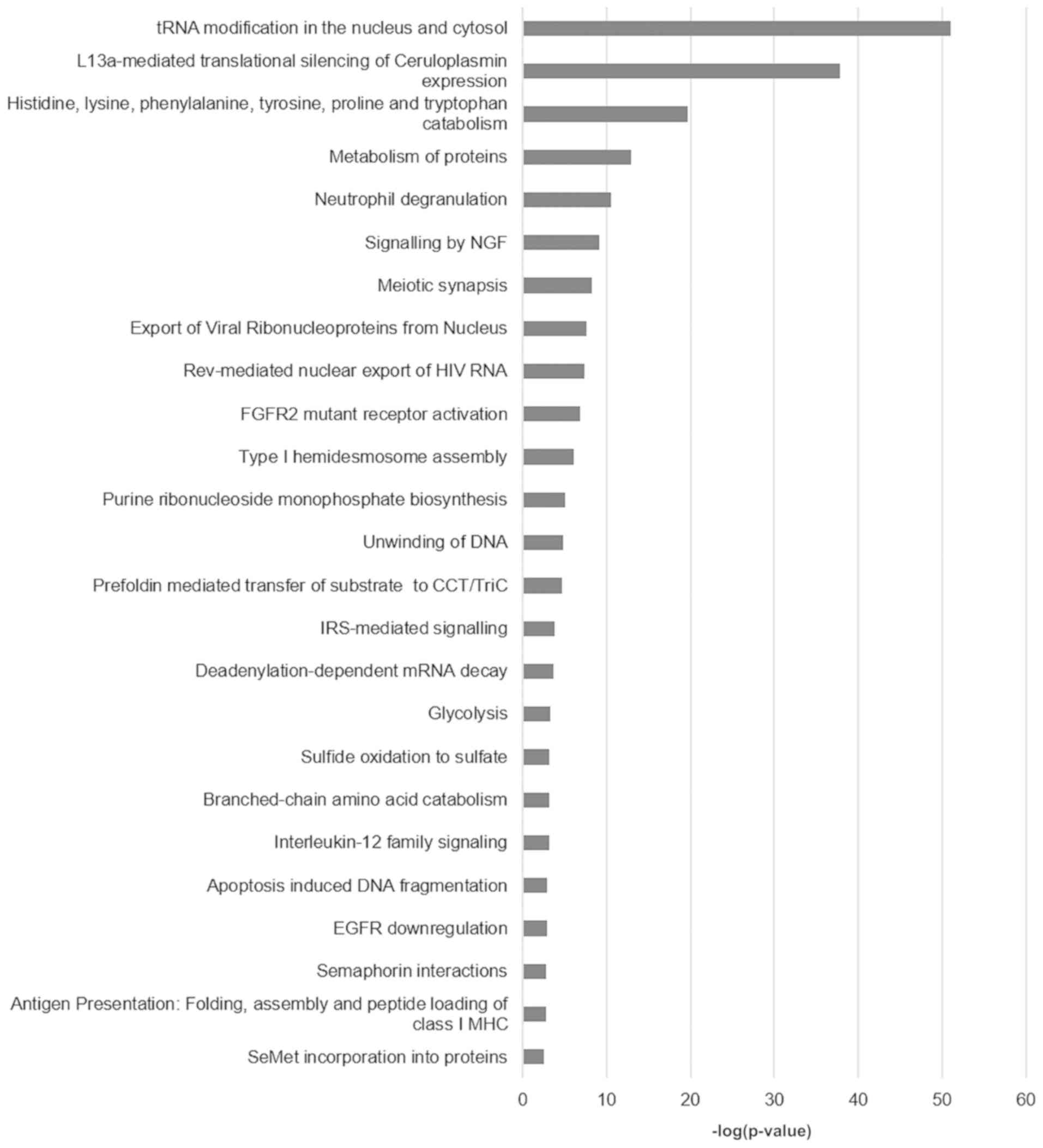
Functional annotation and pathway enrichment of the
differentially expressed proteins per comparison was performed
using the ClueGO plug-in of Cytoscape. The functional analysis
results for the comparisons HeLa vs. HCK1T, SiHa vs. HCK1T, and
C33A vs. HCK1T are presented in Figs.
2–4, respectively, and
Supplementary Table IV (available at Figshare, doi:
10.6084/m9.figshare.7121540; http://figshare.com/articles/Supplementary_data/7121540).
Some of the prominent and significant pathways that appear to be
deregulated between the cervical cancer and the normal cell lines
are metabolism of proteins, metabolism of nucleotides, unwinding of
DNA, glycolysis, gluconeogenesis apoptosis and oxidative stress
induced senescence. These pathways reflect the malignant phenotype
of the cancer cells with high metabolic requirements, increased
cell turnover and damage control mechanisms, as expected.
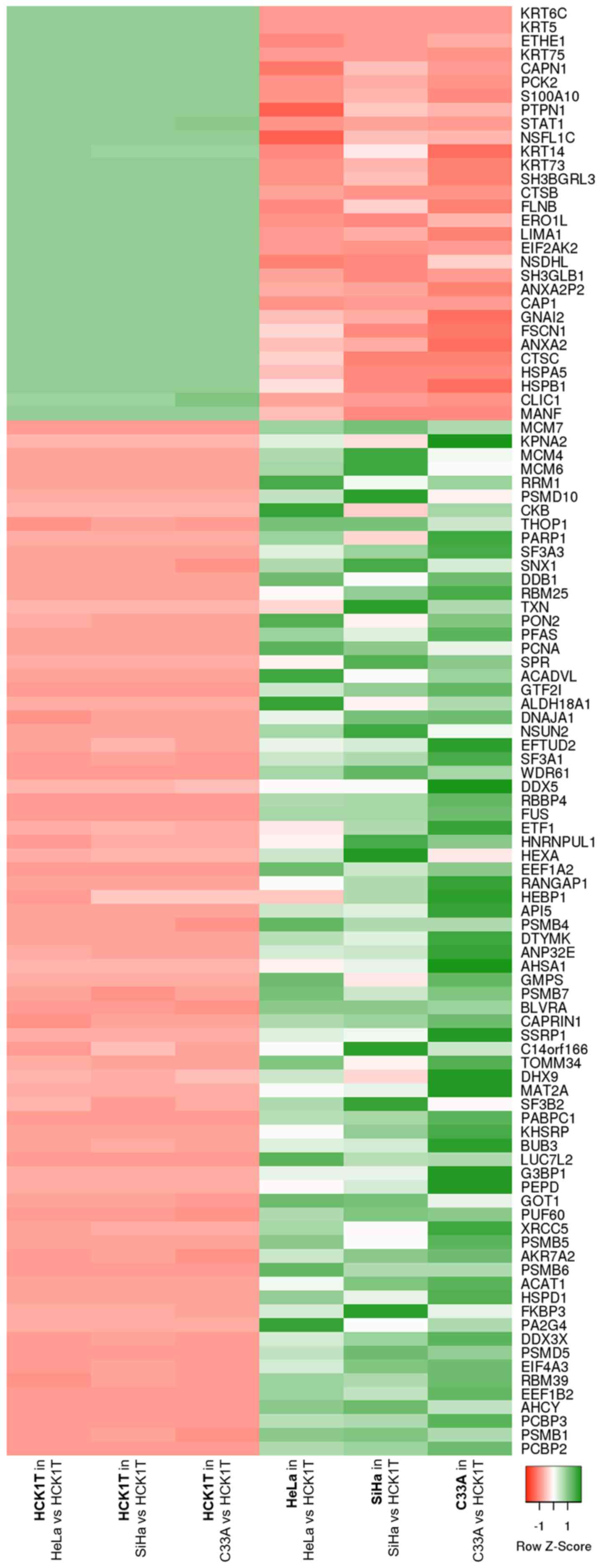
Focusing on the consistent proteomic
changes
In order to focus on the common most prominent
proteomic changes between cervical cancer and normal cervical
keratinocytes, regardless of the cancer origin (presence of HPV
infection, HPV type), a shortlisting of the differentially
expressed proteins that emerged from the three comparisons (HeLa
vs. HCK1T, SiHa vs. HCK1T and C33A vs. HCK1T) was performed. As a
first step, only proteins that followed the same expression trend
in cancer vs. normal cells were selected. Proteins that were
uniquely identified in only one cell line per comparison were
excluded at this point due to probable technical limitations such
as protein quantity being below the limit of detection of the
method used but not completely absent, and challenging
reproducibility, i.e. its absence might not be confirmed with a
different method. This first shortlisting resulted in 105
consistent proteomic changes between the cervical cancer cell lines
and the normal cervical keratinocytes, which are represented by a
heatmap in Fig. 5. Following this, an
extensive literature mining for all the consistently differentially
expressed proteins was performed (Supplementary Table V, available
at Figshare, doi: 10.6084/m9.figshare.7121540; http://figshare.com/articles/Supplementary_data/7121540).
The aim of this bibliographic investigation was to reveal proteins
that are potential key regulators of cervical carcinogenesis but
have not yet been investigated. This search led to a list of 21
proteins (Table I) that are
implicated in cancer but there is no study associating their
expression to cervical cancer, at least to our knowledge, and could
be the basis of further investigations in the context of cervical
cancer.
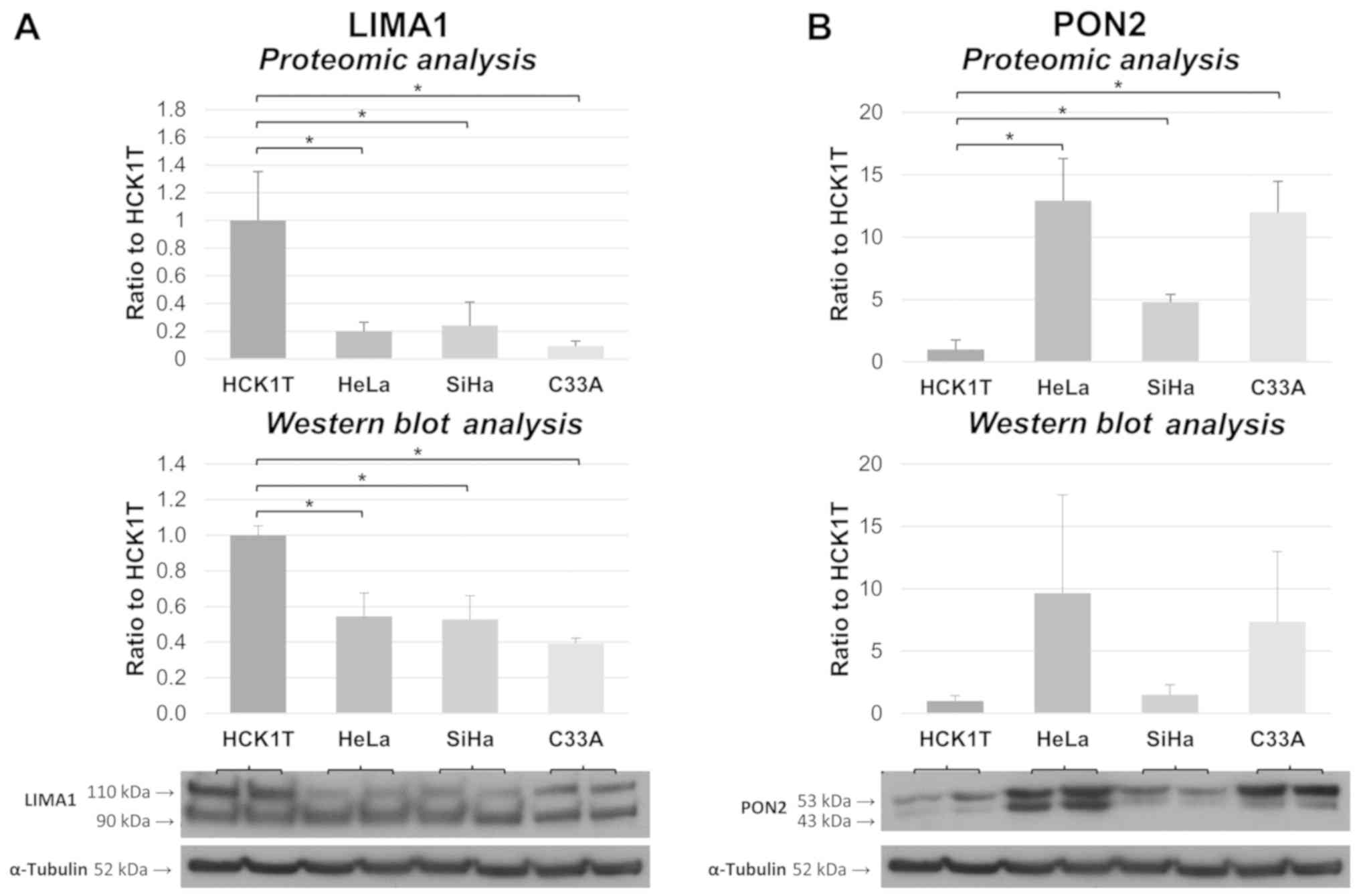
Validation of the expression trend for
two of the shortlisted proteins
LIM domain and actin-binding protein 1 (LIMA1) and
serum paraoxonase/arylesterase 2 (PON2) were also assessed with
western blot analysis in four new biological replicates from each
of the four cervical cell lines. Their expression trend based on
western blot analysis was similar to the proteomics results, as
shown in Fig. 6, validating the
credibility of the method. Specifically, both isoforms of LIMA1
(bands of 90 and 110 kDa in the western blot analysis) are shown to
be downregulated in the cervical cancer cell lines compared to
HCK1T, according to both methodologies. In a similar way, PON2 is
shown to be upregulated in all cervical cancer cell lines, by both
approaches [the double band of PON2 in the Western blot is a common
observation (19)].
Discussion
The comprehensive proteomic analysis of cervical
cancer cell lines and normal cervical keratinocytes, performed
utilizing the LC-MS/MS platform, revealed the proteomic changes
occurring during the malignant transformation of the cervical
epithelium in three subtypes of cervical cancer: HPV18+
(HeLa cells), HPV16+ (SiHa cells) and HPV−
(C33A cells). The cell lines analyzed were chosen carefully for
this study, as they represent the most common high-risk
HPV-infected cervical cancers and HPV-free cervical cancer that,
together, constitute a great percentage of the cervical cancer
incidents. The cancer cell lines were compared to normal cervical
keratinocytes and not keratinocytes of another origin (e.g. skin)
to eliminate differences due to tissue-specific
characteristics.
The differentially expressed proteins in each
comparison (HeLa vs. HCK1T, SiHa vs. HCK1T, and C33A vs. HCK1T) can
be utilized for further analysis and can be the basis for systems
biology approaches. The pathway enrichment analysis of the
differentially expressed proteins from each comparison revealed
that the deregulation processes are associated with the elevated
metabolic demands and increased cell turnover that are common
characteristics of cancer (20).
These findings confirm the biological relevance of our proteomics
results and add to the integrity of the study. Furthermore, the
shortlisting of the differentially expressed proteins performed
based on the existing literature, paves the way for further
investigation of promising candidates, clinical validation and
functional exploration in the context of cervical cancer.
Validation of the expression trend of two of the shortlisted
proteins using an immuno-based technique (western blot analysis)
further confirmed the robustness and accuracy of the proteomics
methodology.
The 21 shortlisted proteins (Table I) have been associated with other
types of cancer in the literature, such as breast, prostate,
ovarian and lung cancer, but have not yet been studied within the
context of cervical cancer, as far as we know. These proteins could
be of great interest for further investigations and could
potentially play pivotal roles in cervical carcinogenesis. Few of
the proteins with functional relevance to cancer are discussed
below.
LIM domain and actin-binding protein 1 (LIMA1),
which is involved in the regulation of actin dynamics, has been
found to be downregulated in various cancer types when compared to
healthy tissue (21). In the present
study, LIMA1 was found to be consistently downregulated in all
three cervical cancer cell lines compared to HCK1T cells in the
proteomic analysis. This downregulation was also confirmed using
western blot analysis. In the literature, it has been shown that
LIMA1 expression significantly decreases concomitantly with cancer
progression and this loss promotes cancer cell migration and
invasion while it is associated with poor prognosis (22). Moreover, induced overexpression of
LIMA1 in cancer cells appears to reverse their invasive phenotype
and to reduce the metastatic potential, indicating that LIMA1 could
be an excellent drug target for cancer treatment (21). In addition, immunohistochemical
staining of LIMA1 in ovarian cancer showed that few cancer samples
expressed the protein while downregulation of LIMA1 in ovarian
cancer cells resulted in increased growth, invasion, adhesion and
migration in vitro (23).
Notably, LIMA1 was identified as a target of the p53 family and the
downregulation of the gene can be a result of p53 mutation
resulting in decreased survival of cancer patients (22).
Importin subunit α-1 (KPNA2) was found to be
significantly upregulated in cervical cancer cell lines when
compared to the normal keratinocytes in our proteomic study. KPNA2,
which is involved in nucleocytoplasmic trafficking, has prognostic
potential in various types of cancer. Specifically, increased
expression of KPNA2 has been associated with poor prognosis in
astrocytic gliomas (24), gastric
adenocarcinoma (25), prostate cancer
(26), cholangiocarcinoma (27) and colorectal cancer (28). Furthermore, in the most recent
studies, KPNA2 was suggested to be a predictive marker of
gemcitabine sensitivity and survival in cholangiocarcinoma
(27) and a predictor of survival
following radical surgery for colorectal cancer (28).
Serum paraoxonase/arylesterase 2 (PON2) is a
ubiquitously expressed cellular antioxidant and was found to be
upregulated in cervical cancer in all three of our comparisons, by
both proteomics and western blot analysis. PON2 has been reported
to play a protective role in oral squamous cell cancer cells
against irradiation-induced apoptosis (29) and higher levels of the protein are
associated with a higher relapse rate in oral squamous cell cancer
patients after either surgery, radiotherapy or chemotherapy
(30). Importantly, the regulation of
PON2-induced therapy resistance is regulated through the
Wnt/glycogen synthase kinase 3β (GSK3β)/β-catenin pathway (30). Increased expression of PON2 has also
been detected in bladder cancer tissues compared to that in normal
tissues, and induced overexpression of the gene in bladder cancer
cells led to higher cell proliferation and oxidative stress
resistance (31). However, a recent
study showed that PON2 can be a tumor suppressor as well (32). Particularly, PON2 expression was
elevated in early stages of ovarian cancer compared to normal
tissue but not in late stages of the disease. In addition, induced
overexpression of PON2 in a mouse xenograft model of ovarian cancer
resulted in reduced cell proliferation (32). Although the findings concerning the
role of PON2 in cancers of different origin vary, it could
certainly be a promising drug target and further investigation is
warranted.
Caprin-1 (CAPRIN1) was also found to be upregulated
in our analysis in cervical cancer cell lines when compared to the
expression level in normal cervical keratinocytes. Caprin-1 protein
is considered to enhance osteosarcoma tumor growth and lung
metastasis in mice via the Akt and ERK1/2 pathways (33). CAPRIN1 was also found to be
overexpressed in breast cancer cells compared to normal breast
cells, and induced overexpression of the gene promoted cell
proliferation and invasion while this phenotype was reversed by
miRNA-223 (34). Finally, based on
immunohistochemical data, CAPRIN1 expression was upregulated in
hepatocellular carcinoma compared to peritumoral tissue and was
significantly associated with worse hepatocellular carcinoma
patient survival (35).
The high resolution proteomic analysis performed in
this study, comparing three different cervical cancer cell lines to
normal cervical keratinocytes, provides a comprehensive dataset of
proteins deregulated in cervical cancer. The generated data provide
new insights into the molecular mechanisms and key regulators of
cervical carcinogenesis and can be the basis for further
investigations and system biology approaches. In particular, the
indicated proteins with potential critical involvement in cervical
malignancy can be used for clinical validation studies and
functional analyses. Overall, our research contributes to a better
understanding of the underlying mechanisms important for cervical
cancer and opens the way for biomarker discovery and drug target
identification.
Acknowledgements
The authors wish to thank Dr Tohru Kiyono (National
Cancer Centre Research Institute, Tokyo, Japan) for his generous
gift of the HCK1T normal cervical cell line.
Funding
This study was funded by the European Union's
European Social Fund (ESF) and Greek National Funds through the
Program THALIS, under the Operational Program Education and
Lifelong Learning of the National Strategic Reference Framework
(NSRF), Grant no. 70-3-11830 to Kalliopi I. Pappa, and by the
Oncology Program of the Central Council of Health of the Ministry
of Health, Grant no. 70-3-9209 to Nicholas P. Anagnou.
Availability of data and materials
The datasets generated and analyzed during the
current study are available in the Figshare repository, doi:
10.6084/m9.figshare.7121540.
Authors' contributions
KIP, JZ and NPA participated in the acquisition of
funding and designed and supervised the study. KIP and VL
interpreted the results and drafted the manuscript. VL performed
the experiments and carried out the statistical analyses. GK
performed the cell culture experiments. KV and MM performed the
mass spectrometry analyses. AS and GD contributed to the
interpretation of the data and drafting of the results. All authors
read and approved the final manuscript and agree to be accountable
for all aspects of the research in ensuring that the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Woodman CB, Collins SI and Young LS: The
natural history of cervical HPV infection: Unresolved issues. Nat
Rev Cancer. 7:11–22. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dewar MA, Hall K and Perchalski J:
Cervical cancer screening. Past success and future challenge. Prim
Care. 19:589–606. 1992.PubMed/NCBI
|
|
4
|
Pimple S, Mishra G and Shastri S: Global
strategies for cervical cancer prevention. Curr Opin Obstet
Gynecol. 28:4–10. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lawrenz B, Mahajan N and Fatemi HM: The
effects of cancer therapy on women's fertility: What do we know
now? Future Oncol. 12:1721–1729. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bentivegna E, Maulard A, Pautier P,
Chargari C, Gouy S and Morice P: Fertility results and pregnancy
outcomes after conservative treatment of cervical cancer: A
systematic review of the literature. Fertil Steril. 106:1195–1211,
e1195. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barra F, Lorusso D, Leone Roberti Maggiore
U, Ditto A, Bogani G, Raspagliesi F and Ferrero S: Investigational
drugs for the treatment of cervical cancer. Expert Opin Investig
Drugs. 26:389–402. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kontostathi G, Zoidakis J, Anagnou NP,
Pappa KI, Vlahou A and Makridakis M: Proteomics approaches in
cervical cancer: Focus on the discovery of biomarkers for diagnosis
and drug treatment monitoring. Expert Rev Proteomics. 13:731–745.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pappa KI, Kontostathi G, Makridakis M,
Lygirou V, Zoidakis J, Daskalakis G and Anagnou NP: High resolution
proteomic analysis of the cervical cancer cell lines secretome
documents deregulation of multiple proteases. Cancer Genomics
Proteomics. 14:507–521. 2017.PubMed/NCBI
|
|
10
|
Pappa KI, Christou P, Xholi A, Mermelekas
G, Kontostathi G, Lygirou V, Makridakis M, Zoidakis J and Anagnou
NP: Membrane proteomics of cervical cancer cell lines reveal
insights on the process of cervical carcinogenesis. Int J Oncol.
53:2111–2122. 2018.PubMed/NCBI
|
|
11
|
Makridakis M, Gagos S, Petrolekas A,
Roubelakis MG, Bitsika V, Stravodimos K, Pavlakis K, Anagnou NP,
Coleman J and Vlahou A: Chromosomal and proteome analysis of a new
T24-based cell line model for aggressive bladder cancer.
Proteomics. 9:287–298. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Narisawa-Saito M, Handa K, Yugawa T, Ohno
S, Fujita M and Kiyono T: HPV16 E6-mediated stabilization of ErbB2
in neoplastic transformation of human cervical keratinocytes.
Oncogene. 26:2988–2996. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yugawa T, Handa K, Narisawa-Saito M, Ohno
S, Fujita M and Kiyono T: Regulation of Notch1 gene expression by
p53 in epithelial cells. Mol Cell Biol. 27:3732–3742. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Makridakis M and Vlahou A: GeLC-MS: A
sample preparation method for proteomics analysis of minimal amount
of tissue. Methods Mol Biol. 1788:165–175. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Filip S, Vougas K, Zoidakis J, Latosinska
A, Mullen W, Spasovski G, Mischak H, Vlahou A and Jankowski J:
Comparison of depletion strategies for the enrichment of
low-abundance proteins in urine. PLoS One. 10:e01337732015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Babicki S, Arndt D, Marcu A, Liang Y,
Grant JR, Maciejewski A and Wishart DS: Heatmapper: Web-enabled
heat mapping for all. Nucleic Acids Res. 44:W147–W153. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bindea G, Mlecnik B, Hackl H, Charoentong
P, Tosolini M, Kirilovsky A, Fridman WH, Pagès F, Trajanoski Z and
Galon J: ClueGO: A cytoscape plug-in to decipher functionally
grouped gene ontology and pathway annotation networks.
Bioinformatics. 25:1091–1093. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Giordano G, Cole TB, Furlong CE and Costa
LG: Paraoxonase 2 (PON2) in the mouse central nervous system: A
neuroprotective role? Toxicol Appl Pharmacol. 256:369–378. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Collins RJ, Jiang WG, Hargest R, Mason MD
and Sanders AJ: EPLIN: A fundamental actin regulator in cancer
metastasis? Cancer Metastasis Rev. 34:753–764. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ohashi T, Idogawa M, Sasaki Y and Tokino
T: p53 mediates the suppression of cancer cell invasion by inducing
LIMA1/EPLIN. Cancer Lett. 390:58–66. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu R, Martin TA, Jordan NJ, Ruge F, Ye L
and Jiang WG: Epithelial protein lost in neoplasm-α (EPLIN-α) is a
potential prognostic marker for the progression of epithelial
ovarian cancer. Int J Oncol. 48:2488–2496. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gousias K, Becker AJ, Simon M and
Niehusmann P: Nuclear karyopherin a2: A novel biomarker for
infiltrative astrocytomas. J Neurooncol. 109:545–553. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li C, Ji L, Ding ZY, Zhang QD and Huang
GR: Overexpression of KPNA2 correlates with poor prognosis in
patients with gastric adenocarcinoma. Tumour Biol. 34:1021–1026.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Grupp K, Habermann M, Sirma H, Simon R,
Steurer S, Hube-Magg C, Prien K, Burkhardt L, Jedrzejewska K,
Salomon G, et al: High nuclear karyopherin α2 expression is a
strong and independent predictor of biochemical recurrence in
prostate cancer patients treated by radical prostatectomy. Mod
Pathol. 27:96–106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tsukagoshi M, Araki K, Yokobori T, Altan
B, Suzuki H, Kubo N, Watanabe A, Ishii N, Hosouchi Y, Nishiyama M,
et al: Overexpression of karyopherin-α2 in cholangiocarcinoma
correlates with poor prognosis and gemcitabine sensitivity via
nuclear translocation of DNA repair proteins. Oncotarget.
8:42159–42172. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yu L, Wang G, Zhang Q, Gao L, Huang R,
Chen Y, Tang Q, Liu J, Liu C, Wang H and Wang X: Karyopherin alpha
2 expression is a novel diagnostic and prognostic factor for
colorectal cancer. Oncol Lett. 13:1194–1200. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Krüger M, Pabst AM, Al-Nawas B, Horke S
and Moergel M: Paraoxonase-2 (PON2) protects oral squamous cell
cancer cells against irradiation-induced apoptosis. J Cancer Res
Clin Oncol. 141:1757–1766. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Krüger M, Amort J, Wilgenbus P,
Helmstädter JP, Grechowa I, Ebert J, Tenzer S, Moergel M, Witte I
and Horke S: The anti-apoptotic PON2 protein is
Wnt/β-catenin-regulated and correlates with radiotherapy resistance
in OSCC patients. Oncotarget. 7:51082–51095. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bacchetti T, Sartini D, Pozzi V,
Cacciamani T, Ferretti G and Emanuelli M: Exploring the role of
paraoxonase-2 in bladder cancer: Analyses performed on tissue
samples, urines and cell cultures. Oncotarget. 8:28785–28795. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Devarajan A, Su F, Grijalva V, Yalamanchi
M, Yalamanchi A, Gao F, Trost H, Nwokedi J, Farias-Eisner G,
Farias-Eisner R, et al: Paraoxonase 2 overexpression inhibits tumor
development in a mouse model of ovarian cancer. Cell Death Dis.
9:3922018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sabile AA, Arlt MJ, Muff R, Husmann K,
Hess D, Bertz J, Langsam B, Aemisegger C, Ziegler U, Born W and
Fuchs B: Caprin-1, a novel Cyr61-interacting protein, promotes
osteosarcoma tumor growth and lung metastasis in mice. Biochim
Biophys Acta. 1832:1173–1182. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gong B, Hu H, Chen J, Cao S, Yu J, Xue J,
Chen F, Cai Y, He H and Zhang L: Caprin-1 is a novel microRNA-223
target for regulating the proliferation and invasion of human
breast cancer cells. Biomed Pharmacother. 67:629–636. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tan N, Dai L, Liu X, Pan G, Chen H, Huang
J and Xu Q: Upregulation of caprin1 expression is associated with
poor prognosis in hepatocellular carcinoma. Pathol Res Pract.
213:1563–1567. 2017. View Article : Google Scholar : PubMed/NCBI
|