Introduction
Osteosarcoma (OS) forms 2.4% of fatal cancer in
children. It accounts for 20% of all primary bone cancers and is a
serious malignancy in pediatric patients (1). The 5-year survival rate is only 50–70%
in osteosarcoma patients due to the lack of effective treatment
options currently (2). OS is a
primary tumor of bone and was reported to derive from malignant
mesenchymal stem cells (3,4). The tumor usually develops in the
metaphysis of the long bones; thus, the proximal tibia, the
proximal humerus and the distal femur are high risk area of tumor
development (5,6). At present, chemotherapy is the first
choice of treatments besides surgical treatment. Chemotherapeutic
drugs, such as cisplatin, ifosfamide and high-dose methotrexate may
result in acquired drug resistance in OS cells (7). Therefore, there is an urgent need for
the development of novel effective therapeutic drugs for the
treatment of this disease (8,9).
For patients who are not candidates for surgery,
immunotherapy is a promising therapeutic option (9,10).
Expansion of autologous tumor-specific effector cells ex
vivo prior to infusion into the host serves an important role
in adoptive cell immunotherapy (11).
CIK cells, lymphokine-activated killer cells, tumor-infiltrating
lymphocytes, cytotoxic T lymphocyte cells and natural killer (NK)
cells are candidate immunological effector cells for treating
cancer, or combined targets following surgery (12,13). There
have been an increasing number of encouraging clinical results in
breast (14), liver (15), and digestive tract (16) cancers by adopting NK cells in
treatment. Thus, treating OS via NK cells has drawn increasing
attention in the field of cancer immunotherapy. Unlike T cells, NK
cells are innate immune lymphocytes, which kill or lyse malignant
cells by producing cytokines and chemokines, independent of antigen
presentation (17,18). Similar to interleukin (IL)-12, IL-15
and IL-18, IL-17 enhances the susceptibility of U2OS osteosarcoma
cells to NK cell lysis (19). NK cell
therapy combined with aerosol IL-2 decreased OS lung metastasis
tumor burden in a mouse model (20,21);
however, the expression of certain inhibitory molecules of tumor
cells negatively regulate NK cell function (22).
With the development of immune checkpoint inhibitors
for the treatment of malignant tumors, cancer immunotherapy has
become an increasingly popular treatment modality in cancer
therapy. Programmed death ligand-1 (PD-L1), a popular immune
checkpoint, serves an important role in escaping tumor
immunosurveillance (23,24). PD-1, a receptor of PD-L1, is expressed
on immune cells, including NK cells, and interacts with tumor
cells, leading to cell apoptosis, anergy or tolerance (25). A PD-L1 antibody therapy clinical trial
(clinical trial no. 2017L04642) was permitted in China after
anti-PD-1 antibodies were approved by the US FDA for the treatment
of melanoma (26,27). In addition, studies have reported that
the tumor response to PD-L1 or PD-1 inhibition is directly
associated with the expression levels of PD-L1 and lymphocytic
infiltration of the tumor (28–30).
Previous studies have begun to examine the role of PD-L1 in OS.
Utilizing reverse transcription-quantitative polymerase chain
reaction (RT-qPCR), PD-L1 mRNA expression was determined to be
associated with lymphocyte infiltration (31). PD-L1 blockade in a mouse model of OS
revealed the initial regression of the tumor followed by growth of
PD-L1 antibody-resistant clones (32). PD-L1 expression was significantly
linked to poor 5-year survival rates (33). The present study aimed to investigate
the function and mechanism of NK cells on the human OS cells.
Whether the difference in the expression ratio of PD-L1 on
distinctive OS cell lines result in the different outcomes was also
explored. Our findings may suggest the potential of anti-PD-L1
combined with NK cell treatment in high PD-L1 expression-OS
cancer.
Materials and methods
Cell isolation and purification
Peripheral blood mononuclear cells (PBMCs) were
freshly isolated from peripheral blood of healthy individuals
(three donors were all males, who were 28 years old at March 03,
2018, 24 years old at July 02, 2018 and 31 years old at September
07, 2018) via Ficoll density gradient separation at the speed of
1,174 × g (raising speed at 9, descent speed at 1) for 30 min at
room temperature. The study was approved by the Ethics Committee of
Changchun Blood Center; written informed consent was obtained from
all patients. NK cells were enriched through negative selection
from outflow cells with an NK cell isolation kit according to the
manufacturer's protocols (Miltenyi Biotec). The purity of the
purified cells was ≥95% as determined by flow cytometry.
Tumor cell lines
MG-63, HOS and U2OS human OS cell lines, as well as
MDA-MB-231 and MCF7 breast cancer cell lines were purchased from
the American Type Culture Collection. The MDA-MB-231 breast cancer
cell line expresses high levels of PD-L1, whereas that of the MCF7
cell is negligible; thus, these two cells served as the positive
and negative controls respectively. The cells were cultured in a
96-well plate containing 200 µl complete medium (Dulbecco's
modified Eagle's medium), 10% fetal calf serum, penicillin (100
U/ml), streptomycin (0.1 mg/ml) and glutamine (Invitrogen; Thermo
Fisher Scientific, Inc.) at a density of 5×104
cells/well, at 37°C.
Cellular cytotoxicity assay
NK cell-mediated cellular cytotoxicity was
determined using a non-radioactive cellular cytotoxicity assay kit
(Techno Suzuta). Tumor cells (5×104) in a round bottom
96-well plate were challenged by 100 µl of NK cells at
effector-to-target ratios of 0:1, 0.2:1, 1.0:1, 5.0:1 and 25.0:1
for 40 min at 37°C with 5% CO2. The detailed procedure
was performed as described previously (34).
Flow cytometry
Annexin V-fluorescein isothiocyanate (FITC) and
propidium iodide (PI) double staining flow cytometry analyses were
employed to assess cell apoptosis. MG-63, HOS and U2OS cells were
collected on the experimental endpoint. Cell apoptosis was analyzed
using a flow cytometer (FACScan; BD Biosciences) with FlowJo 7.6
FACS analysis software (FlowJo LLC). The cells in the different
portions represented the different cell states as follows: The
late-apoptotic cells were present in the upper right portion, the
viable cells were present in the lower left portion, and the early
apoptotic were cells present in the lower right portion. The rate
of apoptosis was obtained from the early apoptotic and the
late-apoptotic cells. Brefeldin A (Sigma-Aldrich; Merck KGaA) was
subsequently added at a final concentration of 5 µg/ml then further
culturing in the 37°C incubator for another 4 h. NK cells were
stained for surface expression of CD107a, and intracellular
expression of granzyme B and interferon (IFN)-γ. To investigate the
involvement of selected molecules, blocking experiments were
performed by adding the following monoclonal antibodies (mAbs):
α-PD-L1 (cat. no. 9049-B7), α-IFN-γ (cat. no. AF-285-SP) and
α-granzyme B (cat. no. MAB2906-SP). Control experiments were
performed using isotype matched mouse antibodies (all mAbs from
R&D Systems). All mAbs were used at a final concentration of 10
µg/ml. The antibodies were added to the cell culture plates and
incubated at 37°C at the beginning of the experiment.
RT-qPCR
Total cellular RNA was isolated using TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA was
reversed transcribed into cDNA by a reverse transcription kit at
42°C for 30 min and 85°C for 5 min (Beijing Transgen Co., Ltd.).
qPCR was conducted using the Fast Start Universal SYBR®
Green Master (ROX) kit (Roche Diagnostics). Reactions were
performed using 3 µl of cDNA in a 20 µl reaction volume and the
following thermal cycles profile: 10 sec for pre-denaturation at
94°C, 5 sec for denaturation at 94°C, and 30 sec for extension at
60°C, for 40 cycles. RT-qPCR was performed using the Power SYBR
Green Master Mix (Takara Bio, Inc.) and an ABI 7300 Real-Time PCR
system (Applied Biosystems, Thermo Fisher Scientific, Inc.) The
primer sequences for PD-L1 were as follows: sense,
5′-TTCCCAGTCCAAACTGAGGAGTCCAAC-3′ and antisense,
5′-TTGTTCGCTACCCGAAACGCTGAG-3′. The GAPDH primers were as follows:
sense, 5′-CCAGGTGGTCTCCTCTGACTT-3′ and antisense,
5′-GTTGCTGTAGCCAAATTCGTTGT-3′.
Western blotting
Cell total protein was extracted using RIPA buffer
(Beyotime Institute of Biotechnology) supplemented with a cocktail
protease inhibitor (Roche Molecular Diagnostics), and the protein
concentration was determined with a BCA kit according to the
manufacturer's protocol. A total of 5–40 µg cell total protein was
separated by 10% SDS-PAGE and then were electrotransferred to
polyvinylidene fluoride membranes (0.45 µm; EMD Millipore) and
blocked at 37°C for 1 h with 5% skim milk in Tris-buffered saline
with Tween-20 (0.1%). Subsequently, membranes were incubated with
monoclonal antibodies against PD-L1 (cat. no. 13684, 1:1,000) and
β-actin (cat. no. 3700, 1:1,000) at 4°C overnight. The membranes
were washed with TBS washing buffer six times, and then incubated
with horseradish peroxidase-conjugated goat anti-mouse (cat. no.
TA130001) or goat anti-rabbit (cat. no. TA130015) secondary
antibodies (1:2,000; OriGene Technologies, Inc.) at 37°C for 1 h.
The detailed procedure was performed as described previously
(33). Protein expression levels were
determined semi-quantitatively by densitometric analysis with the
Quantity One software (V4.62, Bio-Rad Laboratories, Inc.).
Enzyme-linked immunosorbent assay
(ELISA)
The supernatants of the cell cultures were collected
in each experimental condition. Granzyme B (cat. no. DY2906-05,
R&D Systems) and IFN-γ (cat. no. DIF50, R&D Systems)
concentrations were measured by ELISA according to the
manufacturer's instructions.
Statistical analysis
All data and results were calculated from at least
three replicate measurements and are presented as the mean ±
standard deviation. Mean values were compared using one-way ANOVA
multiple comparison tests. P<0.05 was considered to indicate a
statistically significant difference. All statistical tests were
performed with GraphPad Prism software (v5.0, GraphPad Software,
Inc.).
Results
Three human osteosarcoma cell lines
with distinctive susceptibility to NK cells
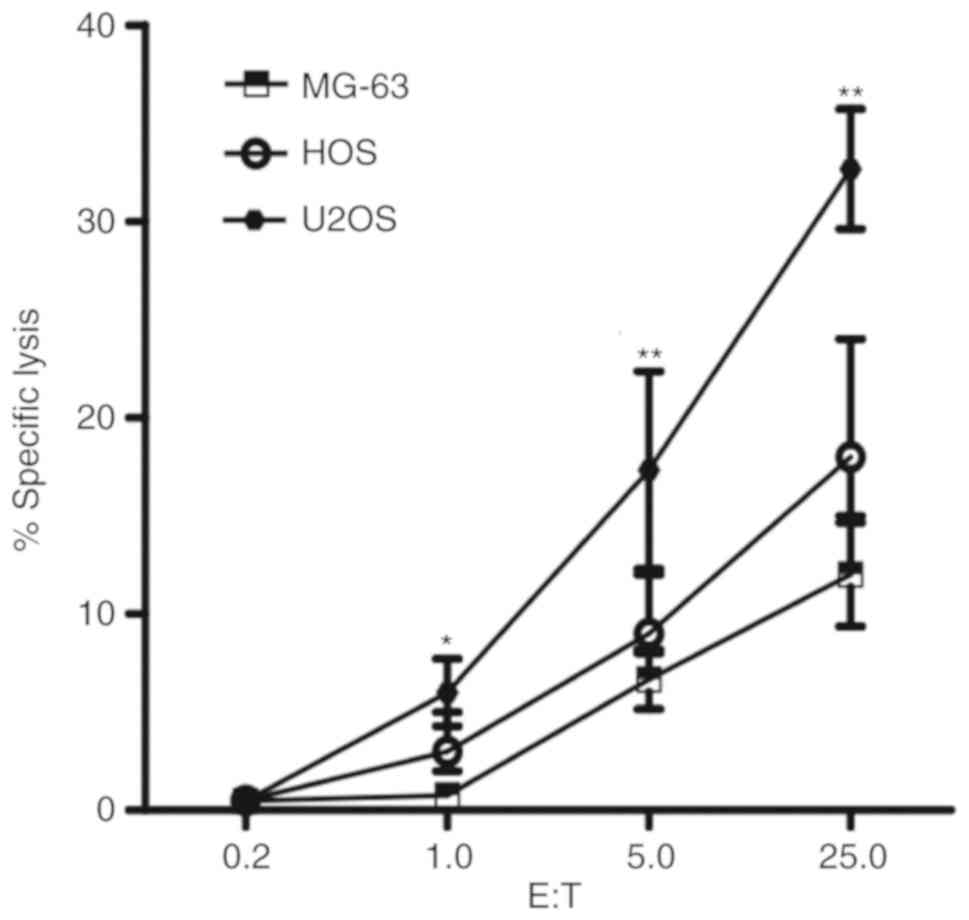
We tested 3 human osteosarcoma cell lines MG-63, HOS
and U2OS, for susceptibility to cytolytic activity of freshly
isolated healthy donor NK cells. All cell lines were lysed by NK
cells in a dose dependent manner (1.0:1, 5.0:1 and 25.0:1). U2OS
cells exhibited significantly increased susceptibility to NK cells
compared with MG-63 and HOS cells at all ratios (P<0.05;
Fig. 1). The susceptibility to the
cytolytic activity of NK cells in MG-63 cells was reduced compared
with the other two cell lines. HOS cells exhibited moderate
cytolysis. The results indicate that different human osteosarcoma
cell lines have distinct susceptibilities to NK cells.
Flow cytometry analysis of human OS
cell apoptosis
Annexin V-FITC and PI double-staining flow cytometry
analyses were performed. The MG-63, HOS and U2OS cells were plated
in 96-well plates containing 200 µl medium at a density of
5×104 cells/well. The induction of apoptosis was
examined with or without NK cells (effector-to-target ratio =
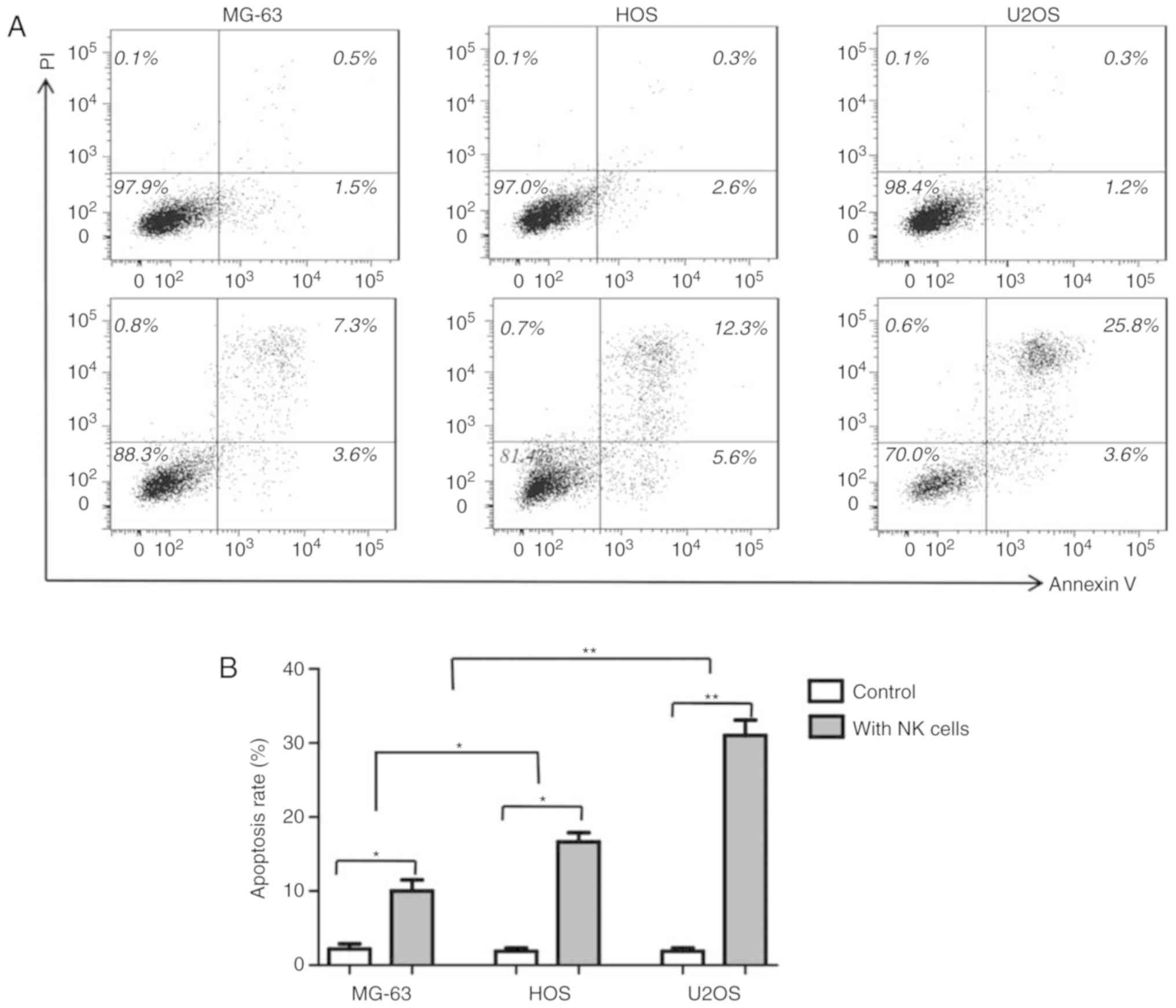
20:1). As presented in Fig. 2, the
number of early- and late-apoptotic cells increased significantly
in the NK cell-treated groups compared with that in the control
group. The apoptotic effects of NK cells on U2OS cells (29.3±3.2%
vs. 1.5±0.45%, P<0.01) and HOS (16.3±2.2% vs. 1.6±0.68%,
P<0.05) cells were significantly increased compared with the
corresponding control cells. Similar to the cellular cytotoxicity
assay results, MG-63 cells exhibited a reduced apoptosis rate than
that of U2OS and HOS cells (P<0.05).
NK cells lyse human OS cells in a
granzyme B-dependent manner
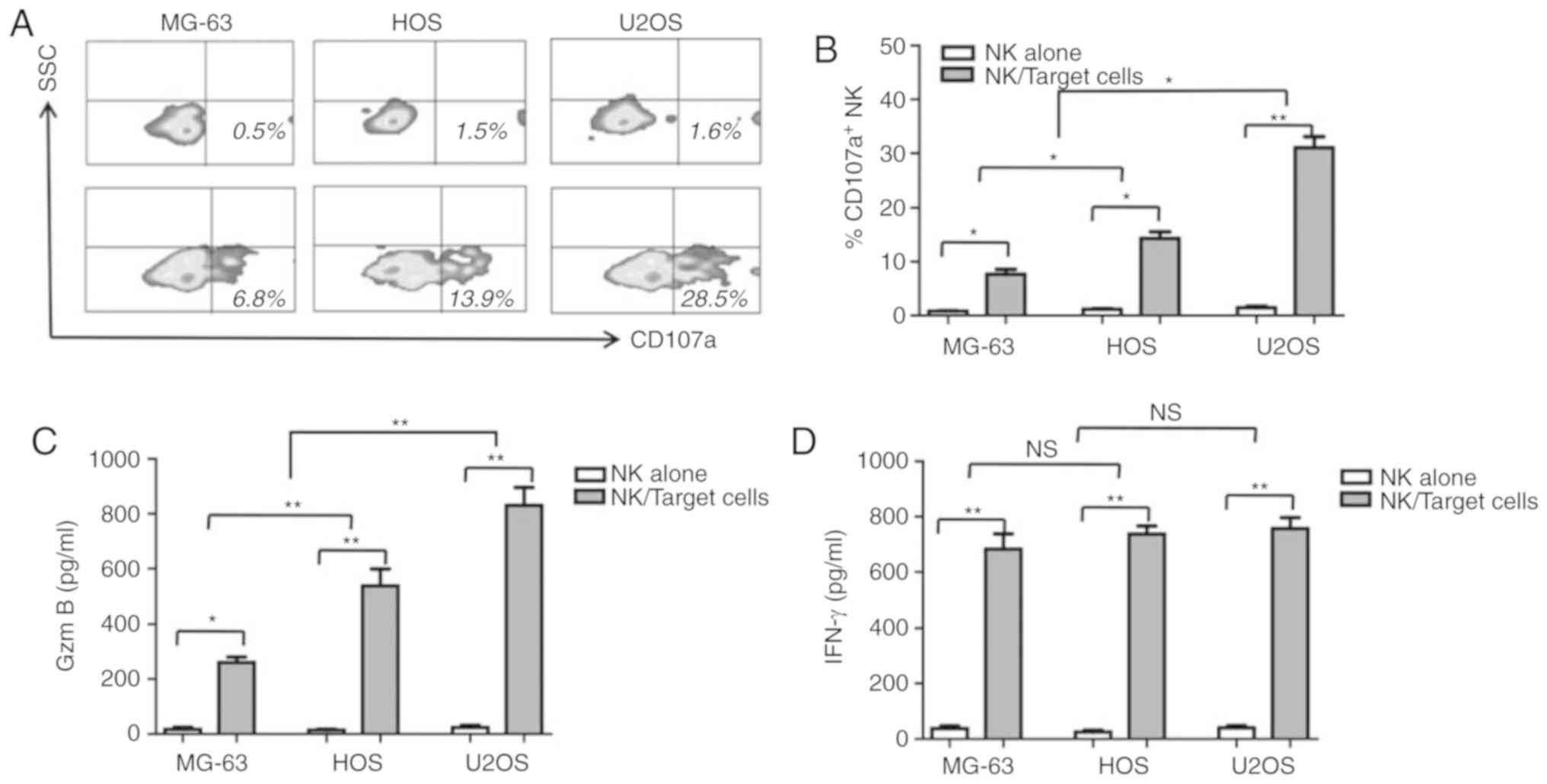
CD107a, a cytotoxicity marker, was determined to be
upregulated in NK cells when cocultured with the human OS cells in
the present study. As shown in Fig. 3A
and B, 28.9±1.3 (P<0.01) and 12.3±2.1% (P<0.05)
CD107a+ NK cells were counted upon coculturing with U2OS
and HOS cells, respectively. Upon culture with MG-63 cells, fewer
CD107a+ NK cells were detected when compared with U2OS
(8.2±1.5% vs. 28.9±1.3%, P<0.01) and HOS cells (8.2±1.5% vs.
12.3±2.1%, P<0.01). Granzyme B and IFN-γ concentrations in the
cell culture supernatant were measured by ELISA. As shown in
Fig. 3C and D, consistent with CD107a
expression, granzyme B and IFN-γ secretion levels were elevated in
response to NK and OD coculturing. Unlike granzyme B and CD107a,
IFN-γ secretion in all three human OS cells was comparable
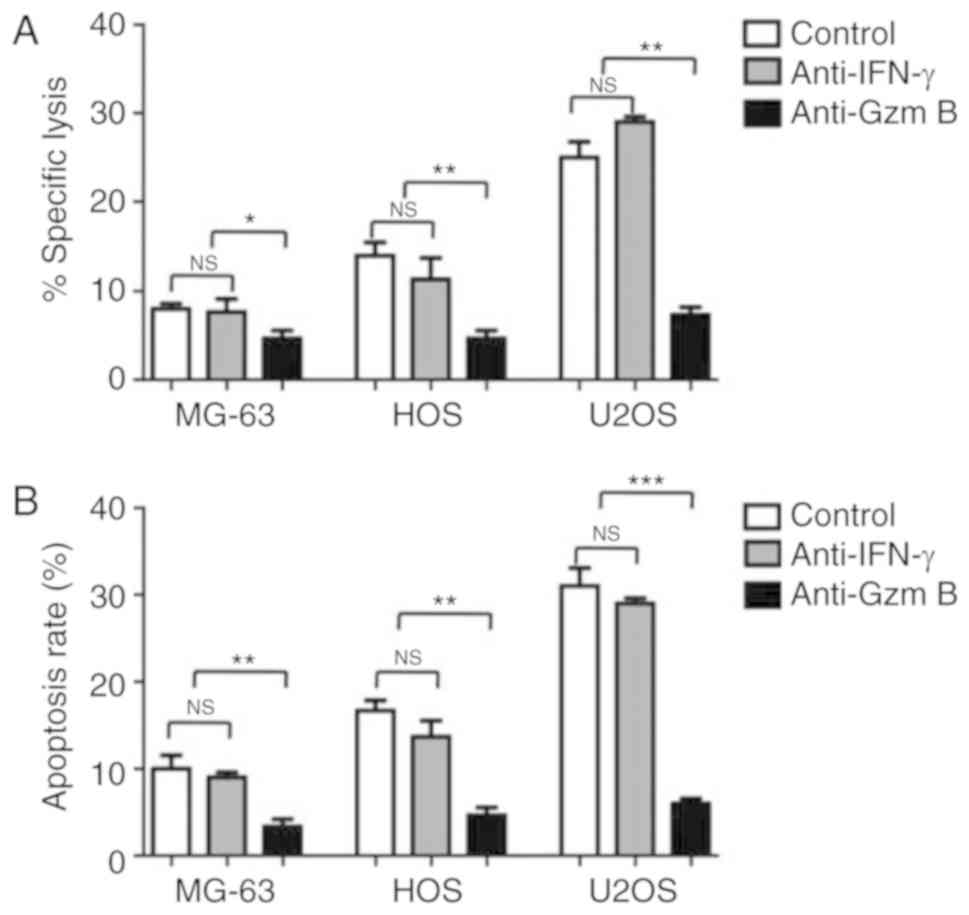
(P>0.05). To determine whether granzyme B may be the key factor
in the cytotoxic effects of NK cells on human OS cells, granzyme B
and IFN-γ antibodies were added to the NK cell/human OS cell
coculture systems. As presented in Fig.
4A, the cytolytic activity of NK cells on MG-63, HOS and U2OS
cells was significantly reduced when granzyme B was blocked
compared with the control (P<0.05). In contrast, when the IFN-γ
antibody was added, no differences in the cytolytic activity of NK
cells on human OS cells were observed (P>0.05). The detection of
cell apoptosis by FACS revealed supporting findings in which the
inhibition of granzyme B led to the significant reduction in
apoptosis compared with the control; significant differences were
not observed between the IFN-γ-inhibited and control groups
(Fig. 4B). These results suggested
that NK cells lyse human OS cells in a granzyme B-dependent
manner.
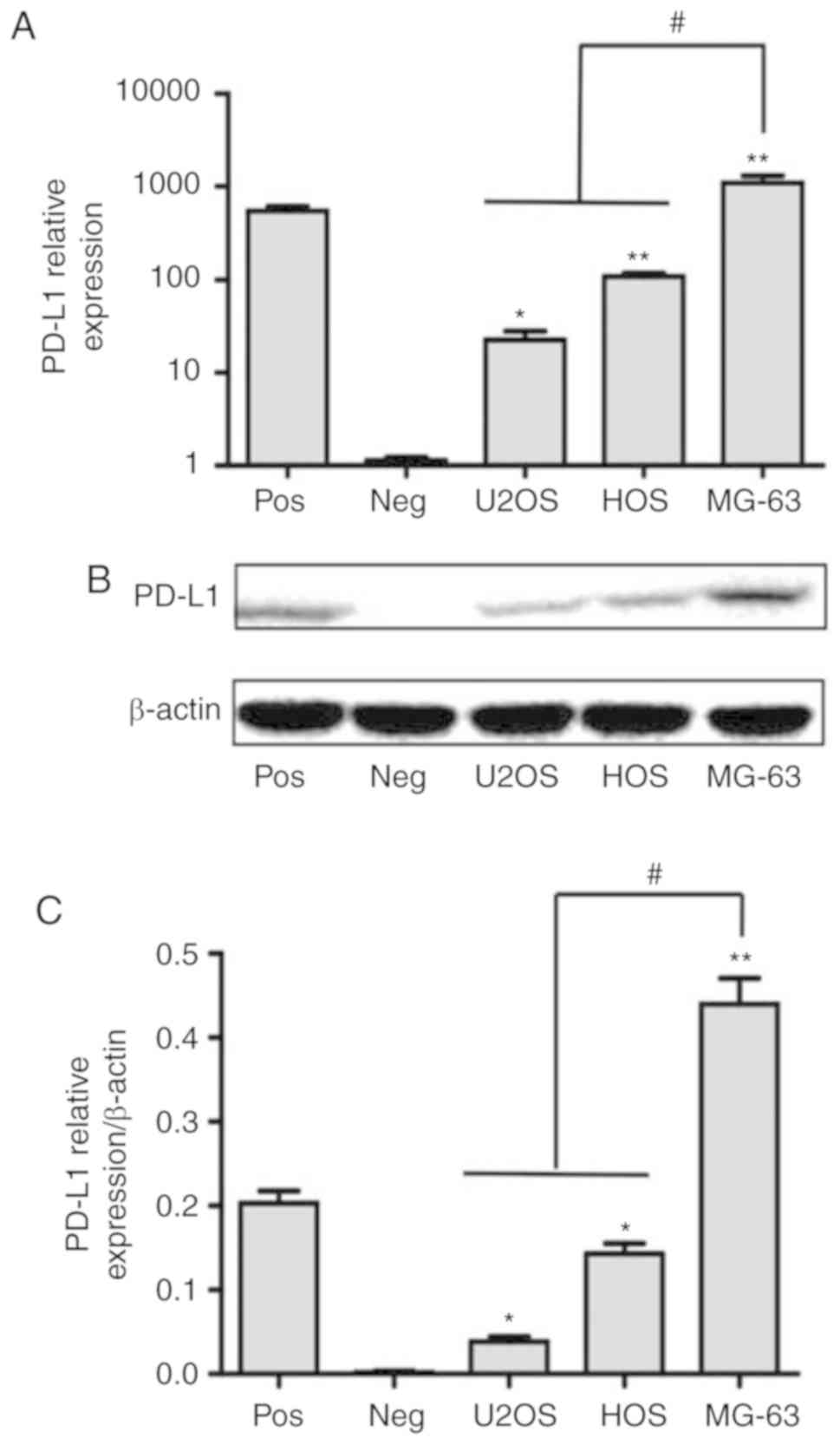
Expression of PD-L1 in MG-63 cell is
greater than that of U2OS and HOS cells
Tumor cells express PD-L1 to inhibit NK cell
cytolytic activity as NK cells express PD-1 (22). In the present study, the expression of
PD-L1 on MG-63, HOS and U2OS cells was investigated. PD-L1
expression was quantified by RT-qPCR and western blotting. The
relative expression of the PD-L1 in MG-63 cell was significantly
greater than that in the HOS and U2OS cells (P<0.01; Fig. 5A). Western blotting revealed that the
protein expression of PD-L1 in MG-63 cells was ~3-5 folds greater
than that in HOS and U2OS cells (P<0.05; Fig. 5B and C).
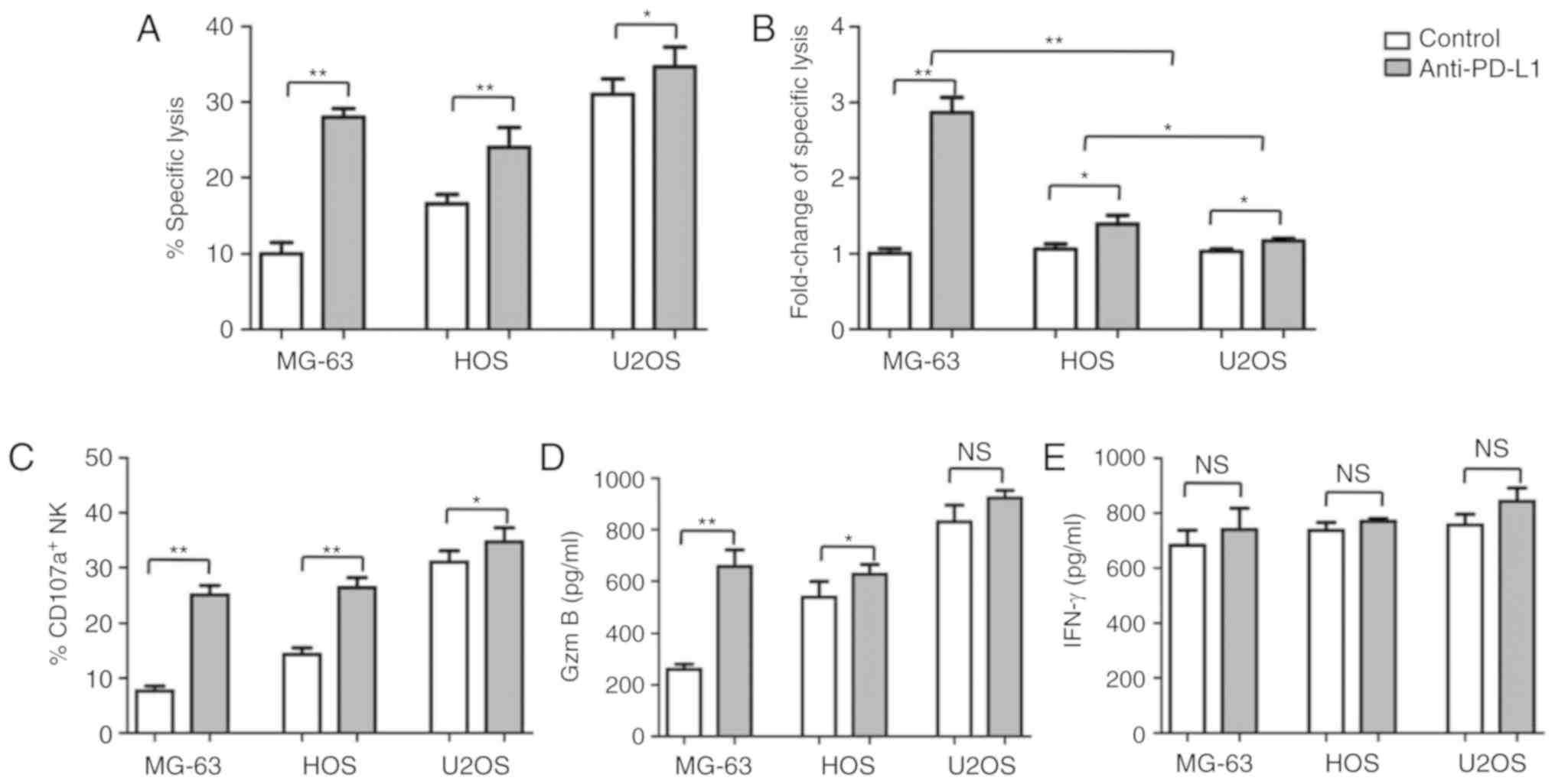
PD-L1 blockage enhances the
cytotoxicity of NK cells in human OS cells
To investigate the pivotal role of PD-L1/PD-1
checkpoint on the cytotoxicity of NK cells in human OS cells, PD-L1
antibody was added to the NK cell/human OS cell coculture systems.
The specific lysis induced by NK cells of human OS cells was
significantly enhanced when PD-L1/PD-1 blocked by PD-L1 antibody,
compared with the control (P<0.05; Fig. 6A). Interestingly, the fold-change in
the specific lysis of MG-63 cells was significantly increased than
that of HOS and U2OS cells (P<0.01; Fig. 6B). This may be due to the notably
upregulated expression of PD-L1 MG-63 cells than in the other two
cell lines. CD107a expression (P<0.05; Fig. 6C) secretion of NK cells was also
enhanced with the coculture with all three cell lines; significant
increases in granzyme B secretion was observed in MG-63 and HOS
cells compared with the control (P<0.05; Fig. 6D). On the contrary, IFN-γ secretion
from NK cells in all coculture systems did not significantly change
in the presence or absence of PD-L1 antibody (P>0.05; Fig. 6E).
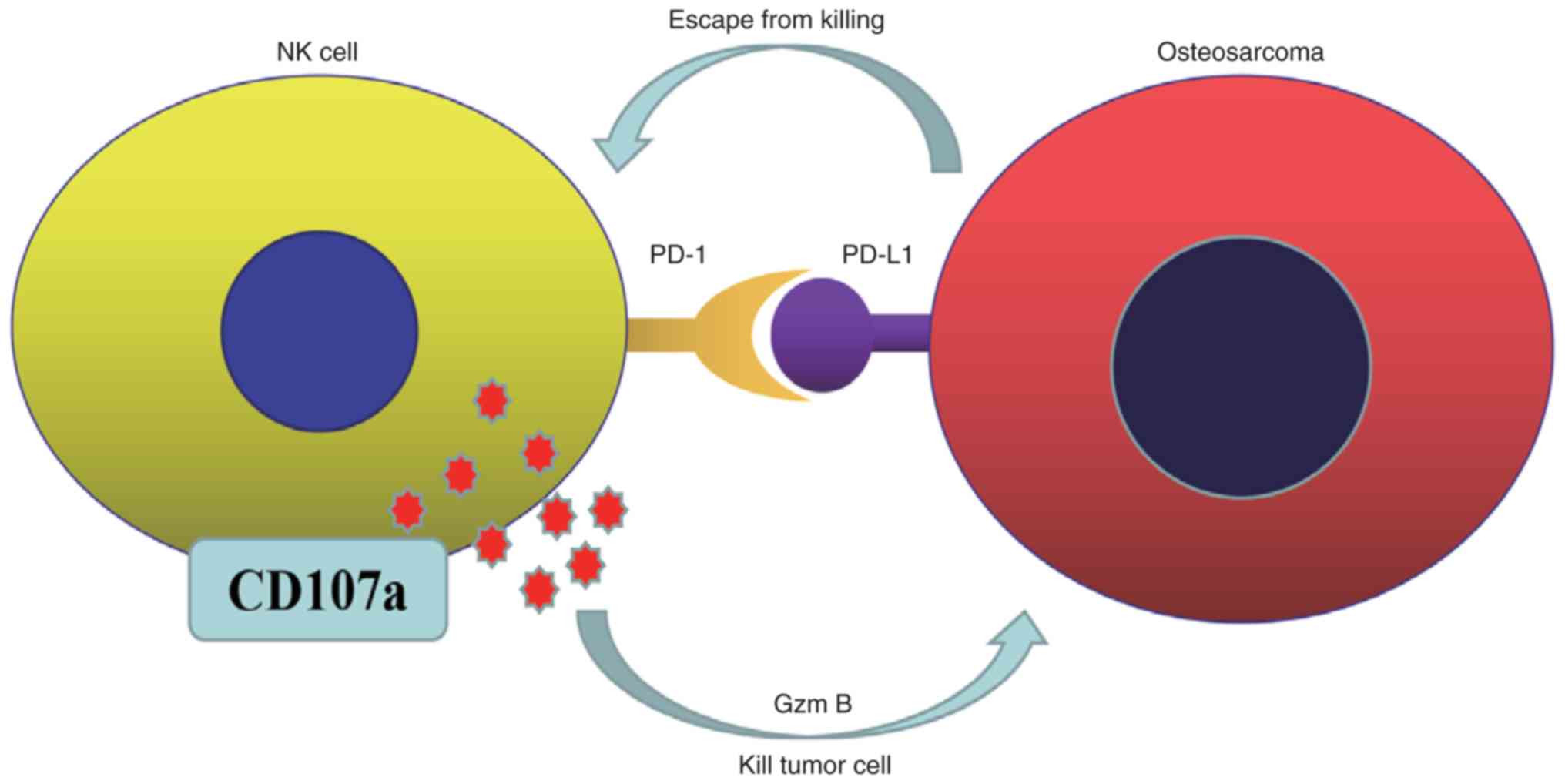
These results indicated that PD-L1 expression in
human OS cell lines plays an important role in the susceptibility
to NK cells. The PD-L1/PD-1 interaction was proposed to serve an
important role in the evasion of tumor immunosurveillance (Fig. 7).
Discussion
The association between the host immune system, the
type of cancer and cancer treatment applied is extremely complex
(29,30). Immunotherapies that include PD-1/PD-L1
blockade have shown prolonged clinical activity against various
human malignancies excluding OS (31), despite the fact that there is evidence
that PD-L1 contributes to OS progression in animal experiments
(32). As the main components of the
innate immune system, NK cells can kill tumor cells or infected
cells directly, independent of antigen presenting cells.
CD56dimCD16bright NK cells (~90%) and
CD56brightCD16dim NK cells (~10%) are two
subsets of NK cells in healthy adult peripheral blood (35). CD56dimCD16bright
NK cells exert mainly cytolytic functions by secreting granzyme B
and perforin, whereas CD56brightCD16dim NK
cells exert primarily immune regulatory functions by secreting
cytokines (33). Cells lacking major
histocompatibility (MHC) molecules can activate NK cells by
interacting with activating receptors on the cell surface,
including NKp30, NKp40, NKG2D and NKp46 (36). Tumor cells are more susceptible to NK
cells due to the lack of MHC class-I molecules (34). In this study, we tested three human OS
cell lines MG-63, HOS and U2OS for susceptibility to cytolytic
activity of freshly isolated healthy donor NK cells. All cell lines
were lysed by NK cells in a dose-dependent manner. MG-63 cells
exhibited reduced susceptibility to NK cells than HOS and U2OS
cells at all ratios. This finding indicated that human OS cells,
similar to the other malignant cells, such as non-small cell lung
cancer and melanoma cells, are more susceptible to NK cells;
however, variations in the expression of certain inhibitory
molecules in different cell lines and patients may account for
differences in analysis.
PD-L1, a systemic immune checkpoint, plays an
important role in escaping tumor immunosurveillance. PD-1, as a
receptor of PD-L1, is expressed on immune cells including NK cells,
and interacts with tumor cells, leading to cell apoptosis, anergy
or tolerance. PD-L1 expression appears to be conserved across a
number of solid tumors and hematologic malignancies (37). The PD-L1 protein is expressed in a
variety of cancers, such as melanoma, non-small cell lung cancer,
lymphomas and osteosarcoma (38). The
detection of PD-L1 protein expression by IHC in the tumors of
patients is a predictor of responses to both anti-PD-L1 and
anti-PD-1 therapy in a variety of cancers (29,30). The
relative gene and protein expression levels of PD-L1 in MG-63 cell
was greater than those in HOS and U2OS cells. PD-L1 antibody was
added to the NK cell/human OS cell coculture system. The specific
lysis of NK cells in human OS cells was enhanced when PD-L1/PD-1
was blocked by the PD-L1 antibody.
To further address the specific mechanism of NK
cells in human OS cells, CD107a, granzyme B and IFN-γ secretion was
detected. When cocultured with human OS cells, CD107a was
significantly expressed. Granzyme B may be the key factor of NK
cell-induced cytotoxicity of human OS cells. To prove this
hypothesis, granzyme B and IFN-γ antibodies were added to the NK
cell/human OS cell coculture system. The cytolytic activity of NK
cells in human OS cells was moderated when granzyme B was blocked.
On the contrary, in the presence or absence of IFN-γ antibody, no
difference in the cytolytic activity of NK cells on human OS cells
was observed. These results suggested that NK cells lyse human OS
cells in a granzyme B-dependent manner.
For patients who are not candidates for surgery,
immunotherapy could be a promising therapeutic option for treating
those with advanced cancer (39). The
expansion of the autologous tumor-specific effector cells ex
vivo prior to infusion into the host serves an important role
in adoptive cell immunotherapy (40).
The clinical success of cancer immunotherapies targeting T-cell
immune checkpoint receptors PD-1/PD-L1 has demonstrated the
importance of immunoevasion as a hallmark of cancer (41). Similar to T cells, NK cell also can
kill tumor cells directly after PD-1/PD-L1 blockage (42). Increased expression of PD-1 on NK
cells prevents NK cell-mediated anti-tumor function via the
secretion of granzyme B and is correlated with poor prognosis in
digestive cancers (43). It has been
well documented that lactate in the tumor microenvironment induces
immunosuppression, which results in NK cell apoptosis and reduces
the frequency of cell infiltration into these tumors (44). NK cells are potential effector cells
against cancer, including blood malignancies and solid tumors, such
as such as non-small cell lung cancer, melanoma. The numbers of
tumor-infiltrating NK cells was positively correlated with the
outcome (45). Lower NK cell activity
in the body has been associated with an increased risk of cancer
development (46). The immunological
characteristics of cancer cells are also important indicators of
the choice of therapy. Although the our findings indicated that the
PD-L1/PD-1 axis regulates granzyme B secretion, the mechanism
requires further investigation. Our future work will involve
investigation into the underlying mechanisms. In summary, the
present study revealed that the PD-L1/PD-1 axis serves an important
role in the NK cell cytotoxicity in OS via granzyme B secretion.
The decision of whether or not to use anti-PD-L1 and anti-PD-1
therapy depends on the detection of the protein expression of PD-L1
by IHC in the tumors of patients. Our findings may contribute to
development of the precise treatment of human OS.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
MLZ, LC and YJL planned and performed the
experiments, and analyzed the data. DLK made substantial
contributions to the design of the present study, interpreted the
data and obtained funding, and wrote the manuscript. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Changchun Blood Center; written informed consent was obtained from
all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bielack SS, Kempf-Bielack B, Delling G,
Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M,
Winkelmann W, et al: Prognostic factors in high-grade osteosarcoma
of the extremities or trunk: An analysis of 1,702 patients treated
on neoadjuvant cooperative osteosarcoma study group protocols. J
Clin Oncol. 20:776–790. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torres K and Horwitz SB: Mechanisms of
Taxol-induced cell death are concentration dependent. Cancer Res.
58:3620–3626. 1998.PubMed/NCBI
|
|
3
|
Anderson ME: Update on survival in
osteosarcoma. Orthop Clin North Am. 47:283–292. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferrari S and Serra M: An update on
chemotherapy for osteosarcoma. Expert Opin Pharmacother.
16:2727–2736. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jaffe N: Osteosarcoma: Review of the past,
impact on the future. The American experience. Cancer Treat Res.
152:239–262. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gai P, Sun H, Wang G, Xu Q, Qi X, Zhang Z
and Jiang L: miR-22 promotes apoptosis of osteosarcoma cells via
inducing cell cycle arrest. Oncol Lett. 13:2354–2358. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim
J, Xie J, Ikenoue T, Yu J, Li L, et al: Inactivation of YAP
oncoprotein by the Hippo pathway is involved in cell contact
inhibition and tissue growth control. Genes Dev. 21:2747–2761.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Steinhardt AA, Gayyed MF, Klein AP, Dong
J, Maitra A, Pan D, Montgomery EA and Anders RA: Expression of
Yes-associated protein in common solid tumors. Hum Pathol.
39:1582–1589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yin J, Dong Q, Zheng M, Xu X, Zou G, Ma G
and Li K: Antitumor activity of dobutamine on human osteosarcoma
cells. Oncol Lett. 11:3676–3680. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kubo M, Morisaki T, Kuroki H, Tasaki A,
Yamanaka N, Matsumoto K, Nakamura K, Onishi H, Baba E and Katano M:
Combination of adoptive immunotherapy with Herceptin for patients
with HER2-expressing breast cancer. Anticancer Res. 23:4443–4449.
2003.PubMed/NCBI
|
|
11
|
Wu C, Jiang J, Shi L and Xu N: Prospective
study of chemotherapy in combination with cytokine-induced killer
cells in patients suffering from advanced non-small cell lung
cancer. Anticancer Res. 28:3997–4002. 2008.PubMed/NCBI
|
|
12
|
Choi D, Kim TG and Sung YC: The past,
present, and future of adoptive T cell therapy. Immune Netw.
12:139–147. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sangiolo D: Cytokine induced killer cells
as promising immunotherapy for solid tumors. J Cancer. 2:363–368.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Geller MA, Cooley S, Judson PL, Ghebre R,
Carson LF, Argenta PA, Jonson AL, Panoskaltsis-Mortari A,
Curtsinger J, McKenna D, et al: A phase II study of allogeneic
natural killer cell therapy to treat patients with recurrent
ovarian and breast cancer. Cytotherapy. 13:98–107. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun C, Sun HY, Xiao WH, Zhang C and Tian
ZG: Natural killer cell dysfunction in hepatocellular carcinoma and
NK cell-based immunotherapy. Acta Pharmacol Sin. 36:1191–1199.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sakamoto N, Ishikawa T, Kokura S, Okayama
T, Oka K, Ideno M, Sakai F, Kato A, Tanabe M, Enoki T, et al: Phase
I clinical trial of autologous NK cell therapy using novel
expansion method in patients with advanced digestive cancer. J
Transl Med. 13:2772015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao Y, Hu J, Li R, Song J, Kang Y, Liu S
and Zhang D: Enhanced NK cell adoptive antitumor effects against
breast cancer in vitro via blockade of the transforming growth
factor-β signaling pathway. Onco Targets Ther. 8:1553–1559.
2015.PubMed/NCBI
|
|
18
|
Cheng M, Chen Y, Xiao W, Sun R and Tian Z:
NK cell-based immunotherapy for malignant diseases. Cell Mol
Immunol. 10:230–252. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Honorati MC, Neri S, Cattini L and
Facchini A: IL-17 enhances the susceptibility of U-2 OS
osteosarcoma cells to NK cell lysis. Clin Exp Immunol. 133:344–349.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guma SR, Lee DA, Ling Y, Gordon N and
Kleinerman ES: Aerosol interleukin-2 induces natural killer cell
proliferation in the lung and combination therapy improves the
survival of mice with osteosarcoma lung metastasis. Pediatr Blood
Cancer. 61:1362–1368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kiany S, Huang G and Kleinerman ES: Effect
of entinostat on NK cell-mediated cytotoxicity against osteosarcoma
cells and osteosarcoma lung metastasis. Oncoimmunology.
6:e13332142017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Makowska A, Braunschweig T, Denecke B,
Shen L, Baloche V, Busson P and Kontny U: Interferon β and
anti-PD-1/PD-L1 checkpoint blockade cooperate in nk cell-mediated
killing of nasopharyngeal carcinoma cells. Transl Oncol.
12:1237–1256. Jul 8–2019.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carreno BM and Collins M: The B7 family of
ligands and its receptors: New pathways for costimulation and
inhibition of immune responses. Annu Rev Immunol. 20:29–53. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dong H and Chen L: B7-H1 pathway and its
role in the evasion of tumor immunity. J Mol Med (Berl).
81:281–287. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pesce S, Greppi M, Grossi F, Del Zotto G,
Moretta L, Sivori S, Genova C and Marcenaro E: PD/1-PD-Ls
checkpoint: Insight on the potential role of NK cells. Front
Immunol. 10:12422019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
McDermott DF and Atkins MB: PD-1 as a
potential target in cancer therapy. Cancer Med. 2:662–673.
2013.PubMed/NCBI
|
|
27
|
Swaika A, Hammond WA and Joseph RW:
Current state of anti-PD-L1 and anti-PD-1 agents in cancer therapy.
Mol Immunol. 67:4–17. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen BJ, Chapuy B, Ouyang J, Sun HH,
Roemer MG, Xu ML, Yu H, Fletcher CD, Freeman GJ, Shipp MA and Rodig
SJ: PD-L1 expression is characteristic of a subset of aggressive
B-cell lymphomas and virus-associated malignancies. Clin Cancer
Res. 19:3462–3473. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Herbst RS, Soria JC, Kowanetz M, Fine GD,
Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger
SN, et al: Predictive correlates of response to the anti-PD-L1
antibody MPDL3280A in cancer patients. Nature. 515:563–567. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wimberly H, Brown JR, Schalper K, Haack H,
Silver MR, Nixon C, Bossuyt V, Pusztai L, Lannin DR and Rimm DL:
PD-L1 expression correlates with tumor-infltrating lymphocytes and
response to neoadjuvant chemotherapy in breast cancer. Cancer
Immunol Res. 3:326–332. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lussier DM, Johnson JL, Hingorani P and
Blattman JN: Combination immunotherapy with α-CTLA-4 α-PD-L1
antibody blockade and prevent immuneescape and leads to complete
control of metastatic osteosarcoma. J Immunother Cancer. 3:212015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Markel JE, Noore J, Emery EJ, Bobnar HJ,
Kleinerman ES and Lindsey BA: Using the Spleen as an in vivo
systemic immune barometer alongside osteosarcoma disease
progression and Immunotherapy with α-PD-L1. Sarcoma.
2018:86943972018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Koirala P, Roth ME, Gill J, Piperdi S,
Chinai JM, Geller DS, Hoang BH, Park A, Fremed MA, Zang X and
Gorlick R: Immune infiltration and PD-L1 expression in the tumor
microenvironment are prognostic in osteosarcoma. Sci Rep.
6:300932016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Senju H, Kumagai A, Nakamura Y, Yamaguchi
H, Nakatomi K, Fukami S, Shiraishi K, Harada Y, Nakamura M, Okamura
H, et al: Effect of IL-18 on the expansion and phenotype of human
natural killer cells: Application to cancer immunotherapy. Int J
Biol Sci. 14:331–340. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li H, Zhai N, Wang Z, Song H, Yang Y, Cui
A, Li T, Wang G, Niu J, Crispe IN, et al: Regulatory NK cells
mediated between immunosuppressive monocytes and dysfunctional T
cells in chronic HBV infection. Gut. 67:2035–2044. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Choi YH, Lim EJ, Kim SW, Moon YW, Park KS
and An HJ: IL-27 enhances IL-15/IL-18-mediated activation of human
natural killer cells. J Immunother Cancer. 7:1682019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Feng M, Xiong G, Cao Z, Yang G, Zheng S,
Song X, You L, Zheng L, Zhang T and Zhao Y: PD-1/PD-L1 and
immunotherapy for pancreatic cancer. Cancer Lett. 407:57–65. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang ZF, Sun WY, Yu DH, Zhao Y, Xu HM, He
YF and Li HJ: Rotundic acid enhances the impact of radiological
toxicity on MCF-7 cells through the ATM/p53 pathway. Int J Oncol.
53:2269–2277. 2018.PubMed/NCBI
|
|
39
|
Von Pawel J, Bordoni R, Satouchi M,
Fehrenbacher L, Cobo M, Han JY, Hida T, Moro-Sibilot D, Conkling P,
Gandara DR, et al: Long-term survival in patients with advanced
non-small-cell lung cancer treated with atezolizumab versus
docetaxel: Results from the randomised phase III OAK study. Eur J
Cancer. 107:124–132. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Corgnac S, Boutet M, Kfoury M, Naltet C
and Mami-Chouaib F: The emerging role of CD8+ tissue
resident memory T (TRM) cells in antitumor immunity: A
unique functional contribution of the CD103 integrin. Front
Immunol. 9:19042018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
O'Donnell JS, Massi D, Teng MWL and
Mandala M: PI3K-AKT-mTOR inhibition in cancer immunotherapy, redux.
Semin Cancer Biol. 48:91–103. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dunai C and Murphy WJ: NK cells for
PD-1/PD-L1 blockade immunotherapy: Pinning down the NK cell. J Clin
Invest. 128:4251–4253. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu Y, Cheng Y, Xu Y, Wang Z, Du X, Li C,
Peng J, Gao L, Liang X and Ma C: Increased expression of programmed
cell death protein 1 on NK cells inhibits
NK-cell-mediatedanti-tumor function and indicates poor prognosis in
digestive cancers. Oncogene. 36:6143–6153. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Brand A, Singer K, Koehl GE, Kolitzus M,
Schoenhammer G, Thiel A, Matos C, Bruss C, Klobuch S, Peter K, et
al: LDHA-associated lactic acid production blunts tumor
immunosurveillance by T and NK cells. Cell Metab. 24:657–671. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Coca S, Perez-Piqueras J, Martinez D,
Colmenarejo A, Saez MA, Vallejo C, Martos JA and Moreno M: The
prognostic significance of intratumoral natural killer cells in
patients with colorectal carcinoma. Cancer. 79:2320–2328. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Imai K, Matsuyama S, Miyake S, Suga K and
Nakachi K: Natural cytotoxic activity of peripheral-blood
lymphocytes and cancer incidence: An 11-year follow-up study of a
general population. Lancet. 356:1795–1799. 2000. View Article : Google Scholar : PubMed/NCBI
|