Introduction
Cancer incidence is increasing worldwide (1). Malignant melanoma (MM) incidence, as
well as mortality, follows this trend in almost all countries
(2). MM is an aggressive skin tumour
arising from melanocytes, cells involved in the protection of skin
against UV-induced damage, including the genotoxic effect of
irradiation on epidermal cells.
Once metastasised, MM frequently remains a fatal
neoplasm with limited therapeutic options and relatively poor
outcomes. There has been notable progress, predominantly in
immunomodulatory therapy, in recent years, but the therapeutic
response is difficult to predict (2).
However, immune checkpoint-oriented therapy is also associated with
a rapid increase in disease-specific healthcare costs (3).
Therefore, there is an urgent need to identify and
validate informative biomarkers to improve the early selection of
at-risk patients suitable for adjuvant therapy, the optimal drug
choice, and possibly for disease progress monitoring (3,4).
Prognostically essential features of primary
melanoma are well known and include tumour thickness [measured in
mm according to Breslow staging (5)]
and the presence of surface epidermal ulceration (6). These parameters are easily acquired by
histologists in routine haematoxylin-eosin-stained paraffin
sections and are indispensable prognostic factors in melanoma
staging (6). More recently,
biomarkers obtained after immunohistochemical and molecular
analysis of the tumour tissue have played a critical role in
melanoma management; namely, the presence of BRAF, GTPase NRas and
c-KIT mutations is predictive of response to small drug inhibitors
(2,7).
At present, no ideal biomarker predicting the therapeutic response
in melanoma patients treated with other modern drugs, such as
immune checkpoint modulators (e.g. anti-programmed cell death
protein 1 or anti-cytotoxic T lymphocyte protein 4), exists
(4).
In all the aforementioned cases, an invasive
surgical procedure (radical excision or limited biopsy) to acquire
a tissue sample is necessary.
From the perspective of a clinical oncologist,
biomarkers determined from a peripheral blood sample would be
extremely beneficial for a patient's regular follow-up. In case of
MM, the detected molecules could include (but not be limited to)
soluble proteins, melanin synthesis-related metabolites,
circulating nucleic acids and/or circulating tumour cells (4,8).
In the American Joint Committee on Cancer (8th
edition) staging system (6), serum
lactate dehydrogenase (LDH) is the only serum biomarker that is
accepted as a robust prognostic parameter for routine clinical use
in melanoma patients (6).
Unfortunately, LDH is not a melanoma-specific enzyme, and increased
LDH is also associated with many other benign and malignant
diseases (9). By contrast, the S100B
protein is highly specific, and its increased levels are detected
in patients with advanced melanoma. Thus, the S100B protein has a
strong association with melanoma prognosis (10). Notably, the European Society of
Medical Oncology, German and Swiss guidelines recommend serum S100B
as the most accurate serological test for follow-up, having better
specificity for progressive disease compared with LDH (11–13).
However, the S100 protein did not prove to be sufficient
(concerning sensitivity) for detecting tumour progression, and it
cannot substitute for imaging methods [e.g. computed tomography
(CT) or positron emission tomography/CT] in long-term follow-up. A
plethora of other biomarkers has been advocated in the literature
for MM; none of these have been broadly accepted for use in a
clinical setting (6,8).
A limited percentage of cells released from a
primary tumour are capable of colonising a new site. This
phenomenon highlights the importance of a permissive tissue
microenvironment during cancer progression and metastatic spread.
This phenomenon was predicted in 1889 by Paget (14), who hypothesised that metastasis does
not occur at particular body sites randomly. Cancer represents a
complicated ecosystem of tumour cells and a variety of other cell
types that form the tumour stroma, such as cancer-associated
fibroblasts (CAFs), tumour-infiltrating leukocytes (TILs),
pericytes and endothelial cells (15).
The microenvironment modifies critical aspects of
tumour biology, such as tumour growth, local aggressiveness,
lymphatic and metastatic spread, and resistance to therapy
(16). The role of the
microenvironment in MM biology has been documented at multiple
levels. CAFs increase tumour cell plasticity and facilitate the
maintenance of the undifferentiated status of tumour cells
(17). CAFs can play a significant
role in the mechanism of primary resistance to targeted therapy via
the production of transforming growth factor-β (18). CAFs and other stromal cells can
produce several other factors with a similar effect, such as
hepatocyte growth factor (HGF), which stimulates the c-Met/PI3K/Akt
signalling pathway. These factors can activate tumour cell growth
in a paracrine manner, thus conferring resistance to targeted
therapy (19). It is evident that the
cancer microenvironment is crucial for MM growth and metastatic
spread, as well as for the emergence of acquired drug
resistance.
It was documented recently (20) that even a minimal number of
circulating tumour cells elicit a systemic inflammatory status
contributing to the promotion of tumour metastasis. These
circulating tumour cells represent a clinically undetectable
disease burden.
On the other hand, a recent study also demonstrated
that the majority of circulating tumour cells are unable to
establish a proliferatively successful metastatic clone (21). This raises several important questions
regarding the timing of therapeutic intervention in cancer,
including MM. It seems likely that the systemic proinflammatory
response could increase the risk of consequent melanoma progression
to metastatic disease, resulting in shorter survival of patients.
Data describing the complexity of the inflammatory landscape in MM
are limited (22). Thus, it may only
be hypothesised whether therapeutic inhibition of the inflammatory
response may reduce the production of cytokines contributing to the
formation of premetastatic niches suitable for disease
dissemination in an organism. A better understanding of the
fundamental components of this inflammatory response may be
important in the design of a therapeutic strategy to prevent tumour
metastasis. More research on the tumour-associated systemic
inflammation blockade may reveal optimal targets to prevent and
treat tumour metastasis.
The present pilot study focused on comparative
multiplex analysis of 31 serum proteins from 12 patients at the
time of melanoma diagnosis, and during the early onset of disease,
progression using Luminex technology (Luminex Corporation). It was
hypothesised that the levels of these proteins could serve as
biomarkers associated with the clinical progression and
pathological features of the disease. These data were also compared
with the immunohistochemical profiles of selected proteins in
primary tumours from the same patients. MM cell lines and a model
of their microenvironment in vitro, using melanoma-specific
CAFs, were also tested.
Materials and methods
Patients
MM patients (n=12) and healthy volunteers (n=5)
participated in the study after giving explicit written consent.
The study was approved by the local ethics committee (Ethics
Committee of the General University Hospital, Prague; no. 15/15).
All tissue and blood samples were obtained strictly with the
explicit informed consent of participants of the study. Samples
from MM patients and healthy volunteers were collected between May
2014 and February 2015.
The patients were diagnosed with MM based on the
clinical appearance of the skin lesion. Histopathological analysis
verified the diagnosis of primary cutaneous MM after tumour
excision. No patient had clinical evidence of metastatic disease at
the time of diagnosis. Based on this favourable status, clinical
follow-up was initiated. Despite radical surgery, some patients
developed metastasis during the follow-up period. The patient
cohort characteristics are summarised in Table I. The healthy volunteers were three
men (35, 40 and 66 years old), and two women (26 and 63 years old)
without any evidence of cancer or chronic disease.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Patient no. | Sex | Age of onset,
years | Location | Breslow | Ulceration | Sentinel lymph node
metastasis | Clinical stage | Therapy at time of
serum collection | Generalization | Mortality caused by
melanoma | PFS, months | OS, months |
|---|
| 1 | F | 65 | Back | 6.5 | Yes | Not | IIC | S, N | Yes | Yes | 12 | 14 |
| 2 | M | 68 | Thigh | 2.5 | Not | Yes (N1a) | IIIB | S, N, L | Not | Not | R | 55 |
| 3 | F | 71 | Lower leg | 0.3 | Not | Not | IA | S, N | Not | Not | R | 54 |
| 4 | M | 61 | Back | 0.8 | Not | Not | IA | S, N | Not | Not | R | 54 |
| 5 | M | 89 | Toe | 3.4 | Yes | Not | IIB | S | Yes | Nota | 12 | 43 |
| 6 | M | 55 | Back | 3.6 | Not | Yes (N1a) | IIIB | S, N, L | Yes | Yes | 14 | 16 |
| 7 | M | 66 | Back | 2.2 | Yes | Yes (N1a) | IIIC | S, N, L | Yes | Yes | 11 | 14 |
| 8 | F | 66 | Thigh | 0.5 | Not | Not | IA | S, N | Not | Not | R | 53 |
| 9 | M | 68 | Back | 2.0 | Not | Yes | IIIB | S, N, L | Not | Not | R | 50 |
| 10 | F | 34 | Lower leg | 1.7 | Not | Not | IB | S, N | Not | Not | R | 51 |
| 11 | F | 71 | Toe | 0.4 | Not | Not | IA | S | Not | Not | R | 51 |
| 12 | F | 68 | Back | 2.7 | Not | Not | IIA | S, N | Not | Not | R | 51 |
Cells
The BLM cell line was kindly provided by Dr L. van
Kempen and Professor J.H.J.M. van Krieken (Department of Pathology,
Radboud University, Nijmegen Medical Centre, Netherlands). The
commercially available A2058 cell line and HP-Mel (HEMn) cell line
was purchased from the American Type Culture Collection
(ATCC® CRL-11147™ and ATCC® PCS-200-012™,
respectively). MP17 melanoma cells were isolated (February 20th,
2015) from pleural ascitic fluid from a patient (74-year-old man)
with tumour generalisation (Tumor-Node-Metastasis stage IV) using a
previously described method (17).
Culture conditions were as described in a previous study (23).
Normal primary dermal fibroblasts (acquired after
breast reduction surgery, designated as HFP4 (from a 55-year-old
female; localisation, chest; collected May 19th. 2014) and CAFs
[two independent isolates designated as MAM (69-year-old female;
localisation, chest; collected July 14th, 2014) and ZAM
(48-year-old male; localisation, abdomen; collected September 9th,
2014)] from skin metastases of MM were prepared and cultured as
previously described (24–26).
MP17, HFP4, MAM and ZAM cells were obtained and
maintained with the informed consent of patients and the approval
of the local ethical committee as indicated above. The authors
confirm that mycoplasma testing was performed on all cell lines
used in the present study.
Serum preparation
Venous blood was collected from MM patients (n=12)
at three time intervals: i) At the time of diagnosis prior to any
surgical treatment; ii) 1 month after the surgery; and iii) ~3
months after the surgery. The blood was left to clot for 30–60 min
at room temperature followed by centrifugation (1,500 × g; 10 min;
4°C). The obtained serum was immediately aliquoted to avoid later
multiple freeze-thaw cycles and stored at −80°C. Control sera were
obtained from healthy individuals (n=5) under identical
conditions.
Analysis of serum samples
The levels of 31 cytokines, chemokines and growth
factors were analysed using Luminex xMAP® bead assays
(Luminex Corporation) in the serum of MM patients and controls. The
Human Cytokine Magnetic 30-Plex Panel (Thermo Fisher Scientific,
Inc.) and CXCL1 Human ProcartaPlex™ Simplex kit (Thermo Fisher
Scientific, Inc.) were used to quantify epidermal growth factor
(EGF), eotaxin, basic fibroblast growth factor, granulocyte-colony
stimulating factor (G-CSF), granulocyte-macrophage
colony-stimulating factor (GM-CSF), growth regulated-α protein
(also known as CXCL1), hepatocyte growth factor (HGF), interferon
(IFN)-α, IFN-γ, interleukin (IL)-1β, IL-1 receptor antagonist
(IL-1RA), IL-2, IL-2 receptor, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10,
IL-12 (p40/p70), IL-13, IL-15, IL-17, IP-10, C-C motif chemokine 1,
MIG, C-C motif chemokine 3, C-C motif chemokine 4 (MIP-1β), RANTES,
tumour necrosis factor-α and vascular endothelial growth factor
(VEGF) levels. The manufacturer validated all antibodies used for
this experiment.
After thawing on ice, the serum samples were gently
mixed, pre-cleaned via centrifugation (16,000 × g; 10 min; 4°C),
and diluted at a 1:1 ratio with Assay Diluent (in the case of the
30-plex assay) or Universal Assay Buffer (in the case of the
Simplex assay) to minimize the matrix effects. The same diluent
buffer was used as a blank and as a diluent for the calibration
standards. The calibration curve was extended at the lower end by
additional dilutions of calibration standards. Reverse pipetting
was used for high accuracy in all liquid handling steps. All
samples, standards and background were analysed in duplicate. The
assay was performed according to the manufacturer's instructions.
The fluorescence intensities of minimum 100 beads/analyte were
recorded using a Luminex 200™ analyser with xPonent software build
3.1.871.0 (Luminex Corporation), adequately calibrated according to
the manufacturer's instructions.
Raw data were exported from xPonent software in. csv
format and processed in the R statistical environment (27) using the drLumi package (28). The median fluorescence intensity was
used for standard curve fitting and quantitation of cytokine
concentrations. Standard curves were fitted using 5-parameter
logistic regression (SSL5) with 4-parameter logistic regression as
a fall-back for occasions where the SSL5 model would not converge.
The concentrations of two technical replicates of each sample were
averaged before further statistical analysis.
Data analysis and statistics
Statistical analysis of the relationship between the
measured protein levels and clinical features of the disease was
conducted using the R statistical environment (27) and tidy verse set of packages (29). Repeated measures ANOVA was used for
statistical comparisons in experiments with more than two groups.
Dunnett's test was used for many-to-one group comparisons of MM
melanoma samples to controls (30–32). The
association between protein levels and Breslow index was evaluated
using Kendall's tau correlation coefficient, and lines representing
the Theil-Sen estimator of a linear relationship were used.
t-Distributed stochastic neighbour embedding (t-SNE)
implemented in the R Rtsne package was used for nonlinear dimension
reduction, to visualise any possible patterns in the
high-dimensional cytokine concentration data (33). Analyte levels below the level of
detection (missing values) were replaced by 80% of the lower limit
of detection of the assay. Data for each analyte were normalized
relative to mean concentration overall data points (all time points
in all patients and controls) for the given analyte. Rtsne was run
with the initial principal component analysis step, perplexity 12
and θ parameter 0.5 over 8,000 iterations to reduce the dimensions
to 2.
Immunohistochemical analysis of
paraffin sections of patient tumours and cultured cells, and
microscopy
Tissue samples were fixed for 24–48 h in 4% neutral
buffered formalin at room temperature and routinely processed to
produce paraffin blocks. Sections (2-µm-thick) were deparaffinised
and rehydrated through xylene and 98% ethanol. Afterwards, the
sections were washed in PBS, and heat-induced epitope retrieval was
performed in citrate buffer, pH 6.0 in an autoclave at 120°C for 3
min, with subsequent gradual cooling to room temperature for 60
min. Non-specific binding of antibodies was inhibited using the
Protein Block system (Dako; Agilent Technologies, Inc.; cat. no.
X0909) followed by treatment with 3% hydrogen peroxide (in PBS;
Sigma-Aldrich; Merck KGaA) for 20 min at room temperature. Sections
were incubated overnight at 4°C with biotinylated primary
antibodies (manufacturer-validated summary antibody information in
Table SI; the manufacturer validated
all employed antibodies for use in these methods).
The following day, the sections were extensively
washed and incubated with secondary (polymer horseradish
peroxidase-tagged) antibody for 30 min at room temperature.
3-Amino-9-ethylcarbazol (AEC; DCS Innovative Diagnostik-Systeme)
chromogen was used for visualisation of the immunohistochemical
reaction, according to the manufacturer's protocol. Nuclei were
counterstained with Gill's haematoxylin for 2 min at room
temperature and mounted in Hydromount (National Diagnostics).
Semi-quantitative analysis (0, negative; +, weakly positive; ++,
moderately positive; and +++, strongly positive) of the
immunohistochemistry reaction was used to express the proportion of
positive staining based on inspection under an optical
microscope.
The cultured cells on coverslips were briefly fixed
in 2% paraformaldehyde in PBS for 5 min at room temperature and
permeabilised with Triton X-100 (Sigma-Aldrich; Merck KGaA). The
coverslips with cultured cells were stained according to the same
protocol using identical primary antibodies and other chemicals
(with the omission of antigen retrieval). Imaging was performed
with a Leica microscope DM2000 equipped with camera (DFC290 HD) and
software package LAS, version 4.3.0 (Leica Microsystems GmbH):
Magnification, ×400; calibration, 1,000 pixels/pixel; capture
format, 2,048×1,536; full frame; γ=1.0; gain, 1.0; auto exposure,
on; nosepiece objective magnification, 40; mag changer
magnification, 1; and aperture, 0.4.
Results
Serological analysis
The present study analysed the levels of a
31-protein panel of cytokines, chemokines and growth factors in
serum prepared from patients suffering from MM compared with
healthy volunteers. Based on the initial hypothesis, these proteins
were investigated for their ability to serve as biomarkers
correlating with certain oncologically-important features of the
disease. Among the analysed proteins, IL-5, IL-7 and GM-CSF were
below the lower limit of detection in most of the samples and were
excluded from further analyses. The levels of the remaining 28
proteins are summarised in Fig. 1 and
Table SII. Data demonstrated that
the levels of five studied proteins, namely IL-1RA, IL-2, IL-13,
RANTES and G-CSF, were significantly different in the serum samples
of healthy volunteers from the melanoma patients, according to
Dunnett's test (Fig. 1). The results
from the ANOVA summarised in Table
SIII indicated that these five proteins were not significantly
different in the serum samples of healthy volunteers compared with
the melanoma patients.
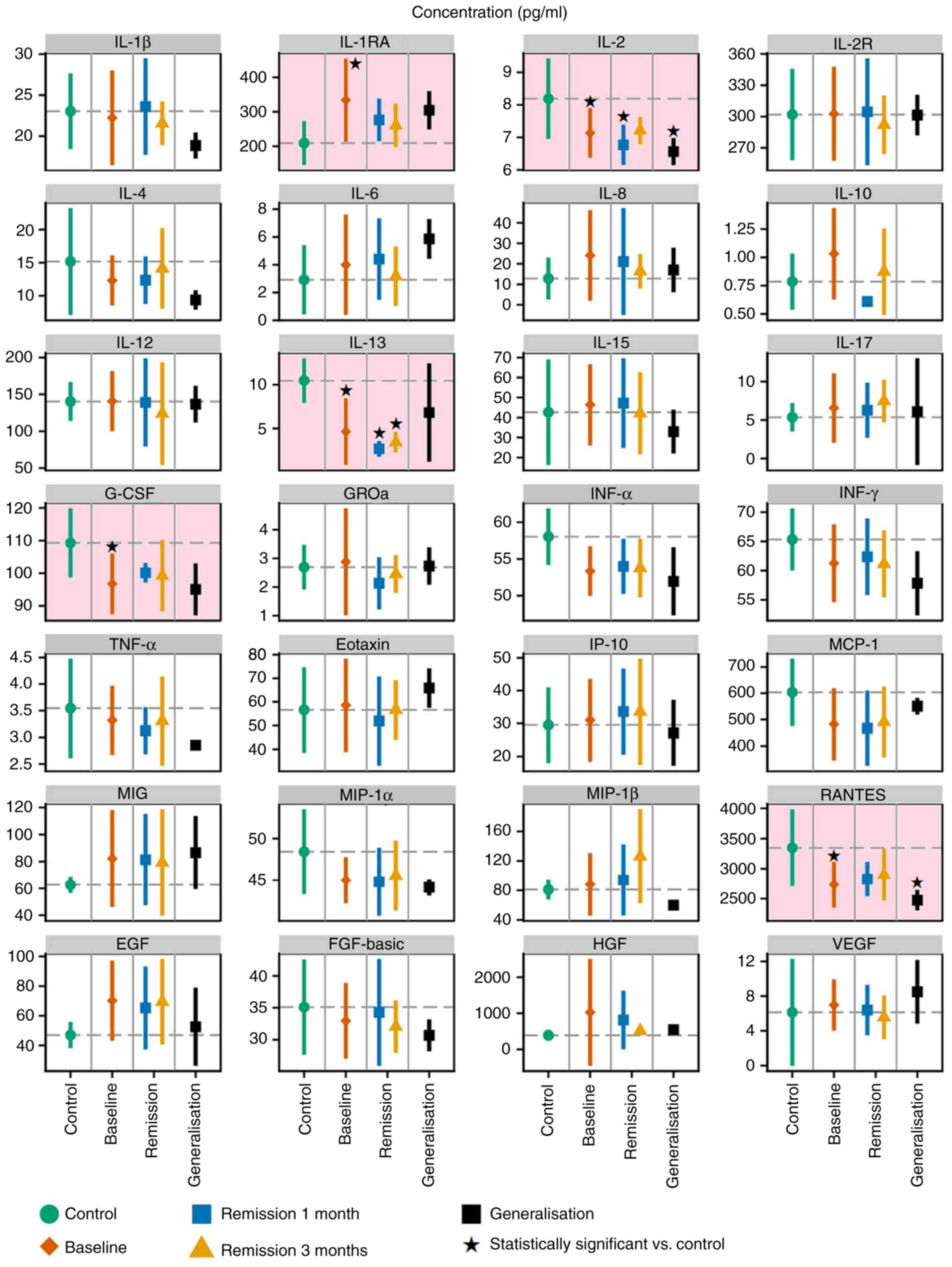 | Figure 1.Levels of 28 proteins in serum
samples from healthy volunteers (control; green) and patients with
MM. MM patient samples were collected at the time of diagnosis
(baseline; red) and again 1 month and 3 months post-surgery. Data
from the two later collections are plotted separately for subsets
of patients without any evidence of disease (remission; blue,
yellow) and patients at the stage of MM generalisation
(progression, in black). The graphs represent the mean ± SD.
Statistical significance (Dunnett's one-to-many test with multiple
testing correction) of comparisons between the control and patient
subsets are highlighted with a pink background. *P<0.05 vs.
control. IL, interleukin; G-CSF, granulocyte-colony stimulating
factor; IL-1RA, IL-1 receptor antagonist; IL-2R, IL-2 receptor;
GROa, C-X-C motif chemokine ligand 1; IFN, interferon; TNF, tumor
necrosis factor; MCP-1, C-C motif chemokine ligand 2; MIG, C-X-C
motif chemokine ligand 9; IP-10, C-X-C motif chemokine ligand 10;
MIP-1α, C-C motif chemokine ligand 3; MIP-1β, C-C motif chemokine
ligand 4; EGF, epidermal growth factor receptor; FGF-basic, basic
fibroblast growth factor; HGF, hepatocyte growth factor; VEGF,
vascular endothelial growth factor. |
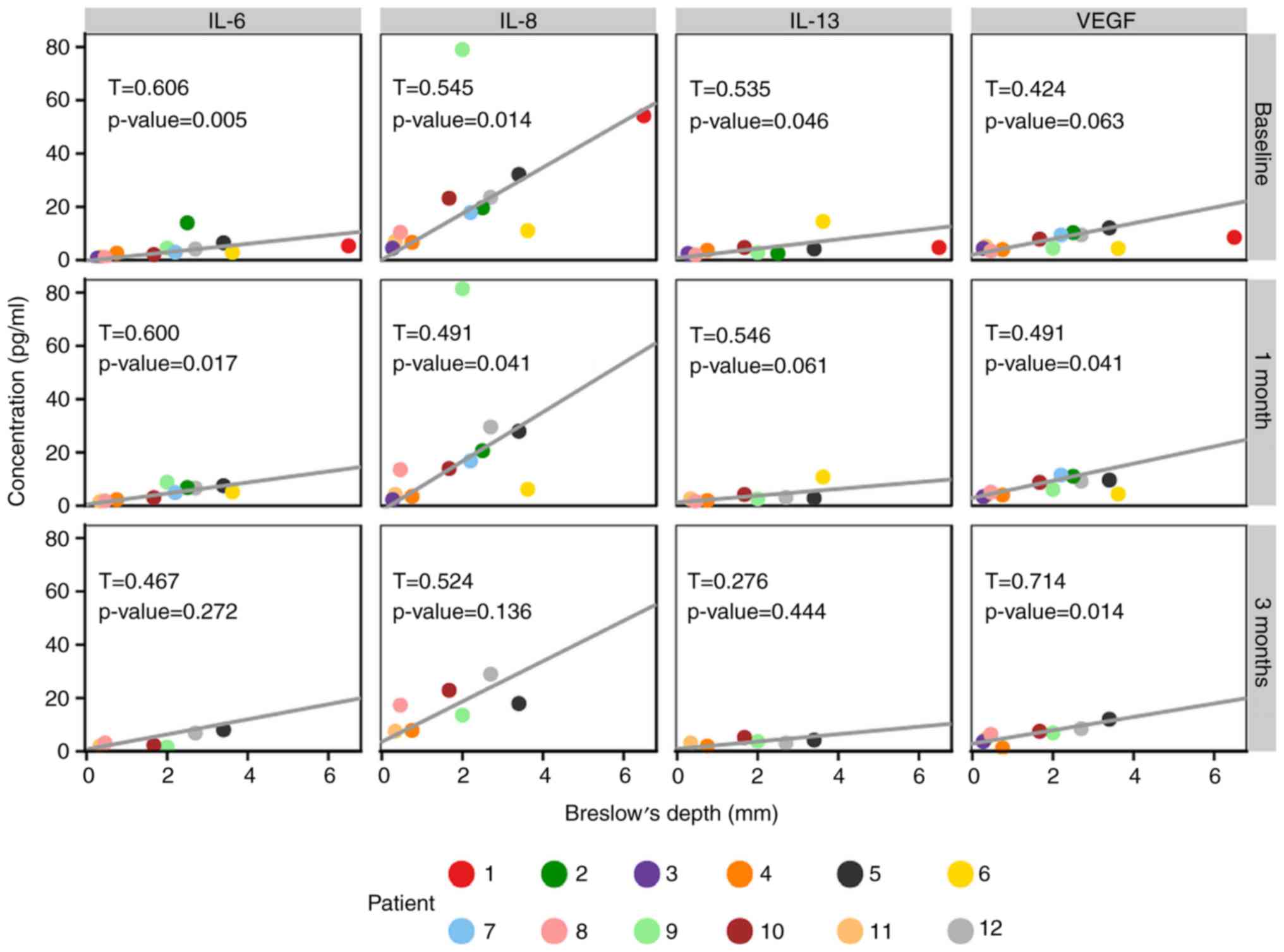
It was observed that the Breslow values of primary
tumours were significantly associated with the serum levels of four
detected proteins, namely IL-6, IL-8, IL-13 and VEGF (Fig. 2).
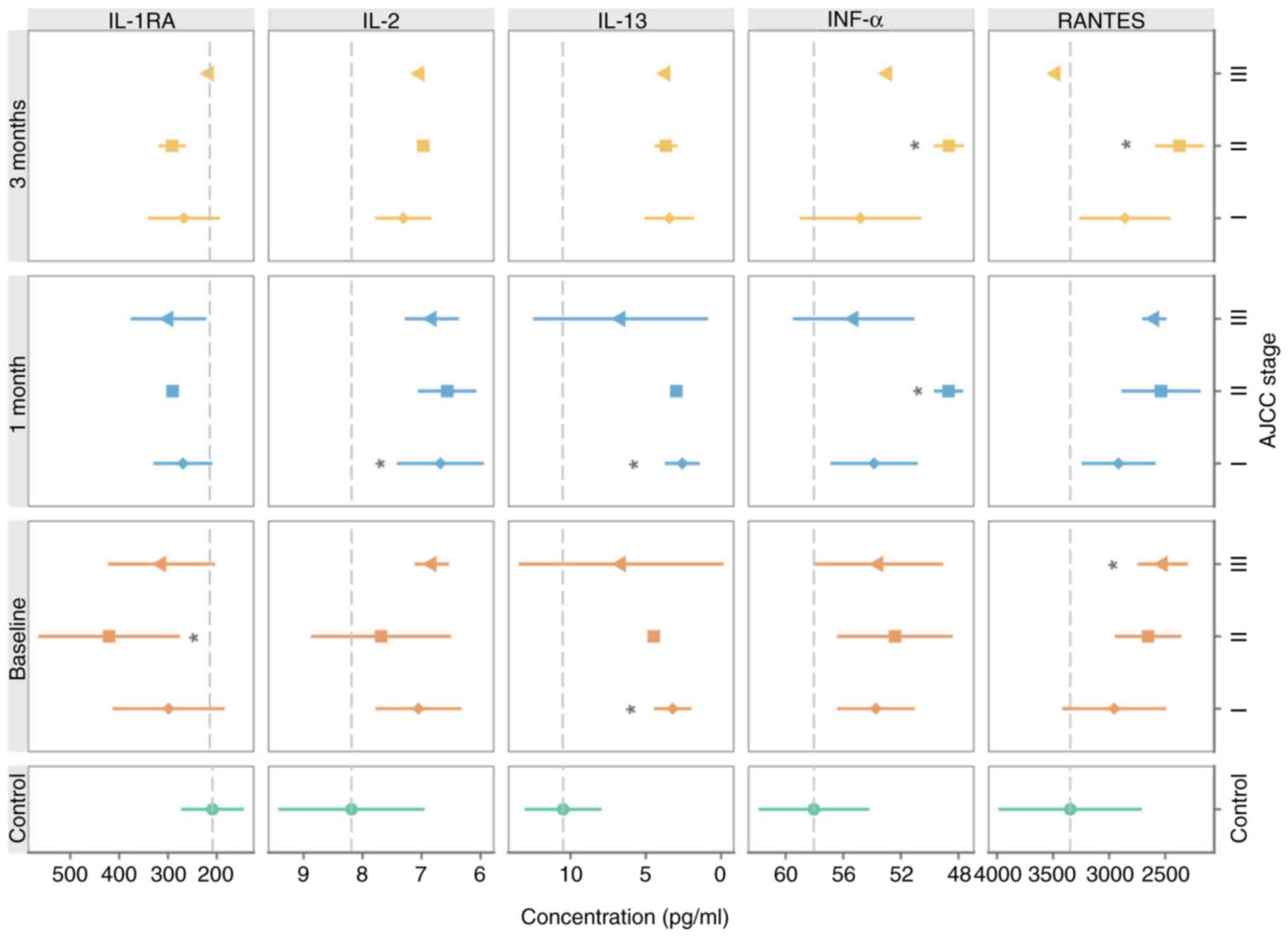
The serum levels of IL-1RA, IL-13, IFNα and RANTES
reflected the clinical stage of the disease at the time of MM
diagnosis (Fig. 3).
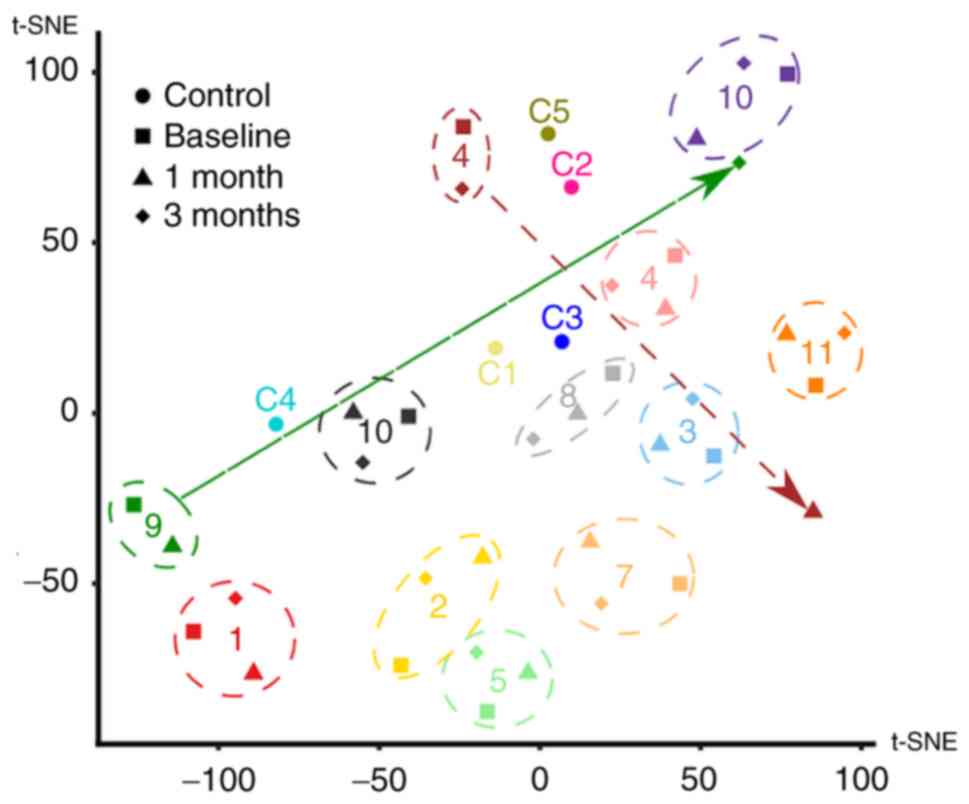
It is important to note that the cluster analysis
demonstrated a vast extent of inter-individual differences among
the tested patients and certain intra-individual level fluctuation
(Fig. 4).
Analysis of primary MM lesions
To assess the contribution of the factors
upregulated in MM patient sera to the MM microenvironment,
immunohistochemistry of the tumours was performed in the same
patients whose serum was analysed (Fig.
5). Immunohistochemical positivity for selected members of the
studied protein families and their receptors in representative
samples of primary tumours is illustrated in Fig. 5A-I and was quantified. Fig. 5J demonstrates the heterogeneity of
primary tumours in the patients from whom the serum samples were
collected. MM cells from all samples were positive for HGF, but the
signal intensity was low. The studied tumours also widely expressed
IL-6 and its receptor IL-6R, and VEGF-R1. The epidermis overlaying
the MM lesions was highly positive for VEGF and VEGF-R2 in all
studied samples (Fig. 5F).
Macrophages widely expressed the studied markers, as demonstrated
in the case of HGF (Fig. 5I).
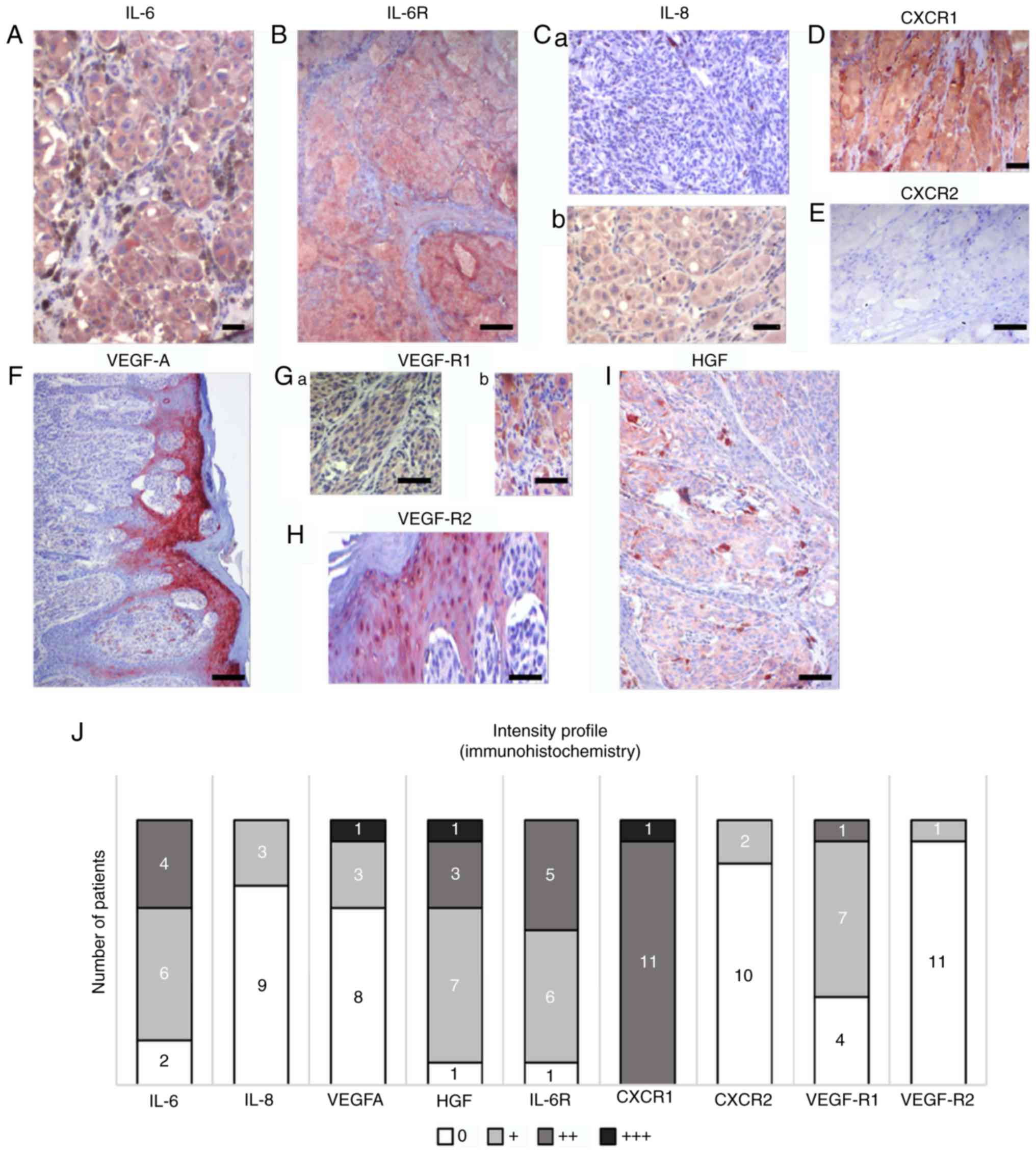 | Figure 5.Characteristic samples of expression
of selected protein markers in sections from primary MM in the same
cohort of patients. Multiple markers were detected by
immunohistochemistry in melanomas. (A) IL-6 and (B) IL-6 receptor
were detected in the majority of tumours in MM cells. (C) IL-8
expression was highly variable in studied samples; representative
sections are included. Differences in expression were also observed
in CXCR1 and CXCR2. (D) CXCR1 with highly positive in all patients,
which contrasted with (E) the low to negative CXCR2 expression. (F)
Notable positivity for VEGF was observed in the epidermis
overlaying the MM lesion. Expression of VEGF receptors (G) VEGF-R1
and (H) VEGF-R2 was also observed beside MM cells in the tumour
microenvironment, represented by stromal macrophages and epidermis.
(I) HGF was also observed in MM cells and macrophages. Scale bar,
50 µm. (J) A summary of the expression of selected markers in
primary MM in the present cohort. Semi-quantitative analysis (0, +,
++, and +++) of the immunohistochemistry reaction was used to
express the proportion of positive staining based on inspection
under an optical microscope. The numbers of positive tumours are
included on the graph. IL, interleukin; CXCR1, C-X-C motif
chemokine receptor 1; HGF, hepatocyte growth factor; CXCR2, C-X-C
motif chemokine receptor 2; VEGF, vascular endothelial growth
factor; VEGF-AR1, VEGF-A receptor 1; VEGF-AR2, VEGF-A receptor 2;
IL-6R, IL-6 receptor. |
Analysis of cultured MM cells (A2058,
BLM and MP17), normal melanocytes (HP-Mel), normal dermal
fibroblasts (HFP4) and CAFs (MAM and ZAM)
The phenotype of melanoma cells and CAFs isolated
from melanoma was analysed by studying the expression of selected
factors detected in the serum of melanoma patients. The aim of this
part of the study was to evaluate the production of these factors
by isolated MM cells and CAFs. Data from cultured MM cells (A2058,
BLM and MP17) demonstrated a similar phenotype to samples from
primary MMs (Fig. 5) with several
exceptions, such as high positivity for IL-8 (Fig. 6Ac, Bc and Cc) and VEGF-R2 (Fig. 6Ah, Bh and Ch). Normal melanocytes were
negative for IL-6 (Fig. 6Da), IL-8
(Fig. 6Dc), CXCR1/2 (Fig. 6Dd and e), VEGF (Fig. 6Df), and the receptors VEGF-R1/R2
(Fig. 6Dg and h). As expected, these
cells were highly positive for protein S100, MiTF and
differentiation marker HMB45 (data not shown). Also as expected,
both normal fibroblasts (HFP4) and fibroblasts isolated from the
cutaneous metastases of MM (CAFs, similarly MAM or ZAM) exhibited a
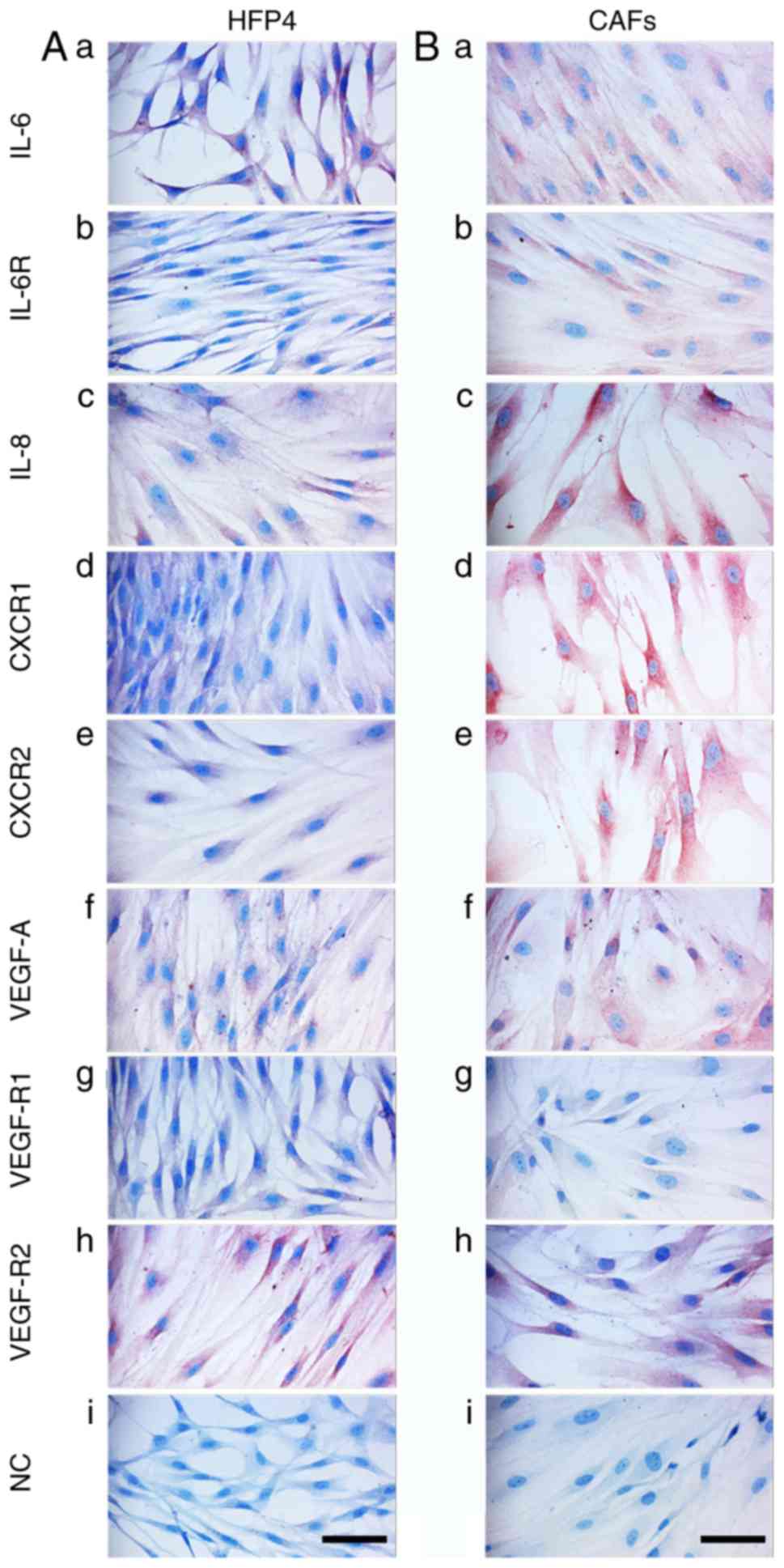
vimentin-rich cytoskeleton (data not shown). The signal for IL-6,
IL-8, CXCR1, and CXCR2 was stronger in MM CAFs (Fig. 7Aa, c, d and e) than in normal
fibroblasts (Fig. 7Ba, c, d and
e).
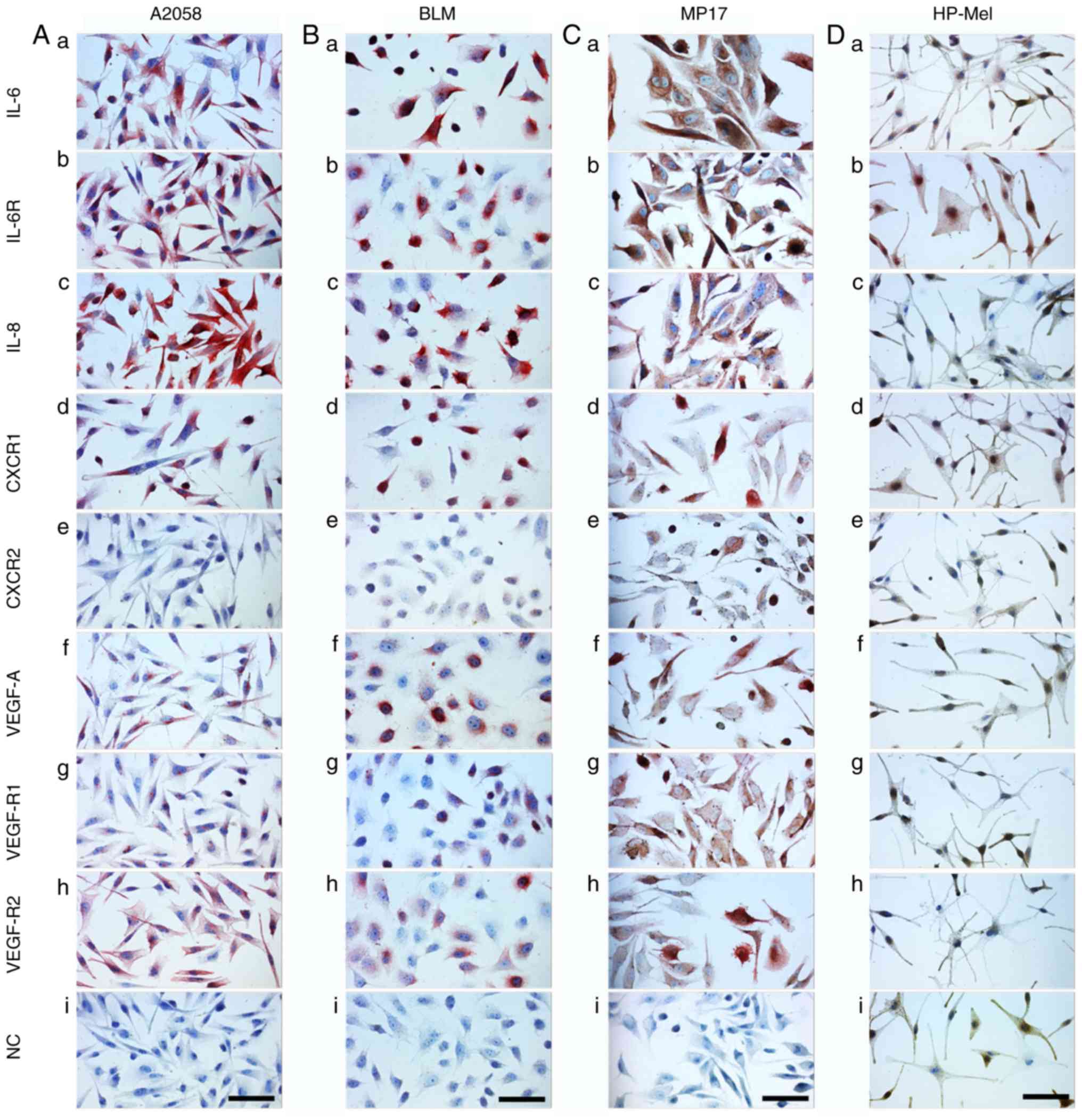 | Figure 6.Detection of selected markers in MM
cell lines. The detection was performed in (A) A2058, (B) BLM and
(C) MP17 cells, and in (D) normal HP-Mel cells. HP-Mel are more
pigmented than MM cell lines and their H2O2
bleaching was not as successful. Therefore, AEC substrate (red) was
used for the immunocytochemical reaction detection. Generally, it
was possible to note that positive staining of HP-Mel was
negligible in comparison with that of MM cell lines. A positive
signal for IL-6 and IL-6R was observed in HP-Mel cells. Scale bar,
100 µm. CXCR1, C-X-C motif chemokine receptor 1; CXCR2, C-X-C motif
chemokine receptor 2; VEGF, vascular endothelial growth factor;
VEGF-AR1, VEGF-A receptor 1; VEGF-AR2, VEGF-A receptor 2; IL-6R,
IL-6 receptor; NC, normal control; HP-Mel, highly pigmented
melanocyte. |
Discussion
The present study demonstrated that the presence of
a primary malignant melanoma tumour and its consequent metastatic
spread is reflected by a profound change in inflammatory molecules
in the serum. Similar findings for individual molecules or smaller
groups have also been reported previously (34,35).
Despite the observed broad inter-individual
variability, the presence of a primary tumour was associated with
significantly decreased levels of IL-2, IL-13, RANTES and G-CSF,
and an increase in IL-1RA detected in the patient sera.
In detail, some of these factors also significantly
differed in certain clinical stages of the disease, wherein IFNα,
IL-13 and RANTES were decreased significantly and IL-1RA was
increased. The cohort of patients was also affected by the healing
of the wound after surgery. However, no complicated courses of
healing were observed in these 12 patients. An association between
the Breslow index of the tumour and the serum levels of tested
proteins was observed in the case of IL-6, IL-8, IL-13 and
VEGF.
The low levels of IFNα in patients with melanoma
seem to be important, because its anticancer effect has been well
documented and recombinant IFNα is employed as a drug in cancer
therapy, including adjuvant therapy for MM (36). Similar therapeutic implications are
true for IL-2 (37).
Both downregulated interleukins IL-2 and IL-13
exhibit anticancer activity, with the potential for application in
anticancer therapy (38–42).
RANTES is also well known as an essential
inflammation-promoting chemokine. However, its role in anticancer
immunity/tumour facilitation requires to be further elucidated
especially in melanoma (43,44).
Attention should also be paid to the cytokine IL-6
and chemokine IL-8. Both molecules exhibit a broad
tumour-supporting effect (23,45). It
should be noted that inflammation-supporting factors such as IL-6
and IL-8 are produced not only by MM cells and TILs, but are also
provided in large quantities by CAFs (23,25,46). This
is apparent from the present immunohistochemical results. The
contribution of particular components of a tumour can be easily
documented through immunocytochemical analysis of isolated cell
populations. Therefore, it may be expected that regardless of the
actual cellular origin of these factors, the protein abundance will
consequently be detected in the serum (8,47–50). These facts highlight the importance of
the tumour microenvironment and support the concept of cancer as a
systemic disease (6,51).
In general, IL-6 and IL-8 stimulate the metastatic
spread of many tumours, including MM (25,46,52), and
participate in the induction of resistance to vemurafenib (18).
In the particular case of IL-6, its effect on MM
seems to be stage-specific. IL-6 has an inhibitory effect in the
initial stages of melanoma. However, IL-6 stimulates the growth and
invasiveness of MM cells in advanced stages of the disease
(53,54). In general, blocking of IL-6 production
seems to be beneficial for MM patients (55) because IL-6 stimulates cancer cachexia
and wasting, a severe and terminal complication of malignant
disease (56–59).
Chemokine IL-8 (CXCL8) and its receptors CXCR1 and
CXCR2 play a notable role in melanoma pathogenesis, particularly in
melanoma progression and metastasis (60). The serum concentration of IL-8 can be
correlated to the disease stage, and changes in the serum IL-8
levels could be used to monitor and predict the clinical benefit of
immune checkpoint inhibitor therapy (61).
In addition to the direct effect of IL-8 on cancer
cells, this chemokine also stimulates the growth of capillaries in
the tumour environment. Thus, it has a synergistic effect with VEGF
(62). The correlation of serum
levels of IL-8 with melanoma tumour mass has been previously
described (63). Breslows depth is
generally accepted as a simple but precise prognostic factor. The
present data demonstrated that the serum levels of IL-6 and IL-8
(both increased) reflect this tumour parameter.
It should be acknowledged here that surgical removal
did not lead to an immediate lowering of the IL-6 and IL-8 levels.
It seems likely that a sustained level of these molecules may
promote another source of this cytokine, other than from a tumour
itself. This can be interpreted as evidence of the systemic
proinflammatory environment in the patient.
The observation of an elevated serum level of VEGF
in the samples from patients with MM is unsurprising, because its
cancer-stimulating effect via the support of tumour vascularisation
is well known (64). Serological
elevation of VEGF is associated with melanoma progression and
adverse immune effects, including elevation of TH2
cytokines (e.g., observed IL-10) and decreased of TH1
cytokines (e.g., observed IL-2 and interferon γ). These changes
result in suppression of anti-tumour immunity (65,66).
Expression of CD114, a surface receptor for VEGF,
has been described in association with melanoma progression
(67), and it can be considered as a
new marker for cancer cells originated from neural crest-derived
stem cells (68).
Besides this, we observed significantly lower levels
of G-CSF. G-CSF is known to stimulate formation of granulocytes,
and its reduction related to cancer progression is therefore
likely. Neutrophils are functionally plastic in the tumour
microenvironment, and N1/N2 functional polarisation has recently
been accepted. Classically activated (N1) neutrophils inhibit
metastatic growth. In contrast, alternatively polarised (N2)
neutrophils have been reported to facilitate colonisation of the
target organ by metastasis-initiating cancer cells.
Comparative analysis of serum samples with
immunohistochemical findings in primary tumours and cultured cells
has shown that both MM cells and cells forming the tumour
microenvironment (CAFs, TILs, keratinocytes, macrophages and
pericytes) participate in the changes of serum proteins. Distinct
clinical stages of MM can induce a specific pattern of serum
proteins that underlines the systemic effect of the disease
(18).
Immunosuppressive properties of the melanoma
microenvironment are responsible for the chronic inflammatory
status of the organism, thus supporting the hypothesis of cancer as
a systemic disease from an early stage. However, it does not solve
the question of the cause and the consequence.
Immunohistochemical analysis of IL-6, IL-8, VEGF and
their receptors was performed in primary melanomas as well as in
several MM cell lines and CAFs. The resulting data harmonised with
the analysis of patient sera. This further demonstrated that the MM
cells and stromal cells (CAFs) participated in the production of
the studied factors. On the other hand, data from individual
patients are highly variable, which is supported by similar
evidence in The Human Protein Atlas (Expression of VEGF, CXCL8, and
IL-6, 2019; https://www.proteinatlas.org/ENSG00000112715-VEGFA/pathology;
https://www.proteinatlas.org/ENSG00000169429-CXCL8/pathology;
http://www.proteinatlas.org/ENSG00000136244-IL6/pathology).
Therefore, a combination of more entries rather than a single
biomarker seems to be necessary. Depression of serum levels of IL-2
and G-CSF in the serum of melanoma patients seems to have some
therapeutic relevance because their participation in anticancer
immunity was established and they were proposed for anticancer
therapy (69).
In conclusion, the aforementioned analysis of the
serum levels of growth factors VEGF and G-CSF, cytokines IL-6,
IL-2, IL-1RA and IFNα, and chemokines IL-8 and RANTES reflect
various aspects of tumour biology in malignant melanoma. It is
necessary to acknowledge that the currently used markers, such as
LDH and S100 proteins, have staging and prognostic significance,
but do not reflect the exact immunopathological actions of the
organism at the time of melanoma diagnosis or during the disease
progression (6,51). Our data also indicated the apparent
deregulation of the anti-tumour immune response, which is an
essential factor for cancer progression. This sustained
proinflammatory environment can significantly contribute to the
clinically important phenomenon of long-lasting minimal residual
disease. As noted earlier, due to the great inter-individual
variability observed by us and by others, strategies based on a
combination of several biomarkers or even multiplexing would be
beneficial in the future. This approach can contribute to more
effective therapy selection, and thus increase the therapeutic
outcomes and the patient survival. This pilot study used a limited
cohort of patients. Broader studies for validation of our
observations are expected in the future. The present study focused
on the soluble molecules detectable in human serum, and those that
were significantly changed after processing using Dunnett's test.
According to the ANOVA, no significant changes were observed
between the control and the studied melanoma patients. This testing
would require a much larger group of patients, which is planned in
a continuation of this experiment. No significant changes were
identified in proteins responsible for coping with oxidative stress
in this study. However, there is well-established evidence that
soluble molecules identified by us (e.g. IL-6) and their signalling
pathways have a tight link to oxidative stress in tissues. The role
of oxidative stress under pathological conditions, including in
cancer biology, has been clearly established (70–72). The
search for these proteins will represent the next step of our
study.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The manuscript represents the outcome of the project
‘Centre for Tumour Ecology-Research of the Cancer Microenvironment
Supporting Cancer Growth and Spread’ (reg. no. CZ.02.1.
01/0.0/0.0/16_019/0000785) supported by the Research, Development
and Education Operational Programme. This publication is also the
result of the implementation of the project: ‘The equipment for
metabolomic and cell analyses’ (reg. no. CZ.1.05/2.1.00/19.0400),
supported by the Research and Development for Innovations
Operational Programme (RDIOP) co-financed by the European Regional
Development Fund and the state budget of the Czech Republic. This
study was supported by the Grant Agency of the Czech Republic
(project no. 16-05534S), Ministry of Health of the Czech Republic
(project nos. 16-29032A and 16-30954A), Charles University project
PROGRESS Q 28, and by the Ministry of Education, Youth and Sports
of CR within the National Sustainability Programme I (no. LO1609)
and II (Project BIOCEV-FAR; reg. no. LQ1604), and by project BIOCEV
(no. CZ.1.05/1.1.00/02.0109).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
KSJ, LL and OK wrote the manuscript. KSJ was also
head of the conception of the study. JK and PV performed the
statistical analysis. JK, OK, IK and JS collected the patient
samples, and HK, HKS and JM performed the proteomic analysis. LL,
OK and PD conducted the histological and immunohistochemical
analysis. KS, BD and LL performed the cell culture and
immunocytochemistry analysis. All authors read and approved the
final manuscript and agree to be accountable for all aspects of the
research, in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
The study was approved by the local ethics committee
(Ethics Committee of the General University Hospital, Prague; no.
15/15). All tissue and blood samples were obtained strictly with
the explicit informed consent of participants of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Global Burden of Disease Cancer
Collaboration, ; Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T,
Alizadeh-Navaei R, Allen C, Alsharif U, Alvis-Guzman N, Amini E, et
al: Global, regional, and national cancer incidence, mortality,
years of life lost, years lived with disability, and
disability-adjusted life-years for 29 cancer groups, 1990 to 2016:
A systematic analysis for the global burden of disease study. JAMA
Oncol. 4:1553–1568. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schadendorf D, van Akkooi ACJ, Berking C,
Griewank KG, Gutzmer R, Hauschild A, Stang A, Roesch A and Ugurel
S: Melanoma. Lancet. 392:971–984. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Verma V, Sprave T, Haque W, Simone CB II,
Chang JY, Welsh JW and Thomas CR Jr: A systematic review of the
cost and cost-effectiveness studies of immune checkpoint
inhibitors. J Immunother Cancer. 6:1282018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang M, Yang J, Hua W, Li Z, Xu Z and
Qian Q: Monitoring checkpoint inhibitors: Predictive biomarkers in
immunotherapy. Front Med. 13:32–44. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Breslow A: Thickness, cross-sectional
areas and depth of invasion in the prognosis of cutaneous melanoma.
Ann Surg. 172:902–908. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gershenwald JE, Scolyer RA, Hess KR,
Sondak VK, Long GV, Ross MI, Lazar AJ, Faries MB, Kirkwood JM,
McArthur GA, et al: Melanoma staging: Evidence-based changes in the
American Joint Committee on Cancer eighth edition cancer staging
manual. CA Cancer J Clin. 67:472–492. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Amann VC, Ramelyte E, Thurneysen S,
Pitocco R, Bentele-Jaberg N, Goldinger SM, Dummer R and Mangana J:
Developments in targeted therapy in melanoma. Eur J Surg Oncol.
43:581–593. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Karagiannis P, Fittall M and Karagiannis
SN: Evaluating biomarkers in melanoma. Front Oncol.
4:3832015.PubMed/NCBI
|
|
9
|
Jurisic V, Radenkovic S and Konjevic G:
The actual role of LDH as tumor marker, biochemical and clinical
aspects. Adv Exp Med Biol. 867:115–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gebhardt C, Lichtenberger R and Utikal J:
Biomarker value and pitfalls of serum S100B in the follow-up of
high-risk melanoma patients. J Dtsch Dermatol Ges. 14:158–164.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dummer R, Hauschild A, Lindenblatt N,
Pentheroudakis G and Keilholz U; ESMO Guidelines Committee, :
Cutaneous melanoma: ESMO clinical practice guidelines for
diagnosis, treatment and follow-up. Ann Oncol. 26 (Suppl
5):v126–v132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dummer R, Siano M, Hunger R, Lindenblatt
N, Braun R, Michielin O, Mihic-Probst D, von Moos R, Najafi Y,
Guckenberger M and Arnold A: The updated Swiss guidelines 2016 for
the treatment and follow-up of cutaneous melanoma. Swiss Med Wkly.
146:142792016.
|
|
13
|
Pflugfelder A, Kochs C, Blum A, Capellaro
M, Czeschik C, Dettenborn T, Dill D, Dippel E, Eigentler T, Feyer
P, et al: Malignant melanoma S3-guideline ‘diagnosis, therapy and
follow-up of melanoma.’. J Dtsch Dermatol Ges. 11 (Suppl
6):S1–S126. 2013.(In English, German). View Article : Google Scholar
|
|
14
|
Paget S: The distribution of secondary
growths in cancer of the breast. Lancet. 1:571–573. 1889.
View Article : Google Scholar
|
|
15
|
Kareva I: What can ecology teach us about
cancer? Transl Oncol. 4:266–270. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lacina L, Plzak J, Kodet O, Szabo P,
Chovanec M, Dvorankova B and Smetana K Jr: Cancer microenvironment:
What can we learn from the stem cell niche. Int J Mol Sci.
16:24094–24110. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kodet O, Dvořánková B, Krejčí E, Szabo P,
Dvořák P, Štork J, Krajsová I, Dundr P, Smetana K Jr and Lacina L:
Cultivation-dependent plasticity of melanoma phenotype. Tumour
Biol. 34:3345–3355. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kodet O, Dvořánková B, Bendlova B,
Sýkorová V, Krajsová I, Štork J, Kučera J, Szabo P, Strnad H, Kolář
M, et al: Microenvironment-driven resistance to B-Raf inhibition in
a melanoma patient is accompanied by broad changes of gene
methylation and expression in distal fibroblasts. Int J Mol Med.
41:2687–2703. 2018.PubMed/NCBI
|
|
19
|
Manzano JL, Layos L, Bugés C, de Los
Llanos Gil M, Vila L, Martínez-Balibrea E and Martínez-Cardús A:
Resistant mechanisms to BRAF inhibitors in melanoma. Ann Transl
Med. 4:2372016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li YC, Zou JM, Luo C, Shu Y, Luo J, Qin J,
Wang Y, Li D, Wang SS, Chi G, et al: Circulating tumor cells
promote the metastatic colonization of disseminated carcinoma cells
by inducing systemic inflammation. Oncotarget. 8:28418–28430.
2017.PubMed/NCBI
|
|
21
|
Brouwer A, De Laere B, Peeters D, Peeters
M, Salgado R, Dirix L and Van Laere S: Evaluation and consequences
of heterogeneity in the circulating tumor cell compartment.
Oncotarget. 7:48625–48643. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mignogna C, Scali E, Camastra C, Presta I,
Zeppa P, Barni T, Donato G, Bottoni U and Di Vito A: Innate
immunity in cutaneous melanoma. Clin Exp Dermatol. 42:243–250.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kodet O, Lacina L, Krejčí E, Dvořánková B,
Grim M, Štork J, Kodetová D, Vlček Č, Šáchová J, Kolář M, et al:
Melanoma cells influence the differentiation pattern of human
epidermal keratinocytes. Mol Cancer. 14:12015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lacina L, Smetana K Jr, Dvoránková B,
Pytlík R, Kideryová L, Kucerová L, Plzáková Z, Stork J, Gabius HJ
and André S: Stromal fibroblasts from basal cell carcinoma affect
phenotype of normal keratinocytes. Br J Dermatol. 156:819–829.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jobe NP, Živicová V, Mifková A, Rösel D,
Dvořánková B, Kodet O, Strnad H, Kolář M, Šedo A, Smetana K Jr, et
al: Fibroblasts potentiate melanoma cells in vitro invasiveness
induced by UV-irradiated keratinocytes. Histochem Cell Biol.
149:503–516. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dvořánková B, Lacina L and Smetana K Jr:
Isolation of normal fibroblasts and their cancer-associated
counterparts (CAFs) for biomedical research. Methods Mol Biol.
1879:393–406. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
R Core Team R: A Language and Environment
for Statistical Computing. 2017, https://www.R-project.org/
|
|
28
|
Sanz H, Aponte JJ, Harezlak J, Dong Y,
Ayestaran A, Nhabomba A, Mpina M, Maurin OR, Díez-Padrisa N,
Aguilar R, et al: drLumi: An open-source package to manage data,
calibrate, and conduct quality control of multiplex bead-based
immunoassays data analysis. PLoS One. 12:e01879012017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Colin B, Clifford S, Wu P, Rathmanner S
and Mengersen K: Using boosted regression trees and remotely sensed
data to drive decision-making. Open J Statistics. 7:859–875. 2017.
View Article : Google Scholar
|
|
30
|
Dunnett CW: A multiple comparison
procedure for comparing several treatments with a control. J Am
Stat Assoc. 50:1096–1121. 1955. View Article : Google Scholar
|
|
31
|
Porshneva K, Papiernik D, Psurski M,
Łupicka-Słowik A, Matkowski R, Ekiert M, Nowak M, Jarosz J, Banach
J, Milczarek M, et al: Temporal inhibition of mouse mammary gland
cancer metastasis by CORM-A1 and DETA/NO combination therapy.
Theranostics. 9:3918–3939. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schou IM and Marschner IC: Design of
clinical trials involving multiple hypothesis tests with a common
control. Biom J. 59:636–657. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rtsne: T-Distributed Stochastic Neighbor
Embedding using a Barnes-Hut Implementation. https://cran.r-project.org/web/packages/Rtsne/index.html
|
|
34
|
Muqaku B, Eisinger M, Meier SM, Tahir A,
Pukrop T, Haferkamp S, Slany A, Reichle A and Gerner C: Multi-omics
analysis of serum samples demonstrates reprogramming of organ
functions via systemic calcium mobilization and platelet activation
in metastatic melanoma. Mol Cell Proteomics. 16:86–99. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Weber JS, Sznol M, Sullivan RJ, Blackmon
S, Boland G, Kluger HM, Halaban R, Bacchiocchi A, Ascierto PA,
Capone M, et al: A serum protein signature associated with outcome
after anti-PD-1 therapy in metastatic melanoma. Cancer Immunol Res.
6:79–86. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ortiz A and Fuchs SY: Anti-metastatic
functions of type 1 interferons: Foundation for the adjuvant
therapy of cancer. Cytokine. 89:4–11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mizui M: Natural and modified IL-2 for the
treatment of cancer and autoimmune diseases. Clin Immunol. Nov
8–201.(Epub ahead of print). doi: 10.1016/j.clim.2018.11.002.
|
|
38
|
Arend WP: Interleukin 1 receptor
antagonist. A new member of the interleukin 1 family. J Clin
Invest. 88:1445–1451. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ma HL, Whitters MJ, Jacobson BA, Donaldson
DD, Collins M and Dunussi-Joannopoulos K: Tumor cells secreting
IL-13 but not IL-13Ralpha2 fusion protein have reduced
tumorigenicity in vivo. Int Immunol. 16:1009–1017. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lavi G, Voronov E, Dinarello CA, Apte RN
and Cohen S: Sustained delivery of IL-1 Ra from biodegradable
microspheres reduces the number of murine B16 melanoma lung
metastases. J Control Release. 123:123–130. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Alva A, Daniels GA, Wong MK, Kaufman HL,
Morse MA, McDermott DF, Clark JI, Agarwala SS, Miletello G, Logan
TF, et al: Contemporary experience with high-dose interleukin-2
therapy and impact on survival in patients with metastatic melanoma
and metastatic renal cell carcinoma. Cancer Immunol Immunother.
65:1533–1544. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Buchbinder EI, Gunturi A, Perritt J,
Dutcher J, Aung S, Kaufman HL, Ernstoff MS, Miletello GP, Curti BD,
Daniels GA, et al: A retrospective analysis of High-Dose
Interleukin-2 (HD IL-2) following Ipilimumab in metastatic
melanoma. J Immunother Cancer. 4:522016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cambien B, Richard-Fiardo P, Karimdjee BF,
Martini V, Ferrua B, Pitard B, Schmid-Antomarchi H and Schmid-
Alliana A: CCL5 neutralization restricts cancer growth and
potentiates the targeting of PDGFRβ in colorectal carcinoma. PLoS
One. 6:e288422011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Aldinucci D and Colombatti A: The
inflammatory chemokine CCL5 and cancer progression. Mediators
Inflamm. 2014:2923762014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kolar M, Szabo P, Dvořánková B, Lacina L,
Gabius HJ, Strnad H, Sáchová J, Vlček C, Plzák J, Chovanec M, et
al: Upregulation of IL-6, IL-8 and CXCL-1 production in dermal
fibroblasts by normal/malignant epithelial cells in vitro:
Immunohistochemical and transcriptomic analyses. Biol Cell.
104:738–751. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jobe NP, Rosel D, Dvořánková B, Kodet O,
Lacina L, Mateu R, Smetana K and Brábek J: Simultaneous blocking of
IL-6 and IL-8 is sufficient to fully inhibit CAF-induced human
melanoma cell invasiveness. Histochem Cell Biol. 146:205–217. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Moretti S, Chiarugi A, Semplici F, Salvi
A, De Giorgi V, Fabbri P and Mazzoli S: Serum imbalance of
cytokines in melanoma patients. Melanoma Res. 11:395–399. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Guida M, Riccobon A, Biasco G, Ravaioli A,
Casamassima A, Freschi A, Palma MD, Galligioni E, Nortilli R,
Chiarion-Sileni V, et al: Basal level and behaviour of cytokines in
a randomized outpatient trial comparing chemotherapy and
biochemotherapy in metastatic melanoma. Melanoma Res. 16:317–323.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yurkovetsky ZR, Kirkwood JM, Edington HD,
Marrangoni AM, Velikokhatnaya L, Winans MT, Gorelik E and Lokshin
AE: Multiplex analysis of serum cytokines in melanoma patients
treated with interferon-alpha2b. Clin Cancer Res. 13:2422–2428.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Jiang H, Gebhardt C, Umansky L, Beckhove
P, Schulze TJ, Utikal J and Umansky V: Elevated chronic
inflammatory factors and myeloid-derived suppressor cells indicate
poor prognosis in advanced melanoma patients. Int J Cancer.
136:2352–2360. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mocellin S, Zavagno G and Nitti D: The
prognostic value of serum S100B in patients with cutaneous
melanoma: A meta-analysis. Int J Cancer. 123:2370–2376. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jayatilaka H, Tyle P, Chen JJ, Kwak M, Ju
J, Kim HJ, Lee JSH, Wu PH, Gilkes DM, Fan R and Wirtz D:
Synergistic IL-6 and IL-8 paracrine signalling pathway infers a
strategy to inhibit tumour cell migration. Nat Commun. 8:155842017.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lu C and Kerbel RS: Interleukin-6
undergoes transition from paracrine growth inhibitor to autocrine
stimulator during human melanoma progression. J Cell Biol.
120:1281–1288. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Armstrong CA, Murray N, Kennedy M, Koppula
SV, Tara D and Ansel JC: Melanoma-derived interleukin 6 inhibits in
vivo melanoma growth. J Invest Dermatol. 102:278–284. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Uemura M, Trinh VA, Haymaker C, Jackson N,
Kim DW, Allison JP, Sharma P, Vence L, Bernatchez C, Hwu P and Diab
A: Selective inhibition of autoimmune exacerbation while preserving
the anti-tumor clinical benefit using IL-6 blockade in a patient
with advanced melanoma and Crohn's disease: A case report. J
Hematol Oncol. 9:812016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Narsale AA and Carson JA: Role of
interleukin-6 in cachexia: Therapeutic implications. Curr Opin
Support Palliat Care. 8:321–327. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Belizario JE, Fontes-Oliveira CC, Borges
JP, Kashiabara JA and Vannier E: Skeletal muscle wasting and
renewal: A pivotal role of myokine IL-6. Springerplus. 5:6192016.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Miller A, McLeod L, Alhayyani S, Szczepny
A, Watkins DN, Chen W, Enriori P, Ferlin W, Ruwanpura S and Jenkins
BJ: Blockade of the IL-6 trans-signalling/STAT3 axis suppresses
cachexia in Kras-induced lung adenocarcinoma. Oncogene.
36:3059–3066. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Pettersen K, Andersen S, Degen S, Tadini
V, Grosjean J, Hatakeyama S, Tesfahun AN, Moestue S, Kim J, Nonstad
U, et al: Cancer cachexia associates with a systemic
autophagy-inducing activity mimicked by cancer cell-derived IL-6
trans-signaling. Sci Rep. 7:20462017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Singh S, Singh AP, Sharma B, Owen LB and
Singh RK: CXCL8 and its cognate receptors in melanoma progression
and metastasis. Future Oncol. 6:111–116. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sanmamed MF, Perez-Gracia JL, Schalper KA,
Fusco JP, Gonzalez A, Rodriguez-Ruiz ME, Oñate C, Perez G, Alfaro
C, Martín-Algarra S, et al: Changes in serum interleukin-8 (IL-8)
levels reflect and predict response to anti-PD-1 treatment in
melanoma and non-small-cell lung cancer patients. Ann Oncol.
28:1988–1995. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Gabellini C, Gómez-Abenza E, Ibáñez-Molero
S, Tupone MG, Pérez-Oliva AB, de Oliveira S, Del Bufalo D and
Mulero V: Interleukin 8 mediates bcl-xL-induced enhancement of
human melanoma cell dissemination and angiogenesis in a zebrafish
xenograft model. Int J Cancer. 142:584–596. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sanmamed MF, Carranza-Rua O, Alfaro C,
Oñate C, Martín-Algarra S, Perez G, Landazuri SF, Gonzalez A, Gross
S, Rodriguez I, et al: Serum interleukin-8 reflects tumor burden
and treatment response across malignancies of multiple tissue
origins. Clin Cancer Res. 20:5697–5707. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Jayson GC, Kerbel R, Ellis LM and Harris
AL: Antiangiogenic therapy in oncology: Current status and future
directions. Lancet. 388:518–529. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Nevala WK, Vachon CM, Leontovich AA, Scott
CG, Thompson MA and Markovic SN; Melanoma Study Group of the Mayo
Clinic Cancer Center, : Evidence of systemic Th2-driven chronic
inflammation in patients with metastatic melanoma. Clin Cancer Res.
15:1931–1939. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ohm JE, Gabrilovich DI, Sempowski GD,
Kisseleva E, Parman KS, Nadaf S and Carbone DP: VEGF inhibits
T-cell development and may contribute to tumor-induced immune
suppression. Blood. 101:4878–4886. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhang L, Agarwal S, Shohet JM and Zage PE:
CD114 expression mediates melanoma tumor cell growth and treatment
resistance. Anticancer Res. 35:3787–3792. 2015.PubMed/NCBI
|
|
68
|
Zage PE, Whittle SB and Shohet JM: CD114:
A new member of the neural crest-derived cancer stem cell marker
family. J Cell Biochem. 118:221–231. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Waldmann TA: Cytokines in cancer
immunotherapy. Cold Spring Harb Perspect Biol. 10(pii):
a0284722018. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Kumari N, Dwarakanath BS, Das A and Bhatt
AN: Role of interleukin-6 in cancer progression and therapeutic
resistance. Tumour Biol. 37:11553–11572. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Elmarakby AA and Sullivan JC: Relationship
between oxidative stress and inflammatory cytokines in diabetic
nephropathy. Cardiovasc Ther. 30:49–59. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Vallée A and Lecarpentier Y: Crosstalk
between peroxisome proliferator-activated receptor gamma and the
canonical WNT/β-catenin pathway in chronic inflammation and
oxidative stress during carcinogenesis. Front Immunol. 9:7452018.
View Article : Google Scholar : PubMed/NCBI
|