Introduction
Chronic myeloid leukemia (CML) is a hematopoietic
clonal disease characterized by the accumulation of myeloid lineage
cells harboring the BCR-ABL1 fusion gene, which results from
the formation of the Philadelphia (Ph) chromosome in hematopoietic
stem cells. Although this disease is regarded as a life-threatening
hematologic malignancy, the advent of tyrosine kinase inhibitors
(TKIs) has revolutionized the management of patients with CML. The
International Randomized Study of Interferon and STI571 trial
conducted in chronic phase (CP) CML patients demonstrated excellent
treatment responses and more durable remissions in patients treated
with imatinib than in those who received the interferon-α plus
cytarabine regimen (1). A recent
long-term observation study also revealed that the 10-year overall
survival (OS) rate in its imatinib arm was >80%, with over half
of the deaths in this arm due to reasons unrelated to CML (1). The efficacy of imatinib has also been
demonstrated in Japanese patients (2,3). Thus,
imatinib therapy has become a standard of care for patients with
CML. In the era of TKI, the OS rate in CML-CP patients who achieve
complete cytogenetic response (CCyR) or better is similar to that
in the general population (4).
However, some patients remain refractory to this therapy and
experience disease progression.
The existence of additional clonal chromosomal
abnormalities in the Ph+ cells (CCA/Ph+) of
patients with CML-CP at diagnosis is associated with poor
prognosis, particularly in those with specific CCAs, referred to as
‘major route abnormalities’ in the pre-imatinib era. Such
abnormalities include trisomy 8, +der(22)t(9;22)(q34;q11),
isochromosome 17 (i(17)(q10)), trisomy 19, and
ider(22)(q10)t(9;22)(q34;q11). The rare CCAs, such as trisomy 21,
t(3;12), t(4;6), t(2;16), and t(1;21), are designated ‘minor route’
CCAs (5–10). Notably, the clinical significance of
harboring CCA/Ph+ has mostly been evaluated in patients
treated with first-line imatinib (7–10).
Patients with CML who concomitantly harbor these abnormalities are
designated as possessing ‘warning’ criteria, according to the
European LeukemiaNet (ELN) 2013 recommendations, and are,
therefore, required to be monitored carefully (6). However, there are no recommended
treatment guidelines for patients with these abnormalities.
Over the past decade, second-generation TKIs, such
as nilotinib, dasatinib, and bosutinib, as well as the
third-generation TKI, ponatinib, were developed for patients
resistant or intolerant to prior TKI therapy. In addition to
imatinib, second-generation TKIs, nilotinib and dasatinib, are now
recommended as first-line therapies for patients with CML-CP
according to the ELN 2013 recommendations, which were based on data
from larger randomized studies (6,11,12). Imatinib, nilotinib, and dasatinib are
currently available as first-line treatments for patients with
CML-CP in Japan. This warrants reevaluation of the impact of the
presence of CCA/Ph+ on patient clinical outcomes in the
era of second-generation TKIs.
In the present study, the treatment responses and
outcomes of CML-CP patients with CCA/Ph+ treated with
second-generation TKIs were investigated. The present data should
help to devise current era TKI treatment regimens that are
optimized according to cytogenetic risk stratification.
Patients and methods
Patients
The data of patients enrolled in the CML Cooperative
Study Group (CML-CSG) database (original study has been reported in
2017 (13)), that encompassed 5
university hospitals (Saitama Medical University International
Medical Center, Nihon University School of Medicine, Saitama
Medical Center Saitama Medical University, Juntendo University
School of Medicine and Kumamoto University Hospital) and 4
university branch hospitals (Juntendo University Nerima Hospital,
Juntendo University Urayasu Hospital, Yokohama Municipal Citizen's
Hospital and Saiseikai Yokohama Nanbu Hospital) was analyzed. The
study included patients diagnosed with CML-CP and treated with a
TKI as first-line therapy. The diagnosis of CML-CP was based on the
ELN criteria as previously described (6). Patients who had received interferon-α or
any other chemotherapeutic agent for CML before TKI administration
were excluded; however, the administration of hydroxyurea prior to
TKI therapy for the purpose of reducing the number of leukocytes
was allowed. Between April 2001 and January 2016, 369 patients
newly diagnosed with a Ph chromosome and/or BCR-ABL1
positive CML were registered in the CML-CSG database. The study was
approved by the institutional review boards of all the above nine
participating facilities and was conducted in accordance with the
Declaration of Helsinki.
Cytogenetic studies
Results of cytogenetic bone marrow analyses obtained
at the time of CML diagnosis were registered in the CML-CSG
database. Twenty metaphases were routinely counted and analyzed in
each patient according to the International System for Human
Cytogenetic Nomenclature recommendations. Major route
CCA/Ph+ was defined as Ph+ cells harboring
trisomy 8, +der(22)t(9;22)(q34;q11), isochromosome 17 (i(17)(q10)),
trisomy 19, or ider(22)(q10)t(9;22)(q34;q11), and the minor route
CCA/Ph+ was defined as Ph+ cells harboring
rare abnormalities such as trisomy 21, t(3;12), t(4;6), t(2;16),
t(1;21), -Y, a variant translocation t(v;22), or other less common
abnormalities. CCA/Ph+ detected in only 1 metaphase was
not considered a clonal cytogenetic abnormality.
Molecular response assessment
The molecular response was assessed by quantifying
the BCR-ABL1 transcript using real-time quantitative PCR or
a transcription-mediated amplification (TMA) and a hybridization
protection assay (14,15). BCR-ABL1 transcript levels ≤0.1%
according to the International Scale (IS) or ≤100 copies/µg RNA as
determined using the TMA assay were considered a major molecular
response (MMR), while BCR-ABL1 IS transcript levels of
≤0.0032% were considered a deep molecular response (DMR), as
previously described (14,15).
Statistical analysis
Fisher's exact and Mann-Whitney U tests were used to
determine statistically significant differences between the groups.
Event-free survival (EFS) was defined as the period between the
date of commencing treatment with TKI and the date of the first
incidence of any event or the last follow-up. An event was defined
as the loss of treatment efficacy after achieving complete
hematologic response, partial cytogenetic response, or CCyR
(6); progression to the accelerated
or blast phase; or death from any cause. Treatment responses were
assessed as the cumulative MMR or DMR achieved at each time-point,
irrespective of switching TKIs. Univariate and multivariate
analyses were performed to identify whether CCA/Ph+ had
a negative impact on outcomes. The Sokal score, type of initial TKI
(imatinib or second-generation TKIs), and presence of CCA/Ph+ were
included in the analysis. A P-value of <0.05 was considered
statistically significant. The statistical analyses were performed
using EZR (Saitama Medical Center, Jichi Medical University), a
graphical user interface for the R programming language (The R
Foundation for Statistical Computing) (16).
Results
Patient characteristics
Between April 2001 and January 2016, 369 patients
newly diagnosed with a Ph chromosome and/or BCR-ABL1
positive CML were registered in the CML-CSG database. Among these
patients, 328 had data on their bone marrow chromosomal analyses at
diagnosis, 9 were in the accelerated phase, and 32 lacked data for
cytogenetic analysis (i.e., cryptic Ph, insufficient number of
mitotic cells obtained, or missing data altogether). Of the 328
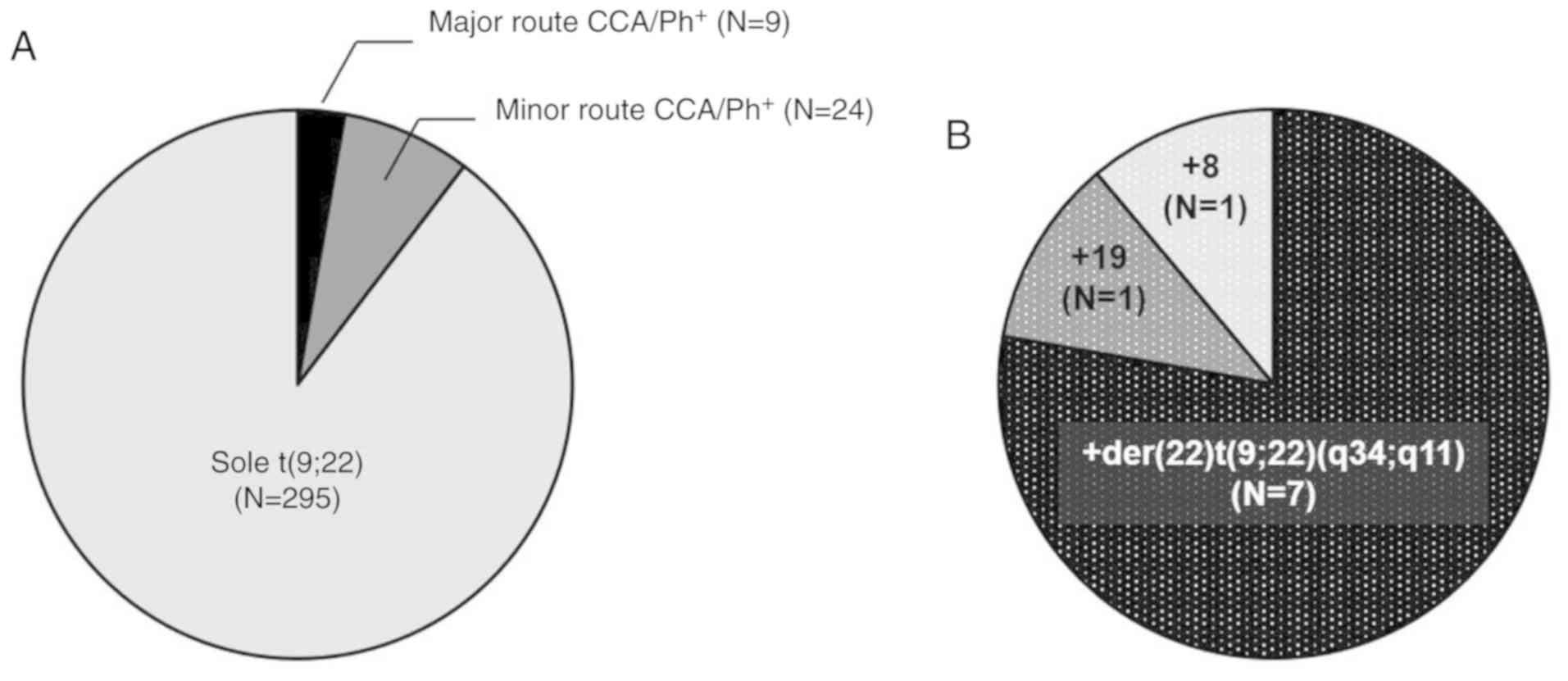
eligible patients, 9 had major route CCA/Ph+, including
7 with +der(22)t(9;22)(q34;q11), 1 with trisomy 19, and 1 with
trisomy 8, and 24 had minor route CCA/Ph+ (Fig. 1). The characteristics of patients with
or without CCA/Ph+ at diagnosis are presented in
Tables I and SI. There were no significant differences
between the groups in terms of median patient age; platelet,
leukocyte, eosinophil, and basophil counts; and spleen size.
However, the proportion of blasts was higher in the
CCA/Ph+ group. Risk stratification by the Sokal,
Hasford, and European Treatment and Outcome Study (EUTOS) scoring
systems were similar between the groups. Furthermore, the use of
second-generation TKIs as the first-line therapies was equally
distributed between the groups.
 | Table I.Baseline characteristics of patients
before treatment according to cytogenetic abnormality. |
Table I.
Baseline characteristics of patients
before treatment according to cytogenetic abnormality.
| Factors | With
CCA/Ph+ (N=33) | Only t(9;22)
(N=295) | P-value |
|---|
| Age (years), median
(range) | 51 (24–82) | 53 (18–86) | 0.508 |
| Sex male, n
(%) | 23 (70) | 176 (60) | 0.348 |
| Hemoglobin (g/dl),
median (range) | 13.0
(8.4–15.6) | 13.0
(5.0–18.8) | 0.932 |
| Platelet number
(×109/l), median (range) | 463
(115–3,417) | 512
(86–4,352)a | 0.382 |
| Leukocyte number
(×109/l), median (range) | 39.1
(13.2–215.9) | 34.1
(5.2–719.8) | 0.927 |
|
Eosinophils (%), median
(range) | 2.0 (0–20.0) | 2.0 (0–24.0) | 0.688 |
|
Basophils (%), median
(range) | 5.0 (0–17.0) | 5.0 (0–19.5) | 0.708 |
| Blasts
(%), median (range) | 0.5 (0–13.5) | 0
(0–13.0) | 0.028 |
| Spleen size (cm),
median (range) | 0 (0–20) | 0 (0–27) | 0.106 |
| Sokal scoring
system, n (%) |
|
|
|
|
Low | 15 (45) | 122 (41) | 0.824 |
|
Intermediate | 10 (30) | 107 (36) |
|
|
High | 6
(18) | 49
(17) |
|
| Hasford scoring
system, n (%) |
|
|
|
|
Low | 14 (42) | 115 (39) | 0.650 |
|
Intermediate | 13 (39) | 135 (46) |
|
|
High | 4
(12) | 28 (9) |
|
| EUTOS scoring
system, n (%) |
|
|
|
|
Low | 24 (73) | 239 (81) | 0.193 |
|
High | 7
(21) | 39
(13) |
|
| Scoring unknown, n
(%) | 2 (6) | 17 (6) |
|
| Initial TKI, n
(%) |
|
|
|
|
Imatinib | 18 (55) | 154 (52) | 0.395 |
|
Dasatinib | 6
(18) | 82
(28) |
|
|
Nilotinib | 9
(27) | 59
(20) |
|
Treatment outcomes according to the
CCA/Ph+ status
The outcomes of patients with CCA/Ph+
were then investigated and compared to those of patients without
additional aberrations. During the follow-up period, 36 events
occurred and 24 patients (7.3%) died. Among these 24, 8 (33.3%)
succumbed due to the progression of CML. With a median follow-up
period of 66 months (range: 14–167 months) in patients with
CCA/Ph+ and 67 months (range: 0–202 months) in those
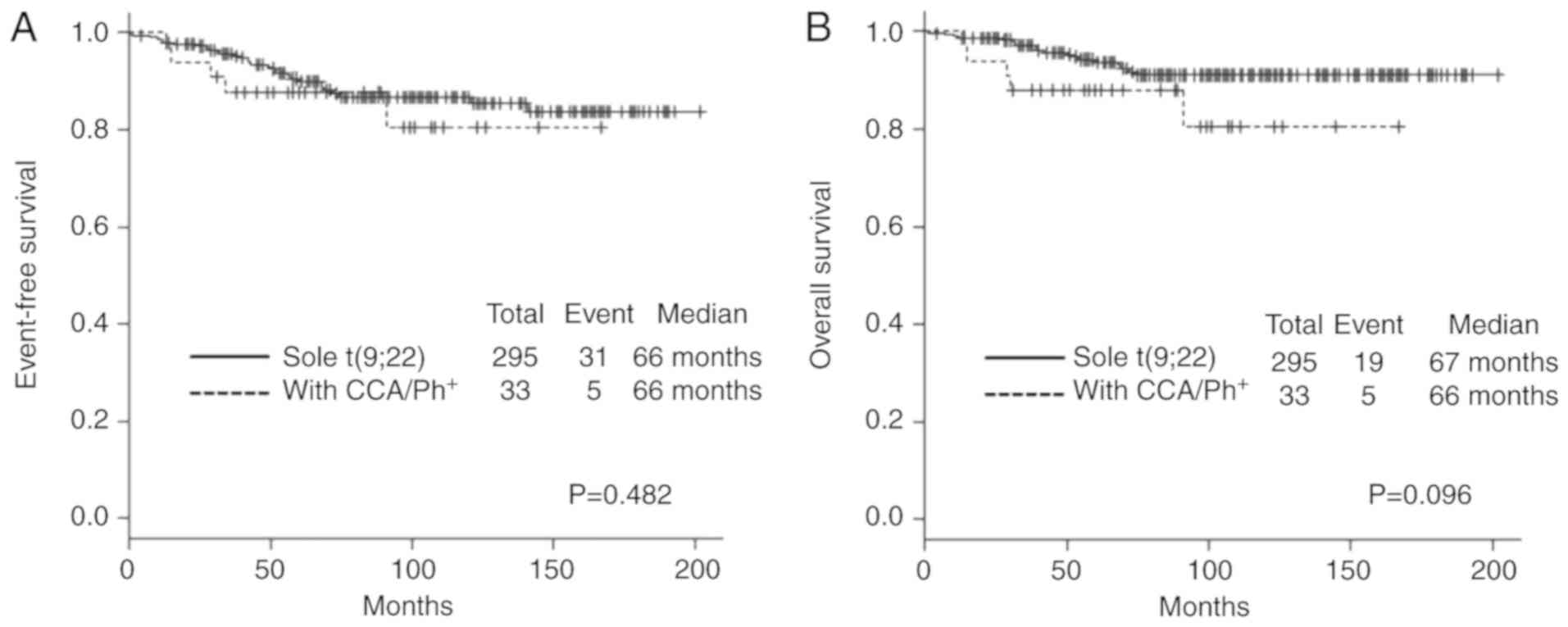
without CCA/Ph+, 5-year EFS rates were 87.8% in the
former group and 90.0% in the latter (P=0.482) (Fig. 2A). Furthermore, 5-year OS rates were
87.9% in the former group and 93.7% in the latter (P=0.096)
(Fig. 2B). Thus, the presence of
CCA/Ph+ was not an adverse prognostic factor among our
patients. Univariate and multivariate analyses revealed that EFS
and OS were shorter in Sokal high-risk patients than in those
considered to be low risk. The analysis confirmed that the presence
of CCA/Ph+ in CML-CP was not a statistically significant
adverse prognostic factor for EFS and OS in our cohort (Table II).
 | Table II.Analysis of the risk factors
associated with the EFS and the OS. |
Table II.
Analysis of the risk factors
associated with the EFS and the OS.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Factor | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| EFS |
| Sokal
high vs. others | 2.24
(1.07–4.70) | 0.032 | 2.26
(1.08–4.72) | 0.043 |
| Initial
TKI imatinib vs. others | 0.69
(0.33–1.41) | 0.307 | 0.62
(0.30–1.31) | 0.210 |
| With
CCA/Ph+ vs. others | 1.40
(0.54–3.61) | 0.485 | 1.55
(0.60–4.02) | 0.365 |
| OS |
| Sokal
high vs. others | 3.06
(1.28–7.31) | 0.012 | 3.09
(1.29–7.36) | 0.011 |
| Initial
TKI imatinib vs. others | 0.78
(0.33–1.88) | 0.587 | 0.68
(0.27–1.69) | 0.405 |
| With
CCA/Ph+ vs. others | 2.26
(0.84–6.04) | 0.106 | 2.64
(0.97–7.16) | 0.057 |
Treatment responses in patients with
CCA/Ph+
The responses to TKI therapy among patients with
CCA/Ph+ (Table III) were
further investigated. Among the 33 patients with
CCA/Ph+, the 12-month and overall MMR rates were 63.6
and 90.9%, respectively, while the 24-month and overall DMR rates
were 15.2 and 57.6%, respectively. Among the 295 patients without
CCA/Ph+, the 12-month and overall MMR rates were 51.2
and 89.8%, respectively, and the 24-month and overall DMR rates
were 15.6 and 57.6%, respectively. None of the differences were
statistically significant, suggesting that the presence of
CCA/Ph+ did not affect the response to TKI therapy.
 | Table III.Outcome of patients according to
CCA/Ph+ status. |
Table III.
Outcome of patients according to
CCA/Ph+ status.
|
| With
CCA/Ph+ (N=33) | Only t(9;22)
(N=295) | P-value |
|---|
| Cumulative MMR rate
(%) |
|
|
|
| 12
months | 63.6 | 51.2 | 0.201 |
|
Overall | 90.9 | 89.8 | 1.000 |
| Cumulative DMR rate
(%) |
|
|
|
| 24
months | 15.2 | 15.6 | 1.000 |
|
Overall | 57.6 | 57.6 | 1.000 |
| Events |
|
|
|
| Loss of
treatment efficacy (%) | 9.1 | 5.8 | 0.438 |
|
Progression to AP/BP (%) | 9.1 | 2.4 | 0.068 |
| Death
(%) | 15.2 | 6.4 | 0.079 |
|
Related to CML,
n | 3 | 5 |
|
|
Unrelated to CML,
n | 2 | 14 |
|
| HSCT (%) | 3.0 | 1.0 | 0.347 |
| Secondary
malignancies (%) | 12.1 | 5.1 | 0.111 |
Treatment responses and prognoses in
patients with major route CCA/Ph+
Finally, it was investigated whether major route
CCA/Ph+ affected the clinical outcomes of these patients
given that the presence of this abnormality at diagnosis is a
critical adverse prognostic factor in patients treated with
first-line imatinib (7,10). Table IV
presents the characteristics and treatment response of the 9
patients with major route and 33 patients with minor route
CCA/Ph+, respectively, their treatment regimens, best
responses to TKI, and outcomes. With respect to initial therapy, in
major route CCA/Ph+, 5 patients were treated with
imatinib, 3 with nilotinib, and 1 with dasatinib; none experienced
disease progression, and all are alive at the date of the writing
of this manuscript. All 9 attained an MMR and retained the
therapeutic benefit of TKI, and 8 attained a DMR during the
observation period. Notably, treatment with second-generation TKIs
resulted in an excellent treatment response; all 4 patients
initially treated with nilotinib or dasatinib achieved MMR within
12 months, which is considered an optimal response according to the
ELN 2013 recommendations (6). The
duration between TKI initiation and the achievement of MMR was
shorter in the second-generation TKI-treated group than in the
imatinib-treated group (median, 5 vs. 10 months) in major route
CCA/Ph+. All 3 patients in minor route
CCA/Ph+ who were unable to achieve MMR experienced
disease progression later.
 | Table IV.Characteristics and treatment
response in patients with CCA/Ph+. |
Table IV.
Characteristics and treatment
response in patients with CCA/Ph+.
|
|
|
|
|
| Best response |
|
|---|
|
|
|
|
|
|
|
|
|---|
| Age (years) | Sex | Subgroup of
CCA/Ph+ | Initial TKI
(mg/day) | MMR (months) | CCyR | MMR | DMR | Disease
progression |
|---|
| 45 | F | trisomy 19 | Imatinib 400 | 22 | √ | √ | √ | No |
| 64 | F |
+der(22)t(9;22)(q34;q11) | Imatinib 400 | 10 | √ | √ | √ | No |
| 49 | M | trisomy 8 | Imatinib 400 | 6 | √ | √ | √ | No |
| 51 | M |
+der(22)t(9;22)(q34;q11) | Imatinib 400 | 5 | √ | √ | √ | No |
| 40 | M |
+der(22)t(9;22)(q34;q11) | Imatinib 400 | 14 | √ | √ | √ | No |
| 73 | F |
+der(22)t(9;22)(q34;q11) | Dasatinib 100 | 3 | √ | √ | √ | No |
| 38 | F |
+der(22)t(9;22)(q34;q11) | Nilotinib 600 | 6 | √ | √ |
| No |
| 72 | F |
+der(22)t(9;22)(q34;q11) | Nilotinib 300 | 3 | √ | √ | √ | No |
| 50 | M |
+der(22)t(9;22)(q34;q11) | Nilotinib 600 | 7 | √ | √ | √ | No |
| 69 | M | t(X;9;22;26)
(p11.2;q34;q11.2;p13.3) | Imatinib 300 | 5 | √ | √ | √ | No |
| 79 | M | inv(8)(p21q22) | Imatinib 300 | 45 | √ | √ |
| No |
| 82 | M |
−14,del(22)(q13) | Imatinib 400 | 37 | √ | √ |
| No |
| 52 | M |
t(9;22;12)(q34;q11;p13) | Imatinib 400 | 6 | √ | √ | √ | No |
| 69 | M |
t(3;9;22)((q21;q34;q11.2) | Imatinib 400 | 3 | √ | √ |
| No |
| 38 | M |
t(8;9;22)(q24+q24+11.2) | Imatinib 400 | 7 | √ | √ |
| No |
| 43 | M |
t(8;12)(p21:p13) | Imatinib 400 | NR | √ |
|
| Yes |
| 24 | M |
t(3;12)(q12;q13),t(4;5) (p34;p15),
add(9)(q34), t(9;22;12)(q34;q11;p13) | Imatinib 400 | NR | √ |
|
| Yes |
| 48 | M |
t(9;22;11)(q34;q11.2;q13) | Imatinib 400 | 19 | √ | √ | √ | No |
| 69 | M |
t(5;7)(q35;q11.2) | Imatinib 400 | 82 | √ | √ |
| No |
| 39 | F |
inv(9)(p12q13),t(9;22;14)
(q34;q11.2;q11.2) | Imatinib 400 | 10 | √ | √ | √ | No |
| 82 | F | -X | Imatinib 400 | 6 | √ | √ | √ | No |
| 34 | F |
t(9;22;21)(q34;q11.2;q22) | Imatinib 400 | 5 | √ | √ | √ | No |
| 64 | M |
t(9;22;14)(q34;q11.2;q32) | Dasatinib 50 | 8 | √ | √ |
| No |
| 64 | M | -Y | Dasatinib 100 | 2 | √ | √ | √ | No |
| 72 | M | inv(3)(p13q27) | Dasatinib 100 | 11 | √ | √ |
| No |
| 50 | M |
t(6;9;22)(p21;q34;q11.2) | Dasatinib 100 | 4 | √ | √ | √ | No |
| 79 | M | -Y | Dasatinib 140 | NR | √ |
|
| Yes |
| 51 | F |
t(9;22;15)(q34;q11.2;q24) | Nilotinib 300 | 6 | √ | √ |
| No |
| 24 | M | del(9)(q?),
add(10)(p11,2) | Nilotinib 600 | 38 | √ | √ |
| No |
| 31 | M |
t(9;22;17)(q34:q11.2:p13) | Nilotinib 600 | 6 | √ | √ | √ | No |
| 65 | M | -Y | Nilotinib 600 | 45 | √ | √ |
| No |
| 49 | M |
t(8;10)(p10;p10) | Nilotinib 600 | 23 | √ | √ | √ | No |
| 42 | F | add(3)(q21),
der(9), add(22)(q11.2) | Nilotinib 600 | 6 | √ | √ | √ | No |
Discussion
To date, studies on the relationship between the
CCA/Ph+ status and treatment outcome have mostly been
conducted in patients treated with the first-in-class TKI imatinib.
Because little is known regarding the impact of the presence of
CCA/Ph+ at diagnosis on clinical outcomes in the current
era of new-generation TKIs, the present study aimed to address this
gap in knowledge using the CML-CSG database. It also focused on
whether major route CCA/Ph+ remains an adverse
prognostic factor in the era of newer TKIs. The present results
revealed no evident differences in EFS and OS between patients with
and without CCA/Ph+. In the entire cohort, only 4
patients received allogeneic stem cell transplantation, including 3
in CP (2 in CP1 and 1 in CP2) and one after blastic transformation;
the last case had CCA/Ph+ at initial diagnosis. During
the follow-up period, 24 patients (7.3%) died. Among them, 8
(33.3%) succumbed to progression of CML, 2 (8.3%) of secondary
malignancies (lung cancer, rectal cancer), 2 of congestive heart
failure, 1 (4.2%) of synchronous pancreatic cancer, 1 from
cardiopulmonary arrest on arrival suspected to be due to a
cardiovascular event, 1 from suicide, and 9 (37.5%) from unknown
causes. Twenty patients experienced loss of treatment efficacy, 3
of whom had CCA/Ph+; 10 of these patients progressed to
accelerated or blast phase, 3 of whom had CCA/Ph+. With
respect to the secondary malignancies, the sites included stomach
(N=4) and colorectal (N=3), with oral, tongue, larynx, esophagus,
breast, lung, liver, gallbladder, renal pelvis, prostate, ovary,
and malignant lymphoma accounting for the remaining 12. We have
already evaluated and published the incidence of secondary
malignancies in our CML-CSG cohort (17). It is of interest that cumulative
incidences of secondary malignancies in our findings are consistent
with those observed in a recent study (18). In terms of the treatment responses in
patients with major route CCA/Ph+, the achievement of
MMR (a critical determinant of patient prognosis) occurred earlier
in those treated with second-generation TKIs than in those treated
with imatinib. Treatment responses in patients with
CCA/Ph+ demonstrated that the achievement of MMR within
12 months has a tendency to sustain DMR later, as previously
reported (19). Although this study
population included 113 patients who were diagnosed before the
approval of the second-generation TKIs in March 2009, all but 8
were followed until approval for nilotinib/dasatinib or thereafter.
Moreover, nearly half of our study's cohort was initially treated
with the second-generation TKIs nilotinib or dasatinib. It should
thus be noted that the outcomes obtained in our study population
reflected the use of various TKIs. We have recently revealed that
the EUTOS score was the most predictive factor for the outcomes of
patients among three scoring systems (Sokal, Hasford, or EUTOS),
however, the use of second-generation TKIs could overcome the
impact of EUTOS high-risk scores (20).
Previous studies conducted with imatinib revealed
that the presence of CCA/Ph+ at diagnosis was an adverse
prognostic factor in patients with CML-CP (7,8). The
overall MMR rate was also reported to be lower in patients with
CCA/Ph+ than in those without CCA/Ph+
(7,8).
Moreover, based on the data of 1,151 patients enrolled in the
German CML-Study IV who received first-line imatinib therapy, those
with major route CCA/Ph+ had significantly poorer
prognoses than those with t(9;22) only, although those with minor
route CCA/Ph+ did not (7). Based on
these data, the ELN 2013 recommendations defined major route
CCA/Ph+ at baseline as a ‘warning’ criterion and
regarded the emergence of CCA/Ph+ during treatment as
‘failure’ (6). Therefore, close
observation and early intervention were warranted for patients with
major route CCA/Ph+ at diagnosis during the imatinib era
(7). In contrast to these collective
data, the latest investigation of the clinical significance of
major route CCA/Ph+ at diagnosis revealed that the
abnormality had no impact on prognosis, which is consistent with
our results (21). Notably, that
study consisted of 603 patients from the MD Anderson Cancer Center,
among whom 324 were initially treated with new-generation TKIs and
207 received high-dose imatinib (21). The discrepancy between the results of
recent studies and older ones is presumably due to the different
treatment modalities used for each lesion or in each institution.
Thus, recent data indicated that the use of new-generation TKIs may
overcome the adverse impact of CCA/Ph+ at diagnosis.
Imatinib, nilotinib, and dasatinib are equally recommended as the
first-line therapy for CML-CP according to the ELN2013
recommendations. Noatbly, the majority of newly diagnosed patients
with CML are now initially treated with the second-generation TKIs
nilotinib or dasatinib in Japan. Although the present study
demonstrated that second-generation TKIs effectively induced faster
and deeper molecular responses even in patients with
CCA/Ph+, the benefits and risks for the use of
second-generation TKIs should be carefully taken into
consideration. In fact, our previous study revealed the frequent
incidence of vascular adverse events from treatment with
second-generation TKIs (22). These
benefits and risks should be investigated in a large well-designed
study population.
Although the etiology of CCA/Ph+ is
uncertain, a higher proportion of blasts was observed in the
peripheral blood of patients with CCA/Ph+ than in those
without CCA/Ph+, which may reflect the aggressive
character of the disease. Some evidence suggests that the
structural alteration of the chromosome is closely linked to an
increase in genomic instability (23,24). Not
only could the numerical chromosomal abnormalities be detected
using standard cytogenetic analysis but also multiple genetic
aberrations were discovered in patients with CML-CP using sensitive
genomic hybridization and single-nucleotide polymorphism analysis
(25). The accumulation of these
additional genetic aberrations represents an underlying genomic
instability and has a detrimental effect on the maintenance of
normal cell physiology, which may explain why the use of TKIs with
more potent and/or broad tyrosine kinase inhibitory activity is
beneficial for patients with CCA/Ph+.
There were some limitations in the present study.
Results of chromosomal analysis of the bone marrow at diagnosis
were only available for 328 of the 360 patients with CML-CP
registered in the CML-CSG database; the unavailability of data from
32 patients may have skewed our findings. Moreover, the results of
chromosomal analyses were reported from each institution without
central review; therefore, a lack of interobserver uniformity may
exist. There may be some bias that could have resulted in the
underestimation of molecular response rates because molecular
evaluations were unavailable in some patients. Furthermore, the
treatment strategy mainly depended on the decisions of the
physicians, which may have led to varying doses and treatment
optimizations for each type of TKI. Thus, the switch to a different
TKI and the loss of molecular response were not considered as an
event in the present study, in accordance with most pivotal
studies. TKI discontinuation is not recommended in the clinical
setting according to the guidelines for CML in Japan; therefore,
this study did not include analysis for TKI discontinuation. A
large-scale observation study for TKI discontinuation is currently
planned by the Japanese Society of Hematology Committee, and
outcomes for TKI discontinuation in our database are going to be
united. Whether the presence of CCA/Ph+ affects the
result of TKI discontinuation will be clarified in the future.
In conclusion, the study revealed no clinical
differences in treatment responses and outcomes between patients
with and without CCA/Ph+ in the era of second-generation
TKIs. The accumulation of more evidence regarding the prognostic
significance of patients with major route CCA/Ph+ in the
era of newer TKIs will provide helpful information on treatment
strategies for patients with this abnormality. Since the proportion
of patients with CCA/Ph+ in the present study was
relatively small, the findings should be validated in larger and
well-established populations.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding information is not applicable.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article. The datasets used and/or
analyzed during the current study are available from the
corresponding author on reasonable request.
Authors' contributions
TK, MT, MI, and NI conceived and designed the study.
MI and NI contributed to analysis and interpretation of data and
wrote the manuscript. All other authors contributed to data
collection and interpretation and have read and approved the final
manuscript.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional review board of all participating facilities and with
the 1964 Helsinki declaration and its later amendments or
comparable ethical standards. The approval number of the
institutional review board of Saitama Medical Center, Saitama
Medical University; as representative facility, is No. 1348,
January 2016.
Patient consent for publication
The requirement for informed consent was not
applicable owing to the study's retrospective nature.
Competing interests
NI received honoraria and speaker fees from
Bristol-Myers Squibb, Novartis Pharma K.K., Otsuka Pharmaceutical,
and Pfizer Inc. MT received honoraria and speaker fees from Pfizer
Inc. and Bristol-Myers Squibb. MK received honoraria and speaker
fees from Bristol-Myers Squibb and Novartis Pharma K.K. TT and YH
received honoraria from Bristol-Myers Squibb and Novartis Pharma
K.K. TK received honoraria and speaker fees from Bristol-Myers
Squibb, Novartis Pharma K.K., and Pfizer Inc. The remaining authors
declare no competing financial interests. None of the authors have
non-financial conflicts of interest to declare.
Glossary
Abbreviations
Abbreviations:
|
CCA/Ph+
|
clonal chromosomal abnormalities in
the Philadelphia chromosome
|
|
CCyR
|
complete cytogenetic response
|
|
CML
|
chronic myeloid leukemia
|
|
CP
|
chronic phase
|
|
DMR
|
deep molecular response
|
|
EFS
|
event-free survival
|
|
ELN
|
European LeukemiaNet
|
|
IS
|
International Scale
|
|
MMR
|
major molecular response
|
|
OS
|
overall survival
|
|
TKI
|
tyrosine kinase inhibitor
|
|
TMA
|
transcription-mediated
amplification
|
References
|
1
|
Hochhaus A, Larson RA, Guilhot F, Radich
JP, Branford S, Hughes TP, Baccarani M, Deininger MW, Cervantes F,
Fujihara S, et al: Long-term outcomes of imatinib treatment for
chronic myeloid leukemia. N Engl J Med. 376:917–927. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kizaki M, Takahashi N, Iriyama N, Okamoto
S, Ono T, Usui N, Inokuchi K, Nakaseko C, Kurokawa M, Sumi M, et
al: Efficacy and safety of tyrosine kinase inhibitors for newly
diagnosed chronic-phase chronic myeloid leukemia over a 5-year
period: Results from the Japanese registry obtained by the New
TARGET system. Int J Hematol. 109:426–439. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tauchi T, Kizaki M, Okamoto S, Tanaka H,
Tanimoto M, Inokuchi K, Murayama T, Saburi Y, Hino M, Tsudo M, et
al: Seven-year follow-up of patients receiving imatinib for the
treatment of newly diagnosed chronic myelogenous leukemia by the
TARGET system. Leuk Res. 35:585–590. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sasaki K, Strom SS, O'Brien S, Jabbour E,
Ravandi F, Konopleva M, Borthakur G, Pemmaraju N, Daver N, Jain P,
et al: Prospective analysis: Relative survival in patients with
chronic myeloid leukemia in chronic phase in the era of tyrosine
kinase inhibitors. Lancet Haematol. 5:e186–e193. 2015. View Article : Google Scholar
|
|
5
|
Mitelman F: The cytogenetic scenario of
chronic myeloid leukemia. Leuk Lymphoma 11:. (Suppl 1):S11–S15.
1993. View Article : Google Scholar
|
|
6
|
Baccarani M, Deininger MW, Rosti G,
Hochhaus A, Soverini S, Apperley JF, Cervantes F, Clark RE, Cortes
JE, Guilhot F, et al: European LeukemiaNet recommendations for the
management of chronic myeloid leukemia: 2013. Blood. 122:872–884.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fabarius A, Leitner A, Hochhaus A, Müller
MC, Hanfstein B, Haferlach C, Göhring G, Schlegelberger B,
Jotterand M, Reiter A, et al: Impact of additional cytogenetic
aberrations at diagnosis on prognosis of CML: long-term observation
of 1,151 patients from the randomized CML study IV. Blood.
118:6760–6768. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Luatti S, Castagnetti F, Marzocchi G,
Baldazzi C, Gugliotta G, Iacobucci I, Specchia G, Zanatta L,
Rege-Cambrin G, Mancini M, et al: Additional chromosomal
abnormalities in Philadelphia-positive clone: Adverse prognostic
influence on frontline imatinib therapy: A GIMEMA Working Party on
CML analysis. Blood. 120:761–767. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee SE, Choi SY, Bang JH, Kim SH, Jang EJ,
Byeun JY, Park JE, Jeon HR, Oh YJ, Kim M and Kim DW: The long-term
clinical implications of clonal chromosomal abnormalities in newly
diagnosed chronic phase chronic myeloid leukemia patients treated
with imatinib mesylate. Cancer Genet 205:. 205:563–571. 2012.
View Article : Google Scholar
|
|
10
|
Fabarius A, Kalmanti L, Dietz CT, Lauseker
M, Rinaldetti S, Haferlach C, Göhring G, Schlegelberger B,
Jotterand M, Hanfstein B, et al: Impact of unbalanced minor route
versus major route karyotypes at diagnosis on prognosis of CML. Ann
Hematol. 94:2015–2024. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kantarjian H, Shah NP, Hochhaus A, Cortes
J, Shah S, Ayala M, Moiraghi B, Shen Z, Mayer J, Pasquini R, et al:
Dasatinib versus imatinib in newly diagnosed chronic-phase chronic
myeloid leukemia. N Engl J Med. 362:2260–2270. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saglio G, Kim DW, Issaragrisil S, le
Coutre P, Etienne G, Lobo C, Pasquini R, Clark RE, Hochhaus A,
Hughes TP, et al: Nilotinib versus imatinib for newly diagnosed
chronic myeloid leukemia. N Engl J Med. 362:2251–2259. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Iriyama N, Tokuhira M, Takaku T, Sato E,
Ishikawa M, Nakazato T, Sugimoto KJ, Fujita H, Fujioka I, Hatta Y,
et al: Incidences and outcomes of therapy-related chronic myeloid
leukemia in the era of tyrosine kinase inhibitors: Surveillance of
the CML Cooperative Study Group. Leuk Res. 54:55–58. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cross NC, White HE, Müller MC, Saglio G
and Hochhaus A: Standardized definitions of molecular response in
chronic myeloid leukemia. Leukemia. 26:2172–2175. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakamae H, Yoshida C, Miyata Y, Hidaka M,
Uike N, Koga D, Sogabe T, Matsumura I, Kanakura Y and Naoe T: A new
diagnostic kit, ODK-1201, for the quantitation of low major BCR-ABL
mRNA level in chronic myeloid leukemia: Correlation of quantitation
with major BCR-ABL mRNA kits. Int J Hematol. 102:304–311. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakazato T, Iriyama N, Tokuhira M,
Ishikawa M, Sato E, Takaku T, Sugimoto KJ, Fujita H, Fujioka I,
Kimura Y, et al: Incidence and outcome of second malignancies in
patients with chronic myeloid leukemia during treatment with
tyrosine kinase inhibitors. Med Oncol. 35:992018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sasaki K, Kantarjian HM, O'Brien S,
Ravandi F, Konopleva M, Borthakur G, Garcia-Manero G, Wierda WG,
Daver N, Ferrajoli A, et al: Incidence of second malignancies in
patients with chronic myeloid leukemia in the era of tyrosine
kinase inhibitors. Int J Hematol. 109:545–552. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sasaki K, Kantarjian H, O'Brien S, Ravandi
F, Konopleva M, Borthakur G, Garcia-Manero G, Wierda W, Daver N,
Ferrajoli A, et al: Prediction for sustained deep molecular
response of BCR-ABL1 levels in patients with chronic myeloid
leukemia in chronic phase. Cancer. 124:1160–1168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sato E, Iriyama N, Tokuhira M, Takaku T,
Ishikawa M, Nakazato T, Sugimoto KJ, Fujita H, Fujioka I, Asou N,
et al: Introduction of second-generation tyrosine kinase inhibitors
may reduce the prognostic impact of high-risk patients, according
to the European treatment and outcome study (EUTOS) score. Leuk
Lymphoma. 59:1105–1112. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Alhuraiji A, Kantarjian H, Boddu P,
Ravandi F, Borthakur G, DiNardo C, Daver N, Kadia T, Pemmaraju N,
Pierce S, et al: Prognostic significance of additional chromosomal
abnormalities at the time of diagnosis in patients with chronic
myeloid leukemia treated with frontline tyrosine kinase inhibitors.
Am J Hematol. 93:84–90. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fujioka I, Takaku T, Iriyama N, Tokuhira
M, Kimura Y, Sato E, Ishikawa M, Nakazato T, Sugimoto KJ, Fujita H,
et al: Features of vascular adverse events in Japanese patients
with chronic myeloid leukemia treated with tyrosine kinase
inhibitors: A retrospective study of the CML Cooperative Study
Group database. Ann Hematol. 97:2081–2088. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fabarius A, Duesberg P, Giehl M, Seifarth
W, Hochhaus A and Hehlmann R: Genomic instability in context of the
chromosomal theory. Cell Oncol. 30:503–504. 2008.PubMed/NCBI
|
|
24
|
Melo JV and Barnes DJ: Chronic myeloid
leukaemia as a model of disease evolution in human cancer. Nat Rev
Cancer. 7:441–453. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Skorski T: Genetic mechanisms of chronic
myeloid leukemia blastic transformation. Curr Hematol Malig Rep.
7:87–93. 2012. View Article : Google Scholar : PubMed/NCBI
|