Introduction
According to Global cancer statistics 2018 (1), colorectal cancer (CRC) ranks third in
terms of incidence and second in terms of mortality among all
cancers types in males and females. Among females, 9.2% of
mortalities associated with cancer are due to CRC. Furthermore, the
top three causes of mortality due to gastrointestinal diseases in
the USA are CRC, followed by pancreatic cancer and liver cancer
(2). Numerous cancer types are
affected by lifestyle and environmental factors, and the incidence
rates of the majority of cancers can be decreased by reducing
potential risk factors. CRC is a potentially preventable cancer,
and approximately 26.7% of CRC cases can be avoided by reducing the
risk factors (3–5). The transformation from normal mucosa
to occult adenoma to adenocarcinoma is a complicated multi-step and
multi-stage process. This long-term pre-cancerous state provides
opportunities to intervene in the development and progression of
CRC. Among the numerous risk factors for CRC, diet factors account
for ~80% (6), which is mainly
associated with a high-fat, high-protein and low-fiber diet. A high
intake of red meat and processed meat is an important factor in the
pathogenesis of CRC (7–9). Heterocyclic amines (HAs), produced by
high-temperature cooking of meat are one of the carcinogens of CRC
(6,10,11). A
study involving 407,270 participants with an overall median
follow-up of 13.8 years demonstrated that the highest quintile of
HAs was associated with increased risk of CRC (12). These HAs may cause chromosomal
translocations, instability of cancer-associated gene
microsatellites, chain mutations and oncogene activation, leading
to the occurrence of CRC (13).
The metabolism of HAs is mainly catalyzed by
metabolism II phase enzymes, and the polymorphic variations in the
detoxifying enzymes may modulate the rate of conversion of toxic or
carcinogenic compounds in the epithelium lining the lumen of the
gastrointestinal tract (14). UDP
glucuronosyltransferase 1A (UGT1A) is an important member of the
family of metabolism II phase enzymes and is considered as an
important system of detoxification. UGT1A metabolizes HAs-DNA
adducts through glucuronidation, and serves a role in gene
protection, which may be of great value in cancer prevention and
therapy (15). Therefore, inducing
the overexpression of metabolism phase II enzymes may aid to
protect cells from the toxicity of carcinogens and DNA damage
caused by the formation of adducts. Our previous study revealed
that UGT1A expression was reduced in adenocarcinoma tissues
compared with normal colonic mucosa tissues, indicating that the
expression of UGT1A is altered in the early stage of colonic
malignant transformation, and UGTlA may be of great significance in
preventing tumor formation (16).
Sulforaphane (SFN), a phytochemical and derivative
of isothiocyanate, is rich in cruciferous plants, and possesses
antioxidant, anti-inflammatory, anticarcinogenic and preventive
effects on CRC (17–19). Our previous study demonstrated that
SFN could upregulate the expression of UGT1A in CRC cells, and
promote cell-cycle arrest and apoptosis in human CRC cells
(20). Based on the aforementioned
detoxifying capacity of UGT1A, it was hypothesized that UGT1A may
be an important molecular target for SFN in the prevention of
CRC.
There is a cis-regulated structure in the
UGT1A gene promoter region termed anti-oxidant responsive element
(ARE). Nrf2, one of the multiple transcription factors that binds
to ARE, can be induced by external factors and is considered to
serve a key role in activating the transcription of various
antioxidant and detoxifying enzymes, which protect cells from
external stress (21,22). Our previous research revealed that
SFN can increase the mRNA expression and nuclear translocation of
Nrf2 in CRC cells, indicating that SFN may induce UGT1A expression
through the activation of Nrf2 (20). However, the specific mechanism by
which SFN induces the expression of Nrf2 and promotes its nuclear
translocation remains unclear. Detailed knowledge concerning the
pharmacological mechanism of action of SFN may aid to explore
strategies for CRC prevention.
The present study provided evidence that
intranuclear Nrf2 is upregulated and the extracellular
signal-regulated kinase (ERK) signaling pathway is activated by
SFN, which induces the expression of UGT1A.
Materials and methods
Cell culture and reagents
The human CRC cell lines HT-29 and SW480 (Shanghai
Zhong Qiao Xin Zhou Biotechnology Co., Ltd.) were used in the
present study and the cell lines were authenticated using STR
profiles. HT-29 and SW480 cells were maintained in Dulbeccos
modified Eagles medium (DMEM) (Gibco; Thermo Fisher Scientific,
Inc.) and RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc.),
respectively, supplemented with 10% fetal bovine serum (FBS)
(Biological Industries) and antibiotics (100 U/ml penicillin and
0.1 mg/ml streptomycin) (EMD Millipore) under 5% CO2 in
air at 37°C. The cell culture medium, including treatments, was
changed every 48 h. All treatments and controls contained a final
dimethyl sulfoxide (DMSO) concentration <0.1%. The anti-β-actin
antibody (product no. 4970; 1:1,000), anti-phosphorylated-p38
(product no. 4511; 1:1,000) and anti-p38 (product no. 8690;
1:1,000) were obtained from Cell Signaling Technology, Inc., while
the anti-Nrf2 (cat. no. ab62352; 1:1,000), anti-Lamin B1 (cat. no.
ab133741; 1:1,000) and anti-phosphorylated-JNK (cat. no. ab124956;
1:1,000), anti-JNK (cat. no. ab179461; 1:1,000) anti-ERK (cat. no.
ab184699; 1:10,000) antibodies were obtained from Abcam. The
anti-phosphorylated-ERK (Thr202/Tyr204) antibody (clone S.812.9;
cat. no. MA5-15173; 1:1,000) was obtained from Invitrogen (Thermo
Fisher Scientific, Inc.). The anti-UGT1A antibody (cat. no.
sc-271268) was obtained from Santa Cruz Biotechnology, Inc. The
horseradish peroxidase (HRP)conjugated goat anti-rabbit
immunoglobulin G (IgG) (cat. no. D110058; 1:5,000) and cyanine-3
(Cy3)-conjugated goat anti-rabbit IgG (cat. no. D111107; 1:100)
antibodies were obtained from Sangon Biotech Shanghai, Co., Ltd.
The peroxidaseconjugated goat anti-mouse IgG (cat. no. ZB2305;
1:2,500) was obtained from Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd. SFN (≥90% purity; product no. S4441;
Fig. 1A) and the ERK inhibitor
PD98059 (≥98% purity; product no. P215) were obtained from
Sigma-Aldrich (Merck KGaA). All protocols of the manufacturers were
followed.
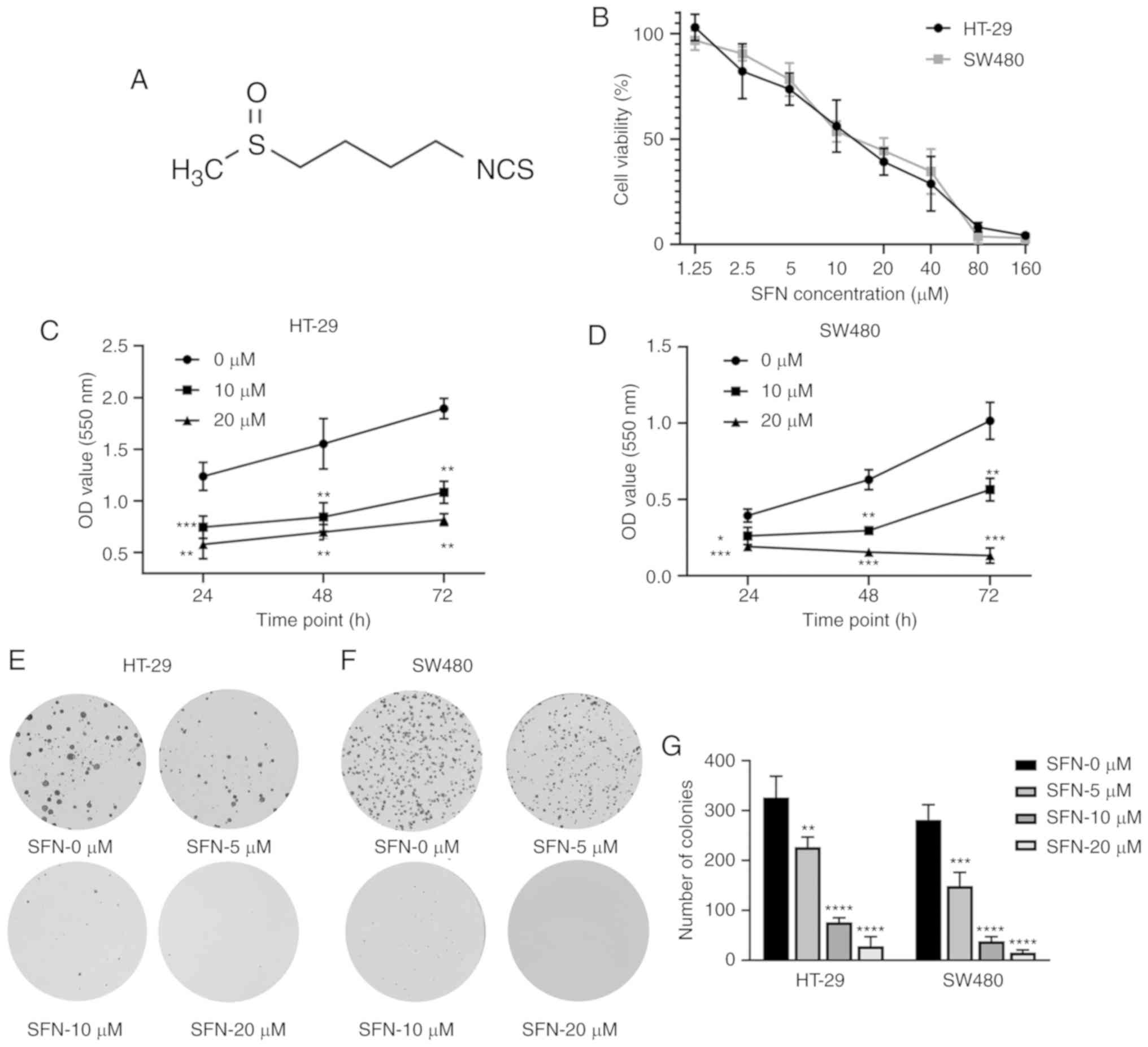 | Figure 1.SFN-induced cytotoxicity in colon
cancer cells. (A) Structure of SFN, also known as
1-isothiocyanato-4-(methylsulfinyl)-butane (CAS, 4478-93-7;
molecular formula, C6H11NOS2). (B) HT-29 and SW480 cells were
incubated with 0, 1.25, 2.5, 5, 10, 20, 40, 80 and 160 µM SFN for
24 h. (C and D) An MTT assay was used to detect cell viability
after treatment with various concentrations of SFN or for different
time-points (24, 48 and 72 h). The statistical significance of the
results was analyzed by two-way ANOVA. (E and F) Colon cancer cells
(HT-29 and SW480) were cultured with various concentrations of SFN
(0, 5, 10 and 20 µM). A representative image of colony formation
from 3 independent experiments is presented. (G) The number of
colonies of various groups presented in the graphs of parts E and F
were quantified. The statistical significance of the results was
analyzed by one-way ANOVA. The results are presented as the mean ±
standard deviation of 3 independent experiments. *P<0.05,
**P<0.01, ***P<0.001, ****P<0.0001. SFN, sulforaphane;
ANOVA, analysis of variance. |
MTT assay
The effect of SFN on HT-29 and SW480 cell viability
was determined by MTT assay. Briefly, cells were seeded at a
density of 5×103 cells in 96-well plates and treated
with various concentrations of SFN (1.25, 2.5, 5, 10, 20, 40, 80
and 160 µM) or blank medium for specific time-points (24, 48 and 72
h). Subsequently, 10 µl MTT reagent (5 mg/ml) was added to each
well of the plates and the cells were incubated for 4 h at 37°C.
Next, the medium was discarded and 100 µl DMSO was added. Upon
agitation at room temperature for 10 min, the absorbance was
measured at 550 nm using an Infinite M200 PRO Microplate Reader
(Tecan Group Ltd.).
Colony formation assay
HT-29 and SW480 cells were inoculated into 6-well
plates at a density of 700 cells/well and treated with various
concentrations of SFN (0, 5, 10 and 20 µM) for 14 days. Finally,
the cells were fixed with methanol at room temperature for 30 min,
washed with PBS and stained with 0.1% crystal violet staining
solution (Beyotime Institute of Biotechnology) at room temperature
for 10 min. Upon washing the cells with PBS, the clusters (≥50
cells) were photographed by a NIKON camera and their number was
counted.
EdU assay
HT-29 and SW480 cells were inoculated into 96-well
plates at a density of 5×103 cells/well and treated with
different concentrations of SFN (0, 10 and 20 µM) for 24 h. An EdU
detection kit (cat. no. C10310-1; Guangzhou RiboBio Co., Ltd) was
used to observe the groups. Cells were incubated with 100 µl medium
and 50 µM EdU for 2 h, and then the medium was discarded. Cells
were washed twice with PBS. The cells were fixed with methanol for
30 min at room temperature and then washed with PBS. Samples were
incubated for 10 min with PBS containing 0.5% Triton X-100 (Beijing
Solarbio Science & Technology Co., Ltd.) in a decolorization
shaker and then washed with PBS. Cells were incubated with 100 µl
1X Apollo staining reaction solution for 30 min on a decolorization
shaker in the dark, and then the staining reaction solution was
discarded. Cells were washed twice with PBS containing 0.5% Triton
X-100 for 10 min. The nucleuses were stained with 1X Hoechst 33342
reaction solution at room temperature for 30 min, prior to
observation and capture of images with a fluorescence microscope
(magnifications, ×100 and ×200).
Analysis of apoptosis and cell cycle
arrest
HT-29 and SW480 cells were cultured in 60-mm dishes
at a density of 60×104 cells/dish and treated with
various concentrations (0, 10, 15 and 20 µM) of SFN for 24 h. An
Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI)
apoptosis detection kit (cat. no. 556547; BD Biosciences) was used
to observe the different states of the cells. Cells were washed
twice with cold PBS and then resuspended in 1X Binding Buffer.
Next, 5 µl Annexin V-FITC conjugate and 5 µl PI solution were added
to the cell suspension, followed by incubation for 15 min at room
temperature in the dark. Subsequently, 400 µl 1X Binding Buffer was
added to each tube. The samples were analyzed by flow cytometry (BD
Biosciences) within 1 h.
Cell cycle arrest was also detected by flow
cytometry. The cells were collected and fixed with 75% ethanol at
4°C for 12 h, followed by flow cytometric analysis on a flow
cytometer after PI staining at 4°C for 30 min.
Wound healing assay
Wound healing assays were carried out to evaluate
the motility and metastatic potential of CRC cells. Cells were
seeded in 6-well plates at a density of 6×105
cells/well. After 24 h, the cell monolayer was scratched with a
10-µl pipette tip. The wound healing assay was performed in
monolayer culture, and then the cells were cultured in serum-free
medium. Wounds were washed with PBS and incubated in serum-free
medium in the presence of various concentrations of SFN (0, 10 and
20 µM). Images were captured at different time-points (0, 24 and 48
h). The wounded area was calculated using ImageJ software
(V1.8.0.112; National Institutes of Health).
Transwell assay
Transwell assays were performed in Transwell
chambers (pore size, 8.0 µm; Costar; Corning, Inc.). HT-29 and
SW480 cells were cultured in medium containing various
concentrations of SFN (0, 10 and 20 µM) for 2 days. Cells
(2×105) were allowed to migrate from the upper chamber
containing medium without FBS to the lower chambers containing
medium with 30% FBS. The migrating cells were fixed with methanol
at room temperature after 48 h of incubation and stained with 0.1%
crystal violet at room temperature for 10 min. The cells that had
migrated through the polycarbonate membrane were counted under a
light microscope (5 random fields/well).
Lentivirus-mediated RNA
interference
Lentiviral particles and Polybrene were purchased
from Shanghai GenePharma Co., Ltd. The short hairpin RNA (shRNA)
target sequence was 5′-GGGAGGAGCTATTATCCATTC-3′, and the negative
control (NC) sequence was 5′-GTTCTCCGAACGTGTCACGT-3′. Nrf2 shRNA
and NC shRNA were cloned into vectors containing the green
fluorescent protein (GFP) gene. Then, the plasmid vectors were
packed into lentiviral particles. Cells were seeded in 6-well
plates at a density of 1×105 cells/well. Lentiviral
particles with a multiplicity of infection of 80 were added to the
cells. The GFP-expressing cells were detected using fluorescence
microscopy, and puromycin was used for 7 days to screen
stable-transfected cells with resistance to puromycin.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from the cells using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturers protocol. RT reactions were carried out with 3 µg
RNA, oligo-dT primer and M-MLV Reverse Transcriptase (Invitrogen;
Thermo Fisher Scientific, Inc.) in a volume of 20 µl with the
Eppendorf Mastercycler nexus PCR instrument. The presence of Nrf2
and UGT1A transcripts was analyzed by qPCR, based on general
fluorescence detection with SYBR-Green (Takara Bio, Inc.). β-actin
was used as an internal control for normalization of the
differences in RNA quantity and quality across samples. The qPCR
conditions were 30 sec at 95°C followed by 40 cycles of 5 sec at
95°C, 10 sec at 60°C and 30 sec at 72°C. The data were quantified
using the 2−ΔΔCq method (23), ΔΔCq=(Cq of target gene-Cq of
β-actin) treatment-(Cq of target gene-Cq of
β-actin)control. The gene-specific primers used were
derived from PrimerBank and are summarized in Table I. All primers were synthesized by
BioSune following sequence alignment with Basic Local Alignment
Search Tool (National Center for Biotechnology Information).
 | Table I.Primer sequences for RT-qPCR. |
Table I.
Primer sequences for RT-qPCR.
| Gene name | Primer sequence
(5′→3′) |
|---|
| Nrf2 | Forward:
TTCCCGGTCACATCGAGAG |
|
| Reverse:
TCCTGTTGCATACCGTCTAAATC |
| UGT1A | Forward:
TCGAATCTTGCGAACAACACG |
|
| Reverse:
ATGAAGGCCACTGTCAGCACG |
| β-actin | Forward:
CATGTACGTTGCTATCCAGGC |
|
| Reverse:
CTCCTTAATGTCACGCACGAT |
Separation of the nucleus from
cytoplasm
A Nucleoprotein extraction kit (BB-3102, BestBio
Science) was used in the separation of the nucleus from cytoplasm.
The cells were washed three times with cold PBS and collected into
1.5 ml-Eppendorf tubes using a cell scraper. Cells were centrifuged
at 500 × g for 3 min at 4°C and then the supernatants were
discarded. Cell lysates were added to the precipitates. The
Eppendorf tubes were then vortexed for 15 sec every 5 min, three
times in total. Subsequently, the suspensions were centrifuged for
5 min at 12,000 × g and the supernatants contained the cytoplasmic
proteins. The nuclear lysates were added to the precipitates. After
vortexing for 15 sec every 10 min, four times in total, the
suspensions were centrifuged for 10 min at 12,000 × g and the
supernatants contained the cytoplasmic proteins.
Immunocytochemistry
Cells were seeded into 24-well cell culture plates
(6×104 cells/well) and divided into three groups: A
control group, an SFN group and a PD98059 + SFN group. PD98059 was
added to the medium 60 min prior to SFN addition, and cells were
incubated for 24 h in the PD98059 + SFN group. Ultimately, the
cells were fixed with methanol (chilled at −20°C) for 15 min and
then washed three times with cold PBS. Samples were incubated for
30 min with PBS containing 0.2% Triton X-100 and then washed three
times with cold PBS. Cells were next incubated in PBS containing 3%
bovine serum albumin for 30 min at room temperature and washed
three times with PBS. Then, cells were incubated with an anti-Nrf2
primary antibody (1:400) overnight at 4°C. The next day, the
solution was removed and the cells were washed three times in PBS.
Cells were then incubated with a Cy3-conjugated goat anti-rabbit
IgG for 1 h at room temperature in the dark, prior to observation
and capture of images with a fluorescence microscope
(magnification, ×400).
Western blotting
Protein were isolated using the protein extraction
kit (R0010; Beijing Solarbio Science & Technology Co., Ltd.).
Protein extracts were heated at 99°C for 5 min and cooled in ice.
The protein concentration was detected using a BCA assay kit
(Tiangen Biotech Co., Ltd.). A total of 30 µg protein of each
sample was loaded and separated on a 10% SDS polyacrylamide gel and
electrophoretically transferred onto a polyvinylidene fluoride
membrane. The membrane was blocked with 5% fat-free milk in
TBS-Tween-20 (TBST) for 2 h and incubated with a specific primary
antibody in Primary Antibody Diluent overnight at 4°C. The blots
were washed three times for 5 min with TBST and then incubated with
a secondary antibody for 1.5 h. After washing the membrane three
times for 5 min with TBST, antibody binding was detected by
ImageQuant LAS 4000 system (GE Healthcare Life Sciences). The
optical density results were calculated using ImageJ 1.52
software.
Measurement of intracellular ROS
Intracellular ROS levels were detected using a
Reactive Oxygen assay kit (Beyotime Institute of Biotechnology).
Dichloro-dihydro-fluorescein diacetate was added to the medium to a
final concentration of 10 µM and incubated for 20 min in a 37°C
incubator. ROS levels were observed directly and images were
obtained with a fluorescence microscope (magnification, ×100).
Statistical analysis
GraphPad Prism 8.0.1 software (GraphPad Software,
Inc.) was used for statistical analysis. The results are presented
as the mean ± standard deviation of ≥3 independent experiments. The
statistical significance of the results was analyzed by Students
t-test, one-way analysis of variance (ANOVA) with Dunnetts post hoc
test and two-way ANOVA. P<0.05 was considered to indicate a
statistically significant difference.
Results
SFN inhibits the proliferation and
clone formation of CRC cells
To evaluate the anticancer effect of SFN on CRC
cells, the CRC cell lines HT-29 and SW480 were treated with
different concentrations of SFN. An MTT assay was used to determine
cell viability under various concentrations of SFN (0, 1.25, 2.5,
5, 10, 20, 40, 80 and 160 µM), as well as different treatment
durations with SFN (24, 48 and 72 h). As presented in Fig. 1B, SFN significantly suppressed the
cell viability relative to the control group in a dose-dependent
and time-dependent manner (Fig.
1B-D). Next, the role of SFN in the colony-forming assay was
assessed. Compared with the control group, the number of colonies
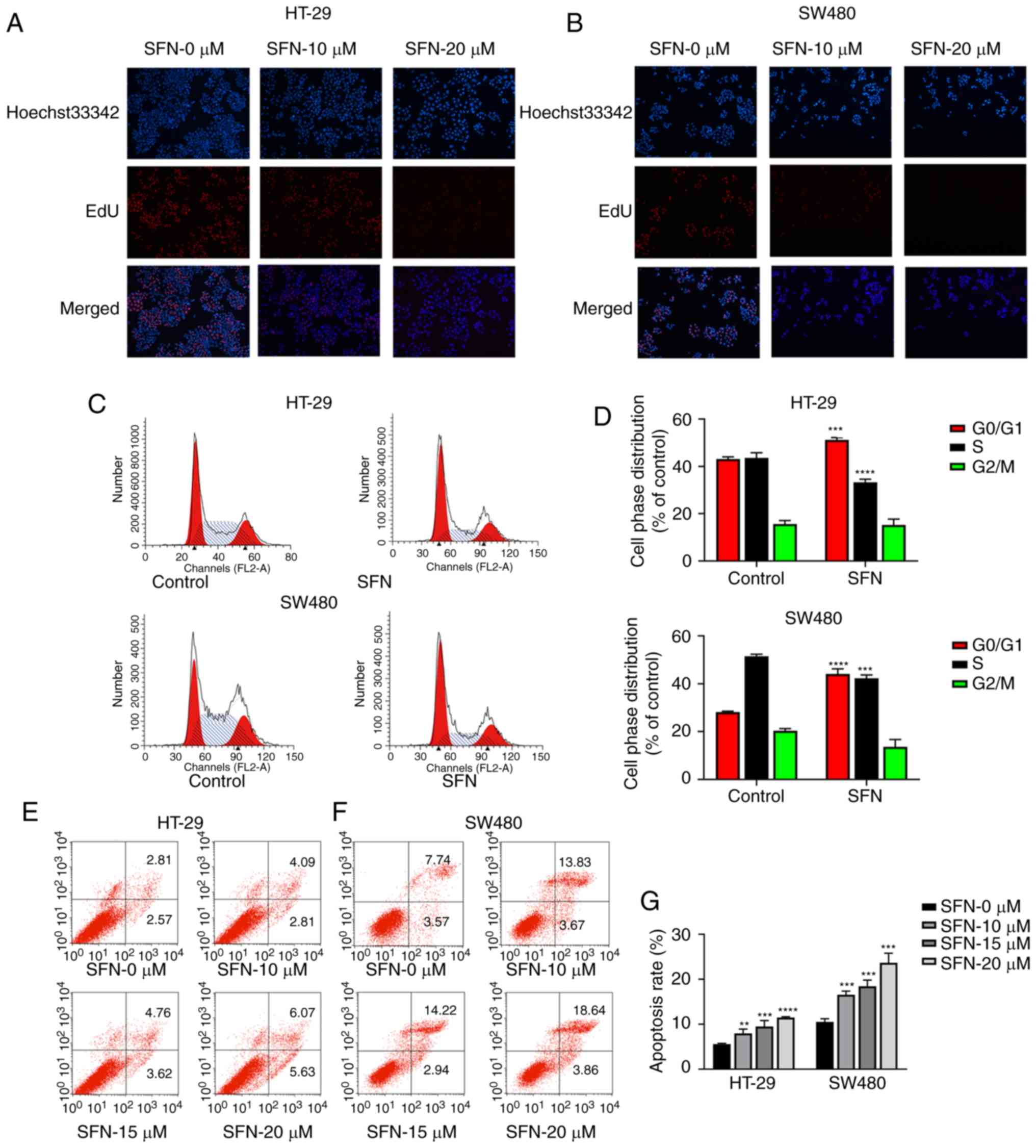
in the SFN groups was significantly reduced (Fig. 1E and G). Furthermore, EdU staining
was used to detect the effect of SFN on the proliferation of CRC
cells (Fig. 2A and B). The results
of EdU assay revealed that the cell proliferation activity in the
SFN groups significantly decreased compared with the control group.
All these results indicated that SFN may inhibit the proliferation
of CRC cells.
SFN induces G0/M1-phase arrest and the
apoptosis of CRC cells
To confirm the effect of SFN in cell cycle and
apoptosis of HT-29 and SW480 cells, cells were treated with various
concentrations (0, 10, 15 and 20 µM) of SFN for 24 h. Cells were
analyzed by flow cytometry, and the results are presented in
Fig. 2C-G. The results indicated
that SFN could induce the G0/G1-phase arrest. Following treatment
with SFN (Fig. 2C and D), there was
a higher percentage of cells in the G0/G1 phase (HT-29: 51.20±0.96;
SW480: 44.09±2.11%) compared with the control group (HT-29:
43.09±0.91; SW480: 28.14±0.37%). The accumulation of cells in the
G0/G1 phase was accompanied by a decrease in the percentage of
cells in the S phase (HT-29: 43.59±2.19 (control), 33.24±1.30
(SFN); SW480: 51.54±0.77 (control), 42.26±1.43% (SFN). The
apoptosis rates (Fig. 2E and G) in
HT-29 cells were 5.56±0.16, 7.97±0.93, 9.49±1.34 and 11.49±0.182%
under treatment of SFN in 0, 10,15 20 µM, respectively. In
addition, the apoptosis rates in SW480 cells were 10.50±0.70,
16.56±0.79, 18.43±1.38 and 23.67±2.12%. SFN could induce G0/G1
phase arrest and apoptosis in colorectal cancer.
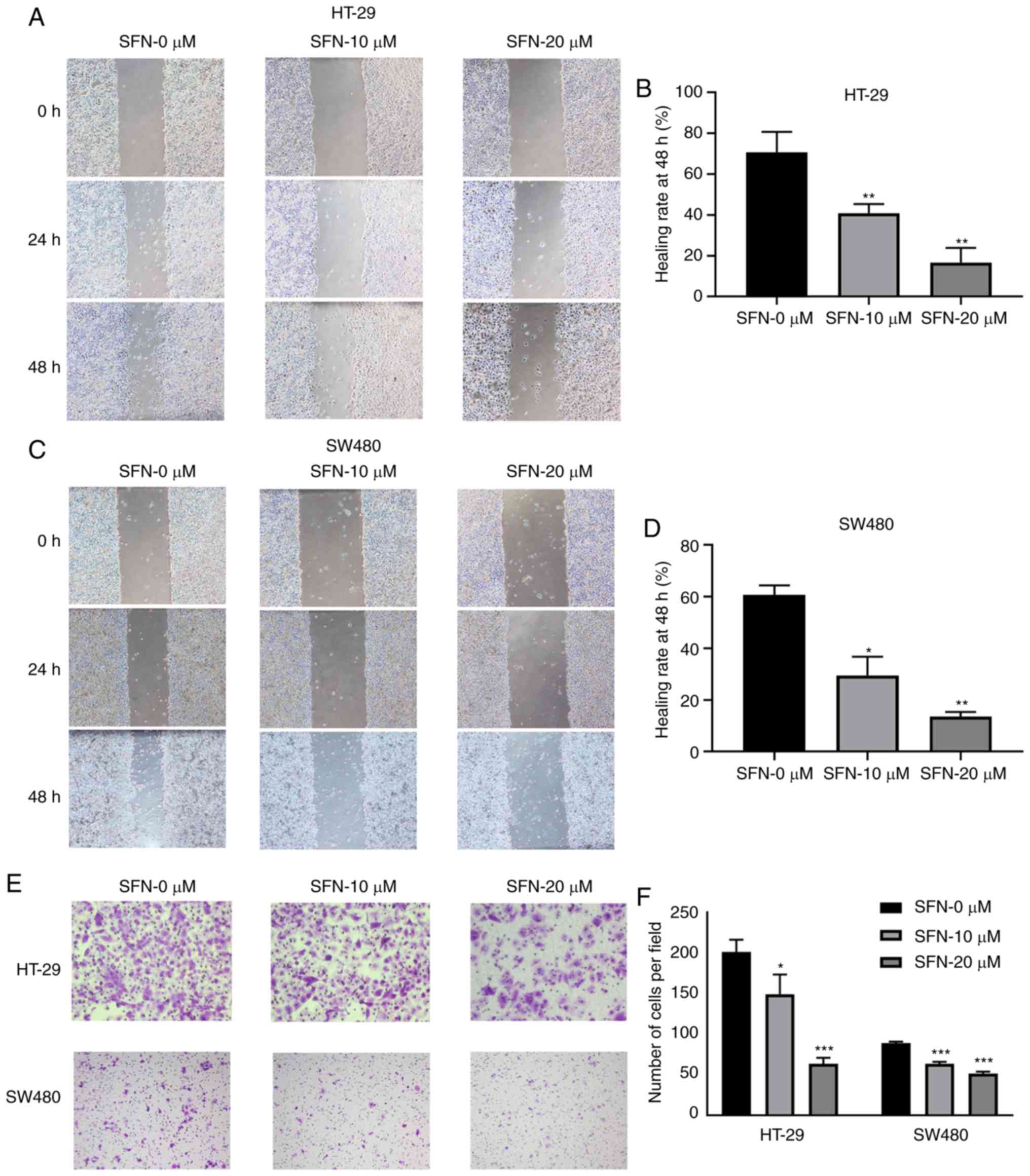
SFN inhibits the motility of CRC
cells
HT-29 and SW480 cells were treated in complete
medium with or without various concentrations of SFN. Changes in
the motility and migration capacity of the CRC cell lines HT-29 and
SW480 were detected and analyzed by wound healing and Transwell
assays. Decreased cell motility was observed in HT-29 and SW480
cell lines treated with SFN (Fig.
3A-D). The 48-h wound healing rates in HT-29 cells were
60.67±3.68, 29.48±7.30 and 13.59±1.76% (for 0, 10, 20 µM SFN,
respectively), while in SW480 cells they were 70.77±9.90,
40.86±4.52 and 16.69±7.15% (for 0, 10, 20 µM SFN, respectively).
The migrating capacity of cells was decreased in the SFN treatment
group compared with the control group (Fig. 3E and F). The number of HT-29 cells
migrating into the lower chamber were 201.33±15.04, 149.33±24.09
and 63.33±7.57 (for 0, 10, 20 µM SFN, respectively). The number of
SW480 cells were 89.00±1.73, 63.33±2.52 and 51.33±2.52 (for 0, 10,
20 µM SFN, respectively). The results demonstrated that the
metastasis of HT-29 and SW480 cells was significantly inhibited
when SFN was added.
Effect of SFN on Nrf2 and UGT1A
expression in CRC cells
To assess the expression of Nrf2 regulated by SFN,
CRC cells were treated with various concentrations (0, 10, 15 and
20 µM) of SFN for 48 h. As presented in Fig. 4A and B, treatment with SFN increased
Nrf2 and UGT1A mRNA levels in a dose-dependent manner. Western
blotting was used to detect the protein levels of Nrf2 and UGT1A
with various concentrations of SFN. Fold-change values of Nrf2
expression in the nucleus (Fig. 4C and
D) and total protein (Fig. 4F and
G) are presented in Fig. 4E and
I. In addition, significant increases in UGT1A expression
(Fig. 4J) were observed in SFN
treatment groups, which coincided with upregulation of Nrf2 levels
in nuclear fractions.
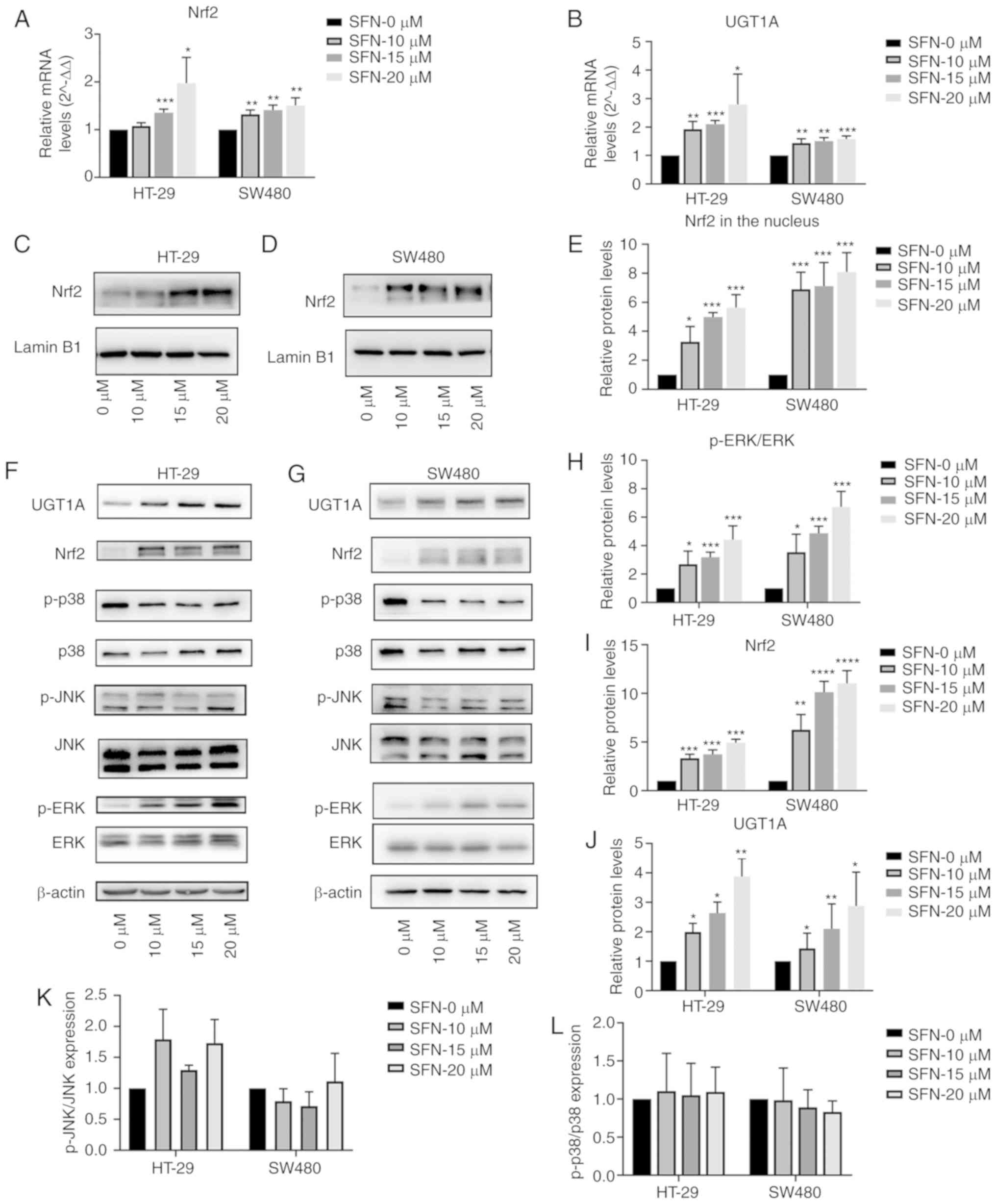 | Figure 4.SFN upregulates Nrf2 and UGT1A in
colon cancer cells. Cells were treated with different
concentrations of SFN. (A and B) The transcriptional levels of Nrf2
and UGT1A were quantified by RT-qPCR and illustrated by a bar
legend. (C-E) The Nrf2 expression level in the nucleus was assessed
by immunoblotting and illustrated by a bar legend. (F-L) The
immunoreactivity of p-ERK was normalized to that of total ERK. The
immunoreactivity of Nrf2 and UGT1A, which was normalized to the
expression of β-actin, and the immunoreactivity of p-JNK and p-p38
were normalized to that of total JNK and p38 was measured by
immunoblotting and represented by respective bar graphs revealing
the mean ± SD. Fold changes in optical density with the 0 h group
normalized to 1. The statistical significance of the results was
analyzed by two-way analysis of variance. The results are expressed
as the mean ± SD of 3 independent experiments. *P<0.05,
**P<0.01, ***P<0.001, ****P<0.0001. SFN, sulforaphane;
Nrf2, nuclear factor, erythroid 2 like 2; UGT1A, UDP
glucuronosyltransferase 1A; ERK, extracellular signal-regulated
kinase; p-ERK, phosphorylated ERK; JNK, c-Jun NH2-terminal kinase;
p-JNK, phosphorylated-JNK; p38, p38 kinase; SD, standard deviation;
RT-qPCR, reverse transcription-quantitative polymerase chain
reaction. |
Nrf2 serves an important role in the
SFN-induced upregulation of UGT1A expression
The expression of Nrf2 in the nucleus and UGT1A were
increased upon treatment with SFN in the present study. Nrf2 is one
of the multiple transcription factors that binds to ARE, which is a
cis-regulated structure in the UGT1A gene promoter region
(22). It was hypothesized that SFN
upregulated UGT1A via Nrf2 binding to ARE. NC shRNA and Nrf2 shRNA
were transfected into HT-29 and SW480 cells with lentiviruses, and
stable-transfected cells were screened by puromycin and used for
subsequent experiments. The levels of Nrf2 mRNA and protein
expression (Fig. 5A-D) were
detected by RT-qPCR and western blotting (P<0.0001). The NC
shRNA and Nrf2 shRNA groups were cultured in complete medium
containing 0, 10 and 20 µM SFN. Protein was collected after 48 h,
and protein expression of UGT1A was detected by western blotting.
The expression of UGT1A increased in the NC shRNA groups following
treatment with SFN. However, the expression level of UGT1A
decreased, and the induction effect of SFN on UGT1A expression
disappeared upon inhibition of the expression of Nrf2 (Fig. 5E-H).
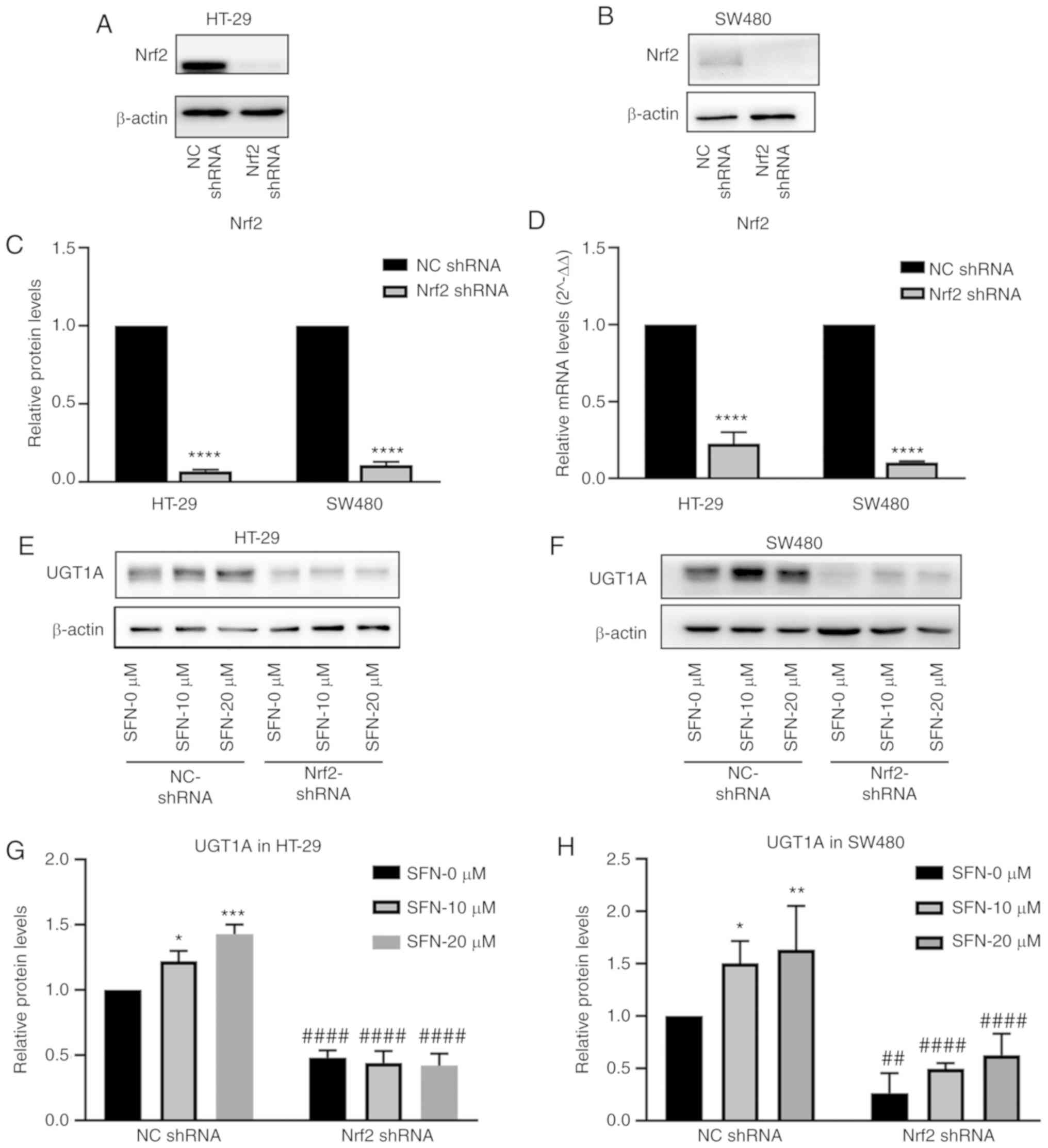 | Figure 5.Effect of Nrf2 on UGT1A expression
induced by SFN. The Nrf2 gene was knocked down in SW480 and HT-29
cells. (A and B) The Nrf2 protein levels in HT-29 and SW480 cells
were assessed by immunoblotting. (C) The expression levels of Nrf2
were quantified by ImageJ and illustrated by a bar legend. The
statistical significance of the results was analyzed by Student's
t-test. ****P<0.0001 vs. NC shRNA. (D) The transcriptional level
of Nrf2 was quantified by RT-qPCR and illustrated by a bar legend.
****P<0.0001 vs. NC shRNA. The statistical significance of the
results was analyzed by Student's t-test. (E and F) The expression
levels of UGT1A in HT-29 and SW480 cells decreased in
Nrf2-knockdown cells, and the induction effect of SFN on UGT1A
expression decreased upon Nrf2-gene knockdown. (G and H) The UGT1A
expression in various groups presented in the images of parts E and
F was quantified by ImageJ. The statistical significance of the
results was analyzed by two-way analysis of variance. *P<0.05,
**P<0.01, ***P<0.001 vs. the SFN 0 µM group;
##P<0.01, #####P<0.0001 vs. the NC
shRNA group. The results are expressed as the mean ± standard
deviation of 3 independent experiments. SFN, sulforaphane; Nrf2,
nuclear factor, erythroid 2 like 2; NC, negative control; shRNA,
short hairpin RNA; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction. |
SFN promotes the expression of Nrf2
and UGT1A, and increases the levels of ROS through ERK
Notably, SFN treatment significantly increased the
phosphorylation of ERK in a dose-dependent manner (Fig. 4F-H). In addition, the effect on JNK
and p38 was not significantly altered (Fig. 4K and L). The results revealed that
SFN may activate the ERK signaling pathway in a dose-dependent
manner. PD98059 is a specific inhibitor of ERK and its inhibitory
ability was verified in the present study (Fig. 6A-C). To confirm whether the
SFN-modulated and Nrf2-induced UGT1A upregulation is associated
with the ERK signaling pathway, cells were treated with PD98059 for
1 h to block the ERK pathway prior to treatment with SFN. Cells
were divided into three groups: a control, an SFN and a PD98059 +
SFN group. The control and SFN groups were treated with medium with
and without SFN, respectively. The PD98059 + SFN group was
pretreated with the ERK inhibitor PD98059 prior to being treated
with SFN. Pretreatment with PD58059 reversed the nuclear
accumulation of Nrf2 and the total protein levels of UGT1A
(Figs. 6D-J and 7A-D). In addition to the aforementioned
detection of the expression of Nrf2 and UGT1A, the levels of ROS
were detected. The ERK inhibitor was able to inhibit the
SFN-induced high levels of ROS in CRC cells (Fig. 7E-H).
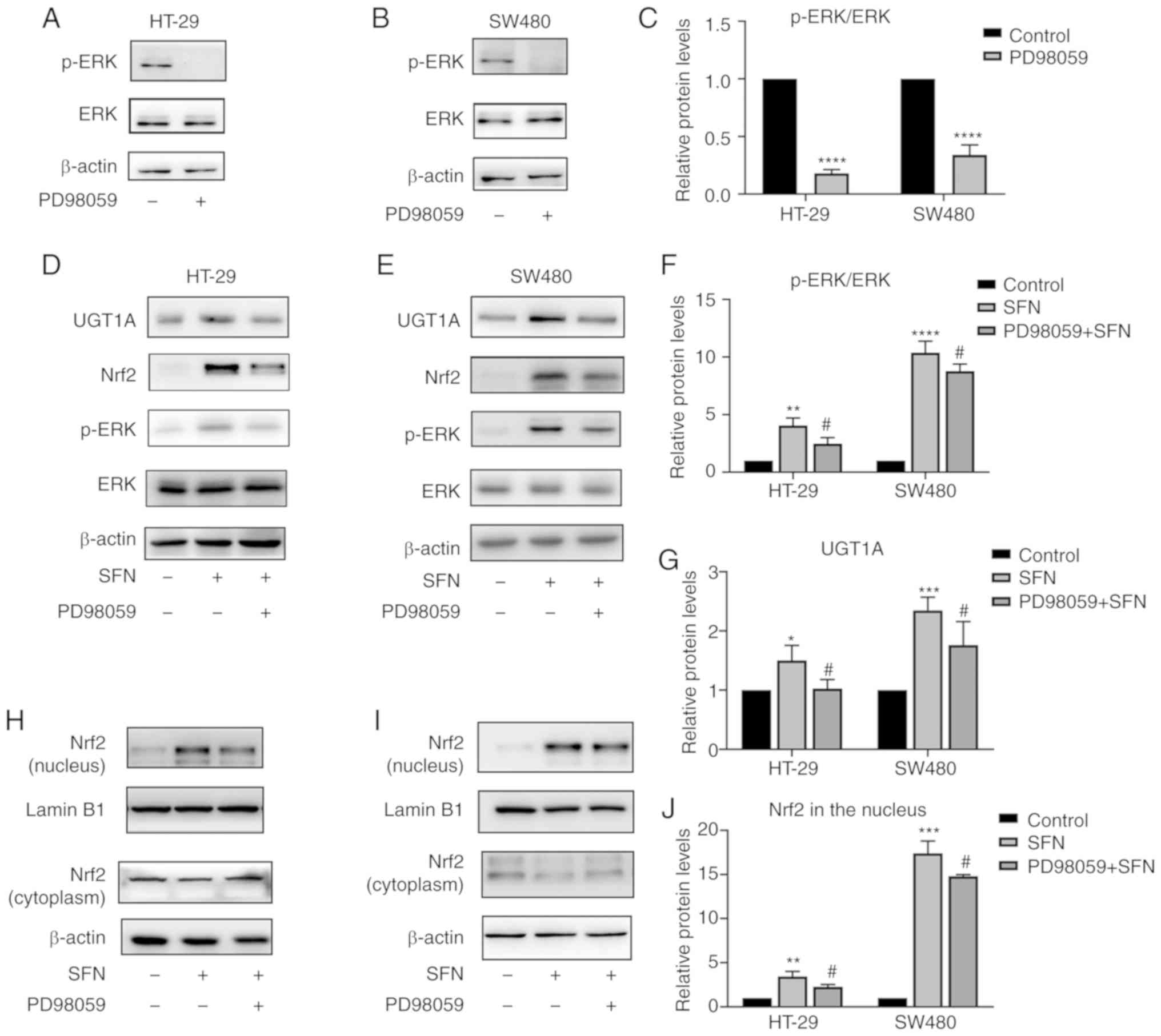 | Figure 6.SFN induces UGT1A expression through
ERK-dependent regulation of Nrf2. HT-29 and SW480 cells were
pretreated with PD98059 (an ERK inhibitor) for 1 h. (A and B) p-ERK
expression levels were assessed by immunoblotting. HT-29 and SW480
cells were pretreated with or without PD98059 for 1 h followed by
24 h of incubation with SFN. (C) The immunoreactivity of p-ERK was
normalized to that of total ERK, and represented by a bar graph
presenting the mean ± SD. Fold changes in optical density compared
with the control group were normalized to 1. The statistical
significance of the results was analyzed by Student's t-test. (D
and E) UGT1A and Nrf2 expression levels of total protein were
assessed by immunoblotting. (F and G) Bar graphs presenting the
mean ± SD fold changes in OD (with the control group set as 1) of
p-ERK normalized to ERK and UGT1A normalized to β-actin. The
statistical significance of the results was analyzed by Student's
t-test. (H and I) Nrf2 expression level in the nucleus and
cytoplasm were assessed by immunoblotting. (J) Bar graph revealing
the immunoreactivities (mean ± SD fold changes in OD, with control
group set at 1) of Nrf2 normalized to Lamin B1 (marker for the
nucleus). The statistical significance of the results was analyzed
by Student's t-test. The results are presented as the mean ± SD of
3 independent experiments. *P<0.05, **P<0.01, ***P<0.001
and ****P<0.0001 vs. the control; #P<0.05, vs.
SFN. SFN, sulforaphane; Nrf2, nuclear factor, erythroid 2 like 2;
UGT1A, UDP glucuronosyltransferase 1A; ERK, extracellular
signal-regulated kinase; SD, standard deviation; OD, optical
density; p-, phosphorylated. |
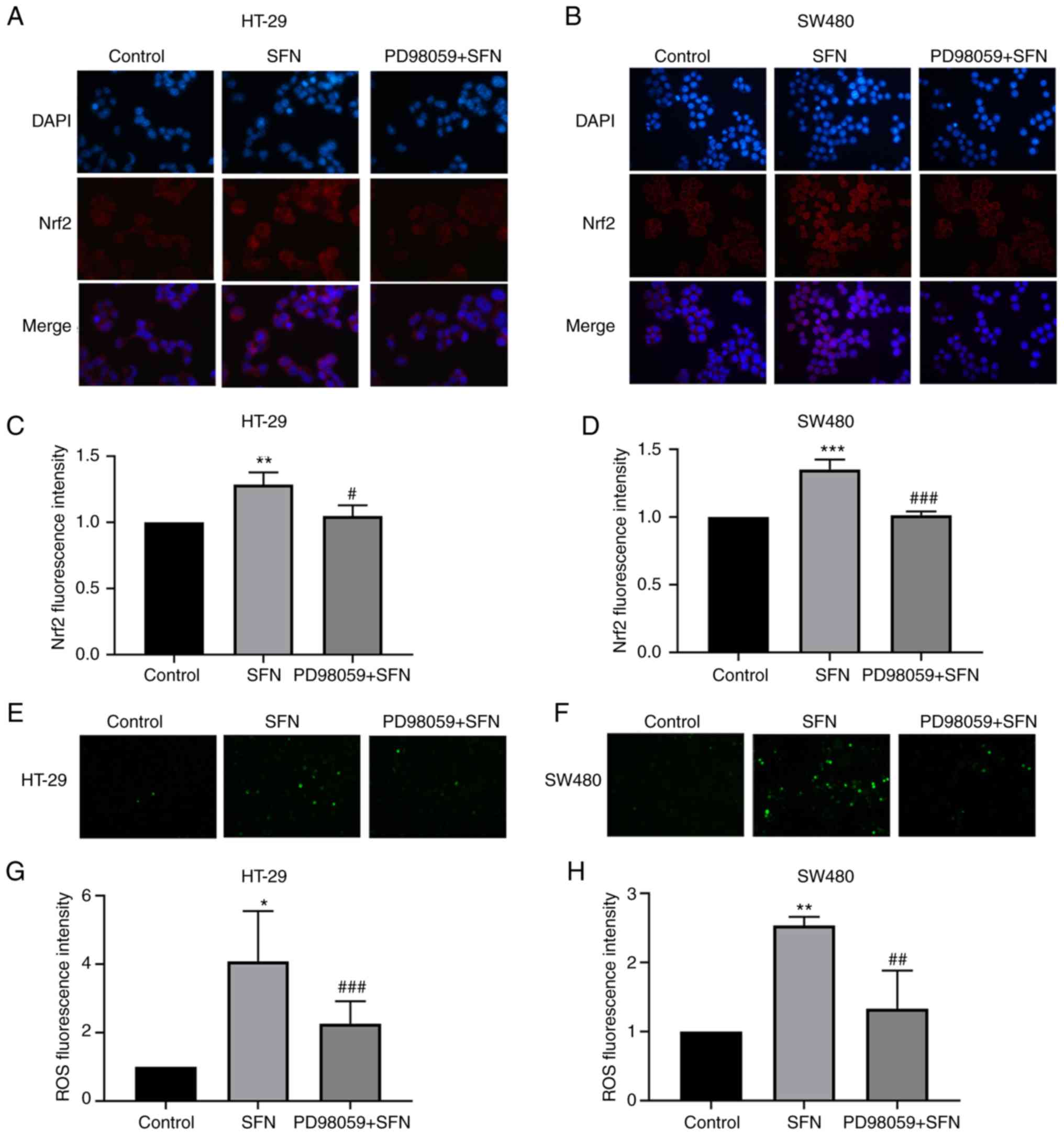 | Figure 7.Nuclear translocation of Nrf2 and ROS
levels observed by fluorescence microscopy. HT-29 and SW480 cells
were pretreated with PD98059 (an ERK inhibitor) for 1 h. (A and B)
Nuclear translocation of Nrf2 in HT-29 and SW480 cells. Fixed cells
were incubated with anti-Nrf2 and fluorescein
isothiocyanate-conjugated anti-rabbit immunoglobulin G antibodies
(magnification, ×400). (C and D) Bar graphs revealing the
fluorescence intensity (mean ± SD fold changes in OD, with control
group set at 1) of Nrf2. (E and F) Intracellular ROS levels were
observed by fluorescence microscopy (magnification, ×100). (G and
H) Bar graphs revealing the fluorescence intensity (mean ± SD fold
changes in OD, with control group set at 1) of ROS. The results are
presented as the mean ± SD of 3 independent experiments.
*P<0.05, **P<0.01, ***P<0.001 vs. the control;
#P<0.05, ##P<0.01,
###P<0.001 vs. SFN. SFN, sulforaphane; Nrf2, nuclear
factor, erythroid 2 like 2; SD, standard deviation. |
Discussion
The roles of various phytochemicals in cancer
prevention and treatment have recently attracted great attention.
The present study investigated the antitumor effect and mechanism
of SFN on the II phase metabolism phase enzyme UGT1A in colorectal
cancer. UGT is one of the II phase metabolism phase enzymes. This
supergene family has two subfamilies, UGT1A and UGT2, which can
encode numerous isoenzymes. UGT1A encodes 9 isoenzymes, UGT1A1 and
UGT1A3-UGT1A10, with different levels of expression in the
intestinal tract, which are involved mainly in the metabolism of
exogenous compounds. Upregulation of the human detoxifying enzyme
UGT1A can metabolize HAs-DNA adducts through glucuronidation
(24), which may aid to prevent the
occurrence and development of CRC in advance. These HAs could be
oxidized by cytochrome P450 family 1 subfamily A member 2 to
N-hydroxyl metabolites along with the blood into the intestinal
mucosa. Then, the N-hydroxyl metabolites could be acetylated by
N-acetylase in intestinal epithelial cells and eventually bind to
the DNA of intestinal epithelial cells to form DNA adducts. These
adducts have been extensively studied and reported to serve a key
role in chemically induced carcinogenesis (25), resulting in a series of cytotoxic
products, including electrophilic groups and oxygen radicals. The
aforementioned changes in DNA may cause chromosomal translocations,
instability of cancer-associated gene microsatellites and chain
mutations, leading to the occurrence of CRC (13). In phase II metabolism, with the
introduction of polar groups, coupling enzymes usually add
endogenous substituents, which greatly increase water solubility
and facilitate excretion (25),
thus increasing cell protection. Therefore, the rate of activation
of HAs in tissues with reduced UGT1A activity is relatively high
and tends to form DNA adducts compared with tissues with normal
UGT1A activity. In the preliminary stages of cancer, the
polymorphism of metabolic enzymes is key in determining the effects
of environmental carcinogens. The high expression of UGT1A in the
intestinal tract indicates that these enzymes may serve an
important role in the metabolism of detoxification.
Our previous research revealed that the
chemopreventive agent SFN upregulates the phase II metabolism
enzyme UGT1A (20), which has a
detoxifying effect on food-borne carcinogenic HAs. However, little
is known concerning the signaling pathway that is involved in
SFN-induced UGT1A expression. Consequently, the mechanism of action
of SFN-induced UGT1A expression must be further explored. Several
studies have revealed that the Nrf2 signaling pathway can be
predictably induced by low concentrations of sulfhydryl-reactive
molecules of various different chemical types, including SFN
(26,27). In the present study, the expression
of Nrf2 and UGT1A exhibited a synchronous increase following
treatment of HT-29 and SW480 cells with SFN. It was observed that
the trend was more obvious in HT-29 cells compared with in SW480
cells. Studies identified four consensus molecular subtypes (CMS)
that expression subtypes have clinical relevance independent of the
cancer stage. HT-29 belongs to CMS-3, and SW480 belongs to CMS-4.
Previous studies have demonstrated that different subtypes of CRC
cell lines have differences in RNA, DNA, and protein levels
(28,29). Colon cell lines were either CMS2 or
CMS3, expressed higher levels of gastro-intestinal marker genes,
including some key transcription factors such as HNF4A and MYB. All
CMS4 models were classified as undifferentiated, consistent with
primary tumors. Concurrently, there are some differences in the
sensitivity of different cell lines to the effects of drugs. This
may explain the different expression levels of Nrf2 and UGT1A in
the two cell lines examined in the present study. Next, the Nrf2
gene was knocked down to demonstrate that Nrf2 is crucial in the
upregulation of UGT1A. The expression of UGT1A increased in the NC
shRNA groups treated with SFN. However, the expression level of
UGT1A decreased and the induction effect of SFN on UGT1A expression
disappeared upon inhibition of the expression of the Nrf2 gene in
the Nrf2 shRNA group. These results demonstrated that Nrf2 serves
an integral role as a transcription factor in the transcriptional
expression of UGT1A.
ERK is a member of the mitogen-activated protein
kinase family. The ERK signaling pathway is known to be involved in
numerous cell biological functions such as proliferation,
migration, differentiation and death (30). A series of studies revealed that the
regulation of Nrf2 may be associated with the activation of the ERK
signaling pathway (31–33). In addition, a study observed that
the expression of UGT1A was downregulated upon inhibition of the
ERK signaling pathway with PD98059 (34). Subsequently, the present study
verified the association between the ERK signaling pathway, Nrf2
and UGT1A through a series of experiments. Activation of ERK by
phosphorylation was observed in the SFN group. To reveal the
mechanism responsible for Nrf2 activation and to detect whether the
activation of Nrf2 and the expression of UGT1A are associated with
changes in the ERK signaling pathway, cells were pretreated with
PD98059 to block the ERK signaling pathway, and then incubated in
complete medium containing SFN. The present results revealed that
SFN exhibited the potential to activate the ERK signaling pathway
in a dose-dependent manner and to increase the protein expression
of Nrf2 and UGT1A, while ERK1/2 inhibition impaired this trend.
These results acknowledge that ERK serves an essential role in
SFN-induced triggering of Nrf2-mediated UGT1A expression in HT-29
and SW480 cells, while inhibition of ERK attenuated the
SFN-mediated upregulation of UGT1A transcription as well as the
Nrf2 accumulation in nuclear compartments. The present study
revealed for the first time that SFN upregulated the expression of
UGT1A, which enhanced the metabolism of carcinogens via the
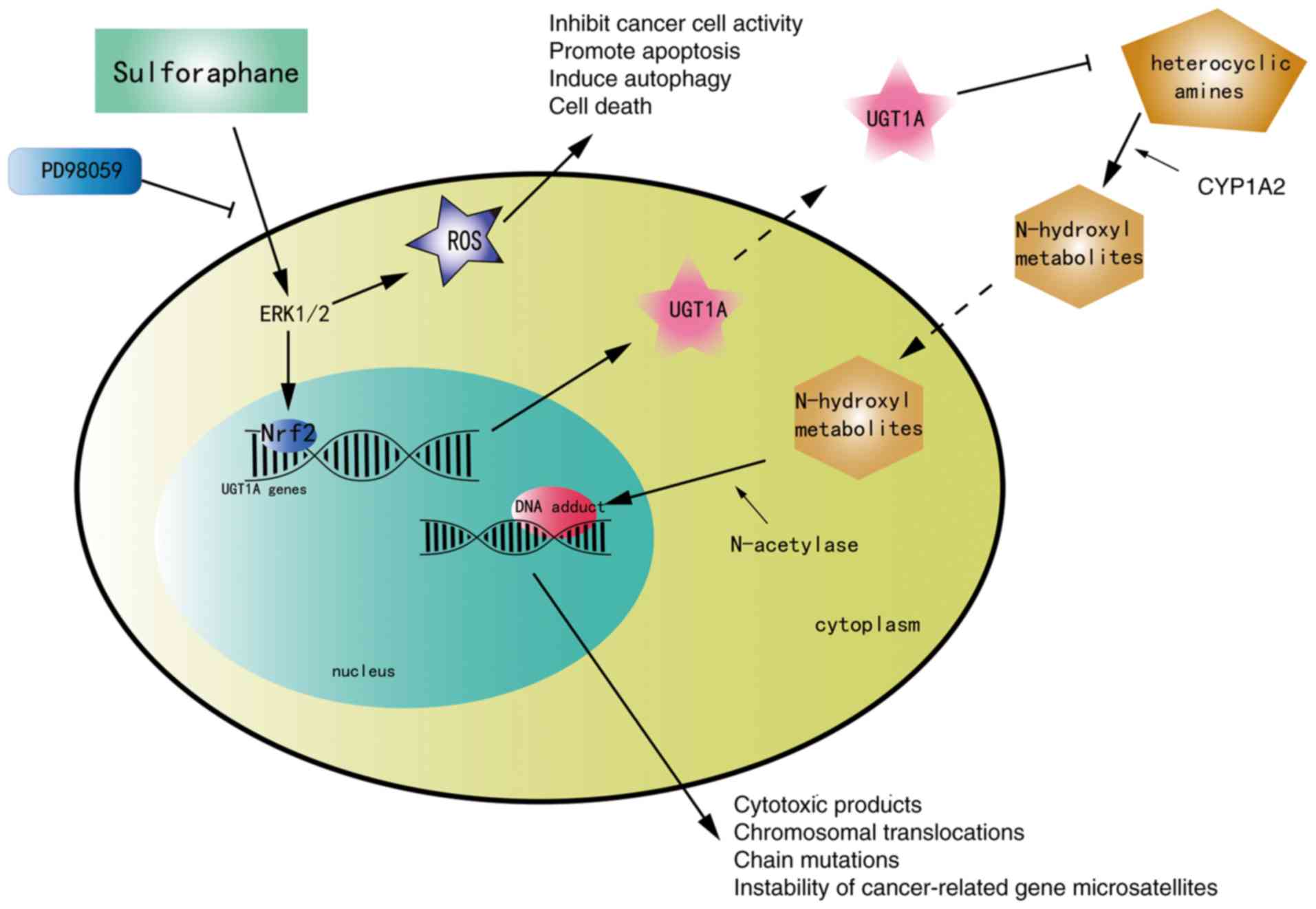
ERK/Nrf2 signaling pathway in CRC cells (Fig. 8). However, blocking the ERK
signaling pathway only partially reversed the upregulation of Nrf2
and UGT1A, and the pathway was not completely blocked. There may be
other signaling pathways and multiple molecular regulatory
mechanisms that may influence this process. It has been reported
that SFN can activate Nrf2 via the PI3K/Akt pathway in
arsenic-induced liver injury and nephrotoxicity (22,35).
This effect may be associated with the high chemical
electrophilicity of the central carbon of the isothiocyanate
(-N=C=S) group. However, there is little research on this
underlying mechanism for the activation of Nrf2 by SFN in colon
cancer; therefore, further investigations are required.
In addition to the aforementioned detection of Nrf2
and UGT1A expression, the present study detected the levels of ROS.
A study on SFN and bladder cancer revealed that SFN induced a
significant increase in ROS levels, which was necessary for
SFN-induced mitochondrial dysfunction, serving an important role in
SFN-induced apoptosis in cancer cells (36). In the present study, ROS levels were
increased in CRC cells when these cells were cultured in
SFN-containing medium. Notably, ERK inhibitors can inhibit
SFN-induced high levels of ROS in CRC cells. This suggests that
SFN-induced high levels of ROS in CRC cells may also be associated
with the ERK signaling pathway. Oxidative stress is involved in
three stages of cancer development: Initiation, promotion, and
progression. Low-to-intermediate levels of ROS promote the
occurrence and progression of tumors, but high levels of ROS can
increase intrinsic oxidative stress of cancer cells, inhibit cancer
cells activity, promote apoptosis, induce autophagy and even cause
cell death (37,38). SFN induces apoptosis in glioblastoma
cells via ROS-dependent inactivation of STAT3 phosphorylation
(39) and has an anti-tumor effect
on bladder cancer cells via the ROS-mediated intrinsic apoptosis
pathway (36). Similar studies have
also been performed in pancreatic cancer and hepatic cancer
(40,41). These findings motivate further
evaluation of SFN as a chemopreventive agent in cancer treatment.
In addition, in studies investigating ROS-induced apoptosis, tumor
necrosis factor-related apoptosis-inducing ligand (TRAIL) can
preferentially activate apoptosis of malignant cells; however, in a
variety of human cancer cells the apoptosis induced by TRAIL is
hard to control, resulting in TRAIL resistance. Notably, SFN
induction of ROS can reduce the resistance and therefore SFN
promotes TRAIL-induced cancer cell death (42). It was hypothesized that the
inhibitory effect of SFN on colon cancer cells may be associated
with the induction of high levels of ROS and may be regulated by
the ERK signaling pathway. Collectively, these results indicated
that the ERK signaling pathway and Nrf2 may represent strategic
targets for the chemopreventive effects of SFN.
In a future experimental design, the mechanism may
be studied and upstream and downstream channels may be further
identified. In addition, given the opportunity, animal experiments
may be added to the future experimental design and the mechanism
may be studied thoroughly. Gene knockout mice would be designed and
HA carcinogens could be used to establish mouse models with
colorectal cancer. Colon tissues would be extracted for analysis at
the gene, protein and cell levels to further explore the chemical
preventive effect of SFN in colorectal cancer.
In addition, SFN is regarded as a sensitizer to
anticancer drugs. A study has revealed that SFN analogs provide a
novel approach to chemically sensitize CRC cells by modulating DNA
damage/repair signaling pathways (43). In addition, SFN was revealed to
inhibit the growth of C666 nasopharyngeal carcinoma cells and
enhance the anti-tumor effect of cisplatin (44). Selectively preconditioning cancer
cells with non-toxic amounts of a natural bioactive compound may
safely enhance drug susceptibility. These compounds often activate
drugs by upregulating the activity of drug metabolic enzymes; thus,
they may significantly affect treatment outcomes despite low
exposure (45–47).
The latest data in CRC revealed that the incidence
rate of CRC has declined in recent years but remains high. CRC can
be treated by surgery as well as radiotherapy and chemotherapy, but
the 5-year survival rate is low, and the quality of life of
patients is greatly reduced. As a multi-step and multi-stage
process disease, CRC has a long precancerous stage. Thus, it is
advisable to prevent it at its origin. Furthermore, the
phytochemical SFN is readily available, and is widely present in
cruciferous plants. Numerous studies have demonstrated the role of
SFN in preventing tumors, inhibiting tumors and sensitizing
anticancer drugs in colorectal tumors. If SFN could be used to
prevent CRC and promote its early commercialization, patients with
cancer and high-risk groups could be better protected. In summary,
phytochemicals may aid to prevent CRC.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant no. 81372681).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors contributions
QH wrote the main manuscript and performed the
experiments. MW and QH designed the study. MW, QH, CZ and WWZ
performed data analysis. MW, YML, NXS, CL and FL contributed to the
manuscript revisions and revised it critically for important
intellectual content. All authors reviewed the manuscript, and read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Peery AF, Crockett SD, Barritt AS, Dellon
ES, Eluri S, Gangarosa LM, Jensen ET, Lund JL, Pasricha S, Runge T,
et al: Burden of gastrointestinal, liver, and pancreatic diseases
in the United States. Gastroenterology. 149:1731–1741.3. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Islami F, Goding Sauer A, Miller KD,
Siegel RL, Fedewa SA, Jacobs EJ, McCullough ML, Patel AV, Ma J,
Soerjomataram I, et al: Proportion and number of cancer cases and
deaths attributable to potentially modifiable risk factors in the
United States. CA Cancer J Clin. 68:31–54. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wilson LF, Antonsson A, Green AC, Jordan
SJ, Kendall BJ, Nagle CM, Neale RE, Olsen CM, Webb PM and Whiteman
DC: How many cancer cases and deaths are potentially preventable?
Estimates for Australia in 2013. Int J Cancer. 142:691–701. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brown KF, Rumgay H, Dunlop C, Ryan M,
Quartly F, Cox A, Deas A, Elliss-Brookes L, Gavin A, Hounsome L, et
al: The fraction of cancer attributable to modifiable risk factors
in England, Wales, Scotland, Northern Ireland, and the United
Kingdom in 2015. Br J Cancer. 118:1130–1141. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
O'Keefe SJ: Diet, microorganisms and their
metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol.
13:691–706. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Turner ND and Lloyd SK: Association
between red meat consumption and colon cancer: A systematic review
of experimental results. Exp Biol Med (Maywood). 242:813–839. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Diallo A, Deschasaux M, Latino-Martel P,
Hercberg S, Galan P, Fassier P, Allès B, Guéraud F, Pierre FH and
Touvier M: Red and processed meat intake and cancer risk: Results
from the prospective NutriNet-Sante cohort study. Int J Cancer.
142:230–237. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Klusek J, Nasierowska-Guttmejer A, Kowalik
A, Wawrzycka I, Chrapek M, Lewitowicz P, Radowicz-Chil A, Klusek J
and Głuszek S: The influence of red meat on colorectal cancer
occurrence is dependent on the genetic polymorphisms of
s-glutathione transferase genes. Nutrients. 11(pii): E16822019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Demeyer D, Mertens B, De Smet S and Ulens
M: Mechanisms linking colorectal cancer to the consumption of
(processed) red meat: A review. Crit Rev Food Sci Nutr.
56:2747–2766. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abid Z, Cross AJ and Sinha R: Meat, dairy,
and cancer. Am J Clin Nutr. 100 (Suppl 1):386S–393S. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Etemadi A, Abnet CC, Graubard BI,
Beane-Freeman L, Freedman ND, Liao L, Dawsey SM and Sinha R:
Anatomical subsite can modify the association between meat and meat
compounds and risk of colorectal adenocarcinoma: Findings from
three large US cohorts. Int J Cancer. 143:2261–2270. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cai T, Yao L and Turesky RJ: Bioactivation
of heterocyclic aromatic Amines by UDP glucuronosyltransferases.
Chem Res Toxicol. 29:879–891. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
van der Logt EM, Bergevoet SM, Roelofs HM,
van Hooijdonk Z, te Morsche RH, Wobbes T, de Kok JB, Nagengast FM
and Peters WH: Genetic polymorphisms in
UDP-glucuronosyltransferases and glutathione S-transferases and
colorectal cancer risk. Carcinogenesis. 25:2407–2415. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu M, Wang Q, Liu F, Cheng X, Wu X, Wang
H, Wu M, Ma Y, Wang G and Hao H: UDP-glucuronosyltransferase 1A
compromises intracellular accumulation and anti-cancer effect of
tanshinone IIA in human colon cancer cells. PLoS One. 8:e791722013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang M, Qi YY, Chen S, Sun DF, Wang S,
Chen J, Li YQ, Han W and Yang XY: Expression of
UDP-glucuronosyltransferase 1A, nuclear factor erythroid-E2-related
factor 2 and Kelch-like ECH-associated protein 1 in colonic mucosa,
adenoma and adenocarcinoma tissue. Oncol Lett. 4:925–930. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yin TF, Wang M, Qing Y, Lin YM and Wu D:
Research progress on chemopreventive effects of phytochemicals on
colorectal cancer and their mechanisms. World J Gastroenterol.
22:7058–7068. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vuong LD, Nguyen QN and Truong VL:
Anti-inflammatory and anti-oxidant effects of combination between
sulforaphane and acetaminophen in LPS-stimulated RAW 264.7
macrophage cells. Immunopharmacol Immunotoxicol. 41:413–419. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mazarakis N, Snibson K, Licciardi PV and
Karagiannis TC: The potential use of l-sulforaphane for the
treatment of chronic inflammatory diseases: A review of the
clinical evidence. Clin Nutr. Mar 25–2019.(Epub ahead of print).
PubMed/NCBI
|
|
20
|
Wang M, Chen S, Wang S, Sun D, Chen J, Li
Y, Han W, Yang X and Gao HQ: Effects of phytochemicals sulforaphane
on uridine diphosphate-glucuronosyltransferase expression as well
as cell-cycle arrest and apoptosis in human colon cancer Caco-2
cells. Chin J Physiol. 55:134–144. 2012.PubMed/NCBI
|
|
21
|
Wakabayashi N, Slocum SL, Skoko JJ, Shin S
and Kensler TW: When NRF2 talks, who's listening? Antioxid Redox
Signal. 13:1649–1663. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thangapandiyan S, Ramesh M, Miltonprabu S,
Hema T, Jothi GB and Nandhini V: Sulforaphane potentially
attenuates arsenic-induced nephrotoxicity via the PI3K/Akt/Nrf2
pathway in albino Wistar rats. Environ Sci Pollut Res Int.
26:12247–12263. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kalthoff S and Strassburg CP: Contribution
of human UDP-glucuronosyltransferases to the antioxidant effects of
propolis, artichoke and silymarin. Phytomedicine. 56:35–39. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ewa B and Danuta MS: Polycyclic aromatic
hydrocarbons and PAH-related DNA adducts. J Appl Genet. 58:321–330.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang L, Palliyaguru DL and Kensler TW:
Frugal chemoprevention: Targeting Nrf2 with foods rich in
sulforaphane. Semin Oncol. 43:146–153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lubelska K, Wiktorska K, Mielczarek L,
Milczarek M, Zbroińska-Bregisz I and Chilmonczyk Z: Sulforaphane
regulates NFE2L2/Nrf2-dependent xenobiotic metabolism phase ii and
phase iii enzymes differently in human colorectal cancer and
untransformed epithelial colon cells. Nutri Cancer. 68:1338–1348.
2016. View Article : Google Scholar
|
|
28
|
Vissenaekens H, Grootaert C, Rajkovic A,
Van De Wiele T and Calatayud M: The response of five intestinal
cell lines to anoxic conditions in vitro. Biol Cell. 111:232–244.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Berg KCG, Eide PW, Eilertsen IA,
Johannessen B, Bruun J, Danielsen SA, Bjørnslett M, Meza-Zepeda LA,
Eknæs M, Lind GE, et al: Multi-omics of 34 colorectal cancer cell
lines-a resource for biomedical studies. Mol Cancer. 16:1162017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cagnol S and Chambard JC: ERK and cell
death: Mechanisms of ERK-induced cell death-apoptosis, autophagy
and senescence. FEBS J. 277:2–21. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jo C, Kim S, Cho SJ, Choi KJ, Yun SM, Koh
YH, Johnson GV and Park SI: Sulforaphane induces autophagy through
ERK activation in neuronal cells. FEBS Lett. 588:3081–3088. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wong SY, Tan MG, Wong PT, Herr DR and Lai
MK: Andrographolide induces Nrf2 and heme oxygenase 1 in astrocytes
by activating p38 MAPK and ERK. J Neuroinflammation. 13:2512016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bucolo C, Drago F, Maisto R, Romano GL,
D'Agata V, Maugeri G and Giunta S: Curcumin prevents high glucose
damage in retinal pigment epithelial cells through ERK1/2-mediated
activation of the Nrf2/HO-1 pathway. J Cell Physiol.
234:17295–17304. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Svehlikova V, Wang S, Jakubikova J,
Williamson G, Mithen R and Bao Y: Interactions between sulforaphane
and apigenin in the induction of UGT1A1 and GSTA1 in CaCo-2 cells.
Carcinogenesis. 25:1629–1637. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Thangapandiyan S, Ramesh M, Hema T,
Miltonprabu S, Uddin MS, Nandhini V and Bavithra Jothi G:
Sulforaphane potentially ameliorates arsenic induced hepatotoxicity
in albino wistar rats: Implication of PI3K/Akt/Nrf2 signaling
pathway. Cell Physiol Biochem. 52:1203–1222. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jo GH, Kim GY, Kim WJ, Park KY and Choi
YH: Sulforaphane induces apoptosis in T24 human urinary bladder
cancer cells through a reactive oxygen species-mediated
mitochondrial pathway: The involvement of endoplasmic reticulum
stress and the Nrf2 signaling pathway. Int J Oncol. 45:1497–1506.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xin Y, Bai Y, Jiang X, Zhou S, Wang Y,
Wintergerst KA, Cui T, Ji H, Tan Y and Cai L: Sulforaphane prevents
angiotensin II-induced cardiomyopathy by activation of Nrf2 via
stimulating the Akt/GSK-3ß/Fyn pathway. Redox Biol. 15:405–417.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chikara S, Nagaprashantha LD, Singhal J,
Horne D, Awasthi S and Singhal SS: Oxidative stress and dietary
phytochemicals: Role in cancer chemoprevention and treatment.
Cancer Lett. 413:122–134. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Miao Z, Yu F, Ren Y and Yang J:
D,L-Sulforaphane induces ROS-dependent apoptosis in human
gliomablastoma cells by inactivating STAT3 signaling pathway. Int J
Mol Sci. 18(pii): E722017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Subramani R, Gonzalez E, Arumugam A, Nandy
S, Gonzalez V, Medel J, Camacho F, Ortega A, Bonkoungou S, Narayan
M, et al: Nimbolide inhibits pancreatic cancer growth and
metastasis through ROS-mediated apoptosis and inhibition of
epithelial-to-mesenchymal transition. Sci Rep. 6:198192016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pocasap P, Weerapreeyakul N and Thumanu K:
Alyssin and iberin in cruciferous vegetables exert anticancer
activity in HepG2 by increasing intracellular reactive oxygen
species and tubulin depolymerization. Biomol Ther (Seoul).
27:540–552. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jin CY, Molagoda IMN, Karunarathne WAHM,
Kang SH, Park C, Kim GY and Choi YH: TRAIL attenuates
sulforaphane-mediated Nrf2 and sustains ROS generation, leading to
apoptosis of TRAIL-resistant human bladder cancer cells. Toxicol
Appl Pharmacol. 352:132–141. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Okonkwo A, Mitra J, Johnson GS, Li L,
Dashwood WM, Hegde ML, Yue C, Dashwood RH and Rajendran P:
Heterocyclic analogs of sulforaphane trigger DNA damage and impede
DNA repair in colon cancer cells: Interplay of HATs and HDACs. Mol
Nutr Food Res. 62:e18002282018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen L, Chan LS, Lung HL, Yip TTC, Ngan
RKC, Wong JWC, Lo KW, Ng WT, Lee AWM, Tsao GSW, et al: Crucifera
sulforaphane (SFN) inhibits the growth of nasopharyngeal carcinoma
through DNA methyltransferase 1 (DNMT1)/Wnt inhibitory factor 1
(WIF1) axis. Phytomedicine. 63:1530582019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Erzinger MM, Bovet C, Hecht KM, Senger S,
Winiker P, Sobotzki N, Cristea S, Beerenwinkel N, Shay JW, Marra G,
et al: Sulforaphane Preconditioning Sensitizes Human Colon Cancer
Cells towards the Bioreductive Anticancer Prodrug PR-104A. PLoS
One. 11:e01502192016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Erzinger MM and Sturla SJ:
Bioreduction-mediated food-drug interactions: Opportunities for
oncology nutrition. Chimia (Aarau). 65:411–415. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Milczarek M, Mielczarek L, Lubelska K,
Dąbrowska A, Chilmonczyk Z, Matosiuk D and Wiktorska K: In vitro
evaluation of sulforaphane and a natural analog as potent inducers
of 5-fluorouracil anticancer activity. Molecules. 23(pii):
E30402018. View Article : Google Scholar : PubMed/NCBI
|