Introduction
Ovarian cancer is associated with the highest
mortality rate among all types of gynecological cancers worldwide
(1). The surgical removal of the
tumor followed by platinum-based chemotherapy are the standard
methods employed for the treatment of the disease (2,3).
Despite these intense surgical and chemotherapeutic treatments, the
recurrence of ovarian cancer is a frequent event (4). Reasonably, progesterone receptor
membrane component (PGRMC)1, which is detected at relatively high
levels in all ovarian tumors and cell lines and exhibits a high
expression in the more advanced stages of ovarian cancer, is
considered responsible for the development and chemoresistance of
ovarian cancer (5,6). Mechanistically, PGRMC1 overexpression
in ovarian cancer cells simultaneously promotes cell survival by
enhancing epithelial growth factor receptor (EGFR) stabilization
(7), and accelerates drug efflux by
inducing cytochrome p450 activation (8). Moreover, PGRMC1 promotes substrate
recycling to help tumor cell survival (9). In fact, PGRMC1 ligands have exhibited
considerable potential in inhibiting tumor invasion and cancer
progression in animal models of cancer (10). Thus, molecules with the capacity of
PGRMC1 inhibition may prove to be beneficial for the treatment of
ovarian cancer.
Flavonoids, the most abundant polyphenols in our
daily diet, have been revealed to possess extensive pharmacological
properties in vivo and in vitro (11). Oroxylin A is a flavonoid isolated
from the root of Scutellaria baicalensis and displays
multiple pharmacological activities, including anti-inflammatory,
anti-viral, antioxidative and antitumor properties (12–14).
Oroxylin A has been previously demonstrated as a competitive
candidate of novel anticancer drugs in certain types of cancers
e.g., breast cancer, glioma, hepatoma, leukemia and colorectal
cancer, although not in ovarian cancer. The mechanisms underlying
the anticancer effects of Oroxylin A vary, including the induction
of cell cycle arrest, the inhibition of metastasis, the induction
of apoptosis, as well as other mechanisms (15,16).
Furthermore, Oroxylin A modulates several signaling pathways,
including nuclear factor-κB (17),
hypoxia-inducible factor-1α/hedgehog (18), the ERK/GSK3β (19) pathways as well as numerous others.
Although Oroxylin A has the potential to dynamically manipulate
signaling pathways, the direct target of the molecule is relatively
unknown. This was until Hui et al (20) observed an interaction between
Oroxylin A and peroxisome proliferator-activated receptor gamma
(PPARγ) in leukemia cells. PPARγ is a ligand-activated
transcription factor of the nuclear receptor superfamily (21,22)
and is related to immune response, inflammation and the
pathogenesis of certain disorders, including obesity,
atherosclerosis, and cancer (23).
Recent studies have focused on the effects of PPARγ ligands
functioning as anticancer agents. There has been a substantial
accumulation of experimental data suggesting that PPARγ ligands
induce the apoptosis of several types of cancer cells (24). As regards ovarian cancer, limited
studies have suggested that PPARγ activation may inhibit the
proliferation and induce the apoptosis of ovarian cancer cells by
modulating multiple pathways (25,26).
Oroxylin A has been revealed to function as an agonist of PPARγ
successfully in leukemia cells, macrophages and endothelial cells
(20,27,28);
however, whether it can also activate PPARγ and induce inhibitory
effects on ovarian cancer cells remains to be determined.
Since the role of PGRMC1 and PPARγ in cell survival
and death in ovarian cancer is not yet fully known, and there is an
urgent need for the development of novel alternative drugs for the
increasing chemoresistance of the tumor, the present study focused
on the effects of Oroxylin A on the proliferation, migration and
apoptosis of ovarian cancer cells. In addition, the detailed
mechanisms involved, including PPARγ and the PGRMC1/2 family were
thoroughly investigated.
Materials and methods
Cells, transfection and reagents
The SKOV-3 ovarian cancer cell line and immortalized
normal ovarian surface epithelial cell line IOSE80 were purchased
from the American Type Culture Collection (ATCC). SKOV-3 or IOSE80
cells were maintained in Dulbecco's modified Eagle's medium (DMEM)
(Invitrogen; Thermo Fisher Scientific, Inc.) with 10% (v/v) fetal
calf serum (FCS; Gibco; Thermo Fisher Scientific, Inc.), 100 IU/ml
penicillin and 100 ng/ml streptomycin (PAA Laboratories GmbH) in a
37°C, 5% CO2 humidified atmosphere. Small interfering
RNAs for PPARγ knockdown were purchased from Shanghai GenePharma
Co., Ltd. and an optimized siRNA for PPARγ was determined by
immunoblotting. The sequences of a scrambled siRNA were
5′-ACGCGUAACGCGGGAAUUUdTdT-3′ (sense) and
5′-AAAUUCCCGCGUUACGCGUdTdT-3′ (antisense), and that of PPARγ siRNA
were: 5′-UCCAUAAAGUCACCAAAAGGCdTdT-3′ (sense)
5′-CUUUUGGUGACUUUAUGGAGCdTdT-3′ (antisense). siRNA transfections
into SKOV-3 cells were performed using Lipofectamine 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Oroxylin A (MW: 284.26, HPLC ≥98%) was
a product of Sigma-Aldrich; Merck KGaA. Oroxylin A was dissolved in
aqueous dimethyl sulfoxide (DMSO) and delivered to the cells in
media containing this solvent at a final concentration of <0.1%
(v/v).
MTT cell viability assay
The viability of the SKOV-3 or IOSE80 cells were
determined by methyl thiazolyl tetrazolium (MTT) assays according
to the standard protocol. Briefly, SKOV-3 or IOSE80 cells were
plated at a density of 2,000 cells/well with 100 µl of medium in
96-well plates with increasing concentrations of Oroxylin A (0, 20,
50, 100, 200, 400 and 800 µM, dissolved in DMSO). Following
incubation for 24, 48, 72 or 96 h, 15 µl MTT (1 µg/µl) reagent were
added into the medium. A total of 200 µl DMSO was added to each
well to dissolve the formazan product. The optical density (OD)
value was examined by a microplate spectrophotometer (Bio-Rad) at
the wavelength of 560 nm.
Wound healing assay
The SKOV-3 ovarian cancer cells at 75% confluency in
12-well plates supplemented with DMEM containing 0.5% FCS was
scratched using a sterilized 10-µl pipette tip, washed and then
subjected to the vehicle or Oroxylin A (20 µM), respectively. The
migration of the SKOV-3 cells into the wound was imaged and
assessed at 0, 24 and 48 h after treatment. The images were
analyzed by Image Pro-Plus version 6.0 (Media Cybernetics, Inc.)
software. The results are representative of three independent
experiments.
Cell invasion assay
The invasion of the SKOV-3 cells through 8-µm pores
was examined using a Matrigel-coated Transwell cell culture chamber
(Corning Costar; Corning Inc.). The lower chamber was filled with
DMEM containing 10% FCS. Cells in a total volume of 100 µl at a
density of 2×105 cells/ml were seeded onto the upper
chamber with the culture containing Oroxylin A at a dosage of 20
µM. Chambers were incubated at 37°C, 5% CO2 humidified
atmosphere for 24 or 48 h. The remaining cells on the top surface
of the membrane were removed using a cotton swab followed by three
washes with PBS. The cells that migrated to the bottom surface of
the chamber were stained with 0.5% crystal violet at room
temperature for 20 min, and quantified by counting 5 fields on the
membrane under a 20X objective.
Cell apoptosis assay
The percentage of ovarian cancer cells actively
undergoing apoptosis was determined by flow cytometry using an
Annexin V/PI assay kit according to the manufacturer's instructions
as previously described (29).
Briefly, SKOV-3 cells were incubated with increasing concentrations
of Oroxylin A (0, 20, 50, 100, 200 and 400 µM) for 48 h, and the
cells were then digested with trypsin and harvested. After washing
with PBS, the number of cells were counted. For each group, a total
of 1×105 cells were resuspended in binding buffer at a
concentration of 1×106 cells/ml. Subsequently, 10 µl
Annexin V and 5 µl propidium iodide (PI) were added to the mixture,
and the cells were incubated at room temperature for ≥15 min in the
dark. Following incubation, the percentage of apoptotic cells was
analyzed by flow cytometry (FACScan; BD Biosciences). For the PPARγ
knockdown assay, SKOV-3 cells transfected with PPARγ siRNA or
scramble siRNA were subjected to treatment with Oroxylin A (100 µM)
for 24 h, and the cells were then harvested. This was followed by
the treatments as aforementioned.
Quantitative PCR
Quantitative PCR was employed to characterize the
expression of PPARγ, PGRMC1 and PGRMC2 in SKOV-3 cells. Total RNA
was isolated from the SKOV-3 cells using the RNeasy Plus Mini kit
(Qiagen, Inc.). Total RNA extracts were treated with DNase I
(Invitrogen; Thermo Fisher Scientific, Inc.) prior to reverse
transcription to avoid DNA contamination, which could lead to
false-positive results. cDNA was synthesized by incubating 1 µg of
RNA with oligo(dT) and Muloney murine leukemia virus reverse
transcriptase (Invitrogen; Thermo Fisher Scientific, Inc). Primers
for the subsequent PCRs were as follows: PPARγ,
5′-TGGAGTTCATGCTTGTGAAG-3′ and reverse, 5′-GCATTATGAGACATCCCCAC-3′
(168 bp); PGRMC1 forward, 5′-GACCAAAGGCCGCAAATTCT-3′ and reverse,
5′-CAGTGCTTCCTTATCCAGGCA-3′ (106 bp); PGRMC2 forward,
5′-AGGGGAAGAACCGTCAGAAT-3′ and reverse, 5′-AAGCCCCACCAGACATTACA-3′
(283 bp); GAPDH forward, 5′-CACCCACTCCTCCACCTTTG-3′ and reverse,
5′-CCACCACCCTGTTGCTGTAG-3′ (110 bp). The reaction times were as
follows: 1 min at 94°C then 35 cycles of 30 sec at 94°C, 30 sec at
60°C and 60 sec at 72°C, and finally 10 min at 72°C. Subsequently,
the relative level of gene expression was expressed as the ratio of
the target gene mean value to the geometric mean value of the
reference gene (2−ΔΔCq) (30).
Flow cytometric analysis
To detect the expression of PPARγ, PGRMC1 or PGRMC2
in ovarian cancer cells, 1×106 cells were harvested and
suspended in cold PBS. After fixing with 80% methanol (5 min) and
permeabilizing with 0.1% PBS-Tween for 20 min, the cells were
incubated with 1X PBS/10% normal goat serum/0.3 M glycine to block
non-specific protein-protein interactions followed by PPARγ mAb
(1:100 dilution; sc-166731), PGRMC1 mAb (1:100 dilution; sc-135720)
or PGRMC2 mAb (1:50 dilution; sc-100904; Santa Cruz Biotechnology,
Inc.) for 30 min at room temperature. The secondary antibody used
was Alexa Fluor® 488 goat anti-mouse IgG (H+L) (product
no. ab150117; Abcam, Inc.) at a dilution of 1:2,000 for 30 min at
room temperature. The acquisition of data for <10,000 events
were collected and analyzed with the FACScan flow cytometer (BD
Biosciences).
Western blot analysis and
antibodies
Western blot analysis was performed as previously
described (29,31). Anti-PGRMC1 (1:1,000 dilution; cat.
no. ab224056) rabbit polyclonal antibody and anti-PGRMC2 (1:1,000
dilution; cat. no. ab241302) rabbit polyclonal antibody were
purchased from Abcam Inc. Anti-GAPDH (1:2,000 dilution; cat. no.
sc-32233) mouse mAb, and horseradish peroxidase (HRP)-conjugated
goat anti-mouse (1:4,000 dilution; cat. no. sc-2004) and
anti-rabbit IgG (1:4,000 dilution; cat. no. sc-2004) were purchased
from Santa Cruz Biotechnology, Inc. Anti-PPARγ (1:1,000 dilution;
cat. no. 95128) mouse monoclonal antibody was purchased from Cell
Signaling Technology, Inc. Primary antibodies were incubated at 4°C
over night, and secondary antibodies were incubated at room
temperature for 1 h.
Statistical analysis
All experiments were carried out at least three
times in triplicate. Numerical data were expressed as the means ±
SD and they were analyzed using one-way ANOVA and a post-hoc
Bonferroni test (α=0.05) to compare the means of all the groups.
Two group comparisons were analyzed by a two-sided Student's
t-test. P-values were calculated using SPSS 22.0 software (IBM
Corp.) and a P-value <0.05 was considered to indicate a
statistically significant difference.
Results
Oroxylin A inhibits the proliferation
of human ovarian cancer cells
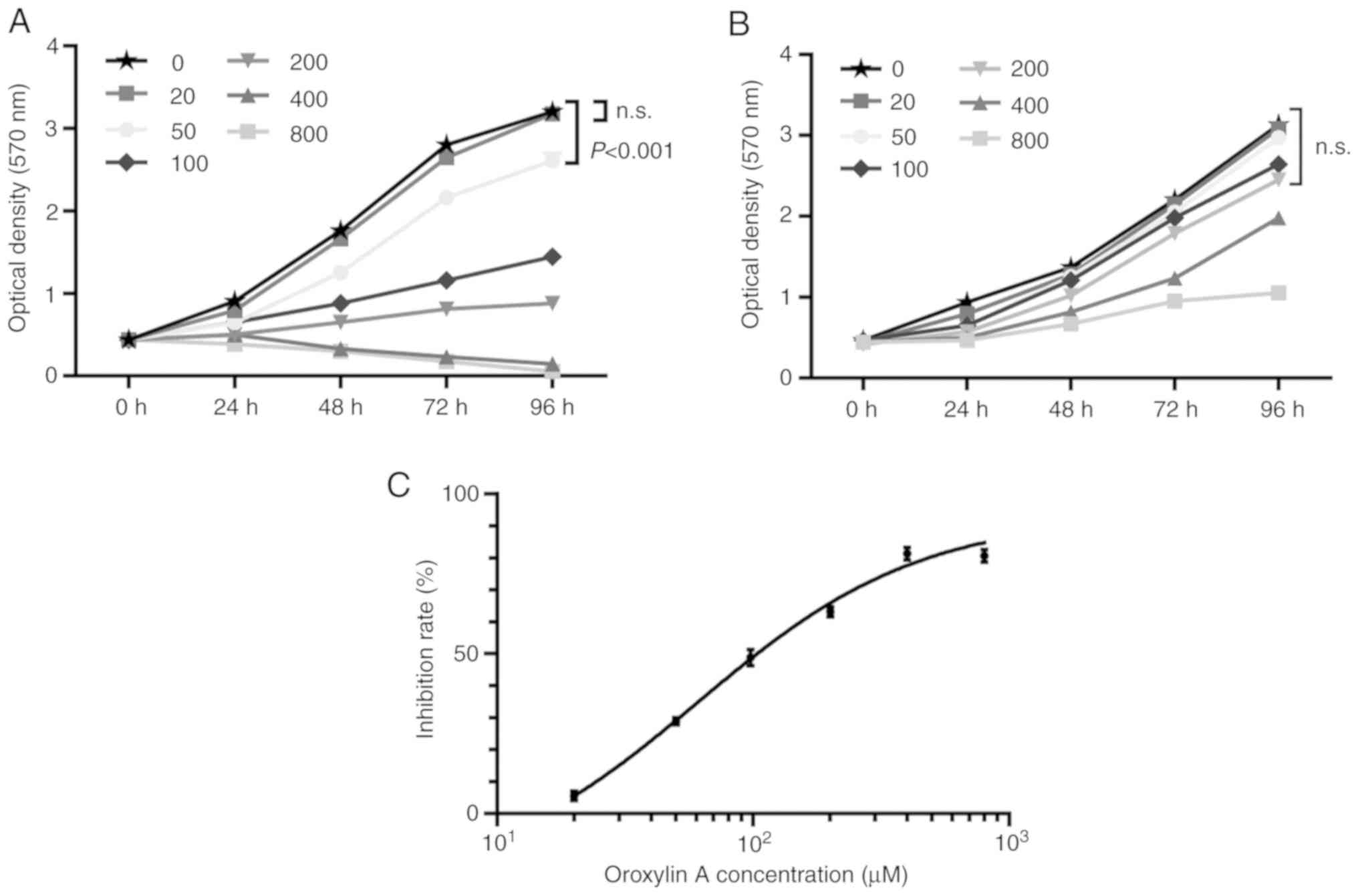
To determine the effects of Oroxylin A on the
proliferation of human ovarian cancer cells, MTT assays with a
series of drug concentrations were first carried out. Using the
SKOV-3 human ovarian cancer cell line, it was observed that
Oroxylin A dose-dependently inhibited the proliferation of these
cells. As revealed in Fig. 1A, when
the drug concentration was <20 µM, no significant inhibitory
effects of Oroxylin A on cell proliferation were observed. However,
when the drug concentration was >50 µM, proliferation of the
SKOV-3 cells decreased significantly, compared to that of the
vehicle-treated cells. Notably, when the concentration of the drug
was increased to 400 µM, Oroxylin A almost entirely abrogated the
proliferation of the ovarian cancer cell line. In addition, the 50%
inhibitory concentration (IC50) of Oroxylin A for the
treatment of the SKOV-3 cells at the time-point of 48 h was also
determined (Fig. 1C), which was
calculated as 60.85 µM (95% CI, 47.72–77.60). Moreover, a
time-dependent association of the drug with the ovarian cancer
cells was also observed. As revealed in Fig. 1A, with a concentration of 200 µM,
the inhibition rates of the SKOV-3 cells treated with Oroxylin A
for 24, 48, 72 and 96 h were 44.39±6.35, 63.91±2.46, 70.97±1.40,
and 72.53±2.95% respectively, indicating a cumulative effect of
Oroxylin A on ovarian cancer cells with time. Oroxylin A has been
revealed to possess an anticancer effect with lower toxicity to
normal cells in some cancers (32,33),
therefore, whether the drug exhibits toxicity to normal ovarian
epithelial cells was determined. As revealed in Fig. 1B, Oroxylin A had no effect on the
cell viability of IOSE80 cells when the dosage was not over 200 µM,
at which concentration cell proliferation was significantly
inhibited in SKOV-3 cells (Fig.
1A), suggesting the lower toxicity of the drug on normal
ovarian cells. These results indicated that Oroxylin A inhibited
the proliferation of and induced cytotoxicity to ovarian cancer
cells.
Oroxylin A inhibits the migration of
human ovarian cancer cells
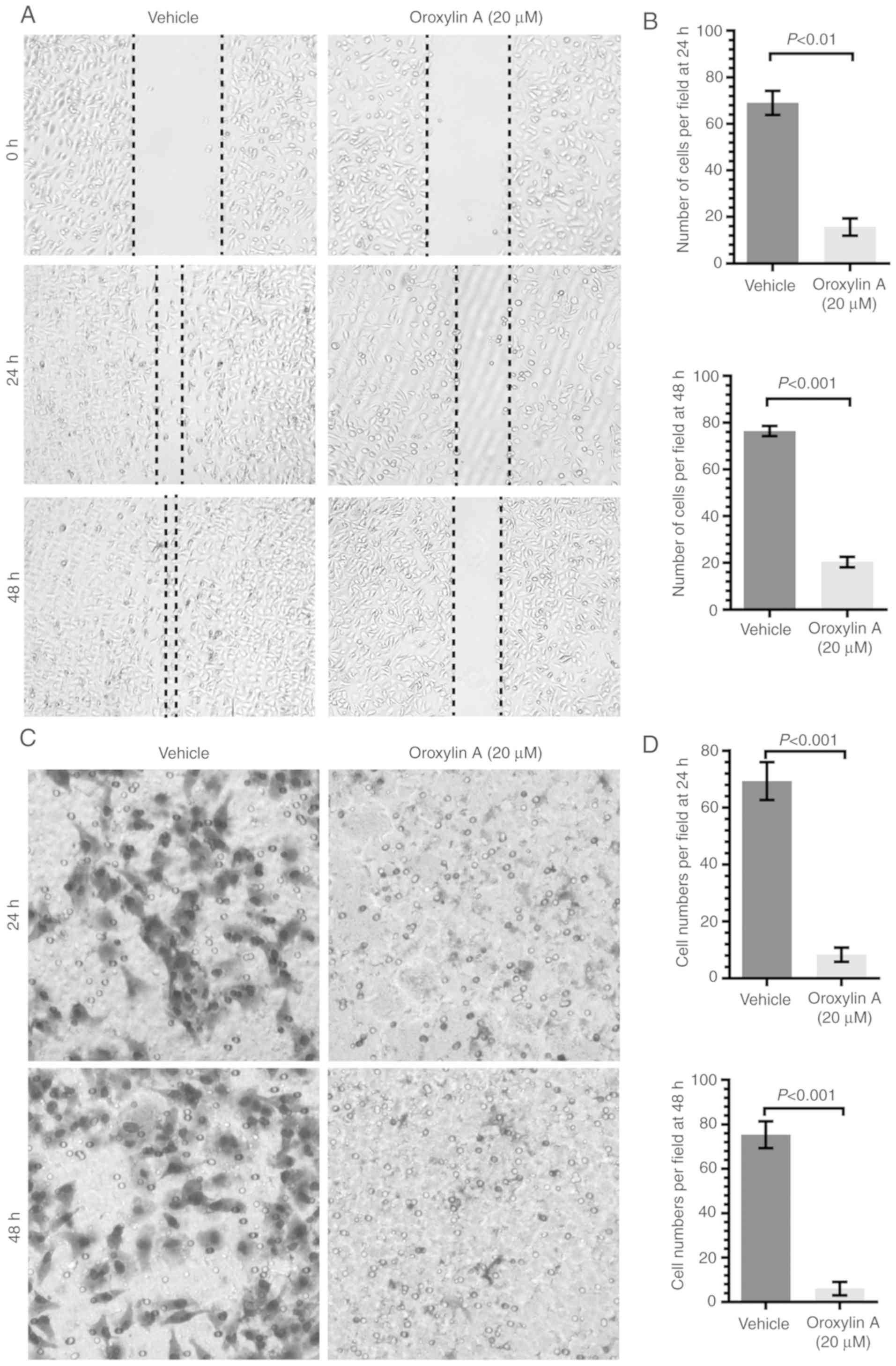
As a hallmark of cell viability, the migration of
ovarian cancer cells was also determined in this study. To avoid
the influence of the killing effect by Oroxylin A on cell
migration, the SKOV-3 cells were cultured with Oroxylin A at a
concentration of 20 µM, where no killing effect was exhibited.
Subsequently, a wound healing assay and a Transwell chamber assay
were employed to determine the migration and invasion of the SKOV-3
cells, respectively. As revealed in Fig. 2A and B, the wound closure of the
SKOV-3 cells exposed to Oroxylin A (20 µM) for 24 and 48 h was
15.67±6.45 and 19.63±2.45%, respectively, whereas that of the
vehicle-exposed cells was 69.00±5.15 and 76.40±2.18%, respectively.
Concordant results were also revealed in the Transwell chamber
assay (Fig. 2C and D). The number
of SKOV-3 cells in the lower chamber treated with Oroxylin A for 24
and 48 h was 8.33±2.52 and 6.03±3.00%, respectively, compared to
that of the vehicle group which was 69.33±6.66 and 75.33±6.00%,
respectively. These results revealed that Oroxylin A exerted a
potent anti-migratory effect on ovarian cancer cells.
Oroxylin A induces the apoptosis of
human ovarian cancer cells
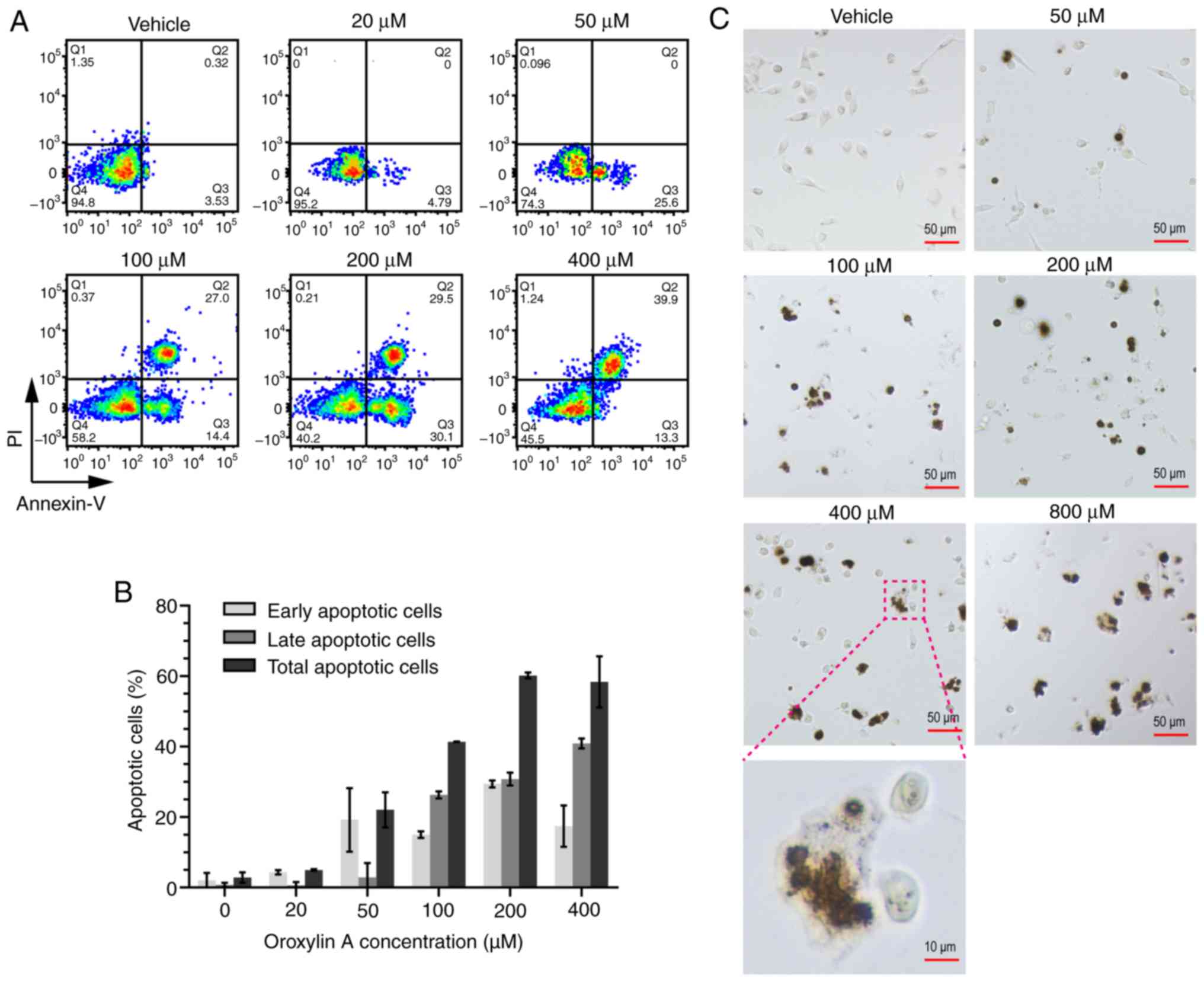
Previous studies have revealed that Oroxylin A
induces the apoptosis of various types of cells (15,34,35).
Thus, an association between apoptosis and the inhibitory effect of
Oroxylin A on ovarian cancer cells may exist. Annexin V/PI
double-staining assay was then carried out to determine the effect
of Oroxylin A on cell apoptosis. As revealed in Fig. 3A, the apoptosis of the SKOV-3 cells
was induced by Oroxylin A in a dose-dependent manner. When the
concentration was >50 µM, cell apoptosis was increased, with the
results indicating that the cells had mainly undergone early
apoptosis. However, along with the increasing drug concentration to
400 µM, the cells began to undergo late apoptosis. As revealed in
Fig. 3B, the total apoptosis of the
SKOV-3 cells exposed to Oroxylin A at concentrations of 20, 50,
100, 200, or 400 µM was 4.97±0.25, 22.08±4.99, 41.35±0.07,
60.20±0.85 and 58.35±7.28%, respectively. It is worth noting that
the morphological alterations observed in Fig. 3C revealed that the cells exhibited
cell shrinkage and chromatin condensation, also indicating the
apoptosis of the SKOV-3 cells induced by Oroxylin A. In sum, the
results of both flow cytometric assay and the morphological
alterations of the cells indicated that Oroxylin A induced the
apoptosis of human ovarian cancer cells.
PPAR gamma and PGRMC1/2 signaling are
involved in Oroxylin A-mediated anticancer activity
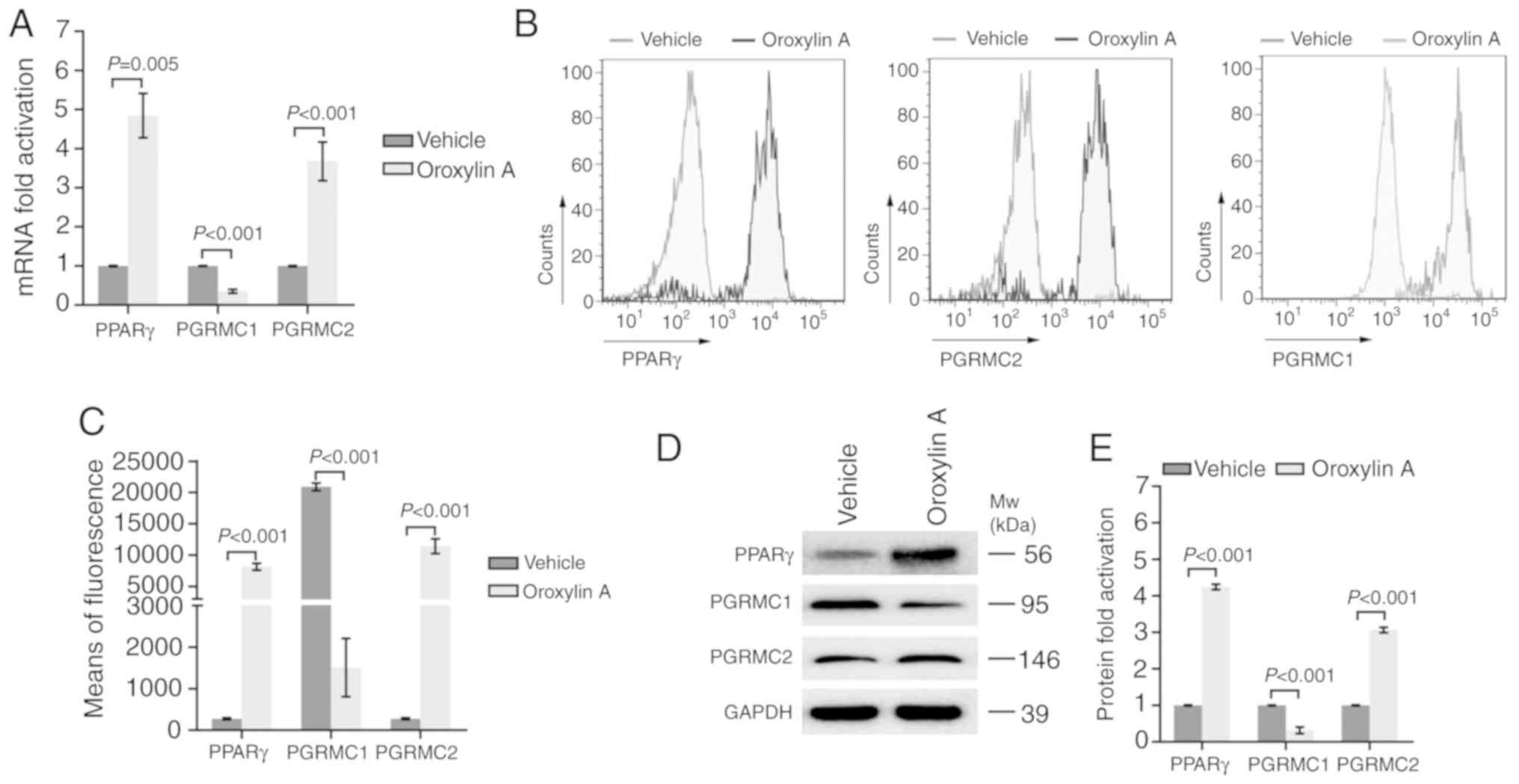
PGRMC1/2 plays a vital role in ovarian cancer
chemical resistance. PPARγ is a key factor modulating cancer
metabolism and survival. Oroxylin A has been revealed as an agonist
of PPARγ previously (20). The
present study further detected the effects of Oroxylin A on the
expression of PPARγ and the PGRMC1/2 family in ovarian cancer
cells. As revealed in Fig. 4A, the
mRNA levels of PPARγ and PGRMC2 were significantly increased in the
SKOV-3 cells treated with Oroxylin A, compared to the cells treated
with the vehicle. However, the mRNA level of PGRMC1 was markedly
decreased following treatment with Oroxylin A. To validate the
results of the mRNA levels, flow cytometry was then carried out to
determine the protein expression. In line with the expression
profiles of mRNAs, the protein levels of PPARγ and PGRMC2 were also
revealed to be increased by Oroxylin A treatment, whereas PGRMC1
expression was also reduced (Fig. 4B
and C). Concordant results were also manifested by western blot
analysis with specific antibodies (Fig.
4D and E). These results indicated that treatment with Oroxylin
A activated PPARγ and altered the expression profile of the
PGRMC1/2 family in SKOV-3 cells.
PPARγ plays a central role in Oroxylin
A-mediated anticancer activity
Considering the important role of PPARγ and the
PGRMC1/2 family in ovarian cancer, whether PPARγ activation has an
effect on PGRMC1/2 expression was determined. Subsequently, PPARγ
knockdown assays were carried out (Fig.
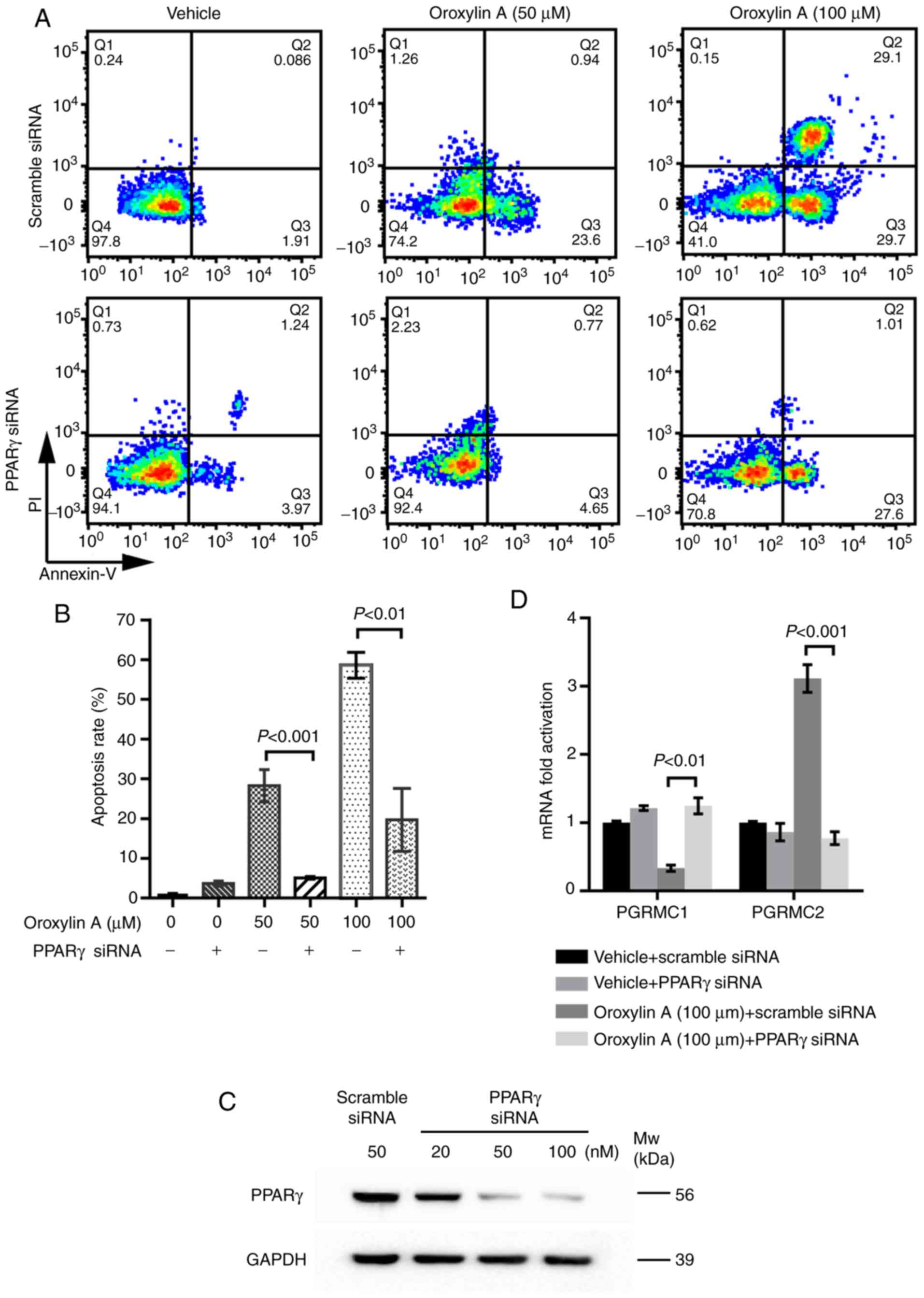
1C). As revealed in Fig. 5A and
B, PPARγ knockdown significantly attenuated the apoptosis of
the SKOV-3 induced by Oroxylin A, compared to that of the scramble
siRNA-treated SKOV-3 by Oroxylin A. These results indicated that
the absence PPARγ attenuated Oroxylin A-induced cell apoptosis and
that PPARγ plays an important role in Oroxylin A-mediated apoptotic
cell death. Then the effects of PPARγ knockdown on the expression
of the PGRMC1/2 family were further detected. As revealed in
Fig. 5D, following treatment with
Oroxylin A, PPARγ knockdown increased the expression of PGRMC1,
while that of PGRMC2 was abolished, indicating a promising
crosstalk between PPARγ and PGRMC1/2 pathways. Collectively, these
results revealed that PPARγ activation mediated PGRMC1/2 expression
and played a central role in the apoptosis of ovarian cancer cells
induced by Oroxylin A.
Discussion
Oroxylin A is a novel anticancer drug used in China.
To date, the anticancer effects of the drug have been determined in
certain types of cancer, such as glioma (36), hepatocellular carcinoma (37), human gastric carcinoma (38), human breast cancer (39), human cervical cancer (40), colorectal adenocarcinoma (41), acute myelogenous leukemia (20), as well as many others. Although
ovarian cancer has the highest mortality rate among all the types
of cancers affecting women worldwide, to the best of our knowledge,
there is no study available to date on the effects of Oroxylin A
treatment on this disease. Herein, substantial experimental
evidence was provided to indicate that Oroxylin A exerts a potent
potential cell-killing effect on ovarian cancer cells in
vitro. The present results indicated that Oroxylin A inhibited
the proliferation of ovarian cancer cells in a dose- and
time-dependent manner. Notably, even at a lower concentration of 20
µM, where no effect of the drug on cell proliferation was observed,
Oroxylin A still inhibited the migration of the cancer cells
robustly. Cell migration and invasion confer to cancer metastasis,
which is the main cause of cancer recurrence. The anti-migratory
effect of Oroxylin A against ovarian cancer cells at a lower
concentration, suggests a promising extensive usage of the agent in
the prevention and cure treatment of ovarian cancer patients.
In addition to its inhibitory effect on cell
migration, Oroxlin A markedly inhibited cell proliferation by
inducing apoptosis, which was more evident with the increasing
concentration. Furthermore, when the drug concentration did not
exceed 100 µM, early apoptotic cell death played a major role;
however, when the concentration increased, late apoptotic cell
death emerged and became dominant. The alteration of the apoptotic
pattern with the increasing drug concentration suggests that there
is an association between the apoptotic pattern and the drug
concentration in Oroxylin A-treated ovarian cancer cells. PPARγ has
been revealed to be a target of Oroxylin A in leukemia cells
(20). Herein, it was also
determined that Oroxylin A activated PPARγ in the SKOV-3 ovarian
cancer cells at both the mRNA and protein levels. This indicated
that the activation of PPARγ by Oroxylin A is receptor-specific and
is independent of the cell type. PPARγ is acknowledged as a
suppressor in various types of cancer, and multiple chemical
compounds exhibit anticancer activities by activating PPARγ
(42–44). The inhibitory effect of Oroxylin A
on ovarian cancer cells by PPARγ activation in the present study
was in line with the findings of other studies on other types of
cancer (43). In addition, PPARγ is
necessary for the effects of Oroxylin A in treatment of the ovarian
cancer cells. PPARγ silencing by specific siRNA significantly
abrogated the apoptotic cell death of the ovarian cancer cells by
Oroxylin A, indicating a central role of PPARγ in the effects of
Oroxylin A.
The PGRMC1/2 family plays crucial role in the
progression of ovarian cancer (6, 29). Both PGRMC1 and PGRMC2
belong to the membrane-associated progesterone receptor family, as
they all contain a cytochrome b5-like heme/steroid binding domain.
Currently, PGRMC1, which is highly expressed in ovarian cancer, has
been extensively characterized as a tumor promoter by facilitating
cancer proliferation and chemoresistance. PGRMC2, sharing an amino
acid identity of ~89%, is strongly homologous to PGRMC1;
contrarily, the limited available studies on PGRMC2 strongly imply
a tumor suppressive function of the protein. The congenital
contradiction of the two proteins indicates the key role of the
PGRMC1/2 family in ovarian cancer progression. In the present
study, it was first demonstrated that Oroxylin A reversed the
expression profile of PGRMC1/2 in ovarian cancer cells by
downregulating the expression of PGRMC1 and upregulating PGRMC2
expression. The present findings indicated that through the
modulation of the expression profile of the PGRMC1/2 family,
Oroxylin A may function as a candidate for the treatment of ovarian
cancer.
Since Oroxylin A can influence both PPARγ signaling
and the PGRMC1/2 family, whether a crosstalk exists between these
two signaling pathways warrants investigation. Although there is no
evidence to reveal the direct interaction between the two signaling
pathways, notably, multiple signaling pathways, including the
Wnt/β-catenin pathway (45,46) and NF-κB pathway (47,48)
have been identified to be involved in modulating both PPARγ
signaling and the PGRMC1/2 family. Considering the function of
PPARγ as a transcription factor activated by Oroxylin A, it is
possible that PPARγ may affect the expression of the PGRMC1/2
family, which is located on the cell membrane by modulating the
aforementioned pathways. As anticipated, PPARγ silencing
significantly restored Oroxylin A-induced PGRMC1 downregulation and
PGRMC2 upregulation. However, whether the aforementioned pathways
or the other mechanisms participate in the modulation of PGRMC1/2
expression by Oroxylin A warrants further investigation.
In conclusion, given the dual effect of Oroxylin A
on both PPARγ elevation and PGRMC1/2 expression profile reversal,
Oroxylin A inhibits the migration and induces apoptosis of ovarian
cancer cells. Thus, Oroxylin A may have potential for use in the
treatment of ovarian cancer.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Nature
Science Foundation of China (81574009 and 81503368), the Jiangsu
Commission of Health (Q201602) and the Taizhou Technology Support
Program (SSF20170218).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JJS, YC and WJG conceived and designed the
experiments. JJS, XFZ, JX and ZFW performed the experiments. YC and
WJG analyzed the data. XFZ, JX and YC contributed the
reagents/materials/analysis tools. JJS and YC wrote the manuscript.
All authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gonzalez-Diego P, Lopez-Abente G, Pollan M
and Ruiz M: Time trends in ovarian cancer mortality in Europe
(1955–1993): Effect of age, birth cohort and period of death. Eur J
Cancer. 36:1816–1824. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Grabowski JP and Sehouli J: Current
management of ovarian cancer. Minerva Med. 106:151–156.
2015.PubMed/NCBI
|
|
3
|
Aletti GD, Gallenberg MM, Cliby WA, Jatoi
A and Hartmann LC: Current management strategies for ovarian
cancer. Mayo Clin Proc. 82:751–770. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Salzberg M, Thurlimann B, Bonnefois H,
Fink D, Rochlitz C, von Moos R and Senn H: Current concepts of
treatment strategies in advanced or recurrent ovarian cancer.
Oncology. 68:293–298. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Peluso JJ, Gawkowska A, Liu X, Shioda T
and Pru JK: Progesterone receptor membrane component-1 regulates
the development and Cisplatin sensitivity of human ovarian tumors
in athymic nude mice. Endocrinology. 150:4846–4854. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peluso JJ, Liu X, Saunders MM, Claffey KP
and Phoenix K: Regulation of ovarian cancer cell viability and
sensitivity to cisplatin by progesterone receptor membrane
component-1. J Clin Endocrinol Metab. 93:1592–1599. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zheng Q, Li Y, Zhang D, Cui X, Dai K, Yang
Y, Liu S, Tan J and Yan Q: ANP promotes proliferation and inhibits
apoptosis of ovarian granulosa cells by NPRA/PGRMC1/EGFR complex
and improves ovary functions of PCOS rats. Cell Death Dis.
8:e31452017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ryu CS, Klein K and Zanger UM: Membrane
associated progesterone receptors: Promiscuous proteins with
pleiotropic functions-focus on interactions with cytochromes P450.
Front Pharmacol. 8:1592017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mir SU, Schwarze SR, Jin L, Zhang J,
Friend W, Miriyala S, St Clair D and Craven RJ: Progesterone
receptor membrane component 1/Sigma-2 receptor associates with
MAP1LC3B and promotes autophagy. Autophagy. 9:1566–1578. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ahmed IS, Rohe HJ, Twist KE, Mattingly MN
and Craven RJ: Progesterone receptor membrane component 1 (Pgrmc1):
A heme-1 domain protein that promotes tumorigenesis and is
inhibited by a small molecule. J Pharmacol Exp Ther. 333:564–573.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Middleton E Jr, Kandaswami C and
Theoharides TC: The effects of plant flavonoids on mammalian cells:
Implications for inflammation, heart disease, and cancer. Pharmacol
Rev. 52:673–751. 2000.PubMed/NCBI
|
|
12
|
Ji S, Li R, Wang Q, Miao WJ, Li ZW, Si LL,
Qiao X, Yu SW, Zhou DM and Ye M: Anti-H1N1 virus, cytotoxic and
Nrf2 activation activities of chemical constituents from
Scutellaria baicalensis. J Ethnopharmacol. 176:475–484. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu L, Zhao L, Wang H, Wang Y, Pan D, Yao
J, Li Z, Wu G and Guo Q: Oroxylin A reverses
P-glycoprotein-mediated multidrug resistance of MCF7/ADR cells by
G2/M arrest. Toxicol Lett. 219:107–115. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen Y, Yang L and Lee TJ: Oroxylin A
inhibition of lipopolysaccharide-induced iNOS and COX-2 gene
expression via suppression of nuclear factor-kappaB activation.
Biochem Pharmacol. 59:1445–1457. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu M, Lu N, Sun Z, Zhang H, Dai Q, Wei L,
Li Z, You Q and Guo Q: Activation of the unfolded protein response
contributed to the selective cytotoxicity of oroxylin A in human
hepatocellular carcinoma HepG2 cells. Toxicol Lett. 212:113–125.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu ZF, Sun XK, Chen G, Han C, Wang F and
Zhang YD: Oroxyloside inhibits human glioma progression by
suppressing proliferation, metastasis and inducing apoptosis
related pathways. Biomed Pharmacother. 97:1564–1574. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun X, Chang X, Wang Y, Xu B and Cao X:
Oroxylin a suppresses the cell proliferation, migration, and EMT
via NF-κB signaling pathway in human breast cancer cells. Biomed
Res Int. 2019:92417692019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wei M, Ma R, Huang S, Liao Y, Ding Y, Li
Z, Guo Q, Tan R, Zhang L and Zhao L: Oroxylin A increases the
sensitivity of temozolomide on glioma cells by hypoxia-inducible
factor 1α/hedgehog pathway under hypoxia. J Cell Physiol.
234:17392–17404. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wei L, Yao Y, Zhao K, Huang Y, Zhou Y,
Zhao L, Guo Q and Lu N: Oroxylin A inhibits invasion and migration
through suppressing ERK/GSK-3β signaling in snail-expressing
non-small-cell lung cancer cells. Mol Carcinog. 55:2121–2134. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hui H, Chen Y, Yang H, Zhao K, Wang Q,
Zhao L, Wang X, Li Z, Lu N and Guo Q: Oroxylin A has therapeutic
potential in acute myelogenous leukemia by dual effects targeting
PPARγ and RXRα. Int J Cancer. 134:1195–1206. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Evans RM: The steroid and thyroid hormone
receptor superfamily. Science. 240:889–895. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schoonjans K, Martin G, Staels B and
Auwerx J: Peroxisome proliferator-activated receptors, orphans with
ligands and functions. Curr Opin Lipidol. 8:159–166. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alarcon de la Lastra C, Sanchez-Fidalgo S,
Villegas I and Motilva V: New pharmacological perspectives and
therapeutic potential of PPAR-gamma agonists. Curr Pharm Des.
10:3505–3524. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kubota T, Koshizuka K, Williamson EA, Asou
H, Said JW, Holden S, Miyoshi I and Koeffler HP: Ligand for
peroxisome proliferator-activated receptor gamma (troglitazone) has
potent antitumor effect against human prostate cancer both in vitro
and in vivo. Cancer Res. 58:3344–3352. 1998.PubMed/NCBI
|
|
25
|
Luo S, Wang J, Ma Y, Yao Z and Pan H:
PPARγ inhibits ovarian cancer cells proliferation through
upregulation of miR-125b. Biochem Biophys Res Commun. 462:85–90.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim S, Lee JJ and Heo DS: PPARγ ligands
induce growth inhibition and apoptosis through p63 and p73 in human
ovarian cancer cells. Biochem Biophys Res Commun. 406:389–395.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang X, Sun Y, Zhao Y, Ding Y, Zhang X,
Kong L, Li Z, Guo Q and Zhao L: Oroxyloside prevents dextran
sulfate sodium-induced experimental colitis in mice by inhibiting
NF-KB pathway through PPARγ activation. Biochem Pharmacol.
106:70–81. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu X, Chen Y, Zhu W, Ji M, Xu J, Guo Y,
Gao F, Gu W, Yang X and Zhang C: Oroxylin A inhibits Kaposi's
sarcoma-associated herpes virus (KSHV) vIL-6-mediated lymphatic
reprogramming of vascular endothelial cells through modulating
PPARγ/Prox1 axis. J Med Virol. 91:463–472. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu X, Ji M, Han Y, Guo Y, Zhu W, Gao F,
Yang X and Zhang C: PGRMC1-dependent autophagy by hyperoside
induces apoptosis and sensitizes ovarian cancer cells to cisplatin
treatment. Int J Oncol. 50:835–846. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu X, Han Y, Fang Z, Wu W, Ji M, Teng F,
Zhu W, Yang X, Jia X and Zhang C: Progesterone protects ovarian
cancer cells from cisplatin-induced inhibitory effects through
progesterone receptor membrane component 1/2 as well as AKT
signaling. Oncol Rep. 30:2488–2494. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wei L, Dai Y, Zhou Y, He Z, Yao J, Zhao L,
Guo Q and Yang L: Oroxylin A activates PKM1/HNF4 alpha to induce
hepatoma differentiation and block cancer progression. Cell Death
Dis. 8:e29442017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ni T, He Z, Dai Y, Yao J, Guo Q and Wei L:
Oroxylin A suppresses the development and growth of colorectal
cancer through reprogram of HIF1α-modulated fatty acid metabolism.
Cell Death Dis. 8:e28652017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Singh J and Kakkar P: Oroxylin A, a
constituent of Oroxylum indicum inhibits adipogenesis and induces
apoptosis in 3T3-L1 cells. Phytomedicine. 21:1733–1741. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qiao C, Wei L, Dai Q, Zhou Y, Yin Q, Li Z,
Xiao Y, Guo Q and Lu N: UCP2-related mitochondrial pathway
participates in oroxylin A-induced apoptosis in human colon cancer
cells. J Cell Physiol. 230:1054–1063. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nakahata N, Kutsuwa M, Kyo R, Kubo M,
Hayashi K and Ohizumi Y: Analysis of inhibitory effects of
scutellariae radix and baicalein on prostaglandin E2 production in
rat C6 glioma cells. Am J Chin Med. 26:311–323. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hu Y, Yang Y, You QD, Liu W, Gu HY, Zhao
L, Zhang K, Wang W, Wang XT and Guo QL: Oroxylin A induced
apoptosis of human hepatocellular carcinoma cell line HepG2 was
involved in its antitumor activity. Biochem Biophys Res Commun.
351:521–527. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang Y, Hu Y, Gu HY, Lu N, Liu W, Qi Q,
Zhao L, Wang XT, You QD and Guo QL: Oroxylin A induces G2/M phase
cell-cycle arrest via inhibiting Cdk7-mediated expression of
Cdc2/p34 in human gastric carcinoma BGC-823 cells. J Pharm
Pharmacol. 60:1459–1463. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sun Y, Lu N, Ling Y, Gao Y, Chen Y, Wang
L, Hu R, Qi Q, Liu W, Yang Y, et al: Oroxylin A suppresses invasion
through down-regulating the expression of matrix
metalloproteinase-2/9 in MDA-MB-435 human breast cancer cells. Eur
J Pharmacol. 603:22–28. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li HN, Nie FF, Liu W, Dai QS, Lu N, Qi Q,
Li ZY, You QD and Guo QL: Apoptosis induction of oroxylin A in
human cervical cancer HeLa cell line in vitro and in vivo.
Toxicology. 257:80–85. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hu R, Chen N, Yao J, Zhao Q, Zhang F, Li
ZY, You QD and Guo QL: The role of Nrf2 and apoptotic signaling
pathways in oroxylin A-mediated responses in HCT-116 colorectal
adenocarcinoma cells and xenograft tumors. Anticancer Drugs.
23:651–658. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lu Y, Sun Y, Zhu J, Yu L, Jiang X, Zhang
J, Dong X, Ma B and Zhang Q: Oridonin exerts anticancer effect on
osteosarcoma by activating PPARγ and inhibiting Nrf2 pathway. Cell
Death Dis. 9:152018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cheng WY, Huynh H, Chen P, Pena-Llopis S
and Wan Y: Macrophage PPARγ inhibits Gpr132 to mediate the
anti-tumor effects of rosiglitazone. Elife. 5:e185012016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lee NJ, Oh JH, Ban JO, Shim JH, Lee HP,
Jung JK, Ahn BW, Yoon DY, Han SB, Ham YW and Hong JT:
4-O-methylhonokiol, a PPARγ agonist, inhibits prostate tumour
growth: p21-mediated suppression of NF-KB activity. Br J Pharmacol.
168:1133–1145. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kim JY, Kim SY, Choi HS, Kim MK, Lee HM,
Jang YJ and Ryu CJ: Progesterone receptor membrane component 1
suppresses the p53 and Wnt/β-catenin pathways to promote human
pluripotent stem cell self-renewal. Sci Rep. 8:30482018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Vallée A, Vallee JN, Guillevin R and
Lecarpentier Y: Interactions between the canonical WNT/Beta-catenin
pathway and PPAR gamma on neuroinflammation, demyelination, and
remyelination in multiple sclerosis. Cell Mol Neurobiol.
38:783–795. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mir SU, Jin L and Craven RJ: Neutrophil
gelatinase-associated lipocalin (NGAL) expression is dependent on
the tumor-associated sigma-2 receptor S2RPgrmc1. J Biol Chem.
287:14494–14501. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu L, Liu X, Bai Y, Tang N, Li J, Zhang
Y, Wu J, Wang X and Wei J: Neuregulin-1β modulates myogenesis in
septic mouse serum-treated C2C12 myotubes in vitro through
PPARγ/NF-KB signaling. Mol Biol Rep. 45:1611–1619. 2018. View Article : Google Scholar : PubMed/NCBI
|